Abstract
Background
Recent case reports have shown the efficacy of apremilast for the treatment of palmoplantar pustulosis (PPP). However, no study has statistically analyzed the clinical efficacy of oral apremilast in patients with PPP.
Objectives
To evaluate the effectiveness of apremilast, a phosphodiesterase 4 inhibitor, for PPP.
Materials and Methods
Among 13 patients who were diagnosed with PPP, 10 patients with PPP with either palmoplantar pustules (>1 mm diameter) or sternoclavicular joint pain were retrospectively analyzed.
Results
Palmoplantar Pustulosis Area and Severity Index (mean ± SD: baseline, 13.4 ± 9.5 vs. after treatment, 5.1 ± 5.6; P = 0.013) and the number of pustules measuring > 1 mm in diameter (3.9 ± 3.9 vs. 1.3 ± 1.9; P = 0.029) significantly improved in 2 (±1) weeks. Moreover, the Dermatology Life Quality Index (9.7 ± 7.0 vs. 3.3 ± 3.6; P = 0.009) and palmoplantar itching (visual analog scale [VAS] score) (5.6 ± 3.5 vs. 2.1 ± 2.2; P = 0.026) significantly improved in 2 weeks, whereas VAS scores of palmoplantar pain (4.8 ± 4.4 vs. 1.1 ± 2.4; P = 0.081) and sternoclavicular joint pain (3.2 ± 3.8 vs. 2.0 ± 2.6; P = 0.194) did not significantly improve. Diarrhea was observed in 60.0% of our patients.
Conclusion
Our study demonstrated that apremilast can effectively treat cutaneous manifestations and arthralgia in Japanese patients with PPP who had apparent pustules and/or clavicular‐sternocostal arthralgia. Owing to the retrospective design of the study and a small sample size, placebo‐controlled clinical trials with a larger number of patients are warranted to confirm the efficacy of apremilast for treatment of PPP.
INTRODUCTION
Palmoplantar pustulosis (PPP) is a chronic inflammatory skin disease characterized by non‐bacterial pustular eruptions accompanied by erythematous scaling on the palms and soles with frequent arthritis and reported 9.4% of patients with PPP have osteoarthritis. 1 PPP and palmoplantar pustular psoriasis are considered as distinct diseases. 2 , 3 , 4 Typically, PPP exanthema presents as pustules, erythema, and scaly appearance on the palms and soles, and most patients do not develop plaque psoriatic lesions. Although both PPP and psoriasis accompany arthritis, sternoclavicular joint arthritis is more frequent and severe in patients with PPP than that in patients with psoriasis. Recently, several studies have revealed the genetic background of PPP is different from that of psoriasis. 3 , 4 The difference in the incidence rates of PPP depending on races and ethnicity suggests the difference in genetic backgrounds may be responsible for susceptibility to PPP. 5 Co‐existence of PPP and psoriasis is known to be rare in the Japanese population; therefore, the majority of Japanese clinicians consider PPP distinct from psoriasis. 5 PPP occurs in 0.12% of the population, more frequently in women aged 30–60 years. 6 Eruptions on the palms and soles greatly deteriorate the patients’ quality of life because of pain, itching, and unfavorable appearance. PPP is a socially significant disease because of its high prevalence and serious impact on patients’ quality of life.
PPP is associated with smoking, chronic upper respiratory tract infection, and oral dysbiosis (tonsillitis, periodontitis, sinusitis, etc.). 7 , 8 , 9 , 10 , 11 Smoking and periodontitis are known to increase serum IL‐17 levels and activate neutrophils in the patient’s peripheral blood, and IL‐17 and neutrophils play a significant role in the etiology of PPP. 7 , 8 , 9 , 10 , 11 Therefore, cessation of smoking, control of chronic oral infections, and adequate oral care are considered the basis of PPP treatment.
PPP is treated with topical steroids, topical vitamin D3, and phototherapy. Oral retinoid, cyclosporine, methotrexate, and colchicine are administered in severe cases. 12 , 13 However, the efficacy of these treatments is limited and highly associated with adverse effects. In 2018, Terui et al. reported in a randomized controlled study that guselkumab, an anti‐IL‐23 monoclonal antibody, effectively treats PPP without apparent severe adverse events. 14 Guselkumab is generally used in severe cases of PPP, although it places a financial burden on patients.
