Abstract
Based on our collective experiences with gap management around immediate dental implants, we have proposed a classification of gap type based on the location in relation to implant periphery. Seven types are proposed, and all but one type should heal without gap grafting provided that flap-less surgery and atraumatic extraction have been achieved. The exception is our Type II gap where the implant has been placed too far buccally leaving a gap only on the lingual/palatal. In this case, the lingual/palatal gap need not be grafted, but the buccal aspect of the implant should best to augmented to avoid the complications.
Key Words: Bone graft, classification, immediate dental implant
INTRODUCTION
The oral cavity has the potential to harbor at least 600 different bacterial species, and surfaces of the teeth can have as many as billion bacteria in the attached bacterial plaque.[1] Periodontitis is an infection of periodontal supporting tissues if not treated can even lead to tooth loss. Dental implants are broadly accepted and greatly predictable management modality in replacing natural teeth.[2] Recently, there is a renewed interest in placing “immediate dental implants” in order to shorten treatment times and improve patient acceptance.[3] While replacing single-rooted teeth with immediate implants have become routine,[4,5,6] the use of immediate IMIs (Immediate Molar Implants) is still not widely practiced because of its difficulty.[5,7] Following IMI placement, there can be significant peri-implant gaps remaining between the implant perimeter and extraction socket walls. These gaps have been termed “jumping distances”[8] and generally have been thought to require some sort of grafting to promote bone fill. In an early investigation, Akimoto et al.[9] studied the impact of gap size in dogs. Following flap elevation, simulated extraction sockets were created by over preparing osteotomies coronally but of appropriate size apically to obtain implant stability. In this way, osteotomy sites were prepared with 0.5 mm, 1.0 mm, and 1.4 mm wide coronal circumferential gaps. After 12 weeks site healing, all gaps appeared clinically to have complete bone fill. However, histological assessment revealed that fibrous connective tissue had developed between newly formed bone and implant surface to variable depths such that the wider the initial gap, the more fibrous tissue. The work had been done with machine-surfaced implants, and the researchers suggested that using moderately rough[10] implants might have given more favorable outcomes. Later, Botticelli et al.[11] proved this point when they published results of a study in dogs comparing machined and roughened implant surfaces as well as submerged and nonsubmerged healing. Gaps of 1.25 mm width were left around all implants, and while no graft materials were used, the gaps were covered with resorbable barrier membranes. Histological assessment after 4 months showed gap bone fill with roughened implants, but once more a fibrous tissue interface with machine-surfaced implants. Following further work by others, the consensus became that gap distances >1.5–2 mm most likely required placement of allograft or xenograft bone particles covered by some sort of membrane to prevent soft-tissue ingrowth.[8,9,12,13] Some investigators even suggested that gaps >0.5 mm should be managed similarly.[14,15] Original techniques favored flap elevation and primary wound closure by flap advancement over immediate implants with or without a nonresorbable barrier applied first,[16,17,18,19] but some authors have now shown that flap-less surgery and nonsubmerged healing after connecting a healing abutment or temporary restoration to the implant may be the preferred approach.[20,21,22,23]
Should the clinician elect to graft wider peri-implant gaps, the recommendation has been that a mineralized, slowly resorbing bone substitute be employed.[24] However, studies have shown that the addition of such materials does delay normal bone healing[8,25] so that if grafting could be avoided, bone healing around immediate implants most likely would be faster and perhaps better. Nevertheless, concern about large gap distances being left ungrafted remains in the minds of many. Recent isolated reports, however, have suggested that gap grafting may in fact not be needed regardless of gap size. For example, Tarnow and Chu[21] reported a case in which a 4.2 mm buccal jumping distance remaining following flap-less placement of a cuspid immediate implant healed with bone fill up to the implant surface (i.e., no fibrous tissue interface had formed) without the use of either grafting or barrier material. Simply adding a healing abutment allowed healing by secondary intention, and the investigators suggested that this was likely dependent on the fact that the procedure was flap-less without interruption of the periosteal blood supply to bone. Other clinicians also have presented the support for the ability of peri-implant gaps to heal under similar conditions following IMI placement in mandible.[26]
Other than the size of jumping distances, little attention has been given to their location and morphology in determining how best to manage them. Indeed, the only classification that could be found in preparing for this paper was that of Schulte[27] who suggested three categories of defect, no-wall, 3-wall, and circumferential. However, in our experiences, gap position in relation to implant perimeter (i.e., buccal, palatal, mesial, distal, or circumferential) can be important, particularly in allowing for the estimation of the future thickness of buccal bone that will remain around the implant. Therefore, our objective in this manuscript is to propose a new classification based upon the gap location in relation to implant periphery. The examples of each gap type from 210 immediate implant cases will be presented.
