Abstract
Aim
To clinically and histologically evaluate in dogs the healing of gingival recessions treated with coronally advanced flap (CAF) with or without cross‐linked hyaluronic acid (HA).
Materials and methods
Gingival recession defects were surgically created on the vestibular side of both maxillary canines in 8 dogs. After 8 weeks of plaque accumulation, the 16 chronic defects were randomly treated with either CAF alone or CAF and HA‐gel (CAF/HA). Clinical and histological outcomes were evaluated at 10 weeks post‐surgically.
Results
Compared to baseline, the clinical measurements at 10 weeks revealed a statistically significant decrease in gingival recession for both CAF (p < 0.01) and CAF/HA (p < 0.001) groups. Statistically significant differences were found in clinical attachment level (p < 0.05) and width of gingival recession (p < 0.01) favouring the CAF/HA group. Bone formation was statistically significantly greater in the CAF/HA group than in the CAF group (1.84 ± 1.16 mm vs., 0.72 ± 0.62 mm, respectively, p < 0.05). Formation of cementum and connective tissue attachment were statistically significantly higher in the CAF/HA group compared with the CAF group (i.e. 4.31 ± 1.78 mm versus 2.40 ± 1.35 mm and 1.69 ± 0.98 mm versus 0.74 ± 0.68 mm, respectively (p < 0.05)).
Conclusions
The present data have for the first time provided histologic evidence for periodontal regeneration of gingival recession defects following treatment with CAF and HA.
Clinical relevance
The use of HA in conjunction with CAF may represent a novel modality for treating gingival recession defects.
Keywords: coronally advanced flap, gingival recession, histological investigation, hyaluronic acid, periodontal regeneration, root coverage
Clinical Relevance.
Scientific rationale for the study: The effect of topical application of cross‐linked hyaluronic acid (HA) on periodontal wound healing/regeneration of gingival recession defects is currently unknown.
Principal findings: The application of HA in conjunction with coronally advanced flaps (CAF) resulted in statistically significantly greater periodontal regeneration (i.e. formation of cementum, periodontal ligament and bone) compared to treatment with CAF alone.
Practical implications: The present findings indicate that the treatment with CAF/HA may represent a novel modality for promoting periodontal wound healing/regeneration in gingival recession defects.
1. INTRODUCTION
Gingival recession is highly prevalent worldwide (Löe et al., 1992), and it results in several problems such as compromised aesthetics, plaque accumulation, gingivitis/periodontitis, root caries and/or dentin hypersensitivity (Löe et al., 1992; Cheng et al. 2015). During the last decades, many surgical therapeutic approaches have been developed to predictably obtain root coverage of gingival recession defects (Hofmänner et al., 2012). The subepithelial connective tissue graft (CTG) with a coronally advanced flap (CAF) is considered as a gold standard (Griffin et al., 2006; Hofmänner et al., 2012) in the treatment of gingival recession because of its high predictability of root coverage and favourable aesthetic outcomes. However, harvesting the autogenous subepithelial connective tissue graft is associated with patient discomfort, donor site morbidity, increased surgical time and pain (Griffin et al., 2006; Aroca et al., 2013). Moreover, substantial evidence indicates that CAF is one of the best documented treatment modalities for single gingival recessions (Cairo et al., 2014).
Hyaluronic acid (HA) is a major natural carbohydrate component of the extracellular matrix in many tissues such as skin, joints, eyes and periodontium (Eliezer et al., 2019; Pilloni et al. 2019) and has unique physiochemical and biological properties including hygroscopic (Dahiya and Kamal 2013; Pilloni et al. 2019), viscoelastic (Dahiya and Kamal 2013), bacteriostatic (Pirnazar et al., 1999; Dahiya and Kamal 2013; Eliezer et al., 2019), anti‐inflammatory (Sasaki & Watanabe, 1995; Moseley et al., 2002; Dahiya and Kamal 2013; Eliezer et al., 2019), anti‐oedematous (Dahiya and Kamal 2013; Eliezer et al., 2019) and osteoinductive (Sasaki & Watanabe, 1995; de Brito BB et al., 2012) nature. Furthermore, extensive studies have demonstrated that HA significantly stimulates clot formation (Pilloni et al. 2019; Scully et al., 1995), induces angiogenesis (West et al., 1985; Pilloni et al. 2019), increases osteogenesis (Pilloni and Bernard 1998; Pilloni et al. 2019; Asparuhova et al., 2020) and plays various pivotal roles in cell adhesion, migration and differentiation mediated by various HA‐binding proteins and cell surface receptors (Oksala et al., 1995). Thus, it has been suggested that HA may represent an ideal material for periodontal wound healing/regeneration in periodontal defects. HA has demonstrated successful results in terms of clinical attachment level gain and probing depth reduction when applied in intrabony defects (Van den Bogaerde., 2009; Fawzy EL‐Sayed et al., 2012; Briguglio et al. 2013). Moreover, it has recently been reported that the adjunctive use of HA in the coronally advanced flap (CAF) is effective in increasing the probability of complete root coverage compared to CAF alone in Miller class Ⅰ recessions in humans (Pilloni et al. 2019). However, to the best of our knowledge, at present there are no histological data evaluating periodontal wound healing/regeneration of recession defects treated with CAF and HA.
Therefore, the present study aimed to evaluate histologically the healing of experimentally created gingival recession defects treated with either CAF alone or CAF and HA‐gel in dogs.
2. MATERIALS AND METHODS
2.1. Animals
Eight healthy male beagle dogs (approximately 16–25 months old – weighing 9 to 14 kg) were chosen in this study. The experimental animals were housed and monitored daily for the duration of the study in the Animal Experimentation Facility Shin Nippon Biomedical Laboratories, Ltd., Kagoshima, Japan. They were kept in individual cages at 20–26℃, relative humidity of 30%‐70% and a 12‐hr light/dark cycle. Approximately 300 g of solid food (NVE‐10, Nippon Pet Food, Co., Ltd.) was provided to each animal daily, and water was available ad libitum. All experimental procedures during the in‐life phase for 20 weeks were reviewed and approved by the ethical committee of the Animal Research Center of Kagoshima University, Japan (Approval No. D18016). This study conformed to the ARRIVE guidelines for pre‐clinical animal studies.
