Abstract
HAMLET is a protein‐lipid complex with a specific and broad bactericidal and tumoricidal activity, that lacks cytotoxic activity against healthy cells. In this study, we show that HAMLET also has general immune‐stimulatory effects on primary human monocyte‐derived dendritic cells and macrophages (Mo‐DC and Mo‐M) and murine RAW264.7 macrophages. HAMLET, but not its components alpha‐lactalbumin or oleic acid, induces mature CD14low/–CD83+ Mo‐DC and M1‐like CD14+CD86++ Mo‐M surface phenotypes. Concomitantly, inflammatory mediators, including IL‐2, IL‐6, IL‐10, IL‐12 and MIP‐1α, were released in the supernatant of HAMLET‐stimulated cells, indicating a mainly pro‐inflammatory phenotype. The HAMLET‐induced phenotype was mediated by calcium, NFκB and p38 MAPK signaling in Mo‐DCs and calcium, NFκB and ERK signaling in Mo‐M as inhibitors of these pathways almost completely blocked the induction of mature Mo‐DCs and M1‐like Mo‐M. Compared to unstimulated Mo‐DCs, HAMLET‐stimulated Mo‐DCs were more potent in inducing T cell proliferation and HAMLET‐stimulated macrophages were more efficient in phagocytosis of Streptococcus pneumoniae in vitro. This indicates a functionally activated phenotype of HAMLET‐stimulated DCs and macrophages. Combined, we propose that HAMLET has a two‐fold anti‐bacterial activity; one inducing direct cytotoxic activity, the other indirectly mediating elimination of bacteria by activation of immune cells of the myeloid lineage.
Keywords: alpha‐lactalbumin, dendritic cells, HAMLET, macrophages, myeloid cells
HAMLET, a human milk protein‐lipid complex induces a pro‐inflammatory phenotype of macrophages and dendritic cells, resulting in augmented release of inflammatory mediators, stimulation of T‐cell proliferation and phagocytosis of pneumococci. We propose that HAMLET's anti‐bacterial activity is two‐fold; one directly bactericidal and the other mediated by activation of myeloid cells.
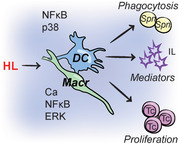
Introduction
HAMLET (Human Alpha‐lactalbumin Made LEthal to Tumor cells) is a complex consisting of the human milk protein alpha‐lactalbumin (αLA) in a partially unfolded state and oleic acid, that exhibit both selective bactericidal activity [1] and broad and potent tumoricidal effects [2, 3]. As of today, HAMLET has shown a direct bactericidal activity against numerous respiratory pathogens [1, 4, 5] as well as efficacy against 40 different cancer cell‐lines and primary tumors of different origin, without exerting cytotoxic effects against healthy, differentiated cells, in vitro or in vivo [2, 6‐10]. Even though HAMLET spares healthy cells, it can still bind to and induce an increased level of intracellular calcium in these cells [2]. However, unlike in bacteria and cancer cells, this effect is temporal and HAMLET is not internalized and do not trigger cell death [2, 9, 10]. In contrast, a study comparing kidney carcinoma cells and healthy primary kidney epithelial cells reported that HAMLET induces signals involved in innate immunity in normal epithelial cells but not cancer cells [11]. Genes, including IL‐6, TNFα and IκB were up‐regulated in response to HAMLET, suggesting that HAMLET could have immunomodulatory effects.
Innate immune cells of the myeloid lineage such as dendritic cells (DCs) and macrophages are key actors in tissue homeostasis, during infection as well as during tumor progression. Myeloid cells are characterized by an enormous plasticity, with extremes stretching from pro‐ to anti‐inflammatory phenotypes, as determined by environmental cues. DCs are professional antigen‐presenting cells that continuously scan the body. Upon exposure to inflammatory stimuli, they migrate to lymph nodes, mature and up‐regulate co‐receptors involved in T‐lymphocyte activation [12]. DCs are thus a crucial component in bridging the innate and adaptive immune system and in eliciting efficient immune responses against pathogens. Macrophages, on the other hand, are specialized in phagocytosis of pathogens as well as of dead cells and debris. Macrophage activation is often simplified according to the M1/M2 dichotomy. Classically activated M1 macrophages are pro‐inflammatory and can eliminate pathogens [13]. In contrast, alternatively activated M2 macrophages possess anti‐inflammatory, pro‐angiogenic and tissue remodeling capacity, depending on the stimuli, and are thus involved in the resolution phase of inflammatory processes [13]. These are, however, the extremes of macrophage activation and polarization, as myeloid cells are very plastic in nature and the phenotypes are highly dependent on environmental signals.
An intriguing field of research is whether therapeutic targeting of myeloid cells could ameliorate immune responses during severe infections or mount anti‐tumor immune responses. Based on current literature, we hypothesized that HAMLET could be a potential new compound with immunomodulatory effects on myeloid cells.
Results
HAMLET induces morphological changes of Mo‐M and Mo‐DCs
In order to assess the potential immunomodulatory effect of HAMLET, we cultured primary human monocyte‐derived DCs (Mo‐DCs) and ‐macrophages (Mo‐M) and stimulated with HAMLET. Native αLA and lipopolysaccharide (LPS) were used as controls. In line with previous observations that HAMLET spares healthy cells [2], HAMLET did not induce toxicity of Mo‐DCs or Mo‐M (Supplemental Fig. 1A‐B) nor of murine RAW264.7 macrophages (Supplemental Fig. 1C). When stimulating Mo‐DCs and Mo‐M, we noticed concentration‐dependent changes in morphology of the cells upon HAMLET treatment (Fig. 1). In Mo‐DC cultures, HAMLET induced prominent morphological changes with enrichment of cells displaying small protrusions, or large aggregates of cells (Fig. 1A‐B). This morphology was similar to that of LPS‐activated Mo‐DCs (Fig. 1A). Similarly, HAMLET‐treated Mo‐M displayed either numerous short protrusions or an elongated morphology, that was distinct from that of LPS‐activated Mo‐M (Fig. 1A‐B). αLA‐treated Mo‐M, on the other hand, were smoother and resembled untreated control cells (Fig. 1A).
Figure 1.
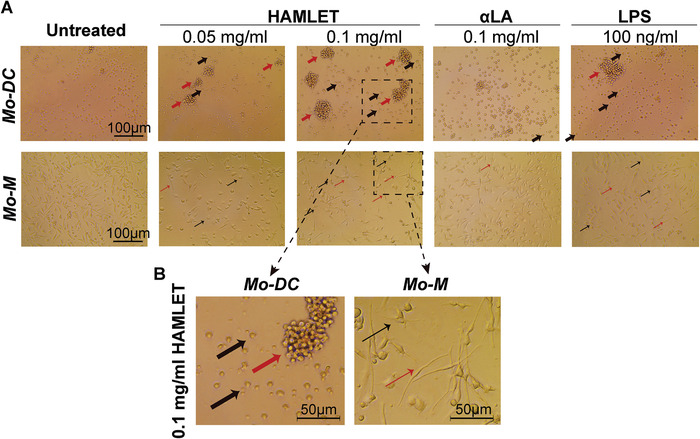
HAMLET induces morphological changes of Mo‐DCs and Mo‐M. (A‐B) Light microscopy images of primary human Mo‐DCs (upper panel) or Mo‐M (lower panel), treated or not with HAMLET, αLA or LPS at indicated concentrations. After 1‐h of treatment, the medium was replaced with fresh differentiation medium and the cells were grown overnight. Thick black arrows indicate Mo‐DCs with short protrusions and thick red arrows indicate aggregates of Mo‐DCs. Thin black arrows indicate Mo‐M with short protrusions and thin red arrows indicate Mo‐M with elongated morphology. (B) Higher magnification (dashed boxes in A) of Mo‐DCs (left panel) and Mo‐M (right panel) treated with 0.1 mg/ml HAMLET. All images were equally corrected for brightness to improve clarity. Images representative of >3 experiments (n = 1‐2 donors per experiment).
