Abstract
Objectives:
Better markers of early response to neoadjuvant chemotherapy (NACT) in patients with breast cancer are required to enable the timely identification of non-responders and reduce unnecessary treatment side-effects. Early functional imaging may better predict response to treatment than conventional measures of tumour size. The purpose of this study was to test the hypothesis that the change in tumour blood flow after one cycle of NACT would predict pathological response.
Methods:
In this prospective cohort study, dynamic contrast-enhanced MRI was performed in 35 females with breast cancer before and after one cycle of epirubicin and cyclophosphamide-based NACT (EC90). Estimates of tumour blood flow and tumour volume were compared with pathological response obtained at surgery following completion of NACT.
Results:
Tumour blood flow at baseline (mean ± SD; 0.32 ± 0.17 ml/min/ml) reduced slightly after one cycle of NACT (0.28 ± 0.18 ml/min/ml). Following treatment 15 patients were identified as pathological responders and 20 as non-responders. There were no relationships found between tumour blood flow and pathological response. Conversely, tumour volume was found to be a good predictor of pathological response (smaller tumours did better) at both baseline (area under the receiver operating characteristic curve 0.80) and after one cycle of NACT (area under the receiver operating characteristic curve 0.81).
Conclusion & advances in knowledge:
The change in breast tumour blood flow following one cycle of EC90 did not predict pathological response. Tumour volume may be a better early marker of response with such agents.
Introduction
Neoadjuvant chemotherapy (NACT) offers an increased rate of breast conserving surgery, can downsize advanced breast tumours to allow surgery and with the addition of human epidermal growth factor receptor 2 (HER2)-targeted therapy (to NACT or adjuvant chemotherapy) can improve survival in HER2-positive breast cancer.1 However, NACT comes at the cost of significant side-effects and it is therefore important to identify non-responders to NACT as early as possible. Typically, a change in tumour size is demonstrated after two or more cycles of NACT2 but several studies have examined functional imaging techniques to assess response after only one cycle.3–5 Amongst the functional imaging techniques 18F-FDG PET has been used to assess changes in tumour glucose metabolism following NACT6 and a number of PET studies have looked specifically at breast tumour blood flow with either [15-O] H2O PET7 or dynamic 18F-FDG PET.8 However, repeated PET imaging is impractical for routine clinical use9 and so MRI techniques that measure parameters related to flow, via dynamic contrast-enhanced (DCE) MRI, have also been studied.10 A difference between these MRI approaches and PET imaging of blood flow is the lack of absolute quantification by MRI. Moreover, there has been a lack of consistency in study methodology and the reporting of pathological response in MRI studies over the years.10–12
In this study, we used a recently developed interleaved high spatial resolution (HSR)/high temporal resolution (HTR) MRI technique13 to measure tumour blood flow at baseline and after one cycle of NACT in order to test the hypothesis that the change in blood flow after one cycle of NACT would predict pathological response as defined according to international recommendations.14 Analysis of the HTR data to estimate blood flow also allowed us to assess related haemodynamic parameters such as blood volume, capillary permeability surface–area product (PS) and interstitial volume and, by using the HSR data, we were able to assess tumour volume.
Methods and materials
Patients
In this prospective study consecutive patients, with newly diagnosed primary invasive carcinoma of the breast due to undergo NACT with curative intent, were approached and asked if they were willing to take part. All those agreeing to join the local Research Ethics Committee approved study provided written informed consent after the nature of the procedures had been fully explained. Each underwent a block sequential regimen of NACT: three 3-weekly cycles of EC90 [epirubicin (90 mg/m2) and cyclophosphamide (600 mg/m2)] followed by three 3-weekly cycles of docetaxel (100 mg/m2). In patients with HER2 positive tumours, docetaxel was accompanied by trastuzumab, and in some (more recent) cases pertuzumab. Blood samples, taken as standard of care during clinic visits, were used to estimate haematocrit at each MRI visit.
