Abstract
Background & Aims
There is intense research for drugs able to reduce disease progression in nonalcoholic fatty liver disease. We aimed to test the impact of novel antidiabetic drugs (dipeptidyl‐peptidase‐4 inhibitors – DPP‐4Is, glucagon‐like peptide‐1 receptor agonists – GLP‐1RAs, sodium‐glucose cotransporter‐2 inhibitors – SGLT‐2Is) on non‐invasive biomarkers of steatosis (fatty liver index, FLI) and fibrosis (Fibrosis‐4 score, FIB‐4) in patients with type 2 diabetes (T2D).
Methods
Clinical, anthropometric and biochemical parameters were retrospectively analysed in 637 consecutive T2D patients switched from metformin w/wo sulfonylureas and/or pioglitazone to DPP‐4Is, GLP‐1RAs and SGLT‐2Is in a tertiary care setting. 165 patients maintained on original treatments served as controls. The effects on FLI and FIB‐4 at 6‐ and 12‐month follow‐up were analysed by logistic regression after adjustment for baseline differences, computed by propensity scores, and additional adjustment for changes in glycosylated hemoglobin (HbA1c) and body mass index.
Results
Body mass index, HbA1c and aminotrasferases significantly decreased following switching to GLP‐1RAs and SGLT2‐Is, compared with both controls and DPP‐4Is, whereas only HbA1c was reduced on DPP‐4Is. FLI and FIB‐4 were reduced on GLP‐1RA and SGLT‐2I; logistic regression analysis confirmed a significant improvement of both biomarkers after adjustment for propensity score. The shift of FIB‐4 values towards the category ruling out advanced fibrosis was maintained after additional adjustment for confounders. These effects were confirmed in a sensitivity analysis on effect size.
Conclusions
Glucagon‐like peptide‐1 receptor agonists and SGLT‐2Is improve biomarkers of steatosis and fibrosis, in keeping with beneficial effects on liver disease progression, and should be considered the treatment of choice in T2D.
Keywords: dipeptidyl‐peptidase‐4 inhibitors, glucagon like peptide‐1 receptor agonists, NAFLD, sodium‐glucose cotransporter‐2 inhibitors, surrogate biomarkers
Abbreviations
- AIFA
Agenzia Italiana del Farmaco
- ANOVA
analysis of variance
- APRI
albumin to platelets ratio index
- AST‐ALT
aspartate and alanine aminotransferases
- BMI
body mass index
- CI
confidence interval
- CTRL
controls
- DPP‐4Is
dipeptidyl‐peptidase‐4 inhibitors
- ELF
enhanced liver fibrosis
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- FIB‐4
Fibrosis‐4 score
- FLI
fatty liver index
- GLP‐1RAs
glucagon‐like peptide‐1 receptor agonists
- HbA1c
glycosylated A1c hemoglobin
- HPLC
High Performance Liquid Chromatography
- MET
metformin
- NAFLD
nonalcoholic fatty liver disease
- OR
odds ratio
- RCT
randomized controlled trial
- SD
standard deviation
- SGLT‐2Is
sodium‐glucose cotransporter‐2 inhibitors
- SULF
sulfonylureas
- T2D
type 2 diabetes mellitus
Key points.
In poorly controlled type 2 diabetes, the impact of switch to novel antidiabetic agents (DPP‐4 inhibitors, GLP‐1 receptor agonists and SGLT‐2 inhibitors) was tested on surrogate biomarkers of steatosis and fibrosis.
Compared to patients maintained on usual treatment, GLP‐1 receptor agonists and SGLT‐2 inhibitors, not DPP‐4 inhibitors, significantly improved steatosis and reduced the risk of significant fibrosis.
GLP‐1 receptor agonists and SGLT‐2 inhibitors qualify for the treatment of NAFLD in subjects with diabetes at risk of disease progression.
1. INTRODUCTION
Non‐alcoholic fatty liver disease (NAFLD), a common disorder closely associated with the metabolic syndrome, 1 causes a high burden to National Health Systems worldwide. 2 Disease progression from fatty liver to steatohepatitis (NASH), fibrosis and cirrhosis is favoured by obesity and type 2 diabetes, 3 , 4 which make lifestyle changes aimed at weight loss the backbone of prevention and treatment. 5 , 6
Several randomized controlled trials (RCT, phase 2‐3) have been completed or are underway to test the effects of pharmaceutical compounds against placebo on liver histology in NAFLD, 7 as required by FDA, EMA or national drug agencies. 8 However, the need for liver biopsy limited enrolment and fostered intensive research for non‐invasive markers for NAFLD diagnosis and staging. 9 Several biomarkers have been developed on clinical and laboratory values 10 ; their use facilitates patients’ selection for RCT, and helps determine the effectiveness of treatment in the real world. The fatty liver index (FLI) 11 is the standard biomarker of pathological liver fat content, following extensive validation in subjects with/without diabetes against ultrasonography and liver biopsy. 12 , 13 Among fibrosis biomarkers, the Fibrosis‐4 (FIB‐4) score 14 has received validation by liver biopsy and transient elastography and is largely used to rule in/out advanced fibrosis. 15 , 16
Subjects with type 2 diabetes constitute a selected cohort in which new classes of drugs could be tested for their potential positive effects on NAFLD progression, outside placebo‐controlled trials. In the past 15 years, both dipeptidyl‐peptidase‐4 inhibitors (DPP‐4Is) and glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) and later gliflozins (sodium‐glucose cotransporter‐2 inhibitors – SGLT‐2Is) have entered the diabetes drug market. Incretin analogs (GLP‐1RAs) exert a direct stimulatory effect on pancreatic β‐cells, whereas DPP‐4Is reduce endogenous incretin degradation. In both cases, glucose disposal is increased via an insulin‐dependent mechanism. On the contrary, SGLT‐2Is promote insulin‐independent glucose control by decreasing glucose reabsorption in the proximal renal tubule. All these drugs are associated with a low risk of hypoglycemia, and both GLP‐1RAs and SGLT‐2Is have been shown to reduce cardiovascular events vs sulfonylureas. 17 , 18 Novel drugs have been recently upgraded as first‐line treatment for type 2 diabetes, 19 but their use is lower than recommended, because of treatment inertia and budget restrictions. 20 , 21 , 22 Few data are available on the effects of novel drugs on liver histology 23 , 24 and limited evidence comes from real‐world experience (phase 4), 25 , 26 or post‐hoc analysis of registration or cardiovascular outcome trials. 27 , 28 The results are generally based on changes in enzymes and surrogate markers of liver steatosis and fibrosis, the factor most closely associated with liver‐ and non‐liver‐related outcomes.
We aimed to analyse the 6‐ and 12‐month impact of the novel drug classes for type 2 diabetes on surrogate markers of both steatosis and fibrosis in a cohort of patients enrolled in a tertiary centre. Results were analysed after adjustment for propensity score, to account for differences in baseline, and for changes in metabolic control and body weight.
