Abstract
The maternal mortality rate in Japan was 3.5 per 100 000 live births in 2017, similar to that reported in other developed countries. To reduce the number of maternal deaths, a Japanese nationwide registration and analysis system was implemented in 2010. Between January 2010 and April 2018, 367 maternal deaths were reported. Among them, by reviewing 80 autopsy records, the direct obstetric causes of death were identified in 52 women. The major causes of deaths were amniotic fluid embolism and acute pulmonary thromboembolism. The other 26 maternal deaths were associated with indirect obstetric causes including invasive Group A Streptococcus infection, aortic dissection, cerebral stroke and cardiomyopathies. This review highlights the importance of autopsy in maternal deaths. On analyzing 42 autopsy specimens obtained from registered cases of maternal death during 2012–2015, the 36% of causes of death by autopsy were discordant with the clinical diagnosis. Moreover, of the 38% of non‐autopsied maternal death, the cause of death could not be clarified from the clinical chart. We emphasized that detailed autopsies are necessary to clarify the precise pathologic evidence related to pregnancy and delivery, especially causes of unexpected death such as amniotic fluid embolism.
Keywords: amniotic fluid embolism, autopsy, maternal death, nationwide registration, pregnancy
Abbreviations
- AFE
amniotic fluid embolism
- JAOG
Japan Association of Obstetricians and Gynecologists
- STN
sialyl Tn antigen
- ZnCP1
zinc coproporphyrin‐1
INTRODUCTION
Maternal deaths are tragic events in obstetric practice. The perinatal mortality rate in Japan was the lowest worldwide in 1985. However, before 1985, the Japanese maternal mortality rate was rather high compared with those reported in other developed countries. Owing to the development of a blood supply system and maternal emergency transports, the maternal death rate has almost halved over the past two decades, dropping from 8.6 per 100 000 live births in 1990 to 4.6 per 100 000 live births in 2010. During this period, the Japanese Ministry of Health, Labour and Welfare registered the number of maternal deaths without analyzing the causes of deaths. 1 The current maternal mortality rate in Japan is estimated to be approximately four per 100 000 deliveries, similar to those reported in other developed countries (Table 1). 1 Maternal deaths in the developed world are uncommon, and therefore, it is difficult to identify factors that can be addressed to prevent deaths based on clinical records obtained from the local communities or hospitals. 2 A comprehensive review of pregnancy‐related deaths could contribute to the prevention of future deaths.
Table 1.
Maternal mortality rate and perinatal mortality rate in developed countries (2016)
| Country | Maternal mortality rate (per 100 000 live births) | Perinatal mortality rate (per 1000 live births) |
|---|---|---|
| Japan (2016) | 3.4 | 2.4 |
| United States (2015) | 28.7 | 6.0 |
| France (2014) | 4.6 | 11.8* |
| Germany (2015) | 3.3 | 5.6 |
| Italy (2012) | 2.1 | 3.8 |
| The Netherlands (2016) | 3.5 | 4.7 |
| Sweden (2016) | 2.6 | 5.0 |
| United Kingdom (2015) | 4.5 | 6.5 |
| *2010 |
‘The Maternal, Newborn and Infant Clinical Outcome Review Programme’, which began in England and Wales in 1952, is the most well‐known nationwide registration and comprehensive review system of maternal deaths. 3 Lower rates of maternal death were achieved in the United Kingdom (UK) after the implementation of the project. The system requires the registration of all maternal deaths in ‘The Confidential Enquiry into Maternal Deaths in the United Kingdom’. 4 The confidential enquiry clarified the real cause of each death based on autopsy findings and accurate pathological diagnoses for all maternal deaths. This program makes recommendations concerning the improvement of clinical care and service and produces a report for all health professionals and funding bodies in the UK every 3 years. 4 , 5 , 6 Many countries have started similar nationwide programs to reduce maternal mortality. 7 , 8 , 9 , 10 , 11 , 12 , 13
In Japan, the Confidential Inquiry into Maternal Deaths Research Group (chaired by Professor Nagaya K) examined all maternal death certificates and analyzed factors contributing to maternal mortality in the Japanese population during 1991–1992. 14
Seventeen years later, in 2008, the maternal mortality rate in Japan began to increase again. To reduce maternal deaths, the Japan Association of Obstetricians and Gynecologists (JAOG) established a registration system for maternal deaths in 2010. The Maternal Death Exploratory Committee chaired by Professor Ikeda T, Mie University, supported by the Japanese Health and Labour Sciences Research Grant (No. 24090101, H30‐healthcare‐general‐014), performs a detailed analysis of maternal deaths. 15 , 16 , 17
In this review article, we discussed the causes of maternal deaths in Japan and presented the importance of maternal autopsies for clarifying the cause of death based on a review of autopsy samples, as a part of the JAOG registration system.