MRI
MRI was performed before initiation of NACT and then repeated in the 2-week period immediately before administration of the second cycle of EC90. All imaging was performed using a 1.5 T MR system (Aera, Siemens Medical Systems, Erlangen, Germany) with the patients positioned head-first and prone. A dedicated 16-channel breast coil and, where possible, an additional flexible array coil placed on the patients back to increase the signal from the descending aorta15 were employed. The flexible array was omitted in eight of the subjects who were too large for its use or found the additional coil too uncomfortable.
The MRI protocol included localizing images, T1-, T2- and diffusion-weighted imaging before a 3D non-selective inversion recovery (IR) prepared spoiled gradient echo sequence (FOV: 340 × 340 × 180 mm, matrix size: 128 × 128 × 36, IR-TR: 3000 ms, TR/TE: 2.8/0.93 ms, flip angle (FA): 8°, GRAPPA parallel factor: 2, acquisition time: 1 min 5 s per inversion time) was used at four inversion times (100, 600, 1200 and 2800 ms) to estimate T1. The volume selected encompassed both breasts, the aortic arch and part of the descending aorta.15 This was followed by interleaved HTR and HSR DCE sequences.13 The DCE series consisted of 93 HTR volumes interleaved with 8 HSR volumes (10 × HTR, 1 × HSR, 43 × HTR, [1 × HSR, 5 × HTR] repeated seven times, 5 × HTR). For the HTR dynamic data, a T1 weighted 3D spoiled gradient echo sequence (FOV: 340 × 340 × 180 mm, matrix size: 128 × 128 × 36, TR/TE: 2.37/0.73 ms, FA: 25°, CAIPIRINHA parallel factor: 2 × 2, acquisition time: 2 s) was employed. The HSR data were acquired using a fat-suppressed T1 weighted 3D spoiled gradient echo sequence (TR/TE: 4.1/1.2 ms, FA: 10°, GRAPPA parallel factor: 3, matrix size: 384 × 384 × 128, acquisition time: 36 s). Both HTR and HSR acquisitions encompassed the same volume as the IR sequence. Gd-DOTA (Dotarem, Guerbet, Laboratories, Aulnays Sous Bois, France) was administered intravenously via the antecubital vein using an automated power injector (Spectris Solaris EP, Bayer, Leverkusen, Germany), at the beginning of the 11th dynamic volume (dose of 0.1 mmol/kg) followed by 20 ml of saline at a rate of 3 ml/s. After all eight HSR volumes had been acquired (and 88 HTR volumes) a second, bookend, IR T1 measurement was made,16 and then the final five HTR volumes were acquired.
Data analysis
Where more than one tumour was identified in a patient, analysis was performed on the largest tumour only. Regions of interest were drawn manually on the HSR images to estimate enhancing tumour volume (these regions were drawn on every slice in which the tumour appeared but excluded areas of necrosis or breast marker clips). These were typically drawn on the first post-contrast volume (acquired approximately 90 s after arrival of contrast agent) and subtracted images were used as a visual aid in the outlining. The multislice regions of interest were drawn by one reader (IMF) and checked by a second (NS; an experienced breast radiologist). Matching regions were drawn on the HTR images and these were used to generate signal-time curves as well as to estimate T1 relaxation times from the IR data. Further regions, drawn in the descending aorta, were used to generate SI-time curves and estimate T1 before and after contrast agent administration to calculate an arterial input function.15 The SI-time curves and bookend T1 estimates were used, with an iterative scheme, to convert signal to contrast agent concentration.15,16 These data were then fitted using locally developed software produced in Matlab (Mathworks; Natick, MA) and a two-compartment exchange model to provide estimates of blood flow, PS, interstitial volume and blood volume.17
Ki-67 and pathological assessment
Each of the patients went on to receive a further two cycles of EC90 and then three cycles of docetaxel, with or without HER2-targeted therapy, before undergoing surgery. The resected surgical specimens were dissected and the histology examined by a specialist pathologist who derived a residual cancer burden (RCB) index.14 Patients with an RCB class of 0 or I (RCB index ≤1.36) were deemed to be pathological responders (pR) and those with an RCB class of II or III (RCB index >1.36) were deemed to be pathological non-responders (pNR). In addition, diagnostic biopsy specimens were retrieved and Ki-67 expression was assessed. Four-micron sections were taken onto X-tra microscope slides (Leica, Wetzlar, Germany) and antigen retrieval was performed using Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA) and heat (pressure cooker for 2 min). Endogenous peroxidase was blocked with 0.3% hydrogen peroxide (10 min). Slides were stained with 1:400 dilution of anti Ki-67 antibody (M7240, Dako; Agilent, Santa Clara, CA) for 1 h at room temperature, and staining was visualised using Impress reagents (Vector Laboratories). Ki67 staining was quantified using the Whole Section Scoring Protocol developed by The International Ki67 in Breast Cancer Working Group.18 Results were reported as a percentage of tumour nuclei expressing Ki-67.
