Abstract
Cytomegalovirus (CMV) viremia occurs in 40% to 80% of CMV-seropositive (R+) recipients of allogeneic hematopoietic cell transplantation (HCT). The preemptive therapy (PET) strategy has reduced the risk of CMV end-organ disease (EOD) and associated mortality but may lead to substantial healthcare resource utilization (HCRU) and costs. Real-world data on the economic impact of PET is relevant for the evaluation of alternative strategies for CMV management. We examined the impact of clinically significant CMV treated with PET on inpatient length of stay (LOS), number of readmissions, and associated costs from day 0 through day 180 post-HCT.
This was a retrospective study of R+ adults who underwent peripheral blood or marrow allogeneic HCT at Memorial Sloan Kettering Cancer Center between March 2013 and December 2017. Patients were routinely screened for CMV by qPCR and received PET according to institutional standards of care. Data were extracted from electronic medical records and hospital databases. Itemized cost data per patient were obtained from the Vizient database, adjusted to 2017 dollars using inflation indices. Study outcomes included HCRU evaluated by inpatient LOS and inpatient cost in patients who received PET for clinically significant CMV (PET group) compared with those who did not receive PET (no PET group) and the frequency and cost of CMV-related readmissions compared with non CMV-related readmissions. We used generalized linear models to examine the incremental HCRU and costs associated with PET controlling for other potential factors. Of 357 patients, PET was initiated in 208 (58.3%), at a median of 35 days after HCT. By day 180, 23 patients (6.4%) had developed CMV EOD and 3 (.8%) had died of CMV. Compared with the no PET group, the PET group had a longer LOS for HCT admission (P = .0276), longer total LOS by day 180 (P = .0001), a higher number of readmissions (P = .0001), a higher mean inpatient cost for HCT admission ($189,389 versus $151,646; P = .0133), and a higher total inpatient cost ($297,563 versus $205,815; P < .0001). Among PET recipients, CMV-related readmissions were associated with higher mean cost per episode compared with non CMV-related readmissions ($165,455 versus $89,419; P = .005). CMV-related readmissions comprised 40.6% of total all-cause readmissions and incurred 55.9% of total all-cause readmission costs in PET recipients. Our data show that patients treated with currently available PET had greater inpatient HCRU and cost, by day 180 compared with patients who did not receive PET. The cost of CMV-related readmissions accounted for 56% of total readmission cost among PET recipients. Future studies are needed to examine the cost-effectiveness of alternative strategies for CMV management.
Keywords: Hematopoietic cell, transplantation, Cytomegalovirus, Preemptive therapy, Readmission, Healthcare resource utilization, Inpatient cost
INTRODUCTION
Cytomegalovirus (CMV) infection is the most common clinically significant viral infection among CMV seropositive (R+) recipients of allogeneic hematopoietic cell transplantation (HCT) [1]. CMV is associated with substantial morbidity and mortality, particularly in recipients of T cell-depleted (TCD) allografts and human leukocyte antigen (HLA)-mismatched or unrelated donor allografts [2–4]. The preemptive therapy (PET) approach is broadly used for CMV management [1]. In this approach, HCT recipients are routinely monitored for CMV post-HCT, and antiviral therapy is initiated on detection of CMV infection. The use of sensitive molecular assays for CMV coupled with PET have contributed to low rates of CMV end-organ disease (EOD) and associated mortality [5]. On the other hand, the administration of (val)ganciclovir or foscarnet for PET often requires or prolongs hospitalization for i.v. infusions, safety monitoring, and/or management of myelosuppression or nephrotoxicity [6–8].
In TCD HCT recipients, CMV infection has been correlated with increased readmissions and prolonged hospital length of stay (LOS) [9]. Jain et al [10] reported that CMV infection was correlated with increased healthcare cost in HCT recipients, largely due to hospitalization. Quantitative data detailing the impact of CMV managed by PET on health care resource utilization (HCRU) after HCT are limited. Here we analyzed HCRU in a cohort of adult CMV R+ recipients in a major cancer center in New York City. The aims of our study were to (1) compare the LOS and inpatient costs among patients who received and did not receive PET for clinically significant CMV infection; (2) assess the impact of PET on HCRU in multivariable models; and (3) estimate the cost of CMV-related hospitalizations by day 180 post-HCT.
METHODS
Study Population
The study cohort consisted of adult CMV R+ recipients of first peripheral blood or bone marrow allograft at Memorial Sloan Kettering Cancer Center (MSKCC) between March 2013 and December 2017. HCT recipients who died in the first 30 days post-HCT, participated in clinical trials of CMV prevention (eg, brincidofovir, letermovir); received a cord blood allograft, or received a CMV antiviral (ie, (val)ganciclovir or foscarnet) before PET initiation for a non-CMV indication (eg, human herpesvirus 6 or resistant herpes simplex virus) were excluded from the analyses. Weekly monitoring by CMV qPCR was performed starting on day 14 and continued through day 180 post-HCT. Patients were followed up until day 180 post-HCT or death, whichever occurred first. Patients with high risk (HR) CMV included recipients of conventional HCT from a mismatched or haploidentical donor, and recipients of TCD HCT regardless of donor HLA match. Low risk (LR) CMV included conventional HCT from matched related donors. Data was extracted from the electronic medical record and hospital databases and linked through the Vizient database to identify the inpatient cost data.
We defined 2 study groups. The PET group included all patients who had clinically significant CMV viremia and received PET with (val)ganciclovir or foscarnet for at least 3 consecutive days by day 100 post-HCT. The remaining patients were included in the no PET group.
HCT Protocols and Supportive Care
Graft manipulation and conditioning regimens were provided in accordance with institutional standard of care and have been described previously [11,12]. In brief, patients with acute leukemia in first complete remission and patients with myelodysplastic syndrome underwent ex vivo TCD/CD34-selected HCT unless deemed ineligible or refused by insurance. TCD was performed with the CliniMACS CD34+ reagent system (Miltenyi Biotec, Gladbach, Germany). Patients not eligible for TCD received unmodified HCT after reduced-intensity conditioning with low-dose total body irradiation or busulfan and fludarabine. Recipients of unmodified HCT received graft-versus-host disease (GVHD) prophylaxis, including tacrolimus/sirolimus plus mycophenolate mofetil with or without methotrexate [13] or post-HCT cyclophosphamide for recipients of haploidentical donor allografts [14]. Bacterial and fungal prophylaxis was administered as described previously [15,16]. All patients received acyclovir prophylaxis for herpes simplex virus and varicella zoster virus in accordance with institutional standards of care [17].
Management of CMV
CMV was monitored by a CMV qPCR assay in plasma (COBAS AmpliPrep/COBAS TaqMan; Roche Molecular Systems, Branchburg Township, NJ), performed at the clinical microbiology laboratory at MSKCC [18]. The lower limit of quantification and linear range was >137 to 9.1 £ 106 IU/mL. PET was initiated according to the MSKCC standard of care. In general, thresholds for PET initiation were at least 2 consecutive viral loads >300 IU/mL for LR patients and at least 2 consecutive positive PCR findings at any level for HR patients. PET consisted of i.v. ganciclovir, valganciclovir, and/or foscarnet. (Val)ganciclovir was the preferred first-line therapy. Foscarnet was generally used in patients with cytopenias (particularly before engraftment) or other contraindications to (val)ganciclovir [8]. PET was initiated with induction doses (valganciclovir 900 mg p.o. every 12 hours, ganciclovir 5 mg/kg i.v. every 12 hours or foscarnet 90 mg/kg i.v. every 12 hours) adjusted for renal function as indicated. Induction was typically given for 2 weeks or until the CMV viral load was <300 IU/mL on at least 2 consecutive measurements. Maintenance with valganciclovir 900 mg p.o. every 24 hours, ganciclovir 5 mg/kg i.v. every 24 hours, or foscarnet 90 mg/kg i.v. every 24 hours) was administered to patients at high risk for recurrence, including recipients of TCD or mismatched allografts and those with GVHD, with duration based on tolerability and immune reconstitution.