Recent case reports have demonstrated the efficacy of apremilast as a treatment for PPP. 15 , 16 , 17 , 18 , 19 Apremilast, a low‐molecular‐weight oral phosphodiesterase 4 (PDE‐4) inhibitor, is used to treat psoriasis vulgaris and psoriatic arthritis. In 2017, Haebich et al. reported a single case of PPP successfully treated with apremilast. 15 In 2019, Eto et al. reported oral apremilast improved the Dermatology Life Quality Index (DLQI) scores of three patients with PPP. 16 A previous study showed that apremilast had the highest median drug survival among non‐biologic systemic agents used in the treatment of PPP. 19 In this study, cutaneous manifestations in patients with PPP were retrospectively evaluated before and after the initiation of oral apremilast using a conventional subjective method (i.e., patient self‐assessment) and an objective measure (i.e., Palmoplantar Pustulosis Area and Severity Index [PPPASI] score and pustule count).
MATERIALS AND METHODS
Participants and inclusion criteria
A total of 13 patients with PPP diagnosed in the Department of Dermatology at Aichi Medical University (Aichi, Japan) between June 12, 2017, and October 25, 2018, were enrolled in this study. Among them, the participants met the following three conditions:
Had taken oral apremilast and had undegone a second or subsequent check‐up.
Had either palmoplantar pustules (>1‐mm diameter) or sternoclavicular joint pain.
Had taken no systemic treatment 3 months prior to introduction of oral apremilast and during the follow‐up period.
Overall, 10 patients met the eligibility critical criteria. All had arthritis and/or nail lesions. Clinical information was retrospectively obtained from the patients’ hospital records. The patients’ clinical characteristics are summarized in Table 1.
Table 1.
Summary of the patients’ basic and clinical information
| Patient characteristics (N = 10) | Number of patients (%) | |
|---|---|---|
| Population/symptom | ||
| Gender | Female | 8 (80.0) |
| Male | 2 (20.0) | |
| Age, years | 51‐60 | 4 (40.0) |
| 61‐70 | 5 (50.0) | |
| 71‐80 | 1 (10.0) | |
| Median (years) | 63.7 | |
| Duration of PPP | Median (months/range) | 75/1‐288 |
| Smoker | 4 (40.0) | |
| Smoking status | Average volume (number/day) | 13.3 |
| Average duration (years) | 35 | |
| Symptoms | Arthritis | 8 (80.0) |
| Sternoclavicular joint pain | 5 (50.0) | |
| Nail lesion | 7 (70.0) | |
| Tonsillitis | 4 (40.0) | |
| Periodontitis | 3 (30.0) | |
| Having some dental metals | 7 (70.0) | |
| Metal allergy | 1 (10.0) | |
| Diabetes | 1 (10.0) | |
| Pretreatment | Topical therapy | |
| Corticosteroid | 6 (60.0) | |
| Vitamin D3 compound steroid | 3 (30.0) | |
| Vitamin D3 formulation | 1 (10.0) | |
| Oral therapy | ||
| Anti‐allergic drugs | 4 (40.0) | |
| Corticosteroid | 2 (20.0) | |
| Cyclosporine | 1 (10.0) | |
| Phototherapy | ||
| 308‐nm excimer lamps | 2 (20.0) | |
| NB‐UVB | 1 (10.0) | |
PPP, palmoplantar pustulosis; NB‐UVB, narrowband ultraviolet B.
Treatment
Apremilast was administered to all patients according to the supplier’s recommended dosing schedule. Day 1: 10 mg in the morning; Day 2: 10 mg in the morning and 10 mg in the evening; Day 3: 10 mg in the morning and 20 mg in the evening; Day 4: 20 mg in the morning and 20 mg in the evening; Day 5: 20 mg in the morning and 30 mg in the evening; and Day 6 and thereafter: 30 mg twice daily.
Ethical considerations
The participation of patients with PPP was obtained through an opt‐out methodology. 20 The study contents were explained, and our contact information was posted on the website with additional information to allow patients the opportunity to refuse participation. This study was approved by the School of Medicine Ethical Review Board of Aichi Medical University.
Endpoints
The primary outcome was PPPASI improvement. The patients’ PPPASI scores were obtained from the clinical records or the calculations from the clinical pictures. To analyze the 2‐week efficacy, the allowable period of 1–3 weeks was defined as 2 weeks. The timing of visits after the first 2 weeks varied among patients. The PPPASI was developed to help clinicians assess and measure the clinical severity of the skin condition associated with PPP. It uses the key clinical aspects of the disease to enable accurate assessment such as pustules, erythema, scaling, and palm and sole areas involvement. 21 Each item is evaluated from 0 to 6 points and is used to track treatment responses of the PPP symptoms. The PPPASI was measured by an experienced dermatologist in all patients.
The secondary outcome included the DLQI score, visual analog scale (VAS) score, and pustule count. DLQI and VAS allow participants to self‐evaluate their quality of life. VAS scores of palmoplantar itching, palmoplantar pain, and sternoclavicular joint pain were evaluated. DLQI and VAS scores were retrospectively collected from clinical records and questionnaires. The pustules measuring > 1 mm in diameter on clinical pictures were counted. Safety was assessed by physical examinations, vital signs, and adverse event evaluations.
