Abstract
Background
Clinically, sleep bruxism is considered to be associated with the presence of tooth wear, but strong evidence is still lacking.
Objective
To examine whether an association exists between polysomnographic parameters, recorded from patients with possible sleep bruxism and tooth wear.
Methods
Sixty‐three possible sleep bruxers (19 males and 44 females, mean ± SD age = 38.5 ± 11.4 years) were recruited among patients attending the Clinic for orofacial pain and dysfunction of the Academic Centre for Dentistry Amsterdam (ACTA). The incisal/occlusal tooth wear was recorded for each tooth clinically, using a 5‐point ordinal scale. Subsequently, all patients underwent an one‐night ambulatory polysomnographic recording, during which the number of bruxism episodes per hour of sleep (Epi/h), the number of bruxism bursts per hour of sleep (Bur/h), and the bruxism time index (BTI) were recorded and analysed. Logistic regression analysis was performed using the presence of tooth wear as the dependent variable, the polysomnographic recordings as independent variables, and corrected for age and gender. The Bur/h and BTI were removed from the analyses due to collinearity with the Epi/h. Additionally, the polysomnographic recordings were also tested for possible association with self‐reported grinding of the teeth during sleep.
Results
No significant correlation was found between tooth wear and Epi/h (P = 0.381). In addition, the presence of tooth wear was not associated with self‐reported parafunctions.
Conclusion
Clinically measured tooth wear and self‐reported parafunction seem not be related to the polysomnographic parameters of possible sleep bruxism.
Keywords: attrition, humans, parafunctions, polysomnography, sleep bruxism, tooth wear
1. INTRODUCTION
Sleep bruxism (SB) is a repetitive jaw‐muscle activity characterised by clenching or teeth grinding and/or bracing or even thrusting the mandible during sleep. 1 The prevalence of frequent bruxism (ie ≥3 times per week) has been estimated to 12.8% in the general adult population and appears to decrease in elderly people, while it does not seem to be affected by gender. 2 However, these estimates may be influenced by the fluctuating character of bruxism and the differences in the criteria applied to set the diagnosis of bruxism. 2 , 3
According to the International Classification of Sleep Disorders (ICSD), which was proposed by the American Association of Sleep Medicine (AASM), the diagnosis of SB is based on the report of regular or frequent tooth grinding sounds, accompanied by clinical signs and symptoms like abnormal tooth wear or transient morning jaw‐muscle pain or fatigue, temporal headache and jaw locking upon awakening. 4 Other non‐specific signs, like tongue and cheek indentation, tooth fracture and mobility, may also be associated with sleep bruxism (SB). 5 These signs alone, however, are insufficient to establish a definite diagnosis of sleep bruxism for different reasons. For example, some of them are only based on the patient's report, while some others can be influenced by factors other than bruxism. Therefore, polysomnography, including audio and video recordings, is currently considered the gold standard for a definite diagnosis of sleep bruxism, with 78% sensitivity and 89% specificity. 6
Tooth wear is a frequently occurring problem, leading to the loss of hard dental tissue. It includes different types of wear, such as attrition, erosion and abrasion. The attrition‐type wear includes facets and sharp edges on opposing teeth, made by tooth‐to‐tooth contact due to function or parafunction. This intrinsic mechanical type of wear can be a natural process attributed to age, or an unnatural process due to day‐time or night‐time parafunction. 7 It is widely believed that SB can amplify the progression of wear, but a clear cause‐effect relationship between SB and attrition‐type tooth wear has not been established yet. 8 , 9 , 10 , 11 Therefore, the aim of the present study was to determine whether an association exists between polysomnographic parameters of sleep bruxism and attrition‐type tooth wear.
2. METHODS
The scientific and ethical aspects of this study were reviewed and approved by the Medical Ethics Committee of the Slotervaart Medical Center in Amsterdam, the Netherlands (approval number 9909), and written informed consent was obtained from all participants in advance.
