Abstract
The evaluation of PD‐L1 expression alone has limitations in predicting clinical outcome in immune‐checkpoint inhibitors (ICI). This study aimed to evaluate the predictive and prognostic effects of the presence of various immune cells in pretreatment tissue samples and to identify determinants associated with response in patients with advanced non‐small cell lung cancer (NSCLC) treated with PD‐1 blockade. Immune cell distribution was heterogeneous and the most dominant immune cell type was T cells. Patients with durable clinical benefit (DCB) showed significantly higher PD‐L1 expression. The ratio of tumor/stroma region of T cell, B cell, and macrophage was significantly higher in patient with DCB. High intratumoral T‐ and B‐cell density (≥median) was associated with DCB in the low PD‐L1 expression (<50%) group. In univariate analyses, the overall survival (OS) benefit was shown according to intratumoral B‐cell density (p = 0.0337). The incidence of hyperprogressive disease (HPD) was 13.0%. The Chi‐square test revealed that HPD was significantly associated with intratumoral B‐cell density but not T‐cell or macrophage density. Our results demonstrate different predictive and prognostic values for infiltrating immune cells in tumor tissue, which may help in selecting patients for ICI.
Keywords: Durable clinical benefit, Hyperprogressive disease, Immune checkpoint inhibitor, Non‐small cell lung cancer, Tumor microenvironment
Pre‐existing immune cells in tumor microenvironment represent key factors in determining clinical response to immunotherapy. High T‐ and B‐cell density has prognostic and predictive potential in combination with PD‐L1. In addition, immune cell proportions are related with risk of hyperprogressive disease.

Introduction
Immune‐checkpoint inhibitors (ICI), such as anti‐PD‐1 therapy, consistently show promising efficacy in non‐small cell lung cancer (NSCLC) and have become the standard of care [1]. Although the recent development of ICI has greatly improved cancer treatment and prolonged patient survival, objective response rates in NSCLC are currently only about 20% [2]. In addition, some patients experience unexpected rapid disease progression called hyperprogressive disease (HPD). Various immune and tumor genomic metrics are associated with sensitivity to ICI, including tumor PD‐L1 expression, tumor mutational burden, existence of tumor‐infiltrating lymphocytes (TILs), and microsatellite instability (MSI) [3]. Tumor PD‐L1 expression in pretreatment tissue is the most widely used single predictive biomarker. However, 11–20% of patients whose tumors have little or no PD‐L1 expression may still exhibit a response to anti‐PD‐1 treatment [3]. Therefore, identification of additional biomarkers that are highly predictive of positive and negative responses to ICI is urgently needed.
The tumor microenvironment (TME) is complex and heterogeneous. Because the TME significantly affects tumor development, the profiling of immune contexture has emerged as a powerful tool for predicting clinical outcome. Recent studies indicated that infiltrating T‐cell status predicts the response to anti‐PD‐1 therapy in several solid tumors including melanoma and NSCLC [4, 5]. The baseline level of CD8+ T cells has been associated with response to PD‐1 blockade in melanoma [4]. In addition, the CD8+/CD4+ TIL ratios in metastatic NSCLC and melanoma tumor biopsies predicted response in patients treated with PD‐1 blockade [6].
In addition to T cells, tumor‐infiltrating B cells have been reported as a prognostic biomarker. High B‐cell infiltration was correlated with better prognosis in the TCGA lung adenocarcinoma dataset [7]. In addition, low CD20+ B‐cell infiltration in nonsmokers with adenocarcinoma was identified as an independent unfavorable prognostic factor in resected NSCLC [8].
Tumor‐associated macrophages (TAMs) are important components of the TME with both anti‐ and protumor effects due to two distinct polarizations: M1 (classically activated) and M2 (alternatively activated). In a meta‐analysis of NSCLC, a high density of CD68+ macrophages was associated with better or poor overall survival (OS) according to pooled studies [9]. Although the prognostic significance of TAMs remains controversial, a recent study reported that CD68+ macrophages are a prognostic factor associated with prolonged survival in NSCLC [10].
The exact nature of the complex interaction between immune cell infiltration and its impact on patient prognosis and OS with NSCLC remains to be elucidated. Exploration of the immune cell composition of baseline NSCLC samples would offer critical insights into the complex and heterogeneous microenvironment landscapes associated with response to ICI. To determine whether pre‐existing immune cells represent key factors in determining clinical response to immunotherapy, we examined pretreatment tissue sections from 100 patients with advanced NSCLC treated with PD‐1 blockade and known response to treatment. The density of tumor‐associated immune cells was measured by multiplex immunohistochemistry (IHC). Multiplex IHC provides objective quantitative data of the tumor immune environment regarding both the numbers of immune subpopulations and their locations such as the tumor and stroma regions [11]. In the current study, the abundance and spatial organization of immune cells were examined along with clinical features, and their correlation with clinical outcome was assessed. This study suggests that integrated immune‐based biomarkers as well as PD‐L1 expression on pretreatment tissue samples may improve patient stratification for PD‐1 blockade immunotherapy.
