Abstract
Background
Hospitalized pediatric hematology‐oncology (PHO) patients have frequent clinical deterioration events (CDE) requiring intensive care unit (ICU) admission, particularly in resource‐limited settings. The objective of this study was to describe CDEs in hospitalized PHO patients in Latin America and to identify event‐level and center‐level risk factors for mortality.
Methods
In 2017, the authors implemented a prospective registry of CDEs, defined as unplanned transfers to a higher level of care, use of ICU‐level interventions on the floor, or nonpalliative floor deaths, in 16 PHO centers in 10 countries. PHO hospital admissions and hospital inpatient days were also reported. This study analyzes the first year of registry data (June 2017 to May 2018).
Results
Among 16 centers, 553 CDEs were reported in PHO patients during 11,536 admissions and 119,414 inpatient days (4.63 per 1000 inpatient days). Event mortality was 29% (1.33 per 1000 inpatient days) but ranged widely across centers (11%‐79% or 0.36‐5.80 per 1000 inpatient days). Significant risk factors for event mortality included requiring any ICU‐level intervention on the floor and not being transferred to a higher level of care. Events with organ dysfunction, a higher severity of illness, and a requirement for ICU intervention had higher mortality. In center‐level analysis, hospitals with a higher volume of PHO patients, less floor use of ICU intervention, lower severity of illness on transfer, and lower rates of floor cardiopulmonary arrest had lower event mortality.
Conclusions
Hospitalized PHO patients who experience CDEs in resource‐limited settings frequently require floor‐based ICU interventions and have high mortality. Modifiable hospital practices around the escalation of care for these high‐risk patients may contribute to poor outcomes. Earlier recognition of critical illness and timely ICU transfer may improve survival in hospitalized children with cancer.
Keywords: clinical deterioration, intensive care, Latin America, pediatric oncology, Pediatric Early Warning Systems (PEWS), resource‐limited settings
Short abstract
In this prospective cohort study of clinical deterioration events among pediatric hematology‐oncology patients in 16 resource‐limited Latin American hospitals, event‐level and center‐level risk factors for mortality are identified. The findings demonstrate that earlier recognition of critical illness and timely intensive care unit transfer may improve survival in hospitalized children with cancer.
Introduction
Hospitalized children with cancer frequently develop critical illness, and up to 40% require intensive care unit (ICU) management during the course of cancer‐directed therapy. 1 , 2 Mortality for pediatric hematology‐oncology (PHO) patients who require critical care, however, is much higher than in other pediatric ICU (PICU) patients, ranging from 6.8% to >50% in high‐resource settings. 3 , 4 Conserningly, a recent meta‐analysis demonstrated 27% overall mortality for PICU admissions in children with cancer, with no clear improvement in mortality over the past 30 years. 5 Emergency medical PICU admissions, particularly clinical deterioration among hospitalized patients, have the highest PICU mortality. 5 , 6 , 7 In line with these findings, a recent international consensus identified defining the optimal timing of life‐sustaining therapies and development of tools to facilitate early recognition of critical illness, such as pediatric early warning systems (PEWS), as the top 2 research priorities to improve outcomes in this patient population. 8
The global burden of childhood cancer, however, is disproportionately shifted toward resource‐limited settings, which represent >90% of childhood cancer deaths worldwide. 9 , 10 Recent emphasis through the World Health Organization Global Initiative in Childhood Cancer 11 and other initiatives 12 has created new frameworks to improve global access to services for children and adolescents with cancer. This focus has made it particularly important to study successful models of supportive care in these patients, including strategies for managing of critical illness. Among PHO patients in resource‐limited settings, up to 50% of deaths are caused by complications of cancer‐directed therapy, 13 , 14 representing a potentially preventable cause of mortality. Although there are limited studies on outcomes of critical illness ]among PHO patients in these settings, available data demonstrate higher mortality ( 32%‐55%) 15 , 16 , 17 , 18 and more frequent inpatient clinical deterioration 19 than in high‐resource hospitals. We recently identified quality and capacity indicators to improve pediatric oncology critical care in resource‐limited settings 20 ; however, the relation between clinical or organizational factors and outcomes of critical illness has not yet been established in these settings.
A better understanding of clinical deterioration in PHO patients in resource‐limited settings is integral to improve outcomes and achieve the global imperative of increased childhood cancer survival. The objectives of this study were to describe clinical deterioration in hospitalized children with cancer in Latin America, a region with a broad spectrum of resource limitations, and to identify event‐level and center‐level risk factors for mortality that could inform future initiatives to improve patient outcomes.
Materials and Methods
Prospective Quality‐Improvement Registry and the EVAT Multicenter Program
Data for this study were obtained from a de‐identified, prospective quality‐improvement registry of clinical deterioration events (CDEs) developed as part of the EVAT (Escala de Valoracion de Alerta Temprana [Early Warning Assessment Scale]) Multicenter Program, a quality‐improvement collaborative of PHO centers in Latin America, with the ultimate goal of improving outcomes for hospitalized children with cancer who develop critical illness. This collaborative aims to improve early identification of clinical deterioration in hospitalized PHO patients through implementation of PEWS using a 3‐phased, structured program of prospective registration of clinical deterioration, formation of a multidisciplinary team, and ultimately, a mentored implementation of PEWS with support from experts at St Jude Children's Research Hospital (SJCRH) and in the region. The program began in 2017 with 16 centers from 10 countries in the region (Dominican Republic [2 centers], Ecuador, El Salvador, Guatemala, Haiti, Honduras, Mexico [6 centers], Nicaragua, Panama, and Peru), representing >2000 annual new pediatric cancer diagnoses (for center characteristics, see Table 1).
TABLE 1.