A PROPOSED CLASSIFICATION OF PERI-IMPLANT GAPS
We propose a classification of peri-implant “jumping distances/gaps” into seven types as follows:
Type I (buccal gap)
The implant has been placed leaving a horizontal gap between the implant and its buccal socket wall. Assuming that the buccal bony wall is intact, this type of gap normally will fill with blood and provided that the surgery was flap-less and the implant surface moderately rough,[8] the clot will be stabilized by the roughened implant surface and the gap will fill with new bone without grafting [Figure 1b].
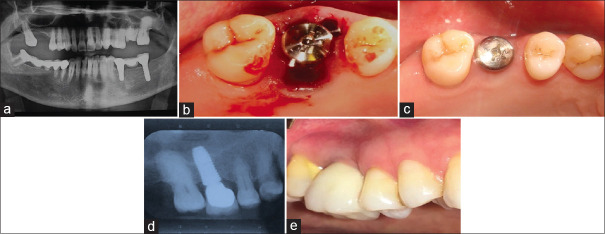
Figure 1.
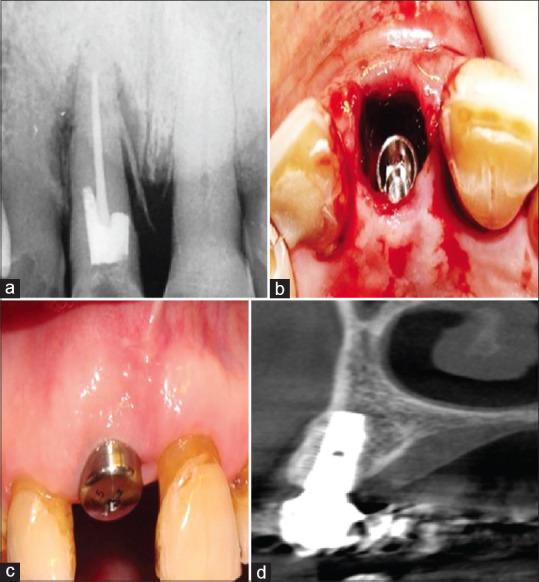
(a) The preoperative radiograph of a hopeless maxillary central incisor. (b) The osteotomy was prepared palatally so that after implant insertion a large buccal gap remained. (c) At 3 months, excellent soft tissue healing was observed. (d) Cone beam computed tomography images after implant restoration showed reformation of a thick buccal plate of bone.
Type II (palatal gap)
The implant has been placed in error too close to the buccal leaving a gap on the palatal aspect only [Figure 2b]. The lack of a corresponding buccal gap most likely will lead to buccal bone loss and implant complications and/or failure unless the remaining buccal bone had been thick (≥3 mm) to begin with.
Figure 2.
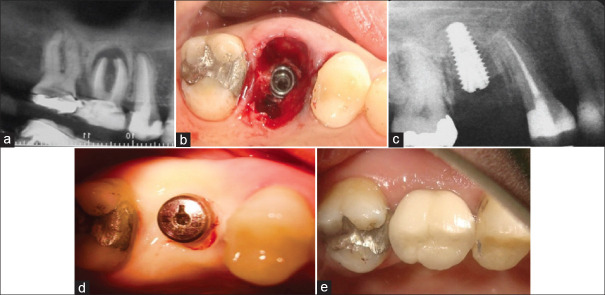
(a) This patient presented with a hopeless maxillary right first molar tooth. (b) While the osteotomy was originally started in the inter-septal bone, in the end the implant ended up too far buccal leaving only a palatal gap. A 6 mm diameter healing abutment was added, but as a flap-less approach was used, no sutures were needed. (c) After 3 months' site healing, it is apparent that the implant had been positioned too far buccally, and while the palatal gap had healed well, there was a loss of buccal bone resulting in a collapse of the buccal ridge anatomy. (d) At the 1-year follow-up visit, excellent bone healing can be seen. (e) This clinical photo taken at 1 year clearly shows that there has been a collapse of the buccal tissues making the implant suspect for the long-term.
Type III (semilunar) gap
After implant placement, a gap exists on two or more aspects, for example, any of buccal, mesial, lingual/palatal, and distal [Figure 3b].
Figure 3.
(a) The patient was missing the left maxillary posterior teeth, while the cuspid was deemed to be nonrestorable. (b) After the cuspid implant placement, a large semilunar gap remained. (c) No grafting was done and the soft tissues loosely sutured around the canine healing abutment. (d) This radiograph was taken at the 1 year follow-up and shows excellent bone healing. (e) A clinical photo corresponding to the radiograph in Figure 3d.