Results
Patient characteristics
To understand the association between baseline TME characteristics, clinical features, and response to ICI, we identified 100 advanced NSCLC patients with available pretreatment archival tumor tissue adequate for multiplex IHC studies. The clinical characteristics of these patients are shown in Table 1. The cohort comprised 74 (74.0%) nonsquamous NSCLC and 26 (26.0%) squamous cell carcinoma. The median age was 61 years (range, 34‐83 years) and 76 (76.0%) patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0‐1. The majority (n = 94, 94.0%) had received more than one previous line of treatment. The patients had been treated with pembrolizumab (n = 43, 43.0%) or nivolumab (n = 57, 57.0%). The best response in this cohort was partial response (PR) in 28 patients (28.0%), stable disease (SD) in 14 patients (14.0%), and progressive disease (PD) in 58 patients (58.0%). Based on an internal database lock on July 2020, the median follow‐up was 6 months (range, 0.1‐45.1 months). Patients with PR and patients with SD > 6 months were classified as durable clinical benefit (DCB; n = 30, 30.0%), while patients with SD ≤ 6 months and patients with PD were classified as nondurable clinical benefit (NDCB; n = 70, 70.0%). Median progression‐free survival (PFS) for DCB and NDCB was 11.3 months (HR 0.22, 95% CI 0.15‐0.34) and 1.2 months (HR 4.45, 95% CI 2.95‐6.73), respectively. Median OS of DCB and NDCB was 23.1 months (HR 0.26, 95% CI 0.18‐0.40) and 2.80 months (HR 3.78, 95% CI 2.50‐5.70), respectively.
Table 1.
Baseline patients’ characteristics
| Category | All (n = 100) |
|---|---|
| Age (years) | |
| Median (range) | 61 (34‐83) |
| Histology | |
| Non‐SQ | 74 (74.0) |
| SQ | 26 (26.0) |
| Gender, n (%) | |
| Male | 73 (73.0) |
| Female | 27 (26.0) |
| Smoking history, n (%) | |
| Never‐smoker | 33 (33.0) |
| Smoker | 67 (67.0) |
| ECOG PS, n (%) | |
| 0‐1 | 76 (76.0) |
| 2‐3 | 24 (24.0) |
| Previous treatment, n (%) | |
| No treatment | 6 (6.0) |
| Chemotherapy | 83 (83.0) |
| Chemoradiotherapy | 11 (11.0) |
| EGFR status, n (%) | |
| Wild‐type | 84 (84.0) |
| Mutant | 16 (16.0) |
| PD‐L1 status, n (%) | |
| Positive | 56 (56.0) |
| Negative | 35 (35.0) |
| Unknown | 9 (9.0) |
| ICI treatment, n (%) | |
| Nivolumab | 57 (57.0) |
| Pembrolizumab | 43 (43.0) |
| Best response*, n (%) | |
| PR | 28 (28.0) |
| SD | 14 (14.0) |
| PD | 58 (58.0) |
| Clinical benefit, n (%) | |
| DCB | 30 (30.0) |
| NDCB | 70 (70.0) |
DCB, durable clinical benefit; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICI, Immune Checkpoint Inhibitors; NDCB, nondurable clinical benefit; PD, progressive disease; PR, partial response; SD, stable disease; SQ, squamous.
Heterogeneous infiltration of immune cell subpopulations in advanced NSCLC
To characterize the TME, including immune cell density and distribution, the entire tissue section was imaged and segmented into tumor and stroma regions. The frequency of immune cell subsets (CD8 T cell, CD4 T cell, B cell, macrophage, and Treg) was analyzed in both the tumor and stroma. As shown in Fig. 1, advanced NSCLC specimens showed various baseline immune cell densities across the tumor and stroma regions regardless of histologic type (Fig. 1A and B). All analyzed immune cells were more prevalent in the stroma than in the tumor. Similar to our previous single‐cell RNA sequencing data, the most dominant immune cell type was T cells in NSCLC, and CD4 T cells were more abundant than CD8 T cells [12].
Figure 1.
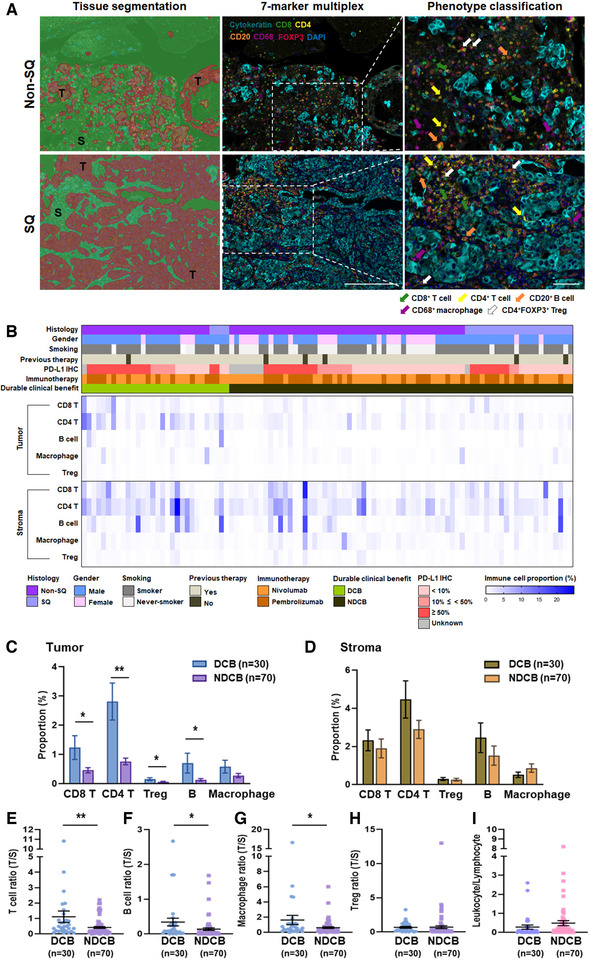