Characteristics of Collaborating Pediatric Hematology‐Oncology Centers
| Site | Country | Country Income Level | Hospital Type | No. of PHO Beds | Average Floor Nursing Ratio: 1 Nurse/X Patients | No. of PICU Beds | PEWS Implemented at Start of Study Period | No. of Annual New Pediatric Cancer Diagnoses, 2018 |
|---|---|---|---|---|---|---|---|---|
| A | Mexico | UMIC | Oncology | 8 | 6 | 4 | No | 23 |
| B | Mexico | UMIC | Pediatric | 26 | 3 | 8 | No | 75 |
| C | Guatemala | UMIC | PHO | 58 | 4 | 9 | Yes | 513 |
| D | Haiti | LIC | Women/children | 13 | 13 | 20 | No | 85 |
| E | Mexico | UMIC | General | 12 | 5 | 8 | No | 8 |
| F | Peru | UMIC | Oncology | 50 | 8 | 16 | No | 800 |
| G | Nicaragua | LMIC | Pediatric | 45 | 15 | 27 | No | 253 |
| H | Panama | HIC | Pediatric | 15 | 9 | 26 | No | 50 |
| I | Mexico | UMIC | PHO | 22 | 4 | 4 | Yes | 61 |
| J | Ecuador | UMIC | Oncology | 46 | 10 | 10 | No | 151 |
| K | Mexico | UMIC | General | 25 | 6 | 5 | No | 58 |
| L | El Salvador | LMIC | Pediatric | 24 | 7 | 16 | No | 200 |
| M | Dominican Republic | UMIC | Pediatric | 23 | 5 | 28 | No | 60 |
| N | Dominican Republic | UMIC | Pediatric | 18 | 12 | 10 | No | 95 |
| O | Honduras | LMIC | General | 22 | 10 | 7 | Yes | 365 |
| P | Mexico | UMIC | General | 10 | 3 | 0 | No | 55 |
Abbreviations: HIC, high‐income country; LIC, low‐income country; LMIC, low‐middle–income country; PHO, pediatric hematology‐oncology; PICU, pediatric intensive care unit; UMIC, upper‐middle–income country.
All participating centers implemented a uniform prospective registry of CDEs in hospitalized PHO patients between April 17 and June 1, 2017, and continued registration before, during, and after the implementation of PEWS. Centers were mentored to create registration systems that capture all eligible events occurring in their inpatient units, with iterative modifications and cross‐referencing other data sources (ICU admission logs, etc), until local leaders were confident they could capture all eligible events. For all identified CDEs, centers filled out a Spanish‐language paper case report form (CRF) describing the CDE (for an English translation, see Supporting Fig. 1) and sent the de‐identified CRF for central electronic data entry into a REDCap database. 21 Before data entry, CRFs were reviewed by a research assistant at SJCRH, and queries were sent to sites with identified errors to optimize data quality. Monthly non‐ICU PHO hospital admissions and PHO hospital inpatient days were reported using hospital statistics or unit admission logs. The current report is based on registry data collected in the first year of the collaborative (June 2017 to May 2018).
Pediatric Early Warning Systems (PEWS)
The PEWS used by all centers in the collaborative (EVAT in Spanish) included a scoring tool with 5 components (neurologic, cardiovascular, respiratory, nursing concern, and family concern) (see Supporting Fig. 2) 7 that was calculated with every set of vital signs by bedside nurses as part of routine care for hospitalized patients and an associated action algorithm (for an example, see Supporting Fig. 3) that guided the medical team how to respond to patients with deterioration. This PEWS has been validated to predict the need for unplanned PICU transfer in pediatric oncology patients in Latin America 22 and in high‐resource settings. 6 Of the 16 collaborating centers, 3 centers already used PEWS at the start of prospective registry data collection; the remaining 13 centers implemented PEWS as part of the collaborative after the start of the data‐collection period. Subsequently, new centers joined the collaborative each year, currently with >60 participating centers.
Definitions
To allow for comparisons between centers, a CDE was uniformly defined as an event in any hospitalized PHO patient requiring an unplanned transfer to a higher level of care (such as an ICU), an ICU‐level intervention on the floor (vasoactive infusion, invasive or noninvasive mechanical ventilation, or cardiopulmonary resuscitation [CPR]), or a floor death in a patient without limitations on resuscitation (nonpalliative death). The CDE began at the time of the first ICU‐level intervention on the floor or an unplanned transfer to a higher level of care, and ended at the time of discharge from the higher level of care or, for patients who remained on the floor, at the time of the last ICU‐level intervention. CDE mortality was defined as a death occurring within 24 hours of the end of the CDE. Sepsis and organ dysfunction were defined using the criteria published by Goldstein et al, 23 and the Pediatric Index of Mortality 2 (PIM2) was calculated using standard criteria. 24 Laboratory data at the start of clinical deterioration included the presence of high lactate, defined as >2 mmol/L, thrombocytopenia, defined as platelets <50/μL, and neutropenia, defined as an absolute neutrophil count <500. For centers with implemented PEWS at the time of the CDE, the use of PEWS in a patient's care during deterioration was documented by recording the highest PEWS score in the 24 hours before the event. Floor cardiopulmonary arrests were defined as any event requiring acute invasive mechanical ventilation, CPR, or a nonpalliative death on the floor. Patients who had limitations on the escalation of care (those with do‐not‐resuscitate orders or those receiving palliative care) were excluded.
Human Subjects Approval
The CDE registry was approved by the SJCRH Institutional Review Board as quality improvement, and the retrospective analysis of de‐identified registry data described in this study was approved as nonhuman subjects research. All collaborating centers obtained formal permission to participate in the EVAT Multicenter Program and approved the prospective de‐identified registry as quality improvement. Additional institutional approval was obtained, where needed, to publish this analysis of registry data.
Statistical Analysis
Because this study focuses on event risk factors for CDE mortality, each deterioration event was used as the unit of analysis. Continuous variables were reported with median and interquartile range (IQR) values, and categorical variables were reported with the frequency and percentage. For situations in which data were missing, only events with available data were used for the analysis. To control for multiple CDEs from 1 patient, we used generalized estimating equations (GEEs) to identify event‐level and center‐level CDE characteristics associated with CDE mortality. The exchangeable correlation structure was used, and the standard errors were estimated using the robust sandwich estimator. To further investigate the robustness of the GEE results, an analysis was also conducted using the first event per patient. The associations between continuous/categorical CDE characteristics and CDE mortality were assessed by using the Wilcoxon rank‐sum test or the Fisher exact test. To control for multiple testing, P values were adjusted using the Benjamini‐Hochberg procedure. Moreover, characteristics at the start of the event and at the time of transfer to a higher level of care that had P values < .05 in the univariate analysis were included in a multivariate GEE model to study their joint effect on CDE mortality. A multivariate GEE model was also used to evaluate organizational characteristics related to CDE mortality by choosing 1 significant characteristic from each group of highly correlated variables. Two‐sided P values < .05 were considered significant. The analysis was performed using SAS software version 9.4 for Windows (SAS Institute Inc) and R version 3.6.3 (R Core Team).