CMV Outcomes
Clinically significant CMV infection was defined as any CMV viremia prompting initiation of PET by the treating physician. CMV EOD was scored by standard criteria where both the presence of signs or clinical symptoms and CMV DNA in a relevant organ are required to definitively diagnose CMV EOD [19].
HCRU
LOS measures consisted of hospital LOS for HCT (index admission) and LOS for readmissions through day 180. Readmission was defined as any admission with LOS >48 hours occurring after discharge from the index hospitalization. The number and proportion of individuals with at least 1 readmission were reported. Readmission LOS was calculated as the sum of the LOS for all readmissions for each patient.
To compute the index admission cost and readmission cost for each patient, inpatient charges were obtained from the Vizient billing database from the date of HCT through day 180 post-HCT or death, whichever came first. Unadjusted charges were converted to adjusted cost for 2017 US dollars (USD) using institutional cost-to-charge ratios, wage index, and the medical component of the Consumer Price Index. Inpatient charges were divided into 6 categories: room and board, laboratory, pharmacy, procedure, imaging services, and others. Readmission cost was calculated as the sum of the costs for all readmissions for each patient.
We next evaluated readmissions at the episode level. Reasons for hospital readmissions were categorized into 2 mutually exclusive groups. Admissions were defined as CMV-related if the reason for admission was i.v. administration of PET (foscarnet or ganciclovir) or workup or management for CMV EOD, or if initiation of PET occurred during the admission. All other admissions were deemed non CMV-related. Reasons for readmission were determined on a hierarchical basis, so that when 2 or more reasons for admission were documented, we preferred CMV-related reasons over non CMV-related reasons and EOD workup or management over CMV treatment.
Statistical Analysis
Descriptive statistics were used to tabulate information on individuals, including demographic information and clinical characteristics among individuals undergoing HCT. Data measured on a continuous scale were expressed as mean (standard deviations, SD) and/or median (interquartile range, IQR), and categorical data were expressed as count and percentage. Differences between the PET and no PET groups were compared using the Student t test and Mann-Whitney Utest for continuous variables and the chi-square test (Fisher’s exact test) for categorical variables. The number and percentage of readmissions were reported and compared by PET use. Right-skewed inpatient LOS and cost data were adjusted using gamma distribution with log link. Mean values and 95% confidence intervals (CIs) of inpatient LOS and costs for index admissions and readmissions were estimated and compared according to PET use and reason for readmission. Adjusted mean total cost and breakdown by cost categories were compared by PET use.
Univariable and multivariable analyses were performed to assess risk factors associated with number of readmissions and total inpatient cost. The variables included in the models were patient demographics (age, sex, race); underlying disease; transplantation characteristics, including stem cell source (peripheral blood or bone marrow), donor CMV seropositivity (D− or D+), donor type (matched related or unrelated, mismatched related or unrelated), conditioning regimen (myeloablative, reduced-intensity, or nonmyeloablative), graft manipulation (TCD or not), antithymocyte globulin (ATG) use, acute GVHD grade by day 100 (0-I or II-IV), EOD (no or yes), and PET use (no or yes). Variables in univariable analysis with P<.3 were entered to the multivariable model for assessment. Forward selection was used to select variables, and those with P< .1 were entered into the final multivariable models. Negative binomial regression was used for readmission counts while adjusting for varying follow-up period for each patient. Risk ratios (RRs) with 95% CIs were calculated for readmission counts between discharge of the index admission to day 180 or death and showed the difference in estimated number of readmissions between patients from various groups. A generalized linear model with log link and gamma distribution was used to estimate incremental total inpatient cost by PET use, accounting for the aforementioned covariates. Cost ratios (CRs) with 95% CIs were calculated for total inpatient cost by day 180 and showed the difference in estimated total inpatient cost between patients from various groups. Stratified analyses were performed to examine CMV outcomes and HCRU by CMV risk. All tests were 2-sided, with a significance level of .05. All statistical analyses were performed using R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria; https://www.rproject.org/).
RESULTS
Study Population
Between March 18, 2013, and December 31, 2017, a total of 917 adults underwent allogeneic HCT at MSKCC. Five hundred and forty-nine patients were excluded from the analyses for reasons noted in Figure 1. The remaining 357 CMV R+ recipients were included in the analyses.
Figure 1.
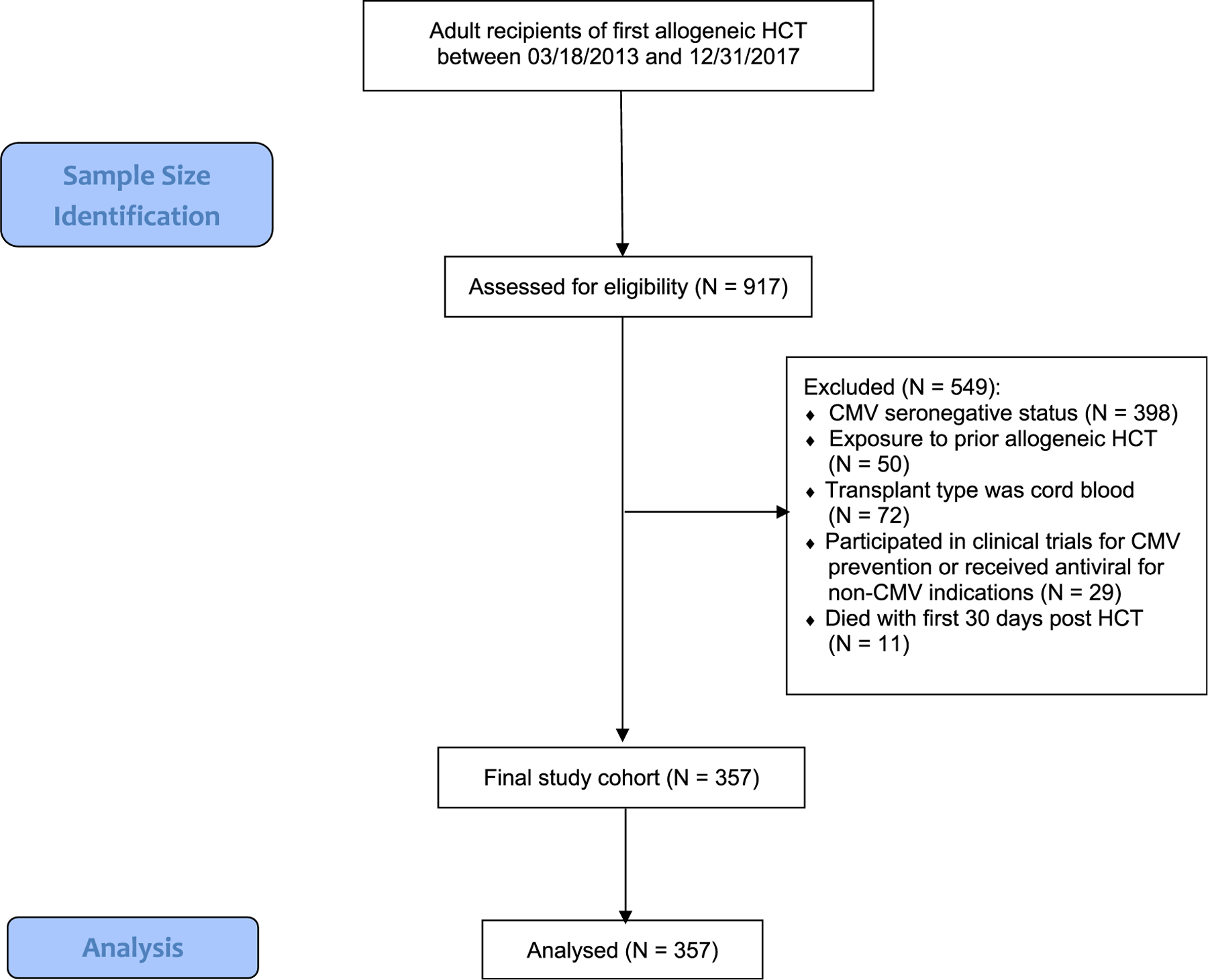
CONSORT diagram of sample size identification.
