Abstract
Purpose
To assess the impact of lymphovascular invasion on the survival of patients with urothelial carcinoma of the renal pelvis.
Materials and Methods
Patients with urothelial carcinoma of the renal pelvis who underwent radical nephroureterectomy from 2010–2015 were identified in the National Cancer Database. Patients were characterized according to demographic and clinical factors, including pathologic tumor stage and lymphovascular invasion. Associations with overall survival were assessed through proportional hazards regression analysis.
Results
4,177 patients were identified; 1,576 had lymphovascular invasion. Patients with category T3 disease and lymphovascular invasion had 5 year survival that was significantly worse than patients with category T3 disease without lymphovascular invasion (34.7% vs. 52.6, p<0.001 by log-rank test), and approached that of patients with category T4 disease without lymphovascular invasion (34.7% vs. 26.5%, p=0.002). On multivariate analysis controlling for age, comorbidities, grade, nodal status, surgical margin status, race, sex, and chemotherapy administration, patients with T3 disease and lymphovascular invasion were also found to have significantly worse survival than patients with T3 disease without lymphovascular invasion (hazard ratio 1.7, 95% confidence interval 1.4–1.91).
Conclusions
Lymphovascular invasion status is a key prognostic marker that can further stratify the risk of patients with pT3 upper tract urothelial carcinoma. Patients with this pathologic feature should be carefully considered for clinical trials exploring existing and novel therapies.
Keywords: lymphovascular invasion, upper tract urothelial carcinoma, prognosis
Precis:
Patients with pT3 renal pelvis cancer and lymphovascular invasion had significantly worse survival than patients with pT3 disease without lymphovascular invasion, approaching the survival of pT4 patients. Lymphovascular invasion can aid in upper tract urothelial carcinoma risk stratification.
Introduction
The incidence of upper tract urothelial cancer (UTUC) has increased over recent decades, with 1.88 cases per 100,000 person-years in 2005.1 The American Joint Committee on Cancer (AJCC) 7th edition staging system classifies UTUC into categories of worsening prognosis2. Staging is based on the TNM system, with node- and metastasis-negative T1, T2, and T3 tumors assigned stages 1, 2, and 3, respectively; T4 tumors and cases with positive nodes or metastases are assigned stage 4.
The AJCC system was constructed from available evidence that T category is highly prognostic of survival outcomes following surgical intervention.3,4 Due to low disease incidence, evidence to guide staging and prognostication has historically been limited to small retrospective series. However, recent multi-institutional data-sharing collaborations have published results reaffirming the centrality of T category to prognosis with greater statistical power.5–7
Also recently, interest has emerged in lymphovascular invasion (LVI) as a prognostic modulator in UTUC. Several studies have found LVI to be an important predictor of survival outcomes, but again, these have largely been confined to small retrospective series.8,9 Since 2010, the National Cancer Database (NCDB) has collected data on LVI status for all newly diagnosed solid tumors, including renal pelvis cancer. Presently, data is available for cases diagnosed through 2015. The broad nature of the data collected by the NCDB provides an excellent opportunity to reassess the influence of LVI in UTUC and potentially affirm findings from previous studies.
The objective of this study is to determine the impact of LVI on survival in patients with UTUC of the renal pelvis, and to determine whether LVI status might usefully inform the UTUC staging system by improving the prognostic utility of T-categorization.
Materials and Methods
The NCDB is a collaborative clinical registry between the American College of Surgeons’ Commission on Cancer and the American Cancer Society. Approximately 70% of incident cancer cases in the United States from more than 1,500 facilities are included.10
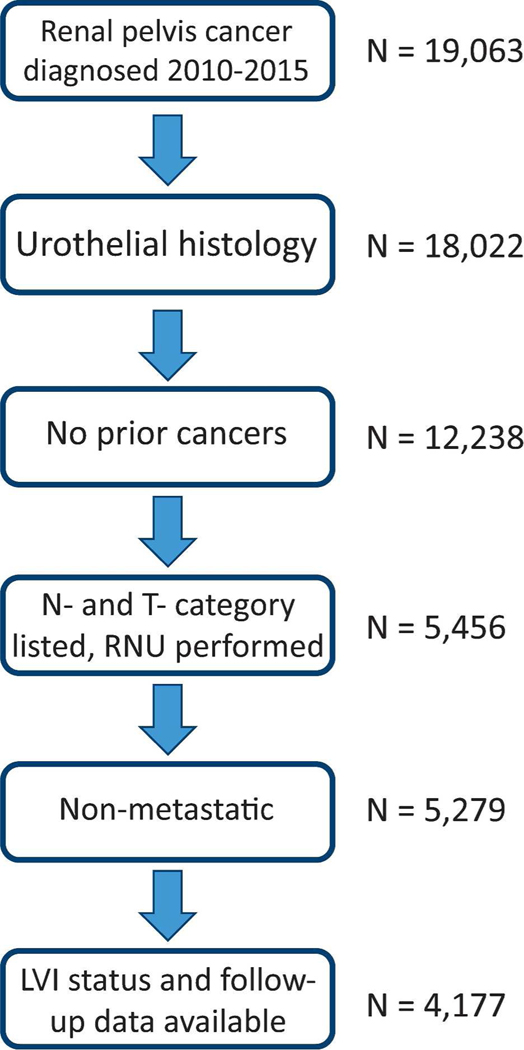
The database was queried for patients 18 years or older diagnosed with renal pelvic cancer from January 1, 2010 to December 31, 2015. Only patients who had undergone radical nephroureterectomy (RNU) were included, both because this is the standard treatment for UTUC of the renal pelvis and because patients undergoing other treatment modalities lacked data on pathologic staging and LVI status in high proportion. Patients with non-urothelial histology or rare urothelial variant histology were excluded; the final cohort had International Classification of Diseases for Oncology – 3 codes 8120 or 8130. Patients were also excluded due to: 1) prior malignancies; 2) known metastatic disease; 3) unknown pathologic T or N category; 4) unknown LVI status; or 5) missing date elements needed to calculate follow up or survival. The selection process is summarized in Figure 1.