2. MATERIALS AND METHODS
2.1. Patients
We retrospectively analysed all patients with type 2 diabetes who were consecutively prescribed DPP‐4Is, GLP‐1RAs, SGLT‐2Is in our metabolic and diabetes unit, in the period 2014‐2017, following the implementation of a new digital registration system. According to initial rules dictated by the Italian Medicines Agency (AIFA), the patients had to fulfil definite criteria and to undergo a monitoring system, only based on body weight, metabolic control and renal function. However, in the majority of cases, other biochemical and clinical data were available and were regularly registered. Liver ultrasonography (usually within 1 year of the visit) was part of their routine check‐up and hence not specifically performed by a dedicated radiologist. We found 637 patients whose treatment was switched from metformin with/without sulfonylureas and/or pioglitazone to the novel antidiabetic drugs, excluding those who had moved to centres outside the metropolitan area of Bologna, had discontinued treatment for adverse events (approximately 10%), or added insulin to their initial treatment (<5%). Switching was dictated by a comprehensive analysis of metabolic control, cardiovascular risk – supposed to be lowered by novel agents –, frailty, patients’ preferences and adherence, life expectancy. In the DPP‐4I group (n = 104), the vast majority of cases had been switched to sitagliptin (n = 88), with a few cases treated by linagliptin (n = 10) and three cases treated by alogliptin and vildagliptin. In the GLP‐1RA group (n = 338), 186 cases had been switched to liraglutide (1.2 or 1.8 mg daily), 70 to dulaglutide (1.5 mg weekly), 82 to weekly exenatide LAR. Finally, among SGLT‐2Is (n = 195), dapagliflozin, empagliflozin and canagliflozin had been used in 81, 72 and 42 cases, respectively. The groups treated by novel antidiabetic agents were compared with a cohort (n = 165) on continuous metformin (MET) treatment (all cases), associated with sulfonylureas (SULF) or glinides (n = 32; mainly gliclazide or repaglinide) or pioglitazone (30 mg/day) (n = 16), who were considered as control group (CTRL). The reasons for not switching were again the comprehensive analysis of patients’ conditions, as well physicians’ or patients’ inertia and fear of adverse events. The clinical data of the whole cohort are reported in Table 1. At all visits, patients received reinforcement for lifestyle changes and adherence to a healthy diet and habitual physical activity. The protocol of the study was approved by the ethical committee of the Azienda Ospedaliero‐Universitaria Sant’Orsola‐Malpighi, protocol 238/2018/Oss/AOUBo, as internal audit. As a result of the retrospective nature of the study and the de‐identified code of patients’ records, no informed consent was required according to Italian law.
TABLE 1.
Baseline data in the whole population and according the drug classes
| Variables | Total (n = 802) | CTRL (n = 165) | DPP‐4 Is (n = 104) | GLP‐1RAs (n = 338) | SGLT‐2Is (n = 195) |
|---|---|---|---|---|---|
| Male gender (n, %) | 476 (59.4) | 91 (55.2) | 59 (56.7) | 188 (55.6) | 138 (70.8) d , e |
| Age (y) | 61.5 ± 10.5 | 60.9 ± 11.6 | 62.7 ± 10.2 | 61.0 ± 10.5 | 62.1 ± 9.7 |
| BMI (kg/m2) | 34.8 ± 7.1 | 32.7 ± 5.3 | 30.9 ± 6.7 d | 37.7 ± 7.0 d , e | 33.6 ± 6.8 e |
| Weight class 1/2/3/4/5 (%) a | 5/20/33/23/19 | 3/30/41/16/10 | 18/38/22/11/11 | 1/8/32/28/31 | 7/25/33/24/11 |
| Glucose (mg/dL) | 162.7 ± 47.8 | 143.6 ± 38.8 | 161.4 ± 46.0 d | 162.9 ± 43.3 d | 177.7 ± 55.5 d , e |
| HbA1c (%) | 8.11 ± 1.20 | 7.48 ± 0.96 | 7.95 ± 1.00 d | 8.25 ± 1.27 d , e | 8.51 ± 1.15 d , e |
| HbA1c (mmol/mol) | 65.1 ± 9.6 | 58.3 ± 7.7 | 63.4 ± 8.0 | 66.7 ± 10.2 | 69.5 ± 9.2 |
| <7; 7‐8; 8‐9; >9 (%) | 16/28/34/22 | 31/23/42/4 | 11/47/32/10 d | 14/28/27/31 d , e | 8/23/41/28 d , e |
| Waist circumference (cm) | 109.3 ± 14.4 | 106.0 ± 13.1 | 101.3 ± 12.6 d | 114.7 ± 13.9 d , e | 107.1 ± 13.9 e |
| AST (U/L) | 26.8 ± 14.4 | 28.7 ± 10.9 | 24.9 ± 12.8 d | 26.4 ± 14.9 | 26.9 ± 16.6 |
| ALT (U/L) | 34.5 ± 20.5 | 36.2 ± 15.0 | 29.9 ± 15.9 d , e | 35.7 ± 22.2 | 34.0 ± 23.0 |
| Normal ALT (n, %) b | 311 (38.8) | 42 (25.5) | 55 (52.9) d | 120 (35.5) d , e | 94 (48.2) d |
| Gamma‐GT (U/L) | 42.0 ± 17.4 | 42.5 ± 10.7 | 39.1 ± 11.4 d | 43.5 ± 22.0 | 40.5 ± 15.1 |
| Platelets (103 × μL) | 211.9 ± 40.7 | 207.7 ± 28.4 | 214.4 ± 39.9 | 209.3 ± 36.8 | 218.6 ± 53.8 d |
| Creatinine (mg/dL) | 0.88 ± 0.27 | 0.94 ± 0.27 | 0.93 ± 0.43 | 0.84 ± 0.23 d , e | 0.89 ± 0.19 |
| Total cholesterol (mg/dL) | 184.0 ± 46.7 | 200.1 ± 45.1 | 183.9 ± 45.9 d | 179.9 ± 45.2 d | 177.4 ± 47.9 d |
| HDL‐cholesterol (mg/dL) | 44.6 ± 10.9 | 47.0 ± 12.1 | 48.1 ± 11.1 | 44.0 ± 10.5 d , e | 41.7 ± 9.6 d , e |
| Triglycerides (mg/dL) | 185.1 ± 112.4 | 184.8 ± 75.7 | 171.7 ± 99.0 | 183.1 ± 102.0 | 195.8 ± 154.2 |
| Systolic Pressure (mmHg) | 134.1 ± 15.8 | 136.8 ± 13.4 | 129.0 ± 13.7 d | 133.4 ± 15.9 d , e | 135.8 ± 18.0 e |
| Diastolic Pressure (mmHg) | 83.7 ± 17.9 | 84.9 ± 9.6 | 79.7 ± 13.1 d | 83.7 ± 12.1 e | 82.4 ± 12.8 d |
| Steatosis at US (%) | 666 (83.0) | 148 (89.8) | 57 (53.9) d | 307 (94.4) e | 140 (71.8) e |
| Fatty liver index (%) | 83.8 ± 17.9 | 81.5 ± 16.3 | 69.7 ± 23.8 d | 90.9 ± 10.7 d , e | 80.5 ± 19.7 e |
| No steatosis (n, %) | 14 (1.7) | 1 (0.6) | 6 (5.8) | 0 (0.0) | 7 (3.6) |
| Undetermined (n, %) | 72 (9.0) | 16 (9.6) | 30 (28.8) | 10 (3.0) | 16 (8.2) |
| Steatosis (n, %) | 716 (89.3) | 148 (89.87) | 68 (65.4) d | 328 (97.0) d , e | 172 (88.2) |
| Fibrosis‐4 (score) | 1.40 ± 0.65 | 1.45 ± 0.56 | 1.40 ± 0.59 | 1.35 ± 0.62 | 1.43 ± 0.78 |
| No advanced fibrosis (n, %) | 391 (40.8) | 61 (37.0) | 52 (50.0) d | 178 (52.7) d | 100 (51.3) d |
| Undetermined (n, %) | 384 (47.8) | 98 (59.4) | 47 (45.2) | 151 (44.6) | 88 (45.1) |
| Advanced fibrosis (n, %) | 27 (3.4) | 6 (3.6) | 5 (4.8) | 9 (2.7) | 7 (3.6) |
| Microalbuminuria (mg/L) c | 11 [29] | 11 [17] | 10 [22] | 12 [35] | 13 [30] |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CTRL, controls; DPP‐4Is, dipeptidyl‐peptidase‐4 inhibitors; GLP‐1RAs, glucagon‐like peptide‐1 receptor agonists; HbA1c, glycosylated A1c haemoglobin; SGLT‐2Is, sodium‐glucose cotransporter‐2 inhibitors.