2.2. Surgical protocol
One experienced surgeon (Yo.S.) performed all surgical procedures under general and local anaesthesia using aseptic routines. Before surgical procedures, analgesics (buprenorphine hydrochloride, 0.1 ml/kg; Leptan, Otsuka Pharmaceutical Co., Ltd, Tokyo, Japan) and antibiotics (Procaine penicillin G and dihydrostreptomycin sulphate aqueous suspension for injection, 0.05 ml/kg; Mycillin Sol Meiji for veterinary use, Meiji Seika Pharma Co., Ltd, Tokyo, Japan) were administered intramuscularly. General anaesthesia was achieved with a pentobarbital sodium (Somunopenchiru, 0.2 ml/kg IV; Kyoritsu Seiyaku Corporation, Tokyo, Japan)/medetomidine hydrochloride (Domitor, 0.08 ml/kg IM; Orion Corporation, Espoo, Finland) combination maintaining spontaneous breathing. Local anaesthesia was performed using lidocaine HCl/epinephrine (2%, 1:80,000; Xylocaine; Fujisawa Inc., Osaka, Japan). Dehiscence‐type gingival recession defects were surgically created bilaterally in the maxillary canines (Figure 1a–d). Two vertical incisions separated by a distance of 5 mm were made from the gingival margin and extending 7 mm apically in order not to reach the mucogingival line to preserve the limited amount of keratinized gingiva. These incisions were connected apically by a horizontal incision and coronally by an intrasulcular incision. The gingival tissue limited by the incisions was removed using a periosteal elevator. The exposed intact bone 1 to 1.5 mm coronally from the horizontal incision line was removed by means of bone chisels to expose the root surface 5 mm from the cement‐enamel junction (CEJ), and the root surfaces were carefully scaled using hand curettes to remove the root cementum (Hu‐Friedy Co., Chicago, IL, USA) (Figure 1b–d). The created defects were exposed for a period of 8 weeks to plaque accumulation. After 8 weeks of plaque accumulation, a regimen of plaque control using a 2% solution of chlorhexidine gluconate (5% HIBITANE®, 25 ml of a 2% solution; Sumitomo Dainippon Pharma Co., Ltd., Osaka, Japan) was instituted for 2 weeks prior to the reconstructive surgeries (Figure 1e,f and i). Full‐thickness flaps were then raised, and the root surfaces were scaled to completely remove the biofilm and the residual inflamed granulation tissue. Reference notches were made using a #1 round bur on the root surface at the base of the defects, at the level of the CEJ and on the crown surface to indicate the precise centre plane of the dehiscence defects and to aid in optimal histologic processing (Figure 1g, j). Bilateral defects were randomly assigned to receive CAF alone (Figure 1e–h) or CAF and cross‐linked HA‐gel (HYADENT BG®: a gel formulation containing BDDE‐cross‐linked HA (1000 kDA HA monomers) and non‐cross‐linked HA (2500 kDA) in a ratio 8:1, made from biotechnologically produced synthetic HA, provided by REGEDENT AG, Zurich, Switzerland) (CAF/HA) (Figure 1i–l). In the CAF/HA group, the HA‐gel was applied to the root surfaces, and the defects were filled up to the adjacent alveolar crest (Figure 1k). A periosteal releasing incision was made to allow tension‐free coronal advancement of the flaps and sutured using non‐resorbable monofilament suture material (Gore‐Tex Suture CV‐6, W. L., Gore and Associates Inc., AZ, USA) with sling and interrupted sutures in the CAF and CAF/HA groups (Figure 1h, l).
Figure 1.
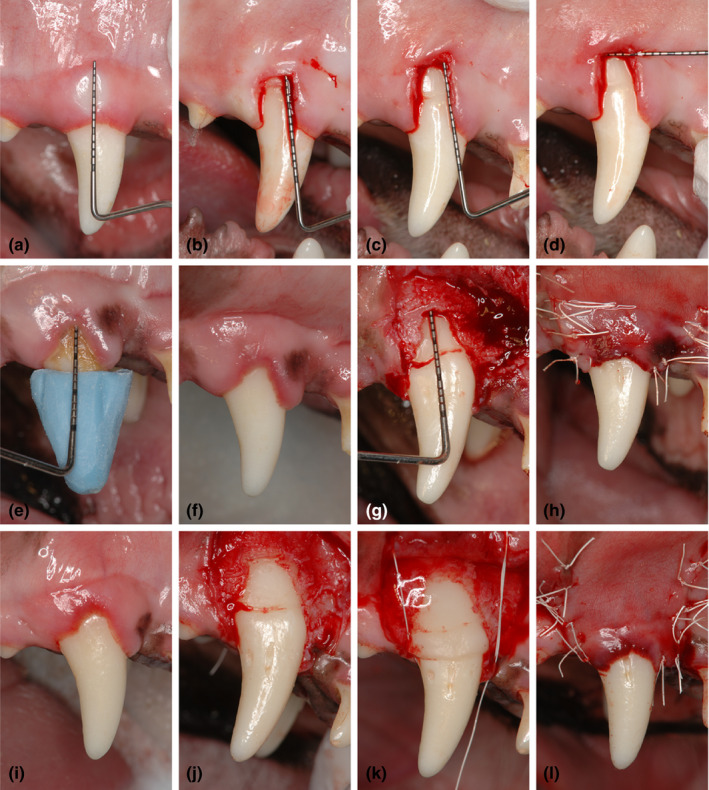
Clinical aspects of the experimental procedures. Creation of standardized gingival recession defect. (a) Buccal view of intact gingival tissue before the first surgery. (b) Denuded root surface after gingivectomy. Note the exposed intact bone at the apical portion of the created gingival recession defect. (c) Cement‐enamel junction (CEJ) is located at 3 mm apical to the native gingival margin. Bone reduction was performed to expose the root surface 5 mm from the CEJ. (d) After creation of soft (7 mm×5 mm) and hard (5 mm×5 mm) tissue recession defect. Buccal gingival recession defect being treated by CAF alone. (e) Clinical measurements at baseline. The customized resin stent was used for the clinical measurements. (f) Before reconstructive surgery after mechanical tooth cleaning. (g) Defect on the root after debridement. (h) Coronal advancement of the flap and suturing. Buccal gingival recession defect being treated by CAF and HA. (i) Gingival clinical aspect before reconstructive surgery. (j) Defect on the root after debridement. (k) HA was applied onto the denuded root surface. (l) A coronally advanced flap totally covered the denuded root surface and HA. CAF, coronally advanced flap; HA, cross‐linked hyaluronic acid gel.
Following reconstructive surgery, the animals were fed a soft diet for 2 weeks. An analgesic of ketoprofen (Capisten IM 50 mg, 2 mg/kg, 0.1 ml/kg; Kissei Pharmaceutical Co., Ltd, Matsumoto, Japan) and an antibiotic (Mycillin Sol) was administered daily for 2 days. Plaque control was maintained by routine (3 times a week) flushing of the oral cavity with 2% solution of chlorhexidine gluconate for 10 weeks after reconstructive surgery. Sutures were removed at 2 weeks after the surgery.
