Summary
What is known and Objective
Meropenem, a broad‐spectrum carbapenem, is frequently used to treat severe bacterial infections in critically ill children. Recommendations for meropenem doses in adult infections are available; however, few studies have been published regarding the use of meropenem in children with sepsis, especially in those receiving continuous renal replacement therapy (CRRT) and extracorporeal membrane oxygenation (ECMO). We aimed to investigate the pharmacokinetic (PK) parameters of meropenem in children with sepsis receiving extracorporeal life support (ECLS).
Methods
This was a prospective observational clinical study of children with sepsis receiving ECMO or CRRT in the paediatric intensive care unit (PICU) of a children's hospital. The enrolled children received 20 mg/kg meropenem infusion over 1 hour, every 8 hours, and were grouped into children receiving ECMO, children receiving CRRT and children receiving neither ECMO nor CRRT. Plasma meropenem concentrations were determined using a validated high‐performance liquid chromatography‐tandem mass spectrometry (HPLC‐MS/MS). The key PK parameters were determined using the non‐compartmental approach.
Results and discussion
Twenty‐seven patients were finally enrolled. The eCLCR of the CRRT group was lower than that of the ECMO group. The values of elimination half‐life (t1/2), area under the plasma concentration‐time curve (AUCtau), area under the plasma concentration‐time curve from time zero to infinity (AUC0‐∞), and total clearance (CL) in the ECMO group were not different from those of the other groups (all p > 0.05). However, the AUCtau (p = 0.0137) and AUC0‐∞ (p = 0.0234) significantly decreased after filtration through a hemofiltration membrane in patients receiving CRRT.
What is new and Conclusion
No significant alterations in the PK parameters of meropenem occurred in children with sepsis administered ECMO and/or CRRT. Further investigations including PK modelling could provide evidence for appropriate meropenem dosing regimens during ECLS administration.
Keywords: AN69 membrane, children, continuous renal replacement therapy, critically ill, extracorporeal life support, extracorporeal membrane oxygenation, meropenem, pharmacokinetic, sepsis
Abbreviations
- AKI
acute kidney injury
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUC
area under the curve
- AUC0‐∞
area under the plasma concentration‐time curve from time zero to infinity
- AUCtau
area under the plasma concentration‐time curve
- CL
clearance
- CLSS
clearance at steady‐state
- CRP
C‐reactive protein
- CRRT
continuous renal replacement therapy
- CVVHDF
continuous veno‐venous haemodialysis
- eCLCR
estimated creatinine clearance
- ECLS
extracorporeal life support
- ECMO
extracorporeal membrane oxygenation
- ECOFF
epidemiological cut‐off value
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- HPLC‐MS/MS
high‐performance liquid chromatography‐tandem mass spectrometry
- LOD
limit of detection
- LOQ
limit of quantification
- MIC
minimum inhibitory concentration
- MRM
multiple reaction monitoring
- PICU
paediatric intensive care units
- PK
pharmacokinetic
- PPK
population pharmacokinetics
- PRISM III
Pediatric Risk of Mortality score III
- Sc
sieving coefficient
- t1/2
the elimination half‐life
- TDM
Therapeutic drug monitoring
- VA‐ECMO
veno‐arterial ECMO
- Vd
volume of distribution
- VV‐ECMO
veno‐veno ECMO
- WAZ
weight adjusted Z score
1. WHAT IS KNOWN AND OBJECTIVE
Severe infection remains a common medical issue in paediatric intensive care units (PICUs) and usually causes high mortality. 1 Broad‐spectrum β‐lactam antibiotics have been widely used to treat severe infections in children. Therapeutic drug monitoring (TDM) of β‐lactam antibiotics focuses on the % fT >MIC—the time, expressed as a percentage of the dosing interval, that the concentration of the antimicrobial agent remains above the minimum inhibitory concentration (MIC) for the microorganism—to achieve adequate therapeutic antibiotic concentrations and exposure, thereby improving clinical outcomes and reducing antibiotic resistance. 2 , 3 , 4 , 5 Recent reports reveal that the pharmacokinetic (PK) characteristics of antibiotics can be altered and their effects on bacteria reduced, in critically ill adults receiving extracorporeal life support (ECLS), including extracorporeal membrane oxygenation (ECMO) and continuous renal replacement therapy (CRRT). 6 , 7 However, PK data of β‐lactam antibiotics in critically ill children receiving ECLS remain limited.
