Abstract
Environmental sampling in Poland and the United States and phylogenetic analyses based on 567 sequences of four genes (155 sequences of nuclear SSU rDNA, 139 of nuclear LSU rDNA, 135 of plastid‐encoded SSU rDNA, and 138 of plastid‐encoded LSU rDNA) resulted in description of the new genus Flexiglena, which has been erected by accommodating Euglena variabilis, and enriching the Discoplastis and Euglenaformis genera with five new species. Four of them have joined the Discoplastis genus, currently consisting of six representatives: D. adunca, D. angusta (=Euglena angusta), D. constricta (=Lepocinclis constricta), D. excavata (=E. excavata), D. gasterosteus (=E. gasterosteus), and D. spathirhyncha. One of them has enriched the Euglenaformis genus, currently represented by two species: Euf. chlorophoenicea (= E. chlorophoenicea) and Euf. proxima. For most studied species, the diagnostic descriptions have been emended and epitypes were designated. Furthermore, the emending of Discoplastis and Euglenaformis diagnoses was performed.
Keywords: Discoplastis, environmental sampling, Euglenaformis, euglenida, Flexiglena, new genus, nSSU and nLSU rDNA, phylogeny, taxonomical revision
Abbreviations
- BI
Bayesian inference
- ML
maximum likelihood
- nt
nucleotide
- pp
posterior probability
- rbs
rapid bootstrap
The molecular and morphological studies conducted in the 21st century have resulted in the description of two new genera of autotrophic euglenids: Discoplastis (Triemer et al. 2006) and Euglenaformis (Bennet et al. 2014). Members of both genera possess numerous small discoid chloroplasts lacking pyrenoids and metabolic cells with a flexible pellicle (diagnostic traits of both genera), which suggests their close relationship. Nevertheless, they form separate clades on a phylogenetic tree (Kim et al. 2015). Discoplastis is an early diverging lineage of the family Phacaceae and thus far contains two species (D. adunca and D. spathirhyncha). Euglenaformis, with only one species (Euf. proxima), is an early‐branching genus of the family Euglenaceae (Linton et al. 2010, Bennett et al. 2014, Kim et al. 2015). Many taxa (mainly from the Euglena and Lepocinclis) that fulfill the morphological criteria of species of Discoplastis and Euglenaformis have been described in the literature. However, they are unavailable in algal culture collections and as such had to be obtained from environmental samples. The research has been conducted without establishing laboratory cultures; a small number of cells were isolated directly from environmental samples and whole‐genome amplification (WGA) was performed, allowing further molecular research (Bennett and Triemer 2012). Isolated cells (=isolates) were morphologically analyzed and documented (photographs and video clips). This approach enabled phylogenetic and morphological analyses of isolates, necessary to identify clades present on phylogenetic trees (Lax and Simpson 2013). Such a procedure has been successfully applied to reconstruct phylogenetic relationships among species of Lepocinclis (Bennett and Triemer 2012, Łukomska‐Kowalczyk et al. 2020a) and Phacus (Łukomska‐Kowalczyk et al. 2015, Łukomska‐Kowalczyk et al. 2020b). It is applied now with an aim to (i) increase the number of taxa on phylogenetic trees that possess numerous small discoid chloroplasts lacking pyrenoids and metabolic cells; (ii) perform comparative morphological and molecular research on new strains (=isolates) together with the literature that will allow verification of morphological diagnostic features for Discoplastis and Euglenaformis and taxonomic verification, emended diagnoses, and designated epitypes for well‐distinguished taxa (species and genera).
MATERIALS AND METHODS
Sampling and morphological study
During nine seasons (2011–2019), plankton samples from 14 eutrophic water bodies located in Poland and one in the United States were collected using a plankton net with a mesh size of 10 µm (Fig. 1). Samples were screened for species diversity, and then, a number of cells (10–300) with similar morphology were isolated from the sample with a micropipette using a micromanipulator (MM‐89 Narishige) installed on a Nikon Ni‐U microscope (Nikon, Tokyo, Japan). Isolates (i.e., morphologically identical cells) were transferred through multiple drops of sterile media to clean the sample and were kept frozen at −80°C until DNA extraction.
Fig. 1.
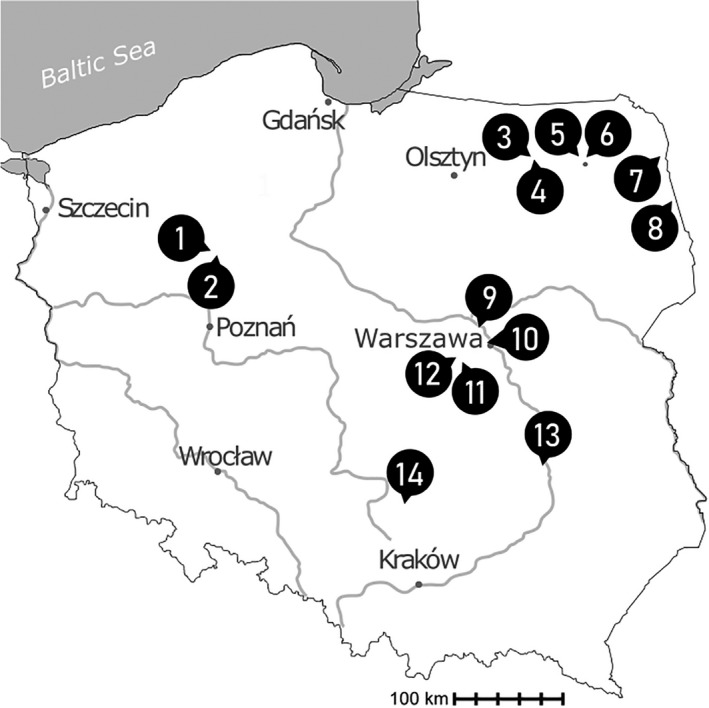
Map of sampling locations in Poland. The names of lakes or of towns, in which the studied water bodies were located, are marked with numbers: (1) Robruch; (2) Kąkolewice; (3) Urwitałt 17; (4) Urwitałt 16; (5) Woszczele; (6) Oracze; (7) Kudrynki; (8) Wojnowce; (9) Kiełpińskie Lake; (10) Warszawa, Moczydło park; (11) Izdebno Nowe; (12) Izdebno Kościelne; (13) Solec; and (14) Łysaków.
Descriptions, measurements, and photographs and video clips of isolated cells were made with a NIKON Eclipse E‐600 microscope with differential interference contrast, equipped with the software NIS Elements Br 3.1 (Nikon) for image recording and processing. Photographs and video clips were taken using a NIKON DX‐1200 digital camera connected to the microscope. The NIS Elements Br measurement program was used for morphometric studies; three parameters were measured for cells of each isolate: cell length, cell width, and tail length that was defined as the hyaline protrusion starting with the bend of curvature at the posterior part of the cell. All observations and measurements were made on live material. Data were analyzed using Statistica (version 9.0) software (StatSoft Inc., Tulsa, OK, USA); means and standard deviations are given in Table 1.
Table 1.
Comparison of morphological traits among the study’s isolates/strains of Discoplastis, Euglenaformis, and Flexiglena. Data based on Kim et al. 2010 are marked with asterisk (*)
| Taxon | Isolate/strain | Number of measured cells | Cell length (μm) Mean ± SD | Cell width (μm) Mean ± SD | Tail length (μm) Mean ± SD | Cell shape | Paramylon grains |
|---|---|---|---|---|---|---|---|
| Discoplastis adunca | CCAC 1602B | 36 | 43.1 ± 2.3 | 6.7 ± 0.9 | 7.1 ± 1.2 | Cylindrical–fusiform | Dimorphic |
| D. angusta | UW2268IK1 | 15 | 53.4 ± 4.0 | 9.3 ± 0.9 | 7.5 ± 1.1 | Narrow cylindrical | Monomorphic |
| D. constricta | UW1963Ora | 7 | 37.8 ± 2.6 | 12.6 ± 1.2 | 5.3 ± 0.5 | Hourglass‐shaped | Monomorphic |
| UW1964Woj | 1 | 36.8 | 13.2 | 4.8 | |||
| UW2111Kak | 11 | 42.3 ± 5.8 | 13.5 ± 1.2 | 4.5 ± 0.8 | |||
| UW2486INo | 3 | 35.9 ± 0.1 | 11.9 ± 0.4 | 4.7 ± 0.2 | |||
| D. excavata | UW1991Sol | 22 | 25.7 ± 1.1 | 8.4 ± 1.1 | 3.9 ± 0.5 | Trumpet‐shaped | Monomorphic |
| UW2233IK1 | 37 | 25.2 ± 2.4 | 8.3 ± 1.9 | 3.4 ± 0.4 | |||
| D. gasterosteus | UW2108Kak | 23 | 39.3 ± 3.2 | 6.8 ± 0.8 | 5.9 ± 1.1 | Cylindrical–fusiform | Monomorphic |
| UW2171Wos | 5 | 46.1 ± 0.7 | 6.2 ± 0.7 | 5.6 ± 0.6 | |||
| UW2434Rob | 1 | 41.0 | 6.6 | 5.8 | |||
| UW2483Lys | 12 | 48.0 ± 1.6 | 8.0 ± 1.1 | 5.7 ± 0.7 | |||
| D. spathirhyncha | SAG 1224‐42 | 20 | 70.0 ± 3.7 | 8.7 ± 1.2 | 9.2 ± 1.5 | ||
| UW1919Rob | 1 | 85.0 | 12.5 | 10.0 | Cylindrical–fusiform | Monomorphic | |
| UW2061UR17 | 2 | 90.0 ± 1.4 | 12.3 ± 1.1 | 12.5 ± 0.7 | |||
| UW2332IK1 | 7 | 75.8 ± 3.8 | 11.0 ± 1.2 | 9.8 ± 1.7 | |||
| Euglenaformis chlorophoenicea | MI 113 | 18 | 155.0 ± 18.2 | 24.6 ± 2.4 | Cylindrical | Monomorphic | |
| Flexiglena variabilis | Gungnamji052507K* | 48.9 ± 4.1* | 19.7 ± 1.5* | ||||
| UW1672Ora | 7 | 90.7 ± 4.7 | 12.3 ± 1.8 | Varies from short Cylindrical by club‐shaped to long fusiform–cylindrical | Dimorphic | ||
| UW1687Ur16 | 7 | 57.2 ± 6.7 | 12.6 ± 1.4 | ||||
| UW1766Gor | 9 | 88.3 ± 1.8 | 10.6 ± 1.0 | ||||
| UW1838Kud | 8 | 78.6 ± 2.4 | 14.8 ± 1.4 | ||||
| UW2078Moc | 3 | 90.7 ± 11.0 | 10.2 ± 1.0 | ||||
| UW2241JKie | 1 | 82.0 | 9.0 |
DNA isolation, amplification, and sequencing
After DNA extraction using the DNeasy Blood & Tissue Kit—Qiagen (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s protocol (with proteinase K addition), the WGA procedure was used to increase the amount of template DNA for PCRs (Bennett and Triemer 2012). For PCR amplification of four genes (nSSU rDNA, nLSU rDNA, cpSSU, and cpLSU rDNA), we used a protocol described previously in Linton et al. (2010). PCR products were sized on agarose gels and purified using the QIAEX II Gel Extraction Kit (Qiagen, Hilden, Germany) or PCR/DNA Clean‐Up Purification Kit (Eurx). Purification and sequencing of PCR products were performed by standard methods as described previously (Zakryś et al. 2002). For nSSU and cpLSU amplification, previously described primers were used (Kim et al. 2010, Łukomska‐Kowalczyk et al. 2020a); for nLSU, we used primers described in Kim et al. (2013) in combination with newly designed nested primers (Table S1 in the Supporting Information). Amplification of cpSSU rDNA was performed with primers from Milanowski et al. (2001) and newly designed primers (Table S1).