Type IV (buccal and lingual/palatal)
The implant has been successfully placed in the center of the socket leaving both buccal and lingual/palatal gaps [Figure 4b].
Figure 4.
(a) The maxillary right first molar suffered endodontic treatment failure and developed a large periapical lesion that had lifted the sinus floor via periosteal reaction. The inter-septal bone had been left intact coronally. (b) The inter-septal bone was sufficient to stabilize the implant but there remained both buccal and palatal bone dehiscence and large gaps. No grafting was done and the site allowed to heal by secondary intention. (c) A radiograph taken at 2 months showed new bone forming in the nongrafted socket. (d) This photo taken 3 months after implant placement showed excellent soft-tissue healing with minimal alveolar ridge remodeling. (e) The clinical situation 1 year after implant restoration showing excellent buccal tissue contours.
Type V
This type of gap is seen where a maxillary IMI has been placed in the palatal root socket leaving both buccal sockets uninvolved [Figure 5a]. In general, no grafting is required as normal healing of the buccal sockets will occur.
Figure 5.
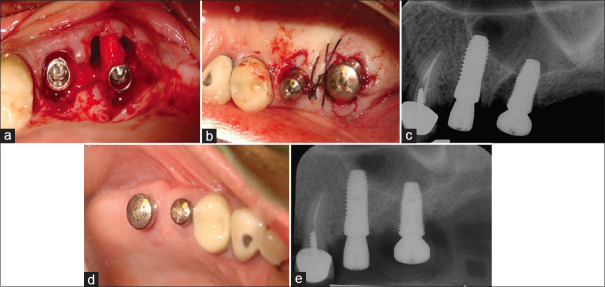
(a) After atraumatic extractions, the clinician opted to place the molar implant in the palatal root socket leaving the two buccal roots un-grafted. (b) Minor flap manipulation was done to rest on and covers the undisturbed inter-septal bone. (c) The immediate postop radiograph shows the large defects left behind by not grafting the two buccal root sockets. (d) Excellent soft tissue healing was documented at 3 months. (e) A radiograph taken after 4 months healing.
Type VI (mesial and distal)
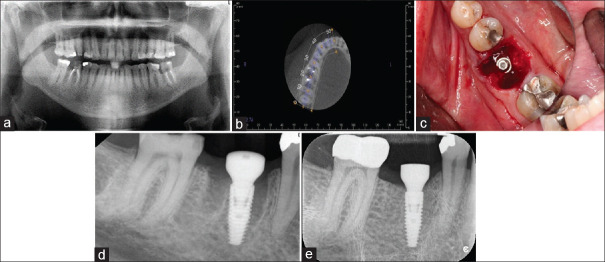
Both mesial and distal gaps exist after implant insertion. This is usually the case at mandibular molar sites where the implant has been successfully stabilized in the inter-septal bone [Figure 6c].
Figure 6.
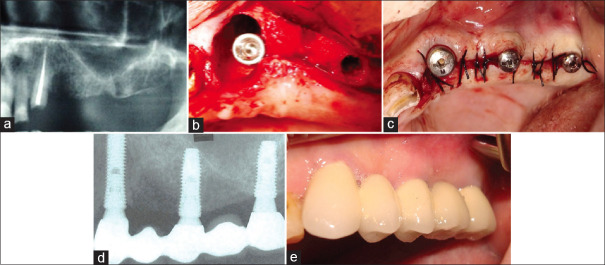
(a) This mandibular right first molar was condemned as nonrestorable. (b) The axial slices of the cone-beam computed tomography scan suggested there to be adequate inter-septal bone to stabilize an IMI. (c) Once the implant had been placed, large gaps remained both mesially and distally. Surgery performed by Dr. Quang Nguyen, Periodontics Resident, Faculty of Dentistry, University of Toronto. (d) The immediate postoperatve radiograph confirms good implant positioning and use of a large diameter healing abutment. (e) A radiograph taken at 4 months revealing excellent bone healing.
Type VII (mesial or distal)
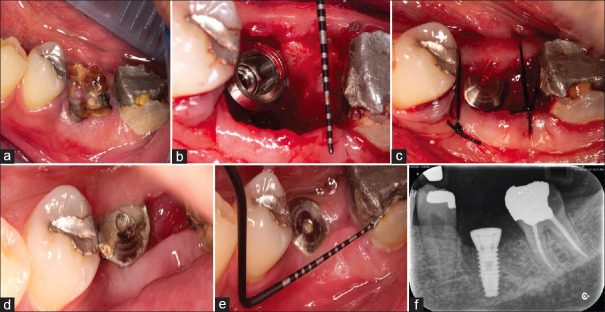
This type of gap remains if a mandibular IMI has been placed in one or other of the existing root sockets [Figure 7b].