Statistical methods
Significant differences in PPPASI, DLQI, VAS scores, and pustule counts were assessed using the paired t‐test after confirmation with the Shapiro–Wilk normality test. Results were recorded as mean and standard deviation (SD). A paired t‐test (with Bonferroni adjustment) was used to compare the data results. A survival analysis was used to assess the clinical outcomes in PPPASI and pustule count. The rate of patients achieving 50%, 75%, 90%, or greater improvement in PPPASI and pustule count were assessed by the Kaplan–Meier method.
RESULTS
Patient characteristics
The patient characteristics are shown in Table 1. The gender distribution showed a female dominance (80.0%; male: female ratio, 0.25). The majority of the patients (90%) were in their 50s and 60s, and the median age was 63.7 years. The median PPP duration was 75 months, and 40.0% of the patients were smokers. The mean smoking volume and duration were 13.3 cigarettes/day and 35 years, respectively. They also had some comorbidities: tonsillitis was observed in 40.0% of patients. Periodontitis was observed in 30.0% of patients. A total of 70.0% of patients with PPP had some dental metals, and 10.0% had a metal allergy. No patients had or have psoriasis vulgaris with the classical psoriatic plaques or family history of psoriasis. All of the patients had skin manifestations. Fifty percent (5 of the 10 patients) had only skin manifestations without sternoclavicular joint pain, and 50% (5 of the 10 patients) had both sternoclavicular joint pain and skin manifestations.
PPPASI scores
PPPASI scores at baseline and week 2 (±1) are shown in Figure 1a. One patient missed the data in week 2 (±1). The administration of oral apremilast improved PPPASI scores in 8 of 9 patients, whereas PPPASI scores were slightly exacerbated in one patient (Fig. 1a). The paired t‐test revealed that the mean (SD) PPPASI score significantly decreased within 2 (±1) weeks after the initiation of oral apremilast (baseline 13.4 ± 9.5 vs. 2 weeks later 5.1 ± 5.6; P = 0.013).
Figure 1.
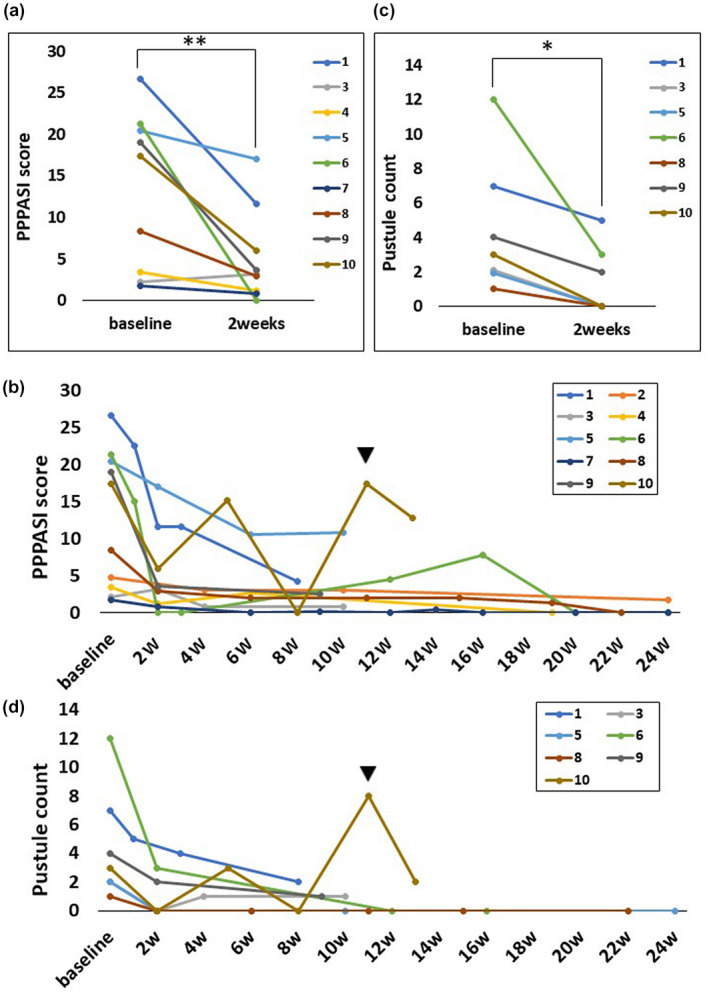
Chronological changes in Palmoplantar Pustulosis Area and Severity Index (PPPASI) score and pustule count (>1 mm) after the initiation of oral apremilast. Both PPPASI score and pustular count were significantly improved in 2 (±1) weeks. Exacerbation because of discontinuation of oral apremilast is observed in case 10 (black arrowhead); however, PPPASI score and pustule count immediately responded to resumption of oral apremilast. (a) Changes in PPPASI score at week 2 (±1) in each patient. PPPASI score improved in eight patients in 2 (±1) weeks and was slightly exacerbated in one patient. (b) The entire course of PPPASI score within 24 weeks after the initiation of apremilast. (c) Changes in the pustule count at week 2 (±1) in each patient. (d) The entire course of pustule counts within 24 weeks. Paired t‐test, *P < 0.05; **P < 0.01
The entire course of each patient’s PPPASI is shown in Figure 1b. The patients’ cutaneous manifestation was immediately relieved by oral apremilast, although transient exacerbation was occasionally observed. Exacerbation was observed 3 weeks after the discontinuation of oral apremilast in case 10, although the PPPASI score immediately responded to the resumption of oral apremilast. Clinical pictures of cases 1 and 6 are shown in Figure 2.
