Abstract
Pentraxin 3 (PTX3) and ficolin are the plasma phase of pattern recognition receptors (PRRs) and can activate complement through classical and lectin pathways, respectively, which may contribute to disease severity. This study aimed to investigate the association between PTX3 and ficolin with disease severity in patients with coronavirus disease-2019 (COVID-19). Seventy-three COVID-19 patients and 25 healthy controls were enrolled in this study. The participants were divided into three groups as follows: 14 patients as the intensive care unit (ICU) group, 59 patients as the non-ICU group, and 25 subjects as the healthy control group. The serum levels of PTX3 and ficolin were measured by enzyme-linked immunosorbent assay (ELISA) kits. Patients in ICU and non-ICU groups had significantly higher levels of PTX3 compared to the healthy control group (p = 0.0002 and p = 0.0072, respectively). Patients in the ICU group also had an increased amount of PTX3 (1957 ± 1769 pg/ml) compared to non-ICU patients (1220 ± 1784 pg/ml). However, this difference was not significant. On the other hand, serum levels of ficolin were not different among the three groups. PTX3, as an acute phase protein, may contribute to disease severity. Its probable inflammatory role could result from the high activation of the complement system. On the other hand, it could be suggested that ficolin has no crucial role in the disease severity of COVID-19 patients.
Keywords: Pentraxin 3, Ficolin, COVID-19, Pattern recognition receptors, Coronavirus
Introduction
New coronavirus disease-19 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is associated with respiratory illnesses varying from mild to life-threatening acute respiratory distress syndrome (ARDS). Several common symptoms, including dry cough, fever, fatigue, dyspnea, myalgia, and shortness of breath, are observed in the COVID-19 patients. Innate immunity, as the first line of defense, protects the body from the pathogens and activates the adaptive immune system through cytokines’ production (Gholipour et al. 2020; Merad and Martin 2020; Taefehshokr et al. 2020; Yousefi et al. 2021).
Innate immunity through pattern recognition receptors (PRRs) recognizes the conserved pattern of microorganisms, such as lipid A portion of lipopolysaccharide (LPS), known as pathogen-associated molecular patterns (PAMPs) (Taefehshokr et al. 2020). Pentraxins belong to the soluble components of PRRs. Pentraxin family consists of short and long pentraxins, which activates the classical pathway of complement. C-reactive protein (CRP) and serum amyloid P (SAP) are short pentraxin subfamily, while pentraxin 3 (PTX3) is a long one (Staubli et al. 2019; Vilahur and Badimon 2015). Ficolin is another family of soluble components of PRRs, which binds to the pathogens and activates the lectin pathway of complement (Garred et al. 2016). Myeloid cells, particularly dendritic cells (DCs), are the main sources of PTX3 among immune cells (Balhara et al. 2013). However, the neutrophils appear to be a main source and reservoir of ficolin, especially ficolin-1 (Garred et al. 2010).
CRP and SAP are produced by liver cells in response to inflammatory mediators, such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α), while PTX3 is released from different innate immune cells and endothelial cells in response to TNF-α or toll-like receptors (TLRs) ligand (Staubli et al. 2019, Vilahur and Badimon 2015). Complement activation via PTX3 and ficolin could result in a high inflammatory condition, activation, and chemotaxis of leukocytes into inflammatory tissue (Vilahur and Badimon 2015). Previous research on the prognostic importance of PTX3 and ficolin in systemic inflammatory diseases, vascular pathology, and also COVID-19 (Brunetta et al. 2021; Latini et al. 2004b; Polycarpou et al. 2020) led the current study, which aimed to learn more about the significance of PTX3 and ficolin in critically ill COVID-19 patients.