Statistical methods
Data were summarised using mean ± standard deviation when they were normally distributed and median [interquartile range] when they were not (assessed using the Shapiro–Wilk test). To determine whether differences existed between the measured parameters, estimates from pR and pNR were compared using the non-parametric Mann–Whitney U test and cycles were compared using the Wilcoxon signed rank test. Response prediction was assessed using receiver operating characteristic (ROC) curves. p < 0.05 was considered to indicate a statistically significant difference.
Results
Between August 2015 and April 2018, 40 female patients (median age 45 years, range 25–69 years) gave written informed consent to enter the study. MRI data were obtained in all 40 patients at baseline (7 [4 - 12] days before the first administration of NACT). However, one patient was unable to tolerate the complete imaging protocol and technical problems in one other (back coil was switched on and off intermittently) precluded the measurement of tumour blood flow. Following a single cycle of EC90, 37 patients were scanned (three patients declined further MRI scans). Technical problems (as previously) affected one further scan, so blood flow estimates were obtained in 36 patients. Paired baseline and cycle one data were available for 35 patients and the results presented below reflect these 35 patients only (Table 1).
Table 1.
Clinical characteristics of the study population
| Characteristic | Number |
|---|---|
| Median age (years) | 45 (range, 25–69) |
| Tumour type | |
| Invasive ductal carcinoma | 33 |
| Invasive (type not specified) | 2 |
| Grade | |
| II | 15 |
| III | 20 |
| Oestrogen receptor status | |
| Positive | 24 |
| Negative | 11 |
| Progesterone receptor status | |
| Positive | 16 |
| Negative | 17 |
| Not evaluable | 2 |
| HER2 status | |
| Positive | 14 |
| Negative | 21 |
| Median Ki-67 expression (%) | 45 (range, 4.3–83.3) |
The immunohistochemical subtype (IHS) of the 35 tumours were: 2 HER2 enriched (oestrogen receptor and progesterone receptor negative and HER2 positive), 9 triple negative and 24 luminal B (oestrogen receptor and/or progesterone receptor positive and HER2 positive or HER2 negative with high levels of Ki-67). Following the completion of NACT and surgery, pathological analysis revealed 6 patients (17%) had a pathological complete response (RCB-0), 9 patients had minimal residual disease (RCB-I), 12 patients moderate (RCB-II) and 8 patients extensive residual disease (RCB-III). Thus, there were deemed to be 15 pR (43%) and 20 pNR (57%).
Pathological complete response to NACT varied as a function of IHS – neither of the patients with HER2 enriched tumours, 3/9 (33%) patients with triple negative tumours and 3/24 (12.5%) patients with luminal B tumours had a complete pathological response. Less variation was seen in pR where 1/2 (50%) patients with HER2 enriched tumours showed pR compared to 3/9 (33%) with triple negative tumours and 11/24 (46%) with luminal B tumours. Luminal B tumours that were HER2 positive responded better to NACT: 9/12 (75%) HER2 positive tumours had a pR compared to only 2/12 (17%) HER2 negative tumours. Median enhancing tumour volume at baseline was 4.8 [2.3 to 22.5] cm3 and patients going on to a pR had tumours that were significantly smaller than those that went on to pNR (p = 0.003). There was a small but significant decrease in tumour volume following one cycle of NACT (median volume after one cycle 5.3 [1.6 to 14.4] cm3, median decrease in volume after one cycle 0.48 [0.0 to 5.03] cm3; p = 0.005) and tumour volume at baseline and after cycle one were good predictors of pR (area under the ROC curve 0.80 (95% CI 0.66 to 0.95) and 0.81 (95% CI 0.66 to 0.95), respectively). Using a volume threshold at cycle one to predict response failure, 12 patients with tumours of 9 cm3 or larger went on to pNR (20 patients scanned after cycle one went on to pNR; the test had a sensitivity of 60%) and all 15 patients who went on to a pR had tumours smaller than 9 cm3 (100% specificity). This test had a positive predictive value of 100%.