Meropenem, a broad‐spectrum carbapenem, is frequently used to treat severe bacterial infections in critically ill children, including those with severe pneumonia and sepsis. 8 Recommendations for meropenem doses in adult infections are available; however, few studies have been published regarding the use of meropenem in children with sepsis, especially those receiving CRRT and ECMO. Hahn et al reviewed PK changes of antibiotics during ECMO in critically ill adult patients and demonstrated that the effects of ECMO on the PK parameters of meropenem are not significant. However, antibiotic clearance (CL) decreased, while the total volume of distribution (Vd) showed uncertain changes in two prospective studies. 9 , 10 Nevertheless, PK data of meropenem in critically ill children remain limited. Rapp et al reported the population pharmacokinetics (PPK) of meropenem in 40 critically ill children, among whom 8 received ECMO and 11 CRRT; the two‐compartment model showed CL, central volume of distribution (V1) and peripheral volume of distribution (V2) of 6.82 L/h, 40.6 L, and 9.2 L, respectively. However, detailed non‐compartmental data for the ECMO and CRRT groups are not available. 11 Cies et al assessed the PPK of meropenem in 9 critically ill children; CL and Vd for this population were 6.99 ± 2.5 mL/(min‧kg) and 0.78 ± 0.73 L/kg, respectively, although the analysis excluded children receiving ECMO or CRRT. 12
In general, PK parameters are influenced by the ECMO device that is used, the physicochemical properties of the drug and the pathological changes in patients. 13 , 14 , 15 Further, CRRT significantly eliminates meropenem, a small molecule with high affinity for water, low volume of distribution and a high unbound fraction. 16 However, CRRT intensity is not a significant modifier of meropenem CL. 16 Moreover, little is known about the influence on the filtration efficacy of β‐lactam antibiotics after inflammatory mediators block the hemofiltration membrane during CRRT. Multiple studies have demonstrated that prolonged infusion of meropenem can improve its PK parameters, with some suggesting a 24‐h continuous infusion at MIC >4 mg/L 11 . However, a 24‐h continuous infusion needs the drug solution to be changed every 3 hours owing to the instability of meropenem under ambient conditions; this may impose a significant financial burden on families or hospitals in developing countries. Therefore, we investigated whether 1‐h infusion is still useful in children with sepsis. The aims of our study were as follows: (i) to quantify PK parameters of meropenem in children with sepsis undergoing CRRT and/or ECMO and (ii) to measure the sieving coefficient (Sc) of meropenem in children with sepsis receiving CRRT with the AN69 filter.
2. METHODS
2.1. Study design
This was a prospective observational study conducted at the PICU of the Children's Hospital of Fudan University, Shanghai, China, between January 2018 and December 2019. The protocol was registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn/index.aspx, ChiCTR‐OPC‐16008703) and approved by the Hospital Committee on Health Research Ethics (registration number 2016‐133, 2016‐311). Written informed consent was obtained from both parents of each child.
2.2. Study participants and eligibility criteria
The PICU in the Children's Hospital of Fudan University is one of the paediatric ECMO and CRRT centres of China, with 5 annual veno‐veno ECMO (VV‐ECMO) runs and 38 veno‐arterial ECMO (VA‐ECMO) runs. Annually, approximately 120 patients undergo CRRT in our PICU. This study enrolled children diagnosed with sepsis who were treated with meropenem. Paediatric sepsis was defined according to ‘Surviving Sepsis Campaign Guidelines for Management of Sepsis and Septic Shock’. 17 The inclusion and exclusion criteria are listed in Appendix Table A1. ECMO was performed to support children with refractory septic shock or severe sepsis‐associated respiratory failure.