Weight class: normal weight/overweight/obesity class I/class II/class III.
ALT < 31 U/L in males and < 19 U/L in females.
Median [interquartile range].
Significantly different from the corresponding value in CTRL group.
Significantly different from the corresponding value in DPP‐4Is.
2.2. Biochemical non‐invasive tests
At time of first visit in the diabetes centre and at all control visits, body weight and height, waist circumference and blood pressure were recorded, as well as blood glucose, lipid profile (total cholesterol, HDL‐cholesterol, triglycerides), HbA1c (HPLC method), renal function (creatinine, glomerular filtration rate [eGFR] and microalbuminuria), liver enzymes, blood cell count, platelets and serum protein. A program for the standardization of biochemical assays had been active in Bologna laboratories since 2008, and was later turned into a Laboratorio Unico Metropolitano, serving the whole area of Bologna.
Based on anthropometric and laboratory data, FLI and FIB‐4 were computed. FLI (%) determines the risk score of having/not having excess liver fat based on a formula including plasma triglycerides, gamma‐GT, waist circumference and BMI. FLI > 60% rules in significant liver fat (specificity, 86%), FLI < 30% rules out (sensitivity, 87%), values between 30% and 60% define a grey zone of indefinite risk. 11 FIB‐4 is a biomarker based on aspartate and alanine transaminases (AST and ALT), age and platelet count optimized to determine the risk of advanced fibrosis in NAFLD patients. 14 FIB‐4 score >2.67 has 97% specificity and rules in advanced fibrosis, whereas FIB‐4 score <1.30 has a high negative predictive value (90%) and rules out advanced fibrosis, thus also leaving a large undetermined area. 29 An upper cut‐off of 2.0 ruling in advanced fibrosis for patients aged ≥65 was also reported. 30
FLI and FIB‐4 were preferred to other biomarkers (eg, NAFLD fibrosis score) considering data availability and their independence of diabetes in validation studies.
2.3. Statistical analysis
After anonymous downloading of records from hospital database, the data were implemented in StatView™ 5.0 program (SAS Institute, Inc) and Stata (StataCorp. 2019. Stata Statistical Software: Release 16.: StataCorp LLC) for analysis. A descriptive statistic was initially carried out (means, standard deviation [SD], prevalence, 95% confidence interval [CI]) on continuous and nominal variables, and data were initially compared by unpaired t‐test, repeated‐measures analysis of variance, Mann‐Whitney or Kruskal‐Wallis test. Considering the significant differences in baseline data among groups treated with different drugs, a propensity score was calculated by logistic regression analyses as the conditional probability to be either maintained on the previous drugs (metformin with/without sulfonylureas) or being assigned to a novel treatment. The following variables were included in the regression: age, gender, BMI, HbA1c. Levels of aminotransferases were not considered, since new treatments were not intentionally favoured in patients with suspected liver disease. A similar procedure was used to calculate a propensity score for being assigned GLP‐1RAs or SGLT‐2Is vs DPP‐4Is.
The effects of the new drugs on steatosis and fibrosis were tested at 6 and 12 months after drug switch. The dependent variables were an improvement of at least one category of FLI and FIB‐4 (FLI from >60% to the intermediate area (30%‐60%) or from the intermediate area to <30%; FIB‐4 from >2.67 to values in the range 1.30‐2.67 or from the intermediate area to <1.30). The odds ratio (OR) and 95% CI were computed in two logistic regression models, having the CTRL group as reference. Model 1 was adjusted for propensity score (ie, for baseline demographic data, obesity grade and metabolic control) and model 2 was additionally adjusted for changes in metabolic parameters (percent changes in BMI and absolute changes in HbA1c). The same models were used to test the difference between DPP‐4I use (the earliest novel treatment, used since 2008) and GLP‐1RAs or SGLT‐2Is, rapidly become the most common treatments, following the accumulated evidence of their advantage on cardiovascular outcomes and the expected impact on body weight, potentially improving metabolic control.
In a sensitivity analysis, the upper FIB‐4 cut‐off of 2.0 was also considered for patients aged ≥65. Additionally, the previous models were tested in a multivariable logistic regression analyses where the dependent variables were absolute reductions of FLI score and FIB‐4 score ≥0.5 SD (effect size, 0.5), calculated at baseline on the total cohort, ie, FLI ≥8 points and FIB‐4 ≥0.325.
All P values refer to two‐tailed tests of significance. P < .05 was considered significant.
3. RESULTS
3.1. Baseline data
The cohort of the study was representative of patients with type 2 diabetes attending a specialized centre. Metabolic control was largely suboptimal; the vast majority of cases fell into the obesity classes, with HBA1c well above the accepted therapeutic targets. In response, patients were invited to adhere to healthier lifestyles, and novel treatments were implemented in 79.4% of cases, with differences in relation to clinical and anthropometric parameters (Table 1). In particular, GLP‐1RAs were more frequently prescribed in subjects with severe obesity, whereas SGLT‐2Is were largely preferred in males, considering the higher risk of genito‐urinary infections associated with their use in females. DPP‐4Is were less commonly prescribed and preferred in lean or moderately overweight, actively working patients. Finally, a considerable number of young patients, in a better metabolic control, maintained their usual treatment.
At initial assessment, ALT exceeded the revised upper normal limits (31 U/L for males and 19 U/L for females) 31 in nearly 40% of cases and were more commonly normal in the DPP‐4I cohort, where the prevalence of steatosis at ultrasonography was much lower than in all other groups. No remarkable differences were observed in lipid levels and renal function. The two surrogate biomarkers of steatosis (FLI) and fibrosis (FIB‐4) confirmed the lower prevalence of steatosis in the DPP‐4I cohort and a low prevalence of advanced fibrosis in all groups (Table 1).
3.2. Follow‐up of anthropometric and biochemical data
Figure 1 shows the changes in BMI, HbA1c at 6‐ and 12‐month follow‐up (Upper two panels). BMI decreased in all groups, with a highly significant reduction in the GLP‐1RA and SGLT‐2I cohorts (with differences also when compared with DPP‐4I‐treated patients). In particular, BMI decreased by 4.0 ± 5.9% in the GLP‐1RA and by 3.9 ± 5.4% in the SGLT‐2I cohorts, respectively, vs only 0.8 ± 3.4% in DPP‐4Is and 1.1 ± 2.6% in CTRL after 12 months, with more limited differences after 6 months. Similarly, HbA1c decreased by 0.69 ± 0.84%, 1.02 ± 1.36 and 1.00 ± 1.13 (5.5 ± 6.7 mmol/mol, 8.0 ± 10.9, 8.0 ± 9.1, respectively) in the DPP‐4I, GLP‐1RA and SGLT‐2I groups after 6 months, vs only 0.08 ± 0.78% (0.6 ± 6.2 mmol/mol) in the control group (all, P < .001). The differences were also significant when GLP‐RA and SGLT‐2I cohorts were compared with DPP‐4I treatment (P < .05) and were all maintained to a similar extent at the 12‐month follow‐up.