Figure 7.
(a) A hopeless mandibular first molar required extraction and the patient wished to have it replaced with an IMI. (b) Following atraumatic extraction and implant placement into the inter-septal bone, a large gap remained distally. Surgery performed by Dr. Armin Khosravi, Periodontics Resident, Faculty of Dentistry, Islamic Azad University, Isfahan Branch, Iran. (c) The gap was left un-grafted and the soft tissues loosely repositioned with two sutures. (d) The healing happening by secondary intention at 10 days postop. (e) Soft-tissue healing of the site at 2.5 months. (f) The radiographic appearance at 2.5 months showing excellent bone fill of the large distal gap.
SAMPLE CASES
Sample case report for type I (buccal gap)
Clinical and radiographic examinations revealed a vertical root fracture of a hopeless maxillary central incisor [Figure 1a]. After flap-less atraumatic extraction, the osteotomy was prepared palatally, and an immediate implant placed toward the palatal left a large buccal gap [Figure 1b]. A healing abutment was added to help shelter the gap, no sutures used, and 3 months later the site had largely healed [Figure 1c]. A cone-beam computed tomography (CBCT) scan of the restored implant site demonstrated excellent new buccal bone formation [Figure 1d]. The total number of cases with buccal gap was 86, in which 99% showed complete bone fill.
Sample case report for Type II (palatal gap)
A hopeless maxillary right first molar [Figure 2a] was atraumatically extracted without raising a flap. Following socket debridement, a 4.5 mm diameter by 12-mm long implant was placed into the inter-septal bone, but ended up unintentionally too far buccal [Figure 2b] leaving no buccal gap but a wide palatal one. No corrective grafting was done, and after placing a large diameter (6 mm) healing abutment the site was left un-sutured. By 3 months, the soft tissues had healed well, but there was obvious collapse of hard and soft tissues buccally [Figure 2c]. A periapical radiograph at 1 year demonstrated satisfactory proximal crestal bone levels [Figure 2d]. However, since the implant had initially been in contact with buccal bone, site remodeling had resulted in collapse of local ridge anatomy with unfavorable esthetics and potential for collecting food debris [Figure 2e]. The total number of cases with palatal gap was 10, in which 76% showed collapse of hard and soft tissue at buccal aspect.
Sample case for Type III (semilunar defect)
The patient had missing of left maxillary bicuspid and molar teeth. Clinical and radiographic examinations revealed the maxillary left cuspid to be un-restorable [Figure 3a]. The tooth was atraumatically extracted using flap-less surgery and a 4 mm diameter by 12 mm long-implant placed immediately with positioning toward the disto-palatal leaving a large semilunar gap affecting the buccal, mesial, and palatal aspects [Figure 3b]. No bone graft or barrier membrane was used, and the flap margins passively sutured with 3-0 silk sutures [Figure 3c]. Two implants were placed more posteriorly to allow the use of a 5-unit fixed implant-supported bridge. At 12 months, a periapical radiograph showed excellent bone healing [Figure 3d], and a clinical photograph confirmed favorable tissue contours [Figure 3e].
The total number of cases with semilunar gap was 8, in which 97% showed complete bone fill.
Sample case for Type IV: (Buccal and palatal)
An example of a Type IV defect is shown in Figure 4. A maxillary right first molar suffered endodontic treatment failure showed a large apical radiolucency of chronic nature. The pathology had been present long enough to put pressure on the sinus floor and cause a periosteal bone reaction [Figure 4a]. Using a flap-less approach, the tooth was removed atraumatically revealing intact inter-septal/furcal bone [Figure 4b]. Following socket debridement, a 12-mm implant was successfully stabilized in the inter-septal bone although dehiscences remained both buccally and palatally. Despite the large gaps, no grafting was done and after placing a healing screw, the site was left to heal without sutures. A radiograph taken at 2 months [Figure 4c] showed the root sockets to be healing well. The implant had been placed without apical grafting, and in this film one can see the layering effect of the new bone forming around the implant apex in the former area of periosteal reaction to the infection. A healing abutment was placed at 3 months after which excellent soft-tissue healing occurred [Figure 4d]. The clinical situation after restoration at the 1-year recall visit showing excellent buccal contours is shown in Figure 4e.
The total number of cases with buccal and palatal gap was 37, in which 98.5 showed complete bone fill.
