Abstract
Neural stem and progenitor cells (collectively termed neural precursor cells [NPCs]) are found along the ventricular neuraxis extending from the spinal cord to the forebrain in regionally distinct niches comprised of different cell types, architecture, and cell‐cell interactions. An understanding of the factors that regulate NPC behavior is critical for developing therapeutics to repair the injured central nervous system. Herein, we demonstrate that myelin basic protein (MBP), the major cytoplasmic protein constituent of the myelin sheath in oligodendrocytes, can regulate NPC behavior. Under physiological conditions, NPCs are not in contact with intracellular MBP; however, upon injury, MBP is released into the neural parenchyma. We reveal that MBP presented in a spinal cord niche is inhibitory to NPC proliferation. This inhibitory effect is regionally distinct as spinal cord NPCs, but not forebrain‐derived NPCs, are inhibited by MBP. We performed coculture and conditioned media experiments that reveal the stem cell niche is a key regulator of MBP's inhibitory actions on NPCs. The inhibition is mediated by a heat‐labile protein released by spinal cord niche cells, but not forebrain niche cells. However, forebrain NPCs are also inhibited by the spinal cord derived factor as revealed following in vivo infusion of the spinal cord niche‐derived conditioned media. Moreover, we show that MBP inhibits oligodendrogenesis from NPCs. Together, these findings highlight the role of MBP and the regionally distinct microenvironment in regulating NPC behavior which has important implications for stem cell‐based regenerative strategies.
Keywords: forebrain, myelin basic protein, neural stem cells, niche, oligodendrocytes, spinal cord
Myelin basic protein (MBP) presented in the spinal cord niche, but not the brain niche, causes the release of an inhibitory factor that regulates neural precursor cell kinetics and oligodendrogenesis. Hence, regionally distinct niches along the neuraxis respond differently to the same protein (MBP) and regulate cell behavior.
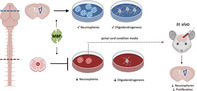
Significance statement.
Neural precursor cells (NPCs) reside in regionally distinct niches in the central nervous system (CNS). The niche regulates NPC behavior in homeostatic and injury conditions. This study shows that myelin basic protein (MBP), a major constituent of myelin sheaths in the CNS, regulates NPC behavior in a niche dependent fashion. in vitro and in vivo studies reveal that MBP presented in the spinal cord niche, but not the brain niche, results in the release of a protein that inhibits NPC proliferation and oligogenesis. Hence, regionally distinct niches respond differently to the same protein (MBP), by releasing factors that regulate NPCs throughout the CNS.
1. INTRODUCTION
Adult neural stem cells (NSCs) arise from neuroepithelial cells that comprise the neural tube during development and give rise to all the cell types of the central nervous system (CNS). 1 , 2 Through development and into adulthood, NSCs persist in the periventricular region lining the lateral ventricles in the forebrain and the central canal of the spinal cord. Both forebrain and spinal cord NSCs and their progeny (together termed neural precursor cells [NPCs]) can be activated in response to injury. A key difference between the forebrain and spinal cord is that forebrain NSCs contribute to ongoing neurogenesis throughout life, whereas the spinal cord becomes aneurogenic in adulthood. 3 , 4 , 5 Furthermore, the periventricular stem cell niche is regionally distinct along the neuraxis; it has been well defined in the forebrain and is composed of stem cells, multiciliated ependymal cells, transient‐amplifying cells, and neuroblasts. All these cells are intricately organized into a well‐characterized pinwheel structure and contribute to the regulation of the neurogenic niche well into adulthood. 6 , 7 The spinal cord niche however is not as well‐characterized and is composed mostly of ependymocytes and a small number of tanycytes. 8 , 9