2.3. Clinical measurements
Customized resin stents were prepared using room temperature curing resin (OSTRONⅡ®,GC Corporation, Tokyo, Japan) before the root coverage surgeries for each animal/tooth. The stents were grooved in occluso‐apical direction with a thin tapered bur, to allow standard NC15 periodontal probe (PCP‐UNC 15, Hu‐Friedy Manufacturing Co., Chicago, IL, USA) to pass through it, which returned to the same deepest point in mid‐facial curve of gingival recessions. One experienced, calibrated and blinded examiner (T.N.) performed all measurements of the following clinical parameters: (a) probing pocket depth (PPD), (b) clinical attachment level (CAL), (c) mid‐facial gingival recession from the mesio‐distal line at the most coronal gingival level (the flat base of the stents) to the most apical gingival margin (GR) (Figure 1e), (d) width of the recession (WR) (measured mesio‐distally at the most coronal level of gingival recession) and (e) width of keratinized tissue (KT). All measurements were recorded at the baseline and repeated before sacrifice.
2.4. Histologic and histometric analysis
Ten weeks after surgery, the animals were euthanized by an overdose injection of sodium thiopental. All the defects were dissected along with the surrounding soft and hard tissues. The tissue blocks were fixed in 10% buffered formalin and trimmed. The samples were dehydrated and embedded in polyester resin. The resin blocks were cut bucco‐lingually to a thickness of 100 to 150 µm with a low‐speed diamond saw. Slides were ground and polished to a final thickness of 35 to 45 µm using a microgrinding system with non‐adhesive abrasive discs and stained with toluidine blue. All the specimens were analysed histomorphometrically under a light microscope (BX51, Olympus Optical Co., LTD, Tokyo, Japan) equipped with a computerized image system (cellSens, Olympus Corp., Tokyo, Japan). For the histometric analysis, 2 sections were selected from the most central area of each gingival recession defect, identified by the coronal and apical notches on the root and the reference notch on the crown. The mean value of each histometric parameter was calculated for each site. The following parameters were measured by the same experienced, calibrated and masked examiner (T.I.). Intra‐examiner reproducibility was ensured by reading sixteen sections from all sites by the examiner and repeating the same procedure forty‐eight hours later. Calibration was accepted, if 90% of the measurements were reproduced within 0.1 mm difference. (a) Gingival recession (GR): distance from the gingival margin to the coronal notch (CEJ) at the sites where the gingival margin was located apically to the coronal notch. Negative values were assigned to these measurements. If the gingival margin was located at the level of the coronal notch or coronal to this notch, a “0” value was applied (Casati et al. 2000; Shirakata et al., 2019). (b) Epithelial length (EL): distance between the apical extent of junctional epithelium and the coronal notch when the gingival margin was coronal to the coronal notch. If gingival recession was present, this measurement was recorded from the gingival margin on the denuded root surface to the apical extent of junctional epithelium, (c) Connective tissue adhesion (CT; without cementum): distance between apical extent of junctional epithelium and coronal extent of newly formed cementum, (d) New cementum formation (NC): distance between apical extent of root planing and coronal extent of newly formed cementum on denuded root surface, (e) New bone formation (NB): distance between apical extent of root planing and coronal extent of newly formed alveolar bone, (f) New attachment formation (NA): linear length of the root surface covered by NC adjacent to newly formed bone, with functionally oriented collagen fibres, (g) Soft tissue thickness (STT): distance from the buccal outermost gingival/mucosal surface to the tooth surface at three different levels, STT‐1: at the top of the coronal notch (CEJ), STT‐2: at the middle between the coronal and apical notches, and STT‐3: at the base of the apical notch (defect), (h) Soft tissue height (STH): distance between apical extent of root planing and gingival margin and (i) Defect height (DH): distance between apical notch and coronal notch.
2.5. Statistical analysis
Due to the limited number of pre‐clinical studies in dogs with a comparable design and the primary outcome, no specific power analysis for sample calculation was performed. However, in order to increase the statistical power, a split‐mouth design was adopted in all 8 animals. For each clinical parameter being evaluated, values were averaged (mean ±SD) at baseline and 10 weeks post‐operatively. Student's t‐tests for paired samples were used to evaluate the differences between baseline and 10‐week values within each treatment group. Student's t‐tests for unpaired samples were used to evaluate the differences between the two treatment groups, both clinically and histologically. The primary outcome variable of this study was the histometric outcome in terms of NA in particular, measured for the CAF and CAF/HA groups at 10 weeks. The clinical parameters were the secondary outcome. P values <0.05 were regarded statistically significant. All calculations were performed using statistical software (BellCurve for Excel, Social Survey Research Information Co., Ltd., Tokyo, Japan).
3. RESULTS
3.1. Clinical observations and clinical parameters
All treated sites (16 sites, 8 sites/group) in both CAF and CAF/HA groups exhibited favourable clinical healing with no complications such as swelling, suppuration or abscess formation throughout the entire experimental period (Figure 2). Post‐operatively, visual gingival redness seemed to remain longer in the CAF group (Figure 2b,c,e and f). The values for clinical parameters at the baseline and 10‐week examinations in both treatment groups are reported in Table 1. At baseline, differences between the CAF and CAF/HA groups for all tested clinical parameters were not statistically significant. At 10 weeks, there was a statistically significant difference (reduction) in the PPD for the CAF/HA group compared to baseline (p < 0.05). In the CAF/HA group, the CAL at 10 weeks was statistically significantly different from baseline (p < 0.05). In addition, the CAL at 10 weeks in the CAF/HA group was statistically significantly different from that in the CAF group (p < 0.05). Both treatment groups showed statistically significant post‐surgical improvements in terms of GR as compared to baseline (CAF group; p < 0.01, CAF/HA group; p < 0.001) (Figure 2c,f). At 10 weeks, the WR in the CAF group was statistically significantly increased compared to baseline (p < 0.01) and the value was statistically significantly different from that in the CAF/HA group (p < 0.01). At 10 weeks the KT in the CAF group was statistically significantly smaller compared to baseline. No statistically significant differences were detected between the CAF and the CAF/HA groups with respect to the following parameters (PPD, GR1, GR2 and KT).
Figure 2.
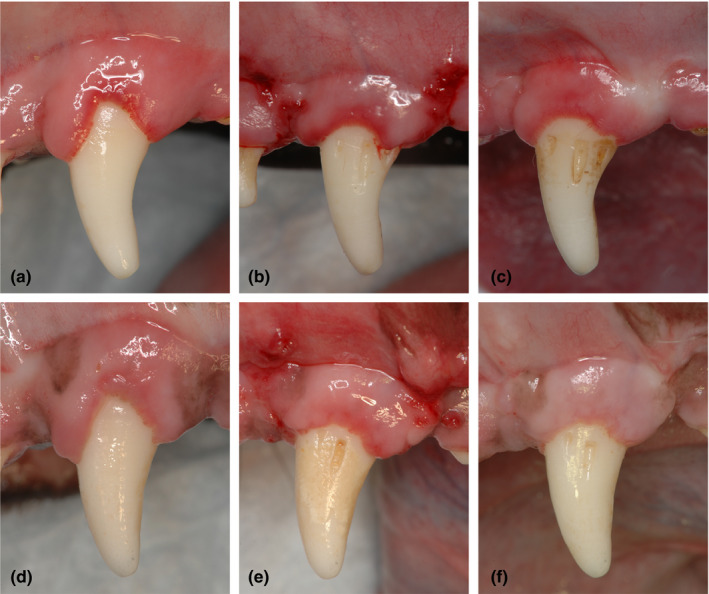
Clinical photographs. CAF group (a) at baseline. (b) after 2 weeks of healing (c) after 10 weeks of healing. CAF with HA group (d) at baseline. (e) after 2 weeks of healing and (f) after 10 weeks of healing. CAF, coronally advanced flap; HA, cross‐linked hyaluronic acid gel.