2.1. Participants
Sixty‐three possible sleep bruxers (19 males, mean ± SD age 37.3 ± 12.9 years and 44 females, mean ± SD age 39.1 ± 10.8 years) were recruited from patients attending the clinic for orofacial pain and dysfunction of the Academic Centre for Dentistry Amsterdam (ACTA).
Criteria for patient selection included: minimum 18 years of age, presence of an entirely natural dentition (no fixed or removable prostheses to restore missing teeth), self‐reported clenching or grinding activity for at least 3 nights per week during the past 6 months, 12 no epilepsy or sleep disorders other than SB (eg obstructive sleep apnea), no use of any medication with a known influence on sleep structure or SB (eg selective serotonin reuptake inhibitors or anti‐Parkinson medication 13 ), no illicit drug abuse, no excessive coffee intake (ie more than 3 cups per day), no alcohol overconsumption (ie more than 5 glasses per day). The appropriate type of glass was associated with the type of alcohol, for example, not whiskey in a beer glass or vice versa, whereby the quantity was standardised.
2.2. Procedure
All patients received a set of questionnaires at intake, which included a standard Dutch medical questionnaire and the Dutch version of the sleep disorders questionnaire (SDQ). 14 , 15 Subsequently, they provided information about their oral and dental history and underwent a clinical examination. Following the intake procedure, the individuals were invited to sleep with an ambulatory polysomnographic (PSG) unit (Monet*) (Medcare Automation BV, Amsterdam, The Netherlands) for home recordings during one night. The montage for the PSG recording took place at the Amsterdam Center for Sleep and Wake Disorders of the Slotervaart Medical Center in Amsterdam, The Netherlands. The montage protocol consisted of the following leads:
Electroencephalography (EEG; C3A2; O2A1)
Electromyography (EMG; right and left masseter muscle; submental area)
Electrooculography (EOG; right and left).
Each masseter EMG signal was recorded at 512 Hz and was appropriately filtered (hardware; 50 Hz notch; 3 Hz high‐pass; 100 Hz low‐pass). No audio or video recordings were carried out.
The PSG recordings were analysed by the REMBRANDT software (Medcare Automation BV, Amsterdam, the Netherlands). The analysis consisted of two parts: a sleep analysis and a bruxism analysis. The sleep analysis was performed to exclude sleep disorders other than SB in addition to the results of the SDQ, to determine any abnormalities in sleep structure and to enable the sole inclusion of masticatory muscle activities during actual sleep in the analysis of SB. Using 30‐s epochs, all sleep analyses were carried out automatically according to the criteria of Rechtschaffen and Kales. 16 An experienced sleep scientist manually checked all analyses.
The analysis of SB was performed in four steps, using an automatic bruxism‐analysing tool incorporated in the REMBRANDT software. The algorithms of this tool are comparable with those described by Gallo et al. 17 The first step of the tool consisted of EMG sample‐rate conversion (to down‐sample the signal to approximately 100 Hz). During the second step, the EMG signals were rectified and low‐pass filtered (time constant 0.1 seconds) to locate areas of increased amplitude. During the third step, periods of elevated EMG activity were detected, using an EMG threshold of 10% of the maximum voluntary EMG activity of the left and right masseter muscles. Finally, during the fourth step, three SB outcome variables were derived during actual sleeping periods only, viz., the number of bruxism episodes per hour of sleep (Epi/h) and the number of bruxism bursts per hour of sleep (Bur/h) according to Lavigne et al 6 , 18 and the bruxism time index (BTI; ie the total time spent in bruxing divided by the total sleep time, times 100%) according to van der Zaag et al 19 Subsequently, all analyses were manually checked. Since no statistically significant differences in SB outcome variables were found between the EMGs of the left and right masseter muscles, the results of both analyses were pooled, and the average value for each patient was used in the statistical analysis.
2.3. Tooth wear scoring
Tooth wear was recorded clinically by an experienced examiner (JvdZ), separately for each tooth using a 5‐point ordinal scale 20 :
0 = no wear.
1 = visible wear limited within the enamel.
2 = visible wear with dentin exposure and loss of clinical crown height < 1/3.