Sequence accession numbers, alignment, and sequence analyses
Eighty new sequences were submitted to GenBank with the following accession numbers: nSSU rDNA: MT591631‐MT591650; nLSU rDNA: MT591651‐MT591672; cpSSU rDNA: MT591613‐MT591630; and cpLSU rDNA: MT591593‐MT591612. See Table S2 in the Supporting Information for collection information and GenBank Accession Numbers for specimens used for phylogenetic analyses.
Sequences of four genes (155 of nSSU rDNA, 137 of nLSU rDNA, 135 of cpSSU rDNA, and 138 of cpLSU rDNA) were aligned separately using FSA 1.15.7 (Bradley et al. 2009) with default options. Alignments were inspected by eye and corrected if necessary. Regions of doubtful homology between sites were removed from the alignments using TrimAl 1.2 with the option “automated1” (Capella‐Gutierrez et al. 2009). After trimming, 1623, 713, 1034, and 1706 base pairs were left for nSSU rDNA, nLSU rDNA, cpSSU rDNA, and cpLSU rDNA, respectively. The final data set, concatenated in SeaView (Gouy et al. 2010), consisted of 5076 positions.
Sequence diversity of nSSU rDNA was calculated using the Mega X software (Kumar et al. 2018) as pair‐wise distance based only on unambiguously aligned positions. Partition homogeneity test was performed by PAUP* (Version 4.0a167).
Phylogenetic analyses
A combined data set of 567 sequences consisting of previously published sequences (487) combined with 80 new sequences representing 159 strains or isolates was generated for the phylogenetic analyses (Table S2). Sequences of one Eutreptia and two Eutreptiella species were used as outgroup taxa to root the tree (Marin et al. 2003, Linton et al. 2010, Kim et al. 2015).
The model of sequence evolution was selected with jModeltest 2.1.7 (Darriba et al. 2012), and the GTR+I+G model was chosen based on AIC, BIC, and DT criteria for each of the four genes (Lanave et al. 1984, Tavare 1986, Rodriguez et al. 1990) and was used to calculate maximum‐likelihood (ML) and Bayesian trees. The ML estimate of the phylogeny was conducted using RAxML 8.2.11 (Stamatakis 2014) employing the GTRGAMMA + I model of sequence evolution with estimated parameters with robustness inferred by rapid bootstrapping (1,000 pseudoreplicates), using four partitions with individual α‐shape parameters, GTR rates, and empirical base frequencies parameters estimated.
Bayesian inference (BI) with default priors was performed in MrBayes 3.2.6 software (Ronquist and Huelsenbeck 2003) with model parameters assigned for four partitions individually. A gamma correction with eight categories and proportion of invariable sites was used. Two independent runs with four Markov chains were performed. In each run, the chains lasted for 10,000,000 generations and trees were sampled every 1,000 generations. The first 25% of trees were discarded as burn‐in. Convergence among runs was assumed as the average standard deviation of split frequencies was below 0.01. Trees were visualized using FigTree v.1.4.2 (available at http://tree.bio.ed.ac.uk/software). The alignment and corresponding phylogenetic tree have been submitted to TreeBase (Study Accession URL: http://purl.org/phylo/treebase/phylows/study/TB2:S26514).
RESULTS
Phylogenetic analyses and morphological characteristics
The partition homogeneity test (p values equal to 0.01) showed that all four data sets could be combined. The obtained Bayesian and ML trees have very similar topology despite the position of the Euglena deses and E. adhaerens subclade in Euglena and the unresolved relationships among clades of Phacus species in both trees (see Fig. 2 and Fig. S1 in the Supporting Information). On both trees, two main clades, corresponding to families, with 12 subclades are present. In family Euglenaceae, there are eight clades (genera) congruent with trees published previously (Kim et al. 2010, 2015, Bennett and Triemer 2012). The newly obtained sequence of strain MI had a strongly supported (rbs = 98; pp = 1.0) sister position to Euglenaformis proxima, and therefore, it is identified as a representative of Euglenaformis chlorophoenica, the second species in this genus. Both species differ in cell size and shape (compare Fig. 3, x and y; 3d).
Fig. 2.
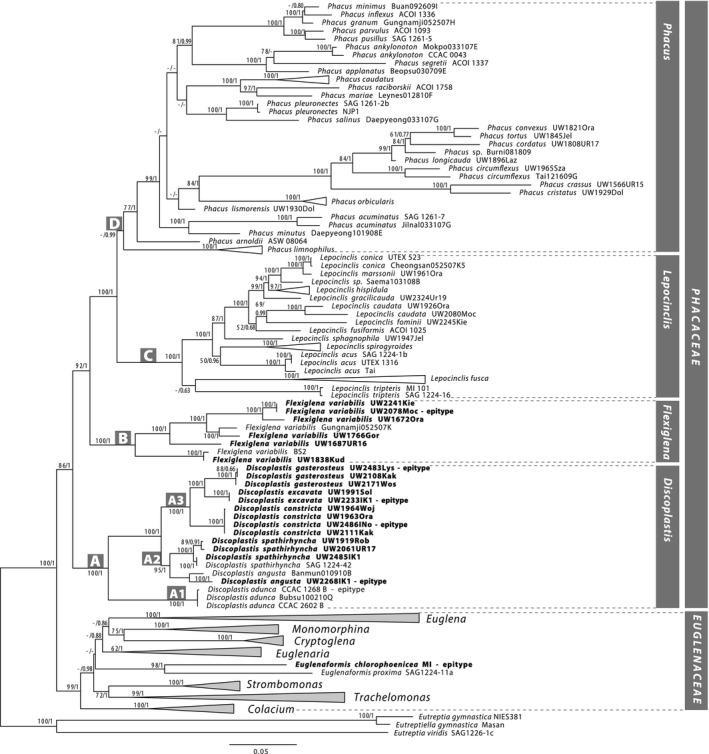
Maximum‐likelihood phylogenetic tree based on 155 of nSSU rDNA, 137 of nLSU rDNA, 135 of cpSSU rDNA, and 138 of cpLSU rDNA representing 159 strains or isolates (represented for the first time are indicated in bold type). Nodes are labeled with the rapid bootstrap (rbs) values and the Bayesian posterior probability (pp) values. The pp < 0.50, rbs values < 50%, and clades not present in the particular analysis are marked with a hyphen (‐). Scale bar represents number of substitutions per site.
Fig. 3.

Light microscopy photographs showing an overview of living cells of the Discoplastis, Euglenaformis, and Flexiglena strains (=isolates): (a‐c) cells of Flexiglena variabilis (isolate UW2078Moc) with pronounced metaboly, so that the shape varies: from fusiform–cylindrical (a) or club‐shaped (b) to short cylindrical with slightly concave sides (c); large paramylon grain visible next to the stigma (arrows); (d) fusiform cell of Euf. proxima (strain SAG 1224‐11a); (e‐g) cells of D. constricta (isolate UW2486INo) resembling an asymmetrical hourglass; (h‐j) cylindrical–fusiform, slightly flattened cells of D. adunca (strain CCAC 1602B = ASW08095) with two visibly larger paramylon grains, with one positioned in front of the nucleus, and the other behind it (arrows); (k‐n) cylindrical–fusiform cells of D. gasterosteus (isolate UW2483Lys) terminated with a sharp hyaline tail; (o, p) cylindrical cells of D. angusta (isolate UW2268IK1); (q‐s) cylindrical–fusiform cells of D. spathirhyncha (isolate UW2485IK1) terminated with a sharp hyaline tail; (s) during the euglenoid movement, the cells create a bulge in the central part, reminiscent of a “ballerina’s tutu skirt”; (t‐w) visibly S‐curved and slightly spirally twisted cells of D. excavata (isolate UW2233IK1); (x, y) metabolic, long cylindrical cells of Euf. chlorophoenicea (isolate MI), posteriorly terminated with a short tail (x) that is very commonly retracted to a blunt rounded end (y). Scale bars 10 µm.