Table 1.
Clinical parameters for each treatment at baseline and 10 weeks (mean ±SD; mm)
| Clinical parameter | Surgical treatment | |
|---|---|---|
| CAF (n = 8) | CAF/HA (n = 8) | |
| PPD | ||
| Baseline | 2.63 ± 0.52 | 2.50 ± 0.54 |
| 10 weeks | 2.88 ± 1.36 | 1.88 ± 0.35* |
| PPD change over 10 weeks | 0.25 ± 1.28 | 0.63 ± 0.52 |
| CAL | ||
| Baseline | 2.69 ± 0.59 | 2.50 ± 0.54 |
| 10 weeks | 3.06 ± 1.21 | 2.06 ± 0.18 * , † |
| CAL change over 10 weeks | 0.38 ± 1.28 | 0.44 ± 0.50 |
| GR | ||
| Baseline | 5.83 ± 0.91 | 5.39 ± 0.68 |
| 10 weeks | 3.56 ± 1.85** | 3.08 ± 1.00*** |
| GR2 change over 10 weeks | 2.27 ± 1.74 | 2.31 ± 0.79 |
| WR | ||
| Baseline | 7.94 ± 1.02 | 7.69 ± 0.88 |
| 10 weeks | 9.25 ± 0.71** | 8.00 ± 0.54 †† |
| WRchange over 10 weeks | 1.31 ± 0.96 | 0.31 ± 1.10 |
| KT | ||
| Baseline | 5.48 ± 1.24 | 5.66 ± 1.51 |
| 10 weeks | 4.72 ± 1.79* | 4.95 ± 1.87 |
| KT change over 10 weeks | 0.77 ± 0.81 | 0.70 ± 1.03 |
The following parameters (PPD, CAL, KT and GR) were measured at the deepest point in mid‐facial curve of gingival recessions.
CAL, clinical attachment level; GR, mid‐facial gingival recession from the mesio‐distal line at the most coronal gingival level (the flat base of the stents) to the most apical gingival margin; KT, width of keratinized tissue; PPD, probing pocket depth; WR, width of gingival recession.
Significantly different from baseline (p < 0.05).
Significantly different from baseline (p < 0.01).
Significantly different from baseline (p < 0.001).
Significantly different from CAF group (p < 0.05).
Significantly different from CAF group (p < 0.01).
3.2. Descriptive histology
Generally, the positions of the gingival margin were located above CEJ in both the CAF and CAF/HA groups and both groups demonstrated comparable epithelial healing by formation of a long junctional epithelium in the subcrevicular area (Figure 3). The surrounding connective tissue showed a healthy and mature fibrous structure with many blood vessels. Minimal new bone formation was observed (Figures 3a, 4a) in 5 sites in the CAF group. Formation of new connective tissue attachment (i.e. new cementum with inserting collagen fibres) was not observed and connective tissue fibres were aligned parallel to the root surface (Figure 4b,c), though new cementum formation was observed in all sites. In the CAF/HA group, bone formation was noted in 5 sites extending from the apical notches towards the coronal region of the defects (Figures 3b, 5). In these defects, dense collagen fibres were seen inserting into the newly formed cementum, oriented oblique to the root surface (Figure 5). The highly vascularized new periodontal ligament‐like tissue, which was formed between the new cementum and new bone, maintained its width up to the coronal portion (Figures 3b, 5). Neither extensive root resorption nor ankylosis was observed in any of the defects.
Figure 3.
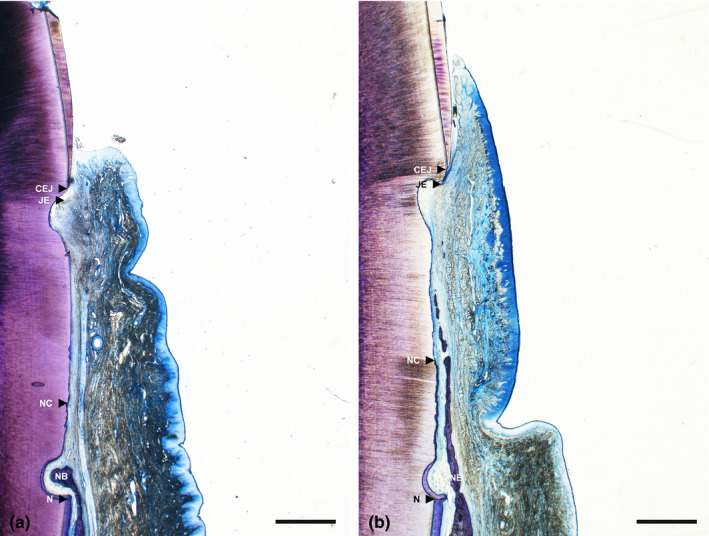
Representative histological photomicrographs of a gingival recession defect treated by CAF (a) and CAF with HA (b). Overview. (scale bar, 1 mm; toluidine blue staining, original magnification ×1.25). CAF, coronally advanced flap; HA, cross‐linked hyaluronic acid gel.
Figure 4.
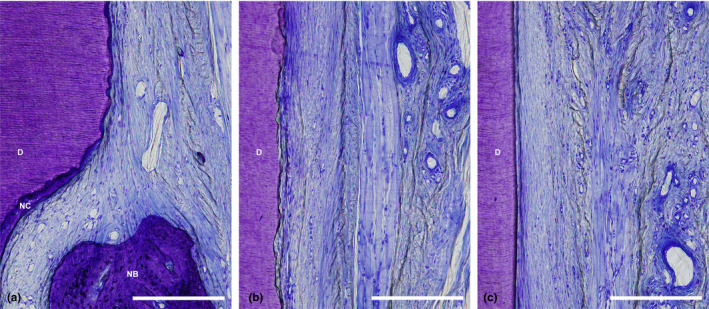
(a) Higher magnification of the apical portion of the defect treated with CAF shown in Figure 3a. (b) Higher magnification of the middle portion of the defect treated with CAF shown in Figure 3a. (c) Higher magnification of the coronal portion of the defect treated with CAF shown in Figure 3a. (scale bar, 200 μm; toluidine blue staining, original magnification ×20). CEJ, cemento‐enamel junction; D, root dentin; JE, apical end of junctional epithelium; N, apical end of apical notch; NC, new cementum; CAF, coronally advanced flap.
Figure 5.