Factors that regulate NSC behavior in response to injury or disease are of particular interest in regenerative medicine. Cell‐cell interactions, released cytokines, and hormones are able to regulate cell kinetics and progenitor fate. For example, resident microglia have been shown to secrete pro‐inflammatory and anti‐inflammatory markers that negatively regulate NSC survival and NSC migration, respectively. 10 Another factor with the potential of regulating cell fate is myelin basic protein (MBP), an essential structural component in the formation of mature myelin in the CNS, specifically the predominant 18.5‐kDa splice isoform. 11 Recently, we demonstrated that extracellular MBP can regulate NSC behavior and more specifically, MBP is inhibitory to spinal cord NSC (spNSC) proliferation in vitro without affecting spNSC survival. 12 MBP is expressed by oligodendrocytes and functions to bring together cytosolic membrane leaflets that create the myelin sheath, which wraps around neuronal axons and enables saltatory conduction. 11 Under normal physiological conditions, MBP is not found in the extracellular space; however, after injury, it is released into the microenvironment. MBP is classified as an intrinsically disordered protein (IDP) and its conformation, and potential activity is highly dependent on the environment where it is found. 13 Hence, we asked if MBP‐mediated inhibition of spNSCs was a common feature of extracellular MBP along the neuraxis or whether it had regionally distinct effects on NSC behavior based on the known differences between the forebrain and spinal cord microenvironments. 6 , 8 , 9 , 14
Herein, we use both in vitro and in vivo assays to demonstrate that the forebrain and spinal cord microenvironments differentially regulate the effects of MBP on NSC proliferation and differentiation. We show that MBP in the presence of the spinal cord microenvironment is inhibitory to NSC proliferation in both the forebrain and spinal cord. In addition, the presence of MBP reduces oligodendrogenesis from spNSCs. Conversely, MBP in the presence of the forebrain microenvironment is not inhibitory to NSC proliferation (brain or spinal cord) through the use of cocultures and conditioned media (CM). We also demonstrate that the inhibitory effects are mediated by a heat‐labile protein released from the spinal cord niche cells in response to MBP. Interestingly, intraventricular infusion of CM from spinal cord cultures which contains the inhibitory factor leads to decreased proliferation of forebrain NSCs (brNSCs) and their progeny in vivo, revealing that regionally distinct NSC populations are responsive to the spinal cord‐derived inhibitory factor. Together, these findings demonstrate that MBP can regulate NPC kinetics in a niche‐dependent manner. Specifically, MBP interacts with the spinal cord niche (but not the forebrain) to release a factor that inhibits proliferation and oligodendrogenesis of sp and brNSCs.
2. MATERIALS AND METHODS
2.1. Mice
Mice were housed within the Department of Comparative Medicine at the University of Toronto. Experiments were conducted following the approval by the Animal Care Committee and in accordance with the Guide to the Care and Use of Experimental Animals. Both sexes from the mutant shiverer mouse model (lacking mature MBP), shi/shi−/− (SHI) (http://jaxmice.jax.org/strain/001428.html; Bar Harbour, Maine), were used in this study as well as the transgenic Rosa26EYFP (http://jaxmice.jax.org/strain/006148; Bar Harbour, Maine) for coculture experiments. C57/Bl6 mice (Charles River) received at 6 weeks of age were acclimated and were used for our in vivo CM infusion studies at 8 weeks of age.