FIGURE 1.
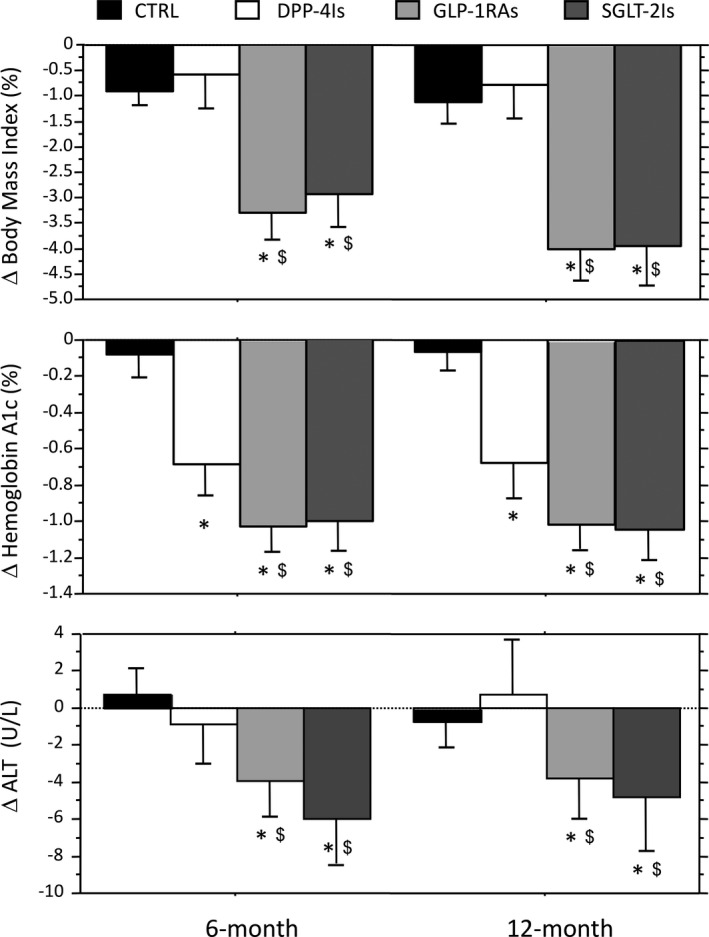
Changes in BMI, glycosylated haemoglobin and alanine aminotransferase levels at 6 and 12 mo in the groups treated by the different glucose‐lowering drug classes. Data are expressed as mean and 95% confidence interval. CTRL represents continuous treatment with metformin ± sulfonylureas and/or pioglitazone. *Significantly different from CTRL values. $Significantly different from the corresponding value of the DPP‐4I group. ALT, alanine transaminase; BMI, body mass index; CTRL, controls; DPP‐4Is, dipeptidyl‐peptidase‐4 inhibitors; GLP‐1RAs, glucagon‐like peptide‐1 receptor agonists; SGLT‐2Is, sodium‐glucose cotransporter‐2 inhibitors
ALT values were unchanged after 6 and 12 months in the CTRL and DPP‐4I group, whereas they decreased significantly with GLP‐1RAs (by 4.0 U/L and 3.8 U/L at 6 and 12 months, respectively; P vs baseline, <.001 for both) and SGLT‐2Is (by 6.0 U/L, P < .01; and by 4.8 U/L; P < .001). Notably, ALT returned within the normal limits of 31 U/L for males and 19 U/L for females in 15% and 16% of cases with elevated values at baseline after 6 months and in 19% and 16% after 12 months in response to GLP‐1RA and SGLT‐2 treatment, respectively, whereas normal values were only achieved in 6%‐7% of CTRL‐ or DPP‐I‐treated patients (χ2 test; P values, <.005). The responses of AST and GGT levels were also different between treatments (not reported).
In the whole population, percent changes in BMI were significantly associated with changes in ALT and AST at 6 months (r = .168 and r = .159; both, P < .001), not with changes in GGT (r = .067; P = .070). Similar data were found at 12‐month follow‐up (r = .147, r = .112 and r = −.004, respectively).
3.3. Effects of anti‐diabetic treatments on non‐invasive biomarkers
The changes in the biomarkers of steatosis and fibrosis are also presented in Figure 2. Both FLI and FIB‐4 decreased significantly in the course of follow‐up following treatment with GLP‐1RAs and SGLT2‐Is, when compared with both the CTRL and the DPP‐4I group. The decrease in FLI observed on GLP‐RAs and SGLT2Is translated into a lower number of cases classified as steatosis or indeterminate at 6 and 12 months in the whole cohort (n = 691 and n = 680, respectively, vs n = 716 at baseline), despite very few passages into more severe FLI classes. The difference being entirely because of changes observed in GLP‐RAs and SGLT2Is (Table 2; Figure 3). Similar improvements were observed in FIB‐4, with the number of cases classified as advanced fibrosis reducing from 27 to 23 and 19 after 6 and 12 months, respectively, and the number classified as no advanced fibrosis increasing from 396 at baseline to 479 after 6 months and 512 after 12 months (both, P < .001).
FIGURE 2.
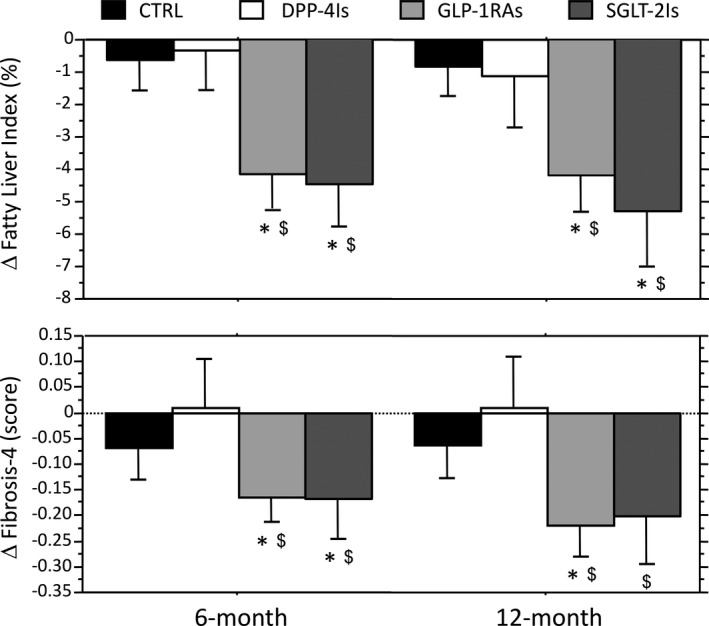
Changes in surrogate biomarkers of steatosis and fibrosis (fatty liver index and Fibrosis‐4 score, respectively) at 6 and 12 mo by the different T2DM drug classes. Data are expressed as mean and 95% confidence interval. CTRL represent treatment with metformin w/wo sulfonylureas and/or pioglitazone. *Significantly different from control values. $Significantly different from the corresponding value of the DPP‐4I group. CTRL, controls; DPP‐4Is, dipeptidyl‐peptidase‐4 inhibitors; GLP‐1RAs, glucagon‐like peptide‐1 receptor agonists; SGLT‐2Is, sodium‐glucose cotransporter‐2 inhibitors
TABLE 2.