Table 1 presents baseline and transplantation characteristics of the study cohort overall and by PET use. The median patient age was 59 years. The most common underlying diseases were acute leukemia and myelodysplastic syndrome, affecting 67.8% of the study cohort; 60.1% of the patients received a myeloablative conditioning regimen, 84.0% received a peripheral blood allograft, and 41.3% received ex vivo TCD HCT; donors were matched unrelated in 50.3% of patients. One hundred eighty-eight patients (51.1%) met the criteria for HR CMV.
Table 1.
Baseline Characteristics of the PET and No PET Groups
| Characteristic | Overall (N = 357) | PET Group (N = 208) | No PET Group (N = 149) | P Value | |||
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Age, yr | |||||||
| Mean (SD) | 55.8 (12.9) | 54.8 (12.8) | 57.3 (12.8) | .06 | |||
| Median (IQR) | 59 (48–66) | 57 (45–66) | 60 (49–67) | .04 | |||
| Age group, N (%) | .41 | ||||||
| 18–39 yr | 50 | (14.0) | 31 | (14.9) | 19 | (12.7) | |
| 40–64 yr | 196 | (54.9) | 118 | (56.7) | 78 | (52.4) | |
| 65+ yr | 111 | (31.1) | 59 | (28.4) | 52 | (34.9) | |
| Sex, N (%) | .69 | ||||||
| Male | 208 | (58.3) | 123 | (59.1) | 85 | (57.0) | |
| Female | 149 | (41.7) | 85 | (40.9) | 64 | (43.0) | |
| Race, N (%) | .001 | ||||||
| White | 255 | (71.4) | 133 | (63.9) | 122 | (81.9) | |
| African American | 31 | (8.7) | 24 | (11.5) | 7 | (4.7) | |
| Hispanic/Latino | 24 | (6.7) | 18 | (8.7) | 6 | (4.0) | |
| Asian | 25 | (7.0) | 21 | (10.1) | 4 | (2.7) | |
| Other/unknown | 22 | (6.2) | 12 | (5.8) | 10 | (6.7) | |
| Transplantation characteristics | |||||||
| Underlying disease, N (%) | <.0001 | ||||||
| Leukemia/MDS | 242 | (67.8) | 133 | (63.9) | 109 | (73.2) | |
| Lymphoma | 49 | (13.7) | 22 | (1.6) | 27 | (18.1) | |
| Multiple myeloma | 39 | (10.9) | 35 | (16.8) | 4 | (2.7) | |
| Other | 27 | (7.6) | 18 | (8.7) | 9 | (6.0) | |
| Donor type, N (%) | .003 | ||||||
| Matched related | 113 | (30.7) | 61 | (29.3) | 52 | (34.9) | |
| Mismatched related | 22 | (6.0) | 19 | (9.1) | 3 | (2.0) | |
| Matched unrelated | 185 | (50.3) | 100 | (48.1) | 85 | (57.0) | |
| Mismatched unrelated | 37 | (10.1) | 28 | (13.5) | 9 | (6.0) | |
| Donor CMV seropositivity, N (%) | .85 | ||||||
| D+ | 216 | (58.7) | 125 | (60.1) | 91 | (61.1) | |
| D− | 141 | (38.3) | 83 | (39.9) | 58 | (38.9) | |
| Stem cell source, N (%) | .75 | ||||||
| Bone marrow | 48 | (13.0) | 29 | (13.9) | 19 | (12.8) | |
| Peripheral blood | 309 | (84.0) | 179 | (86.1) | 130 | (87.2) | |
| Conditioning regimen intensity, N (%) | <.0001 | ||||||
| Myeloablative | 221 | (60.1) | 152 | (73.1) | 69 | (46.3) | |
| Reduced | 105 | (28.5) | 41 | (19.7) | 64 | (43.0) | |
| Nonmyeloablative | 31 | (8.4) | 15 | (7.2) | 16 | (10.7) | |
| ATG use, N (%) | <.0001 | ||||||
| Yes | 185 | (50.3) | 141 | (67.8) | 44 | (29.5) | |
| No | 172 | (46.7) | 67 | (32.2) | 105 | (70.5) | |
| GVHD prophylaxis, N (%) | <.0001 | ||||||
| T cell depletion (CD34+ selection) | 152 | (41.3) | 123 | (59.1) | 29 | (19.5) | |
| Pharmacologic GVHD prophylaxis | 205 | (55.7) | 85 | (40.9) | 120 | (80.5) | |
| CMV risk, N (%) | <.0001 | ||||||
| HR | 188 | (51.1) | 150 | (72.1) | 38 | (25.5) | |
| LR | 169 | (45.9) | 58 | (27.9) | 111 | (74.5) | |
IQR indicates interquartile range; MDS, myelodysplastic syndrome.
Significant P values are in bold type.
PET Utilization
Of the 357 patients, 208 (58.3%) received PET (PET group) and 149 (41.7%) did not receive PET (no PET group). The PET group comprised 150 HR patients (72.1%) and 58 LR patients (27.9%). PET was administered in 150 of 188 HR patients (79.8%) and in 58 of 169 LR patients (34.3%) (P< .0001).
PET included valganciclovir in 158 patients (76.0%), ganciclovir in 63 (30.3%), and foscarnet in 93 (44.7%). Eighty-nine patients (42.8%) received more than 1 PET type by day 180. One hundred and twenty-five patients (60.1%) received PET, at least partially, as i.v. infusion.
CMV Outcomes
CMV EOD
CMV viremia and PET preceded CMV in all EOD cases. By day 180, CMV EOD had developed in 23 patients (6.4% of the entire cohort; 11.1% of the PET group). Gastrointestinal disease was the most common manifestation, occurring in 14 patients, followed by pneumonitis in 5 patients, encephalitis in 3 patients, and retinitis in 1 patient.
Mortality
Forty three of 357 patients (12.0%) died by day 180. The cause of death was relapse or disease progression in 21 patients (5.9%), GVHD in 2 (.6%), infection in 13 (3.6%), and other reasons in 7 (2.0%). CMV was the cause of death in 3 patients in the PET group (.8% of the entire cohort and 1.4% of the PET group). Two of the 3 patients who died of CMV belonged to the HR group.
HCRU
Table 2 compares hospital LOS in days and inpatient costs by day 180 (adjusted to 2017 USD) between the PET and no PET groups. The proportion of patients who had at least 1 readmission was significantly higher in the PET group compared with the no PET group (54.8 versus 34.2%; P= .0001). The PET group also had a longer LOS for the index admission (35.4 versus 31.0 days; P= .0276) and a longer total LOS (49.8 versus 38.1 days; P= .0001). There was no significant between-group difference in readmissions LOS between the 2 groups.
Table 2.