Characterization of immune cell subset infiltration in advanced NSCLC patients treated with PD‐1 blockade. (A) Representative multispectral images of Non‐SQ and SQ NSCLC. Left panels; tissue was segmented tumor (T, red) and stroma (S, green) using InForm image analysis software. Middle panels; FFPE sections of NSCLC were stained by cytokeratin (cyan, tumor cells), CD8 (green, CD8 T cells), CD4 (yellow, CD4 T cells), CD20 (orange, B cells), CD68 (magenta, macrophages), and FOXP3 (red). Right panel; high‐powered filed images showed cell phenotyping. Scale bar = 100 μm and 20× magnification. (B) Heatmap depicting immune cell (CD8 T cells, CD4 T cells, B cells, macrophages, and Treg) densities of 100 patients with advanced NSCLC (lower panel). Clinical characteristics shown at top panel. Each column represents patient, (C‐D) Quantitative analysis of immune cell density between patients with DCB (n = 30) and NDCB (n = 70) in the tumor (C) and stroma (D) regions and measured by Inform software Bar charts represent the mean ± SEM. (E‐H) Tumor to stroma ratio of T cell (E), B cell (F), macrophage (G), and Treg (H) proportion. (I) Leukocyte to lymphocyte ratio in the tumor region. Data are represented as dot plots (bar: mean ± SEM). (A‐I) All the slides of the 100 samples were collected on the same day and the experiments performed on several days *p < 0.05; **p < 0.01 by unpaired t‐test. Non‐SQ, nonsquamous; SQ, squamous; DCB, durable clinical benefit; NDCB, nondurable clinical benefit
To test whether immune context was related to response to PD‐1 blockade, we compared immune cell infiltration in patients with DCB and NDCB. The frequency of T (CD8 T, CD4 T, and Treg) and B cells in the tumor region was higher in patients with DCB than NDCB (Fig. 1C). T and B cells in tumor region had a similar predictive impact (CD8 T AUC, 0.626; CD4 T AUC, 0.698; B cell AUC, 0.697) on the likelihood of DCB (Supporting information Table S1). The intratumoral and intrastromal density of each immune cell showed a similar trend, but statistical significance was only found in the tumor region (Fig. 1C and D). Regarding the relationship between immune cell density of each region and DCB, significant correlations were observed only in tumor T cells (CD8 T and CD4 T, p = 0.0002) and B cells (p = 0.0012) (Supporting information Table S2).
The ratio of tumor/stroma region of T cells (CD8 T and CD4 T), B cells, and macrophages showed a significant difference between DCB and NDCB, but not in Tregs (Fig. 1E to H). When T cells and B cells were combined as lymphocytes, the ratio of leukocytes to lymphocytes in the tumor showed no difference between DCB and NDCB (Fig. 1I). Except for response to ICI, the frequency of each immune cell was not correlated with patient clinicopathologic characteristics such as age, gender, histology, and smoking status (Supporting information Table S3).
Association between PD‐L1 expression and immune cell infiltration and benefit from PD‐1 blockade
The tumor PD‐L1 expression level was retrospectively collected from electronic medical records. Ninety‐one patients had a PD‐L1 IHC score, and 9 patients had no result. PD‐L1 expression was tested by IHC using 22C3 antibody in 90 patients and SP263 antibody in 1 patient. The median PD‐L1 expression was 10%, and 34 of 91 patients (37.4%) had PD‐L1 ≥ 50%. Patients with DCB showed significantly higher PD‐L1 expression compared to NDCB (Fig. 2A). When patients were classified by clinicopathologic features, the PD‐L1 expression level was significantly lower in female than male patients (Supporting information Table S4). Beyond its association with gender, PD‐L1 levels were not significantly associated with any other clinical parameters such as histology, age, and smoking.
Figure 2.
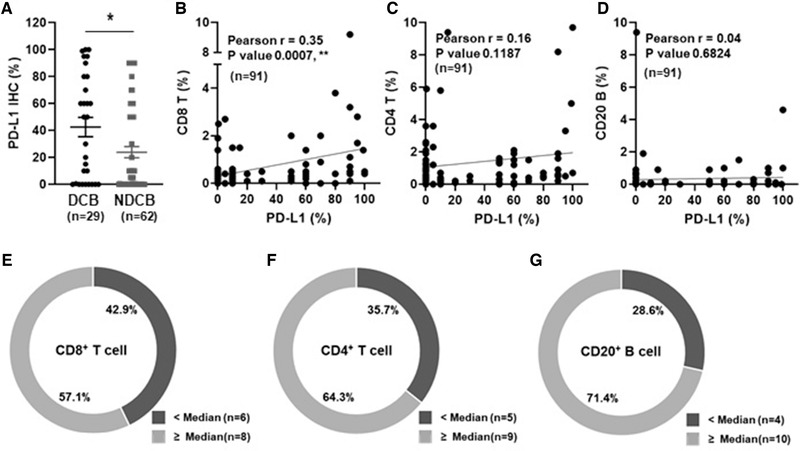
Patient classification based on combination of PD‐L1 and immune cell density. (A) Quantitative analysis of PD‐L1 between patients with durable clinical benefit (DCB, n = 29) and nondurable clinical benefit (NDCB, n = 62) in patients with PD‐L1 result (n = 91) and measured by IHC. (B‐D) Pearson's correlation between PD‐L1 and immune cells (B; CD8 T, r = 0.35, p = 0.0007, C; CD4 T, r = 0.16, p = 0.1187, and D; B cell, r = 0.04, p = 0.6824). Correlation coefficient (r) and significance levels (p value) are calculated using Pearson correlation and unpaired t‐test. (E‐G) Frequency of DCB in the low PD‐L1 group (PD‐L1 < 50%, n = 14) defined by CD8 T (E), CD4 T (F), and B‐ (G) cell density. (A‐G) Data from PD‐L1 IHC experiments. PD‐L1 IHC experiments were performed on 91 slides of the 100 samples and the experiments performed on several days.