Fatty liver index and Fibrosis‐4 score (% of cases in different cells) at baseline and after 6 and 12 mo of treatment by the different drug classes
| CTRL group (n = 165) | DPP‐4I group (n = 104) | GLP‐1RA group (n = 338) | SGLT‐2I group (n = 195) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6‐mo | 12‐mo | Baseline | 6‐mo | 12‐mo | Baseline | 6‐mo | 12‐mo | Baseline | 6‐mo | 12‐mo | |
| Fatty liver index (%) | ||||||||||||
| No steatosis (<30%) | 0.6 | 0.6 | 0.6 | 5.8 | 5.8 | 9.6 | 0.0 | 0.9 | 0.6 | 3.6 | 5.6 | 7.2 |
| Undetermined (30%‐60%) | 9.7 | 11.5 | 9.7 | 25.8 | 27.9 | 24.0 | 3.0 | 3.8 | 6.8 | 8.2 | 14.9 | 15.9 |
| Steatosis (>60%) | 89.7 | 87.9 | 89.7 | 68.4 | 66.3 | 66.4 | 97.0 | 95.3 | 92.6 | 88.2 | 79.5 | 76.9 |
| P vs baseline a | NS | NS | NS | NS | NS | 0.004 | 0.027 | 0.005 | ||||
| Fibrosis‐4 score | ||||||||||||
| No advanced fibrosis (<1.30) | 39.4 | 44.2 | 43.6 | 50.0 | 49.0 | 52.9 | 52.9 | 67.2 | 73.1 | 51.3 | 65.6 | 70.8 |
| Undetermined (1.30‐2.67) | 57.0 | 53.3 | 52.8 | 45.2 | 47.1 | 45.2 | 44.4 | 30.5 | 25.7 | 45.1 | 30.8 | 27.2 |
| Advanced fibrosis (>2.67) | 3.6 | 2.5 | 3.6 | 4.8 | 3.9 | 4.8 | 2.7 | 2.4 | 1.2 | 3.6 | 3.6 | 2.1 |
| P vs baseline b | 0.045 | NS | NS | NS | <0.001 | <0.001 | 0.004 | <0.001 | ||||
Abbreviations: CTRL, controls; DPP‐4Is, dipeptidyl‐peptidase‐4 inhibitors; GLP‐1RAs, glucagon‐like peptide‐1 receptor agonists; NS, not significant; SGLT‐2Is, sodium‐glucose cotransporter‐2 inhibitors.
Calculated on percent of cases positive for steatosis.
Calculated on percent of cases defined as “no advanced fibrosis”.
FIGURE 3.
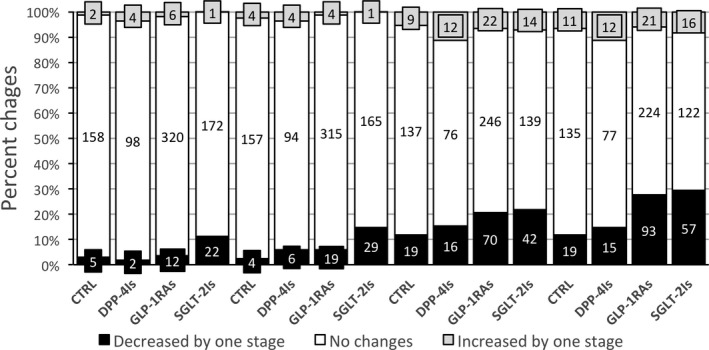
Changes in FLI and FIB‐4 class at 6‐ and 12‐mo follow‐up vs baseline in subjects with and without (CTRL) switch in their antidiabetic treatment. Data are expressed as percent of cases within the class and as absolute numbers. Decreased by 1 class indicates an improvement of at least one category of FLI and FIB‐4 (FLI from > 60% to the intermediate area (30%‐60%) or from the intermediate area to < 30%; FIB‐4 from > 2.67 to intermediate values (1.30‐2.67) or from intermediate values to < 1.30). Increased by 1 class is the opposite. #Changes in FLI status at 6 mo; °Changes in FLI status at 12 mo; ^Changes in FIB‐4 status at 6 mo; *Changes in FIB‐4 status at 12 mo. CTRL, controls; DPP‐4Is, dipeptidyl‐peptidase‐4 inhibitors; FIB‐4, Fibrosis‐4 score; FLI, fatty liver index; GLP‐1RAs, glucagon‐like peptide‐1 receptor agonists; SGLT‐2Is, sodium‐glucose cotransporter‐2 inhibitors
In the whole population, changes in FLI at both 6‐ and 12‐month follow‐up were significantly associated with percent changes in BMI (r = .468 and r = .516; both, P < .001), whereas changes in FIB‐4 showed a less strict correlation (r =.122; P < .01 and r = .078: P < .05). Changes in the two biomarkers maintained a strict correlation with changes in liver enzymes (FLI with GGT; FIB‐4 with AST and ALT as part of their constitutive algorithms).
At logistic regression analysis having CTRL as reference, at 6‐month follow‐up we observed a significant improvement of at least one FLI class only after switch to SGLT‐2Is after adjustment for propensity score (OR, 5.44; 95% CI, 1.87‐15.79) and the improvement was maintained in the fully adjusted model (OR, 3.88; 95% CI, 1.27‐11.83). No intra‐class effects were demonstrated in FIB‐4. After 12 months, the treatment with both GLP1‐RAs and SGLT‐2Is, compared to the CTRL group, induced significant changes on FLI and FIB‐4 when adjusted by propensity score (Figure 4). The improvement in the steatosis biomarker (FLI) was maintained only for SGLT‐2Is in the fully adjusted model, whereas the improvement in FIB‐4 was maintained for both drugs (OR, 2.56; 95% CI, 1.45‐4.50 and OR 2.76; 95% CI, 1.52‐6.06, respectively). Treatment with DPP‐4Is, in all models/adjustments and for both biomarkers, was never associated with significant improvement when compared to CTRL, whereas the effects of treatment with GLP‐1RAs and SGLT‐2Is towards lower grades of steatosis and fibrosis were always significant when compared with DPP‐4I use.
FIGURE 4.

Logistic regression analyses of improvement in surrogate biomarkers of steatosis (A – FLI) and fibrosis (B – FIB‐4 score) at 12 mo after switching treatment to the new classes of glucose‐lowering drugs. Data are expressed as odds ratio (OR) and 95% confidence interval vs the control group (CTRL) and vs DPP‐4I treatment. Analyses were adjusted for propensity score (PS, upper panels), calculated on baseline demographic data, BMI and HbA1c. In the lower panels, the analyses were additionally adjusted for changes in metabolic parameters (percent changes in BMI and absolute changes in HbA1c). BMI, body mass index; CTRL, controls; DPP‐4Is, dipeptidyl‐peptidase‐4 inhibitors; FIB‐4, Fibrosis‐4 score; FLI, fatty liver index; GLP‐1RAs, glucagon‐like peptide‐1 receptor agonists; HbA1c, glycosylated A1c haemoglobin; SGLT‐2Is, sodium‐glucose cotransporter‐2 inhibitors
In the different groups no significant differences were observed in relation to the type of DPP‐4I, GLP‐1RA and SGLT‐2I used (not reported in details).