Figure 1: Selection Criteria.
Descriptive statistics were calculated. Charlson-Deyo comorbidity scores (CCS) were grouped into categories of 0, 1, or ≥2. A proportional hazards model was used to calculate 2-, 3-, 4-, and 5-year overall survival (OS), stratified by pathological T-category and LVI status. Pathological T-categorization was used because clinical T-categorization was unavailable for a large proportion of patients, and because of the significant upstaging that occurs when clinical and pathological T-categorizations are compared.5,11 Patients listed as having N-category N0 or Nx were both considered to be without known node-positive disease. Age-adjusted survival analysis was then performed using proportional hazards regression and represented graphically in Kaplan-Meier plots. Only node-negative patients were included in both age-adjusted and age-unadjusted univariate survival analyses. Survival differences between T and LVI groups were assessed using log-rank comparisons in unadjusted analyses.
Multivariate proportional hazards survival analysis was then performed, adjusting for demographic and clinical covariates. Pathological T-category and LVI status were combined into a single composite category. Both N0 and N+ patients were included in the multivariate proportional hazards regression model; our intention was to demonstrate that LVI is an important predictor of survival independent of nodal status, and is not merely a surrogate marker for node positivity. Hazard ratios were re-calculated using different T and LVI combinations as reference groups. The model was repeated with subsequent primary malignancies as a covariate (the number, site, and other pathologic and clinical details of subsequent malignancies were unavailable). Finally, a separate model was created including T-category and LVI status as separate variables, rather than a single composite variable. This allowed for the inclusion of an interaction term between LVI status and node positivity, to further assess the effect of LVI on survival independent of its relationship to nodal status.
All p-values are the result of two-sided tests and p-values <0.05 were considered significant. Statistics were performed with SAS v9.4 (Cary, NC).
Results
4,177 patients in 963 different facilities met selection criteria. 1,576 (38%) were LVI positive. Baseline demographics are summarized in Table 1. 522 patients (12%) had known node-positive disease with pathologic N category N1-N3. 372 patients (9%) had positive surgical margins. Chemotherapy, of any intent, was administered during the treatment course of 959 patients (23.0%). Subsequent primary malignancies were seen in 14% of patients, including 13% of patients with pathologic T category T3 (15% of pT3 LVI− patients, and 12% of pT3 LVI+ patients). Among patients alive at last contact, median follow-up time was 922 days (interquartile range 513–1,488 days). Median follow-up time for the entire cohort was 723 days (23.7 months).
Table 1 -.