Sample case for Type V (implant placed in palatal root socket)
A maxillary second bicuspid and first molar were atraumatically extracted without raising a flap and two implants placed. An implant of 4.8 mm diameter and 12 mm length was placed into the palatal root socket of the first molar leaving both buccal root sockets un-grafted [Figure 5a]. Minor flap manipulation was done to rest on and covers the undisturbed inter-septal bone. Healing abutments including one of large diameter for the molar implant were placed, and the soft tissues passively stabilized with sutures [Figure 5b]. A periapical radiograph taken immediately after implant placement demonstrated large gaps left as a result of not grafting the buccal root sockets [Figure 5c]. At 3 months postop, the marginal soft tissues showed a wide band of keratinized tissue [Figure 5d]. A periapical radiograph taken at 4 months demonstrated the gaps to have filled with new bone [Figure 5e].
The total number of cases with mesio-buccal and distobuccal gap were 36, in which 98% showed complete bone fill.
Sample case for Type V1
A nonrestorable mandibular first molar tooth [Figure 6a] was planned for extraction, and a preparative CBCT scan suggested that the tooth had sufficient inter-septal bone to stabilize an IMI [Figure 6b]. Flap-less atraumatic extraction was performed by sectioning the tooth through its furcation and removing each root separately following which a 4.1 mm diameter by 12 mm long implant (Straumann BLT® implant) was placed into the inter-radicular septum such that the top of the implant was approximately 1 mm sub-crestal relative to the buccal bone crest height. The distance from the mesial and distal surfaces of the implant to their respective socket walls was 4–5 mm [Figure 6c]. While particulate graft material was not used, autogenous growth factor-containing fibrin clots prepared from the patient's blood were used to fill the peri-implant gaps following which a large diameter 6 mm long-healing abutment was connected to the implant and left exposed for the initial integration period. A radiograph taken immediately after implant placement showed socket gaps both mesially and distally [Figure 6d]. A radiograph taken at 3 months demonstrated the mesial and distal gaps filled with new bone [Figure 6e].
The total number of cases with mesial and distal gap was 30, in which 99% showed complete bone fill.
Sample case for Type VII
The two roots of a hopeless mandibular left first molar [Figure 7a] were atraumatically extracted following sectioning of the tooth through its furcation. The inter-radicular septum was found to be positioned eccentrically toward the mesial, but the operator was able to use it to develop an osteotomy for a 4.3 mm diameter by 12 mm long implant. The final position of implant was slightly more mesial than ideal leaving a large gap distally [buccolingual >9 mm and mesiodistal >7 mm; Figure 7b] which was left un-grafted following placement of a large diameter healing abutment [Figure 7c]. By 10 days, the gap was seen to be healing by secondary intention [Figure 7d]. A clinical photograph [Figure 7e] and periapical radiograph [Figure 7f] taken at 2.5 months demonstrate good soft-tissue healing and crestal bone levels up to the implant platform.
The total number of cases with mesial or distal gap was 6, in which 98% showed complete bone fill.
DISCUSSION
When immediate implants are used to replace extracted teeth, peri-implant gaps can be anticipated and the clinician needs to know how to manage these gaps most efficiently and economically. We have here proposed a classification of peri-implant gaps based on the location relative to implant perimeters. The examples of each gap type from 210 immediate implant cases are shown and supporting the findings of Smith and Tarnow (28), it is stressed once more that provided that flap-less surgery and atraumatic extraction are performed, generally peri-implant gaps regardless of their size do not need grafting or protection with membranes to ensure their complete bone fill.