In the sensitivity analysis having the upper FIB‐4 cut‐off of 2.0 for patients ≥65, the results did not systematically change (Table S1). Considering the old age of the population, the number of patients with FIB‐4 values ruling in significant fibrosis was more than doubled at baseline (n = 70, 8.8%, without differences between treatment groups), and decreased progressively at follow‐up, whereas the number of cases where FIB‐4 ruled out significant fibrosis increased, particularly in the groups switched to GLP‐1RAs and to SGLT‐2Is. Logistic regression analysis confirmed the significant effects of switching to GLP‐1RAs and to SGLT‐2Is on fibrosis at 6 and 12 months, in both models, as well as in comparison to both CTRL and DPP‐4I treatments (Table S2).
In the sensitivity analyses having an effect size of 0.5 for FLI and FIB‐4 in the different treatment cohorts as dependent variables, the results on class shift were largely reproduced (Table S3), with minor differences. In particular, a significant effect was maintained for SGLT‐2Is on FLI at both 6 and 12 months and for GLP‐1RA on FIB‐4 at 12 months, again with superiority of both treatments vs DPP‐4Is.
4. DISCUSSION
The study shows that both GLP‐1RAs and SGLT‐2Is produce significant improvements on steatosis and fibrosis biomarkers of NAFLD in patients with type 2 diabetes, and both drugs are far more effective than DPP‐4Is. Adjustment for changes in metabolic control and BMI maintains a significant effect on fibrosis for GLP‐1RAs and SGLT‐2Is and tends to cancel any significant effect on steatosis improvement, suggesting that the effect on stestosis is principally driven by changes in body weight, further strengthening the importance of lifestyle on hepatic fat accumulation. 32
Data are derived from a systematic analysis of the database of a metabolic and diabetes centre, treating patients addressed by primary care physicians because of insufficient metabolic control. The population is characterized by a high rate of fatty liver, both at ultrasonography and by FLI score, as expected considering the high BMI; by contrast, the prevalence of advanced fibrosis (ruled in by FIB‐4 score) was unexpectedly low compared with literature, 33 frequently biased by selection procedures carried out in liver Units. This prevalence, however, more than doubles using the age‐adjusted cut‐offs proposed for FIB‐4, considering the old age of our population. Our analysis takes advantage of the specificity of diabetes treatment according to the Italian healthcare system, which limits the use of more recently developed antidiabetic drugs to specialist prescription. All analyses were adjusted by propensity score, a technique used mainly in the analysis of real‐world data, 34 likely to balance the effects of treatment for baseline differences.
According to Italian rules and the protocols of our centre, all patients with type 2 diabetes should receive a consultation with a specialist every year or second year, depending on metabolic control, and should be switched to novel anti‐diabetic drugs to improve metabolic control, whenever appropriate. Drug selection, as well as the maintenance of previous treatment, depends on a variety of elements, including frailty, patients’ preferences and acceptance of new treatments, considering the possible change from oral to injectable drugs (daily or weekly GLP‐1RAs), the expected benefits of weight loss, as well as cardiovascular risk, significantly reduced by GLP‐1RAs and SGLT‐2Is. 17 , 18 Patients are also counselled for lifestyle changes, which explains the observed weight loss in CTRL, per se improving metabolic control.
Among novel antidiabetic agents, DPP‐4I prescription produced a systematic improvement in glycosylated hemoglobin, but no effects on weight loss, as expected based on the literature and registration trials. DPP‐4Is are commonly prescribed to individuals scarcely adhering to lifestyle counselling, because of drug safety and lack of side effects (no hypoglycemia risk). According to the present report, their use – namely sitagliptin use, the most commonly employed DPP‐4I – did not produce any relevant improvement in NAFLD biomarkers. Following limited initial experiences, 26 Cui et al reported no differences between sitagliptin and placebo in reducing liver enzymes, liver fat, measured by magnetic resonance‐derived proton density‐fat fraction, and liver fibrosis (by magnetic resonance elastography) in a 24‐week RCT in patients with prediabetes. 35 Only Yan et al found a decreased intrahepatic lipid content in patients with type 2 diabetes treated with sitagliptin, unexpectedly associated with weight loss, 36 which might be the reason for reduced liver fat accumulation. Surprisingly, DPP‐4I treatment was even modestly less effective than the maintenance of original treatment, a result that paralleled changes in BMI achieved by the two strategies. We speculate that the improved metabolic control might be a likely reason for scarce interest and adherence to lifestyle changes, nullifying the effects on body weight and liver fat in this younger, free living cohort.
Many more data support the use of GLP‐1RAs to reduce liver fat in NAFLD. Exenatide, added to pioglitazone, produced a significant decrease in liver fat content after 26‐50 weeks intervention, 37 and liraglutide was more effective than glimepiride combined with metformin for 25 weeks. 38 In both studies, the effects of GLP‐1RAs were fostered by weight loss. Both lixisenatide (a rapid‐acting GLP‐1RA) and dulaglutide, a weekly GLP‐1RA, significantly decreased the levels of aminotransferases or achieved ALT normalization in a higher percentage of cases in post‐hoc analyses of the registration programs. 27 , 28 In the Liraglutide Efficacy and Action in Non‐alcoholic steatohepatitis (LEAN) randomized phase 2 trial, liraglutide at the dose of 1.8 mg/day reached the primary outcome of NASH resolution without worsening of fibrosis after 48 weeks, and fibrosis progression was reduced in patients with/without diabetes. 23 More recently, a phase 2 study of semaglutide, a longer‐acting, weekly dosing GLP‐1RA has also been completed. Semaglutide effectively reduced liver enzymes 39 and also met the primary end‐point of NASH resolution and no worsening in liver fibrosis, after 72 weeks of therapy at the dose of 0.4 mg. 40 However, the study failed to demonstrate any significant improvement in fibrosis stage (F2‐F3 at liver biopsy at entry into the trial). The authors argue that failure to meet the fibrosis end‐point might stem from a longer time needed for NASH resolution to produce effects on fibrosis, particularly in the setting with significant fibrosis, 40 which was in general not the case in our population. Notably, semaglutide produced an important reduction in body weight, and even higher weight losses may be achieved at higher doses (up to 18.2% in subjects who stayed on 2.4 mg semaglutide for 68 weeks). 41 All these results are consistent with the data observed in the present study, where the effects of GLP‐1RAs on steatosis were systematically associated with a remarkable reduction in percent BMI, with minor effects on fibrosis improvement. Interestingly, GLP‐RAs, by favouring weight loss, might also strengthen the beneficial effects of lifestyle counselling, 42 further improving NAFLD biomarkers. Nonetheless, a systematic effect GLP‐1RAs on FIB‐4 is maintained – and confirmed in the sensitivity analyses –, requiring additional validation in unrelated settings and by different fibrosis markers (eg, commercial ELF test or transient elastography).
Also, SGLT‐2Is are receiving increasing attention for NAFLD treatment. By blocking glucose resorption from the proximal tubule, gliflozins promote calorie waste and weight loss, reducing lipid burden to the liver. Approved SGLT‐2Is have been tested for their effects on biomarkers of steatosis and fibrosis, 43 , 44 , 45 and a network meta‐analysis of 29 RCTs confirmed that dapagliflozin, empagliflozin and canagliflozin, the gliflozins used in our cohort, are all significantly likely to induce a ≥5% weight loss compared with placebo. 46 Indeed, compared with CTRL group, gliflozin‐treated patients achieved a mean additional weight loss of 3.1 and 2.8 kg at 6 and 12 months, respectively, a figure of the same magnitude as that achieved by GLP‐1RA treatment.