Hospital LOS and Inpatient Costs by Day 180 in the PET and No PET Groups
| Parameter | Overall (N = 357) | PET Group (N = 208) | No PET Group (N = 149) | P Value |
|---|---|---|---|---|
| HCRU at patient level | ||||
| Readmissions* | ||||
| Number of patients with ≥ 1 readmissions, n (%) | 165 (46.2) | 114 (54.8) | 51 (34.2) | .0001 |
| Inpatient LOS, d, mean (95% CI) | ||||
| HCT (index) admission (N = 357) | 33.5 (31.6–35.6) | 35.4 (32.7–38.3) | 31.0 (28.5–33.7) | .0276 |
| Readmissions (N = 165) | 24.7 (21.0–29.2) | 26.4 (21.8–32.3) | 20.8 (15.5–28.7) | .192 |
| Total inpatient LOS by day 180 post-HCT (N = 357) | 44.9 (42.0–48.1) | 49.8 (45.7–54.4) | 38.1 (34.5–42.2) | .0001 |
| Inpatient cost, USD, mean (95% CI)‡ | ||||
| Index admission (N = 357) | 173,637 (158,664–190,555) | 189,389 (166,162–217,156) | 151,646 (138,920–165,977) | .0133 |
| Readmissions (N = 165) | 185,280 (155,465–223,236) | 197,369 (160,302–246,806) | 158,257 (116,049–223,687) | .268 |
| Total inpatient cost by day 180 post-HCT (N = 357) | 259,270 (236,110–285,563) | 297,563 (263,615–337,600) | 205,815 (180,988–235,401) | <.0001 |
| HCRU per readmission by cause of readmission | ||||
| Number of readmissions | 264 | 187 | 77 | <.0001 |
| CMV-related, N (%) | 76 (28.8) | 76 (40.6) | 0 (0.0) | |
| Non CMV-related, N (%) | 188 (71.2) | 111 (59.4) | 77 (100.0) | |
| Inpatient LOS, d, mean (95% CI) | 15.4 (13.3–18.0) | 16.1 (13.5–19.4) | 13.8 (10.6–18.4) | .362 |
| CMV-related (N = 76) | 22.3 (18.1–27.9) | 22.3 (18.1–27.9) | — | |
| Non CMV-related (N = 188) | 12.6 (10.4–15.6) | 11.9 (9.0–16.1) | 13.8 (10.6–18.4) | .485 |
| Inpatient cost, USD, mean (95% CI) | 115,800 (98,192–137,884) | 120,322 (98,816–148,549) | 104,820 (78,635–144,050) | .468 |
| CMV-related (N = 76) | 165,455 (128,309–218,442) | 165,455 (128,309–218,442) | — | —- |
| Non CMV-related (N = 188) | 95,727 (77,847–119,529) | 89,419 (67,112–122,816) | 104,820 (78,635–144,050) | .481 |
This table summarizes HCRU, including mean inpatient LOS and cost. Among 357 patients analyzed, 165 had at least 1 readmission, with a total of 264 readmissions. Inpatient cost was computed as the sum of all charges during the hospital stay. Readmissions LOS and cost were analyzed among the 165 patients who had ≥1 readmission by day 180. Readmission LOS was calculated per patient as the sum of LOS of all readmissions and readmission cost as the sum of inpatient cost of all readmissions. For analyses by readmission level, each readmission was categorized as CMV-related versus non CMV-related. Significant P values are in bold type.
For index admission LOS and cost are calculated from HCT infusion (day 0) through discharge from the hospital.
Readmission is defined as any hospitalization with LOS >48 hours.
Cost provided in 2017 USD.
The mean inpatient cost for the index admission was greater for the PET group compared with the no PET group ($189,389 versus $151,646; P= .0133). The mean total inpatient cost was also higher for the PET group ($297,563 versus $205,815; P< .0001).
To provide granular data on the types of cost incurred, we also compared the cost by billing category in the 2 groups. For each billing category except imaging, the PET group incurred a higher mean cost (Figure 2). Charges within the category of laboratory studies and procedures were further broken down by department and compared between the 2 groups (Supplementary Figure S1).
Figure 2.
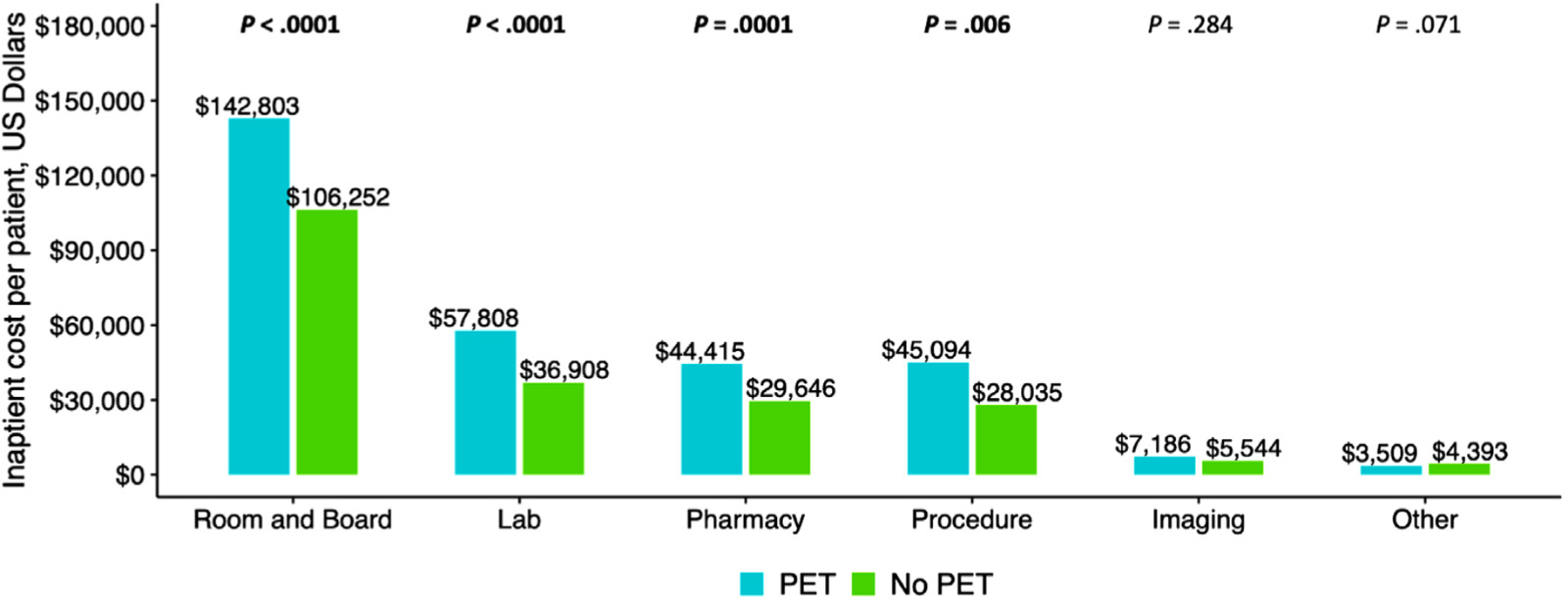
Breakdown of total inpatient cost by major categories. Total inpatient cost was divided into 6 major categories: room and board, clinical laboratory services (Lab), pharmacy, procedure, imaging services, and other. The total cost for room and board, Lab, pharmacy, and procedure were higher in the PET group compared with the no PET group. The total cost for imaging services and other were similar in the 2 groups.
To estimate the LOS and costs directly associated with CMV, we further categorized readmissions into 2 mutually exclusive categories as CMV-related and non CMV-related. Of 264 readmissions, 76 (28.8%) were CMV-related, exclusively in the PET group. The remaining 188 readmissions were non CMV-related, including 111 (59.0%) for the PET group and 77 (41.0%) for the no PET group (Table 2).
Because all CMV-related readmissions occurred within the PET group, we examined the proportion of CMV-related readmissions and associated HCRU in this group. CMV-related readmissions accounted for 40.6% of all readmissions in the PET group. The mean inpatient LOS was significantly longer for CMV-related readmissions compared with non CMV-related readmissions (22.3 days versus 11.9 days; P = .002). Similarly, the mean cost was higher for CMV-related readmissions compared with non CMV-related readmissions ($165,455 versus $89,419; P = .005). There was no significant difference in mean LOS and cost per non-CMV readmission between the PET and no PET groups (Figure 3).
Figure 3.