Figure 2.
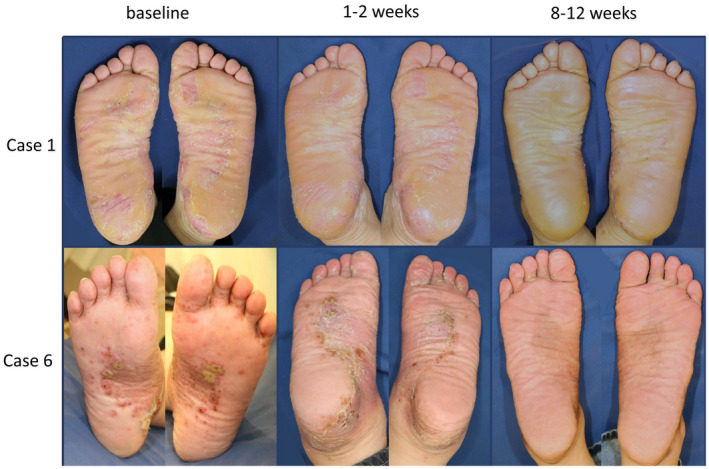
Clinical pictures of cases 1 and 6. Case 1 (upper row): baseline, 2 weeks, and 8 weeks later. Case 6 (lower row): baseline, 10 days, and 12 weeks later
Pustule count
Changes in the pustule count at week 2 (±1) are shown in Figure 1c. Pustules were observed in seven patients. Paired t‐test analysis revealed that the mean (SD) pustule count significantly decreased after 2 (±1) weeks compared with baseline (baseline 3.9 ± 3.9 vs. 2 weeks later 1.3 ± 1.9; P = 0.029).
The entire course of each patient’s pustule count is shown in Figure 1d. Exacerbation because of the discontinuation of oral apremilast was observed in Case 10, although the pustules immediately disappeared after the resumption of oral apremilast.
Kaplan–Meier analysis of PPPASI and pustular count improvements
Because the timing of the patients’ hospital visits varied widely, the PPPASI and pustular count improvement rates were evaluated by Kaplan–Meier method. A comparison of the achievement rates of 50%, 75%, and 90% improvement in PPPASI (PPPASI‐50/75/90%) and achievement rates of 50%, 75%, and 90% improvement in pustule count (PC‐50/75/90%) is shown in Figure 3a‐c.
Figure 3.
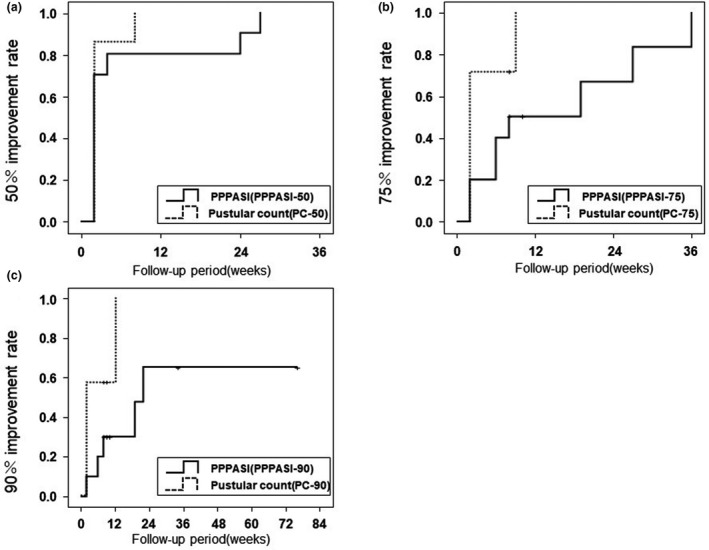
Kaplan–Meier analysis at 50%, 75%, and 90% achievement rates in PPPASI score and pustular count. Achievement rates of 50%, 75%, and 90% improvement in PPPASI scores (PPPASI‐50/75/90%) and achievement rates of 50%, 75%, and 90% improvement in pustule count (PC‐50/75/90%) are compared. Improvement in pustule count preceded improvement in PPPASI score in all three graphs. The Kaplan–Meier method only captures the first occurrence of the study endpoints not transient disease exacerbations. PPPASI, Palmoplantar Pustulosis Area and Severity Index
Kaplan–Meier method revealed that the mean treatment duration at PPPASI‐50 achievement was more than twice as long as that at PC‐50 achievement (median, 6.9 weeks; 95% confidence interval [CI], 0.8–13.0 vs. median 2.9 weeks; 95% CI, 1.2–4.5). The pustule count improvement also preceded the PPPASI improvement in the other Kaplan–Meier graphs (75% improvement [median 16.1 weeks, 95% CI, 7.4–24.7 vs. median 4.0 weeks, 95% CI, 1.4–6.6] and 90% improvement (median 35.4 weeks; 95% CI, 12.7–58.0 vs. median 6.3 weeks; 95% CI, 2.2–10.4). However, the Kaplan–Meier method only captures the first occurrence of the study endpoints not transient disease exacerbations.