Materials and methods
Study populations
A total of 98 participants were enrolled in this study as follows: A number of 14 patients were admitted to intensive care unit (ICU) (mean age ± SD = 53 ± 18, 8 males, 6 females); 59 patients were in the non-ICU group (mean age ± SD = 59 ± 19, 38 males, 21 females); and 25 subjects were in the healthy control group (mean age ± SD = 46 ± 6.5, 16 males, 9 females). All patients (n = 73) with COVID-19 enrolled in this study were confirmed with positive SARS-CoV-2 real-time reverse transcription-polymerase chain reaction (RT-PCR) tests of respiratory secretions and admitted to Ayatollah Rouhani, Shahid Yahya Nezhad, and Shahid Beheshti Hospitals of Babol Medical University (Babol, Iran). The control group consisted of healthy persons with no known history of COVID-19 infection or other infection symptoms, negative RT-PCR, and normal laboratory findings. The study was approved by the Ethics Committee of Babol University of Medical Sciences (Code: IR.MUBABOL.HRI.REC.1399.090).
Sample collection, ficolin, and pentraxin measurements
Five milliliters of peripheral blood was obtained from all participants, and then their sera were considered for the measurement of ficolin and PTX3. The concentrations of ficolin and PTX3 were measured by enzyme-linked immunosorbent assay (ELISA) kits (Boster Bio, Pleasanton, CA, USA). All assays were performed in duplicate according to the manufacturer’s instructions.
Statistical analysis
Data were analyzed via GraphPad Prism 8.0 for Windows (GraphPad Software, Inc., San Diego, USA). The one-way analysis of variance (ANOVA) or the Kruskal–Wallis test was used, and the p value of < 0.05 was considered significant in all statistical tests.
Results
Demographic and clinical characteristics of study subjects
The demographic and clinical features of COVID-19 patients and healthy control subjects are shown in Table 1 (age- and sex-matched). The main clinical and laboratory findings were significantly different between ICU and non-ICU patients. ICU patients had significantly lower O2 saturation (p < 0.0001) and higher respiratory rate (p < 0.0001) compared with non-ICU patients. On the other hand, in ICU patients, lymphocytes were significantly reduced, and neutrophils were significantly increased compared to the non-ICU group (Table 1).
Table 1.
Demographic and clinical characteristics of study subjects
| Variables | Healthy (25) |
Non-ICU (59) |
ICU (14) |
P value |
|---|---|---|---|---|
| Age (years) | 46±6.5 | 59±19 | 53±18 | |
| Gender | ||||
| Female | 9 | 21 | 6 | |
| Male | 16 | 38 | 8 | |
| Temperature | 36.5±0.1 | 37.4±1 | 37.4±0.4 | 0.0001 |
| O2 saturation | - | 90±5 | 79±7 | <0.0001 |
| Respiratory rate | - | 20.5±3 | 27.5±6 | <0.0001 |
| LDH | - | 519±192 | 1241±1029 | <0.0001 |
| CRP | 1.7±1.5 | 87±69 | 176±85 | <0.0001 |
| ESR | - | 56±33 | 62±27 | 0.5407 |
| Ferritin | 103±52 | 638±834 | 507±342 | 0.0227 |
| BUN | 15±3.6 | 22±17 | 34±21 | 0.0040 |
| AST | 21±4 | 51±35 | 81±73 | 0.0059 |
| ALT | 18±7 | 45±28 | 56±48 | 0.0093 |
| WBC | 7489±1931 | 8256±3228 | 9171±2491 | 0.31 |
|
Lymphocytes (% in differential) |
32±5 | 24±12 | 12±5 | <0.0001 |
|
Neutrophil (%in differential) |
67±10 | 71±10 | 83±9 | <0.0001 |
| PLT | 269214±107329 | 252485±138840 | 219643±135754 | 0.5129 |
| NLR | 2.06±0.53 | 4.18±2.18 | 7.17±2.86 | <0.0001 |
| PLR | 114±41 | 155±77 | 190±95 | 0.0507 |