The following data were recorded for each enrolled child: age, gender, weight, height, weight adjusted Z score (WAZ) to evaluate the nutrition status, and Pediatric Risk of Mortality score III (PRISM III) on the day of PICU admission. Further, we recorded several other parameters: C‐reactive protein (CRP) levels, white blood cell count, haemoglobin levels, platelet count, creatinine levels, urea nitrogen levels, bilirubin levels, albumin levels, alanine aminotransferase/aspartate aminotransferase (ALT/AST) levels, estimated creatinine clearance (eCLCR) derived from the Schwartz formula, 18 the ECMO settings, the CRRT intensity, positive results of blood, urine, and sputum cultures, and the dosing history of meropenem. CRRT was performed in the continuous veno‐venous haemodialysis (CVVHDF) mode using the AN69 membrane (Baxter, USA) through a Prismaflex CRRT system (Baxter, USA). The blood flow rate for CRRT was kept at an average of 4 mL/(kg‧min), with a replacement rate of 25 mL/(kg‧h); the dialysis dose was 20 mL/(kg‧h) and an average ultrafiltration rate of 2 mL/(kg‧h) was used. The CRRT and ECMO settings used for the enrolled children are listed in Appendix Table A2 and A3. We initially divided the children into 3 groups as follows: Group I, children receiving ECMO; Group II, children receiving CRRT; and Group III, children receiving neither ECMO nor CRRT (non‐ECLS group).
The MICs were determined in the microbiology laboratory of the Children's Hospital of Fudan University (Shanghai, China). The MIC values of meropenem were determined by the diffusion method using E‐test strips (BioMerieux, France). Among the 27 patients, 9 were confirmed to have an infection; MIC data were available for 4 of them (Table 3). When the MIC data were not available, the epidemiological cut‐off value (ECOFF) was determined as defined by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) data. 19
TABLE 3.
Pharmacokinetic parameters for 1‐h infusion.
| Parameter medium (IQR) | Group | p | ||
|---|---|---|---|---|
| ECMO (n = 6) | CRRT (n = 6) | Non‐ECLS (n = 15) | ||
| t1/2 (h) | 2.27 (1.76–3.55) | 2.22 (1.77–3.76) | 2.25 (1.46–3.06) | 0.45 |
| AUCtau (µg.h/ml) | 24.13 (7.78–86.20) | 24.47 (16.20–109.1) | 23.04 (15.69–34.62) | 0.28 |
| AUC0‐∝ (µg.h/ml) | 26.29 (8.38–64.85) | 37.41 (15.71–124) | 23.39 (19.32–56.21) | 0.25 |
| CL (L/h) | 11.59 (5.92–20.19) | 15.73 (2.11–39.29) | 13.51 (3.71–20.80) | 0.71 |
| Vz (L) | 31.67 (20.95–81.54) | 19.42 (8.76–108.8) | 34.41 (9.57–113.5) | 0.94 |
Abbreviations: AUC0‐∝, the area under the curve area under the plasma concentration‐time curve from time zero to infinity; AUCtau, the area under the curve calculated to the end of a dosing interval (tau) at steady‐state; CL, clearance; IQR, interquartile range; t1/2, half‐life; Vz, the volume in the terminal state.
2.3. Sampling procedures and concentration analysis
Meropenem (Meropen® injection; Sumitomo Dainippon Pharma Co. Ltd, Osaka, Japan) was administered at a dose of 20 mg/kg q8h intravenously for 60 min. After the administration of at least 5 doses of meropenem, the blood samples were first collected prior to meropenem injection (time zero). Subsequently, in children with body weight ≤30 kg, blood samples were collected using a peripheral arterial catheter or CRRT filter port at 5, 45, 90, 180, 360 and 480 min after meropenem infusion. In children with body weight >30 kg, blood samples were collected at 5, 15, 30, 60, 90, 120, 240, 360 and 480 min after meropenem infusion. Plasma was separated immediately and frozen at −80°C until further use. Plasma concentrations were analysed using high‐performance liquid chromatography‐tandem mass spectrometry (HPLC–MS/MS) at the School of Pharmacy, Fudan University (Shanghai, China).
YMeropenem was determined in human plasma based on an internal standard method using HPLC–MS/MS. Antipyrine (internal standard, IS) and meropenem were eluted in the mobile phase (0.1% methanoic acid:acetonitrile, 80:20 v/v) and analysed using HPLC‐ESI‐MS/MS n the multiple reaction monitoring (MRM) mode. The sample was reconstituted in 50 µL of mobile phase, and 5 µL was injected for analysis using HPLC‐MS/MS. The calibration standards were prepared by spiking a standard solution of meropenem into blank human plasma. The IS standard solution was added to each calibration standard in the same way as the sample was pre‐treated. The limit of quantification (LOQ) and the limit of detection (LOD) were determined by analysing multiple concentrations of meropenem in human plasma. The method was found to be precise and accurate based on 5 replicate determinations using human plasma samples.