Tumour blood flow at baseline (0.32 ± 0.17 ml/min/ml) and after cycle one (0.28 ± 0.18 ml/min/ml) showed little variation as a function of IHS (Table 2) or pathological response. PS (0.06 ± 0.04 ml/min/ml at baseline, 0.05 ± 0.03 ml/min/ml after cycle one), blood volume (0.33 ± 0.15 at baseline, 0.34 ± 0.13 after cycle one) and interstitial volume (0.22 ± 0.08 at baseline, 0.23 ± 0.08 after cycle one) similarly showed no significant variation with IHS or pathological response. Tumour T1 at baseline (1287 ± 87 ms) showed no change following one cycle of NACT (1285 ± 92 ms) and showed no significant variation as a function of IHS (Table 2).
Table 2.
Results of the T1 & DCE analysis at baseline as a function of tumour immunohistochemical subtype
| Triple negative | Luminal B | HER2 enriched | |
|---|---|---|---|
| (n = 9) | (n = 24) | (n = 2) | |
| Tumour volume (cm3) median (IQ range) | 10.7 (4.4–23.6) | 4.1 (1.8–24.0) | 6.9 (4.6, 9.2)a |
| Blood flow (ml/min/ml tissue) | 0.31 ± 0.12 | 0.31 ± 0.18 | 0.57 (0.52, 0.62)a |
| Blood volume (no units) | 0.31 ± 0.14 | 0.35 ± 0.16 | 0.25 (0.17, 0.32)a |
| PS (ml/min/ml tissue) | 0.08 ± 0.03 | 0.05 ± 0.03 | 0.12 (0.09, 0.14)a |
| Interstitial volume (no units) | 0.22 ± 0.07 | 0.21 ± 0.08 | 0.26 (0.19, 0.32)a |
| Tumour T1 (ms) | 1315 ± 72 | 1272 ± 92 | 1345 (1336, 1354)a |
mean (both raw values reported).
Patient haematocrit decreased from a baseline value of 0.41 ± 0.03 to 0.39 ± 0.03 (p = 0.007) after one cycle of NACT and this was mirrored in an increase of blood T1 from a baseline value of 1660 ± 64 ms to 1766 ± 109 ms (p = 0.00002) after one cycle of NACT.15
Discussion
At least one previous [15-O] H2O PET study reported a large differential response of tumour blood flow in groups of patients who had pR and pNR, though these data were acquired after 9 weeks (multiple cycles) of NACT.7 We did not see this effect in our study following just one cycle of NACT. There was, on average, a decrease in blood flow of 12% but this was subject to considerable variation, with both increases and decreases seen in both pR and pNR (Figures 1 and 2). Our results reflect those of Humbert et al8 who saw a large variability in blood flow response in HER2 negative tumours after one cycle of EC chemotherapy (HER2 positive patients in that study experienced a large decrease in blood flow but they were selectively treated with docetaxel and trastuzumab). The primary hypothesis of this study - that change in blood flow after one cycle would predict pathological response - was not supported by our results
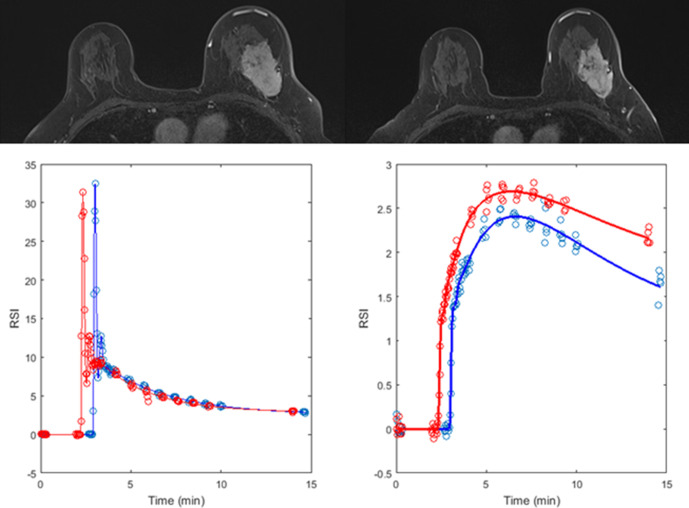
Figure 1.