| Hb | - | 11±1 | 10±1 | 0.1283 |
Increased amount of PTX3 in COVID-19 patients
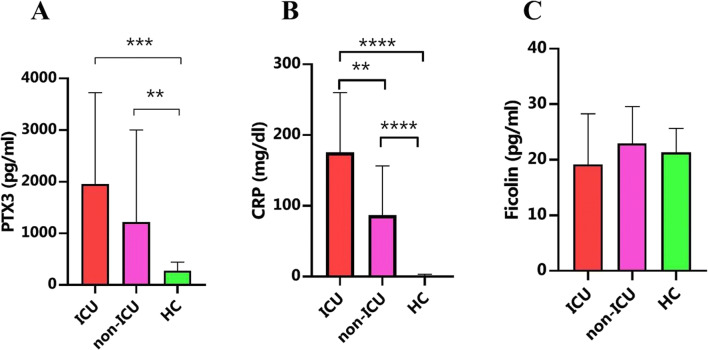
PTX3, as the main component of the pentraxin family, was measured in COVID-19 patients and compared with healthy controls. Both ICU and non-ICU groups had remarkably increased levels of PTX3 compared with healthy controls (p = 0.0002 and p = 0.0072, respectively [Table 2, Fig. 1]). Although the difference between ICU and non-ICU patients was not significant, ICU patients (1957 ± 1769 pg/ml) had higher levels of PTX3 than non-ICU patients (1220 ± 1784 pg/ml [Fig. 1]).
Table 2.
Levels of PTX3 and ficolin in the subjects
| Healthy (25) |
Non-ICU (59) |
ICU (14) |
P value | |
|---|---|---|---|---|
| Pentraxin (pg/ml) | 275±167 | 1220±1784 | 1957±1769 | 0.0002 |
| Ficolin (pg/ml) | 21±4 | 23±6.6 | 19±9 | 0.0523 |
Fig. 1.
Increased level of PTX3 in COVID-19 patients. Serum samples were considered for the measurement of ficolin and PTX3 by enzyme-linked immunosorbent assay (ELISA) kits. Both ICU and non-ICU groups had remarkably increased levels of PTX3 compared with healthy controls (A). These differences were also significant for CRP levels between groups (B). However, ficolin levels did not show any significant difference between the three groups (C). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001
Same level of ficolin in all study subjects
Ficolin was measured using ELISA between study subjects. Kruskal–Wallis analysis did not show any significant difference between the three groups (Table 2, Fig. 1).
Discussion
Several cell types, such as lung epithelial cells, produce PTX3; it was shown that PTX3 gene expression is induced in respiratory tract epithelial cells in response to SARS-CoV-2 (Brunetta et al. 2021). In the inflammatory condition and in response to the microbial recognition, PTX3 is rapidly increased, which could indicate fungal or viral infection (Cunha et al. 2014; Mairuhu et al. 2005). Moreover, in other inflammatory conditions, such as cardiovascular disease (Latini et al. 2004a) and sepsis (Lee et al. 2018), PTX3 is increased and associated with disease severity and mortality (Brunetta et al. 2021). In support, in the setting of sepsis, Song et al. showed that a highly increased amount of PTX3 (> 26 ng/ml) was associated with mortality (Song et al. 2020).
In terms of COVID-19 patients, Brunetta et al. showed that an increased amount of PTX3 could independently predict 28 days mortality. They finally reported that PTX3 is a better prognostic marker compared with CRP, IL-6, ferritin, and D-dimer (Brunetta et al. 2021). In another study, it was found that PTX3 had higher levels in severe COVID-19 patients than in asymptomatic patients and was positively correlated with D-dimer and body math index (BMI) (Krishnamachary et al. 2020). In the current study, our results confirmed the results of previous studies. We showed that both non-ICU and ICU groups had an increased amount of PTX3 compared with healthy controls. Although the difference was not significant between ICU and non-ICU patients, ICU patients showed higher levels of PTX3 with mean rank = 17.04. Moreover, PTX3 is associated with the severity of other lung injuries. In pulmonary contusion patients, PTX3 is remarkably increased and plays the main role in the pathophysiology of pulmonary contusion (Tatli et al. 2017).