2.4. PK parameters
The PK parameters were determined by non‐compartmental analysis conducted using the PKNCA package in R (Version 3.2.4; R Core Team, Vienna, Austria). The key PK parameters investigated were clearance at steady‐state (CLSS), area under the curve (AUC), area under the plasma concentration‐time curve (AUCtau), area under the plasma concentration‐time curve from time zero to infinity (AUC0‐∞) and the elimination half‐life (t1/2).
2.5. Statistic analysis
The demographical and PK outcome data are expressed as median (interquartile range, [IQR]) and were analysed using Prism 8 for macOS (version 8.2.1; GraphPad Software Inc, San Diego, CA). Comparisons between multiple groups were performed using the Kruskal‐Wallis test. P value less than 0.05 was considered significantly statistical difference between groups.
3. RESULTS AND DISCUSSION
3.1. Patient characteristics
Twenty‐seven children (14 males and 13 females) were enrolled in this study. All included children received, on average, 8 (range, 5–10) meropenem doses before blood sample collection. The ECMO treatment duration was of 70.4 h (range, 54.9–85.9 h). A summary of the demographic characteristics of children and the disease severity data are presented in Table 1. No adverse drug reactions were reported.
TABLE 1.
Characteristics for ECMO, CRRT and non‐ECLS groups.
| Non‐ECLS (n = 15) | CRRT (n = 6) | ECMO (n = 6) | |
|---|---|---|---|
| Male/Female | 8/7 | 4/2 | 3/3 |
| Age (years) | 1.29 (0.67–3.25) | 2.00 (1.13–6.88) | 2.50 (0.50–5.25) |
| Body weight (kg) | 11.00 (7.75–12.63) | 12.50 (8.60–27.15) | 14.50 (6.53–21.50) |
| WAZ Score | −0.10 (−2.28 to 1.26) | 0.55 (−1.84 to 1.10) | 0.29 (−1.14 to 1.24) |
| Cases of mechanical ventilation | 12 | 3 | 6 |
| Type of ECMO (VA/VV) | NA | NA | VA |
| Day 1 PRISM‐III Score | 34 (31–36) | 35.5 (32.5–37.75) | 40 (31.5–42.5) |
| Plasma creatinine concentration (µmol/L) | 20.50 (15.25–26.75) | 99.00 (75.00–131.50) | 24.00 (17.75–29.25) |
| eCLCR (ml/min) | 62.68 (33.56–153.7) | 34.65 (28.81–46.45) | 162.5 (110.7–204.5) |
| CRRT mode | NA | CVVHDF | NA |
| Serum bilirubin (µmol/L) | 8.75 (5.00–32.23) | 9.80 (5.25–30.05) | 18.80 (15.05–44.95) |
| Serum albumin (g/L) | 34.10 (27.38–37.03) | 32.50 (28.35–36.60) | 39.95 (34.63–48.08) |
Abbreviations: CRRT, continuous renal replacement therapy; eCLCR, estimated creatinine clearance; ECLS, Extracorporeal Life Support; ECMO, Extracorporeal Membrane Oxygenation; PRISM‐III, Pediatric Risk of Mortality score III; WAZ Score, Weight adjusted Z Score.
3.2. Pharmacokinetic parameters
There were significant differences in the eCLCR among the 3 groups—ECMO, CRRT and non‐ECLS, (p = 0.01) after the 1‐h infusion of meropenem. The eCLCR of the CRRT group was lower than that of the ECMO group. The AUCtau and AUC0‐∞ were lower after filter use during CRRT compared to the pre‐filter condition (all p < 0.05); t1/2 also decreased after filter use, but the difference was not statistically significant (Table 2). There were no significant differences in the remaining PK parameters among the three groups (all p > 0.05; Table 3). The individual meropenem serum concentration‐time profiles are shown in Figures 1, 2, 3.
TABLE 2.