A 51-year-old female with a Grade 3 luminal B invasive ductal carcinoma in the left breast. T1 weighted HSR images (top) highlight a tumour at baseline (left) with a volume of 45 cm3 which reduced to 32 cm3 after one cycle of NACT (right). Analysis of the DCE-MRI data using arterial input functions measured in the descending aorta (bottom left, relative change in signal intensity vs time) produced estimates of blood flow of 0.24 ml/min/ml tissue at baseline (blue data points and curves) and 0.26 ml/min/ml tissue after one cycle (red data points and curves, bottom right). Following completion of NACT, the patient underwent a left mastectomy and pathological assessment of the resected specimen revealed an RCB index of 3.4 (RCB class III, pNR).
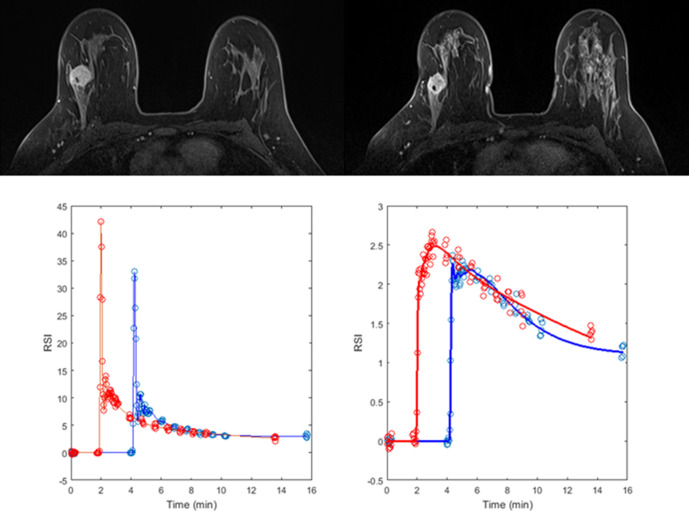
Figure 2.
A 37-year-old female with a Grade 3 triple negative invasive ductal carcinoma in the right breast. T1 weighted HSR images (top) highlight a tumour at baseline (left) with a volume of 3.1 cm3 which reduced to 2.9 cm3 after one cycle of NACT (right). Analysis of the DCE-MRI data using arterial input functions measured in the descending aorta (bottom left) produced estimates of blood flow of 0.46 ml/min/ml tissue at baseline (blue data points and curves) and 0.31 ml/min/ml tissue after one cycle (red data points and curves, bottom right). Following completion of NACT, the patient underwent a wide local excision and pathological assessment of the resected specimen revealed an RCB index of 0 (RCB class 0, pR).
Conversely, tumour volume, which was identified as a more reliable marker of response than tumour diameter in a previous review,10 was predictive of response. This mirrors results seen in the ACRIN 6657 trial that highlighted the importance of enhancing tumour volume, measured at cycle one, to recurrence-free survival.19 At cycle one of our study, 12 of the 35 tumours were 9 cm3 or larger (7 of these tumours were luminal B, 4 were triple negative and 1 was HER2 enriched). These patients could potentially have been spared further NACT since all failed to respond (pNR). Of the remaining 23 patients with tumours smaller than 9 cm3, 15 went on to a pR leaving only 8 non-responding patients. Further work is required to identify a specific imaging phenotype to identify those tumours at the early stages of NACT.