The Phacaceae clade is divided into four clades (A, B, C and D). The earliest branching clade A (fully supported) includes most of the newly obtained sequences and five sequences published earlier belonging to species of Discoplastis (corresponding to clade K in Kim and Shin 2015, except for the sequence labeled as Discoplastis sp. Gungnamji052507K).
The earliest branching of the three subclades in clade A is maximally supported. A1 includes three samples of Discoplastis adunca, two from culture collections and one environmental isolate from South Korea (Beopsu100210Q as Discoplastis sp.). There is no genetic variability of nSSU rDNA in the samples (Table S3 in the Supporting Information). Cells of D. adunca possess dimorphic paramylon grains: two large rod‐like grains (one located in front of the nucleus and the other behind it) and numerous small grains scattered in the cytoplasm (Fig. 3, h–j).
Strongly supported subclade A2 (rbs = 95; pp = 1.0) consists of two species, Discoplastis spathirhyncha and D. angusta. Four sequences of D. spathirhyncha (one of the strain SAG 1224‐42 and three Polish isolates) form a maximally supported clade. Both species are morphologically well distinguished, with D. spathirhyncha characterized by large cells (>65 µm) with long tails (on average 10–12 µm), which during the euglenoid movement expand in the center, creating a characteristic shape similar to a “ballerina’s tutu skirt” (Fig. 3, q–s). The nSSU rDNA genetic variability in the species is 0.0% (Table S3). The other two isolates branching together in subclade A2 are the Korean species of unknown morphology (Banmun010910B) and the Polish isolate, the individuals of which are narrowly cylindrical (48–60 × 4–9 µm) and terminated with a sharp hyaline tail (on average 7.5 µm long). Based on the morphology of the Polish isolate UW2268IK1, the subclade was named D. angusta (Fig. 3, o and p). The genetic variability of nSSU rDNA does not exceed 1.5% for this species (Table S3).
Clade A3 (rbs = 100; pp = 1.0) includes three maximally supported subclades that correspond to three species easily distinguished based on cell shape: Discoplastis excavata (horn‐like; Fig. 3, t–w), D. gasterosteus (fusiform; Fig. 3, k–n), and D. constricta (hourglass; Fig. 3, e–g). All subclades consist exclusively of sequences obtained from environmental isolates from Poland. Genetic diversity of nSSU rDNA in each of the three species is 0.0% (Table S3).
Representatives of all groups in the Discoplastis clade (A) are distinguishable by cell size and shape and presence (or absence) of large paramylon grains; therefore, they are considered distinct species (see the Taxonomic revision and Table 1).
Clade B (rbs = 100; pp = 1.0) includes 8 sequences of Flexiglena variabilis obtained from environmental isolates from Poland (6 sequences), South Korea (Gungnamji052507K of unknown morphology), and an environmental sequence from South Africa (BS2 of unknown morphology). The genetic variability of nSSU rDNA does not exceed 4% for this species (Table S3). Representatives of F. variabilis are characterized by pronounced metaboly, so that the cell shape varies even when swimming, large stigma and the presence of a large paramylon grain located close to the stigma (Fig. 3, a–c). Two sister clades C and D correspond to Lepocinclis and Phacus genera.
Genetic variability
Analysis of 24 nSSU rDNA sequences from members of Flexiglena and Discoplastis revealed that interspecific genetic diversity varies between 2.9% (between D. excavata and D. constricta) and 9.52% (between D. adunca and F. variabilis; UW2078Moc and UW2241Kie). The intraspecific variation was observed only between sequences of D. angusta (1.4%) and F. variabilis (0.5–3.8%). There was no genetic diversity among the nSSU rDNA sequences of other analyzed species even when the strains or isolates originated from diverse parts of the world: D. angusta‐Polish isolate UW2268IK1 and Korean strain Banmun010910B; D. adunca‐Korean strain Bubsu100210Q and both Austrian stains; and D. spathirhyncha‐German strain SAG 1224‐42 and three Polish isolates.
Coexistence of species
The occurrence of more than one species representing the studied group was noted in several places in Poland; however, a small field pond in the Izdebno Kościelne village (see Fig. 1) proved to be the most diverse. For several years, we have noted there the continuous presence of Discoplastis excavata, D. angusta, D. spathirhyncha, and Flexiglena variabilis (no sequence on the tree for F. variabilis from this location). Discoplastis excavata exists there in very dense populations during summer; however, it can also be found in the oxbow of the Vistula River near Solec (see Fig. 1). In Poland, D. constricta, D. gasterosteus, and D. spathirhyncha occur relatively often but at low density, whereas F. variabilis is common and noted in high densities. On the other hand, D. adunca has not been found.
Taxonomic revisions
Phylogenetic and morphological analyses and a review of the literature enabled a reclassification of five taxa from Euglena and Lepocinclis into Discoplastis or Euglenaformis. As a result of these changes, Discoplastis currently contains six species: D. adunca, D. angusta (= E. angusta), D. constricta (= L. constricta), D. excavata (= E. excavata), D. gasterosteus (=E. gasterosteus), D. spathirhyncha, and the genus Euglenaformis two species: Euf. proxima and Euf. chlorophoenicea (= E. chlorophoenicea). Moreover, a new genus in the Phacaceae family‐Flexiglena‐has been described, containing one species (F. variabilis).
Given that the isolated cells were destroyed for DNA extraction, the photographs are designated as epitypes (see International Code of Nomenclature for algae, fungi, and plants [Shenzhen Code]; chapter II, section 2, article 9.9; Turland et al. 2018).
Discoplastis
Triemer in Triemer et al. 2006: 735.
Emended diagnosis
Metabolic cells of differentiated shapes (cylindrical, cylindrical–fusiform, fusiform, hourglass‐shaped, horn‐spaded) ending with a colorless, sharp tail; numerous, small, discoid chloroplasts lacking pyrenoids. Paramylon grains monomorphic (only small) or dimorphic (large and small). Periplast spirally striated.
Discoplastis adunca
(J.Schiller) Triemer in Triemer et al. 2006: 735 (Fig. 3, h‐j).
Emended diagnosis
Cells (38–52 × 5–10 µm) metabolic, cylindrical–fusiform, slightly narrowed and oblique at the front, with a lateral reservoir opening; at the posterior gradually narrowed in a wedge‐like manner and terminated with a sharp, hyaline tail (on average 7 µm); when immobile, they tend to flatten out slightly and twist (Fig. 3, i). Dimorphic paramylon grains: small (rod‐like, oval, ring‐like, or round) scattered in the cytoplasm and two rod‐like grains visibly larger than the rest (one located in front of the nucleus and the other behind it). Periplast spirally striated.
Lectotype
designated herein, Schiller 1956, fig. 57b (see Fig. S2 in the Supporting Information).
Epitype
Figure 3h designated herein that supports the lectotype (Schiller 1956, fig. 57b).
Representative DNA sequence
GenBank MT591631.
Culture representing the epitype
CCAC 1602B (formerly as ASW 08095).
Type locality
Austria, Lake Neusiedler See near Breitenbrunn.
Homotypic synonym
Euglena rostrata Schiller in Hüber‐Pestalozzi 1955: 115, figure 101 (Triemer et al. 2006).
Comments
During our research, we have noticed that the presence of two large paramylon grains, located on either side of the nucleus, is a key diagnostic trait. Meanwhile, no such information is included in the original diagnosis, despite the fact that in most of the drawings by Schiller (as Euglena rostrata in Hüber‐Pestalozzi 1955, fig. 101 [see Fig. S3 in the Supporting Information] and as E. adunca in Schiller 1956, fig. 57b [see Fig. S2] the large paramylon grains are plainly visible, which is why one of the images (fig. 57b in Schiller 1956) has been chosen as the lectotype). Simultaneously, the lectotype previously indicated by Triemer et al. 2006 (the specimen visible in fig. 57a in Schiller 1956 which does not possess the large paramylon grains) is deemed invalid by us as the typification statement does not include the phrase “designated here” (hic designated) or an equivalent (Article 7.11.). In this situation, the designation of an epitype seems justified, particularly that in the literature at least four species are listed of a very similar morphology to Discoplastis adunca (for more details, see Discussion).
Discoplastis angusta
(C.Bernard) Zakryś & M. Łukomska comb. nov. (Fig. 3, o and p).
Emended diagnosis
Cells metabolic (48–60 × 8–11 µm), narrowly cylindrical; wide in the front and oblique, with a lateral reservoir opening; gradually narrowed toward the rear and terminated with a sharp, hyaline tail (on average 7.5 µm). Monomorphic paramylon grains: rod‐like and oval. Periplast spirally striated.
Basionym
Euglena angusta C.Bernard 1908, Protococcacées et Desmidiées: 205, pl. 16, figs. 557, 558.
Lectotype
designated herein, Bernard 1908: 205, pl. 16, fig. 557 (see Fig. S4 in the Supporting information).
Epitype
Figure 3, p designated herein supports the lectotype (Bernard 1908, pl. 16, fig. 557).
Type locality
Island of Java, Buitenzorg Botanical Garden, in vases with aquatic plants.
Representative DNA sequence
GenBank MT591646.
Representative locality
Freshwater field pond in Izdebno Kościelne village (52°08'21.0'' N, 20°32'03.2'' E).
Heterotypic synonyms
Euglena pascheri D.O.Svirenko, 1915: 108, 129; pl. 2, figs. 23‐28; E. subangusta Shi 1999: 81, pl. 26, figs. 2 and 3.