The specific effectiveness of GLP‐1RAs and SGLT‐2Is for the treatment of NAFLD should be discussed, based on evidence that their effects on fibrosis were not cancelled by adjustment for metabolic control and weight loss. Changes of FLI class, although quantitatively important in terms of score, were rare compared with shifting to lower FIB‐4 classes, but FLI scores were largely well above the cut‐off of 60% ruling in steatosis in the GLP‐1RA and SGLT‐2I cohorts. This explains the much larger CIs in the models. The effects of these quantitative changes, largely driven by weight loss, on fibrosis remain a matter of discussion, requiring validation by imaging (transient elastography) or by long‐term analysis of clinical events in the real world. In principle, a decrease in liver fat by GLP‐1RA and SGLT‐2I treatment, also demonstrated by histology or by magnetic resonance spectroscopy in other settings, is expected to reduce necroinflammation – ie, reduced liver enzymes, documented in the present and in other studies – and lower inflammation would in turn stop or reverse fibrosis progression. However, this process is likely to take a lot of time to become clinically relevant. The analysis of large clinical databases may help support this conclusion. At the end of our follow‐up the number of patients of our cohort with FIB‐4 values ruling out advanced fibrosis increased from 398 (49.4%) at baseline to 512 (63.8%; P < .001). Also limiting the analysis to the cohort with evidence of fatty liver by ultrasonography or biomarkers, the number ruling out advanced fibrosis increased to 363 to 474 (+30.6%; P < .001). According to European guidelines, 5 NAFLD patients in this category do not need any additional investigation for their liver disease. Notably, the percent of patients on treatment with pioglitazone – the only drug so far associated with fibrosis regression 47 –, as well as the duration of treatment (from 4 months to over 10 years), was not different between groups. This makes any effect of pioglitazone very unlikely as cause of fibrosis improvement after switching.
The present study has both strengths and limitations. The main strengths are the large sample size, the comparison of different treatments covering the whole spectrum of possible interventions, allocation to treatment according to a defined protocol, and the computation of non‐invasive biomarkers based on data derived from a single, standardized laboratory. A few limitations should also be discussed. Firstly, not all cases could be classified as NAFLD on the basis of ultrasonography and surrogate biomarkers. Although a post‐hoc analysis limited to subjects with ultrasonography‐assessed NAFLD does not qualitatively change the results, the results of imaging should be taken with caution considering that the technique has a high risk of underreporting, as it was not carried out by dedicated radiologists. Secondly, whereas FLI and FIB‐4 may be confidently used to predict or exclude steatosis and fibrosis, based on solid histological evidence, more data are needed to determine their ability to capture longitudinal changes in liver histology. In a post‐hoc analysis of the FLINT trial, a correlation was found between changes in surrogate biomarkers of fibrosis (FIB‐4, APRI and ELF) and changes in histology after 72‐week treatment, 48 and in a 5‐year follow‐up study subjects with worsening FIB‐4 had a higher likelihood of progressive liver disease, 49 but the correlation was poor. Also in this case, validation by elastography might provide important clues. Finally, biomarkers were calculated on a single sample, and their day‐to‐day reproducibility has never been adequately demonstrated. This last limitation applies to individual cases, but it is not likely to affect the final conclusions.
In summary, our results have relevant pathogenic implications. Lifestyle intervention aimed at weight loss, irrespective of the presence of obesity and/or diabetes, is presently the most effective therapeutic strategy for NAFLD patients. In the past few months, several drugs have failed the primary outcome in phase 2 or 3 trials. 50 However, in the presence of type 2 diabetes, the selection of antidiabetic agents may make a difference, promoting a reduction of intrahepatic fat and, possibly, direct or indirect advantages on fibrosis progression. Real‐world data, either based on surrogate biomarkers or imaging techniques (transient elastography coupled with controlled attenuation parameter) will contribute to define the most effective therapeutic strategy.
CONFLICT OF INTEREST
SC, FR, LB, FM, ASS and LP declare no conflicts of interest in relation to the material presented in the study; FAB received honoraria for conference from MSD and Astra‐Zeneca, MLL received honoraria for conference from Novo Nordisk; GM participated in NAFLD advisory boards of Astra‐Zeneca, Pfizer, Gilead, Novartis, and received honoraria for conference from Eli Lilly.
AUTHOR CONTRIBUTIONS
SC, FR, MLP and GM planned the study; SC, LB, FM, FAB, ASS collected data; FR, GM and LP contributed the statistical analysis; SC, FR and GM drafted the manuscript; all authors approved the final version of manuscript; GM acts as guarantor.
Supporting information
Table S1‐S3
ACKNOWLEDGEMENTS
FM and FAB are supported by a contract financed by Italian Ministry of Health and Italian Regions (NET‐2016‐02364191‐4). The authors are indebted to Dr A Maghetti for continuous monitoring of the cohort treated by SGLT‐2 inhibitors.
Colosimo S, Ravaioli F, Petroni ML, et al. Effects of antidiabetic agents on steatosis and fibrosis biomarkers in type 2 diabetes: A real‐world data analysis. Liver Int. 2021;41:731–742. 10.1111/liv.14799
Santo Colosimo and Federico Ravaioli contributed equally to the study.