DLQI scores
The mean DLQI scores at 2, 4, 12, and 24 weeks are shown in Figure 4a. One of the 10 patients was excluded because of a lack of data at 12 and 24 weeks. The mean DLQI significantly decreased at week 2 (9.7 ± 7.0 vs. 3.3 ± 3.6; P = 0.009).
Figure 4.
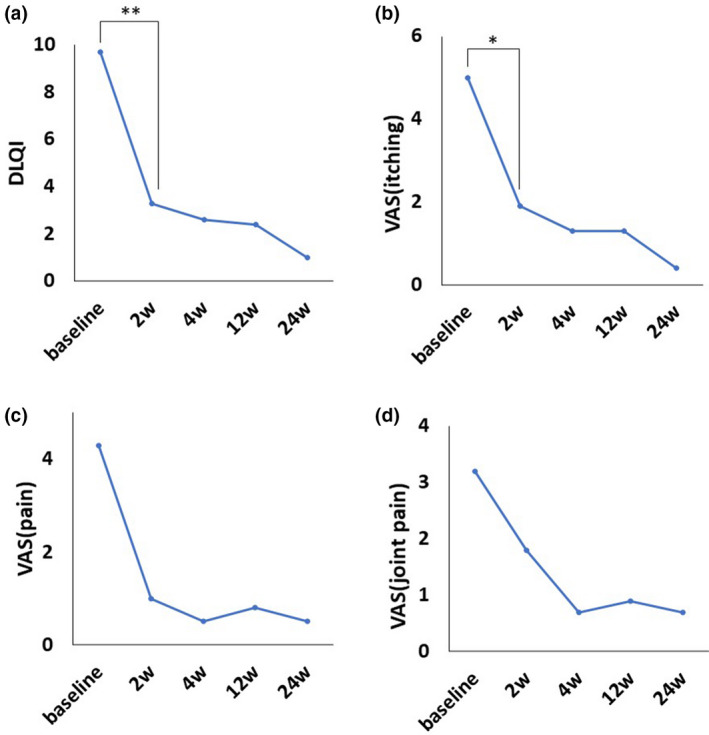
Chronological changes in subjective scores of skin manifestations. (a) Mean DLQI score significantly decreased at week 2. (b) Mean VAS score for palmoplantar itching. (c) Mean VAS score for palmoplantar pain. (d) Mean VAS score for sternoclavicular joint pain. The mean palmoplantar itching was significantly improved at week 2. The mean palmoplantar pain and mean sternoclavicular joint pain were improved from the baseline values at each timepoint; however, the changes were not significant. Sternoclavicular joint pain appeared to improve slowly compared to other cutaneous manifestations. Paired t‐test with Bonferroni adjustment: *P < 0.05, **P < 0.01. DLQI, Dermatology Life Quality Index; VAS, visual analog scale
VAS score
The mean VAS scores of palmoplantar itching, palmoplantar pain, and sternoclavicular joint pain at 2, 4, 12, and 24 weeks are shown in Figures 4b–d respectively. One of the 10 patients was excluded because of a lack of data at 12 and 24 weeks. The mean VAS score of all components decreased from the baseline score at each timepoint. The mean palmoplantar itching (baseline 5.6 ± 3.5 vs. 2 weeks later 2.1 ± 2.2; P = 0.026) was significantly improved at week 2. The mean palmoplantar pain (baseline 4.8 ± 4.4 vs. 2 weeks later 1.1 ± 2.4; P = 0.081) and mean sternoclavicular joint pain (baseline 3.6 ± 3.8 vs. 2 weeks later 2.0 ± 2.6; P = 0.194) were improved from the baseline scores at each timepoint; however, the improvement was not significant. Sternoclavicular joint pain appeared to improve slowly compared to other cutaneous manifestations.