Because intense tumor lymphocytic infiltration was previously validated as a favorable prognostic marker in NSCLC [1, 13, 14], we investigated correlations between PD‐L1 expression and immune cell density and whether they were associated with durable clinical outcomes. When the correlation between PD‐L1 expression and T‐ and B‐cell densities was assessed, different patterns of correlation were found in the tumor region. CD8 T‐cell infiltration was significantly and positively correlated with PD‐L1 expression (Fig. 2B). In contrast, CD4 T‐ and B‐cell infiltration showed no correlation with PD‐L1 expression (Fig. 2C and D).
Although the efficacy of anti‐PD‐1 therapy has been known to be associated with the level of PD‐L1 [6, 8, 15], PD‐L1 expression alone (cutoff level: 50%) could not differentiate between patients with DCB and NDCB in our cohort (Supporting information Fig. S1 and Table S5). To determine whether the combination of PD‐L1 expression and immune cell density has further predictive value than PD‐L1 alone, patients with DCB in the low PD‐L1 expression (<50%) group were divided into two groups based on the median cutoff points for immune cell marker expression. In the low PD‐L1 expression group (<50%), high T‐ and B‐cell density (≥median) was associated with DCB (Fig 2E to G). These results indicate that the combination of PD‐L1 and immune cell density would be helpful to stratify patients who have benefit from PD‐1 blockade.
To test the predictive and/or prognostic effects of baseline clinical factors and immune cell density, the hazard ratio (HR) of progression free survival (PFS) and OS was analyzed after treatment with PD‐1 blockade. In univariate analyses, ECOG PS 0‐1 (p = 0.0002) and B‐cell density (p = 0.0337) in immune cell proportion were associated with longer OS (Fig. 3 and Supporting information Fig. S2). The OS benefit was shown according to ECOG PS (p = 0.0002) in baseline clinical factors (Supporting information Fig. S2) and B‐cell density (p = 0.0337) in immune cell proportion (Fig. 3).
Figure 3.
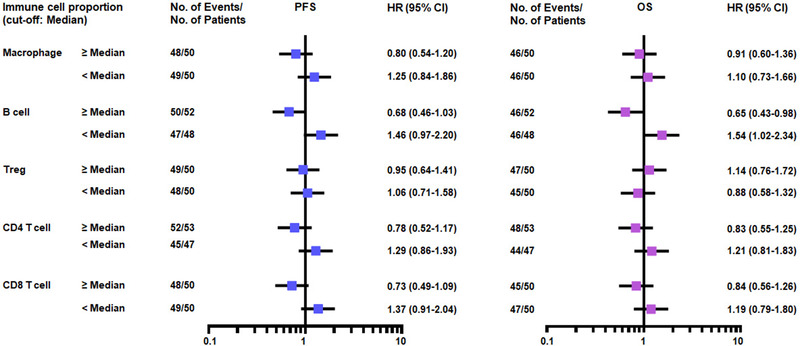
Hazard ratios of advanced NSCLC treated with PD‐1 blockade. Forest plots showing the hazard ratio (HR) of PFS and OS for immune cells.
Predictive role of immune cells in patients with HPD
The identification of patients at risk for developing HPD is a major challenge in the field of immunotherapy. Therefore, we tested whether the baseline TME characteristics were associated with HPD. The incidence of HPD in our cohort was 13.0% (n = 13), which is consistent with a previous report [16]. In HPD patients, the PFS was lower compared to those without HPD (HR, 0.57; 95% CI, 0.28‐1.19, p = 0.05) (Fig. 4A). It is of note that the ratio of leukocytes to lymphocytes in the tumor was significantly higher in patients with HPD (Fig. 4B). However, the ratio of tumor/stroma region of T cells (CD8 T and CD4 T), B cells, macrophages, and Tregs showed no significant difference between patients with HPD and non‐HPD (Fig. 4C to F). Further, in immune cell correlation analysis, CD4 T cells was shown to have an obvious correlation with B cells (r = 0.87) and Tregs (r = 0.89) in the tumor region of patients with HPD (Fig. 4G). In the Chi‐square test, HPD was significantly associated with intratumoral B‐cell density but not T cell or macrophage density (Table 2). These results suggest that the monitoring of B‐cell proportions before treatment allows the prediction of HPD in clinical practice along with complementary radiological evaluation.
Figure 4.