HAMLET induces maturation of Mo‐DCs and an M1‐like surface phenotype of Mo‐M
To further investigate the described changes, we analyzed the surface phenotypes by flow cytometry. When analyzing the effects of HAMLET on Mo‐DC cultures, we noticed a significantly increased population expressing the DC‐maturation marker CD83 that was apparent even at low HAMLET concentrations (Fig. 2). LPS stimulation similarly induced an increase in CD83‐positive Mo‐DCs. In contrast, the human MHC class II receptor HLA‐DR was only modestly, and non‐significantly, affected by stimulation with HAMLET or LPS (Fig. 2A‐B and Supplemental Fig. 2A). The effects were specific for HAMLET‐treated cultures as native αLA had minor to no effects on these markers, although some batch‐dependent variations were observed (Fig. 2 and Supplemental Fig. 2A). Similarly, oleic acid (OA), alone or bound to bovine serum albumin (BSA), at comparable concentration as present in the HAMLET complex showed no significant effect on CD83 expression compared to untreated cells (Supplemental Fig. 2B).
Figure 2.
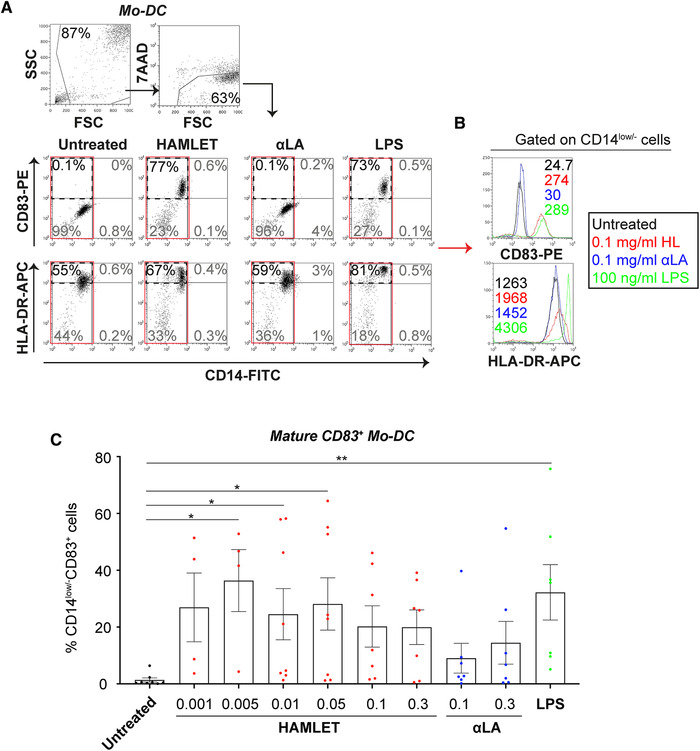
HAMLET induces maturation of Mo‐DCs. Flow cytometric analyses of primary human Mo‐DCs treated in SFM with HAMLET (red), native αLA (blue) or LPS (green) for 1‐h and grown overnight in fresh differentiation medium. (A) Gating strategy and dot plots of CD14, CD83 and HLA‐DR from cells treated with 0.1 mg/ml HAMLET or αLA or 100 ng/ml LPS. Gated on all viable cells, with percentage (%) in gate indicated. (B) Histograms represent mean fluorescence intensity (MFI; values in graphs) of indicated receptors, gated on all CD14low/– Mo‐DCs (red box in A). (C) Individual data points of percentages (%) CD14low/–CD83+ mature Mo‐DCs, with mean and SEM shown. Data from 4–8 individual experiments (n = 4‐8 donors). Statistics by Kruskal‐Wallis with Dunn's multiple comparisons test to untreated control. *P<0.05 and **P<0.01.
In Mo‐M cultures, HAMLET significantly increased the percentage of cells expressing the M1 macrophage‐associated co‐receptor CD86 in a concentration‐dependent manner (Fig. 3). Similarly, concentration‐dependent, yet non‐significant, enrichment of cells expressing the co‐receptor CD80 was observed in HAMLET‐treated Mo‐M cultures, whereas HLA‐DR was only modestly affected (Fig. 3 and Supplemental Fig. 2C‐D). No difference was, however, seen regarding the M2 macrophage marker CD206 (Fig. 3B and Supplemental Fig. 2E). The positive control, LPS failed to induce expression of any of the analyzed markers (Fig. 3 and Supplemental Fig. 2C‐E). This may be due to the low stimulatory capacity of LPS in the absence of serum in Mo‐M cultures, as has previously been described [14]. Similarly, native αLA had little to no effect on the markers investigated, resembling the untreated controls (Fig. 3 and Supplemental Fig. 2C‐E). Furthermore, OA, alone or bound to BSA, did not induce any CD86 expression above the untreated control (Supplemental Fig. 2F). This suggests that Mo‐DCs and Mo‐M treated with HAMLET, but not with either native αLA or OA, consistently acquire a surface phenotype reminiscent of mature Mo‐DCs and classically activated M1‐like macrophages, respectively.
Figure 3.
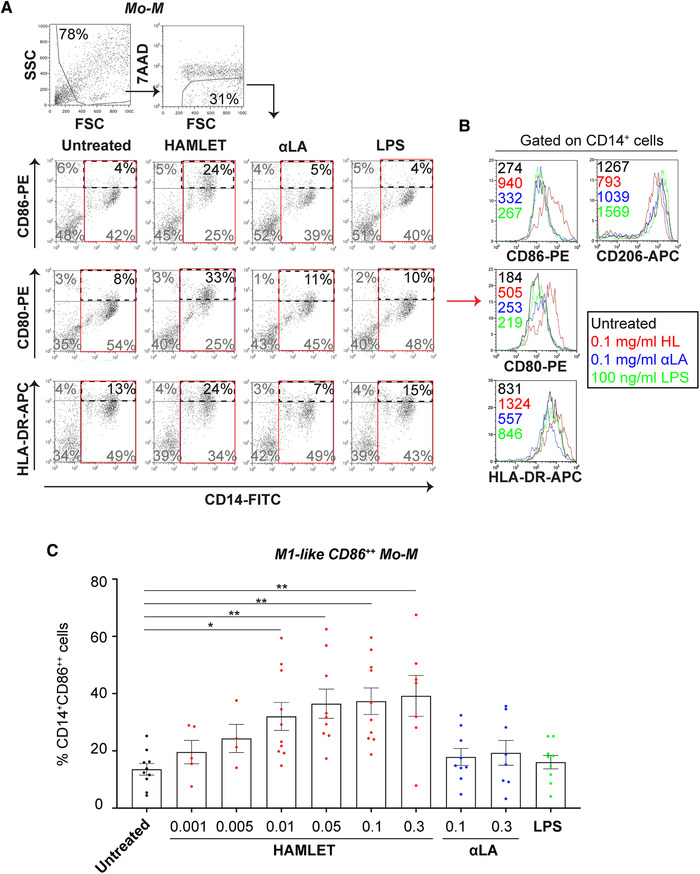
HAMLET induces an M1‐like Mo‐M surface phenotype. Flow cytometric analyses of primary human Mo‐M treated in SFM with HAMLET (red), native αLA (blue) or LPS (green) for 1‐h and grown over night in fresh differentiation medium. (A) Gating strategy and dot plots of CD14, CD86, CD80 and HLA‐DR from cells treated with 0.1 mg/ml HAMLET or αLA or 100 ng/ml LPS. Gated on all viable cells, with percentage (%) of cells in gate indicated. (B) Histograms represent mean fluorescence intensity (MFI; values in graphs) of indicated receptors, gated on all CD14+ Mo‐M (red box in A). (C) Individual data points of percentages (%) CD14+CD86++ M1‐like Mo‐M, with mean and SEM shown. Data from 4–10 individual experiments (n = 4‐10 donors). Statistics by Kruskal‐Wallis with Dunn's multiple comparisons test to untreated control. *P<0.05 and **P<0.01.
HAMLET induces a broad range of inflammatory mediators
Next we measured the levels of cytokines in the supernatants of cells stimulated with HAMLET, native αLA or OA. Bio‐plex Immunoassay revealed that the concentration of most inflammatory mediators investigated e.g., IL‐2, IL‐6, IL‐12, IL‐15, and MIP‐1α were significantly higher in supernatants of HAMLET‐treated Mo‐DCs and Mo‐M as compared to αLA‐treated cells (Supplemental Fig. 3). These results were confirmed using cytometric cytokine bead array (CBA), in which IL‐8, IL‐6, IL‐10 and TNFα, were all markedly higher in supernatants from HAMLET‐treated Mo‐DCs than in supernatants from untreated, αLA‐treated or OA‐treated Mo‐DC (Fig. 4A and Supplemental Fig. 4A), whereas IL‐8, IL‐6 and TNFα were markedly higher in supernatants from HAMLET‐treated Mo‐M than from untreated, αLA‐treated or OA‐treated Mo‐M (Fig. 4B and Supplemental Fig. 4B). LPS showed results similar to HAMLET for Mo‐DCs (Fig. 4A). However, similar to the phenotypic assays above, LPS only induced a moderate release of cytokines in Mo‐M cultures (Fig. 4B). For RAW264.7 cells, IL‐6, MCP‐1 and TNFα were markedly higher in supernatants from HAMLET‐treated RAW264.7 cells than untreated cells (Fig. 4C). Similarly, IL‐6, MCP‐1 and TNFα were higher in supernatants from RAW264.7 cells treated with LPS compared with untreated cells, albeit not significantly so (Fig. 4C).