This is the first published study to report absolute estimates of breast tumour blood flow and related parameters measured using MRI in response to treatment.20 Previous quantitative MRI studies have typically reported parameters such as Ktrans (volume transfer constant) without measuring patient-specific arterial input functions21–23 or by measuring the input function inadequately24 (compare the input functions presented in figures 2 and 3 of Tateishi et al24 with those presented in Georgiou et al15 obtained following very similar injection protocols). It is well recognised that measurement of the arterial input function is very challenging.11 As in the current study, some reports have highlighted the importance of tumour size after a single cycle of NACT as a predictor of response while tracer kinetic parameters have been less useful21,25; others found neither size nor tracer kinetic parameters useful at this timepoint.22 Although the current study lacks direct validation of the blood flow estimates, the values reported very closely reflect previously published estimates obtained using the ‘gold-standard’ of [O-15] H2O PET in a similar group of patients.26 Moreover, the lack of variation in tumour blood flow as a function of IHS seen in the PET study26 is similarly seen in our data (at least in luminal B and triple negative cancers). Despite the well-established variation in response to NACT of the different IHS of breast cancer, there appears to be very little difference in their haemodynamic characteristics at baseline and no clear patterns of change following one cycle of NACT. The decrease of blood flow seen in responders later in the course of NACT by others7 is not yet apparent after one cycle. Measurements of blood flow may prove to be more revealing when assessing NACT regimens with expected antiangiogenic effects.8
Limitations
The additional time required to acquire data for blood flow estimation amounts to less than 10 min (bookend T1 measurements plus a few additional DCE acquisitions) and this could be reduced if a faster B1-insensitive T1 measurement sequence were employed. The interleaving of HTR data with the clinical HSR data did not have a significant impact on clinical reporting.13 Perhaps more significant limitations are the small number of patients studied and the post-processing overhead both for tumour volume estimation and tracer kinetic analysis, which currently rely on in-house software. Further work is required to develop user-friendly tailored software for these applications.
Conclusions
This study has reported, for the first time, absolute estimates of tumour blood flow and related haemodynamic parameters measured using MRI in a cohort of patients with breast cancer undergoing NACT. Those parameters (blood flow, PS, blood volume, interstitial volume) show little variation with tumour IHS at baseline or in response to one cycle of NACT and, contradicting our primary hypothesis, blood flow change following one cycle of NACT did not predict pathological response. In contrast and reflecting the findings of a recent multicentre trial, enhancing tumour volume measured after one cycle of NACT appears to show promise for prediction of pathological response.
Footnotes
Acknowledgements: The study could not have been undertaken without the hard work and support of the following team members: Sue Hartup, Amy Henson, Julie Sharp, and Jane Gibb.
Conflicts of interest: There are no conflicts of interest to report. These data were acquired as part of the CHERNAC study funded by Breast Cancer Now (award 2014MayPR241).
Contributor Information
William Stevens, Email: d.l.buckley@leeds.ac.uk.
Isabelle M Farrow, Email: d.l.buckley@leeds.ac.uk.
Leonidas Georgiou, Email: d.l.buckley@leeds.ac.uk.
Andrew M Hanby, Email: d.l.buckley@leeds.ac.uk.
Timothy J Perren, Email: d.l.buckley@leeds.ac.uk.
Laura M Windel, Email: d.l.buckley@leeds.ac.uk.
Daniel J Wilson, Email: d.l.buckley@leeds.ac.uk.
Nisha Sharma, Email: d.l.buckley@leeds.ac.uk.
David Dodwell, Email: d.l.buckley@leeds.ac.uk.
Thomas A Hughes, Email: d.l.buckley@leeds.ac.uk.
Barbara JG Dall, Email: d.l.buckley@leeds.ac.uk.
David L Buckley, Email: D.L.Buckley@leeds.ac.uk.