Clinical and Pathological Characteristics (n=4,177)
| Parameter | Number of patients | Percent of patients |
|---|---|---|
| Year of diagnosis | ||
| 2010 | 681 | 16.3 |
| 2011 | 597 | 14.3 |
| 2012 | 690 | 16.5 |
| 2013 | 730 | 17.5 |
| 2014 | 815 | 19.5 |
| 2015 | 664 | 15.9 |
| Age at diagnosis | ||
| 18–39 | 32 | 0.8 |
| 40–49 | 142 | 3.4 |
| 50–59 | 564 | 13.5 |
| 60–69 | 1,108 | 26.5 |
| 70–79 | 1,384 | 33.1 |
| 80+ | 948 | 22.7 |
| Race | ||
| White (incl. Hispanic) | 3,763 | 90.1 |
| Black | 215 | 5.1 |
| Other/Unknown | 199 | 4.8 |
| Sex | ||
| Male | 2,379 | 56.95 |
| Female | 1,796 | 43.0 |
| Other | 2 | .05 |
| Charlson-Deyo Score | ||
| 0 | 2,854 | 68.3 |
| 1 | 956 | 22.9 |
| 2+ | 367 | 8.8 |
| WHO Grade | ||
| Low | 451 | 10.8 |
| High | 3,495 | 83.7 |
| Unknown | 231 | 5.5 |
| Pathologic T Category | ||
| 1 | 1,183 | 28.3 |
| 2 | 481 | 11.5 |
| 3 | 2,076 | 49.7 |
| 4 | 437 | 10.5 |
| Pathologic N Category | ||
| No known node-positive disease | 3,655 | 87.5 |
| Known node-positive disease | 522 | 12.5 |
| Lymphovascular Invasion | ||
| Present | 1,576 | 37.7 |
| Absent | 2,601 | 62.3 |
| Surgical Margins | ||
| Positive | 372 | 8.9 |
| Negative | 3,754 | 89.9 |
| Unknown | 51 | 1.2 |
| Chemotherapy Administered | ||
| Yes | 959 | 23.0 |
| No | 3,077 | 73.7 |
| Unknown | 141 | 3.4 |
| Status at Last Follow-up | ||
| Deceased | 1,529 | 36.6 |
| Alive | 2,648 | 63.4 |
During follow-up 1,529 (37%) patients died from any cause; 2,648 were alive at last contact. Unadjusted OS for the 3,655 patients without known node-positive disease is shown in Table 2. 95% confidence intervals for OS overlapped for the T1 LVI+ and the T2 LVI− groups, for the T2 LVI+ and the T3 LVI− groups, and for the T3 LVI+ and T4 LVI− groups. 95% confidence intervals did not overlap for the T3 LVI− and T3 LVI+ groups. OS rates were re-calculated in an age-adjusted fashion. These are shown in Supplemental Table S1. Age adjustment did not substantially alter the calculated survival rates and did not affect which groups did or did not have overlapping confidence intervals.
Table 2.
Overall survival in patients without known node-positive disease (n=3,655).
| Cohort | Patients | 2-year OS (%) | 95% CI | 3-year OS (%) | 95% CI | 4-year OS (%) | 95% CI | 5-Year OS (%) | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| T1 LVI− | 1,065 | 92.5 | 90.8–94.2 | 86.5 | 84.0–89.0 | 80.4 | 77.3–83.7 | 74.8 | 70.9–79.0 |
| T1 LVI+ | 105 | 83.5 | 76.4–91.3 | 77.4 | 69.0–87.0 | 73.9 | 64.7–84.4 | 64.8 | 52.8–79.5 |
| T2 LVI− | 349 | 89.3 | 85.8–92.9 | 79.4 | 74.3–84.9 | 70.3 | 63.9–77.4 | 58.9 | 50.9–68.1 |
| T2 LVI+ | 102 | 76.4 | 68.0–85.9 | 59.9 | 49.7–72.1 | 52.2 | 41.5–65.7 | 44.4 | 33.1–59.6 |
| T3 LVI− | 989 | 76.9 | 74.1–79.8 | 67.4 | 64.1–70.9 | 60.9 | 57.2–64.9 | 52.6 | 48.2–57.4 |
| T3 LVI+ | 768 | 56.7 | 53.1–60.6 | 48.3 | 44.4–52.5 | 40.7 | 36.5–45.3 | 34.7 | 30.1–40.0 |
| T4 LVI− | 72 | 35.4 | 25.2–49.7 | 30.5 | 20.5–45.4 | 26.5 | 16.3–43.0 | 26.5 | 16.3–43.0 |
| T4 LVI+ | 205 | 25.5 | 19.9–32.7 | 16.6 | 11.7–23.5 | 12.6 | 8.0–19.7 | 9.1 | 4.8–17.3 |
OS = overall survival. CI = confidence interval. LVI = lymphovascular invasion. Pathologic T categorization is used.
Among pT3 patients without known node positive disease, 8% had positive surgical margins, including 3% of pT3 LVI− patients and 10% of pT3 LVI+ patients. 4-year OS for pT3 LVI− patients was 62.3 months (95% CI: 58.5–66.4) without positive margins and 40.1 months (95% CI 23.5–68.3) with positive margins. 4-year OS for pT3 LVI+ patients was 43.6 months (95% CI 39.1–48.7) without positive margins and 12.5 months (95% CI 5.9–26.8) with positive margins.
Among pT3 LVI− patients, chemotherapy was administered to 4% of patients prior to surgery and 20% of patients after surgery. Among pT3 LVI+ patients, chemotherapy was administered to 4% of patients prior to surgery and 33% of patients after surgery.