Data collection and assessment processes by the Maternal Death Exploratory Committee in Japan
The registration system of the JAOG has been described in a previous study. 16 When a maternal death occurs at a hospital or clinic, reports including clinical details are submitted to the registration system of the JAOG by the relevant medical institution. The number of deaths registered in this system has been nearly equal to or greater than that reported by the Japanese Ministry of Health, Labour and Welfare since 2013.
A registration form for maternal death during pregnancy or within a year post‐delivery comprised approximately 100 questions addressing the clinical course of the illness, characteristics of the maternity unit where care was provided and characteristics of the caregivers. All medical records related to the death were also collected, such as records of anesthetic procedures, medical images, laboratory data and pathological and autopsy reports, including serum samples from women with postpartum hemorrhage.
Regarding the pathological diagnosis of maternal death, we published and distributed a manual to standardize the autopsy procedure and the criteria for diagnosis in 2010. In particular, for amniotic fluid embolism (AFE), patients were diagnosed using the Japanese consensus criteria for AFE, based on the US/UK criteria (Table 2). 18 In suspected cases of AFE, we measured the serum levels of the sialyl Tn antigen (STN) and zinc coproporphyrin‐1 (ZnCP1) to confirm the presence of fetal components. In addition to hematoxylin–eosin (HE), Alcian blue (Merck) staining and immunohistochemistry with a broad‐spectrum anti‐pancytokeratin cocktail (AE1/AE3), anti‐cytokeratin 1 (CK1) were performed for specimens from the lung and uterus to detect fetal components (Figs. 1, 2, 3). AE1/AE3 and CK1 staining were used to detect the squamous cells of fetal skin in maternal capillaries. Alcian blue staining was performed to identify the presence of amniotic mucin in the maternal circulation.
Table 2.
Japanese criteria for the diagnosis of amniotic fluid embolism (AFE)
| (1) If symptoms appeared during pregnancy or within 12 h of delivery. |
| (2) If any intensive medical intervention was conducted to treat one or more of the following symptoms/diseases: |
| (A) Cardiac arrest |
| (B) Severe bleeding of unknown origin within 2 h of delivery (≥1500 mL) |
| (C) Disseminated intravascular coagulation |
| (D) Respiratory failure |
| (3) If the findings or symptoms cannot be explained by other diseases. |
| A clinical diagnosis of AFE can be made if the pathological condition meets the above three criteria. Because these diagnostic criteria serve the purpose of making a clinical diagnosis and being able to promptly provide treatment, the pathological conditions that meet them may include those other than AFE. |
Figure 1.
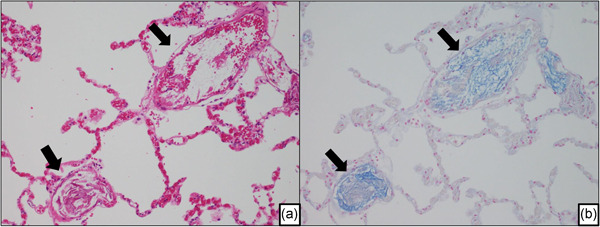
Amniotic fluid embolism in an autopsied maternal lung. (a) Image showing the fetal debris in the pulmonary arterioles. (arrow). (b) Serial section of Fig. 1a stained with Alcian blue. In the pulmonary artery, mucin as fetal components were identified (arrow).
Figure 2.
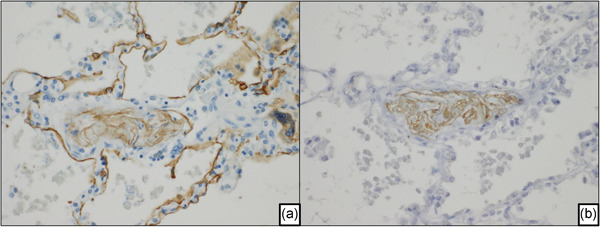
Immunohistochemistry of amniotic fluid embolism in an autopsied lung. (a) Fetal skin fragments stained positively with the broad‐spectrum anti‐pancytokeratin cocktail (AE1/AE3) are shown, and alveolar pneumocytes are shown as a positive control. (b) Cytokeratin‐1‐positive fetal skin fragments in pulmonary arterioles.
Figure 3.