Comments
Following the nomenclatural priority rule, this morphological form (cells small, metabolic, narrowly cylindrical, and terminated with a sharp hyaline tail) has been assigned the name Discoplastis angusta (=Euglena angusta). The individual seen in Bernard’s drawing (1908, pl. 16, fig. 557; see Fig. S4) corresponds with the aforementioned characteristic, similarly as the cells from the Polish population (isolate UW2268IK1), from which the epitype originates. However, due to the high morphological similarity to D. adunca, D. gasterosteus, and other taxa described in the literature, the designation of an epitype is justified (for more details, see Discussion). Euglena pascheri (see Fig. S5 in the Supporting Information) and E. subangusta have been included as D. angusta synonyms, as no diagnostic traits have been found that would distinguish the three taxa.
Discoplastis constricta
(Matvienko) Zakryś & M. Łukomska comb. nov. (Figs. 3 , e‐g).
Emended diagnosis
Cells (26–50 × 11.5–22 µm) metabolic, wide‐fusiform, visibly concave in the center (resembling an asymmetrical hourglass in general overview); elongated in the front and oblique, with a lateral reservoir opening; gradually narrowed toward the rear in a wedge‐like manner and terminated with a sharp hyaline tail (on average 4.5 µm). Paramylon grains monomorphic (only small), rod‐like and oval; no visibly larger grains have ever been observed. Periplast spirally striated.
Basionym
Lepocinclis constricta, Matvienko 1938, Uczen. Zap. Charkov. Derż. Univ. 14: 69, pl. II, figs. 17, 18.
Lectotype
Designated herein, Matvienko 1938, pl. 2, fig. 17 (see fig. 17 in Fig. S6 in the Supporting Information).
Epitype
Figure 3e designated herein supports the lectotype (Matvienko 1938, pl. 2, fig. 17).
Type locality
Ukraine, Kharkiv District, Sphagnum‐swamp “Klukvennoye”.
Representative DNA sequence
GenBank MT591641.
Representative locality
Freshwater pond in Izdebno Nowe village (52°08'01.3'' N, 20°32'52.9'' E).
Comments
In the diagnosis of Lepocinclis constricta, Matvienko mentions the atypical cell shape, which resembles an hourglass (“both parallel sides with a waist‐like contraction”). This allowed the identification of the Polish representatives of this species. However, of the two individuals visible in Matvienko’s drawings only one (fig. 17) bears the characteristic shape, which is why it has been designated as the lectotype (see Fig. S6). Also, due to the high morphological similarity to two other species of such shape, the designation of an epitype is justified (for more details, see Discussion).
Discoplastis excavata
(Schiller) Zakryś & M. Łukomska comb. nov. (Fig. 3, t‐w )
Emended diagnosis
Cells small (on average 20–30 × 6–11 µm), of a shape resembling a “trumpet” or a “cone,” heavily S‐curved and slightly longitudinally twisted; narrowed in a wedge‐like manner in the rear and terminated with a sharp hyaline tail (on average 3–4.5 µm). When immobile, the cells elongate visibly; however, they still maintain the characteristic shape. Paramylon grains oval or short rod‐like, mostly located in the center of the cell. Periplast delicately spirally straited.
Basionym
Euglena excavata Schiller in Huber‐Pestalozzi 1955, Das Phytoplancton des Süsswassers 4: 117, fig. 105 (three drawings).
Lectotype
Designated herein, Schiller in Huber‐Pestalozzi 1955, fig. 105 (first individual on the left, see Fig. S7 in the Supporting Information).
Epitype
Figure 3t designated herein supports the lectotype (Schiller in Huber‐Pestalozzi 1955, fig. 105, first specimen on the left).
Syntype localities
Austria, the canal Rust of the Lake Neusiedler See and farm ponds in Marchfeld.
Representative DNA sequence
GenBank MT591643.
Representative locality
Freshwater field pond in Izdebno Kościelne village (52°08'21.0'' N, 20°32'03.2'' E).
Comments
Each of the three individuals visible in Schiller’s drawing (fig. 105 in Huber‐Pestalozzi 1955‐see Fig. S7) has a different shape, and only one, the individual first from the left, is designated as the lectotype and is characteristically S‐curved. Schiller does draw attention to this in his description, and it was this feature that allowed the identification of the Polish strains. As the photograph shows the complex shape much more clearly than a hand‐drawn picture, we believe that designating an epitype in the form of a photograph seems justified (for more details, see Discussion).
Discoplastis gasterosteus
(Skuja) Zakryś & M.Łukomska comb. nov. (Fig. 3, k‐n ).
Emended diagnosis
Cells (30–57 × 5–11 µm) metabolic, cylindrical–fusiform, and oblique in the front; terminate in the rear with a sharp hyaline tail (on average 5–6 µm). Monomorphic paramylon grains (rod‐like and oval).
Basionym
Euglena gasterosteus Skuja 1948, Symbole Botanicae Upsaliensis 9: 195, pl. 22, figs. 21‐25.
Lectotype
Designated herein, Skuja 1948, pl. 22, fig. 23 (see Fig. S8 in the Supporting Information).
Epitype
Figure 3l designated herein supports the lectotype (Skuja 1948, pl. 22, fig. 23).
Type locality
Latvia, known from several lakes.
Representative DNA sequence
GenBank MT591647.
Representative locality
Freshwater pond in Łysaków village (50°43'28.04" N, 19°42'30.06" E).
Heterotypic synonym
Euglena gentilis Skuja, 1956: 233, pl. 41, figures 14‐16.
Comments
Each of the four individuals visible in Skuja’s drawings has a different shape (see Fig. S8). This emphasizes the metabolic character of the cell; however, it does make the identification much more difficult, due to high resemblance to other taxa of a similar morphology. Of the four drawings, figure 23 was chosen as the lectotype, as it represents a swimming cell of a cylindrical–fusiform shape. It was this trait that allowed the identification of the Polish strains. Euglena gentilis has been included as a Discoplastis gasterosteus synonym as no diagnostic traits have been found that would distinguish the two taxa.
Discoplastis spathirhyncha
(Skuja) Triemer in Triemer et. al. 2006: 735 (Fig. 3, q‐s ).
Emended diagnosis
Cell cylindrical–fusiform (60–96 × 6.5–13 µm), very metabolic; during the euglenoid movement, a characteristic bulge is formed in the center of the cell (“ballerina’s tutu skirt”). Anterior end elongated in a snout‐like manner and diagonally cut. Posterior end with a wedge‐like narrowing and terminated with a sharp hyaline tail (on average 9–12 µm). Monomorphic paramylon grains (oval or short rod‐like).
Lectotype
Designated herein, Skuja 1948, pl. 22, fig. 19 (see Fig. S9 in the Supporting Information).
Representative DNA sequence
GenBank AJ532454.
Representing culture
Strain SAG 1224‐42.
Type locality
Sweden, Säbysjön Lake.
Representative locality
Freshwater field pond in Izdebno Kościelne village (52°08'21.0'' N, 20°32'03.2'' E).
Homotypic synonym
Euglena spathirhyncha Skuja 1948: 196, pl. 22, figures 17‐20 (Triemer et al. 2006).
Heterotypic synonym
Euglena phacoides Nygaard 1949: 163, fig. 100 (Triemer et al. 2006).
Comments
The ability to create a bulge in the center of the cell during euglenoid movement is the key trait of Discoplastis spathirhyncha. The lectotype (pl. 22, fig. 19 in Skuja 1948‐see Fig. S9) shows the final stage of the movement (“ballerina’s tutu skirt”). Simultaneously, the lectotype indicated by Triemer et al. 2006 (the same fig. 19 in Skuja 1948) is deemed invalid by us, because the typification statement does not include the phrase “designated here” (hic designated) or an equivalent (Article 7.11). Euglena phacoides has been included as a D. spathirhyncha synonym as no diagnostic traits have been found that would distinguish the two taxa.
Euglenaformis
M.S. Bennett & Triemer in Bennett et al. 2014: 68
Emended diagnosis
Cells metabolic, spindle‐shaped, or cylindrical. Chloroplasts parietal, numerous, small, discoid, without pyrenoids. Paramylon grains monomorphic (only small: short rod‐like, oval, or round), scattered in the cytoplasm. Periplast spirally striated.
Euglenaformis chlorophoenicea
(Schmarda) Zakryś & M.Łukomska comb. nov. (Fig. 3, x and y ).
Emended diagnosis
Cells metabolic, long cylindrical (103–196 × 17–39 µm) slightly tapered anteriorly, posteriorly terminated with a short point that is very commonly retracted to a blunt rounded end. Protoplasm red‐colored (which is not a permanent characteristic) produces a lot of transparent mucus, especially during cell division. Monomorphic paramylon grains (only small), rod‐like and oval, scattered in the cytoplasm. Periplast spirally striated.
Basionym
Euglena chlorophoenicea Schmarda 1846, Kleine Beiträge zur Naturgeschichte der Infusorien, p. 18, pl. 1, figs. III. 1‐7.
Lectotype
Designated herein, Schmarda 1846, pl. 1, fig. III. 6 (see Fig. S10 in the Supporting Information).
Epitype
Figure 3x designated herein supports the lectotype (Schmarda 1846, pl. 1, fig. III. 6).
Type locality
Germany, small pond near Rondeau.
Representative DNA sequence
GenBank MT591632.
Representative locality
Freshwater, Michigan, USA, shallow roadside pond in Holt (42°36'66.9" N, 34°53'90" E).
Heterotypic synonym
Euglena demulcens Gojdics 1953: 92, pl. 9, figure 1, a and b.