3 = loss of crown height 1/3 to 2/3.
4 = loss of crown height > 2/3.
The median score per participant was used as a summary measure of tooth wear, and it was further converted into a binary variable (ie 0 or 1), using a cut‐off between scores 1 and 2 in order to facilitate the statistical analysis.
2.4. Statistical analysis
A logistic regression model was used to evaluate the possible association between the Epi/h, Bur/h and BTI (independent variables) and the presence of tooth wear (dependent variable). For reasons of collinearity, verified by checking the scaled uncentred cross‐products matrix of the regression model and the variance proportion for each predictor, 21 Bur/h and BTI were removed from the regression model. Age and gender were included in the model as covariates. The null‐hypothesis was that there is no significant association between the polysomnographic parameters of sleep bruxism and the presence of tooth wear. In addition, a second logistic regression was performed between the Epi/h variable and the self‐reported grinding and clenching of the teeth. Due to the collinearity between the self‐reported grinding and clenching, only the variable of grinding was used for the analysis. The alpha level was set to 0.05. Statistical analysis was conducted using SPSS 24 (IBM, Armonk, New York, NY, USA).
3. RESULTS
In total, 63 patients were included in this study, with a mean ± SD age of 38.5 ± 11.4 years. The mean ± SD Epi/h was 4.5 ± 6.06 (males: 3.0 ± 4.1; females: 5.2 ± 6.7). The median tooth wear score was 2, both for males and for females. Following binarization, 42/63 patients had a tooth‐wear score of 1 (14/19 males and 27/44 females). The distribution of Epi/h values over men and women with and without tooth wear is shown in Figure 1. In Table 1 the descriptives of the predictors of the regression model are presented. The logistic regression did not reveal any significant association between the Epi/h and tooth wear (X2 (3) = 3.071, P = 0.381). Nagelkerke's R2 was equal to 0.066. The presence of episodes of bruxism/hour of sleep was not associated with self‐reported grinding of the teeth (X2 (1) = 2.365, P = 0.124). The Nagelkerke's R2 was equal to 0.067.
FIGURE 1.
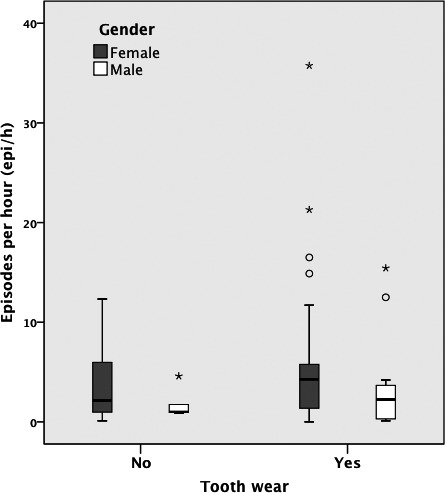
Box‐whisker plot depicting the number of sleep bruxism episodes per hour of sleep (Epi/h) for patients with and without tooth wear, separately for women and men
TABLE 1.