Inflammatory factors, such as cytokines (IL-17, IL-1β, and IL-6), acute phase proteins, and neutrophils, are associated with disease severity, and some of these factors may be reduced after the current treatment protocol (Kataržytė et al. 2020). PTX3 is the major acute phase protein, which remarkably increases in COVID-19 patients and may contribute to disease severity. One of the possible functions of PTX3 may result from the increased activation of the complement cascade, which therefore increases the inflammation. Although it was reported that PTX3 could also reduce inflammation (Slusher et al. 2016; Vilahur and Badimon 2015), in terms of COVID-19 patients, it may not regulate uncontrolled inflammation.
Conclusion
In summary, PTX3 in addition to CRP could be considered as an important factor, which contributes to disease severity. Furthermore, the early assessment of PTX3 along with CRP could be used as a prognostic factor for severe COVID-19 patients, which could be controlled by a modified therapeutic strategy.
Acknowledgements
We thank the staffs of the Ayatollah Rouhani, Shahid Yahya Nezhad, and Shahid Beheshti, Hospitals (Babol, Iran) for the collection of patient samples.
Author contributions
ZM, MMA, MS: Investigation, writing - original draft writing – review and editing. MB, MM, AR: Writing – review and editing.
Funding
Not applicable
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This project has been registered in Babol University of Medical Sciences with the code of ethics of MUBABOL.HRI.REC.1399.09
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mousa Mohammadnia-Afrouzi, Email: m.mohammadnia@mubabol.ac.ir.
Mehdi Shahbazi, Email: m.shahbazi@mubabol.ac.ir.
References
- Balhara J, Koussih L, Zhang J, Gounni AS. Pentraxin 3: an immuno-regulator in the lungs. Front Immunol. 2013;4:127. doi: 10.3389/fimmu.2013.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetta E, Folci M, Bottazzi B, De Santis M, Gritti G, Protti A, Mapelli SN, Bonovas S, Piovani D, Leone R. Macrophage expression and prognostic significance of the long pentraxin PTX3 in COVID-19. Nat Immunol. 2021;22:19–24. doi: 10.1038/s41590-020-00832-x. [DOI] [PubMed] [Google Scholar]
- Cunha C, Aversa F, Lacerda JF, Busca A, Kurzai O, Grube M, Loeffler J, Maertens JA, Bell AS, Inforzato A. Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation. N Engl J Med. 2014;370:421–432. doi: 10.1056/NEJMoa1211161. [DOI] [PubMed] [Google Scholar]
- Garred P, Honoré C, Ma YJ, Rørvig S, Cowland J, Borregaard N, Hummelshøj T. The genetics of ficolins. J Innate Immun. 2010;2:3–16. doi: 10.1159/000242419. [DOI] [PubMed] [Google Scholar]
- Garred P, Genster N, Pilely K, Bayarri-Olmos R, Rosbjerg A, Ma YJ, Skjoedt MO. A journey through the lectin pathway of complement-MBL and beyond. Immunol Rev. 2016;274:74–97. doi: 10.1111/imr.12468. [DOI] [PubMed] [Google Scholar]
- Gholipour S, Nikaeen M, Manesh RM, Aboutalebian S, Shamsizadeh Z, Nasri E, Mirhendi H. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) contamination of high-touch surfaces in field settings. Biomed Environ Sci. 2020;33:925–929. doi: 10.3967/bes2020.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataržytė M, Balčiūnas A, Haseler M, Sabaliauskaitė V, Lauciūtė L, Stepanova K, Nazzari C, Schernewski G. Cigarette butts on Baltic Sea beaches: monitoring, pollution and mitigation measures. Mar Pollut Bull. 2020;156:111248. doi: 10.1016/j.marpolbul.2020.111248. [DOI] [PubMed] [Google Scholar]