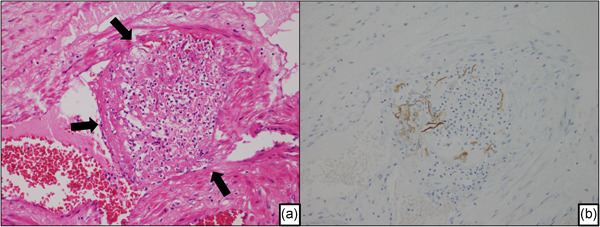
A representative case of amniotic fluid embolism in an autopsied uterus. (a) Thrombus around the fetal component in a venule of the uterus (arrow). The image shows intravascular coagulation due to the fetal debris. (b) The serial section of Fig. 3a. The fetal debris in the venules of the uterus show positive staining with the broad‐spectrum anti‐pancytokeratin cocktail (AE1/AE3).
Therefore, we proposed two types of AFE according to clinical symptoms. One is mainly characterized by cardiopulmonary collapse, dyspnea, chest pain, symptoms of shock and cardiac arrest, which are well‐known symptoms of classical AFE (cardiopulmonary collapse type AFE). The other type involves the presence of disseminated intravascular coagulation (DIC) and uterine atonic bleeding (uterine focused AFE); it is characterized by atonic bleeding and fibrinolysis‐dominant DIC. 18 , 19
The medical records were anonymized by the JAOG and sent to the evaluation committee of the Maternal Death Exploratory Committee. This evaluation committee includes 15 obstetricians, five anesthetists, two pathologists, one forensic pathologist and other specialists. The committee carefully examined and discussed all data, confirmed the diagnosis and clarified the factors associated with each maternal death.
RESULTS AND DISCUSSION
Autopsy data related to maternal deaths in Japan (Tables 3 and 4)
Table 3.
Summary of the analyzed cases between January 2010 and April 2018
| Pathological autopsy | 80 cases |
|---|---|
| Age | 34.6 ± 4.8 years |
| Range | 23–45 years |
| Direct obstetric causes | 52 cases |
| Indirect obstetric causes | 26 cases |
| Unknown causes | 2 cases |
Table 4.
Causes of maternal deaths in Japan (autopsy cases: 2010–2018)
| Cause of death | Number of cases |
|---|---|
| Direct deaths | 52 |
| Amniotic fluid embolism (cardiopulmonary collapse type) | 15 |
| Amniotic fluid embolism (uterine focused) | 18 |
| Cervical and vaginal laceration | 2 |
| Uterine rupture | 3 |
| Uterine inversion | 2 |
| Placental abruption | 3 |
| Atonic bleeding | 2 |
| Pulmonary embolism | 7 |
| Indirect deaths | 26 |
| Cerebral stroke | 1 |
| Subarachnoid hemorrhage | 1 |
| Vertebral artery dissection | 2 |
| Aortic dissection | 3 |
| Cardiomyopathy | 3 |
| Malignant lymphoma | 1 |
| Ureteral cancer | 1 |
| Hemophagocytic syndrome | 1 |
| Systemic lupus erythematosus | 1 |
| Right subclavian venous rupture (neurofibromatosis type 1) | 1 |
| Chlamydia psittaci infection (parrot disease) | 1 |
| Bacterial meningitis | 1 |
| Septic shock | 1 |
| Group A Streptococcus infection | 8 |
| Unknown causes | 2 |
Between January 2010 and April 2018, 367 cases of maternal deaths were reported in the maternal death registry. The Maternal Death Exploratory Committee analyzed 361 of these cases. Autopsy was performed in 138 cases (37.6%), pathological autopsy in 86 cases (23.4%), judicial autopsy in 41 cases (11.2%) and administrative autopsy in 11 cases (3.0%). Fig. 4 shows the recent tendency of autopsy rates.
Figure 4.
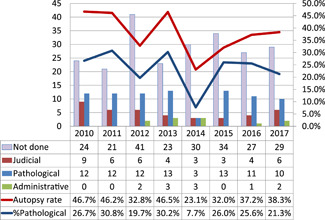
Recent trends in autopsy rates (2010–2017: analyzed cases).
Reports given by the committee during the aforementioned period were used in this study. Among the 86 cases that performed pathological autopsies for 8 years and 3 months, there were 80 cases available for satisfied analysis of specimens and autopsy records. The median age was 34.7 ± 4.8 years (range, 23–45 years). The pathological diagnoses of the 80 analyzed cases are summarized in Table 4. We identified that 52 maternal deaths had occurred due to direct obstetric causes and 26 had occurred due to indirect obstetric causes. In the remaining two cases, the precise causes of maternal death could not be identified even after autopsy.
The leading cause of death was AFE. All 33 patients with AFE were diagnosed based on the detection of fetal components, such as fetal keratin, and/or amniotic fluid components in mothers’ lungs and/or uterus.