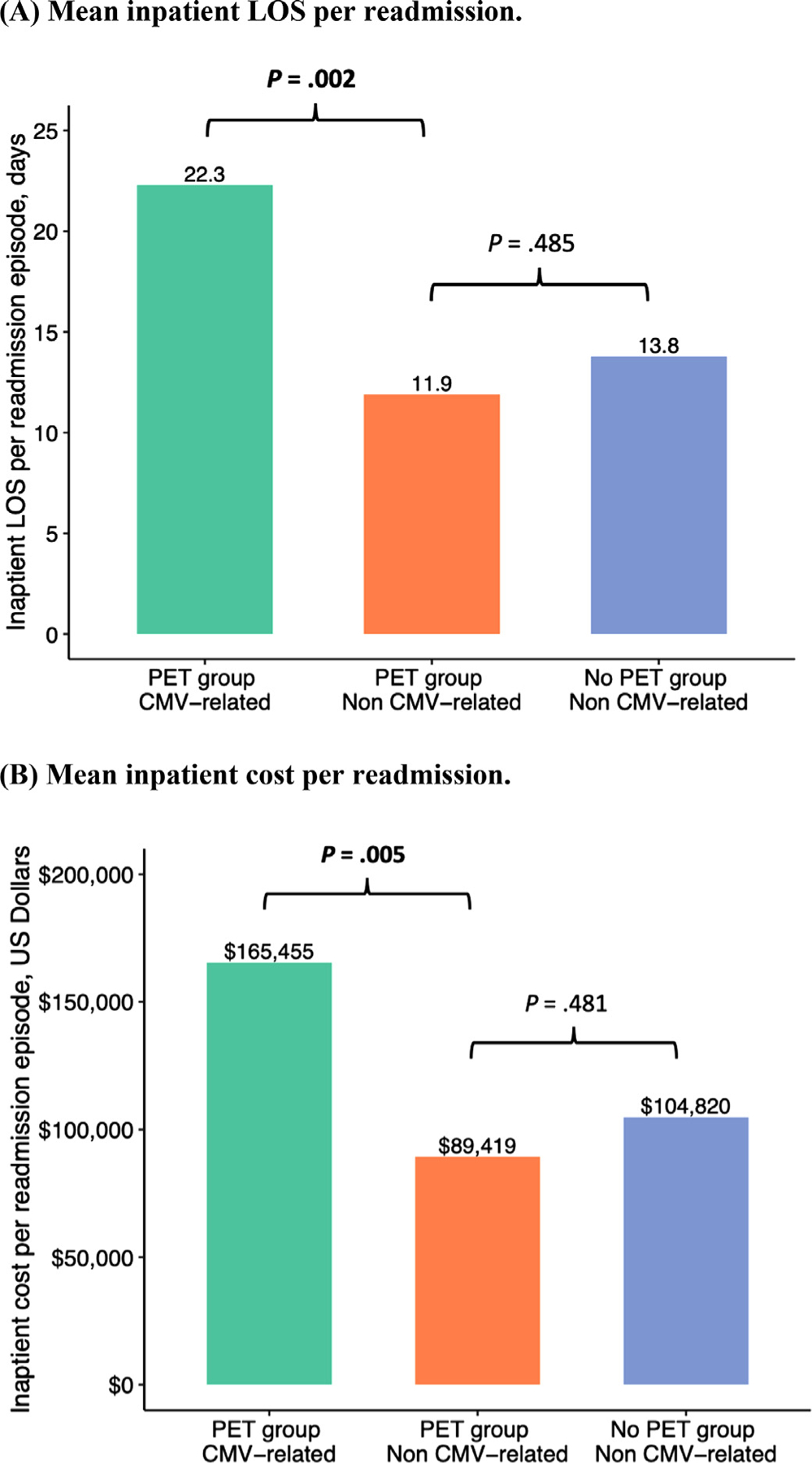
Comparison of mean inpatient LOS and cost per readmission between CMV-related and non CMV-related readmissions. (A) Mean inpatient LOS per readmission. In the PET group, the mean LOS per CMV-related readmission was longer than that per non CMV-related readmission (22.3 days versus 11.9 days; P = .002). In contrast, the mean LOS per non CMV-related readmission was similar in the PET and no PET groups (11.9 days versus 13.8 days; P= .485). (B) Mean inpatient cost per readmission. In the PET group, the mean cost per CMV-related readmission was higher than that per non CMV-related readmission ($165,455 versus $89,419; P= .005). In contrast, the mean cost per non CMV-related readmission was similar in the PET and no PET groups ($89,419 versus $104,820; P= .481).
Figure 4 shows the relative proportion of CMV-related readmissions (CMV treatment or CMV EOD) and the relative contribution of CMV-related readmissions to the total readmission cost in the PET group. A total of 187 readmissions occurred within the PET group, accounting for a total cost of 22.5 million USD. CMV-related readmissions represented 40.6% of total readmissions and 55.9% of total inpatient costs for readmissions.
Figure 4.
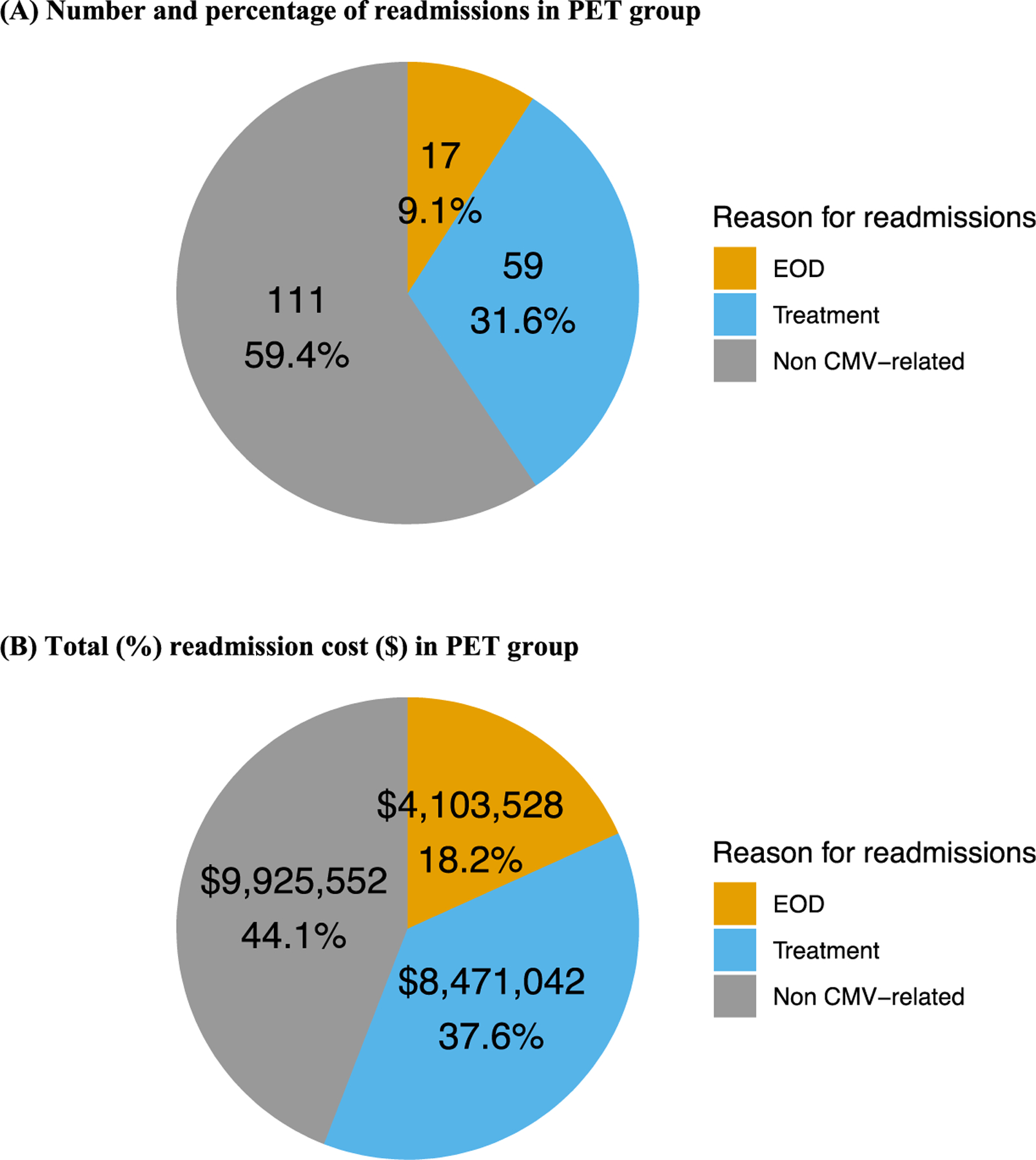
Number and percentage of readmissions and readmission cost by reason for readmission in the PET group. Shown is the relative proportion of readmissions that were CMV-related (CMV treatment or CMV EOD) and the relative contribution of CMV-related readmissions to the total readmission cost for the PET group. A total of 187 readmission episodes occurred in the PET group, accounting for a total cost of 22.5 million USD. CMV-related readmissions accounted for 40.6% of all readmissions and 55.9% of the total cost for readmissions. (A) Number and percentage of readmissions in PET group. (B) Total (%) readmission cost (USD) in the PET group.