Comments
Schmarda described the shape of the red and relatively large (108.5–120.5 µm long) cells of Euglenaformis chlorophoenicea as “cylindrical, elongated.” However, only one individual of the 7 depicted in his drawings is, in fact, cylindrical in shape, which is why it has been designated as the lectotype (fig. III. 6 in Schmarda 1846, see Fig. S10). The lack of any information regarding the chloroplasts (their number, shape, pyrenoid presence) in Schmarda’s description makes correct identification of this species very difficult, especially when other “red” euglenids are known from the literature (for more details, see Discussion). As the shape and size of the individuals from the American strain are consistent with the original diagnoses of E. chlorophoenicea and E. demulcens, the name chlorophoenicea has been chosen following the priority rule. As a result, E. demulcens is now considered a synonym. Due to the aforementioned, the designation of an epitype is justified.
Euglenaformis proxima
(P.A.Dangeard) M.S.Bennett & Triemer in Bennett et al. 2014: 68 (Fig. 3d ).
Emended diagnosis
Cells metabolic, fusiform (52–90 × 13–25 µm) slightly tapered, and rounded at the anterior end, with a conical posterior part. Paramylon grains monomorphic (only small), rod‐like and oval, scattered across the cytoplasm. Periplast spirally striated.
Lectotype
Representative DNA sequence
Representing culture
Strain SAG 1224‐11a (Bennett et al. 2014).
Flexiglena
Zakryś & M.Łukomska gen. nov.
Diagnosis
Cells free‐living, solitary, with one emergent flagellum, with a flexible pellicle and such pronounced metaboly (cell plasticity) that the shape of the cell varies even when swimming. Chloroplasts parietal, numerous, small, discoid, without pyrenoids. Paramylon grains dimorphic—small ones (oval and rod‐like) scattered in the cytoplasm and one visibly larger than the rest located near the stigma. The stigma is relatively large compared with the size of the organism. Periplast spirally striated.
Type species
Flexiglena variabilis (G.A. Klebs) Zakryś & M.Łukomska comb. nov.
Etymology
The name Flexiglena is derived from the combination of the Latin “flexi” (meaning “flexible”) and the generic name Euglena, which is to emphasize the pronounced metaboly (cell flexibility, plasticity).
Flexiglena variabilis
(G.A. Klebs) Zakryś & M.Łukomska comb. nov. (Fig. 3, a‐c ).
Emended diagnosis
The shape of the cells (50–98 × 9–21 µm) varies from short cylindrical (with slightly concave sides) through club‐shaped to fusiform with a cylindrical central part; ending with a more or less conical posterior part and rounded anterior. Paramylon grains dimorphic—small ones (oval and short rod‐like) scattered in the cytoplasm and one visibly larger than the rest (short rod‐like) located near the stigma. The stigma is relatively large compared with the size of the organism.
Basionym
Euglena variabilis, Klebs 1883, Unters. Bot. Inst. Tübingen. 1: 300, pl. 3, figures 4, 8.
Lectotype
Designated herein, Klebs 1883, figure 8 (see Fig. S11 in the Supporting Information).
Epitype
Figure 3b designated herein that supports the lectotype (Klebs 1883, fig. 8).
Representative DNA sequence
GenBank MT591636.
Type locality
Germany, pools in the Botany Garden at Tübingen.
Representative locality
Freshwater, pond in Moczydło park, Warsaw (52°14'32.1" N, 20°56'59.6" E).
Heterotypic synonym
Euglena proxima var. anglesia Pringsheim 1953: 160, figure 11.
Comments
The Polish strains have been identified based on the presence of a large paramylon grain located near the stigma and strong metaboly of the cells. The strong metaboly explains the name variabilis, while the atypical localization of the large paramylon grain is mentioned in the original diagnosis and visible in Klebs’s drawings. Klebs’s drawings (1883, fig. 8) showed a cell with a flagellum that has been designated as the lectotype (see Fig. S11). The cell is shaped like a short, wide cylinder with slightly concave sides, a rounded anterior end, and a wedge‐like posterior. Our research has shown that swimming individuals are mostly club‐shaped or cylindrical–fusiform, and they appear to “constrict” when swimming slowly or not moving—an effect of the pronounced metaboly. Due to the aforementioned, the designation of an epitype is justified, as F. variabilis is morphologically very alike many taxa known from the literature (for more details, see Discussion). Euglena proxima var. anglesia (with a large paramylon grain next to the stigma in fusiform–cylindrical cells) has been included as a F. variabilis synonym as no diagnostic traits have been found that distinguish the two taxa.
DISCUSSION
The study of both Polish and American strains has not only allowed the enrichment of the representation of species of Discoplastis and Euglenaformis on contemporary phylogenetic trees, but also confirmed the presence of a new genus, Flexiglena, in the Phacaceae, visible on phylogenetic trees as a single sequence Gungnamji052507K labeled as Discoplastis sp. (Kim et al. 2010, Kim and Shin 2014).
Flexiglena variabilis
This species was known as Euglena variabilis since its original description by Klebs (1883). However, it is difficult to identify due to its resemblance to many other taxa described later of a similar morphology, that is, metabolic cells and numerous, small pyrenoid‐less chloroplasts (E. chlamydophora, E. choretes, E. cylindrica, E. gibbosa, E. hemipellucida, E. multiformis, E. proxima var. anglesia, E. proxima var. dangeardii, and many more). Euglena variabilis was described from pools in the Botanical Garden in Tübingen. In the description, Klebs drew attention to the robust metaboly of the cells (i.e., the ability to change their shape). He also noted the relatively large stigma and the presence of a large paramylon grain (visibly bigger than the other grains) located next to it. Our careful observations have revealed that the cell shape varies even when swimming‐in one moment, cells can be club‐shaped or broadly cylindrical with a slight waist‐like contraction (Fig. 3, b and c) and then suddenly assume a new, narrow fusiform–cylindrical or fusiform shape (Fig. 3a). In this situation, the description of a shape or even the measurements of the cells are tedious (and as a result, few exist) and laden with a huge error margin even when modern measurement technology is applied (one that allows the measurement of a maximally elongated cell in motion; Table 1). Pringsheim arrived at a similar conclusion (1956, p. 64 and fig. 9) after he conducted a detailed study on four strains isolated in England. The best diagnostic trait is the presence of a visibly larger paramylon grain located next to the stigma. Pringsheim (1956) noted this trait, although earlier describing E. proxima var. anglesia Pringsheim (1953) based on the same feature (the presence of a large paramylon grain next to the stigma) and cell size (65–70 × 7–17 µm) similar to variabilis (stretched cells: 50–98 × 9–16.5 µm). Due to the aforementioned, E. proxima var. anglesia has been placed in synonymy under Flexiglena variabilis.
According to Pringsheim, Flexiglena variabilis (as Euglena variabilis) is uncommon (1956, p. 63), but later was described as cosmopolitan by Popova (1966), Popova and Safonova (1976), Starmach (1983), Tell and Conforti (1986), and Shi et al. (1999). In Poland, F. variabilis occurs often in eutrophic small water bodies (fish ponds, field ponds, farm ponds, park ponds) and in high densities. The presence of environmental sequences of F. variabilis from South Africa (BS2) and South Korea (Gungnamji052507K) on the tree (Fig. 2) confirms its cosmopolitan nature.
In the present study, the Flexiglena variabilis clade forms a separate lineage in the Phacaceae (clade B in Fig. 2 and Fig. S1), despite being morphologically (through strong metaboly) similar to the Discoplastis species. A similar situation occurs in Phacus, where two species (P. arnoldii and P. limnophilus) are located in an unstable position in regard to the other Phacus species. One difference is that morphologically these two are better “suited” to the Lepocinclis genus (Kim et al. 2015, Łukomska‐Kowalczyk et al. 2020b). The position of P. limnophilus is particularly unstable, as in some of our analyses it forms the earliest branch of the Lepocinclis clade (B. Zakryś, M. Łukomska‐Kowalczyk, K. Chaber, A. Fells & R. Milanowski, unpub. data).
Genetic variability of nSSU rDNA sequences of specimens of Flexiglena variabilis is high (up to 3.8%) in comparison with morphological diversity, as none of the four lineages located in the clade can be distinguished based on morphological features. A similar situation was observed in many other autotrophic euglenid species, for example, Lepocinclis fusiformis up to 7.8%, L. hispidulus up to 5.1% (Łukomska‐Kowalczyk et al. 2019), Phacus circumflexus up to 4.9% (Łukomska‐Kowalczyk et al. 2015), and Phacus salinus up to 11.3% (Łukomska‐Kowalczyk et al. 2020).
Discoplastis adunca
This morphological form was originally described by Schiller under the name Euglena rostrata (Schiller in Huber‐Pestalozzi 1955). However, as this name had already been taken (E. rostrata Ehrenberg 1838), it was changed to E. adunca (Schiller 1956) a year later. It was found in Austria, in the same place as Discoplastis excavata (the Rust canal of the Lake Neusiedler). Two contemporary isolated strains from Austria of E. adunca (in years 1981 and 1992) are deposited in the Cologne Culture Collection (CCAC, Germany). One of the strains originates from the Neusiedler Lake near Breitenbrunn (strain CCAC 2602 B, formerly as ASW 0895), while the other (CCAC 1268 B, formerly as ASW 08039) was isolated from a fish pond near Gresten (Lower Austria). The third report of a D. adunca (as Discoplastis sp. Bubsu100210Q) comes from South Korea (Kim et al. 2015), but neither the strain nor any documentation of it (microscope images, drawings) is available. Only the nuclear and plastid sequences (SSU, LSU) are known, which locate the strain in the adunca clade (A1 in Figs. 2 and S1). Due to the aforementioned, in our studies only the Austrian strain was analyzed morphologically (strain CCAC 2602 B), the nSSU rDNA sequence of which is identical with that of CCAC 1268 B. The individuals from the strain CCAC 2602 B are the same size (38‐48 × 5–9.5 µm) as those described by Schiller (40–52 × 9–10 µm) and have dimorphic paramylon grains (two rods larger than the rest; usually one placed in the anterior of the cell and the other in the posterior; Fig. 3, h–j). In the two descriptions by Schiller (as E. rostrata Schiller in Huber‐Pestalozzi 1955 and as E. adunca Schiller 1956), only very general comments are made about paramylon grains (“several short, oval or elongated rods”). Meanwhile, in all of the drawings from 1955 (four specimens in fig. 101 in Huber‐Pestalozzi 1955) and in one of the three from 1956 (Schiller 1956, fig. 57b), two large paramylon grains are clearly visible (see Figs. S2 and S3). The literature mentions at least four more species similar to D. adunca morphology (small metabolic cells: 40–50 × 5–10 µm, with numerous chloroplasts without pyrenoids and dimorphic paramylon grains): E. neglecta (described from Donieck), E. limnophila var. minor (from Poland), E. limnophila var. swirenkoi (from Russia), and E. parvula (from Crimea), which is why the designation of an epitype seems justified.