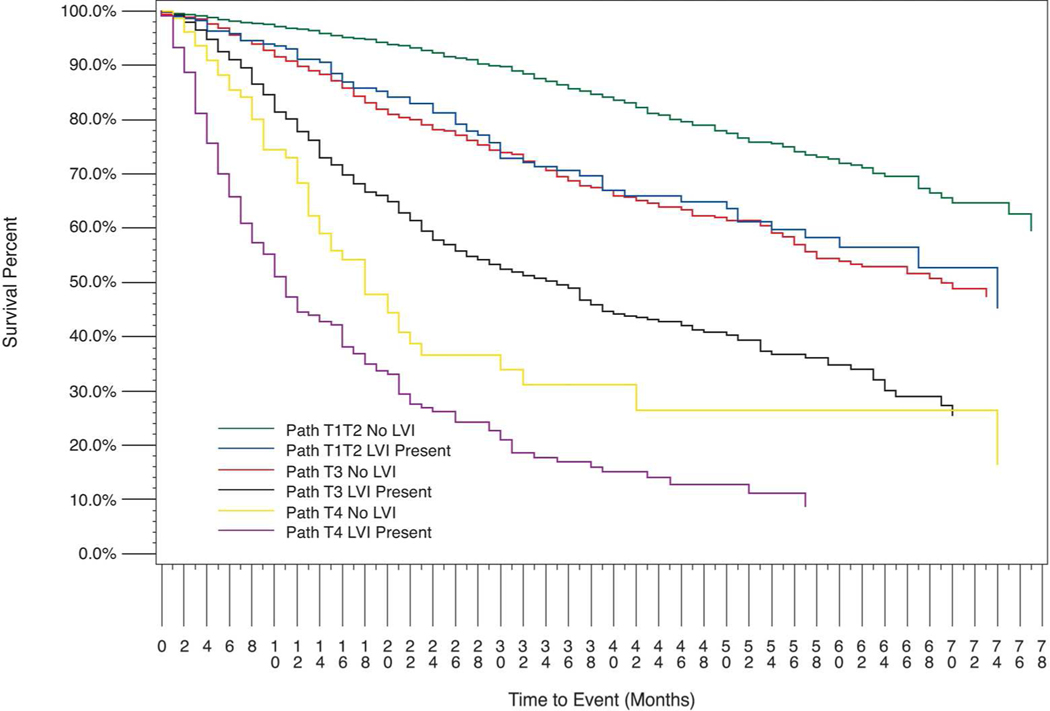
Age adjusted proportional hazards survival for patients without known node-positive disease, stratified by pathological T- category and LVI status, is shown in Figure 2. Log-rank unadjusted comparisons between selected pairs of groups without known node-positive disease are as follows: T1T2 LVI+ vs. T1T2 LVI−, p=<.0001; T1T2 LVI+ vs. T3 LVI−, p=0.6069; T1T2 LVI+ vs. T3 LVI+, p<.0001 T1/T2 LVI+ vs. T3 total (not shown), p<0.0041; T3 LVI+ vs. T3 LVI−, p<0.0001; T3 LVI+ vs. T4 LVI−, p=0.0019; T3 LVI+ vs. T4 LVI+, p<0.0001; and T3 LVI+ vs. T4 total, p<0.0001.
Figure 2: Kaplan-Meier Analysis of Age Adjusted Overall Survival for Patients Without Known Node-positive Disease (n= 3,655).
Number at risk: T1/T2 LVI−, n=1,414; T1/T2 LVI+, n=207; T3 LVI−, n=989; T3 LVI+, n=768; T4 LVI−, n=72; T4 LVI+, n=205
The same graphical survival analysis was performed with T1 and T2 patients separated; this is shown in Supplemental Figure S1. The T1 and T2 groups are also graphed separately for greater visual clarity; this is included as Figure S2. The following additional log-rank comparisons were performed to further elucidate the relative impact of categories T1 and T2: T1 LVI+ vs. T1 LVI−, p=0.0132; T1 LVI+ vs. T2 LVI−, p=0.9000; T2 LVI+ vs. T2 LVI−, p=0.0007; T2 LVI+ vs. T3 LVI−, p=0.2229. Table 3 shows risk adjusted hazard ratios (HRs) for the effect of LVI and pathological T- category on OS, controlling for demographic and pathological cofactors in a multivariate proportional hazards model. Substantially overlapping confidence intervals were observed for T1/T2 LVI+ and T3 LVI−, and also for T3 LVI+ and T4 LVI−. The model was also calculated with subsequent primary malignancy included as a cofactor. Subsequent primary malignancy did not affect survival (p=0.45), and we opted to omit this variable from our primary reported model due to the lack of specific detail available as previously noted. The analysis was also repeated with T1 and T2 patients considered separately (Supplemental Table S2). Confidence intervals for T2 LVI+ and T3 LVI− overlapped substantially, and confidence intervals for T1 LVI+ and T3 LVI− overlapped as well.