The pattern of bone healing following tooth extraction has been well documented. Rapid loss of alveolar ridge height and particularly buccolingual/palatal width normally occurs within the first 3 months postextraction.[28,29] Despite speculations that immediate implant placement would reduce these losses, this has not turned out to be the case.[30,31] Nevertheless, flap-less surgery will slightly reduce buccal bone loss, particularly if the buccal plate thickness after extraction is at least 2 mm, but leaving buccal gaps (Type I) to fill in naturally with new bone also is a prerequisite.[32,33] If these conditions are met, in our collective experiences, it is not necessary to graft most defects. The only situation where some grafting will be needed is with Type II defects, i.e., where only a palatal peri-implant gap is left and the implant is in contact with a thin buccal plate. In this situation, either the implant procedure should be aborted or some form of buccal contour augmentation grafting done to minimize loss of buccal bone. Provided that the overlying soft tissues are thick and well keratinized, this augmentation grafting could be as simple as creating a small buccal pouch and inserting xenograft particles.[34,35] However, in cases where a dehiscence or fenestration of the buccal bone exists or if the buccal bone thickness is <2 mm, making further bone loss likely, should an immediate implant be placed, it will need to be accompanied by some sort of regenerative procedure.[36]
The main conclusion drawn from our experiences with peri-implant gap management is that most gaps will heal naturally without grafting. Others have previously reported single cases of large gaps not needing grafting,[21,26] even without soft tissue closure, but we confirm the recent findings of Smith et al.[37] with 300 immediate molar implants (IMIs) placed over an 11-year period that gap size appears to be irrelevant provided that flap-less surgery and healing by secondary intention are accomplished. Earlier work by Smith and Tarnow[38] had established a classification of molar extraction sockets based on the amounts of inter-septal/furcal bone inter radicular bone (IRB) remaining after extraction. Three types of socket were defined and it was suggested that only Type A sockets (i.e., having sufficient IRS to completely contain the implant) could receive IMIs without the need for gap grafting. In the present paper following our experiences for over 200 immediate implant cases placed without gap grafting, we have proposed a classification of peri-implant gaps into 7 types based on their locations. Our protocol has been to perform flap-less surgery so as not to disturb the periosteal blood supply to bone, to ensure adequate initial implant stability, and to add wide-diameter healing abutments or temporary crowns without suturing of soft tissues. This approach without coronal soft tissue advancement has been proposed to reduce the likelihood for gingival connective tissue reaching the implant surface prior to bone being formed in the granulation tissue arising from initial blood clots formed in the gaps.[39] The other advantage of leaving gingival margins intact and undisturbed by using flap-less surgery and avoiding subsequent flap advancement is an obvious gain in the amount of keratinized tissue during healing.
Questions have risen regarding the possibility of food particles and bacterial contamination affecting gaps when soft tissues are not co-apted tightly around an immediate implant. However, experience indicates that early clot formation with attachment to a moderately rough implant surface will prevent these untoward events provided that the clot is not dislodged. In most instances, placement of a healing abutment wider than the implant platform at the time of immediate implant placement will be all that is needed for clot protection. This approach also will provide some early “nonocclusal loading” which is known to stimulate osteogenesis during osseointegration.[40]
CONCLUSION
When immediate implants are placed, inevitably there will be gaps remaining at one or more locations around the implant periphery. In this paper, we have presented a classification of peri-implant gaps based on their location. Provided that flap-less surgery and atraumatic extraction have been accomplished and either an expanded healing abutment or customized, temporary crown has been connected to the implant, gap location is irrelevant and new bone will fill the gaps without the need for particulate grafting and membrane protection. The exception is what we have called the Type II gap, i.e., where the implant has been placed too close to the buccal plate with a gap remaining lingually/palatally rather than buccally. In this situation, particularly if the buccal bone is thin, complications can be expected unless some form of buccal guided bone regeneration augmentation is included in the procedure.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Saini R, Saini S, Sharma S. Oral sex, oral health and orogenital infections. J Glob Infect Dis. 2010;2:57–62. doi: 10.4103/0974-777X.59252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saini R. Oral biofilm and dental implants: A brief. Natl J Maxillofac Surg. 2011;2:228–9. doi: 10.4103/0975-5950.94490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ketabi M, Deporter D, Atenafu EG. A systematic review of outcomes following immediate molar implant placement based on recently published studies. Clin Implant Dent Relat Res. 2016;18:1084–94. doi: 10.1111/cid.12390. [DOI] [PubMed] [Google Scholar]