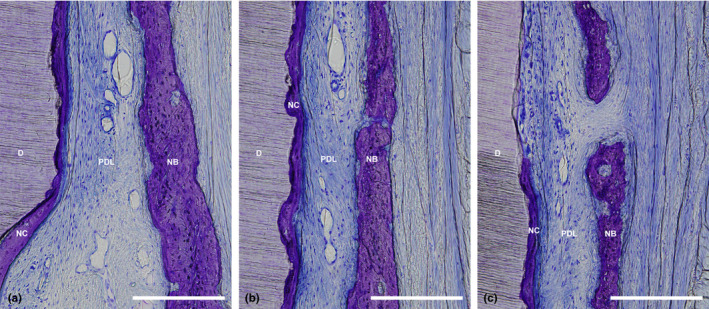
(a) Higher magnification of the apical portion of the defect treated with CAF/HA shown in Figure 3b. (b) Higher magnification of the middle portion of the defect treated with CAF/HA shown in Figure 3b. (c) Higher magnification of the coronal portion of the defect treated with CAF shown in Figure 3b. (scale bar, 200 μm; toluidine blue staining, original magnification ×20). CEJ, cemento‐enamel junction; D, root dentin; JE, apical end of junctional epithelium; N, apical end of apical notch; NC, new cementum; PDL, periodontal ligament; CAF, coronally advanced flap; HA, cross‐linked hyaluronic acid gel.
3.3. Histomorphometric analysis
The results of the histometric analysis are described in Table 2. No statistically significant differences were detected between the CAF and CAF/HA groups with respect to any of the following parameters (GR, EL, CT, STH, STT‐1, −2, −3 and DH). Formation of cementum (NC, p < 0.05) with a continuous periodontal ligament‐like tissue and new bone (i.e. NA, p < 0.05) was statistically significantly greater in the CAF/HA group compared to the CAF group. Formation of NB was statistically significantly greater in the CAF/HA group compared to the CAF group (p < 0.05).
Table 2.
Histomorphometric parameters for 10 weeks after reconstructive surgery (mean ±SD; mm)
| Histometric parameter | Surgical treatment | |
|---|---|---|
| CAF (n = 8) | CAF/HA (n = 8) | |
| DH | 6.13 ± 0.41 | 6.43 ± 1.20 |
| GR | 0.03 ± 0.11 | 0.12 ± 0.35 |
| EL | 2.05 ± 1.75 | 1.17 ± 1.11 |
| CT | 1.70 ± 1.04 | 0.93 ± 0.81 |
| NC | 2.40 ± 1.35 | 4.31 ± 1.78* |
| NA | 0.74 ± 0.68 | 1.69 ± 0.98* |
| NB | 0.72 ± 0.62 | 1.84 ± 1.16* |
| STH | 7.90 ± 1.08 | 8.23 ± 1.58 |
| STT−1 | 1.14 ± 0.38 | 0.89 ± 0.43 |
| STT−2 | 1.60 ± 0.59 | 1.84 ± 0.49 |
| STT−3 | 2.69 ± 0.55 | 2.94 ± 0.50 |
CR, complete periodontal regeneration; CT, connective tissue adhesion (without cementum); DH, defect height; EL, epithelial length; GR, gingival recession; NA, new attachment formation; NB, new bone formation; NC, new cementum formation; STH, soft tissue height; STT, soft tissue thickness (STT‐1) at the top of the coronal notch, (STT‐2) at the middle between the coronal and apical notches, (STT‐3) at the base of the apical notch.
Significantly different from CAF group (p < 0.05).
4. DISCUSSION
The present study has demonstrated that the additional application of HA to CAF led to superior results both clinically and histologically compared to CAF treatment in buccal recession defects in dogs. At 10 weeks, the recession depth decreased statistically significantly in both treatment groups. However, regarding the clinical measurement of GR, the alternative gingival recession (defined as mid‐facial gingival recession from the mesio‐distal line at the most coronal gingival level (the flat base of the stents) to the most apical gingival margin) was employed in this specific model in dogs. Since there are great anatomical differences in the primary location of CEJ, the intact alveolar crest and gingival margin and morphology of gingiva/tooth between humans and dogs. Thus, caution must be exercised when trying to make direct comparison of the results and it may only mean the profile changes from scalloped to flat one with small root coverage. Particularly, the CAF/HA group revealed statistically significant differences in PPD and CAL with less visible redness at 10 weeks when compared with baselines. In addition, the comparison between CAF and CAF/HA groups revealed that the CAF group exhibited a far greater degree of variation with the worse results in PPD and CAL than the CAF/HA group and a statistically significant difference in terms of CAL, which was in favour of the CAF/HA treatment. The clinical results in the CAF group were also characterized by statistically significantly greater WR than that in the CAF/HA group, which indicate the greater post‐operative shrinkage of the gingival margin and/or the frequent existence of the thinner gingival tissue which may influence the clinical outcome of gingival recession defects (Berlucchi et al. 2005; Huang et al. 2005). On the other hand, the present positive results in the CAF/HA group are in agreement with the previous clinical studies demonstrating that the adjunctive use of HA yielded statistically significant improvement of CAL and reduction of GR compared to treatments without HA in classⅠsingle gingival recession (Pilloni et al. 2019) and intrabony defects (Fawzy EL‐Sayed et al., 2012). This may be attributed to the fact that HA is a potent anti‐inflammatory agent, which can modulate wound healing due to its ability to scavenge the inflammatory cell‐derived reactive oxygen species (Sasaki & Watanabe, 1995; Moseley et al., 2002; Dahiya and Kamal 2013; Eliezer et al., 2019) and has also the antibacterial effect of high molecular weight HA‐gel on periodontal pathogens (Eick et al., 2013). However, a recent randomized clinical trial comparing CAF/HA with CAF alone has reported no statistically significant differences in the clinical parameters between the treatment groups (Kumar et al., 2014). Furthermore, Engström et al (Engström et al., 2001) have reported that no statistically significant differences in clinical parameters were found between sites treated by means of guided tissue regeneration (GTR) in conjunction with HA compared to sites treated with GTR alone. These discrepancies in clinical outcomes between the studies referred to may be explained by the variations in hyaluronic acid gel formulation used in the different studies, defect characteristics (e.g. morphology, location and size), treatment modalities, surgical techniques and follow‐up intervals.