Table 3.
Effect of Pathological T-category and Lymphovascular Invasion on Overall Survival (n=4,177)
| Group | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| T1/T2 LVI− | referent | referent | |
| T1/T2 LVI+ | 1.8 | 1.4–2.3 | <0.001 |
| T3 LVI− | 2.1 | 1.8–2.5 | <0.001 |
| T3 LVI+ | 3.7 | 3.1–4.3 | <0.001 |
| T4 LVI− | 5.3 | 4.0–7.1 | <0.001 |
| T4 LVI+ | 7.1 | 5.8–8.7 | <0.001 |
Multivariate competing-risks regression analysis adjusted for age, sex, race, Charlson–Deyo score, WHO grade, pathological N category, surgical margin status, and chemotherapy administration. Cofactors with statistically significant independent effects included (hazard ratios, 95% confidence intervals in parentheses): older age (continuous) (1.03, 1.02–1.04), Charlson–Deyo score ≥2 (1.5, 1.3–1.8), pathologic N+ (1.5, 1.3–1.7), high grade (1.9, 1.5–2.4), unknown grade (2.0, 1.4–2.7), positive surgical margins (2.0, 1.8–2.4), unknown surgical margins (1.5, 1.0–2.2), and chemotherapy administered (0.8, 0.7–0.9).
When T3 LVI− was set as the statistical referent, a direct comparison of T3 LVI+ vs. T3 LVI− yielded a HR of 1.7 (95% CI: 1.5–2.0). T1T2 LVI+ vs. T3 LVI− yielded a HR of 0.9 (95% CI: 0.7–1.1). Finally, when T3 LVI+ was set as the statistical referent, a direct comparison of T4 LVI− vs. T3 LVI+ yielded a HR of 1.4 (95% CI: 1.0–1.9).
An additional multivariate competing-risks survival model was created to assess the interaction between LVI and pathologic node status. In this model T category and LVI were entered as independent variables, rather than being combined into a composite variable as in Figure 2 and Tables 2 and 3. T-category, nodal status, and LVI status were all independent predictors of survival (p<0.001, for each). Category T3 vs. T1/T2 carried an HR of 2.1 (95% CI 1.8–2.5). Category T4 vs. T1/T2 carried an HR of 5.3 (95% CI 4.0–7.1–5.4). The interaction between LVI and node status was statistically significant (p=0.003). Table 4 shows the calculated HRs.
Table 4.
Effect of Interaction between Pathological N-category and Lymphovascular Invasion on Overall Survival (n=4,177).
| N0 | N+ | |||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| LVI | 1.8 | 1.6–2.0 | 1.1 | 0.8–1.5 |
| LVI− | LVI + | |||
| HR | 95% CI | HR | 95% CI | |
| Node positivity | 2.2 | 1.6–2.9 | 1.3 | 1.1–1.6 |
Multivariate competing-risks regression analysis adjusted for age, sex, race, Charlson-Deyo score, WHO grade, pathological T category, surgical margin status, and chemotherapy administration. Cofactors with statistically significant independent effects included (hazard ratios, 95% confidence intervals in parentheses): older age (continuous) (1.03, 1.02–1.04), high grade (1.9, 1.5–2.4), unknown grade (2.0, 1.4–2.7); Charlson–Deyo score ≥2 (1.5, 1.3–1.8), positive surgical margins (HR 2.1, 1.8–2.4), and chemotherapy administered (0.8, 0.7–0.9).
Discussion
In the current study, LVI was a key predictor of poor prognosis in patients with UTUC of the renal pelvis treated with RNU. Patients with pT3, LVI+ tumors represented a particularly aggressive category of disease, with survival that was substantially worse than pT3 LVI− patients and which approached that of pT4 LVI− patients. Survival differences between pT3 LVI− and pT3 LVI+ groups were both clinically meaningful (with an 18% 5-year OS difference) and statistically robust owing to the large sample size.
There is a known interaction between LVI and node positivity.12 In our multivariate competing-risks regression analysis, LVI maintained significance when controlling for node status. Additionally, the interaction term between LVI and node status was statistically significant when added to the model, indicating synergism of risk beyond the additive effect of the two variables. Both findings suggest that LVI has an independent impact on survival, distinct from its relationship with nodal status. Our data does suggest that the impact of LVI is substantially greater in patients without known node-positive disease, which is consistent with prior evidence.9,12,13 This finding is rational given the significance of LVI as an early step in tumor dissemination; it is logical that the impact of LVI would be diminished when overt nodal spread is present.