- 4.Chen ST, Buser D. Esthetic outcomes following immediate and early implant placement in the anterior maxilla-a systematic review. Int J Oral Maxillofac Implants. 2014;29(Suppl):186–215. doi: 10.11607/jomi.2014suppl.g3.3. [DOI] [PubMed] [Google Scholar]
- 5.Canellas JVDS, Medeiros PJD, Figueredo CMDS, Fischer RG, Ritto FG. Which is the best choice after tooth extraction, immediate implant placement or delayed placement with alveolar ridge preservation.A systematic review and meta-analysis? J Craniomaxillofac Surg. 2019;47:1793–802. doi: 10.1016/j.jcms.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Tarnow DP, Chen SJ. Batavia: Quintessence; 2020. The Single-Tooth Implant: A Minimally Invasive Approach for Anterior and Posterior Extraction Sockets. [Google Scholar]
- 7.Dawson A, Chen ST. Berlin: Quintessence; 2009. The SAC Classification in Implant Dentistry. [Google Scholar]
- 8.Botticelli D, Berglundh T, Buser D, Lindhe J. The jumping distance revisited: An experimental study in the dog. Clin Oral Implants Res. 2003;14:35–42. doi: 10.1034/j.1600-0501.2003.140105.x. [DOI] [PubMed] [Google Scholar]
- 9.Akimoto K, Becker W, Persson R, Baker DA, Rohrer MD, O'Neal RB. Evaluation of titanium implants placed into simulated extraction sockets: A study in dogs. Int J Oral Maxillofac Implants. 1999;14:351–60. [PubMed] [Google Scholar]
- 10.Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1-review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004;17:536–43. [PubMed] [Google Scholar]
- 11.Botticelli D, Berglundh T, Lindhe J. The influence of a biomaterial on the closure of a marginal hard tissue defect adjacent to implants.An experimental study in the dog. Clin Oral Implants Res. 2004;15:285–92. doi: 10.1046/j.1600-0501.2003.01008.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen ST, Wilson TG, Jr , Hämmerle CH.Immediate or early placement of implants following tooth extraction: Review of biologic basis, clinical procedures, and outcomes. Int J Oral Maxillofac Implants. 2004;19(Suppl):12–25. [PubMed] [Google Scholar]
- 13.Fugazzotto PA. Treatment options following single-rooted tooth removal: A literature review and proposed hierarchy of treatment selection. J Periodontol. 2005;76:821–31. doi: 10.1902/jop.2005.76.5.821. [DOI] [PubMed] [Google Scholar]
- 14.Carlsson L, Röstlund T, Albrektsson B, Albrektsson T. Implant fixation improved by close fit.Cylindrical implant-bone interface studied in rabbits. Acta Orthop Scand. 1988;59:272–5. doi: 10.3109/17453678809149361. [DOI] [PubMed] [Google Scholar]
- 15.Knox R, Caudill R, Meffert R. Histologic evaluation of dental endosseous implants placed in surgically created extraction defects. Int J Periodontics Restorative Dent. 1991;11:364–75. [PubMed] [Google Scholar]
- 16.Warrer L, Gotfredsen K, Hjørting-Hansen E, Karring T. Guided tissue regeneration ensures osseointegration of dental implants placed into extraction sockets.An experimental study in monkeys. Clin Oral Implants Res. 1991;2:166–71. doi: 10.1034/j.1600-0501.1991.020402.x. [DOI] [PubMed] [Google Scholar]
- 17.Becker W, Becker BE. Replacement of maxillary and mandibular molars with single endosseous implant restorations: A retrospective study. J Prosthet Dent. 1995;74:51–5. doi: 10.1016/s0022-3913(05)80229-x. [DOI] [PubMed] [Google Scholar]
- 18.Fugazzotto PA. Implant placement at the time of maxillary molar extraction: Treatment protocols and report of results. J Periodontol. 2008;79:216–23. doi: 10.1902/jop.2008.070338. [DOI] [PubMed] [Google Scholar]
- 19.Fugazzotto PA. Implant placement at the time of mandibular molar extraction: Description of technique and preliminary results of 341 cases. J Periodontol. 2008;79:737–47. doi: 10.1902/jop.2008.070293. [DOI] [PubMed] [Google Scholar]
- 20.Kan JY, Rungcharassaeng K, Lozada J. Immediate placement and provisionalization of maxillary anterior single implants: 1-year prospective study. Int J Oral Maxillofac Implants. 2003;18:31–9. [PubMed] [Google Scholar]
- 21.Tarnow DP, Chu SJ. Human histologic verification of osseointegration of an immediate implant placed into a fresh extraction socket with excessive gap distance without primary flap closure, graft, or membrane: A case report. Int J Periodontics Restorative Dent. 2011;31:515–21. [PubMed] [Google Scholar]