Fifteen deaths were caused by cardiopulmonary collapse type AFE, and 18 were caused by uterine focused AFE. In cardiopulmonary collapse type AFE, the initial symptom was sudden cardiac arrest; two patients experienced this initial symptom during scheduled cesarean section (Table 5). In all 18 patients with uterine focused AFE, the initial symptom was massive obstetric hemorrhage; three patients experienced this initial symptom during the scheduled cesarean section before the onset of labor. The 14 mothers showed the symptoms and signs in the fourth stage of labor (Table 5).
Table 5.
Occurrence of signs and symptoms and types of delivery associated with amniotic fluid embolism (AFE)
| Cardiopulmonary collapse type AFE | Uterine focused AFE | |
|---|---|---|
| N = 15 (mode of delivery) | N = 18 (mode of delivery) | |
| Before the onset of labor | 1 | 0 |
| First stage of labor | 5 | 0 |
| Second stage of labor | 1 | 0 |
| Third stage of labor | 3 | 1 (forceps 1) |
| Puerperium | 3 (C/S 1, vaginal 2) | 14 (vacuum 8, vaginal 6) |
| During scheduled C/S | 2 | 3 |
Abbreviation: C/S, cesarean section.
The birth canal injuries such as cervical laceration, vaginal laceration and uterine rupture occurred in five cases. The seven patients died of postpartum pulmonary thromboembolism.
Autopsies in cases of maternal deaths were performed for approximately 37.8% of patients with obstetric hemorrhage, 40% of patients with cardiopulmonary arrest and 37.9% of patients with infectious disease; however, autopsies were rarely performed in cases of cerebral stroke and malignancies (4.3% and 20%, respectively). The causes of death for these cases could be diagnosed by imaging modalities.
Computed tomography or other imaging modalities could also diagnose aortic dissection or aortic rupture in sudden onset; however, underlying diseases causing sudden events, such as Marfan syndrome and Ehlers‐Danlos syndrome, were not revealed before autopsy. To clarify the actual pathogenesis, genetic analysis should be also performed at autopsy.
The importance of autopsies especially for maternal death
In Japan, the recent autopsy rate has been low. In 2013, the autopsy rate was reported to be 4.97% in hospitals certificated by the Japan Council for Quality Health Care (https://www.jq-hyouka.jcqhc.or.jp/wp-content/uploads/2016/10/databook_h23.pdf). The annual report of the Japanese Society of Pathology showed decreasing trends in autopsy rates. Winters et al. conducted a systematic review of autopsy results from ICU cases. 20 They reported that 28% of autopsies revealed at least one misdiagnosis, and 8% of autopsies revealed major and potentially lethal diagnostic errors. In 2016, Marshall and Milikowski compared the clinical diagnosis with anatomic findings in a single institution during 2009–2014. 21 They reported that in 19.5% of autopsy cases, major significant findings that had not been clinically detected were found. Based on these reports, it was considered that 20–30% of cases from other medical fields require autopsy.
The analysis of 361 cases of maternal death in our registry during 2010–2017 revealed that the incidence of direct obstetric deaths was 61% in 2017, and the proportion of direct obstetric causes for maternal deaths exceeded the proportion of indirect causes. 15
In the United Kingdom, according to the report for 2013–2015, the number of direct maternal deaths was lower than that of indirect maternal deaths. The major causes of maternal death in the United Kingdom were heart disease, pneumonia and thrombosis. A similar trend applies to reports from four Nordic countries; the causes of maternal deaths were cardiovascular disease, hypertensive disorders of pregnancy, thromboembolism and suicide. 22 A report from the United States that analyzed the data for 2011–2015 showed that the major causes of maternal death were cardiovascular conditions, infectious diseases and obstetric hemorrhage. 13 In general, in developed countries, the proportion of maternal deaths due to indirect causes may exceed that of maternal deaths due to direct causes.
However, obstetric bleeding remained the most common cause of maternal death in Japan in 2017. 15 Obstetric hemorrhage is a clinical symptom that may be related to several initial manifestations, including uterine focused AFE, placental abruption, uterine rupture, inversion of the uterus and uterine atony. 15 Although precise diagnosis might be difficult, nearly half of the maternal deaths due to obstetric hemorrhage may be avoided through improvements in medical practices, obstetric care, maternal transport and blood supply systems. 8 , 9 , 10 , 12 , 23 The pathophysiology of obstetric hemorrhage may differ among cases. Additionally, to reduce the number of deaths due to obstetric hemorrhage, it is necessary to determine the underlying cause of hemorrhage. For this purpose, we emphasize the necessity of autopsy in all cases of maternal death.