Estimates from the logistic regression analysis for the presence of tooth wear
| Predictive variables | B | df | Sig. | Exp (B) | 95% CI for Exp (B) |
|---|---|---|---|---|---|
| Episodes/ hour | 0.001 | 1 | 0.308 | 1.001 | 0.999‐1.002 |
| Gender | 0.683 | 1 | 0.281 | 1.980 | 0.146‐1.749 |
| Age | 0.027 | 1 | 0.284 | 1.027 | 0.978‐1.079 |
4. DISCUSSION
The main objective of the current study was to investigate whether the outcomes of ambulatory PSG recordings of possible sleep bruxers are associated with tooth wear. No significant association between sleep bruxism and attrition‐type tooth wear could be detected, hence the null‐hypothesis (ie there is no significant association between the polysomnographic parameters of sleep bruxism and the possibility of having tooth wear) could not be rejected. The outcome that the sleep bruxism activity is independent of gender and age and is in line with the current literature in adults. 2
The association between the amount of wear and SB could also have been examined through several consecutive nights of polysomnographic monitoring, in order to assess the actual magnitude of the events in a sleep laboratory setting by taking into consideration the time‐variant nature of SB. 3 However, the night‐to‐night variability of SB is not strongly supported by the literature. 22 In addition, such longitudinal assessment of SB would have presented additional difficulties due to the costs related to a polysomnographic study and the requirement for long‐term patient commitment. A possible disadvantage of a single‐night recording may also have been the fact that sleep during the first night is considered to be lighter ("first night" effect), 23 although the association of this effect on sleep bruxism has not been demonstrated unequivocally. 3 , 24 In order to minimise this effect, ambulatory rather than hospital‐based PSG was employed in the present study. Although this system lacks audio and video control, it is a relatively low‐cost option that allows monitoring in the patient's natural environment and it is considered to yield reliable and high‐quality recordings compared with sleep laboratory setting. 25
The 5‐point ordinal scale 20 employed here for the assessment of tooth wear is widely used to assess wear on dental cast or intraorally. 8 , 9 More refined tooth wear scales, such as the Tooth Wear Evaluation System (TWES 7 ), could have been more accurate to evaluate the amount of tooth wear. However, for the purpose of the present explorative study, more details were deemed redundant.
Earlier studies using electromyographic recordings have concluded that tooth wear is not a good indicator for sleep bruxism. 9 , 10 These findings are generally in agreement with the present study findings, although a slightly different method was used in the present study, viz., polysomnography. On most occasions, the electromyographic recordings came from a single‐channel device. Using these devices makes the discrimination between sleep bruxism and other oral activities during sleep more difficult and can possibly yield an overestimation of the outcomes.
In an earlier case‐control study, 8 young patients were placed in three groups by the report of their sleep partner and their EMG recording and were then tested for tooth wear. The first group consisted of moderate to high‐frequency sleep bruxers (SBr); the second group, low‐frequency SBr. Both groups had a positive sleep partner report of current tooth grinding. The third group had low RMMA (Rhythmic Masticatory Muscle Activity) frequency and negative sleep partner report and was used as control. The authors of this study found a significant difference in tooth wear scores in both the SBr groups in comparison with the scores of the control group, but not between the two SBr subgroups. Thus, they concluded that the tooth wear scores alone are not a good indicator for sleep bruxism yielding the same results like the present study.
It is noteworthy that in the literature, there are studies reporting that patients can be differentiated as being bruxers based on their self‐report. 9 , 26 , 27 , 28 , 29 Such subjective data may be poorly correlated to instrument‐based recordings of jaw‐muscle activity. Contrary to these earlier findings, no significant association was found in the present study between self‐reported grinding and the polysomnographic recordings.
Attrition is intrinsic mechanical wear as a result of function and/or parafunction (eg bruxism), due to tooth‐to‐tooth contact. However, many different factors can play a role in the process of wear, such as intrinsic‐chemical (reflux activity) and extrinsic‐mechanical (abrasion) components. 7 It is now generally accepted that tooth wear has a multifactorial aetiology. Although the patients of this study were included for having attrition‐type of wear (ie sharp edges on opposing occluding surfaces), an erosive component (dietary or intrinsic) cannot be excluded. Day‐time parafunctions can also be associated with the presence of wear, such as fingernail biting, biting on hard objects, day‐time clenching and sometimes even grinding. As a consequence, we cannot conclude that the presence of tooth wear is mainly attributed to sleep bruxism. In the future, a prospective study should take into account the influences of various factors for the presence or absence of tooth wear studying not only attrition but also erosion and abrasion variables.