NCA Pharmacokinetic parameters for pre‐filter and post‐filter during CRRT.
| Parameters medium (IQR) | Subgroups | p | |
|---|---|---|---|
| Pre‐filter | Post‐filter | ||
| Cmax (µg/ml) | 20.43 (14.49–27.79) | 14.15 (8.92–18.80) | 0.002 |
| t1/2 (h) | 1.97 (1.23–4.30) | 1.91 (1.75–3.12) | 0.742 |
| AUCtau (µg.h/ml) | 28.29 (23.91–63.83) | 17.73 (11.23–49.64) | 0.0137 |
| AUC0‐∝ (µg.h/ml) | 40.57 (24.19–129.6) | 18.99 (11.83–95.08) | 0.0234 |
Abbreviations: AUC0‐∝, area under the plasma concentration‐time curve from time zero to infinity; AUCtau, area under the plasma concentration‐time curve calculated to the end of a dosing interval (tau) at steady‐state; Cmax, Maximum plasma concentration; IQR, interquartile range; NCA, non‐compartmental analysis; t1/2, the elimination half‐life.
FIGURE 1.
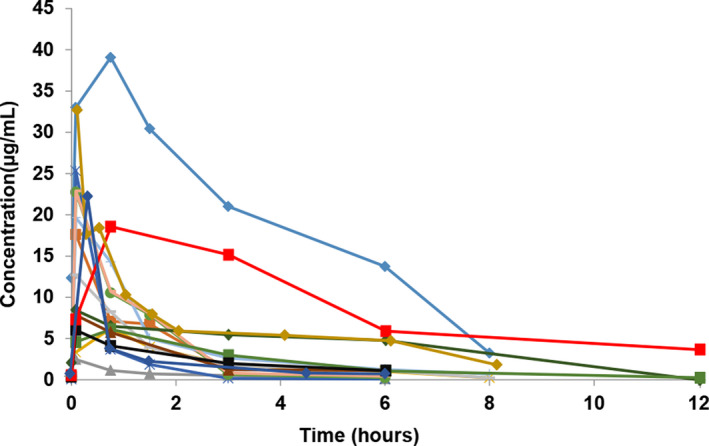
Individual meropenem serum concentration‐time profiles after 1‐h infusion of meropenem in the non‐ECLS group (n = 15).
FIGURE 2.
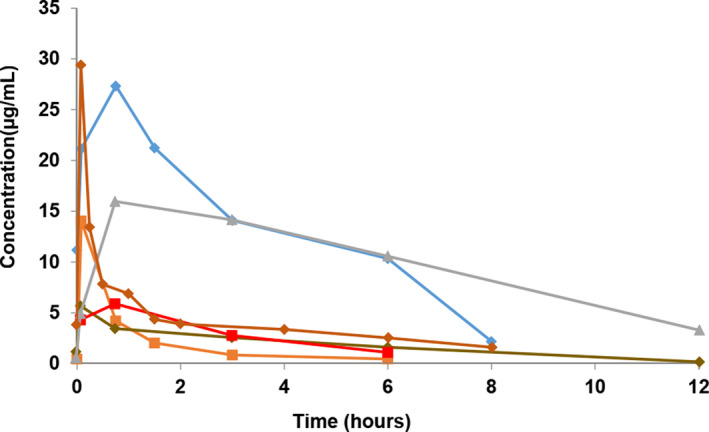
Individual meropenem serum concentration‐time profiles after 1‐h infusion of meropenem in patients administered CRRT (n = 6).
FIGURE 3.
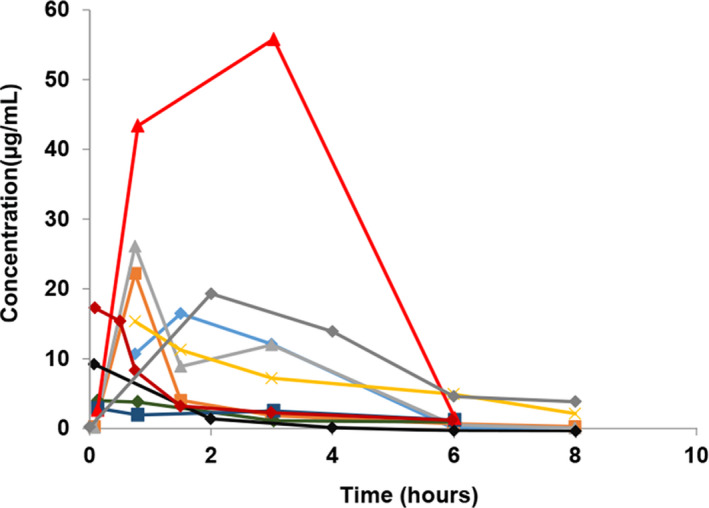
Individual meropenem serum concentration‐time profiles after 1‐h infusion of meropenem in patients administered ECMO (n = 6).