Necessity of autopsies to clarify the factors associated with maternal death
According to the ‘Annual of pathological autopsy cases of Japan’, the number of registered Japanese cases at the Japanese Society of Pathology was 24 575 in 2002 compared to 11 089 in 2017. Some clinicians argue that the pathophysiology is clear and that detailed reports of radiological studies or blood tests are sufficient and accurate, therefore there is no need to perform autopsies. However, autopsy is still considered the most valuable tool to determine the cause of maternal death by the Maternal Death Exploratory Committee. Based on autopsy reports, we estimated the pathophysiological changes in mothers, the need for or the effect of treatments and the direct causes of death. Our committee could not determine the direct cause of death when autopsy reports were not available; consequently, it was not possible to provide clear recommendations to caregivers in Japan on how to prevent maternal death in similar cases in Japan.
We analyzed the necessity and importance of performing an autopsy in determining the exact cause of maternal death in Japan. 24 Reports pertaining to 127 women who died during pregnancy or within a year post‐delivery were analyzed by the Maternal Death Exploratory Committee during 2012–2015 in Japan. Maternal deaths were analyzed to verify the need for autopsy in cases in which autopsy was and was not performed (Table 6). Autopsies were performed in 42 cases (33%); among them, the final diagnosis was compatible with the clinical course in 24 cases (55%), while the autopsy diagnosis was incompatible with the clinical course in 15 cases (36%). A reasonable cause of death could not be identified in only three cases, even after autopsy. For the 85 cases in which autopsy was not performed, we concluded that a reasonable cause of death could not be identified in 33 cases (38%). On assessing significance using the Chi‐square test, the incidence of cases in which a reasonable cause of death could not be identified was significantly more frequent compared to the cases in which the autopsy was performed (P < 0.001).
Table 6.
Final diagnosis of maternal deaths and their evidence in cases where autopsy was performed or not (2012–2015)
| Autopsy performed, N = 42 | ||
|---|---|---|
| Pathological diagnosis | ||
| Compatible with the clinical course | 24 | (57%) |
| Incompatible with the clinical course | 15 | (36%) |
| Diagnosis based on autopsy findings | 12 | (29%) |
| Diagnosis based on surgical specimen findings | 1 | (2%) |
| Clinical diagnosis after the exclusion of other causes based on autopsy findings | 2 | (5%) |
| Unexplained even after autopsy | 3 | (7%) |
| Autopsy not performed, N = 85 | ||
| Diagnosis based on ante‐mortem surgical specimen findings | 7 | (8%) |
| Clinical diagnosis during operation | 14 | (16%) |
| Diagnosis made by post‐mortem imaging | 3 | (4%) |
| Diagnosis based on the results of ante‐mortem examinations | 18 | (21%) |
| Coroner inspection | 10 | (12%) |
| Autopsy should be performed | 33 | (39%) |
| Original cause was unclear | 25 | (29%) |
| Unexplained by clinical diagnosis | 8 | (9%) |
New concept of AFE and problems for diagnosis
Amniotic fluid embolism is an acute, severe, and unexpected complication in obstetrics. It is thought to arise from a tear in the fetal membrane, which gives way for the passage of amniotic fluid into the maternal circulation via uterine veins. The clinical course is characterized by the rapid onset of cardiopulmonary arrest and coagulopathies. The mortality rate of patients with AFE remains high, and AFE accounts for 5–15% of all maternal deaths in developed countries. 18 , 19 , 25 , 26 The reported risk factors of AFE include maternal age of ≥35 years, cesarean delivery, forceps/vacuum delivery, placenta previa, placenta abruption, eclampsia and fetal distress. 24
A definite histopathological diagnosis is based on the identification of fetal keratin and/or amniotic fluid components in the uterine vessels and maternal pulmonary arteries. Microscopic fibrin thrombi were found in mothers who died rapidly (Figs. 1, 3).
In 1926, Meyer first described the physical obstruction of the maternal circulation by amniotic fluid. In 1941, Steiner and Lushbaugh emphasized that emboli of fetal components constitute the main cause of maternal death, based on the results of 32 autopsy cases. 27 In the 1950s, it was believed that the mechanical obstruction of pulmonary vessels was caused by amniotic fluid/fetal components in AFE.