5. CONCLUSION
Clinically measured tooth wear and self‐reported parafunction seem not be related to the polysomnographic parameters of possible sleep bruxism.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Despoina Kapagiannidou, contributed to the design of the study, data analysis and interpretation, performed all statistical analysis, and drafted and critically revised the manuscript. Koutris Michail, contributed to the data analysis, interpreted and critically revised the manuscript. Peter Wetselaar, contributed to the design and critically revised the manuscript. Corine Miriam Visscher, contributed to data analysis and interpreted and critically revised the manuscript. Jacques van der Zaag, contributed to the conception and design of the study, collected the data and critically revised the manuscript. Frank Lobbezoo, contributed to the conception and design, and critically revised the manuscript. All authors, gave their final approval and agreed to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
The author is grateful to Darrel J. Wicks for his assistance in the analysis of the data.
Kapagiannidou D, Koutris M, Wetselaar P, Visscher CM, van der Zaag J, Lobbezoo F. Association between polysomnographic parameters of sleep bruxism and attrition‐type tooth wear. J Oral Rehabil. 2021;48:687–691. 10.1111/joor.13149
DATA AVAILABILITY STATEMENT
The datasets generated and analysed during the current study are not publicly available due to restrictions in the METC approval but are available from the corresponding author on reasonable request.
REFERENCES
- 1. Lobbezoo F, Ahlberg J, Glaros AG, et al. Bruxism defined and graded: an international consensus. J of Oral Rehabil. 2013;40:2‐4. [DOI] [PubMed] [Google Scholar]
- 2. Manfredini D, Winocur E, Guarda‐Nardini L, Paesani D, Lobbezoo F. Epidemiology of bruxism in adults: a systematic review of the literature. J Orofac Pain. 2013;27:99‐110. [DOI] [PubMed] [Google Scholar]
- 3. van der Zaag J, Lobbezoo F, Visscher CM, Hamburger HL, Naeije M. Time‐variant nature of sleep bruxism outcome variables using ambulatory polysomnography: implications for recognition and therapy evaluation. J of Oral Rehabil. 2008;35:577‐584. [DOI] [PubMed] [Google Scholar]
- 4. Sateia MJ. International classification of sleep disorders‐third edition: highlights and modifications. Chest. 2014;146:1387‐1394. [DOI] [PubMed] [Google Scholar]
- 5. Paesani DA. Bruxism: theory and practice. New Malden, UK: Quintessence Publishing; 2010. [Google Scholar]
- 6. Lavigne GJ, Rompré PH, Montplaisir JY. Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dent Res. 1996;75:546‐552. [DOI] [PubMed] [Google Scholar]
- 7. Wetselaar P, Lobbezoo F. The tooth wear evaluation system: a modular clinical guideline for the diagnosis and management planning of worn dentitions. J Oral Rehabil. 2016;43:69‐80. [DOI] [PubMed] [Google Scholar]
- 8. Abe S, Yamaguchi T, Rompré PH, De Grandmont P, Chen YJ, Lavigne GJ. Tooth wear in Young Subjects: a discriminator Between Sleep Bruxers and Controls? Int J Prosthodont. 2009;22:342‐350. [PubMed] [Google Scholar]
- 9. Jonsgar C, Hordvik PA, Berge ME, Johansson AK, Svensson P, Johansson A. Sleep bruxism in individuals with and without attrition‐type tooth wear: An exploratory matched case‐control electromyographic study. J Dent. 2015;43:1504‐1510. [DOI] [PubMed] [Google Scholar]
- 10. Baba K, Haketa T, Clark GT, Ohyama T. Does tooth wear status predict ongoing sleep bruxism in 30‐year‐old Japanese subjects? Int J Prosthodont. 2004;17:39‐44. [PubMed] [Google Scholar]