Results
The 16 collaborating PHO centers reported 553 CDEs in 467 individual patients and 504 unique hospital admissions during the initial 12 months of prospective data collection. These events occurred among a total of 11,536 PHO hospital admissions and 119,414 PHO inpatient days, representing a CDE rate of 4.63 per 1000 inpatient days (for individual center data, see Supporting Table 1).
CDE Characteristics
CDE characteristics are described in Table 2 and in Supporting Tables 2, 3, and 4. Events occurred among patients with a median age of 8 years who most commonly had a primary diagnosis of leukemia (70%). Only 1 event occurred in a patient who had a history of hematopoietic cell transplantation; all others were oncology patients. The most common reasons for hospital admission were for diagnostic workup (55%) and treatment of acute infection (23%).
TABLE 2.
Event Risk Factors for Mortality During Clinical Deterioration Events
| Characteristic | All CDEs, n = 553 | CDE Survival, n = 394 | CDE Mortality, n = 159 | P value | Adjusted P value a |
|---|---|---|---|---|---|
| Patient characteristic | |||||
| Age: median [IQR], y | 8.1 [3.8‐13.1] | 8.5 [3.8‐13.3] | 7.3 [3.7‐12.4] | .2841 | .3934 |
| Male, n (%) | 306 (55.3%) | 219 (55.6%) | 87 (54.7%) | .8566 | .8566 |
| Oncologic diagnosis n (%) | .8020 | .8566 | |||
| Hematologic malignancy | 425 (76.8%) | 305 (77.4%) | 120 (75.5%) | ||
| Solid tumor | 120 (21.7%) | 84 (21.3%) | 36 (22.6%) | ||
| Other | 8 (1.5%) | 5 (1.3%) | 3 (1.9%) | ||
| Characteristic at start of CDE | |||||
| Event time of day b n (%) | .5057 | .6247 | |||
| Daytime: 7 am to 7 pm | 339 (61.3%) | 238 (60.4%) | 101 (63.5%) | ||
| Nighttime: 7 pm to 7 am | 214 (38.7%) | 156 (39.6%) | 58 (36.5%) | ||
| Day of the week n (%) | .8226 | .8566 | |||
| Weekday | 424 (76.7%) | 303 (76.9%) | 121 (76.1%) | ||
| Weekend | 129 (23.3%) | 91 (23.1%) | 38 (23.9%) | ||
| Any ICU‐level interventions on the floor n (%) | 162 (29.3%) | 99 (25.1%) | 63 (39.6%) | .0012 | .0028 |
| PEWS implemented at time of event? n (%) | 277 (50.1%) | 232 (58.9%) | 45 (28.3%) | <.0001 | <.0001 |
| ICU consult? n (%) | 478 (86.4%) | 353 (89.6%) | 125 (78.6%) | .0032 | .0066 |
| Transfer to higher level of care? n (%) | 498 (90.1%) | 363 (92.1%) | 135 (84.9%) | .0209 | .0313 |
| Reason for deterioration n (%) | |||||
| Sepsis/septic shock | 352 (63.7%) | 250 (63.5%) | 102 (64.2%) | .8137 | .8566 |
| Respiratory distress | 224 (40.5%) | 127 (32.2%) | 97 (61.0%) | <.0001 | <.0001 |
| Other CV dysfunction | 121 (21.9%) | 66 (16.8%) | 55 (34.6%) | <.0001 | <.0001 |
| Neurologic deterioration | 97 (17.5%) | 56 (14.2%) | 41 (25.8%) | .0036 | .0066 |
| High lactate: >2 mmol/L n (%) | 146 (26.4%) | 93 (23.6%) | 53 (33.3%) | .0008 | .0021 |
| Missing | 199 | 135 | 64 | ||
| Thrombocytopenia, <50/μL: n (%) | 248 (44.9%) | 162 (41.1%) | 86 (54.1%) | .0038 | .0066 |
| Missing | 26 | 19 | 7 | ||
| Neutropenia, ANC <500 n (%) | 280 (50.6%) | 196 (49.8%) | 84 (52.8%) | .3378 | .4434 |
| Missing | 31 | 20 | 11 | ||
| Any organ dysfunction | 390 (70.5%) | 245 (62.2%) | 145 (91.2%) | <.0001 | <.0001 |
| No. of organs with dysfunction: median [IQR] | 1 [0‐2] | 1 [0‐2] | 2 [1‐3] | <.0001 | <.0001 |
| Interventions required during CDE | |||||
| Vasoactive infusions n (%) | 319 (57.7%) | 177 (44.9%) | 142 (89.3%) | .0098 | .0158 |
| Any invasive mechanical ventilation c n (%) | 237 (42.9%) | 92 (23.4%) | 145 (91.3%) | <.0001 | <.0001 |
| Cardiopulmonary resuscitation n (%) | 77 (13.9%) | 2 (0.5%) | 75 (47.2%) | <.0001 | <.0001 |
| At the Time of Transfer to a Higher Level of Care | P value | Adjusted P value a | |||
|---|---|---|---|---|---|
| All Transfers, n = 498 | CDE Survival, n = 363 | CDE Mortality, n = 135 | |||
| PIM2 at time of transfer: median [IQR] | 6.3 [4.4‐11.4] | 5.75 [4.1‐8.5] | 11.66 [6‐33.2] | <.0001 | <.0001 |
| Sepsis at time of transfer n (%) | <.0001 | <.0001 | |||
| No | 154 (30.9%) | 120 (33.1%) | 34 (25.2%) | ||
| Sepsis | 118 (23.7%) | 100 (27.6%) | 18 (13.3%) | ||
| Severe sepsis | 47 (9.4%) | 34 (9.4%) | 13 (9.6%) | ||
| Septic shock | 179 (35.9%) | 109 (30.0%) | 70 (51.9%) | ||
| Any organ dysfunction at time of transfer n (%) | 367 (73.7%) | 232 (63.9%) | 135 (100.0%) | <.0001 | <.0001 |
| No. of organs with dysfunction: median [IQR] | 1 [0‐3] | 1 [0‐2] | 3 [2‐4] | <.0001 | <.0001 |
Abbreviations: ANC, absolute neutrophil count; CDE, clinical deterioration event; CV, cardiovascular; ICU, intensive care unit; IQR, interquartile range; PEWS, Pediatric Early Warning System; PIM, Pediatric Index of Mortality.
P values were adjusted for the false‐discovery rate.
For day (8 am to 4 pm) versus night (4 pm to 8 am), P = .788 (not significant).
Intubation or tracheostomy with mechanical ventilation was categorized as invasive.