However, in the 1990s, two hypotheses based on the maternal immune response to fetal antigens were considered. The first hypothesis was that clinical symptoms indicated an anaphylactoid reaction resulting from mast cell degranulation and the release of histamine (the underlying mechanism of the anaphylactoid reaction), 28 , 29 , 30 and the other hypothesis was that the symptoms resulted from massive activation of the complement pathway. 30
In 2001, Benson et al. reported that maternal complement levels (C3 and C4 levels) were significantly decreased in AFE. 29 These findings suggested that disorders involving the coagulofibrinolytic system and complement system might play an important role in the pathogenesis of AFE. However, pulmonary vasospasm due to an anaphylactoid reaction to fetal components is more frequently reported. 19 Currently, it is considered to be an immune/inflammatory reaction as it is called an ‘anaphylactoid syndrome of pregnancy’. 30
However, the pathophysiology of this condition is still not well understood. To date, AFE is diagnosed clinically when pregnant women suddenly become extremely ill without an obvious explanation, with abrupt onset of hypoxia, hypotension, seizures or DIC during labor or delivery. 18 , 31 , 32 AFE, in Japan, is defined according to the Japanese consensus criteria for the diagnosis of AFE, based on the US/UK criteria (Table 2).
Currently, the detection of amniotic materials when performing an autopsy is the only reliable diagnostic clue for AFE. Several peripheral blood tests have been proposed for the diagnosis of AFE, including tests for tryptase, 29 STN, complements C3 and C4, 29 , 33 and ZnCP1. 34 , 35 However, these methods have limitations, and they are still considered as research tools rather than reliable diagnostic tools for clinical practice.
The mortality rate of AFE remains high. 25 The rate of maternal mortality in Japan, due to AFE was estimated to be 24.3%. 36 In the United States, the incidence of AFE was 5.1 per 100 000 deliveries, with a fatality rate of 13.2% from 2001 to 2007. 37 The difference in mortality rates is thought to be due to the variation in AFE definitions among countries. 32
In many countries, including Japan, the diagnosis of AFE is based on clinical symptoms only, and histopathological examination is not always deemed necessary. However, on using criteria based on clinical symptoms alone, patients presenting with severe DIC due to causes other than AFE might be included as having AFE or conversely, some atypical AFE cases might be excluded. 29 Therefore, for the accurate analysis of AFE pathophysiology, it is necessary to perform research based on autopsy cases.
Limitations of the Japanese maternal death registration system
The Japanese registration system is based on voluntary reports from obstetricians, and enrollment in the system is not mandatory for clinicians. Most events that occur during pregnancy or immediately after delivery may be reported by obstetricians. However, we could not analyze the maternal death cases treated by clinicians other than obstetricians at a chronic stage. We believe that a comprehensive reporting system is needed in Japan for the identification of all maternal deaths, with the aim of reducing its incidence.
Instructions for performing maternal autopsy
Autopsy plays an important role in clarifying the causes of maternal death. The surgeon should recognize the pathogenesis of pregnancy‐related diseases such as AFE, hypertensive disorders of pregnancy or infarction of the pituitary gland.
Pathologists should ask obstetricians for advice about the physiological and anatomical changes associated with pregnancy and on surgical procedures performed before maternal death. It is important to examine the resected uterus, placenta and the stillborn baby.
In addition, in an autopsy case involving a woman of childbearing age, the pathologists should check the age of her youngest child. When the child is aged under 1 year, the doctors should register this case in the registry as a case of late maternal death.
When examining the uterus, it is important to measure the uterine weight. Based on our data regarding uterine weight immediately after delivery, the uterus weighed more than 700 g in AFE cases. The main pathogenesis of AFE is increased blood vessel permeability, resulting in interstitial edema, which may increase uterine weight. The abrasion or laceration of the uterus should be examined in detail to identify the potential entry site of amniotic fluid.
Serological methods for diagnosing AFE include tests for Zn‐CP1 and STN. Zn‐CP1 is the most specific marker of AFE and can be measured at the Department of Obstetrics and Gynecology, Hamamatsu University School of Medicine. The serum must be stored at −20°C, wrapped with aluminum foil, and sent to Hamamatsu University School of Medicine. For histological analysis, we routinely performed HE staining, Alcian blue staining (Merck), and immunohistochemical staining using a broad‐spectrum anti‐pan‐cytokeratin cocktail (AE1/AE3), to confirm the existence of fetal components in the mother (Figs. 1, 2, 3). Alcian blue staining is very convenient for detecting amniotic fluid mucin in the capillaries of the lungs and uterus of the mother. Anti‐cytokeratin 1(CK1) is useful for detecting fragmented fetal components in the maternal tissue.
Regarding the number of sections, we recommend at least one section from each lobe of lungs, total of five blocks, and six blocks for the uterus (from the left and right sides of the cervix, corpus and fundus). For detailed information, Japanese pathologists can refer to the ‘Autopsy manual for maternal death cases’ edited by our committee (in Japanese).
CONCLUSION
Pregnancy and parturition are physiological events, but the pathogenesis of an abnormal pregnancy has not been elucidated. The characteristics of pregnancy in women with complications are still obscure. Certainly, the maternal mortality rate in Japan has been very low in the past years, but it is unacceptable that young women may lose their lives during pregnancy or childbirth; there should become no maternal death by pathologist's efforts of pathological analysis.