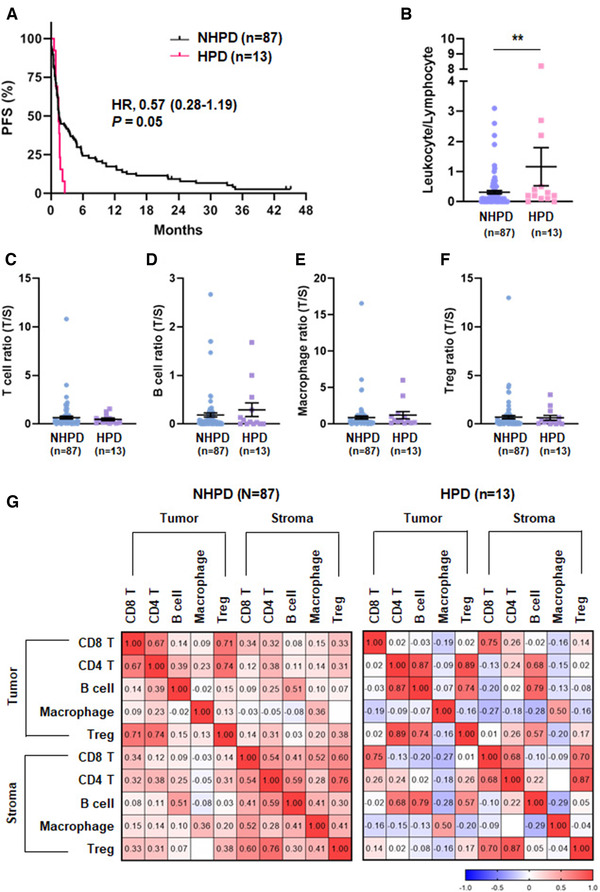
Association of tumor microenvironment and HPD. (A) Kaplan–Meier plots of PFS according HPD (n = 13) and NHPD (n = 87). (B) Leukocyte to lymphocyte ratio in the tumor region and measured by multiplex IHC. (C‐F) Tumor to stroma ratio of T cell (C), B cell (D), macrophage (E), and Treg (F) proportion and measured by multiplex IHC. Data are represented as dot plots (bar: mean ± SEM) (*p < 0.05; **p < 0.01 by unpaired t‐test). (G) Pearson‐correlation matrix of five immune cell types in the tumor and stromal regions according HPD. HPD, hyperprogressive disease; NHPD, nonhyperprogressive disease. (B‐G) Data from multiplex IHC experiments. All the slides of the 100 samples were collected on the same day and the experiments performed on several days.
Table 2.
Comparison between HPD and non‐HPD
| All (n = 100), n | |||
|---|---|---|---|
| NHPD | HPD | p value# | |
| CD8 T cell | |||
| <median | 42 | 8 | |
| ≥median | 45 | 5 | 0.37 |
| CD4 T cell | |||
| <median | 41 | 9 | |
| ≥median | 46 | 4 | 0.14 |
| Treg | |||
| <median | 42 | 8 | |
| ≥median | 45 | 5 | 0.37 |
| B cell | |||
| <median | 40 | 10 | |
| ≥median | 47 | 3 | 0.04* |
| Macrophage | |||
| <median | 45 | 5 | |
| ≥median | 42 | 8 | 0.37 |
HPD, hyperprogressive disease; NHPD, nonhyperprogressive disease.
#Chi‐square test.
*P < 0.05.
Discussion
To identify TME characteristics associated with ICI responsiveness, we used multiplex IHC to investigate the immune cell composition of pretreatment tumor specimens obtained from patients with advanced NSCLC treated with PD‐1 blockade.
Similar to previous studies [17], the PD‐L1 expression level showed a correlation with response to PD‐1 blockade and intratumoral density of CD8 T cells, suggesting that PD‐L1 expression is induced by local inflammatory signals such as interferon gamma (IFN‐γ) by CD8 T cells. Although we found that PD‐L1 expression was correlated with gender, the association between PD‐L1 expression and clinicopathologic features remains controversial.
Although high PD‐L1 expression has been used as a criterion to identify patients who have a chance to benefit from ICI, this does not fully predict their response. Therefore, additional biomarkers have also been considered to predict responses to ICI such as immune cells. The prognostic significance of TILs has been demonstrated in various cancers including NSCLC, melanoma, and pancreatic cancer. Detection of pre‐existing CD8 T cells has been associated with response to ICI in melanoma and pancreatic cancer [4]. Increased CD8 T‐cell infiltration was a positive predictor for clinical outcomes in NSCLC regardless of histology [18]. In addition, based on transcriptome sequencing data, classifying the TME based on PD‐1/PD‐L1 and CD8+ TIL combination successfully stratified patients with different clinical outcomes in adenocarcinoma but not in squamous NSCLC [19]. Another study reported that the combination of low CD4+/CD8+/CD68+ cell density and high PD‐L1 expression on tumor cells was a worse prognostic factor in the adenocarcinoma subtype of NSCLC [5]. Furthermore, a combination of PD‐L1 expression level and CD8 T‐cell density showed a prognostic effect in patients with NSCLC receiving concurrent chemoradiotherapy (CCRT) [20]. Although the immunologic function of CD20+ B cells during immunotherapy has not been fully elucidated, several studies demonstrated that B‐cell infiltration in tumors may represent a favorable prognostic biomarker for cancers including NSCLC. In contrast, a low accumulation of CD20+ B cells was an unfavorable prognostic factor in nonsmokers with adenocarcinoma [8]. Our data showed that high intratumoral T‐ and B‐cell density was associated with response to PD‐1 blockade, which is consistent with previous results. Intriguingly, in patients with low PD‐L1 expression (<50%), high intratumoral T and B cells were enriched in DCB. This observation was also found in melanoma treated with ICI [21].