Impact of PET on Number of Readmissions and Inpatient Costs
PET and EOD were entered as categorical variables in our univariable and multivariable models. Table 3 shows the results of univariable and multivariable analyses for number of readmissions. Patients who died during the index admission for HCT did not contribute to the number of readmissions. The remaining 349 patients who were discharged alive from the index admission were included in the analyses for the number of readmissions. Of the 349 patients, 204 (58.5%) were in the PET group and 145 (41.5%) in the no PET group. In multivariable analysis, PET was associated with a 63% increase in number of readmissions (RR, 1.63; 95% CI, 1.41 to 1.88; P< .0001), and EOD with an 84% increase in the number of readmissions (RR, 1.84; 95% CI, 1.53 to 2.21; P< .0001). Additional risk factors for higher number of readmissions were Hispanic/Latino race (RR, 1.52; 95% CI, 1.25 to 1.84; P< .0001), mismatched unrelated donor (RR, 1.37; 95% CI, 1.13 to 1.65; P= .001), peripheral blood as stem cell source (RR, 1.26; 95% CI, 1.02 to 1.57; P= .03) and grade II-IV acute GVHD (RR, 1.58; 95% CI, 1.39 to 1.80; P< .0001). In contrast, male sex (RR, .84; 95% CI, .74 to .94; P= .003), mismatched related donor (RR, .40; 95% CI, .28 to .59; P< .0001), reduced-intensity conditioning (RR, .70; 95% CI, .58 to .84; P= .0001), and ATG use (RR, .79; 95% CI, .66 to .94; P= .01) were associated with a decreased number of readmissions.
Table 3.
Univariable and Multivariable Analyses of Factors Associated with Number of Readmissions (N = 3491)*
| Factor | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| RR† | 95% CI | P Value | RR | 95% CI | P Value | |
| Age groups | ||||||
| 18–39 yr | ||||||
| 40–64 yr | 1.01 | .84–1.22 | .89 | |||
| 65+ yr | .90 | .73–1.10 | .29 | |||
| Sex | ||||||
| Female | ||||||
| Male | .79 | .69-.89 | .0002 | .84 | .74-.94 | .003 |
| Race | ||||||
| White | ||||||
| African American | 1.12 | .90–1.40 | .31 | 1.07 | .87–1.33 | .52 |
| Asian | 1.05 | .82–1.35 | .70 | .96 | .75–1.21 | .70 |
| Hispanic/Latino | 1.86 | 1.51–2.29 | <.0001 | 1.52 | 1.25–1.84 | <.0001 |
| Other/unknown | .97 | .75–1.27 | .85 | 1.15 | .90–1.48 | .26 |
| Underlying disease | ||||||
| Leukemia/MDS | ||||||
| Lymphoma | 1.29 | 1.08–1.53 | <.0001 | |||
| Multiple myeloma | 1.24 | 1.02–1.50 | .03 | |||
| Other | .80 | .61–1.04 | .09 | |||
| Donor type | ||||||
| Matched related | ||||||
| Mismatched related | .61 | .43-.86 | .004 | .40 | .28-.59 | <.0001 |
| Matched unrelated | 1.03 | .89–1.19 | .68 | 1.02 | .89–1.17 | .75 |
| Mismatched unrelated | 1.45 | 1.19–1.78 | .0003 | 1.37 | 1.13–1.65 | .001 |
| Donor CMV seropositivity | ||||||
| D− | ||||||
| D+ | .94 | .83–1.07 | .38 | |||
| Stem cell source | ||||||
| Bone marrow | ||||||
| Peripheral blood | 1.40 | 1.14–1.73 | <.0001 | 1.26 | 1.02–1.57 | .03 |
| Conditioning regimen intensity | ||||||
| Myeloablative | ||||||
| Reduced intensity | .84 | .73-.97 | .02 | .70 | .58-.84 | .0001 |
| Nonmyeloablative | .90 | .72–1.13 | .36 | 1.23 | .97–1.55 | .08 |
| ATG use | ||||||
| No | ||||||
| Yes | 1.17 | 1.03–1.33 | .01 | .79 | .66-.94 | .01 |
| GVHD prophylaxis | ||||||
| Pharmacologic GvHD prophylaxis | ||||||
| T cell depletion (CD34+ selection) | 1.37 | 1.21–1.55 | <.0001 | |||
| Acute GVHD grade | ||||||
| 0-I | ||||||
| II-IV | 1.44 | 1.27–1.64 | <.0001 | 1.58 | 1.39–1.80 | <.0001 |
| EOD | ||||||
| No | ||||||
| Yes | 2.44 | 2.02–2.94 | <.0001 | 1.84 | 1.53–2.21 | <.0001 |
| PET | ||||||
| No | ||||||
| Yes | 1.73 | 1.52–1.97 | <.0001 | 1.63 | 1.41–1.88 | <.0001 |
The univariable and multivariable analyses for number of readmissions were performed using negative binomial regression, adjusted for varying follow-up periods from discharge of HCT admission to day 180 or death. Number of readmissions during follow-up periods was entered as count data. Forward selection was used. Significant P values are in bold type.
Eight patients who died during the index admission were removed from this analysis.
The RR was estimated using a generalized linear model with negative binomial distribution. The ratio shows the difference in estimated number of readmissions between patients in one category of a covariate compared with patients in the reference category of the covariate.
We performed subgroup analyses by TCD versus unmodified HCT. The PET group included 123 of 152 (80.9%) TCD HCT recipients, and 85 of 205 (41.5%) unmodified HCT recipients. PET was associated with increased readmissions in TCD (RR, 1.70; 95% CI, 1.31 to 2.21; P< .0001) and unmodified HCT (RR, 1.45; 95% CI, 1.20 to 1.74; P< .0001). Similarly, CMV EOD was associated with increased readmissions in TCD and unmodified HCT (RR, 1.99; 95% CI, 1.63 to 2.43; P< .0001 and RR, 1.56; 95% CI, 1.09 to 2.25; P= .02, respectively).
We next examined the impact of PET and CMV EOD on the total inpatient cost. All 357 patients were included in the cost analyses. Both PET and EOD were independently associated with higher inpatient cost, associated with a 29% (CR, 1.29; 95% CI, 1.10 to 1.51; P= .002) and a 41% (CR, 1.41; 95% CI, 1.02 to 1.94; P= .04) increase in total cost, respectively. PET was associated with a $61,220 incremental per patient cost, and CMV EOD was associated with an $87,550 incremental per patient cost.
Additional factors associated with increased cost were mismatched unrelated donor (CR, 1.40; 95% CI, 1.07 to 1.84; P= .02) and acute GVHD (CR, 1.55; 95% CI, 1.32 to 1.82; P < .0001). Male sex was associated with lower inpatient cost (CR, .79; 95% CI, .68 to .93; P= .003) (Table 4).
Table 4.