Handling editor: Salvatore Petta
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844‐1850. [DOI] [PubMed] [Google Scholar]
- 2. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11‐20. [DOI] [PubMed] [Google Scholar]
- 3. Harris R, Card TR, Delahooke T, Aithal GP, Guha IN. Obesity is the most common risk factor for chronic liver disease: results from a risk stratification pathway using transient elastography. Am J Gastroenterol. 2019;114:1744‐1752. [DOI] [PubMed] [Google Scholar]
- 4. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hepatol. 2019;71:793‐801. [DOI] [PubMed] [Google Scholar]
- 5. European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity . EASL‐EASD‐EASO clinical practice guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64:1388‐1402. [DOI] [PubMed] [Google Scholar]
- 6. Petroni ML, Brodosi L, Barbanti FA, di Domizio S, Petta S, Marchesini G. Lifestyle changes for the treatment of nonalcoholic fatty liver disease – a 2015–19 update. Curr Pharm Des. 2020;26:1110‐1118. [DOI] [PubMed] [Google Scholar]
- 7. Younossi ZM, Loomba R, Rinella ME, et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2018;68:361‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2019;16:377‐386. [DOI] [PubMed] [Google Scholar]
- 9. Wong VW, Adams LA, de Ledinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH – current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15:461‐478. [DOI] [PubMed] [Google Scholar]
- 10. Younossi ZM, Loomba R, Anstee QM, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. 2018;68:349‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gastaldelli A, Kozakova M, Hojlund K, et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology. 2009;49:1537‐1544. [DOI] [PubMed] [Google Scholar]
- 13. Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population‐based study. Clin Gastroenterol Hepatol. 2013;11:1201‐1204. [DOI] [PubMed] [Google Scholar]
- 14. Vallet‐Pichard A, Mallet V, Nalpas B, et al. FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32‐36. [DOI] [PubMed] [Google Scholar]
- 15. Anstee QM, Lawitz EJ, Alkhouri N, et al. Noninvasive tests accurately identify advanced fibrosis due to NASH: baseline data from the STELLAR trials. Hepatology. 2019;70:1521‐1530. [DOI] [PubMed] [Google Scholar]
- 16. McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non‐invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non‐alcoholic fatty liver disease. Gut. 2010;59:1265‐1269. [DOI] [PubMed] [Google Scholar]
- 17. Kristensen SL, Rorth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP‐1 receptor agonists in patients with type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776‐785. [DOI] [PubMed] [Google Scholar]
- 18. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393:31‐39. [DOI] [PubMed] [Google Scholar]
- 19. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes‐2020. Diabetes Care. 2020;43:S98‐S110. [DOI] [PubMed] [Google Scholar]
- 20. Bonora E, Cataudella S, Marchesini G, et al. A view on the quality of diabetes care in Italy and the role of diabetes clinics from the 2018 ARNO Diabetes Observatory. Nutr Metab Cardiovasc Dis. 2020;30:1945‐1953. [DOI] [PubMed] [Google Scholar]
- 21. Heintjes EM, Overbeek JA, Hall GC, et al. Factors associated with type 2 diabetes mellitus treatment choice across four european countries. Clin Ther. 2017;39:2296‐2310. [DOI] [PubMed] [Google Scholar]
- 22. Wilkinson S, Douglas I, Stirnadel‐Farrant H, et al. Changing use of antidiabetic drugs in the UK: trends in prescribing 2000–2017. BMJ Open. 2018;8:e022768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (LEAN): a multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet. 2016;387:679‐690. [DOI] [PubMed] [Google Scholar]
- 24. Seko Y, Sumida Y, Tanaka S, et al. Effect of 12‐week dulaglutide therapy in Japanese patients with biopsy‐proven non‐alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res. 2017;47:1206‐1211. [DOI] [PubMed] [Google Scholar]
- 25. Bajaj HS, Brown RE, Bhullar L, Sohi N, Kalra S, Aronson R. SGLT2 inhibitors and incretin agents: associations with alanine aminotransferase activity in type 2 diabetes. Diabetes Metab. 2018;44:493‐499. [DOI] [PubMed] [Google Scholar]
- 26. Kato H, Nagai Y, Ohta A, et al. Effect of sitagliptin on intrahepatic lipid content and body fat in patients with type 2 diabetes. Diabetes Res Clin Pract. 2015;109:199‐205. [DOI] [PubMed] [Google Scholar]
- 27. Cusi K, Sattar N, Garcia‐Perez LE, et al. Dulaglutide decreases plasma aminotransferases in people with Type 2 diabetes in a pattern consistent with liver fat reduction: a post hoc analysis of the AWARD programme. Diabet Med. 2018;35:1434‐1439. [DOI] [PubMed] [Google Scholar]
- 28. Gluud LL, Knop FK, Vilsboll T. Effects of lixisenatide on elevated liver transaminases: systematic review with individual patient data meta‐analysis of randomised controlled trials on patients with type 2 diabetes. BMJ Open. 2014;4:e005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McPherson S, Hardy T, Dufour JF, et al. Age as a confounding factor for the accurate non‐invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112:740‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1‐10. [DOI] [PubMed] [Google Scholar]
- 32. Hallsworth K, Adams LA. Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep. 2019;1:468‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Younossi ZM, Tampi RP, Racila A, et al. Economic and clinical burden of nonalcoholic steatohepatitis in patients with type 2 diabetes in the U.S. Diabetes Care. 2020;43:283‐289. [DOI] [PubMed] [Google Scholar]
- 34. Birkeland KI, Jorgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs (CVD‐REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5:709‐717. [DOI] [PubMed] [Google Scholar]
- 35. Cui J, Philo L, Nguyen P, et al. Sitagliptin vs. placebo for non‐alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2016;65:369‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yan J, Yao B, Kuang H, et al. Liraglutide, sitagliptin, and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology. 2019;69:2414‐2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sathyanarayana P, Jogi M, Muthupillai R, Krishnamurthy R, Samson SL, Bajaj M. Effects of combined exenatide and pioglitazone therapy on hepatic fat content in type 2 diabetes. Obesity. 2011;19:2310‐2315. [DOI] [PubMed] [Google Scholar]
- 38. Jendle J, Nauck MA, Matthews DR, et al. Weight loss with liraglutide, a once‐daily human glucagon‐like peptide‐1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009;11:1163‐1172. [DOI] [PubMed] [Google Scholar]
- 39. Newsome P, Francque S, Harrison S, et al. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment Pharmacol Ther. 2019;50:193‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Newsome PN, Buchholtz K, Cusi K, et al. A placebo‐controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2020. 10.1056/NEJMoa2028395 [DOI] [PubMed] [Google Scholar]
- 41. NOVO Nordisk . Semaglutide 2.4 mg demonstrates superior and sustained weight loss versus placebo and in addition a 17.4% weight loss after 68 weeks in STEP 4 trial. GlobeNewswire; 2020. https://ml‐eu.globenewswire.com/Resource/Download/4951d1a2‐3bd1‐47ea‐840a‐a1234109c018. Accessed December 26, 2020. [Google Scholar]
- 42. Petroni ML, Montesi L, Colosimo S, Caletti MT, Mazzotti A, Marchesini G. Combination of GLP‐1 receptor agonists and behavioural treatment in type 2 diabetes elicits synergistic effects on body weight: a retrospective cohort study. Endocrinol Diab Metab. 2019;2:e00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shimizu M, Suzuki K, Kato K, et al. Evaluation of the effects of dapagliflozin, a sodium‐glucose co‐transporter‐2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non‐alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21:285‐292. [DOI] [PubMed] [Google Scholar]
- 44. Kuchay MS, Krishan S, Mishra SK, et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E‐LIFT trial). Diabetes Care. 2018;41:1801‐1808. [DOI] [PubMed] [Google Scholar]
- 45. Cusi K, Bril F, Barb D, et al. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes Metab. 2019;21:812‐821. [DOI] [PubMed] [Google Scholar]
- 46. Wang H, Yang J, Chen X, Qiu F, Li J. Effects of sodium‐glucose cotransporter 2 inhibitor monotherapy on weight changes in patients with type 2 diabetes mellitus: a Bayesian network meta‐analysis. Clin Ther. 2019;41:322‐334. [DOI] [PubMed] [Google Scholar]
- 47. Musso G, Cassader M, Paschetta E, Gambino R. Pioglitazone for advanced fibrosis in nonalcoholic steatohepatitis: new evidence, new challenges. Hepatology. 2017;65:1058‐1061. [DOI] [PubMed] [Google Scholar]
- 48. Chalasani N, Abdelmalek MF, Loomba R, et al. Relationship between three commonly used non‐invasive fibrosis biomarkers and improvement in fibrosis stage in patients with non‐alcoholic steatohepatitis. Liver Int. 2019;39:924‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hagstrom H, Talback M, Andreasson A, Walldius G, Hammar N. Repeated FIB‐4 measurements can help identify individuals at risk of severe liver disease. J Hepatol. 2020;73:1023‐1029. [DOI] [PubMed] [Google Scholar]
- 50. Ratziu V, Friedman SL. Why do so many NASH trials fail? Gastroenterology. 2020. 10.1053/j.gastro.2020.05.046 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