HPD is unexpectedly accelerated tumor growth during ICI treatment and is associated with prompt clinical deterioration. So far, there is no validated biomarker to identify patients at risk of developing HPD. Similar to the results (8‐30%) from previous studies [22, 23], we found a 13% incidence of HPD in our cohort. Although recent studies reported that some clinicopathological features, strong expansion of CD28–CD4 lymphocytes in peripheral blood, and tumor infiltration by M2‐like CD163+CD33+PD‐L1+ macrophages are associated with HPD in NSCLC [22, 24, 25], a predictive biomarker has not been clearly defined. In this study, we found that high intratumoral leukocyte/lymphocyte ratio and low intratumoral B‐cell density were associated with HPD in advanced NSCLC. Furthermore, intratumoral B cells correlated with intratumoral CD4 T cells in patients with HPD. In a previous study, tumor‐infiltrating B cells presented antigen to CD4+ TILs in vitro, and activated tumor‐infiltrating B cells were associated with an effector CD4+ TIL phenotype (IFN‐γ+ CD4+) in NSCLC [26]. In addition, B cells showed close correlation with T cells in both tumor center and tumor margin, and a high density of B cells correlated with prolonged survival in colorectal cancer [27]. Thus, more studies are needed to reveal the precise role of B cells in the TME to identify new strategies that efficiently target these cells by immunotherapy.
This study has several limitations. First, immune cell analysis was conducted by retrospective staining of archival small biopsy specimens collected before anti‐PD‐1 therapy. Therefore, these small biopsy samples may not fully reflect the characteristics of the entire tumor, and some cases did not contain enough stroma. Second, since biopsy samples were obtained from either primary or metastatic tumors from various tumor sites, the proportion of immune cells may be both heterogeneous and dynamic, as reflected by discrepancies of the biopsy site. Third, the threshold for positivity of each marker may differ because PD‐L1 and immune cell marker staining were not conducted using the same method. Despite these limitations, our results showed that the pre‐existing immune cell context may result in the differing efficacy of immunotherapies in NSCLC, regardless of histologic type.
In conclusion, we showed that high T‐ and B‐cell density in combination with PD‐L1 expression is a positive prognostic indicator for patients with advanced NSCLC treated with anti‐PD‐1 therapy. Therefore, this combination of immune cell density and PD‐L1 expression has more promising prognostic and predictive potential than each factor alone. In addition, immune cell proportions are related with risk of HPD. Thus, evaluation of the TME using pretreatment tissue, even in small biopsy samples, may lead to the establishment of immunologic diagnostic methods and shed light on individualized immunotherapy for patients with advanced NSCLC.
Materials and methods
Patients, specimens, and ethical statement
Patients treated with anti‐PD‐1 at Samsung Medical Center were retrospectively identified based on FFPE tissue availability. FFPE sections of pretreatment biopsies from 100 patients were obtained from archived specimens for multiplex IHC on the same day. All procedures involving tumor specimens were reviewed and approved by the Institutional Review Board (IRB) of Samsung Medical Center (No. SMC 2013‐10‐112). Written informed consent was provided by patients; however, in some cases, a waiver of consent was obtained. Data from patients treated with pembrolizumab or nivolumab between December 2015 and August 2018 were analyzed, and the data cutoff was in July 2020. Clinicopathologic information including, ECOG PS, commonly used to assess how a patient's disease is progressing, and PD‐L1 IHC score were retrospectively collected from electronic medical records. Patient response was determined using RECIST 1.1 criteria. DCB was categorized as patients with a RECIST response of CR, PR, or SD of greater than 6 months with no progression, while non‐DCB (NDCB) were categorized as PD or SD for less than or equal to 6 months before disease progression. The definition of HPD was as follows: (I) time to treatment failure < 2 months; (II) tumor growth kinetics (TGK) ratio ≥ 2; and (III) volume increase 50% compared to baseline. Measured diameters (2r) of the target lesions were extrapolated to spherical volume using the mathematical formula 4/3ᴨr3. Sums of the estimated volumes were used. Per the RECIST system, patients with nonmeasurable disease only at baseline could not be assessed for TGK. For patients who had disease progression with new lesions, TGK was computed on the target lesions, and new lesions were included in the RECIST sum.
Multiplex immunohistochemistry (OPALTM) staining
FFPE tissues were sectioned at 4 μm, and multiplex immunofluorescence staining was performed using the following antibodies: CD8 (Bio‐Rad, Hercules, CA), CD4 (Abcam, Cambridge, UK), CD20 (Abcam), CD68 (DAKO, Carpinteria, CA), FOXP3 (Abcam), and CK (NOVUS, Centennial, CO). Staining was performed on several days using same experimental condition. Slides were scanned using the Perkin‐Elmer Vectra 3.0 Automated Quantitative Pathology Imaging System (Perkin‐Elmer, Waltham, MA). Scanned images were analyzed using Inform 2.2 software and TIBCO Spotfire™ (Perkin‐Elmer). Tumor (CK‐positive) and stroma (CK‐negative) areas were designated by an algorithm which was designed based on pattern recognition. The proportion of each immune cell type (CD8+ T cell, CD4+ T cell, CD4+/FOXP3+ Treg, CD20+ B cell, and CD68+ macrophage) was calculated as the percentage of positively staining cells against all nucleated cells (% positive cells/all nucleated cells) in the tumor and stromal regions.
Statistical analysis
An unpaired two‐tailed Student's t‐test was used to examine the significance of differences between samples, and p values less than 0.05 indicated significant difference. Relationships between paired data were analyzed using Pearson's correlation coefficient (r). The χ 2 test was used to examine differences in categorical variables. Survival analysis was conducted using Kaplan–Meier curves, and statistical significance was determined using the log‐rank test.
Authors’ contributions
Ahn MJ designed and supervised the study. Ku BM, Kim Y, Lee KY, and Kim SY performed all the experiments and analyzed the data. Ku BM and Kim Y wrote the manuscript. Sun JM, Lee SH, Ahn JS, Park K, and Ahn MJ diagnosed and recruited the patients and provided their clinical records. All authors contributed to revision of the manuscript.