Discoplastis angusta (=Euglena angusta)
This species was described from containers with aquatic plants in a botanical garden on Java Island (Bernard 1908). It is best known from the literature under the name Euglena pascheri (Svirenko 1915, from ponds in Ukraine; see Fig. S5), likely due to the fact that the description made by Bernard had been deemed inadequate (see the checklist in Gojdics 1953, p. 169). Meanwhile, both the size of the representatives of the two species (E. angusta: 50–60 × 5 µm, E. pascheri: 51–53 × 4–5 µm) and the shape (narrowly cylindrical, ending with sharp hyaline tail) leave no doubt that it refers to the same morphological form (see Figs. S4 and S5). The Latin name angusta (=narrow) refers to the cell shape. Discoplastis angusta is reported to be common in Europe and Asia as E. pascheri (e.g., Popova 1966, Popova and Safonova 1976, Starmach 1983, Shi et al. 1999). It is just as frequent in Poland, but it occurs in very low densities and as such is difficult to isolate. Only one sequence was obtained which is placed with the Korean sequence (Banmun010910B as Discoplastis sp.) on the phylogenetic tree. Its closest relation is D. spathirhyncha (sister taxa). On the phylogenetic tree, the sequences of both species occur in a common clade (clade A2 in Figs. 2 and S1), despite being morphologically very different (Fig. 3, o, p and q‐s).
Discoplastis constricta (= Lepocinclis constricta)
This species was described from Ukraine under the name Lepocinclis constricta (Matvienko 1938). The most characteristic diagnostic trait of this species is the shape, atypical for euglenids in that it resembles an hourglass (with a cinch in the center of the cell). The literature mentions only two other species of such shape: Euglenaria clepsydroides and Euglena undulata, which differ from D. constricta by having several large chloroplasts with pyrenoids (Kato 1983, Zakryś et al. 2013). The conducted phylogenetic analyses prove, that despite the unique cell shape, these species are not closely related (Zakryś et al. 2013 and the present study).
The morphology of the Polish strains of Discoplastis constricta corresponds with the literature data. This includes the cell size (on average 33–50 × 11.5–15.5 µm; Table 1), which does not differ significantly from the data given by other authors: Matvienko (1938): 33 × 21 µm; Asaul (1975), Popova and Safonova (1976): 26.5–35 × 15.9−22 µm; and Shi et al. (1999): 26–33 × 14–18 µm.
So far, Discoplastis constricta (as Lepocinclis constricta) has been noted only a few times: in Ukraine and Siberia (Matvienko 1938, Asaul 1975, Popova and Safonova 1976), in Japan (Kato 1983), in China and Taiwan (Shi et al. 1999), and in Slovakia (Wołowski and Hindák 2005, as E. gymnodinioides‐figs. 66 and 67). This is the first report ever from Poland, where it has been found several times, but in very low densities. Most likely, the issues with correct identification and the occurrence in low densities cause this species to be considered very rare.
Discoplastis excavata (=Euglena excavata)
Described from Austria‐from the Rust canal of the Lake Neusiedler See and from farm ponds in Marchfeld (Schiller in Huber‐Pestalozzi 1955). The two Polish populations of this species constitute the first occurrence outside of Austria of this very characteristic horn‐shaped euglenid (Fig. 3, t–w). A particularly dense population exists in a small, eutrophic water body in a field outside of Warsaw (Izdebno Kościelne village), where its constant occurrence during the summer has been noted for the past few years (Fig. 1). The size of the cells from the Austrian population (25 × 10–12 µm) and the Polish one (in average 25 × 8 µm; Table 1) is practically identical.
Discoplastis gasterosteus (=Euglena gasterosteus)
In less than a decade, Skuja described two morphologically impossible to distinguish species (Euglena gasterosteus [Skuja 1948] from Latvia: cells metabolic, fusiform, or cylindrical–fusiform [30–57 × 6–11 µm] and E. gentilis [Skuja 1956] from Sweden: cells metabolic, fusiform [55–60 × 8–10 µm]), which he commented on as “common among freshwater, inland water bodies” of the two countries. However, even a detailed analysis of the diagnostic descriptions and drawings of the two species did not allow us to distinguish them, which is why we named the Polish strains Discoplastis gasterosteus based on the principle of priority, and included Euglena gentilis as a synonym of D. gasterosteus.
Literature studies and the morphological–molecular research presented herein have shown that Discoplastis gasterosteus is morphologically very similar to D. angusta and D. adunca. Those three species have a pronounced metaboly, which is why minimal differences in cell shape or size cannot be used as diagnostic features. Similarly, the paramylon grain dimorphism observed in Euglena adunca is not always helpful for identification, as the large paramylon grains may be obscured by numerous smaller ones. Due to the aforementioned, only DNA sequences are diagnostic; each of the listed species represents an independent lineage (see Figs. 2 and S1). Discoplastis gasterosteus has been noted multiple times in Sweden (Skuja 1948), Ukraine (Asaul 1975), Hungary (Uherkovich 1982), Czech Republic (Wołowski 1992), and China (Shi et al. 1999). It is common in Poland, but it does not occur in dense populations (Burchardt 1976, 1977, Dąmbska 1976, Wołowski 1998 and our studies).
Discoplastis spathirhyncha
This species is very characteristic due to a tendency to visibly widen the central bulge of the cell (resembling a “ballerina’s tutu skirt”) that appears during euglenoid movement (Fig. 3, q–s). The morphology (including the cell size) of the Polish strains is consistent with the literature (e.g., Skuja 1948: 66–85 × 12–16 µm, Tell and Conforti 1986: 60–96 × 12–20 µm; Polish strains: on average 70–90 × 10–12 µm; Table 1). The species is considered to be cosmopolitan, but relatively uncommon (Popova 1966, Starmach 1983, Tell and Conforti 1986). In Poland, D. spathirhyncha occurs often in small eutrophic water bodies such as farm ponds, fish ponds, or field ponds, but never in high densities (B. Zakryś, pers. obs.), which is why it might have been reported rarely (Bucka 1958, Wołowski 1998).
Euglenaformis chlorophoenicea
This species was described by Schmarda (1846) from a small pond near Rondeau (Germany) where it occurred in low densities. The cells, however, drew attention due to their red‐green coloring. Since then, it has been found only a handful of times. Westberg (1895) reported this species from near Riga (Latvia) from a gelatinous red surface bloom; Gojdics (1953) collected it from a pond near Beaufort (North Carolina, USA) and from Adamstown (Pennsylvania, USA) as Euglena demulcens. The only report from Asia comes from China (Shi et al. 1999 as E. demulcens). The material studied in this work was collected from a shallow roadside pond in Holt, Michigan, USA (42°36.669' N, 84°34.539’ W; Table S2). A red, gelatinous surface bloom with this taxon is periodically observed in this pond. The red pigmentation of Euglenaformis chlorophoenicea (caused by the presence of astaxanthin) is not a permanent characteristic, but one dependent on environmental conditions, albeit the exact mechanism by which this happens is unknown (Gojdics 1953 and present study). The appearance of astaxanthin has also been observed in the cells of many other euglenoid species (e.g., Gojdics 1953, Pringsheim 1956, Popova 1966) leading some authors to be very skeptical about distinguishing this species. For example, Popova (1966) recognizes E. chlorophoenicea as a synonym of E. sanguinea (p. 249); Pringsheim (1956 p. 134) states that this species is “not recognizable (…) It is not clear, why the form, to which Gojdics 1953 gives the name should be different from her E. demulcens or identical with Schmarda’s which is barely described at all (…). Its relation to E. sanguinea ought to be established.” In our opinion, the descriptions and drawings of both species, E. chlorophoenicea made by Schmarda (1846) and E. demulcens made by Gojdics (1953), represent the same species, which is why we recognize the priority of the name E. chlorophoenicea and consider E. demulcens a synonym (see drawings of both species in Gojdics 1953, pl. 9, figs. 1, 2). The size of the individuals from the population from Michigan (130–183 × 20–29 µm) is comparable with Schmarda’s description of chlorophoenicea (108.5–120.5 µm long) and Gojdics’s description of demulcens (130–196 × 35–39 µm).
CONCLUSIONS
Of the six studied Discoplastis species, three of them are difficult to distinguish morphologically (D. adunca, D. gasterosteus, and D. angusta). Their cells are small (on average 40–55 × 6–9 µm) with a pronounced metaboly and terminate with a colorless, sharp tail (6–7 µm long). The cells' shape (narrowly cylindrical, cylindrical–fusiform, or fusiform) may be described only when the cell is swimming. Moreover, two large paramylon grains present in D. adunca are often invisible, due to the cover provided by numerous smaller grains. In this case, molecular identification is by far the most reliable.
This work was supported by the OPUS 2016/23/B/NZ8/00919 grant from the National Science Centre, Poland. The Open access for this publication was funded by the University of Warsaw. We thank Prof. Richard Triemer, MI, USA, for providing the strain of Euf. chlorophoenicea (strain MI). We are grateful for the input of three anonymous reviewers who have been a great help in improving the manuscript.