Figure 4.
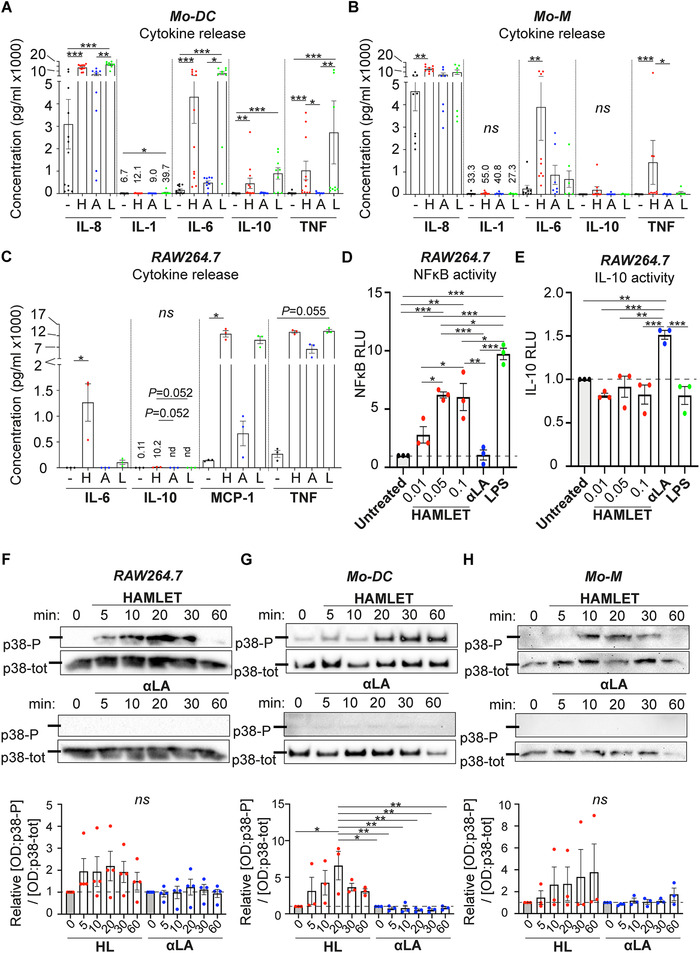
HAMLET induces NFκB and p38 activation. (A‐C) Supernatants from primary human Mo‐DCs (A), Mo‐M (B) and RAW264.7 cells (C) stimulated in SFM with 0.1 mg/ml HAMLET (red) or native αLA (blue) or with 100 ng/ml LPS (green) for 1‐h and grown overnight in fresh differentiation medium, were analyzed by cytometric cytokine bead array (CBA). Individual data points of respective cytokine concentration (pg/ml) with mean and SEM are shown. Data from 7–12 individual experiments (n = 7‐12 donors) (A‐B) or N = 3 individual experiments with one technical replicate per experiment (C). Statistics by Kruskal‐Wallis with Dunn's multiple comparison test between all samples. ns = not significant, *P<0.05, **P<0.01, ***P<0.001. (D‐E) Dual luciferase NFκB (D) or IL‐10 (E) promoter assay using murine RAW264.7 macrophages. The relative dual luciferase units (RLU) were measured using pTK‐Renilla and pCMV‐Renilla vector, respectively, as controls. Individual data points of relative promoter activity (RLU) to untreated control with mean and SEM are shown. N = 3 individual experiments with mean of two technical replicates per experiment. Statistics by one‐way ANOVA with Tukey's multiple comparison test. *P<0.05, **P<0.01 and ***P<0.001. (F‐H) RAW264.7 cells (F), primary human Mo‐DCs (G) and Mo‐M (H) were treated with 0.1 mg/ml HAMLET or native αLA for indicated time points and analyzed by Western blot for phosphorylated p38. Total p38 are used as loading control. Lines indicate the 40 kD molecular weight marker. Blots are representative of N = 3‐4 individual experiments (one donor per experiment). The blots have been cropped for presentation purposes (raw data shown in Supplemental Fig. 10). Graphs depict relative [OD of p38‐P]/[OD of p38‐tot] to 0‐min untreated control, with mean and SEM shown. N = 3‐4 experiments. Statistics by one‐way ANOVA with Tukey's multiple comparison test between all samples. ns = not significant, *P<0.05 and **P<0.01.
HAMLET induces NFκB and p38 signaling
The NFκB and MAPK pathways are well‐known mediators of inflammatory signaling. To analyze whether HAMLET could activate either of these pathways we performed NFκB‐luciferase assays using RAW264.7 macrophages. In line with the previous results, HAMLET, but not αLA, significantly induced NFκB activity in a concentration‐dependent manner (Fig. 4D). As the anti‐inflammatory cytokine IL‐10 was released upon HAMLET treatment (Fig. 4A‐C), we also investigated IL‐10 promoter activity by luciferase assay. Interestingly, HAMLET did not induce IL‐10 promoter activity, whereas αLA did (Fig. 4E). Furthermore, when analyzing p38 MAPK signaling in RAW264.7 cells and primary human Mo‐DCs and Mo‐M, HAMLET, but not native αLA, consistently induced phosphorylation of p38 (Fig. 4F‐H). Thus, HAMLET induces both NFκB and p38 MAPK signaling.
HAMLET‐induced activation is not blocked by PmB, but is affected by TAK242
Given that LPS is a common contaminant of lab reagents and has profound effects on inflammatory cells, we next evaluated any potential contribution of LPS to the HAMLET‐induced Mo‐DC and Mo‐M phenotypes. LPS was detected in all HAMLET‐batches (mean±SEM, 0.86±0.13 EU/ml), and the concentrations were equal to those present in the corresponding native αLA batches (mean±SEM, 0.92±0.22 EU/ml). Despite the presence of similar amounts of LPS, the morphology of αLA‐treated cells were similar to that of untreated control cells and αLA overall failed to induce Mo‐DC and Mo‐M activation and cytokine release (Figs. 1, 2, 3, 4). Nevertheless, as we detected low levels of LPS in HAMLET, we next stimulated Mo‐DCs and Mo‐M with HAMLET that was boiled for 1‐h at 98°C (heat‐inactivated) or treated with endotoxin‐removal resin. Neither boiled nor resin‐treated HAMLET differed significantly in their capacity to induce maturation/activation of Mo‐DCs and Mo‐M as compared with untreated HAMLET (Supplemental Fig. 5A‐B). At low concentrations, resin‐treated HAMLET, similar to resin‐treated LPS, displayed reduced capacity to stimulate Mo‐DC maturation (Supplemental Fig. 5A). However, at higher concentrations, resin‐treatment did not affect HAMLET's capacity to induce maturation/activation of Mo‐DC and Mo‐M (Supplemental Fig. 5A‐B). Resin‐treatment reduced the LPS content in HAMLET by 93%, however, it also reduced the fatty acid content in HAMLET by 60%. This affected HAMLET's bactericidal activities on Streptococcus pneumoniae strain D39, increasing the minimum inhibitory concentration (MIC) value 3.75 times, from 20 μg/ml to 75 μg/ml (Supplemental Fig. 5C). Consequently, resin‐treatment efficiently remove LPS from HAMLET, but also affects HAMLET's structural components and its activities.