Side effects
The frequencies of the main side effects are shown in Table 2. The main side effects were frequent bowel movements (eight patients), diarrhea (six patients), weight loss (three patients), palpitations (1 patient) and headache (1 patient).
Table 2.
Frequencies of adverse events in the 10 patients
| Adverse event | Number of patients (%) |
|---|---|
| Frequent bowel movements | 8 (80.0) |
| Diarrhea | 6 (60.0) |
| Weight loss | 3 (30.0) |
| Nausea | 1 (10.0) |
| Palpitation | 1 (10.0) |
| Headache | 1 (10.0) |
Six of 10 patients discontinued treatment or required a dose reduction because of side effects; all of them had diarrhea. Three patients discontinued the apremilast treatment, and two of them resumed oral apremilast at a reduced dose few weeks later because of recurrence (Case 4: treatment was discontinued on day 4 and resumed in week 6 with half dose; Case 10: treatment was reduced to half dose in week 3 and discontinued in week 13.) The other patient discontinued treatment in week 2 and did not resume apremilast treatment (Case 9). Three other patients required a 50% dose reduction because of diarrhea (Case 5: week 6, Case 6: week 12, Case 7: week 9). However, one of them increased back to the original dose to control itchiness at week 12 (Case 7).
DISCUSSION
To our knowledge, this is the first study statistically showing the effectiveness of apremilast for cutaneous manifestations, especially for pustules, in patients with PPP. Treatment with oral apremilast achieved significant improvements in PPPASI scores and pustule counts at week 2 (±1). The patients’ manifestations continued improving during the observation period with occasional transient exacerbation. Oral apremilast also improved the patient’s DLQI and VAS scores of itching.
In our study, 80% of patients with severe skin manifestations (PPPASI, >15; average PPPASI, 20.94) achieved PPPASI‐50 at week 16. In contrast, a previous randomized controlled trial (n = 49; 25 patients treated with guselkumab; average PPPASI, 19.1) showed 60% of patients treated with guselkumab achieved PPPASI‐50 at week 16. 14 Although this study is limited by its retrospective design and small cases, our data might suggest the non‐inferiority of oral apremilast to guselkumab in terms of immediateness and effectiveness.
Response to apremilast was more immediate in patients with PPP than in those with psoriasis. According to the previous data reported by Ohtsuki et al., oral apremilast gradually improved the cutaneous manifestations in patients with Japanese psoriasis, and the improvement continued for 3 months. 22 , 23 In comparison, our data showed that response to oral apremilast was observed within 2–4 weeks. Furthermore, improvement in the pustule count (>1 mm in diameter) preceded improvement in the PPPASI score in the Kaplan–Meier analysis. This result suggests that pustule improvement preceded the improvement in erythema and scale because PPPASI measures the patients’ pustules, erythema, and scale. Thus, we assume that patients with PPP respond faster to apremilast than those with psoriasis because apremilast immediately controls pustules.
The immediate effect of oral apremilast on the symptoms of PPP appears to be similar to that on the symptom of Behçet’s disease, in contrast to the gradual response of symptoms of psoriasis which takes several months to improve. In the recent randomized, placebo‐controlled trial, improvement of the number of oral ulcers and pain in patients with Behçet’s syndrome were already seen one week after the administration of oral apremilast. 24 We speculate that the rapid response of the symptoms of Behçet’s disease and PPP is caused by the effect of apremilast on neutrophils, because the pathogenic mechanisms of both diseases is closely related to the activation of neutrophils. It is known that neutrophils from patients with Behçet's disease show increased superoxide production, enhanced chemotaxis, and excessive production of lysosomal enzymes, indicating that the neutrophils are overactive and work in tissue injuries. 25
Evidence from molecular studies also support the effects of apremilast on pustules. Regarding neutrophils, PDE‐4 has been shown to be involved in the production of IL‐8, leukotriene B4, and superoxide anions that facilitate degranulation and chemotaxis of neutrophils. Moreover, PDE‐4 mediates the adhesion of neutrophils by inducing the expression of β2‐integrin Mac‐1, which mediates adhesion to the vascular wall endothelium. 26 , 27 Apremilast was also reported to be effective in preventing ulcer formation in the oral cavity of patients with Behçet’s disease, 24 , 28 with neutrophils playing an essential role in its pathogenesis. 29 However, because apremilast modulates a wide array of inflammatory mediators, many other processes also affect the PPP activities.
Furthermore, low‐dose apremilast in the first two weeks was effective to treat PPP in the present study. We speculate that even a low dose of apremilast might be sufficiently efficient to treat PPP via inactivation effects of apremilast on activated neutrophils, although we do not have enough evidence to support our hypothesis.