3.3. Discussion
To the best of our knowledge, this is the first study to focus on PK parameters of meropenem in children with sepsis receiving ECLS. We found that the PK parameters of meropenem did not change significantly in children with sepsis who had normal nutrition status and who were undergoing ECMO or CRRT with the AN69 haemofilter.
The impact of ECMO on PK parameters varies among drugs in adult patients. In a case‐matched control study of adult patients, meropenem had slightly lower CL and Vd (125 mL/min and 0.46 L/kg, respectively) in the ECMO‐treated group than in the non‐ECMO group (144 mL/min and 0.60 L/kg, respectively). 9 However, another matched cohort study of adults who were administered meropenem reported discordant data; CL decreased (7.9 L/h vs 11.7 L/h), while Vd increased (0.45 L/kg vs 0.41 L/kg) in ECMO patients compared to non‐ECMO patients. 10 In our study, the effect on meropenem CL upon ECMO initiation was similar to that reported in previous studies. ECMO did not influence the CL of meropenem in the studied children. Further, nutritional status is an important factor affecting the PK of antibiotics; therefore, we calculated the WAZ scores and found that all patients had normal nutrition status. We also assessed disease severity in the subjects with PRISM‐III.
Theoretically, CRRT increases Vd and CL. However, in this study, the CRRT group showed no significant increase in the total CL of meropenem. Three reasons could explain this finding. First, according to the ‘Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016’, CRRT was performed in patients with stage 2 and stage 3 acute kidney injury (AKI); glomerular filtration rates in these patients are lower than those of healthy volunteers. Second, dialysate and filtration flow rates in CRRT affect drug elimination. In this study, the CRRT mode was not high‐flow filtration, which may have prevented high CRRT clearance (CLCRRT). Third, the obtained Sc was lower than that reported previously, which could also reduce CLCRRT. The Sc can be influenced by the pathological and physiological conditions of patients, the molecular weight of the drug and properties of the hemofiltration membrane. The AN69 hemofiltration membrane achieves the removal of such solutes primarily by adsorption. 20 After the first few hours of filter use, the efficacy of adsorptive clearance may decrease because of the peptides and proteins clogging the membrane. This would also decrease the filtration efficiency of small molecules such as meropenem. More detailed data will be shown in a follow‐up study, by our team, based on the PK model.
Changes in PK parameters are complex and affected by multiple factors during ECMO and CRRT in children with sepsis. There were no significant differences in the PK parameters, as determined using the non‐compartment model, in children with sepsis administered ECMO compared to those in the non‐ECLS group in this study. Further, no significant differences were found in the PK parameters even in children with sepsis who received CRRT compared to those who did not; this might be associated with the AKI status of the paediatric patients, the low Sc of the AN69 filter, and the CRRT mode. The values of Cmax, AUCtau and AUC0‐∞ were significantly lower in blood samples collected after filtration through the hemofiltration membrane compared to those collected before filtration; meanwhile, the Sc of meropenem through the AN69 membrane (0.26) was lower than that reported for patients during CVVH (0.95–1.00), which may be related to the pathophysiological characteristics of the children with sepsis as well as the physicochemical properties of this hemofiltration membrane. However, this requires confirmation using further analytical models.
Our study had a few limitations. We compared PK parameters between different groups, but their sample sizes varied significantly. In addition, some results showed a trend, but without a statistically significant difference, because of the small number of patients. Considering the characteristics of the haemofilter membrane and positively‐charged meropenem, the results of this study apply to children with sepsis administered ECMO and/or CRRT with the AN69 hemofilter and infered 1‐h meropenem infusion was still useful in children with sepsis receiving ECLS. However, we need further PK/PD modelling to investigate when 1‐h meropenem infusion is still useful in children with sepsis under situations with different body weight, eCRCL and MIC.
4. WHAT IS NEW AND CONCLUSION
This study accurately describes the PK properties of meropenem in paediatric patients with sepsis receiving ECMO or CRRT—ECMO and CRRT intensity did not alter the PK parameters of meropenem; therefore, dosage adjustment might not be needed in these patients. Further investigation on PK modelling will give the answer whether actual meropenem dosing regimens are appropriate in septic children with ECLS.