Conflict of Interest
The authors declare no commercial or financial conflict of interest.
Peer review
The peer review history for this article is available at https://publons.com/publon/10.1002/eji.202048966
Abbreviations
- ICI
immune‐checkpoint inhibitor
- NSCLC
non‐small cell lung cancer
- DCB
durable clinical benefit
- OS
overall survival
- HPD
hyperprogressive disease
- TIL
tumor‐infiltrating lymphocytes
- TME
tumor microenvironment
- TAM
tumor‐associated macrophage
- IHC
immunohistochemistry
- PR
partial response
- SD
stable disease
- PD
progressive disease
- PFS
progression‐free survival
- HR
hazard ratio
- TGK
tumor growth kinetics
Supporting information
Figure S1. AssociationofclinicalbenefitbetweenPD‐L1andimmunecells.Dotplotofcolor‐codedclinicalbenefitofthepatients.Dashedlinesrepresentthecutoffvalues,whichwere50%forPD‐L1andmedianforimmunecells.DCB,durableclinicalbenefit;NDCB,nondurableclinicalbenefit
Figure S2. HazardratiosofadvancedNSCLCtreatedwithPD‐1blockade. Forestplotsshowingthehazardratio(HR)ofPFSandOSforbaselineclinicalfactors.
Table S1. ROC analysis of DCB at immune cell proportion in tumor region.
Table S2. Comparison of immune cells between DCB and NDCB in NSCLC.
Table S3. Association between immune cell proportion in tumor and clinicopathological features
Table S4. Association between PD‐L1 and clinicopathological features (n = 91)
Table S5. Comparison of PD‐L1 between DCB and non‐DCB. (n = 91)
Acknowledgments
We are thankful to all the patients involved in the study. This work was supported by the Collaborative Genome Program for Fostering New Post‐Genome Industry of the National Research Foundation (NRF) funded by the Ministry of Science and ICT (MSIT) (No. NRF‐2017M3C9A6044633 to M.J. Ahn).
Data availability statement
Data available on request due to privacy/ethical restrictions
References
- 1. Borghaei, H. , Paz‐Ares, L. , Horn, L. , Spigel, D. R. , Steins, M. , Ready, N. E. , Chow, L. Q. et al., Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N. Engl. J. Med. 2015. 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stankovic, B. , Bjorhovde, H. A. K. , Skarshaug, R. , Aamodt, H. , Frafjord, A. , Muller, E. , Hammarstrom, C. et al., Immune cell composition in human non‐small cell lung cancer. Front. Immunol. 2018. 9: 3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prelaj, A. , Tay, R. , Ferrara, R. , Chaput, N. , Besse, B. and Califano, R. , Predictive biomarkers of response for immune checkpoint inhibitors in non‐small‐cell lung cancer. Eur. J. Cancer 2019. 106: 144–159. [DOI] [PubMed] [Google Scholar]
- 4. Tumeh, P. C. , Harview, C. L. , Yearley, J. H. , Shintaku, I. P. , Taylor, E. J. , Robert, L. , Chmielowski, B. et al., PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014. 515: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parra, E. R. , Behrens, C. , Rodriguez‐Canales, J. , Lin, H. , Mino, B. , Blando, J. , Zhang, J. et al., Image analysis‐based assessment of PD‐L1 and tumor‐associated immune cells density supports distinct intratumoral microenvironment groups in non‐small cell lung carcinoma patients. Clin. Cancer Res. 2016. 22: 6278–6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uryvaev, A. , Passhak, M. , Hershkovits, D. , Sabo, E. and Bar‐Sela, G. , The role of tumor‐infiltrating lymphocytes (TILs) as a predictive biomarker of response to anti‐PD1 therapy in patients with metastatic non‐small cell lung cancer or metastatic melanoma. Med. Oncol. 2018. 35: 25. [DOI] [PubMed] [Google Scholar]
- 7. Ho, K. H. , Chang, C. J. , Huang, T. W. , Shih, C. M. , Liu, A. J. , Chen, P. H. , Cheng, K. T. et al., Gene landscape and correlation between B‐cell infiltration and programmed death ligand 1 expression in lung adenocarcinoma patients from The Cancer Genome Atlas data set. PLoS One 2018. 13: e0208459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kinoshita, T. , Muramatsu, R. , Fujita, T. , Nagumo, H. , Sakurai, T. , Noji, S. , Takahata, E. et al., Prognostic value of tumor‐infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non‐small‐cell lung cancer. Ann. Oncol. 2016. 27: 2117–2123. [DOI] [PubMed] [Google Scholar]
- 9. Mei, J. , Xiao, Z. , Guo, C. , Pu, Q. , Ma, L. , Liu, C. , Lin, F. et al., Prognostic impact of tumor‐associated macrophage infiltration in non‐small cell lung cancer: A systemic review and meta‐analysis. Oncotarget 2016. 7: 34217–34228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rakaee, M. , Busund, L. R. , Jamaly, S. , Paulsen, E. E. , Richardsen, E. , Andersen, S. , Al‐Saad, S. et al., Prognostic value of macrophage phenotypes in resectable non‐small cell lung cancer assessed by multiplex immunohistochemistry. Neoplasia 2019. 21: 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stack, E. C. , Wang, C. , Roman, K. A. and Hoyt, C. C. , Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 2014. 70: 46–58. [DOI] [PubMed] [Google Scholar]