The most frequent initial CDE was an unplanned transfer to a higher level of care; however, 29% of events required an ICU‐level intervention (vasoactive infusions, mechanical ventilation, or CPR) on the floor. Ultimately, 90% of patients were transferred to a higher level of care; however, 55 events (10%) that required ICU‐level interventions never had access to a higher level of care due to a lack of ICU resources in their hospital or mortality prior to transfer. Among those who were transferred to a higher level of care, greater than one‐third (37%) reported no ICU bed availability at the time of transfer request, with a median wait of 4 hours (IQR, 3.0‐6.2 hours).
The most common reasons for deterioration were sepsis or septic shock (64%) and respiratory distress (41%). Approximately one‐half of patients were neutropenic (51%) and thrombocytopenic (45%) at the time of the CDE, and the majority had at least 1 organ with dysfunction (71%) (for details, see Supporting Table 5). Most events (70%) required at least 1 ICU‐level intervention. Over one‐half required vasoactive infusions (58%; median, 2.5 days; IQR, 0.7‐6.5 days), and 42% required invasive mechanical ventilation (median, 4.6 days; IQR, 1.3‐10.6 days). Of the events that resulted in transfer to a higher level of care, the median PIM2 score at the time of transfer was 6.3% (IQR, 4.4%‐11.4%). Most patients met criteria for sepsis (69%) and had organ dysfunction (74%) at the time of transfer.
CDEs lasted a median of 4.2 days (IQR, 2‐9 days), with an event mortality of 29% (159 events), representing a CDE mortality rate of 1.33 per 1000 inpatient days, and one‐third of mortalities (32%) occurred within the first 24 hours.
Event Risk Factors for CDE Mortality
Table 2 lists the event characteristics associated with mortality during CDEs. Using the deterioration event as the unit of analysis and controlling for multiple sampling, patient characteristics (age, sex, or oncologic diagnosis) and the timing of the event (time of day or day of the week) were not associated with event mortality. Significant risk factors for event mortality included receiving any ICU‐level intervention on the hospital floor, not having PEWS implemented in the hospital at the time of the event, not receiving an ICU consult, and not being transferred to a higher level of care. Deterioration caused by respiratory distress, neurologic, and non‐sepsis cardiovascular dysfunction, and with a high lactate, low platelets, and organ dysfunction at event recognition were associated with an increased risk of mortality. Patients requiring ICU interventions, such as vasoactive infusions (45% mortality) or invasive mechanical ventilation (61% mortality), had a higher risk of mortality (Table 2 and Fig. 1). Of 77 events requiring CPR only 2 patients survived the deterioration event (3%), and 1 survived to hospital discharge (1%). Among patients who were transferred to a higher level of care, those with higher PIM2 scores, severe sepsis or septic shock, and more organ dysfunction at the time of transfer had a higher risk of mortality. These results were consistent when using only the first event per patient (see Supporting Table 6) and when controlling for multiple testing (Table 2). In multivariate analysis of characteristics at the start of CDE not having PEWS implemented at the time of event, not receiving an ICU consultation, deterioration caused by respiratory distress, neurologic, and non‐sepsis cardiovascular dysfunction, and presence of organ dysfunction at event recognition were associated with event mortality (see Supporting Table 7). Of the characteristics that were present at the time of transfer to a higher level of care, the PIM2 score and the presence of any organ dysfunction were associated with event mortality (see Supporting Table 8).
Figure 1.
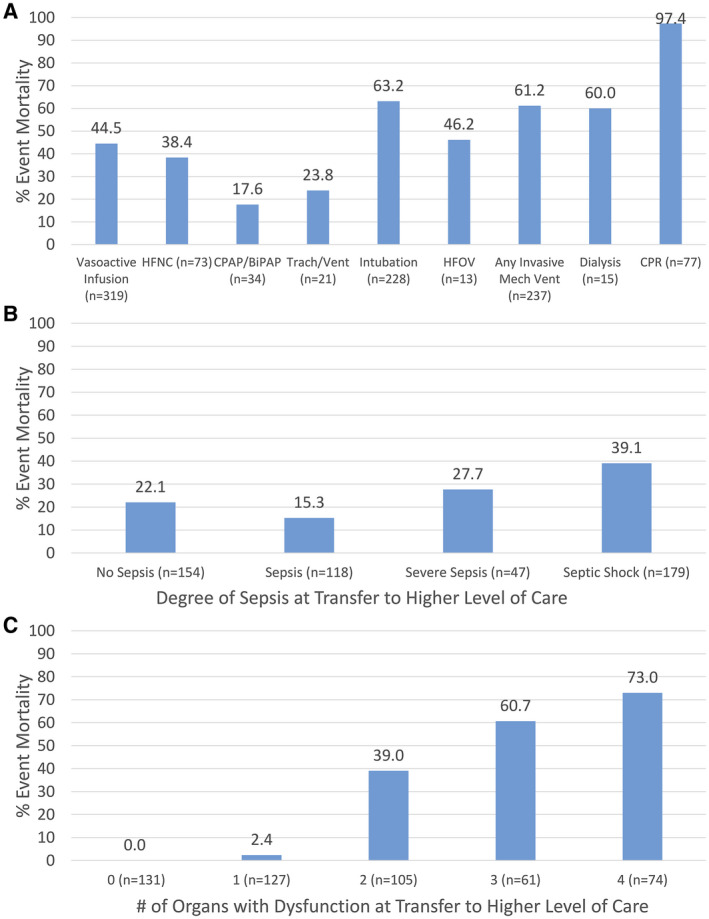
Clinical deterioration event (CDE) characteristics and the percent mortality are illustrated among hospitalized pediatric hematology‐oncology patients who had CDEs according to (A) the requirement for intensive care unit‐level interventions during the CDE (n = 553), (B) categories of sepsis at the time of transfer to a higher level of care (n = 498), and (C) the number of organs with dysfunction at the time of transfer to a higher level of care (n = 498). BiPAP indicates bilevel positive airway pressure; CPAP, continuous positive airway pressure; CPR, cardiopulmonary resuscitation; HFNC, high‐flow nasal cannula; HFOV, high‐flow oscillatory ventilation; Mech, mechanical.
Organizational Risk Factors for Higher CDE Mortality
Our analysis demonstrated large variation in CDE rates, characteristics, and outcomes among the 16 collaborating centers (Fig. 2; see Supporting Table 1). The frequency of deterioration ranged from 1.44 to 9.13 per 1000 inpatient days, and event mortality ranged from 11% to 79% across centers, or 0.36 to 5.80 per 1000 inpatient days (16‐fold difference) (see Supporting Table 1).