To the best of our knowledge, this is the first study that histologically evaluated periodontal wound healing/regeneration following HA application combined with CAF in single gingival recession defects. The results have for the first time provided histologic evidence for periodontal regeneration following the application of HA in recession‐type defects and demonstrated that the additional application of HA to CAF resulted to a greater extent in periodontal regeneration compared to the use of CAF alone. The histomorphometric analysis revealed that the amounts of NC, NA and NB in the CAF/HA group were statistically significantly greater than that in the CAF group. Furthermore, in contrast to the CAF group, dense collagen fibres inserted into the newly formed cementum were oriented obliquely to the root surface, and highly vascularized new periodontal ligament‐like tissue was found in the area between new cementum and new bone in the CAF/HA group. These findings may be explained by the numerous biological roles of HA in wound healing (Briguglio et al. 2013; Dahiya and Kamal 2013). In the initial inflammatory stages, HA bonds with the fibrin clot; activates inflammatory cells such as leucocytes and macrophages at the inflamed sites, stimulating the phagocytosis and killing of invading microbes (Håkansson et al., 1980); and induces production of proinflammatory cytokines by fibroblasts, keratinocytes, cementoblasts and osteoblasts which promote the inflammatory response and consequently stimulate HA synthesis by endothelial cells (Larjava et al., 1989). Next, HA is transiently elevated during the formation of granulation tissue and the re‐establishment of the epithelium in non‐mineralized tissues (Bartold & Page, 1986), and in mineralized tissues HA is gradually replaced by provisional mineralized callus (Bertolami & Messadi, 1994). In the later stage of the granulation phase, HA synthesis ceases and existing HA is degraded by hyaluronidase to produce low molecular weight HA, which in turn promotes the formation of blood vessels (angiogenesis) within wound sites (Ruggiero et al., 1987; Weigel et al., 1988; Moseley et al., 2002). Specifically, the bone formation obtained following treatment with CAF/HA may be explained by the findings that HA significantly induced earlier bone deposition, resulting in a more organized/mineralized bone compared to clot alone in extraction sockets in experimental animals (Mendes et al., 2008; Kim et al., 2016), by promoting the expression of bone morphogenetic protein‐2, osteopontin (Mendes et al., 2008) and alkaline phosphatase activity (Huang et al., 2003). In addition, recent in vitro studies using the present HA reported that in contact with HA, human palatal/gingival fibroblasts significantly increased expression of the growth factors including PDGFB, FGF‐2 and EGF which are known to stimulate cell proliferation and migration for soft tissue wound healing (Asparuhova et al. 2019), and HA strongly induces the growth of osteoprogenitors and maintains their stemness, thus potentially regulating the balance between self‐renewal and differentiation capabilities (Asparuhova et al., 2020). All these effects may be responsible together or in turn for periodontal wound regeneration following CAF/HA treatment.
The amounts of STT in the CAF/HA group were comparable to that in the CAF group, without statistically significant differences. On the other hand, the measurements of STT values in the CAF combined with CTG or with porcine‐derived acellular dermal collagen matrix treatments were consistently greater than that in the CAF and CAF/HA groups when compared in the same design of our previous studies (Shirakata et al., 2016, 2019). These results also indicate that CTG or connective tissue alternatives may increase and maintain the volume of soft tissue in root coverage of multiple gingival recessions especially in thin biotype (Schlee & Esposito, 2011; Garces‐McIntyre et al., 2017; Stähli et al. 2020).
Taken together, the favourable results obtained in the CAF/HA group support the use of HA in combination with CAF for the treatment of gingival recession defects and are in line with results from two very recent cases series which have demonstrated excellent clinical outcomes following treatment of single and multiple gingival recessions by means of the modified coronally advanced tunnel in conjunction with HA and palatal subepithelial tissue grafts (Guldener et al., 2020; Lanzrein et al., 2020). However, since the present results were obtained in single gingival recession defects in dogs, further studies are required to determine whether the adjunctive use of HA to CAF treatment has beneficial effects on multiple gingival recession defects and the CAF/HA treatment ensure long‐term stability of the clinical outcomes by achieving periodontal wound regeneration.
5. CONCLUSION AND CLINICAL RELEVANCE
In conclusion, the present data indicate that: (a) the application of HA in conjunction with CAF promotes periodontal wound healing/regeneration in gingival recession defects, and (b) the use of HA in conjunction with CAF may represent a novel modality for treating gingival recession defects.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR'S CONTRIBUTION
Conceptualization: Yo.S. and A.S.; methodology: Yo.S. and A.S.; validation: Yo.S. and A.S.; formal analysis: T. N.; investigation: Yo.S., T.N., Y.K., T.I. and Yu.S; data curation: T.N. and T.I.; writing – original draft preparation: Yo.S. and A.S.; writing – review and editing: all authors.; visualization: Yo.S. and Yu.S.; supervision: K.N.; project administration: Yo.S. and A.S.; funding acquisition: T.N. and Y. K. All authors have read and agreed to the published version of the manuscript.
Ethical approval
All applicable international, national and/ or institutional guidelines for the care and use of animals were followed, and all procedures performed in studies involving animals were in accordance with the ethical standards of the ethical committee of the Animal Research Center of Kagoshima University, Japan (Approval No. D18016).
Informed consent
For this type of study, formal consent is not required.
ACKNOWLEDGEMENTS
This study was partly funded by REGEDENT AG (Zurich, Switzerland) and by Grants‐in‐Aid for Scientific Research C (No.18K09604 & No. 18K09620) from the Japan Society for the Promotion of Science. REGEDENT AG kindly provided the hyaluronic acid gel used in this study.