2.2. Dissection and in vitro assays
The protocol for isolation and culturing of NSCs has been previously described. 15 , 16 Briefly, mice were euthanized with an overdose of sodium pentobarbital and the spinal cord and/or forebrain was removed. The tissue surrounding the central canal of the spinal cord and/or tissue lining the lateral ventricle of the forebrain was carefully dissected and dissociated into single cells using enzymatic treatment (trypsin [40 mg/30 mL] and hyaluronidase [24.9 mg/30 mL]; T1005‐1G/H6254‐1G, Sigma, Canada) and mechanical trituration. Cells were plated at 1 to 10 cells/μL in Neurobasal‐A medium (10888022, Invitrogen, Canada) containing L‐glutamine (2 mM, 25030164, Invitrogen), penicillin/streptavidin (100 U/0.1 mg/mL, 15140163, Invitrogen), mitogens (epidermal growth factor [20 ng/mL]; fibroblast growth factor [10 ng/mL]; heparin [2 μg/mL], Sigma) with or without MBP (0‐100 μg/mL, Invitrogen). Primary neurospheres were plated at clonal density (10 cells/μL). 15 For spinal cord samples, cells were plated in media with the appropriate mitogens for 24 hours, then collected and replated for primary neurosphere formation. All primary neurosphere cultures (forebrain and spinal cord) contain niche cells as a result the dissection protocol previously referenced. Primary neurospheres were counted after 7 days. For passaging, neurospheres were collected and mechanically dissociated into single cells then replated at clonal density (1‐10 cells/μL) 15 in the same media conditions. For all experiments using passaged neurospheres, NPCs were twice passaged. For cocultures, the total numbers of neurospheres were counted using brightfield microscopy and the total numbers of YFP positive (YFP+) neurospheres were counted using fluorescence imaging. All YFP+ neurospheres were derived from wild type (WT) (MBP+ mice). YFP negative spheres were from shiverer mice (SHI) lacking MBP. The cumulative plating densities of the YFP+ and YFP negative cells were at, or below, clonal density (10 cells/μL); hence, the resulting neurospheres were not the result of mixing of the YFP+ and YFP negative populations.
2.3. Neurosphere culture myelin depletion
Percoll Plus solution (23%, Millipore‐Sigma, GE17‐5445‐01) dissolved in 1X sterile phosphate‐buffered saline (PBS) was added to primary enzymatically digested tissue (brain and spinal cord) and triturated until tissue was homogenized in the solution. The solution was then centrifuged for 15 minutes at 1800 RPM. Myelin supernatant was removed and myelin depleted tissue was washed with serum‐free media (37°C). The resuspended pellet was triturated until homogenized and centrifuged at 1500 RPM for 3 minutes. Supernatant was removed and cells were counted and plated at clonal density in the neurosphere assay as described. 12
2.4. CM experiments
CM was generated from primary spinal cord and forebrain dissections by plating cells at 50 cells/μL for 48 hours. CM was collected following centrifugation and filtered through a 0.22‐μm syringe driven filter unit (Millipore, Toronto, Canada, http://www.cedarlanelabs.com/). Heat inactivation of CM was further performed by boiling samples at 100°C for 30 minutes, after which they were allowed to cool, sterile‐filtered (0.22‐μm syringe driven filter unit (Millipore), and resupplemented with fresh media at a 1:1 dilution.
2.5. ELISA
Filtered and nonfiltered CM was assayed using an ELISA kit for MBP (Cloud‐Cone Corp, Texas, http://www.cloud-cone.us/) in accordance with the manufacturer's protocol and as described previously. 12
2.6. Neurosphere differentiation
Following 7 days of culture for primary and 14 days of culture for passaged neurospheres, the neurospheres (100‐150 μm) were picked using a P20 pipette and plated onto Neural Basal Media (21103049, Thermo Fisher Scientific, Canada) containing laminin (L2020‐1MG, Millipore‐Sigma) (50 μL in 10 mL). Forty‐eight well plates were used and each sphere was plated in a single well. Neurospheres were differentiated for an additional 7 days.