REFERENCES
- 1.Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010; 375: 377–84. doi: 10.1016/S0140-6736(09)61964-4 [DOI] [PubMed] [Google Scholar]
- 2.Pickles MD, Gibbs P, Lowry M, Turnbull LW. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging 2006; 24: 843–7. doi: 10.1016/j.mri.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 3.Wasser K, Klein SK, Fink C, Junkermann H, Sinn HP, Zuna I, et al. Evaluation of neoadjuvant chemotherapeutic response of breast cancer using dynamic MRI with high temporal resolution. Eur Radiol 2003; 13: 80–7. doi: 10.1007/s00330-002-1654-1 [DOI] [PubMed] [Google Scholar]
- 4.Kolesnikov-Gauthier H, Vanlemmens L, Baranzelli M-C, Vennin P, Servent V, Fournier C, et al. Predictive value of neoadjuvant chemotherapy failure in breast cancer using FDG-PET after the first course. Breast Cancer Res Treat 2012; 131: 517–25. doi: 10.1007/s10549-011-1832-4 [DOI] [PubMed] [Google Scholar]
- 5.Hylton NM, Blume JD, Bernreuter WK, Pisano ED, Rosen MA, Morris EA, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy-results from ACRIN 6657/I-SPY TRIAL. Radiology 2012; 263: 663–72. doi: 10.1148/radiol.12110748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunnwald LK, Gralow JR, Ellis GK, Livingston RB, Linden HM, Specht JM, et al. Tumor metabolism and blood flow changes by positron emission tomography: relation to survival in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. J Clin Oncol 2008; 26: 4449–57. doi: 10.1200/JCO.2007.15.4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mankoff DA, Dunnwald LK, Gralow JR, Ellis GK, Schubert EK, Tseng J, et al. Changes in blood flow and metabolism in locally advanced breast cancer treated with neoadjuvant chemotherapy. J Nucl Med 2003; 44: 1806–14. [PubMed] [Google Scholar]
- 8.Humbert O, Lasserre M, Bertaut A, Fumoleau P, Coutant C, Brunotte F, et al. Breast cancer blood flow and metabolism on dual-acquisition 18F-FDG PET: correlation with tumor phenotype and neoadjuvant chemotherapy response. J Nucl Med 2018; 59: 1035–41. doi: 10.2967/jnumed.117.203075 [DOI] [PubMed] [Google Scholar]
- 9.Fowler AM, Mankoff DA, Joe BN. Imaging neoadjuvant therapy response in breast cancer. Radiology 2017; 285: 358–75. doi: 10.1148/radiol.2017170180 [DOI] [PubMed] [Google Scholar]
- 10.Marinovich ML, Sardanelli F, Ciatto S, Mamounas E, Brennan M, Macaskill P, et al. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast 2012; 21: 669–77. doi: 10.1016/j.breast.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 11.Prevos R, Smidt ML, Tjan-Heijnen VCG, van Goethem M, Beets-Tan RG, Wildberger JE, et al. Pre-Treatment differences and early response monitoring of neoadjuvant chemotherapy in breast cancer patients using magnetic resonance imaging: a systematic review. Eur Radiol 2012; 22: 2607–16. doi: 10.1007/s00330-012-2653-5 [DOI] [PubMed] [Google Scholar]
- 12.Cheng Q, Huang J, Liang J, Ma M, Ye K, Shi C, et al. The diagnostic performance of DCE-MRI in evaluating the pathological response to neoadjuvant chemotherapy in breast cancer: a meta-analysis. Front Oncol 2020; 10: 93. doi: 10.3389/fonc.2020.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgiou L, Sharma N, Broadbent DA, Wilson DJ, Dall BJ, Gangi A, et al. Estimating breast tumor blood flow during neoadjuvant chemotherapy using interleaved high temporal and high spatial resolution MRI. Magn Reson Med 2018; 79: 317–26. doi: 10.1002/mrm.26684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007; 25: 4414–22. doi: 10.1200/JCO.2007.10.6823 [DOI] [PubMed] [Google Scholar]