Funding information
This study was partly funded by REGEDENT AG (Zurich, Switzerland) and by Grants‐in‐Aid for Scientific Research C (No.18K09604 & No. 18K09620) from the Japan Society for the Promotion of Science.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aroca, S. , Molnár, B. , Windisch, P. , Gera, I. , Salvi, G. E. , Nikolidakis, D. , & Sculean, A. (2013). Treatment of multiple adjacent Miller class I and II gingival recessions with a Modified Coronally Advanced Tunnel (MCAT) technique and a collagen matrix or palatal connective tissue graft: a randomized, controlled clinical trial. Journal of Clinical Periodontology, 40(7), 713–720. 10.1111/jcpe.12112. [DOI] [PubMed] [Google Scholar]
- Asparuhova, M. B. , Chappuis, V. , Stähli, A. , Buser, D. , & Sculean, A. (2020). Role of hyaluronan in regulating self‐renewal and osteogenic differentiation of mesenchymal stromal cells and pre‐osteoblasts. Clinical Oral Investigations, 24(11), 3923–3937. 10.1007/s00784-020-03259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asparuhova, M. B. , Kiryak, D. , Eliezer, M. , Mihov, D. , & Sculean, A. (2019). Activity of two hyaluronan preparations on primary human oral fibroblasts. Journal of Periodontal Research, 54(1), 33–45. 10.1111/jre.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartold, P. M. , & Page, R. C. (1986). The effect of chronic inflammation on gingival connective tissue proteoglycans and hyaluronic acid. Journal of Oral Pathology and Medicine, 15(7), 367–374. 10.1111/j.1600-0714.1986.tb00643.x. [DOI] [PubMed] [Google Scholar]
- Berlucchi, I. , Francetti, L. , Del Fabbro, M. , Basso, M. , & Weinstein, R. L. (2005). The Influence of Anatomical Features on the Outcome of Gingival Recessions Treated With Coronally Advanced Flap and Enamel Matrix Derivative: A 1‐Year Prospective Study. Journal of Periodontology, 76(6), 899–907. 10.1902/jop.2005.76.6.899. [DOI] [PubMed] [Google Scholar]
- Bertolami, C. N. , & Messadi, D. V. (1994). The Role of Proteoglycans in Hard and Soft Tissue Repair. Critical Reviews in Oral Biology & Medicine, 5(3), 311–337. 10.1177/10454411940050030601. [DOI] [PubMed] [Google Scholar]
- Briguglio, F. , Briguglio, E. , Briguglio, R. , Cafiero, C. , & Isola, G. (2013). Treatment of infrabony periodontal defects using a resorbable biopolymer of hyaluronic acid: a randomized clinical trial. Quintessence International, 44(3), 231–240. [DOI] [PubMed] [Google Scholar]
- Cairo, F. , Nieri, M. , & Pagliaro, U. (2014). Efficacy of periodontal plastic surgery procedures in the treatment of localized facial gingival recessions. A systematic review. Journal of Clinical Periodontology, 41, S44–S62. 10.1111/jcpe.12182. [DOI] [PubMed] [Google Scholar]
- Casati, M. Z. , Sallum, E. A. , Caffesse, R. G. , Nociti, F. H. Jr , Sallum, A. W. , & Pereira, S. L. (2000). Guided tissue regeneration with a bioabsorbable polylactic acid membrane in gingival recessions A histometric study in dogs. Journal of Periodontology, 71(2), 238–248. 10.1902/jop.2000.71.2.238. [DOI] [PubMed] [Google Scholar]
- Cheng, G. L. , Fu, E. , Tu, Y. K. , Shen, E. C. , Chiu, H. C. , Huang, R. Y. , Yuh, D. Y. , & Chiang, C. Y. (2015). Root coverage by coronally advanced flap with connective tissue graft and/or enamel matrix derivative: a meta‐analysis. Journal of Periodontal Research, 50(2), 220–230. 10.1111/jre.12199. [DOI] [PubMed] [Google Scholar]
- Dahiya, P. , & Kamal, R. (2013). Hyaluronic acid: a boon in periodontal therapy. North American Journal of Medicine and Science, 5(5), 309–315. 10.4103/1947-2714.112473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito, B. B. , Mendes Brazao, M. A. , de Campos, M. L. , Casati, M. Z. , Sallum, E. A. , & Sallum, A. W. (2012). Association of hyaluronic acid with a collagen scaffold may improve bone haling in critical‐size bone defects. Clinical Oral Implants Research, 23(8), 938–942. [DOI] [PubMed] [Google Scholar]
- Eick, S. , Renatus, A. , Heinicke, M. , Pfister, W. , Strarul, S. , & Jentsch, H. (2013). Hyaluronic acid as an adjunct after scaling and root planning: a prospective randomized clinical trial. Journal of Periodontology, 84(7), 941–949. 10.1902/jop.2012.120269. [DOI] [PubMed] [Google Scholar]
- Eliezer, M. , Lmber, J. C. , Sculean, A. , Pandis, N. , & Teich, S. (2019). Hyaluronic acid as adjunctive to non‐surgical and surgical periodontal therapy: a systematic review and meta‐analysis. Clinical Oral Investigations, 23(9), 3423–3435. 10.1007/s00784-019-03012-w. [DOI] [PubMed] [Google Scholar]
- Engström, P. E. , Shi, X. Q. , Tronje, G. , Larsson, A. , Welander, U. , Frithiof, L. , & Engstrom, G. N. (2001). The effect of hyaluronon on bone and soft tissue and immune response in wound healing. Journal of Periodontology, 72(9), 1192–1200. 10.1902/jop.2000.72.9.1192. [DOI] [PubMed] [Google Scholar]
- Fawzy El‐Sayed, K. M. , Dahaba, M. A. , Aboul‐Ela, S. , & Darhous, M. S. (2012). Local application of hyaluronan gel in conjunction with periodontal surgery: a randomized controlled trial. Clinical Oral Investigations, 16(4), 1229–1236. 10.1007/s00784-011-0630-z. [DOI] [PubMed] [Google Scholar]
- Garces‐McIntyre, T. , Carbonell, J. M. , Vallcorba, L. , Santos, A. , Valles, C. , & Nart, J. (2017). Coronal advanced flap in combination with a connective tissue graft. Is the thickness of the flap a predictor for root coverage? A prospective clinical study. Journal of Clinical Periodontology, 44(9), 933–940. 10.1111/jcpe.12769. [DOI] [PubMed] [Google Scholar]
- Griffin, T. J. , Cheung, W. S. , Zavras, A. I. , & Damoulis, P. D. (2006). Postoperative complications following gingival augmentation procedures. Journal of Periodontology, 77(12), 2070–2079. 10.1902/jop.2006.050296. [DOI] [PubMed] [Google Scholar]
- Guldener, K. , Lanzrein, C. , Eliezer, M. , Katsaros, C. , Stähli, A. , & Sculean, A. (2020). Treatment of single mandibular recessions with the modified coronally advanced tunnel or laterally closed tunnel, hyaluronic acid, and subepithelial connective tissue graft: a report of 12 cases. Quintessence International, 51(6), 456–463. 10.3290/j.qi.a44492. [DOI] [PubMed] [Google Scholar]
- Håkansson, L. , Hällgren, R. , & Venge, P. (1980). Regulation of granulocyte function by hyaluronic acid. In vitro and in vivo effects on phagocytosis, locimotion, and metabolism. Journal of Clinical Investigation, 66(2), 298–305. 10.1172/JCI109857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmänner, P. , Alessandri, R. , Laugisch, O. , Aroca, S. , Salvi, G. E. , Stavropoulos, A. , & Sculean, A. (2012). Predictability of surgical techniques used for coverage of multiple adjacent gingival recessions‐A systematic review. Quintessence International, 43(7), 545–554. [PubMed] [Google Scholar]