Supporting information
Figure S1. Maximum likelihood phylogenetic tree based on 155 sequences of nSSU rDNA, 139 of nLSU rDNA, 135 of cpSSU rDNA and 138 of cpLSU rDNA representing 159 strains or isolates (strains/isolates represented for the first time are indicated in bold type).
Figure S2. The original drawings of Euglena adunca Schiller 1956, fig. 57, a‐c; the individual on fig. 57b is the lectotype of Discoplastis adunca.
Figure S3. The original drawing of Euglena rostrata (=E. adunca) Schiller in Huber‐Pestalozzi 1955, fig. 101.
Figure S4. The original drawings of Euglena angusta Bernard 1908, pl. 16, figs. 557 and 558; the individual on fig. 557 is the lectotype of Discoplastis angusta.
Figure S5. The original drawing of Euglena pascheri Svirenko 1915, pl. 2, figs. 23‐28.
Figure S6. The original drawing of Euglena constricta Matvienko 1938, pl. II, figs. 17, 18; the individual on fig. 17 is the lectotype of Discoplastis constricta.
Figure S7. The original drawing of Euglena excavata Schiller in Huber‐Pestalozzi 1955, fig. 105; the first individual from left is the lectotype of Discoplastis excavata.
Figure S8. The original drawing of Euglena gasterosteus Skuja 1948, pl. 22, figs. 21‐25; the individual on fig. 23 is the lectotype of Discoplastis gasterosteus.
Figure S9. The original drawing of Euglena spathirhyncha Skuja 1948, pl. 22, figs. 17‐20; the individual on fig. 19 is the lectotype of Discoplastis spathirhyncha.
Figure S10. The original drawing of Euglena chlorophoenicea Schmarda 1846, pl. 1, figs. III. 1‐7; the individual on fig. 6 is the lectotype of Euglenaformis chlorophoenicea.
Figure S11. The original drawing of Euglena variabilis Klebs 1883, pl. 3, figs. 4, 8; the individual on fig. 8 is the lectotype of Flexiglena variabilis.
Table S1. Newly designed primers used for PCR amplification and sequencing of cpSSU rDNA and nLSU rDNA; specific primers, based on cpSSU rDNA sequence of Flexiglena variabilis strain Gungnamji052507K are marked with asterisk (*).
Table S2. List of species and sampling data of isolates/strains used in this study. GenBank accession numbers are given with new sequences indicated in bold type. Isolate/Strain codes are those used in the phylogenetic tree (Fig. 2).
Table S3. The nuclear SSU rDNA pair‐wise sequence distances (%) among studied strains and isolates of Discoplastis and Flexiglena.
References
- Asaul, Z. I. 1975. Viznachnik evglenovikh vodorostej ukrainskoj RSR. [Survey of the euglenophytes of the Ukrainian SSR] Kiiv, Izd. Naukova Dumka, Kiev, 407 pp. (in Ukrainian).
- Bennett, M. S. & Triemer, R. E. 2012. A new method for obtaining nuclear gene sequences from field samples and taxonomic revisions of the photosynthetic euglenoids Lepocinclis (Euglena) helicoideus and Lepocinclis (Phacus) horridus (Euglenophyta). J. Phycol. 48:254–60. [DOI] [PubMed] [Google Scholar]
- Bennett, M. S. , Wiegert, K. E. & Triemer, R. E. 2014. Characterization of Euglenaformis gen. nov. and the chloroplast genome of Euglenaformis [Euglena] proxima (Euglenophyta). Phycologia 53:66–73. [Google Scholar]
- Bernard, C. 1908. Protococcacées et Desmidiées d'eau douce, récoltées à Java. Departm. De l’agriculture aux Indes Neerlandaises, Batavia, 230 pp. [Google Scholar]
- Bradley, R. K. , Roberts, A. , Smoot, M. , Juvekar, S. , Do, J. , Dewey, C. , Holmes, I. & Pachter, L. 2009. Fast statistical alignment. PLoS Comput. Biol. 5:e1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucka, H. 1958. The appearance of Euglena species in postregulation ponds at the banks of the Vistula near Cracaw. Fragm. Flor. Geobot. 3:161–80. [Google Scholar]
- Burchardt, L. 1976. Nowe dla Polski taksony glonów. [New for Poland taxa of algae]. Fragm. Flor. Geobot. 22:248–50 (in Polish). [Google Scholar]
- Burchardt, L. 1977. Zmiany w składzie fitoplanktonu Jeziora Pątnowskiego odbiornika wód podgrzewanych i ścieków z cukrowni (1972/73). [Changes in the phytoplankton of the Lake Pątnowskie collector of warmed water and sewage from the sugar plant (1972/73)]. UAM Poznań. Ser. Biologia 8:1–117 (in Polish with English summary). [Google Scholar]
- Capella‐Gutierrez, S. , Silla‐Martinez, J. M. & Gabaldon, T. 2009. TrimAl: a tool for automated alignment trimming in large‐scale phylogenetic analyses. Bioinformatics 25:972–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dąmbska, I. 1976. Roślinność rezerwatu “Dębina” pod Wągrowcem w Wielkopolsce. 2. Glony. [Vegetation of the “Dębina” reserve in Wielkopolska Region. 2. Algae]. Bad. Fizjogr. Pol. Zach. Ser. B‐Botanika 29:51–5 (in Polish with English summary). [Google Scholar]
- Dangeard, P. A. 1901. Recherches sur les Eugléniens. Le Botaniste 8:97–360. [Google Scholar]
- Darriba, D. , Taboada, G. L. , Doallo, R. & Posada, D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg, C. G. 1838. Die Infusionsthierchen als Vollkommene Organismen. Ein Blick in Das Tiefere organische Leben der Natur. Nebst einem Atlas von vierundsechszig colorirten Kupfertafeln, gezeichnet vom Verfasser. Verlag von Leopold Voss, Leipzig, 547 pp. [Google Scholar]
- Gojdics, M. 1953. The genus Euglena. The University of Wisconsin Press, Madison, WI, 268 pp. [Google Scholar]
- Gouy, M. , Guindon, S. & Gascuel, O. 2010. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27:221–4. [DOI] [PubMed] [Google Scholar]
- Hüber‐Pestalozzi, G. 1955. Das Phytoplankton des Süsswassers; Systematik und Biologie: 4 Teil; Euglenophyceen. E. Schweizerbartsche Verlagsbuchhandlung, Stuttgart, Germany, 606 pp. [Google Scholar]
- Kato, S. 1983. A new species of Euglena (Euglenophyceae) from Japan. J. Japan. Bot. 58:237–9. [Google Scholar]
- Kim, J. I. , Shin, W. & Triemer, R. E. 2010. Multigene analyses of photosynthetic euglenoids and new family, Phacaceae (Euglenales). J. Phycol. 46:1278–87. [Google Scholar]
- Kim, I. J. , Shin, W. & Triemer, R. E. 2013. Cryptic speciation in the genus Cryptoglena (Euglenaceae) revealed by nuclear and plastid SSU and LSU rDNA gene. J. Phycol. 49:92–102. [DOI] [PubMed] [Google Scholar]
- Kim, J. I. & Shin, W. 2014. Molecular phylogeny and cryptic diversity of the genus Phacus (Phacaceae, Euglenophyceae) and the descriptions of seven new species. J. Phycol. 50:948–59. [DOI] [PubMed] [Google Scholar]
- Kim, J. I. , Linton, E. & Shin, W. 2015. Taxon‐rich multigene phylogeny of the photosynthetic euglenoids (Euglenophyceae) . Front. Ecol. Evol. 3:1–11. [Google Scholar]
- Klebs, G. 1883. Über die Organisation einiger Flagellaten‐Gruppen und ihre Beziehungen zu Algen und Infusorien. Untersuchung Botan. Inst. Tübingen 1:233–362. [Google Scholar]
- Kumar, S. , Stecher, G. , Li, M. , Knyaz, C. & Tamura, K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35:1547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanave, C. , Preparata, G. , Saccone, C. & Serio, G. 1984. A new method of calculating evolutionary substitution rate. J. Mol. Evol. 20:86–93. [DOI] [PubMed] [Google Scholar]
- Lax, G. & Simpson, A. G. B. 2013. Combining molecular data with classical morphology for uncultured phagotrophic Euglenids (Excavata): a single‐cell approach. J. Eukaryot. Microbiol. 60:615–25. [DOI] [PubMed] [Google Scholar]
- Linton, E. , Karnkowska‐Ishikawa, A. , Kim, J. I. , Shin, W. , Bennett, M. S. , Kwiatowski, J. , Zakryś, B. & Triemer, R. E. 2010. Reconstructing euglenoid evolutionary relationships using three genes: nuclear SSU and LSU, and chloroplast SSU rDNA sequences and the description of Euglenaria gen. nov. (Euglenophyta). Protist 161:603–19. [DOI] [PubMed] [Google Scholar]
- Łukomska‐Kowalczyk, M. , Karnkowska, A. , Łach, Ł. , Milanowski, R. & Zakryś, B. 2015. Delimiting species boundaries within the Phacus longicauda complex (Euglenida) through morphological and molecular analyses. J. Phycol. 51:1147–57. [DOI] [PubMed] [Google Scholar]
- Łukomska‐Kowalczyk, M. , Chaber, K. , Fells, A. , Milanowski, R. & Zakryś, B. 2020a. Molecular and morphological delimitation of species in the group of Lepocinclis ovum‐like taxa (Euglenida). J. Phycol. 56:283–99. [DOI] [PubMed] [Google Scholar]
- Łukomska‐Kowalczyk, M. , Fells, A. , Chaber, K. , Milanowski, R. & Zakryś, B. 2020b. Taxon‐rich phylogeny and taxonomy of the genus Phacus (Euglenida) based on morphological and molecular data. J. Phycol. 56:1135–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin, B. , Palm, A. , Klingberg, M. & Melkonian, M. 2003. Phylogeny and taxonomic revision of plastid‐containing euglenophytes based on SSU rDNA sequence comparisons and synapomorphic signatures in the SSU rRNA secondary structure. Protist 154:99–145. [DOI] [PubMed] [Google Scholar]
- Matvienko, O. M. 1938. Materiały do vyvchennia vodorostej URSR. I. Vodorosti Klukvennoho bołota [Contribution to the study of the algae of the Ukraine USSR. I. Algae of the sphagnum‐swamp “Klukvennoye”]. Uczen. Zapiski Charkov. Derż. Univ. 14:29–70. [Google Scholar]
- Milanowski, R. , Zakryś, B. & Kwiatowski, J. 2001. Phylogenetic analysis of chloroplast small‐subunit rDNA genes of the genus Euglena Ehrenberg. Int. J. Syst. Evol. Microbiol. 51:773–81. [DOI] [PubMed] [Google Scholar]
- Nygaard, G. 1949. Hydrobiological studies on some Danish ponds and lakes; Part II: The quotient hypothesis and some new or little known phytoplankton organisms. Det. Kon. Danska Vid. Selsk. Biol. Skr. 7:1–293. [Google Scholar]
- Popova, T. G. 1966. Flora Sporovych Rastenij SSSR, 8. [Flora plantarum cryptogamarum URSS, 8]. Euglenophyta 1. Nauka, Moskva‐Leningrad 411 pp. (in Russian).