The limitation was the lack of placebo and limited sample size. To evaluate the placebo effect on our study, especially in the first two weeks, we reviewed previous randomized, double‐blind, placebo‐controlled studies using other agents. Data from the previous study comparing the effect of guselkumab to placebo injection for PPP treatment showed that use of placebo for 2 weeks did not affect each subscore of erythema, pustule/vesicle, or desquamation/scale of palmoplantar pustulosis severe index (PPSI) at the baseline and week 2. 14 Another study comparing the effect of maxacalcitol to placebo‐ointment for PPP treatment showed 2 weeks use of placebo‐ointment achieved about 16% decrease in the skin score of pustules/vesicles. 30 Because the improvement rates of PPPASI and the pustule count were 61.5% and 67.7% in our study, oral apremilast appears to decrease PPPASI and pustule counts within 2 weeks, even considering the data of placebo effect from the previous studies.
Although no severe adverse effects were observed in this study, oral apremilast for treating PPP versus psoriasis is associated with treatment adherence difficulty because of frequent diarrhea. In our study, 60.0% of patients reported having diarrhea, while 20.0% discontinued the oral apremilast. In a previous randomized controlled study of Japanese patients, 10% of patients with psoriasis who were treated with apremilast had diarrhea, and only 1.7% discontinued treatment because of diarrhea. 22 We successfully controlled both diarrhea and manifestations of PPP by reducing the apremilast dose (8.3–50% of the default dose) in four of six patients with diarrhea. Therefore, the reduced treatment dose of apremilast (e.g., start with 5 mg/day and set 30 mg/day as the target dose 2 weeks later, considering a dose increase for partial responders) may be preferred for patients with PPP.
The effectiveness of oral retinoid, cyclosporine, methotrexate, and colchicine for treatment of PPP is limited. 13 Guselkumab was recently used to treat severe PPP, but treatment with guselkumab can create a financial burden for patients. Therefore, treatment with oral apremilast, especially at a lower dose, is reasonable, as it is less expensive.
CONCLUSIONS
Our study demonstrated apremilast can effectively treat cutaneous manifestations and arthralgia in Japanese patients with PPP with apparent pustules and/or clavicular‐sternocostal arthralgia. Although more than half of the patients needed discontinuation of treatment or dose reduction owing to adverse events (frequent bowel movements and diarrhea), apremilast may be appropriate for patients without unbearable adverse events. However, a placebo‐controlled trial is necessary to confirm our findings. Besides, we recommend a reduced‐dose administration of apremilast to prevent diarrhea and minimize the patient’s financial burden.
Acknowledgments
We thank Editage (www.editage.jp) for English language editing.
Financial support: This work was supported by JSPS KAKENHI (grant number JP17K16355).
Conflict of interest: None.
REFERENCES
- 1. Sonozaki H, Kawashima M, Hongo O, et al. Incidence of arthro‐osteitis in patients with pustulosis palmaris et plantaris. Ann Rheum Dis 1981; 40: 554–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Griffiths CE, Christophers E, Barker JN, et al. A classification of psoriasis vulgaris according to phenotype. Br J Dermatol 2007; 156: 258–262. [DOI] [PubMed] [Google Scholar]
- 3. Kati A, Mahreen A, Sari S, et al. Genetic analysis of PSORS1 distinguishes guttate psoriasis and palmoplantar pustulosis. J Invest Dermatol. 2003; 120: 627–632. [DOI] [PubMed] [Google Scholar]
- 4. Huang CM, Tsai TF. Clinical characteristics, genetics, comorbidities and treatment of palmoplantar pustulosis: A retrospective analysis of 66 cases in a single center in Taiwan. J Dermatol 2020; 47(9): 1046–1049 [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto T. Clinical characteristics of Japanese patients with palmoplantar pustulosis. Clin Drug Investig 2019; 39: 241–252. [DOI] [PubMed] [Google Scholar]
- 6. Hu SC, Chen GS, Tu HP. Epidemiology of depression in patients with psoriasis: a nationwide population‐based cross‐sectional study. Acta Derm Venereol 2019; 99: 530–538. [DOI] [PubMed] [Google Scholar]