As the results were inconclusive regarding the potential role of LPS in HAMLET‐induced maturation/activation, we next evaluated whether polymyxin B (PmB, a classic inhibitor of LPS), TAK242 (an intracellular inhibitor of LPS‐TLR4 signaling), L48H37 (an MD‐2 inhibitor that blocks TLR4 signaling extracellularly) or a neutralizing antibody of the LPS co‐receptor CD14 could block the HAMLET‐induced maturation/activation of Mo‐DCs and Mo‐M (Supplemental Fig. 5D‐F). Representative dose responses are shown in Supplemental Fig. 6. PmB had no effect on the HAMLET‐induced enrichment of CD83+ mature Mo‐DCs, whereas it significantly reduced the LPS‐induced maturation of Mo‐DCs (Supplemental Fig. 5D). Nor did PmB have any effect on HAMLET‐induced activation of Mo‐M (Supplemental Fig. 5E). Interestingly, the HAMLET‐induced maturation of Mo‐DCs was significantly affected by the TLR4 inhibitor TAK242, but not by L48H37 or the CD14 neutralizing antibody (Supplemental Fig. 5D). None of the inhibitors had any significant effect on HAMLET‐ or LPS‐induced activation of CD86++ M1‐like Mo‐M (Supplemental Fig. 5E), likely explained by the fact that the cells were differentiated in serum‐free media [14]. TAK242 was the only inhibitor that significantly affected HAMLET‐ and LPS‐induced release of cytokines from Mo‐DC and Mo‐M (Supplemental Fig. 5F). Furthermore, when analyzing the effect of PmB on HAMLET‐ and LPS‐induced NFκB activity in RAW264.7 cells (which are grown in medium with serum), PmB had a minute, yet significant, effect on HAMLET‐induced NFκB activity, and a more substantial inhibitory effect on LPS‐induced NFκB activity (1.2‐ and 2.7‐fold decrease compared to the control, respectively, Supplemental Fig. 5G). Altogether, these results indicate that the major HAMLET‐induced effects are not mediated by LPS, although the results cannot completely rule out a potential contribution of LPS.
As TAK242 seemed to affect the HAMLET‐induced phenotype, we next assessed whether HAMLET may engage TLRs and affect the cellular localization of TLR4 and TLR2, as has previously been reported for LPS‐stimulation of e.g., alveolar macrophages [15, 16]. As shown in Supplemental Fig. 7, HAMLET‐ and LPS‐treatment, and to a minor degree αLA‐treatment of Mo‐DC and Mo‐M affected the cellular localization of TLR4, where TLR4 re‐located to the cell periphery or aggregated in clusters. Similar results were also seen for TLR2 (data not shown). To further address this, we also co‐stained Mo‐DCs and Mo‐M treated with Alexa Fluor 568‐labelled HAMLET or αLA with antibodies against TLR4 or TLR2. HAMLET, and to a lesser extent αLA, localized in close proximity to TLR4 and TLR2 (Supplemental Fig. 8). In some areas, the signals overlapped, indicating that HAMLET may co‐localize with these TLRs.
The HAMLET‐induced effect is partly mediated by NFκB signaling
To delineate the specific pathway(s) involved in the HAMLET‐induced phenotypes, we next used a panel of inhibitors to assess the potential involvement of NFκB, p38 and ERK MAPK signaling (Fig. 5). Representative dose responses are shown in Supplemental Fig. 6A‐B. As calcium signals previously have been shown to be involved in HAMLET‐induced tumor cell death [17, 18] as well as HAMLET's bactericidal activity [19], we also included the calcium‐chelator BAPTA‐AM. The HAMLET‐induced phenotype was significantly blocked in the presence of the NFκB inhibitors MG132 and BOT‐64 for both Mo‐DCs and Mo‐M (Fig. 5A and B). Similarly, MG132 and BOT‐64 significantly blocked LPS‐induced maturation/activation of Mo‐DCs and Mo‐M (Fig. 5A‐B and Supplemental Fig. 6A‐B). BAPTA‐AM significantly reduced the HAMLET‐induced effect on Mo‐DCs and Mo‐M and had some effect on the LPS‐induced phenotype of Mo‐DCs (Fig. 5A‐B, Supplementary Fig. 6). The p38 inhibitors SB202190 and SB208580 both significantly reduced the HAMLET‐ and LPS‐induced effects on Mo‐DCs but had no inhibitory effect on Mo‐M (Fig. 5A and B), despite HAMLET's induction of p38 phosphorylation in both cell types. No effect was observed for either ERK inhibitor in Mo‐DC cultures, whereas U0126 significantly blocked the HAMLET‐ but not LPS‐induced activation of Mo‐M (Fig. 5A and B). Treatment with combination of inhibitors did not appear to have any additive effect in Mo‐DC or Mo‐M cultures (Supplemental Fig. 6C and D).
Figure 5.
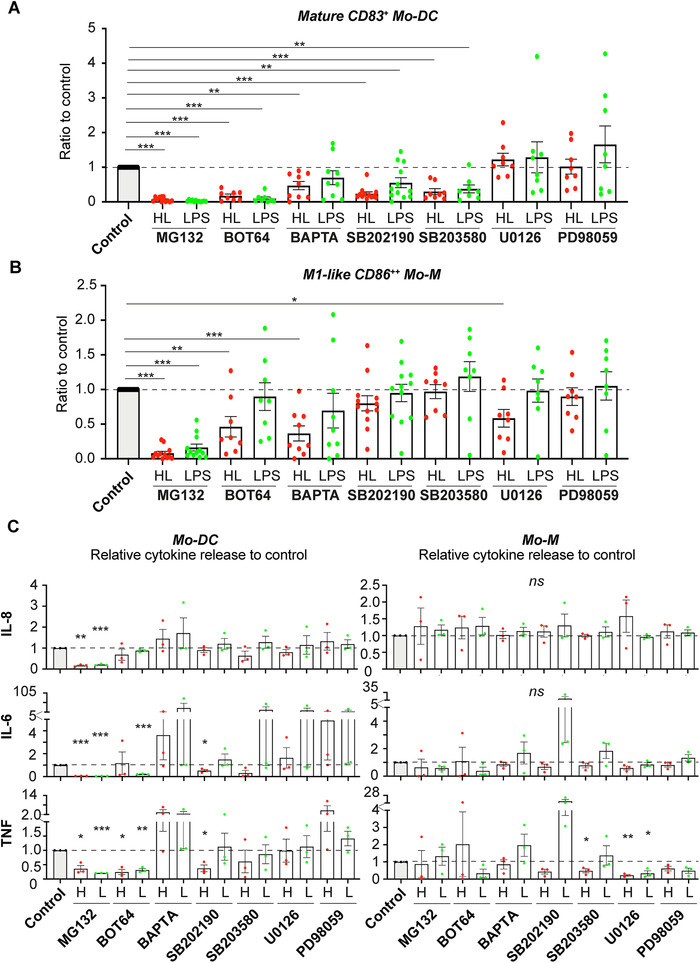
The HAMLET‐induced phenotypes are mediated by calcium, NFκB and p38 MAPK signaling. Primary human Mo‐DCs (A) or Mo‐M (B) were pre‐treated with indicated inhibitor for 2‐h, stimulated with 0.1 mg/ml HAMLET (HL; red) or 100 ng/ml LPS (green) for 1‐h and subsequently grown overnight in fresh differentiation medium. The cells were analyzed by flow cytometry. Individual data points of relative % CD14low/– CD83+ Mo‐DC or CD14+CD86++ M1‐like Mo‐M to respective control (HL or LPS without inhibitor, but with PBS, ethanol or DMSO), with mean and SEM shown. Data from 8–12 individual experiments (n = 8‐12 donors). (C) Supernatants from Mo‐DCs (left) and Mo‐M (right) from A‐B were analyzed by CBA. Individual data points of relative cytokine concentration to respective control (HL or LPS without inhibitor, but with PBS, ethanol or DMSO), with mean and SEM shown. Data from 3 individual experiments (n = 3 donors). All statistics by paired t‐test to respective control. ns = not significant, *P<0.05, **P<0.01 and ***P<0.001.
In line with these results, the HAMLET‐ and LPS‐induced release of cytokines was significantly blocked in the presence of MG132 in Mo‐DC cultures (Fig. 5C). BOT‐64 significantly inhibited the LPS‐, but not HAMLET‐induced release of IL‐6, whereas SB202190 significantly inhibited HAMLET‐, but not LPS‐induced, IL‐6 and TNFα release. In contrast, the cytokine release was only modestly affected by inhibitors in Mo‐M cultures, with the exception of significantly reduced TNFα in HAMLET‐, but not LPS‐treated Mo‐M in the presence of SB203580, and a reduction of HAMLET‐ and LPS‐induced TNFα when treated with U0126 (Fig. 5C). These results indicate that LPS‐ and HAMLET‐induced activation of Mo‐DCs, but not Mo‐M involved p38 signaling and that LPS‐induced activation of both Mo‐DCs and Mo‐M is mainly mediated by NFκB signaling, whereas NFκB and calcium are involved in HAMLET‐induced maturation/activation of Mo‐DCs and Mo‐M.