Univariable and Multivariable Analyses of Factors Associated with Total Inpatient Cost (N = 357)
| Factor | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| Cost Ratio* | 95% CI | P Value | Cost Ratio | 95% CI | P Value | Adjusted Incremental Cost, USD | |
| Age group | |||||||
| 18–39 yr | |||||||
| 40–64 yr | .85 | .64–1.13 | .26 | ||||
| 65+ yr | .94 | .69–1.27 | .68 | ||||
| Sex | |||||||
| Female | |||||||
| Male | .77 | .64-.93 | .01 | .79 | .68-.93 | .003 | −44,298 |
| Race | |||||||
| White | |||||||
| African American | 1.15 | .82–1.63 | .41 | ||||
| Asian | 1.03 | .71–1.51 | .87 | ||||
| Hispanic/Latino | 1.26 | .86–1.85 | .24 | ||||
| Other/unknown | .91 | .61–1.36 | .66 | ||||
| Underlying disease | |||||||
| Leukemia/MDS | |||||||
| Lymphoma | .96 | .72–1.28 | .78 | ||||
| Multiple myeloma | 1.04 | .76–1.42 | .81 | ||||
| Other | .96 | .66–1.38 | .81 | ||||
| Donor type | |||||||
| Matched related | |||||||
| Mismatched related | 1.82 | 1.22–2.72 | .004 | 1.28 | .88–1.88 | .20 | 60,690 |
| Matched unrelated | 1.25 | 1.02–1.53 | .04 | 1.13 | .95–1.34 | .16 | 28,022 |
| Mismatched unrelated | 1.64 | 1.19–2.27 | .003 | 1.40 | 1.07–1.84 | .02 | 85,875 |
| Donor CMV seropositivity | |||||||
| D− | |||||||
| D+ | .93 | .76–1.13 | .45 | ||||
| Stem cell source | |||||||
| Bone marrow | |||||||
| Peripheral blood | .72 | .54-.95 | .02 | .81 | .63–1.04 | .10 | −40,615 |
| Conditioning regimen intensity | |||||||
| Myeloablative | |||||||
| Reduced intensity | 1.17 | .95–1.44 | .14 | ||||
| Nonmyeloablative | .91 | .65–1.27 | .56 | ||||
| ATG use | |||||||
| No | |||||||
| Yes | .86 | .71–1.04 | .11 | ||||
| GVHD prophylaxis | |||||||
| Pharmacologic GVHD prophylaxis | |||||||
| T cell depletion (CD34+ selection) | .89 | .74–1.07 | .22 | ||||
| Acute GVHD grade | |||||||
| 0-I | |||||||
| II-IV | 1.70 | 1.44–2.02 | <.0001 | 1.55 | 1.32–1.82 | <.0001 | 116,639 |
| EOD | |||||||
| No | |||||||
| Yes | 1.78 | 1.21–2.62 | .004 | 1.41 | 1.02–1.94 | .04 | 87,550 |
| PET | |||||||
| No | |||||||
| Yes | 1.45 | 1.21–1.75 | <.0001 | 1.29 | 1.10–1.51 | .002 | 61,220 |
The univariable and multivariable analyses for total inpatient cost were performed using a generalized linear model with gamma distribution and log link function, adjusted for varying follow-up periods from discharge of HCT admission to day 180 or death. Total inpatient cost was calculated for the sum of charges during HCT admission and all readmissions per patient and was entered as a continuous variable. Forward selection was used. Significant P values are in bold type.
Cost ratio was estimated using a generalized linear model with gamma distribution and log link function. The ratio shows the difference in estimated total inpatient cost between patients in one category of a covariate compared with patients in the reference category of that covariate.
In a subgroup analysis based on HCT manipulation, PET was associated with higher cost in unmodified HCT recipients ($146,407 incremental cost; CR, 1.37; 95% CI, 1.10 to 1.7; P= .01), but not in TCD recipients. EOD was associated with higher cost in TCD recipients ($108,406 incremental cost; CR, 1.62; 95% CI, 1.14 to 2.28; P = .01), but not in unmodified HCT recipients.
DISCUSSION
CMV infection is associated with increased morbidity and mortality in HCT recipients [20]. The strategy of PET is broadly used, and although effective in reducing rates of CMV EOD and CMV-related mortality, a survival disadvantage still persists for CMV-seropositive compared with CMV-seronegative HCT recipients [5,21,22]. CMV infection and CMV treatment are inextricably linked with regard to CMV outcomes and HCRU. The currently available antivirals for PET, (val)ganciclovir or foscarnet, are associated with myelosuppression or nephrotoxicity, respectively, and although uncommon, some patients still develop CMV EOD and may die of CMV or treatment-related toxicities [8]. CMV treatment is associated with increased risk for readmissions, prolonged hospitalization, and increased healthcare costs [9,10,23,24]. As safe and effective CMV prophylaxis has become available and new options for treatment of CMV are entering late stages of development, studies quantifying the impact of CMV and PET on clinical outcomes and HCRU are relevant for programmatic decision making.
We evaluated PET utilization, CMV outcomes, and HCRU including LOS and costs in 357 R+ HCT recipients managed by PET in a single center. By day 180 post-HCT, 58% of the patients had received PET, 6% had developed CMV EOD, and the CMV-attributable mortality was 1%. CMV-related complications were more common in the recipients at HR for CMV. Compared with the no PET group, the PET group had longer LOS for the index admission for HCT, a greater proportion requiring readmission, and had higher mean costs for the index admission and total mean inpatient costs through day 180. In multivariable analyses, PET and EOD were independently associated with more readmissions and higher inpatient costs. After adjusting to other variables, PET was associated with approximately $60,000 extra cost per patient and EOD with nearly $90,000 incremental cost. When we looked at readmissions based on reason for readmission, 41% of readmissions in the PET group were directly related to the management of CMV, and such readmissions incurred longer LOS and higher cost compared non CMV-related readmissions.
Our results align with published studies associating PET with higher HCRU and cost post-HCT. CMV infection following HCT has been associated with increased economic burden in HCT recipients. Studies evaluating patient-level cost and HCRU showed increased total medical cost and longer LOS for patients receiving PET compared with patients not receiving PET [10,24,25]. El Haddad et al [23] showed that the mean direct cost per patient admitted for PET in MD Anderson Cancer Center, was significantly higher compared to patients admitted for management of GVHD. Our study further highlights the economic burden associated with PET showing on average higher total inpatient cost through day 180 post-transplantation. Higher cost was noted in the PET group for most inpatient billing categories, including room and board, laboratory workup, procedures, and pharmacy. In addition, CMV-related readmissions accounted for 56% of the total readmission cost in the PET group by day 180.
The mean cost per CMV-related readmission in our study was estimated at $165,455, which is higher compared with previous reports. El-Haddad et al [23] reported an average cost of $116,976 per PET admission at MD Anderson Cancer Center and $42,327 when pooling costs from 19 US cancer centers. Factors possibly contributing to this discrepancy include differences in definitions for CMV admissions, in costs included in the analyses, or in study populations. For example, our PET group had a higher rate of EOD compared with the cohort reported by El Haddad et al (11% versus 4%, respectively), which could be explained, at least in part, by a higher proportion of HR patients in our cohort. Variability in delivery of care and clinical practices across geographic areas and institutions or differences in insurers or other factors also might have contributed to the high variability in cost per admission across centers in the aforementioned study [23].
Some HCRU differences between the PET and no PET groups could be attributed to differences in the baseline characteristics between the 2 groups. Patients who require PET are generally at risk for other post-HCT complications and may require hospitalizations for reasons other than CMV [4,26]. In our cohort, patients in the PET group were more likely to have a mismatched donor, undergo ex vivo TCD HCT, receive ATG, receive a myeloablative conditioning regimen, and have GVHD. These characteristics are associated with CMV infection and a lower viral load threshold for PET initiation on detection of CMV viremia. Nevertheless, after adjusting for these variables in our multivariable analyses, PET remained an independent predictor for a greater number of readmissions and higher total inpatient cost. The fact that within the PET group, CMV-related readmissions incurred higher cost compared with non CMV-related readmissions further supports the notion that at least part of the excess cost of readmissions in the PET group was directly attributed to the management of CMV. Indeed, in the PET group, although CMV-related readmissions represented 41% of readmissions, they accounted for 56% of the total readmission cost. Readmissions for EOD diagnosis and workup were associated with higher costs, and although relatively uncommon (9% of readmissions), they accounted for 18% of the readmission cost. The overall cost of EOD might have been underestimated in our study, because we only included the readmission during which EOD was diagnosed. EOD is often associated with extensive laboratory workup and prolonged antiviral treatment preceding the diagnosis of EOD, and associated costs may extend beyond day 180 [27].