- 12. Kim, N. , Kim, H. K. , Lee, K. , Hong, Y. , Cho, J. H. , Choi, J. W. , Lee, J. I. et al., Single‐cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nat. Commun. 2020. 11: 2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brahmer, J. , Reckamp, K. L. , Baas, P. , Crino, L. , Eberhardt, W. E. , Poddubskaya, E. , Antonia, S. et al., Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N. Engl. J. Med. 2015. 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herbst, R. S. , Baas, P. , Kim, D. W. , Felip, E. , Perez‐Gracia, J. L. , Han, J. Y. , Molina, J. et al., Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet 2016. 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 15. Fumet, J. D. , Richard, C. , Ledys, F. , Klopfenstein, Q. , Joubert, P. , Routy, B. , Truntzer, C. et al., Prognostic and predictive role of CD8 and PD‐L1 determination in lung tumor tissue of patients under anti‐PD‐1 therapy. Br. J. Cancer 2018. 119: 950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Denis, M. , Duruisseaux, M. , Brevet, M. and Dumontet, C. , How can immune checkpoint inhibitors cause hyperprogression in solid tumors? Front. Immunol. 2020. 11: 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu‐Lieskovan, S. , Lisberg, A. , Zaretsky, J. M. , Grogan, T. R. , Rizvi, H. , Wells, D. K. , Carroll, J. et al., Tumor characteristics associated with benefit from pembrolizumab in advanced non‐small cell lung cancer. Clin. Cancer Res. 2019. 25: 5061–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meng, X. , Gao, Y. , Yang, L. , Jing, H. , Teng, F. , Huang, Z. and Xing, L. , Immune microenvironment differences between squamous and non‐squamous non‐small‐cell lung cancer and their influence on the prognosis. Clin. Lung Cancer 2019. 20: 48–58. [DOI] [PubMed] [Google Scholar]
- 19. Lin, Z. , Gu, J. , Cui, X. , Huang, L. , Li, S. , Feng, J. , Liu, B. et al., Deciphering microenvironment of NSCLC based on CD8+ TIL density and PD‐1/PD‐L1 expression. J. Cancer 2019. 10: 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tokito, T. , Azuma, K. , Kawahara, A. , Ishii, H. , Yamada, K. , Matsuo, N. , Kinoshita, T. et al., Predictive relevance of PD‐L1 expression combined with CD8+ TIL density in stage III non‐small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur. J. Cancer 2016. 55: 7–14. [DOI] [PubMed] [Google Scholar]
- 21. Sade‐Feldman, M. , Yizhak, K. , Bjorgaard, S. L. , Ray, J. P. , de Boer, C. G. , Jenkins, R. W. , Lieb, D. J. et al., Defining t cell states associated with response to checkpoint immunotherapy in melanoma. Cell 2018. 175: 998–1013 e1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen, Y. , Hu, J. , Bu, F. , Zhang, H. , Fei, K. and Zhang, P. , Clinical characteristics of hyperprogressive disease in NSCLC after treatment with immune checkpoint inhibitor: a systematic review and meta‐analysis. BMC Cancer 2020. 20: 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrara, R. , Mezquita, L. , Texier, M. , Lahmar, J. , Audigier‐Valette, C. , Tessonnier, L. , Mazieres, J. et al., Hyperprogressive disease in patients with advanced non‐small cell lung cancer treated with PD‐1/PD‐L1 inhibitors or with single‐agent chemotherapy. JAMA Oncol. 2018. 4: 1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arasanz, H. , Zuazo, M. , Bocanegra, A. , Gato, M. , Martinez‐Aguillo, M. , Morilla, I. , Fernandez, G. et al., Early detection of hyperprogressive disease in non‐small cell lung cancer by monitoring of systemic T cell dynamics. Cancers (Basel) 2020. 12: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lo Russo, G. , Moro, M. , Sommariva, M. , Cancila, V. , Boeri, M. , Centonze, G. , Ferro, S. et al., Antibody‐Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non‐small cell lung cancer subsequent to PD‐1/PD‐L1 blockade. Clin. Cancer Res. 2019. 25: 989–999. [DOI] [PubMed] [Google Scholar]
- 26. Bruno, T. C. , Ebner, P. J. , Moore, B. L. , Squalls, O. G. , Waugh, K. A. , Eruslanov, E. B. , Singhal, S. et al., Antigen‐presenting intratumoral B cells affect CD4(+) TIL phenotypes in non‐small cell lung cancer patients. Cancer Immunol. Res. 2017. 5: 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bindea, G. , Mlecnik, B. , Tosolini, M. , Kirilovsky, A. , Waldner, M. , Obenauf, A. C. , Angell, H. , et al., Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013. 39: 782–795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. AssociationofclinicalbenefitbetweenPD‐L1andimmunecells.Dotplotofcolor‐codedclinicalbenefitofthepatients.Dashedlinesrepresentthecutoffvalues,whichwere50%forPD‐L1andmedianforimmunecells.DCB,durableclinicalbenefit;NDCB,nondurableclinicalbenefit
Figure S2. HazardratiosofadvancedNSCLCtreatedwithPD‐1blockade. Forestplotsshowingthehazardratio(HR)ofPFSandOSforbaselineclinicalfactors.
Table S1. ROC analysis of DCB at immune cell proportion in tumor region.
Table S2. Comparison of immune cells between DCB and NDCB in NSCLC.
Table S3. Association between immune cell proportion in tumor and clinicopathological features
Table S4. Association between PD‐L1 and clinicopathological features (n = 91)
Table S5. Comparison of PD‐L1 between DCB and non‐DCB. (n = 91)
Data Availability Statement
Data available on request due to privacy/ethical restrictions