PATIENT CONSENT
Written informed consent was obtained from both parents of each child.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
The paper had no material reproduced from other sources.
CONFLICTS OF INTEREST
All authors have completed the ICMJE uniform disclosure form. The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Dr. YX Wang and Professor C Chen conceptualized and designed the study, analysed and interpreted the data, had full access to all the data, and took responsibility for the integrity of the data and the accuracy of the data analysis. Dr. WM Chen, Dr. GF Yan, Dr. GF Wang and Professor GP Lu contributed to drafting of the manuscript. Professor GP Lu, ZP Li and C Chen contributed to the critical revision of the manuscript and provided administrative support. Professor C Chen supervised the study.
ACKNOWLEDGEMENTS
The authors thank Mr. Ding Junjie of the Centre for Tropical Medicine and Global Health, Nuffield Department of Clinical Medicine, University of Oxford, for technical support. They also thank Sumitomo Pharmaceuticals (Suzhou) Co. Ltd. for consultation and language editing.
Appendix 1.
TABLE A1.
Inclusion and exclusion criteria
| Inclusion criteria |
|
| Exclusion criteria |
|
Abbreviation: CRRT, continuous renal replacement therapy.
TABLE A2.
Characteristics for enrolled children receiving ECMO
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Sex (Male/Female) | F | M | F | M | F | M |
| Age (years) | 0.5 | 0.5 | 9 | 4 | 2.5 | 0.75 |
| Weight (kg) | 6 | 6.7 | 29 | 19 | 14 | 9 |
| WAZ | −1.22 | −1.11 | 0.12 | 2.16 | 0.45 | 1.32 |
| PRISM‐III | 32 | 42 | 44 | 30 | 38 | 36 |
| Pump speed (LPM) | 0.45 | 0.61 | 0.92 | 0.52 | 1.2 | 0.8 |
| Centrifugal pump speed (RPM) | 1230 | 1800 | 1600 | 1330 | 1500 | 1600 |
| Sweep gas flow (LPM) | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.5 |
| Heparin dose (IU/kg/h) | 20 | 25 | 20 | 25 | 23 | 20 |
| ECMO circuit volume (ml) | 275 | 265 | 320 | 275 | 300 | 320 |
Abbreviations: ECMO, Extracorporeal Membrane Oxygenation; PRISM‐III, Pediatric Risk of Mortality score III; WAZ, Weight adjusted Z Score.
TABLE A3.
Characteristics for enrolled children receiving CRRT
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Sex (Male/Female) | M | F | F | M | M | M |
| Age (years) | 1.83 | 3.75 | 2 | 10 | 0.42 | 3.5 |
| Weight (kg) | 12 | 18 | 12.5 | 36.3 | 5.2 | 17.5 |
| WAZ | −0.14 | 0.97 | 0.55 | 1.23 | −3.54 | 0.86 |
| PRISM‐III | 43 | 37 | 42 | 35 | 42 | 36 |
| CRRT blood flow speed (ml/kg/min) | 4 | 3 | 5 | 4.5 | 3.5 | 4 |
| Predilution replacement flow rate (ml/kg/h) | 30 | 20 | 20 | 30 | 25 | 25 |
| Dialysate flow rate (ml/h) | 19 | 22 | 21 | 20 | 18 | 20 |
| Ultrafiltrate flow rate (ml/h) | 3 | 0 | 2 | 1 | 1.5 | 2 |
| residual urine output (ml/kg/24 h) | 0.8 | 1.0 | 0.5 | 0.7 | 0.9 | 1.0 |
Abbreviations: CRRT, continuous renal replacement therapy; PRISM‐III, Pediatric Risk of Mortality score III; WAZ, Weight adjusted Z Score.
Yixue Wang, Zhiping Li and Weiming Chen contributed equally to this work
Chen Chao and Lu Guoping share senior authorship.
Funding information
This work was supported by the Science and Technology Innovation Plan in Shanghai, China, under Grant of Clinical Medicine Project (no. 18411950700).
Contributor Information
Guoping Lu, Email: 13788904150@163.com.
Chao Chen, Email: chen6010@163.com.