Figure 2.
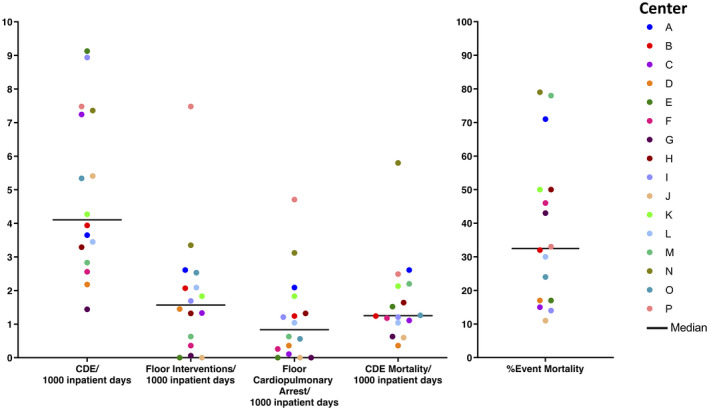
Center variations in the rates of clinical deterioration events (CDEs) and mortality are illustrated. Variations in the rates of CDE per 1000 inpatient days, floor intensive care unit‐level interventions per 1000 inpatient days, floor cardiopulmonary arrests per 1000 inpatient days, CDE mortality per 1000 inpatient days, and the percent mortality of all documented CDEs are depicted. Each dot color represents 1 center (n = 16), and the dark black line indicates the median in each category.
Center characteristics associated with increased CDE mortality are described in Table 3. Events that occurred in specialized oncology centers, centers with higher PHO volumes (total PHO hospital patient days, new annual pediatric oncology diagnoses, number of pediatric oncology beds), centers with increased capacity for patient monitoring (fewer patients per nurse on the PHO floor, implemented PEWS at the start of the study period), those with less use of ICU interventions on the floor (rate of floor interventions and floor cardiopulmonary arrests per 1000 inpatient days, more frequent transfer to a higher level of care), and those with a lower severity of illness on transfer (PIM2 score, the number of organs with dysfunction) had lower mortality. Interestingly, CDEs in centers with more PICU beds had higher mortality. CDE mortality was not related to the country income level or patient case mix (percentage of events with leukemia). In multivariate analysis, the floor nursing ratio, the rate of floor cardiopulmonary arrests, and having PEWS implemented at the start of the study period were associated with event mortality (see Supporting Table 9).
TABLE 3.
Associations Between Center Clinical Deterioration Event Mortality and Center Characteristics
| Center Characteristic | Correlation Coefficient | OR (95% CI) | P | Adjusted P a |
|---|---|---|---|---|
| UMIC/HIC vs LIC/LMIC | −0.0737 | 0.93 (0.58‐1.49) | .7606 | .7606 |
| Pediatric hospital vs all ages | −0.1734 | 0.84 (0.57‐1.23) | .3743 | .401 |
| Oncology hospital vs pediatric/general multidisciplinary | −1.0345 | 0.36 (0.24‐0.52) | <.0001 | <.0001 |
| Total PHO hospital patient‐days: June 2017 to May 2018 | −0.0431 | 0.96 (0.94‐0.98) | <.0001 | <.0001 |
| No. of annual new pediatric cancer diagnoses | −9.00E‐04 | 0.999 (0.998‐1) | .045 | .0562 |
| No. of PHO beds | −0.0261 | 0.97 (0.96‐0.98) | <.0001 | <.0001 |
| No. of PICU beds | 0.0537 | 1.06 (1.02‐1.09) | .0004 | .0007 |
| Case mix, % of events with leukemia | −0.7872 | 0.46 (0.11‐1.93) | .283 | .3265 |
| Average floor nursing ratio: 1 nurse/x patients | 0.1251 | 1.13 (1.07‐1.20) | <.0001 | <.0001 |
| Rate of floor interventions per 1000 patient‐days | 0.1283 | 1.14 (1.01‐1.27) | .0278 | .0379 |
| Rate of floor cardiopulmonary arrest per 1000 patient‐days | 0.3822 | 1.46 (1.24‐1.72) | <.0001 | <.0001 |
| PEWS implemented at start of study period | −1.2685 | 0.28 (0.19‐0.42) | <.0001 | <.0001 |
| Percentage of CDEs with transfer to higher level of care b | −2.1371 | 0.12 (0.2‐0.76) | .024 | .036 |
| Median PIM2 on PICU admission b | 0.9396 | 2.56 (2.01‐3.26) | <.0001 | <.0001 |
| Median no. of organs with dysfunction on transfer | 0.2138 | 1.24 (1.16‐1.37) | <.0001 | <.0001 |
Abbreviations: CDEs, clinical deterioration events; HIC, high‐income country; LIC, low‐income country; LMIC, low‐middle–income country; OR, odds ratio; PHO, pediatric hematology‐oncology; PEWS, Pediatric Early Warning System; PICU, pediatric intensive care unit; PIM2, Pediatric Index of Mortality 2; UMIC, upper‐middle–income country.
P values were adjusted for the false‐discovery rate.
These included 15 centers with ICUs.
Discussion
The current study represents the first multicenter initiative to describe clinical deterioration and outcomes in hospitalized children with cancer in resource‐limited settings. The pediatric oncology centers included in this prospective cohort varied by center type, organization, size of PHO service, and human and ICU resources available to treat critical illness. Our findings demonstrate frequent deterioration with high event mortality (29%), which increases in patients who require ICU‐level interventions. Although this represents higher mortality than reported in critically ill children with cancer in the United States (7%‐15%), 3 , 6 , 25 it is similar to findings from a large international meta‐analysis of oncology PICU outcomes in high‐resource settings (33% mortality among medical PICU admissions). 5 While it is likely that data from high‐resource settings include higher risk patients, such as hematopoietic cell transplantation recipients, compared with our cohort, these results demonstrate that, despite resource limitations, reasonable survival rates are possible in these patients with the provision of appropriate critical care.
Unlike typical practice in high‐resource settings, nearly one‐third of deterioration events (29%) in our cohort required ICU‐level interventions on the hospital floor, and not all patients with critical illness had access to ICU admission (10% remained on the floor). Not surprisingly, this resulted in a higher rate of floor cardiopulmonary arrest than reported in PHO patients in high‐resource settings (0.68 vs 0.22 per 1000 inpatient days). 25 Our data suggest that this occurred due to a lack of ICU bed availability and delays in recognizing critical illness, both of which are challenges that must be addressed to improve overall hospital survival in these high‐risk patients.