HAMLET‐treated macrophages display increased phagocytic activity against pneumococci
Finally, we investigated whether the HAMLET‐treated cells were functionally affected. We therefore evaluated the phagocytic capacity of RAW264.7 macrophages after treatment with HAMLET, αLA or LPS. In accordance with the activated phenotype, HAMLET‐treated macrophages were significantly more efficient in phagocytosis of S. pneumoniae, as assessed by determining the number of internalized bacteria after co‐culture (Fig. 6A). LPS‐activated macrophages similarly displayed increased phagocytosis, whereas αLA‐treated macrophages resembled untreated controls (Fig. 6A). Similarly, HAMLET‐treated primary human Mo‐M were significantly more efficient in phagocytosis of S. pneumoniae compared to untreated Mo‐M (Fig. 6B). No difference was, however, observed in the capacity to kill the internalized pneumococci for either RAW264.7 or Mo‐M (data not shown).
Figure 6.
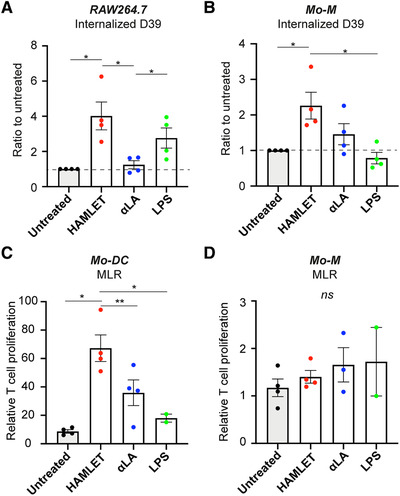
HAMLET induces functional changes to macrophages and DCs. (A‐B) Murine RAW264.7 macrophages (A) or primary human Mo‐M (B) were stimulated with 0.1 mg/ml HAMLET or native αLA, or 100 ng/ml LPS for 1‐h and grown overnight in fresh medium without stimulation. 1×107 CFU/ml S. pneumoniae D39 were added to pre‐stimulated cells and the number of internalized bacteria was analyzed by viable plate counts after 1‐h of co‐culture. Individual data points of relative number (CFU/ml) of internalized bacteria, with mean and SEM are shown from 4 individual experiments with mean of 3 technical replicates per experiment (A) or from 4 individual experiments (n = 4 donors) (B). Statistics by paired t‐test, *P<0.05. (C‐D) Primary human Mo‐DCs (C) and Mo‐M (D) were stimulated or not with 0.1 mg/ml HAMLET, 0.1 mg/ml native αLA or 100 ng/ml LPS for 1‐h and grown overnight in fresh differentiation medium. The cells were re‐seeded with allogeneic T‐lymphocytes and co‐cultured for 5 days in a mixed lymphocyte reaction (MLR). Relative proliferation of T‐lymphocytes to unstimulated T‐cell control was measured by [3H] incorporation. Individual data points with mean and SEM are shown. Data from 2–4 independent experiments (n = 2‐4 donors) . Statistics by paired t‐test, *P<0.05, **P<0.01.
Lastly, we evaluated the potential effect of HAMLET‐treatment on the T‐cell stimulatory capacity of primary human Mo‐DCs and Mo‐M. HAMLET‐treated Mo‐DCs displayed a significantly increased capacity to stimulate allogeneic T‐lymphocyte proliferation in a mixed lymphocyte reaction (MLR) compared to untreated and αLA‐treated controls (Fig. 6C). In contrast, only a slight and variable increase in T‐cell proliferation was seen in Mo‐M cultures, regardless of stimulation (Fig. 6D). When analyzing the T‐cell phenotype in the Mo‐DC MLR by flow cytometry, the vast majority of T‐cells were of CD4+CD183+ Th1 phenotype whereas few cells were CD4+CD294+ Th2 cells (Supplemental Fig. 9A). Compared to untreated Mo‐DCs, HAMLET‐treated Mo‐DCs tended to induce more Th1 cells and αLA‐treated Mo‐DC induce more Th2 cells, although not significantly so (Supplemental Fig. 9B‐C). Altogether, these results indicate that HAMLET induces functional changes in macrophages and DCs that result in both increased phagocytosis of bacteria and T‐lymphocyte proliferation.
Discussion
HAMLET as a treatment strategy is unique in that it has no cytotoxic effect on normal, healthy cells, but possesses a broad bactericidal and tumoricidal activity [2, 6]. Since its discovery, anti‐bacterial effects against several respiratory pathogens, including S. pneumoniae, Streptococcus pyogenes, Mycobacterium tuberculosis and Haemophilus influenzae have been described [1, 4, 5]. Additionally, anti‐tumor activity against more than 40 different tumor types have been determined and HAMLET is currently in Phase I/II trial for treatment of bladder cancer. Apart from the lack of cytotoxic effects on healthy cells, it is not well known how HAMLET affects non‐transformed cells. One study showed that HAMLET induces signals involved in innate immunity in healthy kidney epithelial cells [11]. Thus, we hypothesized that HAMLET may activate innate immune cells.
Here, we show that HAMLET has immune‐stimulatory effects on myeloid cells in vitro. HAMLET, unlike native αLA or oleic acid, consistently induced an activated morphology of Mo‐DCs and Mo‐M, mature Mo‐DC and M1‐like Mo‐M surface phenotypes and concomitant release of inflammatory mediators. This is in accordance with the HAMLET‐induced upregulation of genes involved in innate immune regulation (including IL‐1 and IL‐6) in healthy kidney epithelial cells [11]. Functionally, HAMLET‐treated cells more efficiently phagocytosed pneumococci and stimulated T‐cell proliferation than unstimulated or αLA‐stimulated cells. The effect was partially or almost completely blocked by the calcium chelator BAPTA‐AM and NFκB inhibitors MG132 and BOT‐64 in Mo‐DCs and Mo‐M, as well as the p38 inhibitors SB202190 and SB203580 in Mo‐DCs, indicating that HAMLET‐induced maturation/activation of Mo‐DC and Mo‐M is partially dependent on calcium signals, NFκB and p38 signaling. A role for p38 signaling in the HAMLET‐induced tumoricidal effects has previously been proposed, where SB202190 was shown to attenuate HAMLET‐induced death of kidney carcinoma cells [11].
LPS is a potent activator of myeloid cells. A previous study has reported that commercial αLA frequently is contaminated with LPS (333‐4228 EU/ml in 5 mg/ml αLA), which mediates a pro‐inflammatory effect on RAW264.7 macrophages [20]. LPS was also detected in our purified native αLA, as well as in HAMLET. However, although native αLA and HAMLET have equal amount of LPS, αLA had little to no effect on the morphology, induction of surface receptors, cytokine release and signaling pathways analyzed. Endotoxin‐removal resin efficiently removed LPS from HAMLET, but also affected HAMLET's structural components and its activities. The effect was, however, less pronounced at higher HAMLET concentrations, likely attributed to the finding that HAMLET induces phenotypic changes already at low concentrations. Furthermore, in our hands, PmB had no inhibitory activity on the HAMLET‐induced effects whereas it significantly blocked LPS‐induced maturation of Mo‐DCs and NFκB activity in RAW264.7 cells. Conversely, BAPTA‐AM blocked HAMLET‐, but not LPS‐induced maturation/activation of Mo‐DCs and Mo‐M. Despite this, we cannot fully rule out any role of LPS. It is possible that HAMLET, unlike native αLA, could function as an LPS‐binding protein, delivering LPS to TLR4 and mediate its signaling in an MD‐2 independent manner. That would be in accordance with the failure of the MD‐2 inhibitor L48H37 to block activity and the significant inhibitory effect of TAK242, that blocks TLR4 signaling intracellularly, on the HAMLET‐induced activation of Mo‐DCs and Mo‐M. HAMLET‐treatment, similar to LPS‐treatment, affected the cellular localization of TLR4 in Mo‐DCs and Mo‐M. Furthermore, HAMLET and to a minor extent αLA, appeared to localize in close proximity with TLR2 and TLR4, which warrants further study. It is also noteworthy that HAMLET continuously had greater phenotypic effects on the myeloid cells than LPS, indicating that the effect is, at least in part, HAMLET‐specific. Even if HAMLET would mediate parts of its effects via LPS, the net‐result is likely still an activation of immune responses.