DATA AVAILABILITY STATEMENT
The data sets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kempker JA, Martin GS. The changing epidemiology and definitions of sepsis. Clin Chest Med. 2016;37:165‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levy Hara G, Kanj SS, Pagani L, et al. Ten key points for the appropriate use of antibiotics in hospitalised patients: a consensus from the Antimicrobial Stewardship and Resistance Working Groups of the International Society of Chemotherapy. Int J Antimicrob Agents. 2016;48:239‐246. [DOI] [PubMed] [Google Scholar]
- 3. Kumar A. Early antimicrobial therapy in severe sepsis and septic shock. Curr Infect Dis Rep. 2010;12:336‐344. [DOI] [PubMed] [Google Scholar]
- 4. Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003;115:529‐535. [DOI] [PubMed] [Google Scholar]
- 5. MacArthur RD, Miller M, Albertson T, et al. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin Infect Dis. 2004;38:284‐288. [DOI] [PubMed] [Google Scholar]
- 6. Hahn J, Choi JH, Chang MJ. Pharmacokinetic changes of antibiotic, antiviral, antituberculosis and antifungal agents during extracorporeal membrane oxygenation in critically ill adult patients. J Clin Pharm Ther. 2017;42:661‐671. [DOI] [PubMed] [Google Scholar]
- 7. Hanberg P, Öbrink‐Hansen K, Thorsted A, et al. Population pharmacokinetics of meropenem in plasma and subcutis from patients on extracorporeal membrane oxygenation Treatment. Antimicrob Agents Chemother. 2018;62:e02390‐e2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Datapharm . Meronem IV 500 mg & 1 g. Updated 9 Mar 2017. Available from https://www.medicines.org.uk/emc/medicine/11215.
- 9. Donadello K, Antonucci E, Cristallini S, et al. beta‐Lactam pharmacokinetics during extracorporeal membrane oxygenation therapy: a case‐control study. Int J Antimicrob Agents. 2015;45:278‐282. [DOI] [PubMed] [Google Scholar]
- 10. Shekar K, Fraser JF, Taccone FS, et al. The combined effects of extracorporeal membrane oxygenation and renal replacement therapy on meropenem pharmacokinetics: a matched cohort study. Crit Care. 2014;18:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rapp M, Urien S, Foissac F, et al. Population pharmacokinetics of meropenem in critically ill children with different renal functions. Eur J Clin Pharmacol. 2020;76:61‐71. [DOI] [PubMed] [Google Scholar]
- 12. Cies JJ, Moore WS, Enache A, Chopra A. Population pharmacokinetics and pharmacodynamic target attainment of meropenem in critically ill young children. J Pediatr Pharmacol Ther. 2017;22:276‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel K, Roberts JA, Lipman J, Tett SE, Deldot ME, Kirkpatrick CM. Population pharmacokinetics of fluconazole in critically ill patients receiving continuous venovenous hemodiafiltration: using Monte Carlo simulations to predict doses for specified pharmacodynamic targets. Antimicrob Agents Chemother. 2011;55:5868‐5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roberts DM, Roberts JA, Roberts MS, et al. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: a multicentre pharmacokinetic study. Crit Care Med. 2012;40:1523‐1528. [DOI] [PubMed] [Google Scholar]
- 15. Boucher BA, Wood GC, Swanson JM. Pharmacokinetic changes in critical illness. Crit Care Clin. 2006;22:255‐271. [DOI] [PubMed] [Google Scholar]
- 16. Ulldemolins M, Vaquer S, Llauradó‐Serra M, et al. Beta‐lactam dosing in critically ill patients with septic shock and continuous renal replacement therapy. Crit Care. 2014;18:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858‐873. [DOI] [PubMed] [Google Scholar]
- 18. Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832‐1843. [DOI] [PubMed] [Google Scholar]
- 19. Polsfuss S, Bloemberg GV, Giger J, Meyer V, Hombach M. Comparison of European Committee on Antimicrobial Susceptibility Testing (EUCAST) and CLSI screening parameters for the detection of extended‐spectrum β‐lactamase production in clinical Enterobacteriaceae isolates. J Antimicrob Chemother. 2012;67:159‐166. [DOI] [PubMed] [Google Scholar]
- 20. Clark WR, Gao D, Lorenzin A, Ronco C. Membranes and Sorbents. Contrib Nephrol. 2018;194:70‐79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analysed during the current study are available from the corresponding author upon reasonable request.