- 15.Georgiou L, Wilson DJ, Sharma N, Perren TJ, Buckley DL. A functional form for a representative individual arterial input function measured from a population using high temporal resolution DCE MRI. Magn Reson Med 2019; 81: 1955–63. doi: 10.1002/mrm.27524 [DOI] [PubMed] [Google Scholar]
- 16.Cron GO, Santyr G, Kelcz F. Accurate and rapid quantitative dynamic contrast-enhanced breast MR imaging using spoiled gradient-recalled echoes and bookend T(1) measurements. Magn Reson Med 1999; 42: 746–53. doi: [DOI] [PubMed] [Google Scholar]
- 17.Sourbron SP, Buckley DL. Tracer kinetic modelling in MRI: estimating perfusion and capillary permeability. Phys Med Biol 2012; 57: R1–33. doi: 10.1088/0031-9155/57/2/R1 [DOI] [PubMed] [Google Scholar]
- 18.Leung SCY, Nielsen TO, Zabaglo LA, Arun I, Badve SS, Bane AL, et al. Analytical validation of a standardised scoring protocol for Ki67 immunohistochemistry on breast cancer excision whole sections: an international multicentre collaboration. Histopathology 2019; 75: 225–35. doi: 10.1111/his.13880 [DOI] [PubMed] [Google Scholar]
- 19.Hylton NM, Gatsonis CA, Rosen MA, Lehman CD, Newitt DC, Partridge SC, et al. Neoadjuvant chemotherapy for breast cancer: functional tumor volume by MR imaging predicts recurrence-free Survival-Results from the ACRIN 6657/CALGB 150007 I-SPY 1 trial. Radiology 2016; 279: 44–55. doi: 10.1148/radiol.2015150013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brix G, Kiessling F, Lucht R, Darai S, Wasser K, Delorme S, et al. Microcirculation and microvasculature in breast tumors: pharmacokinetic analysis of dynamic Mr image series. Magn Reson Med 2004; 52: 420–9. doi: 10.1002/mrm.20161 [DOI] [PubMed] [Google Scholar]
- 21.Padhani AR, Hayes C, Assersohn L, Powles T, Makris A, Suckling J, et al. Prediction of clinicopathologic response of breast cancer to primary chemotherapy at contrast-enhanced MR imaging: initial clinical results. Radiology 2006; 239: 361–74. doi: 10.1148/radiol.2392021099 [DOI] [PubMed] [Google Scholar]
- 22.Cho N, Im S-A, Park I-A, Lee K-H, Li M, Han W, et al. Breast cancer: early prediction of response to neoadjuvant chemotherapy using parametric response maps for MR imaging. Radiology 2014; 272: 385–96. doi: 10.1148/radiol.14131332 [DOI] [PubMed] [Google Scholar]
- 23.Li X, Abramson RG, Arlinghaus LR, Kang H, Chakravarthy AB, Abramson VG, et al. Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer. Invest Radiol 2015; 50: 195–204. doi: 10.1097/RLI.0000000000000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tateishi U, Miyake M, Nagaoka T, Terauchi T, Kubota K, Kinoshita T, et al. Neoadjuvant chemotherapy in breast cancer: prediction of pathologic response with PET/CT and dynamic contrast-enhanced MR imaging--prospective assessment. Radiology 2012; 263: 53–63. doi: 10.1148/radiol.12111177 [DOI] [PubMed] [Google Scholar]
- 25.Yu HJ, Chen J-H, Mehta RS, Nalcioglu O, Su M-Y, HJ Y, MY S. MRI measurements of tumor size and pharmacokinetic parameters as early predictors of response in breast cancer patients undergoing neoadjuvant anthracycline chemotherapy. J Magn Reson Imaging 2007; 26: 615–23. doi: 10.1002/jmri.21060 [DOI] [PubMed] [Google Scholar]
- 26.Specht JM, Kurland BF, Montgomery SK, Dunnwald LK, Doot RK, Gralow JR, et al. Tumor metabolism and blood flow as assessed by positron emission tomography varies by tumor subtype in locally advanced breast cancer. Clin Cancer Res 2010; 16: 2803–10. doi: 10.1158/1078-0432.CCR-10-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]