- 11. Stuginski‐Barbosa J, Porporatti AL, Costa YM, Svensson P, Conti PC. Agreement of the International Classification of Sleep Disorders Criteria with polysomnography for sleep bruxism diagnosis: a preliminary study. J Prosthet Dent. 2017;117:61‐66. [DOI] [PubMed] [Google Scholar]
- 12. Kato T, Thie NM, Montplaisir JY, Lavigne GJ. Bruxism and orofacial movements during sleep. Dent North Am. 2001;45:657‐684. [PubMed] [Google Scholar]
- 13. Winocur E, Gavish A, Voikovitsh M, Emodi‐Perlman A, Eli I. Drugs and bruxism: a critical review. J Orofac Pain. 2003;17:99‐111. [PubMed] [Google Scholar]
- 14. Douglass AB, Bornstein R, Nino‐Murcia G, Keenan S. Creation of the ASDC Sleep Disorders Questionnaire. Sleep Res. 1986;15:117. [DOI] [PubMed] [Google Scholar]
- 15. Sweere Y, Kerkhof GA, de Weerd AW, Kamphuisen HAC, Kemp B, Schimsheimer RJ. The validity of the Dutch Sleep Disorders Questionnaire (SDQ). J Psychosom Res. 1998;45:549‐555. [DOI] [PubMed] [Google Scholar]
- 16. Kales A, Rechtschaffen A. A manual of standardized terminology: techniques and scoring system for sleep stages of human subjects. Los Angeles, CA: UCLA Brain Information Service ⁄ Brain Research Institute; 1968. [Google Scholar]
- 17. Gallo LM, Lavigne G, Rompré P, Palla S. Reliability of scoring EMG orofacial events: polysomnography compared with ambulatory recordings. J Sleep Res. 1997;6:259‐263. [DOI] [PubMed] [Google Scholar]
- 18. Lavigne GJ, Rompré PH, Poirier G, Huard H, Kato T, Montplaisir JY. Rhythmic masticatory muscle activity during sleep in humans. J Dent Res. 2001;80:443‐448. [DOI] [PubMed] [Google Scholar]
- 19. van der Zaag J, Lobbezoo F, Wicks DJ, Visscher CM, Hamburger HL, Naeije M. Controlled assessment of the efficacy of occlusal stabilization splints on sleep bruxism. J Orofac Pain. 2005;19:151‐158. [PubMed] [Google Scholar]
- 20. Lobbezoo F, Naeije M. A reliability study of clinical tooth wear measurements. J Prosthet Dent. 2001;86:597‐602. [DOI] [PubMed] [Google Scholar]
- 21. Field A. Discovering statistics using SPSS, 3rd edn. SAGE Publications; 2009:279 p. [Google Scholar]
- 22. Lavigne GJ, Guitard F, Rompré PH, Montplaisir JY. Variability in sleep bruxism activity over time. J Sleep Res. 2001;10:237‐244. [DOI] [PubMed] [Google Scholar]
- 23. Virtanen I, Kalleinen N, Urrila AS, Polo‐Kantola P. First‐night effect on sleep in different female reproductive states. Behav Sleep Med. 2018;16:437‐447. [DOI] [PubMed] [Google Scholar]
- 24. Hasegawa Y, Lavigne G, Rompré P, Kato T, Urade M, Huynh N. Is there a first night effect on sleep bruxism? a sleep laboratory study. J Clin Sleep Med. 2013;9:1139‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doering S, Boeckman JA, Hugger S, Young P. Ambulatory polysomnography for the assessment of sleep bruxism. Journal of Oral Rehabil. 2008;35:572‐576. [DOI] [PubMed] [Google Scholar]
- 26. Johansson A, Fareed K, Omar R. Analysis of possible factors influencing the occurrence of occlusal tooth wear in a young Saudi population. Acta Odontol Scand. 1991;49:139‐145. [DOI] [PubMed] [Google Scholar]
- 27. Ekfeldt A, Hugoson A, Bergendal T, Helkimo M. An individual tooth wear index and an analysis of factors correlated to incisal and occlusal wear in an adult Swedish population. Acta Odontol Scand. 1990;48:343‐349. [DOI] [PubMed] [Google Scholar]
- 28. Xhonga FA. Bruxism and its effect on the teeth. J of Oral Rehabil. 1977;4:65‐76. [DOI] [PubMed] [Google Scholar]
- 29. Pintado MR, Anderson GC, DeLong R, Douglas WH. Variation in tooth wear in young adults over a two‐year period. J Prosthet Dent. 1997;77:313‐320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due to restrictions in the METC approval but are available from the corresponding author on reasonable request.