- Huang, L. , Cheng, Y. Y. , Koo, P. L. , Lee, K. M. , Qin, L. , Cheng, J. C. , & Kumta, S. M. (2003). The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial‐derived cell cultures. Journal of Biomedical Materials Research Part A, 66(4), 880–884. 10.1002/jbm.a.10535. [DOI] [PubMed] [Google Scholar]
- Huang, L. H. , Neiva, R. E. , & Wang, H. L. (2005). Factors affecting the outcomes of coronally advanced flap root coverage procedure. Journal of Periodontology, 76(10), 1729–1734. [DOI] [PubMed] [Google Scholar]
- Kim, J. J. , Song, H. Y. , Ben Amara, H. , Kyung‐Rim, K. , & Koo, K. T. (2016). Hyaluronic acid improves bone formation in extraction sockets with chronic pathology: A pilot study in dogs. Journal of Periodontology, 87(7), 790–795. 10.1002/jop.2016.150707. [DOI] [PubMed] [Google Scholar]
- Kumar, R. , Srinivas, M. , Pai, J. , Suragimath, G. , Prasd, K. , & Polepalle, T. (2014). Efficacy of hyaluronic acid (hyaluronan) in root coverage procedures as an adjunct to coronally advanced flap in Miller classⅠrecession: a clinical study. Journal of Indian Society of . Periodontology, 18(6), 746–750. 10.4103/0972-124X.147411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzrein, C. , Guldener, K. , Imber, J. C. , Katsaros, C. , Stähli, A. , & Sculean, A. (2020). Treatment of multiple adjacent recessions with the modified coronally advanced tunnel or laterally closed tunnel in conjunction with cross‐linked hyaluronic acid and subepithelial connective tissue graft: a report of 15 cases. Quintessence International, 5, 2–11. [DOI] [PubMed] [Google Scholar]
- Larjava, H. , Heino, J. , Kähäri, V. M. , Krusius, T. , & Vuorio, E. (1989). Characterization of one phenotype of human periodontal granulation‐tissue fibroblasts. Journal of Dental Research, 68(1), 20–25. 10.1177/00220345890680010301. [DOI] [PubMed] [Google Scholar]
- Löe, H. , Anerud, A. , & Boysen, H. (1992). The natural history of periodontal disease in man: prevalence, severity, and extent of gingival recession. Journal of Periodontology, 63(6), 489–495. 10.1902/jop.1992.63.6.489. [DOI] [PubMed] [Google Scholar]
- Mendes, R. M. , Silva, G. A. , Lima, M. F. , Calliari, M. V. , Almeida, A. P. , Alves, J. B. , & Ferreira, A. J. (2008). Sodium hyaluronate accelerates the healing process in tooth sockets of rats. Archives of Oral Biology, 53(12), 1155–1162. 10.1016/j.archoralbio.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Moseley, R. , Waddington, R. J. , & Embery, G. (2002). Hyaluronan and its potential role in periodontal healing. Dental Update, 29(3), 144–148. 10.12968/denu.2002.29.3.144. [DOI] [PubMed] [Google Scholar]
- Oksala, O. , Salo, T. , Tammi, R. , Hakkinen, L. , Jalkanen, M. , Inki, P. , & Larjava, H. (1995). Expression of proteoglycans and hyaluronan during wound healing. Journal of Histochemistry & Cytochemistry, 43(2), 125–135. 10.1177/43.2.7529785. [DOI] [PubMed] [Google Scholar]
- Pilloni, A. , & Bernard, G. W. (1998). The effect of hyaluronan on mouse intramembranous osteogenesis in vitro. Cell and Tissue Research, 294(2), 323–333. 10.1007/s004410051182. [DOI] [PubMed] [Google Scholar]
- Pilloni, A. , Schmidlin, P. R. , Sahrmann, P. , Sculean, A. , & Rojas, M. A. (2019). Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class Ⅰ single gingival recession sites: a randomized controlled clinical trial. Clinical Oral Investigations, 23(3), 1133–1141. [DOI] [PubMed] [Google Scholar]
- Pirnazar, P. , Wolinsky, L. , Nachnani, S. , Haake, S. , Pilloni, A. , & Bernard, G. W. (1999). Bacteriostatic effects of hyaluronic acid. Journal of Periodontology, 70(4), 370–374. 10.1902/jop.1999.70.4.370. [DOI] [PubMed] [Google Scholar]
- Ruggiero, S. L. , Bertolami, C. N. , Bronson, R. E. , & Damiani, P. J. (1987). Hyaluronidase activity of rabbit skin wound granulation tissue fibroblasts. Journal of Dental Research, 66(7), 1283–1287. 10.1777/00220345870660071301. [DOI] [PubMed] [Google Scholar]
- Sasaki, T. , & Watanabe, C. (1995). Stimulation of osteoinduction in bone wound healing by high‐molecular hyaluronic acid. Bone, 16(1), 9–15. 10.1016/s8756-3282(94)00001-8. [DOI] [PubMed] [Google Scholar]
- Schlee, M. , & Esposito, M. (2011). Human dermis graft versus autogenous connective tissue grafts for thickening soft tissue and covering multiple gingival cessesions: 6‐month results from a preference clinical trial. European Jouranal of Oral Implantology, 4(2), 119–125. [PubMed] [Google Scholar]
- Scully, M. F. , Kakkar, V. V. , Goodwin, C. A. , & O’Regan, M. (1995). Inhibition of fibrinolytic activity by hyaluronan and its alcohol ester derivatives. Thrombosis Research, 78(3), 255–258. 10.1016/0049-3848(95)90876-h. [DOI] [PubMed] [Google Scholar]
- Shirakata, Y. , Nakamura, T. , Shinohara, Y. , Nakamura‐Hasegawa, K. , Hashiguchi, C. , Takeuchi, N. , Imafuji, T. , Sculean, A. , & Noguchi, K. (2019). Split‐mouth evaluation of connective tissue graft with or without enamel matrix derivative for the treatment of isolated gingival recession defects in dogs. Clinical Oral Investigations, 23(8), 3339–3349. 10.1007/s00784-018-2750-1. [DOI] [PubMed] [Google Scholar]
- Shirakata, Y. , Sculean, A. , Shinohara, Y. , Sena, K. , Takeuchi, N. , Bosshardt, D. D. , & Noguchi, K. (2016). Healing of localized gingival recessions treated with a coronally advanced flap alone or combined with an enamel matrix derivative and a porcine acellular dermal matrix: a preclinical study. Clinical Oral Investigations, 20(7), 1791–1800. 10.1007/s00784-015-1680-4. [DOI] [PubMed] [Google Scholar]
- Stähli, A. , Imber, J. C. , Raptis, E. , Salvi, G. E. , Eick, S. , & Sculean, A. (2020). Effect of enamel matrix derivative on wound healing following gingival recession coverage using the modified coronally advanced tunnel and subepithelial connective tissue graft: a randomised, controlled, clinical study. Clinical Oral Investigations, 24(2), 1043–1051. 10.1007/s00784-019-03008-6. [DOI] [PubMed] [Google Scholar]
- Van den Bogaerde, L. (2009). Treatment of infrabony periodontal defects with esterified hyaluronic acid: clinical report of 19 consecutive lesions. The International Journal of Periodontics and Restorative Dentistry, 29(3), 315–323. [PubMed] [Google Scholar]
- Weigel, P. H. , Frost, S. J. , McGary, C. T. , & LeBoeuf, R. D. (1988). The role of hyaluronic acid in inflammation and wound healing. International Journal of Tissue Reactions, 10(6), 355–365. [PubMed] [Google Scholar]
- West, D. C. , Hampson, I. N. , Arnold, F. , & Kumar, S. (1985). Angiogenesis induced by degradation products of hyaluronic acid. Science, 228(4705), 1324–1326. 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