In summary, we propose that HAMLET potentially exerts its anti‐bacterial activity (and possibly also tumoricidal activity) through dual mechanisms; a direct cytotoxic effect and an indirect effect mediated by activated immune cells. This adheres well with the current thinking on treatment strategies against infectious diseases that suggest that both elimination of microbes and bolstering host inflammation is required for optimal treatment efficacy [21, 22]. For example, using rIL‐2 as an adjuvant to antimicrobial tuberculosis treatment improved outcome and was associated with expansion of CD4+ and NK cell populations in the lung [23] and use of interferons as adjunct therapy during treatment of hepatitis B and C is currently in clinically use [24]. Similar to some antibiotics and antimicrobial peptides [25, 26], HAMLET has the benefit of being a two‐in‐one treatment with both antibacterial and immunomodulatory effects that can be used to eliminate bacteria at the site of infection. Due to its broad effect it is unlikely that pathogens develop resistance to HAMLET, a feature that is unprecedented among current treatment strategies and evoke hope for HAMLET as an upcoming drug candidate.
Materials and methods
Reagents and production of HAMLET
All recombinant human cytokines were from R&D Systems (Minneapolis, MN, USA). rhGM‐CSF and rhIL‐4 were used at a concentration of 10 ng/ml and 20 ng/ml, respectively. Oleic acid and LPS from Salmonella enterica serotype typhimurium were purchased from Sigma Aldrich (St Louis, MO, USA) and used at a concentration of 30 μg/ml and 100 ng/ml, respectively. Inhibitors used were all purchased from Sigma Aldrich unless otherwise stated: Polymyxin B sulfate salt (PmB; binds to and inactivates LPS, 1x:10 μg/ml), BAPTA‐AM (cell permeable calcium chelator, 1x:50 μM), SB202190 (p38 MAPK inhibitor, 1x:20 μM), SB203580 (ATP competitive inhibitor of p38 and Akt, 1x:15 μM), U0126 (MEK‐1/2 ERK inhibitor, 1x:20 μM, purchased from ThermoFisher Scientific), PD98059 (MEK/ERK inhibitor, 1x:10 μM, purchased from ThermoFisher), MG‐132 (proteasome inhibitor, that inhibits NFκB signaling by blocking the degradation of inhibitor of kappa B alpha, IκBα; 1x:10 μM), BOT‐64 (inhibits NFκB signaling by blocking IKKβ‐mediated IκBα phosphorylation, 1x:10 μM), TAK‐242 (TLR4 inhibitor that acts intracellularly by blocking Cys747 on TLR4 to the adaptor proteins TIRAP/TRAM; 1x:3 μM) and L48H37 (blocks TLR4 extracellularly by acting as an MD‐2 inhibitor; 1x:10 μM). The CD14 neutralizing antibody (clone 61D3; used at 1x:1:100 dilution) was from Novus Biologicals. The 1x concentration used represent concentrations based on the manufacturer's recommendation and according to the current literature [11, 27‐34]. Inhibition experiments were also conducted using inhibitors at different concentrations to titrate their activity.
αLA was enriched from breast milk from human donors. HAMLET was produced by converting EDTA‐treated αLA in the presence of oleic acid (C18:1) on an anion‐exchange matrix, as previously described [3]. Unconverted, native αLA has no bactericidal or tumoricidal effect and was used in all experiments as a control. For all batches, the bactericidal activity of HAMLET and αLA was measured against the Streptococcus pneumoniae lab strain D39 to validate any inter‐batch variability of activity. Minimal inhibitory concentration (MIC) of HAMLET was determined by the microdilution method, as described [4]. All batches were also assessed for LPS (endotoxin) using Limulus amebocyte lysate assay (ThermoFisher) according to manufacturer's instructions; mean±SEM endotoxin in HAMLET and αLA 0.86±0.13 EU/ml and 0.92±0.22 EU/ml, respectively. Where indicated, HAMLET and LPS were treated with Pierce High‐Capacity Endotoxin Removal Resin (ThermoFisher) according to the manufacturer's instructions.
Isolation of primary human monocytes and T‐lymphocytes
Leukocytes from healthy blood donors were collected from leukocyte concentrates. Approval was obtained from the Regional Ethics Committee in Lund, Sweden (Dnr 2016/433). The leukocyte concentrate was diluted 1:4 in PBS supplemented with 2.5% w/v sucrose and 2 mM EDTA and overlaid on Ficoll‐Paque Plus (GE Healthcare). Peripheral blood mononuclear cells (PBMCs) were collected by density centrifugation and washed once in PBS. Monocytes or T‐lymphocytes were isolated with Pan‐Monocyte isolation kit or Pan‐T‐cell isolation kit (both from Miltenyi Biotec, Bergisch Gladback, Germany).
Cell culture
Murine RAW264.7 macrophages were routinely grown in DMEM with addition of 10% heat‐inactivated fetal bovine serum (FBS) at 37°C, 5% CO2. Primary human monocytes were differentiated for 6 days into dendritic cells (Mo‐DCs) or macrophages (Mo‐M) as previously described [35, 36]. Briefly, Mo‐DCs were grown in RPMI‐1640 supplemented with 10% heat‐inactivated FBS, penicillin/streptomycin, rhGM‐CSF and rhIL‐4 and Mo‐M where grown in OptiMEM supplemented with penicillin/streptomycin and rhGM‐CSF. Medium was replaced on day 3 and 5 of differentiation. RAW264.7 cells or differentiated Mo‐M and Mo‐DCs were washed once in serum‐free medium before stimulation for up to 1‐h at 37°C. For inhibition experiments, the cells were pre‐incubated with indicated inhibitor or relevant controls 2‐h before stimulation with HAMLET, αLA or LPS. For flow cytometry and functional assays, the medium was replaced with fresh differentiation medium (containing serum and/or cytokines) after 1‐h of stimulation, and the cells were cultured for another 18‐20‐h, after which cells and supernatants were harvested. All media and supplements were from ThermoFisher.
Viability assays
Cell viability was assessed according to the manufacturers’ instructions using trypan blue exclusion assay (ThermoFisher) or WST‐1 assay (Sigma Aldrich). For WST‐1 assay, primary human monocytes were seeded in triplicates in 96‐well plates at a density of 10,000 cells per well and differentiated into Mo‐DCs or Mo‐M. RAW264.7 cells were seeded in triplicates at a density of 8000 cells per well the day before stimulation. The cells were stimulated with HAMLET or controls as described above and viability was assessed 18‐h after stimulation.
Flow cytometry
Mo‐DCs and Mo‐M were detached using non‐enzymatic cell dissociation solution (Sigma Aldrich) and washed once in FACS buffer prior to staining for flow cytometry. Antibodies used were; FITC‐CD14 clone M5E2 (1:10), APC‐HLA‐DR clone L243 (1:50), PE‐CD80 clone L307.4 (1:15), PE‐CD86 clone IT2.2 (1:15), PE‐CD83 clone HB15e (1:15), PE‐CD1a clone HI149 (1:15), APC‐ CD206 clone 19.2 (1:20), APC‐CD209 clone DCN46 (1:15) and APC‐CD33 clone WM53 (1:10), all from BD Biosciences (San Jose, Ca, USA). All analyses were performed using 7AAD as a cell death discriminator. Cells were analyzed using a FACS Calibur (BD Biosciences). All antibodies and dilutions were optimized previously [35, 36], adhering to the guidelines by Cossarizza et al [37].
Cytokine analyses
The Human Cytokine 27‐plex assay (Bio‐PlexTM Pro, Bio‐Rad Laboratories) with two supplemental analytes was used to evaluate the concentration of the following inflammatory mediators: FGF basic, Eotaxin, G‐CSF, GM‐CSF, IFN‐γ, IL‐1β, IL‐1ra, IL‐2, IL‐4, IL‐5, IL‐6, IL‐7, IL‐8, IL‐9, IL‐10, IL‐12 (p70), IL‐13, IL‐15, IL‐17A, IP‐10, MCP‐1 (MCAF), MIP‐1α, MIP‐1β, PDGF‐BB, RANTES, TNFα,VEGF, M‐CSF and IL‐18. For these specific analyses BSA was added to the supernatants at a final concentration of 0.5 % before analysis, according to the manufacturer's instructions. The concentrations of IL‐8, IL‐6, IL‐12, IL‐10, TNFα and IL‐1β were further validated using Human Inflammatory Cytokine Cytometric Bead Array (CBA; BD Biosciences, San Diego, CA; USA) according to the manufacturer's instructions. IL‐12 was largely undetectable by this method and hence excluded in this study.