- 22.Amato F, Polara G. immediate implant placement in single-tooth molar extraction sockets: A 1- to 6-year retrospective clinical study. Int J Periodontics Restorative Dent. 2018;38:495–501. doi: 10.11607/prd.3179. [DOI] [PubMed] [Google Scholar]
- 23.Bittner N, Planzos L, Volchonok A, Tarnow D, Schulze-Späte U. Evaluation of horizontal and vertical Buccal ridge dimensional changes after immediate implant placement and immediate temporization with and without bone augmentation procedures: Short-term, 1-year results.A randomized controlled clinical trial. Int J Periodontics Restorative Dent. 2020;40:83–93. doi: 10.11607/prd.4152. [DOI] [PubMed] [Google Scholar]
- 24.Hämmerle CH, Araújo MG, Simion M; Osteology Consensus Group 2011. Evidence-based knowledge on the biology and treatment of extraction sockets. Clin Oral Implants Res. 2012;23(Suppl 5):80–2. doi: 10.1111/j.1600-0501.2011.02370.x. [DOI] [PubMed] [Google Scholar]
- 25.Santos FA, Pochapski MT, Martins MC, Zenóbio EG, Spolidoro LC, Marcantonio E., Jr Comparison of biomaterial implants in the dental socket: Histological analysis in dogs. Clin Implant Dent Relat Res. 2010;12:18–25. doi: 10.1111/j.1708-8208.2008.00126.x. [DOI] [PubMed] [Google Scholar]
- 26.Gober DD, Fien MJ. Flapless Extraction Socket Healing Around an Immediate Implant Placed into a Mandibular Molar Site Without the Use of Regenerative Materials: A Case Report. Int J Periodontics Restorative Dent. 2016;36:e26–32. doi: 10.11607/prd.2516. [DOI] [PubMed] [Google Scholar]
- 27.Schulte W. The intraosseous Al2O3 (Frialit) Tuebingen implant.Developmental status after eight years. Quintessence Int. 1984;15:19–35. [Google Scholar]
- 28.Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23:313–23. [PubMed] [Google Scholar]
- 29.Araujo MG, Lindhe J. Dimensional ridge alterations following tooth extraction.An experimental study in the dog. J Clin Periodontol. 2005;32:212–218. doi: 10.1111/j.1600-051X.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 30.Clementini M, Tiravia L, De Risi V, Vittorini Orgeas G, Mannocci A, de Sanctis M. Dimensional changes after immediate implant placement with or without simultaneous regenerative procedures: A systematic review and meta-analysis. J Clin Periodontol. 2015;42:666–77. doi: 10.1111/jcpe.12423. [DOI] [PubMed] [Google Scholar]
- 31.Botticelli D, Berglundh T, Lindhe J. Hard-tissue alterations following immediate implant placement in extraction sites. J Clin Periodontol. 2004;31:820–8. doi: 10.1111/j.1600-051X.2004.00565.x. [DOI] [PubMed] [Google Scholar]
- 32.Ferrus J, Cecchinato D, Pjetursson EB, Lang NP, Sanz M, Lindhe J. Factors influencing ridge alterations following immediate implant placement into extraction sockets. Clin Oral Implants Res. 2010;21:22–9. doi: 10.1111/j.1600-0501.2009.01825.x. [DOI] [PubMed] [Google Scholar]
- 33.Pluemsakunthai W, Le B, Kasugai S. Effect of buccal gap distance on alveolar ridge alteration after immediate implant placement: A microcomputed tomographic and morphometric analysis in dogs. Implant Dent. 2015;24:70–6. doi: 10.1097/ID.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 34.Brugnami F, Caiazzo A. Efficacy evaluation of a new buccal bone plate preservation technique: A pilot study. Int J Periodontics Restorative Dent. 2011;31:67–73. [PubMed] [Google Scholar]
- 35.Birang E, Deporter D, Birang R, Mahabadi M, Atenafu E, Ketabi M. Effectiveness of buccal pouch grafting in minimizing loss of alveolardimension: A canine investigation. Dent Res J. 2019;16:338–45. [PMC free article] [PubMed] [Google Scholar]
- 36.Sicilia-Felechosa A, Pereira-Fernández A, García-Lareu J, Bernardo-González J, Sicilia-Blanco P, Cuesta-Fernández I. Flapless immediate implant placement and provisionalization in periodontal patients: A retrospective consecutive case-series study of single-tooth sites with dehiscence-type osseous defects. Clin Oral Implants Res. 2020;31:229–38. doi: 10.1111/clr.13559. [DOI] [PubMed] [Google Scholar]
- 37.Smith RB, Tarnow DP, Sarnachiaro G. Immediate placement of dental implants in molar extraction sockets: An 11-year retrospective analysis. Compend Contin Educ Dent. 2019;40:166–70. [PubMed] [Google Scholar]
- 38.Smith RB, Tarnow DP. Classification of molar extraction sites for immediate dental implant placement: Technical note. Int J Oral Maxillofac Implants. 2013;28:911–6. doi: 10.11607/jomi.2627. [DOI] [PubMed] [Google Scholar]
- 39.Aukhil I. Biology of wound healing. Periodontol 2000. 2000;22:44–50. doi: 10.1034/j.1600-0757.2000.2220104.x. [DOI] [PubMed] [Google Scholar]
- 40.Sommer M, Zimmermann J, Grize L, Stübinger S. Marginal bone loss one year after implantation: A systematic review of different loading protocols. Int J Oral Maxillofac Surg. 2020;49:121–34. doi: 10.1016/j.ijom.2019.03.965. [DOI] [PubMed] [Google Scholar]