In our current analysis, non‐sepsis cardiovascular dysfunction, respiratory failure, and neurologic deterioration were associated with higher event mortality, similar to other studies that evaluated risk factors for mortality in critically ill children with cancer. 3 , 4 , 15 , 16 , 17 , 18 , 26 , 27 Importantly, event‐specific factors, such as a higher severity of illness (organ dysfunction) at event recognition, the use of ICU‐level interventions on the floor, lack of an implemented PEWS, and no ICU consultation or ICU transfer, were identified as risk factors for mortality. Severity of illness on event recognition, lack of implemented PEWS, and no ICU consultation were significant in multivariate analysis. Similarly, among patients who were transferred to the ICU, those who had a higher severity of illness (higher PIM2 scores and the presence of organ dysfunction) at ICU admission had higher event mortality.
Among the 16 collaborating PHO centers, the rate of deterioration and event mortality varied widely, with a difference >16‐fold in the CDE mortality rate between the highest and lowest performing centers. These findings were expected given the known variation in PICU resources in Latin American hospitals. 28 , 29 CDEs occurring in specialized oncology centers with a higher volume of PHO patients had lower mortality in our cohort, likely because of greater institutional familiarity with managing these patients. This differs from findings in recent studies on volume‐outcome relations in high‐resource PHO hospitals. 30 Interestingly, events occurring in centers that had more PICU beds had higher mortality, possibly representing larger multidisciplinary pediatric hospitals without a dedicated focus on pediatric oncology.
Hospital practices around the management of critical illness in PHO patients also were significantly related to mortality in our analysis. Events occurring in centers with fewer PHO patients per nurse and with PEWS implemented at the start of the study, representing improved ability to monitor for clinical changes in hospitalized patients, had lower mortality. Similarly, events in centers using less floor‐based ICU interventions and earlier ICU transfer of critically ill patients at a lower severity of illness had lower mortality. Not surprisingly, events occurring in centers with lower rates of floor cardiopulmonary arrests, a standard quality measure, also had lower mortality. These findings likely represent the impact of different floor monitoring, ICU use, and transfer practices for PHO patients on patient outcomes. It is likely that some centers more proactively identify critically ill PHO patients and expeditiously transfer them to a higher level of care, whereas others delay transfer, resulting in more organ failure, the use of floor‐based ICU interventions, floor cardiopulmonary arrest, and event mortality. These differences highlight the fact that PICU bed capacity does not guarantee actual PICU access for certain patient populations. Because these identified indicators represent modifiable hospital characteristics driven by policies and clinician practice, the current analysis is helpful to guide best practices in the identification and management of critically ill PHO patients and is consistent with published baseline standards for PHO floor nursing ratios 31 and pediatric oncology critical care quality indicators in resource‐limited settings. 20
Our data suggest that interventions that aid in earlier recognition of critical illness, prompt ICU consultation, and timely ICU transfer may improve hospital outcomes in these high‐risk patients. PEWS implementation can achieve these goals, potentially explaining why events at centers using PEWS had lower mortality in our analysis. Importantly, PEWS allow for the early identification of abnormal vital signs and other warning signs, such as staff and family concern. 22 , 32 For centers in this cohort without PEWS at the time of the study, the implementation of these and other quality‐improvement measures may improve survival. Beyond the direct patient benefit, institutional interest in PEWS implementation signals a broader commitment to improving the quality of hospital care. However, these findings are preliminary, and the impact of PEWS must be confirmed through further study. We plan to prospectively explore the impact of PEWS implementation on patient outcomes at collaborating centers in future work.
The current study has several limitations. This was a retrospective analysis of data collected through a prospective quality‐improvement registry. Although deterioration events were identified prospectively, most centers retrospectively extracted clinical data from patient charts either during or after the event. It is possible that not all centers captured all elements of deterioration events, and some characteristics may have been difficult to extract from the medical record. Laboratory data, such as lactate levels and blood counts, were not collected for all events, resulting in some missing data. It is also possible that some deterioration events were missed in registration. However, the centers received extensive mentorship on strategies to capture all deterioration events, including cross‐checking with other data sources, and these are typically memorable because they require acute medical interventions. Similarly, our internal data review minimized missing data, and data quality was optimized by central data entry. For these reasons, we are confident that our findings represent a valid assessment of clinical deterioration in PHO patients among collaborating centers.
We were also challenged by the heterogeneity among collaborating PHO centers regarding institutional capacity and practices. Similarly, centers that were not using PEWS at the start of the study were planning implementation during the data collection period. The change in the use of PEWS in these centers may have led to earlier identification of deterioration, decreasing the frequency of risk factors related to CDE mortality. However, this should not change the underlying relation between risk factors and mortality, and the variation between centers allowed us to explore associations between hospital practices and event mortality and to make important suggestion for best practices in managing critically ill children with cancer. The inclusion of centers with different organizational structures, resources, and practices also makes our findings more generalizable to other PHO centers in Latin America and in other resource‐limited settings.
Conclusion
We present a large, international, multicenter study of characteristics and outcomes of clinical deterioration in pediatric oncology patients hospitalized in resource‐limited centers in Latin America, demonstrating frequent deterioration with high mortality and significant variability in outcomes across centers. Our analysis identified modifiable hospital practices that may contribute to higher mortality in these settings. Improvements in early identification, timely ICU consultation, and ICU access for children with cancer who develop critical illness through implementation of quality‐improvement systems like PEWS may improve hospital survival in these patients. These findings, however, must be confirmed through studies evaluating the impact of PEWS implementation and other quality initiatives on patient outcomes. International collaboration to develop best practices in pediatric oncology critical care, such as our collaborative, can aid in this objective and help meet the World Health Organization imperative to improve childhood cancer outcomes globally.
Funding Support
This work was supported by a Conquer Cancer Foundation Global Oncology Young Investigator Award (Asya Agulnik) and by the American Lebanese Syrian Associated Charities (ALSAC).