Dual luciferase reporter assay
RAW264.7 cells were transfected with 0.5 μg pNFκB‐luciferase plasmid (BD Biosciences) and 0.05 μg TK‐Renilla luciferase (Promega, Madison, WI) or with 0.5 μg pIL‐10‐luciferase plasmid (a kind gift from Prof. L. Ziegler‐Heitbrock) and CMV‐Renilla luciferase (Promega), using Turbofect transfection reagent (Thermo Fisher). At 24‐h post‐transfection, the cells were stimulated for 1‐h with HAMLET, αLA or LPS, as described above. All experiments were performed in duplicates. Relative luciferase activity was assessed 24‐h after stimulation using Fluostar Omega microplate reader (BMG labtech).
Western blot
Primary human Mo‐DCs or Mo‐M, or RAW264.7 macrophages were stimulated with HAMLET or αLA in serum‐free medium, harvested at indicated time points and directly lysed in boiling Laemmli sample buffer supplemented with 100 mM DTT. Protein lysates were run on 10% SDS‐PAGE gels, transferred to PVDF membranes and blotted using p38‐P (Thr180/Tyr182, clone 12F8) and p38 MAPK antibodies from Cell Signaling Technology Inc (Danvers, MA, USA). Densitometry of bands was conducted using ImageJ (National Institutes of Health, MD, USA) and the relative [OD of p38‐P]/[OD of p38‐tot] to 0‐min untreated control was calculated.
Immunofluorescence
0.1×106 RAW264.7 cells or primary human monocytes were seeded per well in chamber slides and differentiated to Mo‐DCs and Mo‐M. In one set of experiments, the cells were treated or not with 10 μg/ml PmB for 2‐h and stimulated with HAMLET, αLA or LPS, as described above and incubated a further 18‐h. In a second set of experiments, the cells were treated with 0.2 mg/ml of Alexa Fluor 568‐conjugated HAMLET or αLA (labeled using the Alexa Fluor 568 labeling kit from ThermoFisher according to the manufacturer's instructions) for 2‐h and washed. For both sets of experiments, the cells were then fixed in 4% paraformaldehyde (PFA), permeabilized in 0.1% Triton‐X100 in PBS and stained with TLR4 (clone 25) 1:100 or TLR2 (clone A‐9) 1:100 (both from Santa Cruz Biotechnology) overnight at 4°C after which the cells were incubated with goat‐anti‐mouse Alexa Fluor 488‐conjugated secondary antibody 1:200 for 1‐h. Vectashield DAPI mounting medium (Vector Laboratories, Inc.) was used for nuclear staining. The cells were visualized using an Olympus BX41 microscope and images were collected by the cellSense Dimension v1.9 software using an Olympus DP72 CCD camera (Olympus).
Phagocytosis assay
RAW264.7 cells were seeded at a density of 300,000 cells per 24‐well on the day before stimulation with HAMLET, αLA or LPS, as described above. Primary human monocytes were seeded at a density of 1.2×106 cells per 24‐well and differentiated to Mo‐M, as described above and stimulated with HAMLET, αLA or LPS. 18‐h post‐stimulation, the cells were washed in fresh cell culture media (without antibiotics) and exposed to 1×107 CFU/ml Streptococcus pneumoniae lab strain D39 in 500 μl fresh cell culture media for 1‐h at 37°C. To remove extracellular bacteria, the cells were then washed three times with PBS and incubated for 15‐min in fresh media supplemented with 500 μg/ml gentamicin (ThermoFisher). The cells were subsequently lysed in 500 μl 0.1% Triton X‐100 and the number of internalized bacteria was analyzed by viable plate counts on blood agar plates.
Allogeneic mixed lymphocyte reaction
Primary human Mo‐DCs or Mo‐M treated, or not, with HAMLET, αLA or LPS for 1‐h were harvested 24‐h post‐stimulation and re‐seeded in 96‐well plates. Freshly isolated allogeneic T‐lymphocytes were added in a stimulator:responder ratio of 1:1‐1:100 and the cells were incubated for a total of five days. 1 μCi [methyl‐3H]thymidine was added for the last 18‐h and incorporation was determined in a Microbeta Counter (PerkinElmer). For analyses of T‐cell phenotype, the cells were detached on day 5 of co‐culture using non‐enzymatic cell dissociation solution and washed once in FACS buffer prior to staining for flow cytometry. Antibodies used were; APC‐CD4 clone RPA‐T4 (1:25), PE‐CD183 clone 1C6/CXR3 (1:20) and FITC‐CD294 clone BM16 (1:20) as described in [38], and analysis was performed as described above.
Statistical analysis
Statistical analyses were performed using Prism 8 (GraphPad Software, San Diego, CA, USA). For multiple comparisons, Kruskal‐Wallis test with Dunn's multiple comparison test (for non‐parametric analyses) and one‐way ANOVA with Tukey's multiple comparison test (for parametric analyses) were performed. For comparison between two groups of paired samples, paired t test was used. A P‐value of <0.05 was deemed significant.
Author contributions
GV performed, analyzed and interpreted experiments. APH designed, analyzed and interpreted experiments. CB was responsible for designing the study, performing, analyzing and interpreting experiments as well as for writing the first draft of the manuscript. All authors contributed significantly to the study as well as read and approved the final manuscript.
Conflict of interest
The authors declare no financial or commercial conflict of interest
Peer review
The peer review history for this article is available at https://publons.com/publon/10.1002/eji.202048813.
Abbreviations
- αLA
alpha‐lactalbumin
- BSA
bovine serum albumin
- CD
cluster of differentiation
- DCs
dendritic cells
- HAMLET
human alpha‐lactalbumin made lethal to tumor cells
- HLA
human leukocyte antigen
- IκBα
inhibitor of kappa B alpha
- IL
interleukin
- LPS
lipopolysaccharide
- MAPK
mitogen‐activated protein kinase
- Mo‐DCs
monocyte‐derived dendritic cells
- Mo‐M
monocyte‐derived macrophages
- OA
oleic acid
- PBMCs
peripheral blood mononuclear cells
Supporting information
Supporting information
Acknowledgements
The authors thank Dr. A. Mossberg for purification of αLA and preparation of HAMLET as well as for providing helpful suggestions for improvements, and Professor L. Ziegler‐Heitbrock for the IL‐10 promoter‐luciferase vector. This work was supported by grants from The Swedish Medical Research Council (VR; Grants K2015‐99X‐22878, 2018/05947, 2018/05795 (APH), The Royal Physiographic Society in Lund (GV and CB) and The Swedish Society for Medical Research (SSMF; CB).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Hakansson, A. , Svensson, M. , Mossberg, A. K. , Sabharwal, H. , Linse, S. , Lazou, I. , Lonnerdal, B. et al., A folding variant of alpha‐lactalbumin with bactericidal activity against Streptococcus pneumoniae. Mol Microbiol 2000. 35: 589–600. [DOI] [PubMed] [Google Scholar]
- 2. Hakansson, A. , Zhivotovsky, B. , Orrenius, S. , Sabharwal, H. and Svanborg, C. , Apoptosis induced by a human milk protein. Proc Natl Acad Sci U S A 1995. 92: 8064–8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Svensson, M. , Hakansson, A. , Mossberg, A. K. , Linse, S. and Svanborg, C. , Conversion of alpha‐lactalbumin to a protein inducing apoptosis. Proc Natl Acad Sci U S A 2000. 97: 4221–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alamiri, F. , Riesbeck, K. and Hakansson, A. P. , HAMLET, a protein complex from human milk has bactericidal activity and enhances the activity of antibiotics against pathogenic Streptococci. Antimicrob Agents Chemother 2019. 63: e01193–e01219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meikle, V. , Mossberg, A. K. , Mitra, A. , Hakansson, A. P. and Niederweis, M. , A Protein Complex from Human Milk Enhances the Activity of Antibiotics and Drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother 2019. 63: e01846–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Svanborg, C. , Agerstam, H. , Aronson, A. , Bjerkvig, R. , Duringer, C. , Fischer, W. , Gustafsson, L. , et al., HAMLET kills tumor cells by an apoptosis‐like mechanism–cellular, molecular, and therapeutic aspects. Adv Cancer Res 2003. 88: 1–29. [DOI] [PubMed] [Google Scholar]