DISCLOSURE STATEMENT
None declared.
FUNDING
This study was funded by a grant from the Ministry of Health, Labour and Welfare, Japan (No. 24090101, H30‐healthcare‐general‐014).
AUTHOR CONTRIBUTIONS
TW, MT and HI‐U reviewed and interpreted the pathological data, contributed to writing the manuscript and approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank all members of the Maternal Death Exploratory Committee and the members of the Japan Association of Obstetricians and Gynecologists. The author (TW) has been announced as the winner of The Japanese Society of Pathology; Case Research Award in 2018.
REFERENCES
- 1. National Institute of Population and Social Security Research . Latest Demographic Statistics, 2019. Population Research Series, No. 341. Tokyo: National Institute of Population and Social Security Research, 2019. [Google Scholar]
- 2. Willcox ML, Price J, Scott S et al. Death audits and reviews for reducing maternal, perinatal and child mortality. Cochrane Database Syst Rev 2020; 3: CD012982. 10.1002/14651858.CD012982.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewis G. Saving Mothers’ Lives: The continuing benefits for maternal health from the United Kingdom (UK) confidential enquires into maternal deaths. Semin Perinatol 2012; 36: 19–26. [DOI] [PubMed] [Google Scholar]
- 4. Knight M, Nair M, Tuffnell D et al. Saving Lives, Improving Mothers’ Care. Lessons Learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2013–15. Oxford: National Perinatal Epidemiology Unit, University of Oxford, 2017. [Google Scholar]
- 5. Lewis G, ed. Saving Mothers’ Lives: Reviewing Maternal Deaths to Make Motherhood Safer −2003‐2005, The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. London: The Confidential Enquiry into Maternal and Child Health (CEMACH), 2007. [Google Scholar]
- 6. Centre for Maternal and Child Enquiries (CMACE) . Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006–08. The Eighth Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG 2011; 118: 1–203. [DOI] [PubMed] [Google Scholar]
- 7. Wu TP, Liang FW, Huang YL, Chen LH, Lu TH. Maternal mortality in Taiwan: A nationwide data linkage study. PLoS One 2015; 10: e0132547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vangen S, Bødker B, Ellingsen L et al. Maternal deaths in the Nordic countries. Acta Obstet Gynecol Scand 2017; 96: 1112–19. [DOI] [PubMed] [Google Scholar]
- 9. Sullivan EA, Ford JB, Chambers G, Slaytor EK. Maternal mortality in Australia, 1973‐1996. Aust N Z J Obstet Gynaecol 2004; 44: 452–7. [DOI] [PubMed] [Google Scholar]
- 10. Schutte JM, de Jonge L, Schuitemaker NW, Santema JG, Steegers EA, van Roosmalen J. Indirect maternal mortality increases in the Netherlands. Acta Obstet Gynecol Scand 2010; 89: 762–8. [DOI] [PubMed] [Google Scholar]
- 11. Saucedo M, Deneux‐Tharaux C, Bouvier‐Colle MH. Ten years of confidential inquiries into maternal deaths in France, 1998‐2007. Obstet Gynecol 2013; 122: 752–60. [DOI] [PubMed] [Google Scholar]
- 12. Fässler M, Zimmermann R, QuackLötscher KC. Maternal mortality in Switzerland 1995‐2004. Swiss Med Wkly 2010; 140: 25–30. [DOI] [PubMed] [Google Scholar]
- 13. Petersen EE, Davis NL, Goodman D et al. Vital signs: Pregnancy‐related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. Morb Mortal Wkly Rep 2019; 68: 423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nagaya K, Fetters MD, Ishikawa M et al. Causes of maternal mortality in Japan. JAMA 2000; 283: 2661–7. [DOI] [PubMed] [Google Scholar]
- 15. Hasegawa J, Katsuragi S, Tanaka H et al. Decline in maternal death due to obstetric haemorrhage between 2010 and 2017 in Japan. Sci Rep 2019; 9: 11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hasegawa J, Ikeda T, Sekizawa A et al. Recommendations for saving mothers’ lives in Japan: Report from the Maternal Death Exploratory Committee (2010‐2014). J Obstet Gynaecol Res 2016; 42: 1637–43. [DOI] [PubMed] [Google Scholar]