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Asya Agulnik: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, software, supervision, validation, visualization, writing–original draft preparation, and writing–reviewing and editing. Adolfo Cárdenas: Investigation, methodology, validation, and writing–reviewing and editing. Angela K. Carrillo: Data curation, formal analysis, project administration, software, validation, visualization, and writing–reviewing and editing. Purva Bulsara: Formal analysis, software, visualization, and writing–reviewing and editing. Marcela Garza: Data curation, project administration, and writing–reviewing and editing. Yvania Alfonso Carreras: Investigation, validation, and writing–reviewing and editing. Manuel Alvarado: Investigation, validation, and writing–reviewing and editing. Patricia Calderón: Investigation, validation, and writing–reviewing and editing. Rosdali Díaz: Investigation, validation, and writing–reviewing and editing. Claudia de León: Investigation, validation, and writing–reviewing and editing. Claudia del Real: Investigation, validation, and writing–reviewing and editing. Tania Huitz: Investigation, validation, and writing–reviewing and editing. Angélica Martínez: Investigation, validation, and writing–reviewing and editing. Scheybi Miralda: Investigation, validation, and writing–reviewing and editing. Erika Montalvo: Investigation, validation, and writing–reviewing and editing. Octavia Negrín: Investigation, validation, and writing–reviewing and editing. Alejandra Osuna: Investigation, validation, and writing–reviewing and editing. Clara Krystal Perez Fermin: Investigation, validation, and writing–reviewing and editing. Estuardo Pineda: Investigation, validation, and writing–reviewing and editing. Dora Soberanis: Investigation, validation, and writing–reviewing and editing. Maria Susana Juárez Tobias: Investigation, validation, and writing–reviewing and editing. Zhaohua Lu: Formal analysis, methodology, software, supervision, visualization, writing–original draft preparation, and writing–reviewing and editing. Carlos Rodriguez‐Galindo: Conceptualization, funding acquisition, methodology, supervision, and writing–reviewing and editing. The EVAT Study Group: Investigation and validation.
Supporting information
Supplementary Material
Agulnik A, Cárdenas A, Carrillo AK, Bulsara P, Garza M, Alfonso Carreras Y, Alvarado M, Calderón P, Díaz R, de León C, del Real C, Huitz T, Martínez A, Miralda S, Montalvo E, Negrín O, Osuna A, Perez Fermin CK, Pineda E, Soberanis D, Juárez Tobias MS, Lu Z, Rodriguez‐Galindo C; the EVAT Study Group . Clinical and organizational risk factors for mortality during deterioration events among pediatric oncology patients in Latin America: A multicenter prospective cohort. Cancer.2021. 10.1002/cncr.33411
Members of the Early Warning Assessment Scale (Escala de Valoración de Alerta Temprana) [EVAT]) Study Group include the following: Daniela Covarrubias, Gabriela Pérez, and Deysi Cahuich (Campeche); Maite Echavarría, Lety Cabreros, and Edgardo Tostado (Culiacán); Alejandra Méndez and Ricardo Mack (Guatemala); Carmitude Michel and Madonie Raydmound (Haiti); Eduardo Altamirano and Faviola Castillo (La Paz); Roxana Morales, Carlos Villasante, Esmenia Pérez, and Zulma Carpio (Lima); Grania Obando, Sabrina Alguera, Angela Alvarado, and Reyna Jirón (Managua); Karina Quintero, Kenia Miller, Almida de Rosas, Gloria Ceballo, and Hercilia Gómez (Panamá); Jocelyn Rivera, Silvana Espinoza, and Cinthia Hernández (Querétaro); Ivón Sánchez, Erika Villanueva, and Jeaneth Quelal (Quito); Yolanda Galarza, Angélica Cortéz, Blanca Cuellar, Verónica Medina, and Francisco Alejo (San Luis Potosí); Angélica Hernández, Soad Fuentes, and Mónica Palma (San Salvador); Lucy de León, Gretchen Fernández, Rosa Almonte, and Mayra Jimenez (Santiago); Wendy Gómez, Emma Almonte, Evelyn Ramos, Octavia Negrín, and Johanna P. Gil (Santo Domingo); Ligia Fu and Blanca Maradiaga (Tegucigalpa); and Miriam Armenta and Alicia Sánchez (Tijuana).
Contributor Information
Asya Agulnik, Email: asya.agulnik@stjude.org.
EVAT Study Group:
Daniela Covarrubias, Gabriela Pérez, Deysi Cahuich, Maite Echavarría, Lety Cabreros, Edgardo Tostado, Alejandra Méndez, Ricardo Mack, Carmitude Michel, Madonie Raydmound, Eduardo Altamirano, Faviola Castillo, Roxana Morales, Carlos Villasante, Esmenia Pérez, Zulma Carpio, Grania Obando, Sabrina Alguera, Angela Alvarado, Reyna Jirón, Karina Quintero, Kenia Miller, Almida de Rosas, Gloria Ceballo, Hercilia Gómez, Jocelyn Rivera, Silvana Espinoza, Cinthia Hernández, Ivón Sánchez, Erika Villanueva, Jeaneth Quelal, Yolanda Galarza, Angélica Cortéz, Blanca Cuellar, Verónica Medina, Francisco Alejo, Angélica Hernández, Soad Fuentes, Mónica Palma, Lucy de León, Gretchen Fernández, Rosa Almonte, Mayra Jimenez, Wendy Gómez, Emma Almonte, Evelyn Ramos, Johanna P. Gil, Ligia Fu, Blanca Maradiaga, Miriam Armenta, and Alicia Sánchez
References
- 1. Demaret P, Pettersen G, Hubert P, Teira P, Emeriaud G. The critically‐ill pediatric hemato‐oncology patient: epidemiology, management, and strategy of transfer to the pediatric intensive care unit. Ann Intensive Care. 2012;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenman MB, Vik T, Hui SL, Breitfeld PP. Hospital resource utilization in childhood cancer. J Pediatr Hematol Oncol. 2005;27:295‐300. [DOI] [PubMed] [Google Scholar]
- 3. Zinter MS, DuBois SG, Spicer A, Matthay K, Sapru A. Pediatric cancer type predicts infection rate, need for critical care intervention, and mortality in the pediatric intensive care unit. Intensive Care Med. 2014;40:1536‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faraci M, Bagnasco F, Giardino S, et al. Intensive care unit admission in children with malignant or nonmalignant disease: incidence, outcome, and prognostic factors: a single‐center experience. J Pediatr Hematol Oncol. 2014;36:e403‐e409. [DOI] [PubMed] [Google Scholar]