We believe this data, particularly the strong effect of LVI within the pT3 category, has implications for pathological staging. While a reorganization of risk stratification schema to merge pT3 LVI+ patients with pT4 patients may be premature, a subcategorization of the AJCC pT3 category into two categories based on the presence of LVI may be reasonable. While our data also suggests a role for LVI in sub-stratifying other T categories besides T3, the relatively small number of patients in the T1/T2 and T4 subgroups limited analytic power and limits the strength of the conclusions which can be drawn from our data. However, it is notable that patients with category pT1/T2, LVI+ disease had significantly worse prognosis than their pT1/T2, LVI− counterparts. Furthermore, neither pT1/T2 LVI+ patients nor pT2 LVI+ patients had survival that differed significantly from that of pT3 LVI− patients on proportional hazards survival analysis. The relatively smaller sample sizes in these subgroups preclude overly strong conclusions; for this reason we grouped pT1 and pT2 together in our main analyses and included sub-analyses with pT1 and pT2 separated in our supplemental material. Despite these caveats the results are provocative, and further investigation into the clinical behavior of these subgroups is warranted, as is consideration of a role for LVI in the selection of patients for adjuvant systemic therapy.
LVI is known to be of prognostic significance in bladder urothelial cell carcinoma.14 In a multi-center review of 750 patients, LVI predicted local and distant recurrence, OS, and cause-specific survival after cystectomy.13 In a 2013 meta-analysis of 21 studies, LVI predicted recurrence free-, overall-, and cancer-specific survival following cystectomy.14 This relationship is seen in non-urothelial cell solid organ tumors as well.15,16
While prior studies have investigated the prognostic significance of LVI in UTUC, the low incidence of UTUC has limited study quality; most analyses are small, retrospective, single-center series. Thus, in 2009 the Upper Tract Urothelial Carcinoma Collaboration combined data from 8 countries to produce a cohort of 1,453 patients undergoing RNU for UTUC.9 LVI was present in 24% of patients and was associated with lower 5-year recurrence-free survival (77% vs. 44%) and cancer-specific survival (79% vs. 47%). Another multicenter study from 2010 combined data from six countries to produce a cohort of 762 different patients undergoing RNU for UTUC. LVI was present in 19.4% of patients and was associated with lower 5-year recurrence-free survival (79.3% vs. 45.1%) and cancer-specific survival (82.1% vs. 45.8%). That group included LVI in a nomogram predicting recurrence and survival after RNU.17 Other large collaborative studies and a nearly 5000- patient meta-analysis further support the importance of LVI in predicting survival in UTUC.6,18
These findings have led investigators to suggest utilizing LVI status to risk stratify post-RNU UTUC patients. Godfrey et al. found LVI to predict worse survival in their cohort of 211 patients, and noted similar survival between patients with T1 or less, LVI+ disease and patients with muscle-invasive, LVI− disease.8 The authors suggested consideration of including LVI in the TNM system for UTUC pending larger studies. Data from the current study, emanating from a broadly inclusive national database, further supports sub-stratification of the UTUC TNM system using LVI status.
Developments in pathologic staging and post-operative risk stratification in oncology have important implications for selecting patients for receipt of adjuvant therapy or participation in clinical trials. In UTUC, there has been mixed evidence supporting adjuvant chemotherapy in patients with high risk (Stage III-IV) tumors.2,19–22 However, when only cisplatin-based regimens are considered, disease-free and overall survival may be improved,23 as seen in bladder UCC.24 The POUT trial, a randomized controlled trial of adjuvant chemotherapy vs. surveillance after RNU for UTUC, opened in 2012.25 Historically, neoadjuvant therapies for UTUC have received greater emphasis than adjuvant therapies, due to the limitation on cisplatin administration to patients with reduced renal function following RNU.26 However, novel non-platinum agents such as checkpoint inhibitors and immunotherapies being tested in UCC may expand our ability to deliver adjuvant treatment to high-risk patients.27–29 This would elevate the utility of enhanced risk stratification using variables such as LVI.
Strengths of the present study include its large sample size and generalizability, capturing a majority of incident cases of UTUC in the United States during the study period, including cases managed at community centers. Notably, many prior studies represent institutional cohorts from tertiary referral centers, with an inherent risk of selection bias. The prospective nature of the data collection, and the NCDB’s rigorous and standardized methodology for data collection, ensures robust data quality and reduces the potential for measurement bias.
Limitations include limited follow-up duration; however, prior studies suggest that a large proportion of UTUC mortality occurs early in the disease course. Margulis et al. found a median time to cancer-specific mortality of 18.5 months.5 Under-representation of certain sub-groups is discussed above. No centralized pathologic re-review to verify LVI status was performed; however, we contend that this reflects real world practice. Incomplete availability of data on specific patient and pathologic factors resulted in a diminished cohort size and a less extensive set of covariates for analytical models which ultimately included age, sex, race, CCS, WHO grade, pathological N- category, surgical margin status, and chemotherapy administration. Specific pathologic features contributing to pT categorization, such as renal parenchyma invasion versus peripelvic fat involvement in pT3 patients, were unavailable, as was data on tumor multifocality. Specifics of the location and nature of subsequent malignancies were unavailable, and we could not determine their clinical significance, or if they represented unrelated malignancies versus bladder or contralateral upper tract recurrences.