- 17. Hasegawa J, Sekizawa A, Tanaka H et al. Current status of pregnancy‐related maternal mortality in Japan: A report from the Maternal Death Exploratory Committee in Japan. BMJ Open 2016; 6: e010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanayama N, Tamura N. Amniotic fluid embolism: Pathophysiology and new strategies for management. J Obstet Gynaecol Res 2014; 40: 1507–17. [DOI] [PubMed] [Google Scholar]
- 19. Tamura N, Farhana M, Oda T, Itoh H, Kanayama N. Amniotic fluid embolism: Pathophysiology from the perspective of pathology. J Obstet Gynaecol Res 2017; 43: 627–32. [DOI] [PubMed] [Google Scholar]
- 20. Winters B, Custer J, Galvagno SM et al. Diagnostic errors in the intensive care unit: A systematic review of autopsy studies. BMJ Qual Saf. 2012; 21: 894–902. [DOI] [PubMed] [Google Scholar]
- 21. Marshall HS, Milikowski C. Comparison of clinical diagnoses and autopsy findings: Six‐year retrospective study. Arch Pathol Lab Med 2017; 141: 1262–6. [DOI] [PubMed] [Google Scholar]
- 22. Nair M, Knight M. Maternal Mortality in the UK 2012–14: Surveillance and Epidemiology Saving Lives, Improving Mothers’ Care – Lessons Learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2013–15. Oxford: National Perinatal Epidemiology Unit, University of Oxford, 2017. [Google Scholar]
- 23. Bødker B, Hvidman L, Weber T et al. Maternal deaths in Denmark 2002‐2006. Acta Obstet Gynecol Scand 2009; 88: 556–62. [DOI] [PubMed] [Google Scholar]
- 24. Hasegawa J, Wakasa T, Matsumoto H et al. Analysis of maternal death autopsies from the nationwide registration system of maternal deaths in Japan. J Matern Fetal Neonatal Med 2018; 31: 333–8. [DOI] [PubMed] [Google Scholar]
- 25. Conde‐Agudelo A, Romero R. Amniotic fluid embolism: An evidence‐based review. Am J Obstet Gynecol 2009; 201: e1–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bonnet MP, Zlotnik D, Saucedo M, Chassard D, Bouvier‐Colle MH, Deneux‐Tharaux C. Maternal death due to amniotic fluid embolism: A national study in France. Anesth Analg 2018; 126: 175–82. [DOI] [PubMed] [Google Scholar]
- 27. Steiner PE, Lushbaugh CC, Frank HA. Fatal obstetric shock for pulmonary emboli of amniotic fluid. Am J Obstet Gynecol 1949; 58: 802–5. [DOI] [PubMed] [Google Scholar]
- 28. Attwood HD. Fatal pulmonary embolism by amniotic fluid. J Clin Pathol 1956; 9: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benson MD, Kobayashi H, Silver RK, Oi H, Greenberger PA, Terao T. Immunologic studies in presumed amniotic fluid embolism. Obstet Gynecol 2001; 97: 510–14. [DOI] [PubMed] [Google Scholar]
- 30. Benson MD. Anaphylactoid syndrome of pregnancy. Am J Obstet Gynecol 1996; 175: 749. [PubMed] [Google Scholar]
- 31. Benson MD. Current concepts of immunology and diagnosis in amniotic fluid embolism. Clin Dev Immunol 2012; 2012: 946576. 10.1155/2012/946576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clark SL, Romero R, Dildy GA et al. Proposed diagnostic criteria for the case definition of amniotic fluid embolism in research studies. Am J Obstet Gynecol 2016; 215: 408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benson MD. A hypothesis regarding complement activation and amniotic fluid embolism. Med Hypotheses 2007; 68: 1019–25. [DOI] [PubMed] [Google Scholar]
- 34. Kobayashi H, Ooi H, Hayakawa H et al. Histological diagnosis of amniotic fluid embolism by monoclonal antibody TKH‐2 that recognizes NeuAc alpha 2‐6GalNAc epitope. Hum Pathol 1997; 28: 428–33. [DOI] [PubMed] [Google Scholar]
- 35. Kanayama N, Yamazaki T, Naruse H, Sumimoto K, Horiuchi K, Terao T. Determining zinc coproporphyrin in maternal plasma – A new method for diagnosing amniotic fluid embolism. Clin Chem 1992; 38: 526–9. [PubMed] [Google Scholar]
- 36. Kanayama N, Inori J, Ishibashi‐Ueda H et al. Maternal death analysis from the Japanese autopsy registry for recent 16 years: Significance of amniotic fluid embolism. J Obstet Gynaecol Res 2011; 37: 58–63. [DOI] [PubMed] [Google Scholar]
- 37. Fong A, Chau CT, Pan D, Ogunyemi DA. Amniotic fluid embolism: Antepartum, intrapartum and demographic factors. J Matern Fetal Neonatal Med 2015; 28: 793–8. [DOI] [PubMed] [Google Scholar]