- 5. Wosten‐van Asperen RM, van Gestel JPJ, van Grotel M, et al. PICU mortality of children with cancer admitted to pediatric intensive care unit a systematic review and meta‐analysis. Crit Rev Oncol Hematol. 2019;142:153‐163. [DOI] [PubMed] [Google Scholar]
- 6. Agulnik A, Forbes PW, Stenquist N, Rodriguez‐Galindo C, Kleinman M. Validation of a pediatric early warning score in hospitalized pediatric oncology and hematopoietic stem cell transplant patients. Pediatr Crit Care Med. 2016;17:e146‐e153. [DOI] [PubMed] [Google Scholar]
- 7. Agulnik A, Mora Robles LN, Forbes PW, et al. Improved outcomes after successful implementation of a pediatric early warning system (PEWS) in a resource‐limited pediatric oncology hospital. Cancer. 2017;123:2965‐2974. [DOI] [PubMed] [Google Scholar]
- 8. Soeteman M, Potratz J, Nielsen JSA, et al. Research priorities in pediatric onco‐critical care: an international Delphi consensus study. Intensive Care Med. 2019;45:1681‐1683. [DOI] [PubMed] [Google Scholar]
- 9. Bhakta N, Force LM, Allemani C, et al. Childhood cancer burden: a review of global estimates. Lancet Oncol. 2019;20:e42‐e53. [DOI] [PubMed] [Google Scholar]
- 10. Rodriguez‐Galindo C, Friedrich P, Morrissey L, Frazier L. Global challenges in pediatric oncology. Curr Opin Pediatr. 2013;25:3‐15. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization . Global Initiative for Childhood Cancer. Accessed March 2, 2021. http://www.who.int/cancer/childhood‐cancer/en/
- 12. St Jude Children's Research Hospital . St Jude Global. Accessed March 2, 2020. https://www.stjude.org/global.html
- 13. Bansal D, Davidson A, Supriyadi E, Njuguna F, Ribeiro RC, Kaspers GJL. SIOP PODC adapted risk stratification and treatment guidelines: recommendations for acute myeloid leukemia in resource‐limited settings. Pediatr Blood Cancer. 2019;2019:e28087. [DOI] [PubMed] [Google Scholar]
- 14. Gupta S, Antillon FA, Bonilla M, et al. Treatment‐related mortality in children with acute lymphoblastic leukemia in Central America. Cancer. 2011;117:4788‐4795. [DOI] [PubMed] [Google Scholar]
- 15. Dursun O, Hazar V, Karasu GT, Uygun V, Tosun O, Yesilipek A. Prognostic factors in pediatric cancer patients admitted to the pediatric intensive care unit. J Pediatr Hematol Oncol. 2009;31:481‐484. [DOI] [PubMed] [Google Scholar]
- 16. Akhtar N, Fadoo Z, Panju S, Haque A. Outcome and prognostic factors seen in pediatric oncology patients admitted in PICU of a developing country. Indian J Pediatr. 2011;78:969‐972. [DOI] [PubMed] [Google Scholar]
- 17. Ali AM, Sayed HA, Mohammed MM. The Outcome of critically ill pediatric cancer patients admitted to the pediatric intensive care unit in a tertiary university oncology center in a developing country: a 5‐year experience. J Pediatr Hematol Oncol. 2016;38:355‐359. [DOI] [PubMed] [Google Scholar]
- 18. Khan Sial GZ, Khan SJ. Pediatric cancer outcomes in an intensive care unit in Pakistan. J Glob Oncol. 2019;5:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agulnik A, Johnson S, Wilkes R, Faughnan L, Carrillo A, Morrison R. Impact of implementing a pediatric early warning system (PEWS) in a pediatric oncology hospital. Pediatr Qual Saf. 2018;3:e065. [Google Scholar]
- 20. Arias AV, Garza M, Murthy S, et al. Quality and capacity indicators for hospitalized pediatric oncology patients with critical illness: a modified delphi consensus. Cancer Med. 2020;9:6984‐6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agulnik A, Mendez Aceituno A, Mora Robles LN, et al. Validation of a pediatric early warning system for hospitalized pediatric oncology patients in a resource‐limited setting. Cancer. 2017;123:4903‐4913. [DOI] [PubMed] [Google Scholar]
- 23. Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis . International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2‐8. [DOI] [PubMed] [Google Scholar]
- 24. Slater A, Shann F, Pearson G, Paediatric Index of Mortality (PIM) Study Group . PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29:278‐285. [DOI] [PubMed] [Google Scholar]
- 25. Agulnik A, Gossett J, Carrillo A, Kang G, Morrison R. Abnormal vital signs predict critical deterioration in hospitalized pediatric hematology‐oncology and post‐hematopoietic cell transplant patients. Front Oncol. 2020;10:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dalton HJ, Slonim AD, Pollack MM. Multicenter outcome of pediatric oncology patients requiring intensive care. Pediatr Hematol Oncol. 2003;20:643‐649. [PubMed] [Google Scholar]
- 27. Haase R, Lieser U, Kramm C, et al. Management of oncology patients admitted to the paediatric intensive care unit of a general children's hospital—a single center analysis. Klin Padiatr. 2011;223:142‐146. [DOI] [PubMed] [Google Scholar]
- 28. Campos‐Mino S, Sasbon JS, von Dessauer B. [Pediatric intensive care in Latin America]. Med Intensiva. 2012;36:3‐10. [DOI] [PubMed] [Google Scholar]
- 29. Diaz F, Carvajal C, Gonzalez‐Dambrauskas S, et al. Abstract O‐44: Organizational characteristics and resources in Latin‐American pediatric intensive care units. Preliminary report of REAL‐CIP (Realidad en America Latina de Cuidados Intensivos Pediatricos) study. Pediatr Crit Care Med. 2018;19(6S):19. [Google Scholar]
- 30. Wilkes JJ, Hennessy S, Xiao R, et al. Volume‐outcome relationships in pediatric acute lymphoblastic leukemia: association between hospital pediatric and pediatric oncology volume with mortality and intensive care resources during initial therapy. Clin Lymphoma Myeloma Leuk. 2016;16:404‐410.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Day S, Hollis R, Challinor J, Bevilacqua G, Bosomprah E. Baseline standards for paediatric oncology nursing care in low to middle income countries: position statement of the SIOP PODC Nursing Working Group. Lancet Oncol. 2014;15:681‐682. [DOI] [PubMed] [Google Scholar]
- 32. Brady PW, Zix J, Brilli R, et al. Developing and evaluating the success of a family activated medical emergency team: a quality improvement report. BMJ Qual Saf. 2015;24:203‐211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