The cohort is in some ways unrepresentative of the US population, though not dissimilar from previously reported populations with UTUC; it is 90% White (including Hispanic) and almost 23% is 80 years of age or older. As noted, age adjustment did not meaningfully alter survival rates.
We were also limited in our ability to assess the impact of chemotherapy within this cohort for two reasons. The first is a low utilization rate of chemotherapy, which is reflective of real-world clinical practice.30 The second is insufficient data capture, as the NCDB identifies the timing of chemotherapy but lacks sufficient detail to accurately determine therapeutic intent.
A persistent difficulty in studying UTUC is the large proportion of pNx patients due to low rates of lymphadenectomy during RNU; well below 50% in the United States and Europe.31–33 We elected to categorize pN0 and pNx patients as those without known node-positive disease, in contrast to pN1-pN3 patients who we identified as node-positive, since the alternative of excluding pNx patients would artificially enrich the cohort for node-positive patients and distort the data.
Conclusion
In this study LVI was able to risk-stratify patients following RNU for UTUC of the renal pelvis and identify those at highest risk of death. LVI therefore represents a key factor in the selection of optimal candidates for adjuvant treatment, clinical trials, or heightened surveillance. Although the survival benefits of adjuvant chemotherapy for UTUC are incompletely proven, many novel agents are in development, with demonstrated activity in the metastatic setting and potential effectiveness in the adjuvant setting as well.27–29 In the future a wide pool of effective and tolerable adjuvant therapy options will render a thorough understanding of each patient’s risk profile more valuable. Pathological features such as LVI may be important contributors to that risk stratification.
Supplementary Material
Footnotes
Conflicts: Per disclosures.
Publisher's Disclaimer: This is the author manuscript accepted for publication and has undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of record. Please cite this article as doi:10.1002/cncr.31372.
References
- 1).Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int. 2011;107(7):1059–64. [DOI] [PubMed] [Google Scholar]
- 2).Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual (7th ed). New York, NY: Springer; 2010. [Google Scholar]
- 3).Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52(4):594–601. [DOI] [PubMed] [Google Scholar]
- 4).Novara G, De marco V, Gottardo F, et al. Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract: multi-institutional dataset from 3 European centers. Cancer. 2007;110(8):1715–22. [DOI] [PubMed] [Google Scholar]
- 5).Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115(6):1224–33. [DOI] [PubMed] [Google Scholar]
- 6).Rouprêt M, Hupertan V, Seisen T, et al. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. J Urol. 2013;189(5):1662–9. [DOI] [PubMed] [Google Scholar]
- 7).Fradet V, Mauermann J, Kassouf W, et al. Risk factors for bladder cancer recurrence after nephroureterectomy for upper tract urothelial tumors: results from the Canadian Upper Tract Collaboration. Urol Oncol. 2014;32(6):839–45. [DOI] [PubMed] [Google Scholar]
- 8).Godfrey MS, Badalato GM, Hruby GW, Razmjoo M, Mckiernan JM. Prognostic indicators for upper tract urothelial carcinoma after radical nephroureterectomy: the impact of lymphovascular invasion. BJU Int. 2012;110(6):798–803. [DOI] [PubMed] [Google Scholar]
- 9).Kikuchi E, Margulis V, Karakiewicz PI, et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol. 2009;27(4):612–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).About the National Cancer Database. Retrieved October 02, 2016, from https://www.facs.org/qualityprograms/cancer/ncdb/about
- 11).Margulis V, Youssef RF, Karakiewicz PI, et al. Preoperative multivariable prognostic model for prediction of nonorgan confined urothelial carcinoma of the upper urinary tract. J Urol. 2010;184(2):453–8. [DOI] [PubMed] [Google Scholar]
- 12).Novara G, Matsumoto K, Kassouf W, et al. Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: an international validation study. Eur Urol. 2010;57(6):1064–71. [DOI] [PubMed] [Google Scholar]
- 13).Lotan Y, Gupta A, Shariat SF, et al. Lymphovascular invasion is independently associated with overall survival, cause-specific survival, and local and distant recurrence in patients with negative lymph nodes at radical cystectomy. J Clin Oncol. 2005;23(27):6533–9. [DOI] [PubMed] [Google Scholar]