- 7. Fischer, W. , Gustafsson, L. , Mossberg, A. K. , Gronli, J. , Mork, S. , Bjerkvig, R. and Svanborg, C. , Human alpha‐lactalbumin made lethal to tumor cells (HAMLET) kills human glioblastoma cells in brain xenografts by an apoptosis‐like mechanism and prolongs survival. Cancer Res 2004. 64: 2105–2112. [DOI] [PubMed] [Google Scholar]
- 8. Gustafsson, L. , Leijonhufvud, I. , Aronsson, A. , Mossberg, A. K. and Svanborg, C. , Treatment of skin papillomas with topical alpha‐lactalbumin‐oleic acid. N Engl J Med 2004. 350: 2663–2672. [DOI] [PubMed] [Google Scholar]
- 9. Mossberg, A. K. , Hou, Y. , Svensson, M. , Holmqvist, B. and Svanborg, C. , HAMLET treatment delays bladder cancer development. J Urol 2010. 183: 1590–1597. [DOI] [PubMed] [Google Scholar]
- 10. Mossberg, A. K. , Wullt, B. , Gustafsson, L. , Mansson, W. , Ljunggren, E. and Svanborg, C. , Bladder cancers respond to intravesical instillation of HAMLET (human alpha‐lactalbumin made lethal to tumor cells). Int J Cancer 2007. 121: 1352–1359. [DOI] [PubMed] [Google Scholar]
- 11. Storm, P. , Klausen, T. K. , Trulsson, M. , Ho, C. S. J. , Dosnon, M. , Westergren, T. , Chao, Y. , et al., A unifying mechanism for cancer cell death through ion channel activation by HAMLET. PLoS One 2013. 8: e58578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boltjes, A. and van Wijk, F. , Human dendritic cell functional specialization in steady‐state and inflammation. Front Immunol 2014. 5: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sica, A. and Mantovani, A. , Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012. 122: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kato, A. , Ogasawara, T. , Homma, T. , Saito, H. and Matsumoto, K. , Lipopolysaccharide‐binding protein critically regulates lipopolysaccharide‐induced IFN‐beta signaling pathway in human monocytes. J Immunol 2004. 172: 6185–6194. [DOI] [PubMed] [Google Scholar]
- 15. Sender, V. , Lang, L. and Stamme, C. , Surfactant protein‐A modulates LPS‐induced TLR4 localization and signaling via beta‐arrestin 2. PLoS One 2013. 8: e59896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGettrick, A. F. and O'Neill, L. A. , Localisation and trafficking of Toll‐like receptors: an important mode of regulation. Curr Opin Immunol 2010. 22: 20–27. [DOI] [PubMed] [Google Scholar]
- 17. Kohler, C. , Gogvadze, V. , Hakansson, A. , Svanborg, C. , Orrenius, S. and Zhivotovsky, B. , A folding variant of human alpha‐lactalbumin induces mitochondrial permeability transition in isolated mitochondria. Eur J Biochem 2001. 268: 186–191. [DOI] [PubMed] [Google Scholar]
- 18. Hakansson, A. P. , Roche‐Hakansson, H. , Mossberg, A. K. and Svanborg, C. , Apoptosis‐like death in bacteria induced by HAMLET, a human milk lipid‐protein complex. PLoS One 2011. 6: e17717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clementi, E. A. , Marks, L. R. , Duffey, M. E. and Hakansson, A. P. , A novel initiation mechanism of death in Streptococcus pneumoniae induced by the human milk protein‐lipid complex HAMLET and activated during physiological death. J Biol Chem 2012. 287: 27168–27182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin, I. C. and Kuo, C. D. , Pro‐inflammatory effects of commercial alpha‐lactalbumin on RAW 264.7 macrophages is due to endotoxin contamination. Food Chem Toxicol 2010. 48: 2642–2649. [DOI] [PubMed] [Google Scholar]
- 21. Pirofski, L. A. and Casadevall, A. , Immunomodulators as an antimicrobial tool. Curr Opin Microbiol 2006. 9: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Casadevall, A. , The third age of antimicrobial therapy. Clin Infect Dis 2006. 42: 1414–1416. [DOI] [PubMed] [Google Scholar]
- 23. Zhang, R. , Xi, X. , Wang, C. , Pan, Y. , Ge, C. , Zhang, L. , Zhang, S. et al., Therapeutic effects of recombinant human interleukin 2 as adjunctive immunotherapy against tuberculosis: A systematic review and meta‐analysis. PLoS One 2018. 13: e0201025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forton, D. and Karayiannis, P. , Established and emerging therapies for the treatment of viral hepatitis. Dig Dis 2006. 24: 160–173. [DOI] [PubMed] [Google Scholar]
- 25. Labro, M. T. , Immunomodulatory effects of antimicrobial agents. Part I: antibacterial and antiviral agents. Expert Rev Anti Infect Ther 2012. 10: 319–340. [DOI] [PubMed] [Google Scholar]
- 26. Soehnlein, O. , Zernecke, A. , Eriksson, E. E. , Rothfuchs, A. G. , Pham, C. T. , Herwald, H. , Bidzhekov, K. , et al., Neutrophil secretion products pave the way for inflammatory monocytes. Blood 2008. 112: 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou, S. , Yuan, X. , Liu, Q. , Zhang, X. , Pan, X. , Zang, L. and Xu, L. , BAPTA‐AM, an intracellular calcium chelator, inhibits RANKL‐induced bone marrow macrophages differentiation through MEK/ERK, p38 MAPK and Akt, but not JNK pathways. Cytokine 2010. 52: 210–214. [DOI] [PubMed] [Google Scholar]
- 28. Shi, Q. , Cheng, L. , Liu, Z. , Hu, K. , Ran, J. , Ge, D. and Fu, J. , The p38 MAPK inhibitor SB203580 differentially modulates LPS‐induced interleukin 6 expression in macrophages. Cent Eur J Immunol 2015. 40: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monick, M. M. , Powers, L. S. , Barrett, C. W. , Hinde, S. , Ashare, A. , Groskreutz, D. J. , Nyunoya, T. et al., Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. J Immunol 2008. 180: 7485–7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blumenthal, A. , Ehlers, S. , Ernst, M. , Flad, H. D. and Reiling, N. , Control of mycobacterial replication in human macrophages: roles of extracellular signal‐regulated kinases 1 and 2 and p38 mitogen‐activated protein kinase pathways. Infect Immun 2002. 70: 4961–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rydberg, C. , Mansson, A. , Uddman, R. , Riesbeck, K. and Cardell, L. O. , Toll‐like receptor agonists induce inflammation and cell death in a model of head and neck squamous cell carcinomas. Immunology 2009. 128: e600–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim, B. H. , Roh, E. , Lee, H. Y. , Lee, I. J. , Ahn, B. , Jung, S. H. , Lee, H. , et al., Benzoxathiole derivative blocks lipopolysaccharide‐induced nuclear factor‐kappaB activation and nuclear factor‐kappaB‐regulated gene transcription through inactivating inhibitory kappaB kinase beta. Mol Pharmacol 2008. 73: 1309–1318. [DOI] [PubMed] [Google Scholar]
- 33. Piccinini, A. M. , Zuliani‐Alvarez, L. , Lim, J. M. and Midwood, K. S. , Distinct microenvironmental cues stimulate divergent TLR4‐mediated signaling pathways in macrophages. Sci Signal 2016. 9: ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang, Y. , Shan, X. , Dai, Y. , Jiang, L. , Chen, G. , Zhang, Y. , Wang, Z. , et al., Curcumin Analog L48H37 Prevents Lipopolysaccharide‐Induced TLR4 Signaling Pathway Activation and Sepsis via Targeting MD2. J Pharmacol Exp Ther 2015. 353: 539–550. [DOI] [PubMed] [Google Scholar]
- 35. Bergenfelz, C. , Medrek, C. , Ekstrom, E. , Jirstrom, K. , Janols, H. , Wullt, M. , Bredberg, A. et al., Wnt5a induces a tolerogenic phenotype of macrophages in sepsis and breast cancer patients. J Immunol 2012. 188: 5448–5458. [DOI] [PubMed] [Google Scholar]
- 36. Bergenfelz, C. , Janols, H. , Wullt, M. , Jirstrom, K. , Bredberg, A. and Leandersson, K. , Wnt5a inhibits human monocyte‐derived myeloid dendritic cell generation. Scand J Immunol 2013. 78: 194–204. [DOI] [PubMed] [Google Scholar]
- 37. Cossarizza, A. , Chang, H. D. , Radbruch, A. , Acs, A. , Adam, D. , Adam‐Klages, S. , Agace, W. W. , et al., Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur J Immunol 2019. 49: 1457–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pianta, S. , Bonassi Signoroni, P. , Muradore, I. , Rodrigues, M. F. , Rossi, D. , Silini, A. and Parolini, O. , Amniotic membrane mesenchymal cells‐derived factors skew T cell polarization toward Treg and downregulate Th1 and Th17 cells subsets. Stem Cell Rev Rep 2015. 11: 394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