Our study has several limitations inherent to its retrospective and observational design. First, as a single-center study it reflects our institutional practices, and our results might not be generalizable in other settings. Second, although we controlled for known potential covariates that may have affected LOS or cost in multivariable analyses, there may be additional confounders not included in our models. For example, bacterial and fungal infections are often associated with GVHD and CMV infection following HCT and may indirectly influence HCRU. Third, the follow-up in our study was through day 180; however, EOD may be a late CMV manifestation occurring later than day 180 [5], and thus we might have underestimated the total LOS and cost associated with CMV, as well as CMV-attributable mortality. More recently, letermovir prophylaxis has been implemented in many centers, including ours. In these centers, case-control studies may enable a direct comparison between PET and prophylaxis and their impact on HCRU and cost.
In conclusion, 6% of CMV R+ HCT recipients in our cohort composed of 50% HR CMV patients developed EOD by day 180. Preemptive management with currently available antivirals was associated with increased readmissions, prolonged LOS, and higher total inpatient costs by day 180. Our real-world data highlight the need to optimize management strategies for CMV infection in R+ HCT recipients.
Supplementary Material
ACKNOWLEDGMENTS
Financial disclosure: This study was sponsored by a grant from MerckSharp & Dohme Corp, a subsidiary of Merck & Co, Inc, to G.A.P. This work was also supported in part by the National Cancer Institute at the National Institutes of Health (P30 CA008748).
Footnotes
Conflict of interest statement: A.D.R. and Y.T. are employees of MerckSharp & Dohme Corp, a subsidiary of Merck & Co, Inc, the market authorization holder of Prevymis (letermovir). G.A.P. is an investigator for Merck and Shire and has received grant support and consulting and other fees from Merck & Co. S.G. serves on advisory board for Amgen, Actinuum, Celgene, Johnson & Johnson, Jazz Pharmaceutical, Takeda, Novartis, Kite, and Spectrum Pharma and has received research funding from Amgen, Actinuum, Celgene, Johnson & Johnson, Miltenyi Biotec, and Takeda Pharmaceutical. M.A.P. has received honoraria from Abbvie, Bellicum, Bristol-Myers Squibb, Incyte, Merck, Novartis, Nektar Therapeutics, Omeros, and Takeda; serves on Data Safety and Monitoring Boards for Servier and Medigene and scientific advisory boards of MolMed and NexImmune; has received research support for clinical trials from Incyte, Kite/Gilead, and Miltenyi Biotec; serves in a volunteer capacity as a member of the Board of Directors of the American Society for Transplantation and Cellular Therapy and Be The Match (National Marrow Donor Program), as well as on the CIBMTR Cellular Immunotherapy Data Resource Committee.
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.bbmt.2020.06.025.
REFERENCES
- 1.Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol 2016;3:e119–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood 2009;113:5711–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang YT, Neofytos D, Foldi J, et al. Cytomegalovirus infection after CD34(+)-selected hematopoietic cell transplantation. Biol Blood Marrow Transplant 2016;22:1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dziedzic M, Sadowska-Krawczenko I, Styczynski J. Risk factors for cytomegalovirus infection after allogeneic hematopoietic cell transplantation in malignancies: proposal for classification. Anticancer Res 2017;37:6551–6556. [DOI] [PubMed] [Google Scholar]
- 5.Erard V, Guthrie KA, Seo S, et al. Reduced mortality of cytomegalovirus pneumonia after hematopoietic cell transplantation due to antiviral therapy and changes in transplantation practices. Clin Infect Dis 2015;61:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahata M, Hashino S, Nishio M, et al. Occurrence of adverse events caused by valganciclovir as pre-emptive therapy for cytomegalovirus infection after allogeneic stem cell transplantation is reduced by low-dose administration. Transpl Infect Dis 2015;17:810–815. [DOI] [PubMed] [Google Scholar]
- 7.Avery RK, Arav-Boger R, Marr KA, et al. Outcomes in transplant recipients treated with foscarnet for ganciclovir-resistant or refractory cytomegalovirus infection. Transplantation 2016;100. e74–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zavras P, Su Y, Fang J, et al. Impact of preemptive therapy for cytomegalovirus on toxicities after allogeneic hematopoietic cell transplantation in clinical practice: a retrospective single-center cohort study. Biol Blood Marrow Transplant 2020;26(8):1482–1491. 10.1016/j.bbmt.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang YT, Su Y, Kim SJ, et al. Cytomegalovirus infection in allogeneic hematopoietic cell transplantation managed by the preemptive approach: estimating the impact on healthcare resource utilization and outcomes. Biol Blood Marrow Transplant 2019;25:791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain NA, Lu K, Ito S, et al. The clinical and financial burden of pre-emptive management of cytomegalovirus disease after allogeneic stem cell transplantation-implications for preventative treatment approaches. Cytotherapy 2014;16:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobbs GS, Kaur N, Hilden P, et al. A novel reduced intensity conditioning regimen for patients with high-risk hematological malignancies undergoing allogeneic stem cell transplantation. Bone Marrow Transplant 2016;51:1010–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montoro J, Ceberio I, Hilden P, et al. Ex vivo T cell-depleted hematopoietic stem cell transplantation for adult patients with acute myelogenous leukemia in first and second remission: long-term disease-free survival with a significantly reduced risk of graft-versus-host disease. Biol Blood Marrow Transplant 2020;26:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceberio I, Devlin SM, Sauter C, et al. Sirolimus, tacrolimus and low-dose methotrexate based graft-versus-host disease prophylaxis after non-ablative or reduced intensity conditioning in related and unrelated donor allogeneic hematopoietic cell transplant. Leuk Lymphoma 2015;56:663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed S, Kanakry JA, Ahn KW, et al. Lower graft-versus-host disease and relapse risk in post-transplant cyclophosphamide-based haploidentical versus matched sibling donor reduced-intensity conditioning transplant for Hodgkin lymphoma. Biol Blood Marrow Transplant 2019;25:1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosillo C, Avila AM, Huang YT, et al. Sequential systematic anti-mold prophylaxis with micafungin and voriconazole results in very low incidence of invasive mold infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 2018;20:e12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo SK, Xiao K, Huang YT, et al. Impact of peri-transplant vancomycin and fluoroquinolone administration on rates of bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients: a 12-year single institution study. J Infect 2014;69:341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009;15:1143–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babady NE, Cheng C, Cumberbatch E, Stiles J, Papanicolaou G, Tang YW. Monitoring of cytomegalovirus viral loads by two molecular assays in whole-blood and plasma samples from hematopoietic stem cell transplant recipients. J Clin Microbiol 2015;53:1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017;64:87–91. [DOI] [PubMed] [Google Scholar]
- 20.Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood 2016;127:2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Einsele H, Ehninger G, Hebart H, et al. Polymerase chain reaction monitoring reduces the incidence of cytomegalovirus disease and the duration and side effects of antiviral therapy after bone marrow transplantation. Blood 1995;86. 2815–282. [PubMed] [Google Scholar]
- 22.Green ML, Leisenring W, Stachel D, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012;18:1687–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Haddad L, Ghantoji SS, Park AK, et al. Clinical and economic burden of pre-emptive therapy of cytomegalovirus infection in hospitalized allogeneic hematopoietic cell transplant recipients. J Med Virol 2020;92: 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saullo JL, Li Y, Messina JA, et al. Cytomegalovirus in allogeneic hematopoietic transplantation: impact on costs and clinical outcomes using a preemptive strategy. Biol Blood Marrow Transplant 2020;26. 568–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueno R, Nishimura S, Fujimoto G, Piao Y, Takenaka K, et al. The clinical and economic burden of cytomegalovirus management post allogeneic hematopoietic stem cell transplantation in Japan - a retrospective database study. Curr Med Res Opin 2019;35:2089–2096. [DOI] [PubMed] [Google Scholar]
- 26.Huang YT, Kim SJ, Lee YJ, et al. Co-infections by double-stranded DNA viruses after ex vivo T cell-depleted, CD34+ selected hematopoietic cell transplantation. Biol Blood Marrow Transplant 2017;23:1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ariza-Heredia EJ, Nesher L, Chemaly RF. Cytomegalovirus diseases after hematopoietic stem cell transplantation: a mini-review. Cancer Lett 2014;342:1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