- 7. Olsson P, Skogstrand K, Nilsson A, et al. Smoking, disease characteristics and serum cytokine levels in patients with primary Sjögren’s syndrome. Rheumatol Int 2018; 38: 1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zou Y, Chen X, Liu J, et al. Serum IL‐1β and IL‐17 levels in patients with COPD: associations with clinical parameters. Int J Chron Obstruct Pulmon Dis 2017; 12: 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schenkein HA, Koertge TE, Brooks CN, et al. IL‐17 in sera from patients with aggressive periodontitis. J Dent Res 2010; 89: 943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Torii K, Saito C, Furuhashi T, et al. Tobacco smoke is related to Th17 generation with clinical implications for psoriasis patients. Exp Dermatol 2011; 20: 371–373. [DOI] [PubMed] [Google Scholar]
- 11. Murakami M, Hagforsen E, Morhenn V, et al. Patients with palmoplantar pustulosis have increased IL‐17 and IL‐22 levels both in the lesion and serum. Exp Dermatol 2011; 20: 845–847. [DOI] [PubMed] [Google Scholar]
- 12. Engin B, Aşkın Ö, Tüzün Y. Palmoplantar psoriasis. Clin Dermatol 2017; 35: 19–27. [DOI] [PubMed] [Google Scholar]
- 13. Reich K, Graff O, Mehta N. Oral alitretinoin treatment in patients with palmoplantar pustulosis inadequately responding to standard topical treatment: a randomized phase II study. Br J Dermatol 2016; 174: 1277–1281. [DOI] [PubMed] [Google Scholar]
- 14. Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti‐interleukin 23 monoclonal antibody, for palmoplantar pustulosis: A randomized clinical trial. J Dermatol 2018; 154: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haebich G, Kalavala M. Successful treatment of refractory palmoplantar pustulosis with apremilast. Clin Exp Dermatol 2017; 42: 471–473. [DOI] [PubMed] [Google Scholar]
- 16. Eto A, Nakao M, Furue M. Three cases of palmoplantar pustulosis successfully treated with apremilast. J Dermatol 2019; 46: e29–e30. [DOI] [PubMed] [Google Scholar]
- 17. Carrascosa R, Conde Montero E, de la Cueva DP. Refractory palmoplantar pustulosis succesfully treated with apremilast. Dermatol Ther. 2020; 33: e13230. [DOI] [PubMed] [Google Scholar]
- 18. Haller C, Cozzio A, von Kempis J, et al.Successful treatment of rituximab‐associated palmoplantar pustulosis with apremilast in a patient with seropositive rheumatoid arthritis. J Clin Rheumatol. 2020; May 22. [DOI] [PMC free article] [PubMed]
- 19. Kromer C, Wilsmann‐Theis D, Gerdes S, et al. Drug survival and reasons for drug discontinuation in palmoplantar pustulosis: a retrospective multicenter study. J Dtsch Dermatol Ges. 2019; 17: 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vellinga A, Cormican M, Hanahoe B, et al. Opt‐out as an acceptable method of obtaining consent in medical research: a short report. BMC Med Res Methodol 2011; 11: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhushan M, Burden AD, McElhone K, et al. Oral liarozole in the treatment of palmoplantar pustular psoriasis: a randomized, double‐blind, placebo‐controlled study. Br J Dermatol 2001; 145: 546–553. [DOI] [PubMed] [Google Scholar]
- 22. Ohtsuki M, Okubo Y, Komine M, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of Japanese patients with moderate to severe plaque psoriasis: efficacy, safety and tolerability results from a phase 2b randomized controlled trial. J Dermatol 2017; 44: 873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti‐interleukin‐23 monoclonal antibody, for the treatment of moderate to severe plaque‐type psoriasis in Japanese patients: efficacy and safety results from a phase 3, randomized, double‐blind, placebo‐controlled study. J Dermatol 2018; 45: 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hatemi G, Mahr A, Ishigatsubo Y, et al. Trial of apremilast for oral ulcers in Behçet’s syndrome. N Engl J Med 2019; 381: 1918–1928. [DOI] [PubMed] [Google Scholar]
- 25. Sakane T, Takeno M, Suzuki N, et al. Behçet’s disease. N Engl J Med. 1999; 341: 1284–1291. [DOI] [PubMed] [Google Scholar]
- 26. Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase‐4 as a therapeutic target. Drug Discov Today 2005; 10: 1503–1519. [DOI] [PubMed] [Google Scholar]
- 27. Jones NA, Boswell‐Smith V, Lever R, et al. The effect of selective phosphodiesterase isoenzyme inhibition on neutrophil function in vitro. Pulm Pharmacol Ther 2005; 18: 93–101. [DOI] [PubMed] [Google Scholar]
- 28. Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet's syndrome ‐ a phase 2, placebo‐controlled study. N Engl J Med 2015; 372: 1510–1518. [DOI] [PubMed] [Google Scholar]
- 29. Eksioglu‐Demiralp E, Direskeneli H, Kibaroglu A, et al. Neutrophil activation in Behçet’s disease. Clin Exp Rheumatol 2001; 19: S19–S24. [PubMed] [Google Scholar]
- 30. Umezawa Y, Nakagawa H, Tamaki K. Phase III clinical study of maxacalcitol ointment in patients with palmoplantar pustulosis: A randomized, double‐blind, placebo‐controlled trial. J Dermatol 2016; 43: 288–293. [DOI] [PubMed] [Google Scholar]