- Krishnamachary B, Cook C, Spikes L, Chalise P, Dhillon NK (2020): The potential role of extracellular vesicles in COVID-19 associated endothelial injury and pro-inflammation. medRxiv. doi: 10.1101/2020.08.27.20182808 [DOI] [PMC free article] [PubMed]
- Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, Vago L, Pasqualini F, Signorini S, Soldateschi D. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–2354. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, Vago L, Pasqualini F, Signorini S, Soldateschi D, Tarli L, Schweiger C, Fresco C, Cecere R, Tognoni G, Mantovani A. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–2354. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- Lee YT, Gong M, Chau A, Wong WT, Bazoukis G, Wong SH, Lampropoulos K, Xia Y, Li G, Wong MC. Pentraxin-3 as a marker of sepsis severity and predictor of mortality outcomes: a systematic review and meta-analysis. J Infect. 2018;76:1–10. doi: 10.1016/j.jinf.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Mairuhu AT, Peri G, Setiati TE, Hack CE, Koraka P, Soemantri A, Osterhaus AD, Brandjes DP, Van Der Meer JW, Mantovani A. Elevated plasma levels of the long pentraxin, pentraxin 3, in severe dengue virus infections. J Med Virol. 2005;76:547–552. doi: 10.1002/jmv.20397. [DOI] [PubMed] [Google Scholar]
- Merad M, Martin JC (2020) Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20:355–362. [DOI] [PMC free article] [PubMed]
- Polycarpou A, Howard M, Farrar CA, Greenlaw R, Fanelli G, Wallis R, Klavinskis LS, Sacks S. Rationale for targeting complement in COVID-19. EMBO Mol Med. 2020;12:e12642. doi: 10.15252/emmm.202012642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusher AL, Mischo AB, Acevedo EO. Pentraxin 3 is an anti-inflammatory protein associated with lipid-induced interleukin 10 in vitro. Cytokine. 2016;86:36–40. doi: 10.1016/j.cyto.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Song J, Moon S, Park DW, Cho H-J, Kim JY, Park J, Cha JH. Biomarker combination and SOFA score for the prediction of mortality in sepsis and septic shock: a prospective observational study according to the Sepsis-3 definitions. Medicine. 2020;99:e20495. doi: 10.1097/MD.0000000000020495. [DOI] [PubMed] [Google Scholar]
- Staubli SM, Schäfer J, Rosenthal R, Zeindler J, Oertli D, Nebiker CA. The role of CRP and pentraxin 3 in the prediction of systemic inflammatory response syndrome and death in acute pancreatitis. Sci Rep. 2019;9:1–7. doi: 10.1038/s41598-019-54910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taefehshokr N, Taefehshokr S, Hemmat N, Heit B (2020) Covid-19: perspectives on innate immune evasion. Front Immunol 11:580641. 10.3389/fimmu.2020.580641 [DOI] [PMC free article] [PubMed]
- Tatli O, Kurt NBK, Karaca Y, Sahin A, Aygün A, Sahin E, Katipoglu B, Eryigit U, Turkmen S. The diagnostic value of serum pentraxin 3 levels in pulmonary contusion. Am J Emerg Med. 2017;35:425–428. doi: 10.1016/j.ajem.2016.11.030. [DOI] [PubMed] [Google Scholar]
- Vilahur G, Badimon L. Biological actions of pentraxins. Vasc Pharmacol. 2015;73:38–44. doi: 10.1016/j.vph.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Yousefi M, Oskoei V, Jafari AJ, Farzadkia M, Firooz MH, Abdollahinejad B, Torkashvand J (2021) Municipal solid waste management during COVID-19 pandemic: effects and repercussions. Environ Sci Pollut Res 28:32200–32209 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.