- Popova, T. G. & Safonova, T. A. 1976. Flora Sporovych Rastenij SSSR, 9. [Flora plantarum cryptogamarum URSS, 9]. Euglenophyta 2. Nauka, Moskva‐Leningrad, 286 pp. (in Russian).
- Pringsheim, E. G. 1953. Salzwasser‐Eugleninen. Arch. Microbiol. 18:149–64. [PubMed] [Google Scholar]
- Pringsheim, E. G. 1956. Contribution towards a monograph of the genus Euglena . Nova Acta Leopold. 18:1–168. [Google Scholar]
- Rodriguez, F. , Oliver, J. L. , Marin, A. & Medina, J. R. 1990. The general stochastic model of nucleotide substitution. J. Theor. Biol. 142:485–501. [DOI] [PubMed] [Google Scholar]
- Ronquist, F. & Huelsenbeck, J. P. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–4. [DOI] [PubMed] [Google Scholar]
- Schiller, J. 1956. Untersuchungen an den planktischen Protophyten des Neusiedler Sees 1950–1954. III Teil. Euglenen. Sitzungsber. Österr. Akad. Wiss. Abt. 1:546–83. [Google Scholar]
- Schmarda, L. K. 1846. Kleine Beiträge zur Naturgeschichte der Infusorien. Verlag der Carl Haas’schen Buchhandlung, Wien, 61 pp. [Google Scholar]
- Shi, Z. X. , Wang, Q. , Xie, S. & Dai, I. 1999. Flora Algarum Sinicarum Aquae Dulcis. Tomus VI. Euglenophyta. Science Press, Beijing, 414 pp. (in Chinese). [Google Scholar]
- Skuja, H. 1948. Taxonomie des Phytoplanktons einiger Seen in Uppland. Schweden. V. Euglenophyta. Symb. Bot. Upsal. 9:183–238. [Google Scholar]
- Skuja, H. 1956. Taxonomische und biologische Studien über das Phytoplankton schwedischer Binnengewässer. V. Euglenophyta. Nova Acta Reg. Soc. Sci. Upsal. Series IV 16:228–400. [Google Scholar]
- Stamatakis, A. 2014. RAxML version 8: a tool for phylogenetic analysis and post‐analysis of large phylogenies. Bioinformatics 30:1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmach, K. 1983. Euglenophyta ‐ eugleniny. In Starmach, K. & Sieminńska, J. (Eds.) Flora Słodkowodna Polski 3. P. W. N, Warsaw, 594 pp. [Google Scholar]
- Svirenko, D. O. 1915. Matérial pour servir à l’étude des algues de la Russie. Étude systématique et géographique sur les Euglénacées. Trav. Inst. Bot. Univ. Charkov 48:67–143 (in Russian). [Google Scholar]
- Tavare, S. 1986. Some probabilistic and statistical problems on the analysis of DNA sequences. Lec. Math. Life Sci. 17:57–86. [Google Scholar]
- Tell, G. & Conforti, V. 1986. Euglenophyta pigmentadas de la Argentina. Bibliotheca Phycologica, J. CRAMER, Berlin, Stuttgart, 301 pp. [Google Scholar]
- Triemer, R. E. , Linton, E. , Shin, W. , Nudelman, A. , Monfils, A. , Bennett, M. & Brosnan, S. 2006. Phylogeny of the Euglenales based upon combined SSU and rDNA sequence comparisons and description of Discoplastis gen. nov. (Euglenophyta). J. Phycol. 42:731–40. [Google Scholar]
- Turland, N. J. , Wiersema, J. H. , Barrie, F. R. , Greuter, W. , Hawksworth, D. L. , Herendeen, P. S. , Knapp, S. et al. 2018. International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) Regnum Vegetabile 159. Koeltz Botanical Books, Glashütten, Glashütten. [Google Scholar]
- Uherkovich, G. 1982. A Fekete‐hegy (Balaton‐felvidèk) Kerek‐tava algavegetaciója [Die Algenvegetation des Kerk (Rund) – Sees vom Fekete (Schwarz)‐Berg (Balaton‐Oberland)]. Bakonyi Term. Tud. Muzeum Közl. 1:81–110 (in Hungarian with German and English summaries). [Google Scholar]
- Westberg, P. 1895. Ueber Euglena chlorophoenicea Schmarda. Korrespondenzblatt des Naturf. Ver. zu Riga 38:98–104. [Google Scholar]
- Wołowski, K. 1992. Occurrence of Euglenophyta in the Třebon Biosphere Reserve (Czechoslovakia). Algol. Stud. 66:73–98. [Google Scholar]
- Wołowski, K. 1998. Taxonomic and environmental studies on Euglenophytes of the Kraków‐Częstochowa upland (Southern Poland). Fragm. Florist. Geobot. 6:3–192. [Google Scholar]
- Wołowski, K. & Hindák, F. 2005. Atlas of euglenophytes. VEDA, Publishing House of the Slovak Academy of Science, Bratislava, 136 pp. [Google Scholar]
- Zakryś, B. , Karnkowska‐Ishikawa, A. , Łukomska‐Kowalczyk, M. & Milanowski, R. 2013. A new photosynthetic euglenoid isolated in Poland: Euglenaria clepsydroides sp. nov. (Euglenea). Eur. J. Phycol. 48:260–7. [Google Scholar]
- Zakryś, B. , Milanowski, R. , Empel, J. , Borsuk, P. , Gromadka, R. & Kwiatowski, J. 2002. Two different species of Euglena, E. geniculata and E. myxocylindracea (Euglenophyceae), are virtually genetically and morphologically identical. J. Phycol. 38:1190–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Maximum likelihood phylogenetic tree based on 155 sequences of nSSU rDNA, 139 of nLSU rDNA, 135 of cpSSU rDNA and 138 of cpLSU rDNA representing 159 strains or isolates (strains/isolates represented for the first time are indicated in bold type).
Figure S2. The original drawings of Euglena adunca Schiller 1956, fig. 57, a‐c; the individual on fig. 57b is the lectotype of Discoplastis adunca.
Figure S3. The original drawing of Euglena rostrata (=E. adunca) Schiller in Huber‐Pestalozzi 1955, fig. 101.
Figure S4. The original drawings of Euglena angusta Bernard 1908, pl. 16, figs. 557 and 558; the individual on fig. 557 is the lectotype of Discoplastis angusta.
Figure S5. The original drawing of Euglena pascheri Svirenko 1915, pl. 2, figs. 23‐28.
Figure S6. The original drawing of Euglena constricta Matvienko 1938, pl. II, figs. 17, 18; the individual on fig. 17 is the lectotype of Discoplastis constricta.
Figure S7. The original drawing of Euglena excavata Schiller in Huber‐Pestalozzi 1955, fig. 105; the first individual from left is the lectotype of Discoplastis excavata.
Figure S8. The original drawing of Euglena gasterosteus Skuja 1948, pl. 22, figs. 21‐25; the individual on fig. 23 is the lectotype of Discoplastis gasterosteus.
Figure S9. The original drawing of Euglena spathirhyncha Skuja 1948, pl. 22, figs. 17‐20; the individual on fig. 19 is the lectotype of Discoplastis spathirhyncha.
Figure S10. The original drawing of Euglena chlorophoenicea Schmarda 1846, pl. 1, figs. III. 1‐7; the individual on fig. 6 is the lectotype of Euglenaformis chlorophoenicea.
Figure S11. The original drawing of Euglena variabilis Klebs 1883, pl. 3, figs. 4, 8; the individual on fig. 8 is the lectotype of Flexiglena variabilis.
Table S1. Newly designed primers used for PCR amplification and sequencing of cpSSU rDNA and nLSU rDNA; specific primers, based on cpSSU rDNA sequence of Flexiglena variabilis strain Gungnamji052507K are marked with asterisk (*).
Table S2. List of species and sampling data of isolates/strains used in this study. GenBank accession numbers are given with new sequences indicated in bold type. Isolate/Strain codes are those used in the phylogenetic tree (Fig. 2).
Table S3. The nuclear SSU rDNA pair‐wise sequence distances (%) among studied strains and isolates of Discoplastis and Flexiglena.