- 14).Kim H, Kim M, Kwak C, Kim HH, Ku JH. Prognostic significance of lymphovascular invasion in radical cystectomy on patients with bladder cancer: a systematic review and meta-analysis. PLoS ONE. 2014;9(2):e89259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Dicken BJ, Saunders LD, Jhangri GS, et al. Gastric cancer: establishing predictors of biologic behavior with use of population-based data. Ann Surg Oncol. 2004;11(6):629–35. [DOI] [PubMed] [Google Scholar]
- 16).Woo CS, Silberman H, Nakamura SK, et al. Lymph node status combined with lymphovascular invasion creates a more powerful tool for predicting outcome in patients with invasive breast cancer. Am J Surg. 2002;184(4):337–40. [DOI] [PubMed] [Google Scholar]
- 17).Cha EK, Shariat SF, Kormaksson M, et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol. 2012;61(4):818–25. [DOI] [PubMed] [Google Scholar]
- 18).Ku JH, Byun SS, Jeong H, Kwak C, Kim HH, Lee SE. Lymphovascular invasion as a prognostic factor in the upper urinary tract urothelial carcinoma: a systematic review and meta-analysis. Eur J Cancer. 2013;49(12):2665–80. [DOI] [PubMed] [Google Scholar]
- 19).Bamias A, Deliveliotis Ch, Fountzilas G, et al. Adjuvant chemotherapy with paclitaxel and carboplatin in patients with advanced carcinoma of the upper urinary tract: a study by the Hellenic Cooperative Oncology Group. J Clin Oncol. 2004;22(11):2150–4. [DOI] [PubMed] [Google Scholar]
- 20).Huang YC, Chen MF, Shi CS, et al. The Efficacy of Postoperative Adjuvant Chemotherapy for Patients with pT3N0M0 Upper Tract Urothelial Carcinoma. J Urol. 2015;194(2):323–9. [DOI] [PubMed] [Google Scholar]
- 21).Vassilakopoulou M, De la motte rouge T, Colin P, et al. Outcomes after adjuvant chemotherapy in the treatment of high-risk urothelial carcinoma of the upper urinary tract (UUT-UC): results from a large multicenter collaborative study. Cancer. 2011;117(24):5500–8. [DOI] [PubMed] [Google Scholar]
- 22).Hellenthal NJ, Shariat SF, Margulis V, et al. Adjuvant chemotherapy for high risk upper tract urothelial carcinoma: results from the Upper Tract Urothelial Carcinoma Collaboration. J Urol. 2009;182(3):900–6. [DOI] [PubMed] [Google Scholar]
- 23).Leow JJ, Martin-doyle W, Fay AP, Choueiri TK, Chang SL, Bellmunt J. A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol. 2014;66(3):529–41. [DOI] [PubMed] [Google Scholar]
- 24).Leow JJ, Martin-doyle W, Rajagopal PS, et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66(1):42–54. [DOI] [PubMed] [Google Scholar]
- 25).Birtle AJ, Lewis R, Johnson M, Hall E. Time to define an international standard of postoperative care for resected upper urinary tract transitional cell carcinoma (TCC) - opening of the peri-operative chemotherapy versus surveillance in upper tract urothelial cancer (POUT) Trial. BJU Int. 2012;110(7):919–21. [DOI] [PubMed] [Google Scholar]
- 26).Kaag MG, O’malley RL, O’malley P, et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol. 2010;58(4):581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–62. [DOI] [PubMed] [Google Scholar]
- 28).Colombel M, Heidenreich A, Martinez-Pineiro L, et al. Perioperative chemotherapy in muscle-invasive bladder cancer: overview and the unmet clinical need for alternative adjuvant therapy as studied in the MAGNOLIA trial. Eur Urol. 2014;65(3):509–11. [DOI] [PubMed] [Google Scholar]
- 29).Bellmunt J, Fougeray R, Rosenberg JE, et al. Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapy. Ann Oncol. 2013;24(6):1466–72. [DOI] [PubMed] [Google Scholar]
- 30).Reardon ZD, Patel SG, Zaid HB, et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: a sign of changing tides. Eur Urol. 2015;67(1):165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Moschini M, Foerster B, Abufaraj M, et al. Trends of lymphadenectomy in upper tract urothelial carcinoma (UTUC) patients treated with radical nephroureterectomy. World J Urol. 2017;35(10):1541–1547. [DOI] [PubMed] [Google Scholar]
- 32).Zareba P, Rosenzweig B, Winer AG, Coleman JA. Association between lymph node yield and survival among patients undergoing radical nephroureterectomy for urothelial carcinoma of the upper tract. Cancer. 2017;123(10):1741–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Pearce SM, Pariser JJ, Patel SG, Steinberg GD, Shalhav AL, Smith ND. The effect of surgical approach on performance of lymphadenectomy and perioperative morbidity for radical nephroureterectomy. Urol Oncol. 2016;34(3):121.e15–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.