2.7. Immunocytochemistry — Neurospheres
Following differentiation, cells were fixed with 4% paraformaldehyde (PFA). To stain, cells were blocked in 10% normal goat serum (NGS; 1:10, 005‐000‐121, Jackson Immunoresearch, Europe) for 1 hour and incubated in O4 primary antibody overnight at 4°C (mouse monoclonal, 1:1000, R&D systems, MAB1326, Canada). On the second day, cells were washed (3x 5 minutes in 1X PBS), and a secondary antibody was added for 1 hour at room temperature (1:400, Alexa 568 goat‐anti mouse IgM, Thermo Fisher Scientific, A21043). Cells were then washed, permeabilized for 20 minutes (0.3% Triton‐X), and washed again. The second block (10% NGS) was added for 1 hour at room temperature; following this, primary antibodies for GFAP (rabbit, 1:500, Sigma, G9269) and BIII‐Tubulin (mouse monoclonal, 1:1000, Sigma, T8660) were added and incubated at 4°C overnight. On the third day, secondaries for GFAP (1:400, Alexa 647 goat‐anti‐rabbit IgG, Thermo Fisher Scientific, A21245) and BIII‐Tubulin (1:400, Alexa 488 goat‐anti mouse IgG, Thermo Fisher Scientific, A11029) were added for 1 hour at room temperature, followed by Hoechst 33258 (1:1000) for 10 minutes at room temperature. Wells were covered with 1 mL of PBS and imaged.
2.8. Tissue section immunohistochemistry
Mice were overdosed with sodium pentobarbital (intraperitoneally injected [i.p.]) and transcardially perfused with 4°C PBS followed by 4% PFA. Tissue was post‐fixed in 4% PFA, then transferred and stored in 30% sucrose in PBS until sectioning. Tissue was cryosectioned at 20 μm and mounted on SuperFrost slides (Fisherbrand, Canada). For staining, slides were rehydrated with PBS for 10 minutes followed by 20 minutes of permeabilization with 0.3% Triton‐X (Thermo Fisher Scientific, 28313). Slides are then washed 3x 5 minutes in 1X PBS. The EdU Click‐iT (Thermo Fisher Scientific; C10340 [Alexa fluor 647]) reaction was performed according to the manufacturer's instructions. Slides were then blocked in a blocking solution for 1 hour (9.5 mL of 0.3% Triton‐X, 0.5 mL normal goat serum (005‐000‐121, Jackson Immunoresearch) and 100 mg of Bovine Serum Albumin (A96‐47‐100G, Sigma). Anti‐sox2 (rabbit polyclonal, 1:200, ab97959, Abcam, USA) was used as our primary antibody and left on the slides overnight at 4°C. The following day, slides were washed (3x 5 minute PBS) and then incubated with Alexa fluor 568 for Sox2 (goat anti‐rabbit, 1:400, A32723, Thermo Fisher Scientific) as a secondary antibody for 1 hour at room temperature. Slides were washed and then counterstained with DAPI (1:10000, D1306, Invitrogen). Final washes were performed before adding mounting medium (Dako, S3023) to the slides, which were then cover‐slipped with 24 × 60 microscope cover glass (Fisherbrand) and left to dry overnight at room temperature.
2.9. Imaging
Cells were imaged at ×20 using a Zeiss Microscope (Axio Observer, Canada). The O4+ oligodendrocytes were counted from four regions of the 48‐well plate. The percentage of O4+DAPI+/DAPI+ cells was calculated as the sum of the four regions per sphere and then averaged across the total number of spheres counted.
Sections were imaged at ×20 on the Zeiss Microscope. Four to five brain sections extending from the genu of the corpus callosum to the crossing of the anterior commissure were counted per animals. The percent of Sox2+ EdU+/Edu+ cells counted per area (200 × 250 microns, i.e., 205 K pixels) in the dorsolateral corner of the subventricular zone in both hemispheres. The average of both hemispheres was taken per section, and then this number was averaged per brain (ie, per slide).
2.10. Spinal cord injury
A spinal cord injury (SCI) was performed as previously described. 16 , 17 Briefly, mice were anesthetized with 5% isoflurane (inhalation) and ketoprofen (5 mg/kg; injected i.p.). A laminectomy was performed at level T8/9 and the spinal cord exposed. A 30‐gauge needle was used to lesion the dorsal funiculus from rostral to caudal, sparing the central canal, with the aid of a surgical microscope. Control mice received laminectomy but no needle injury.
2.11. In vivo Conditioned Media (CM) infusion
Mice were anesthetized as per above. Alzet 1007D mini osmotic pumps (0.05 μL/hour, Direct Corp, California, http://www.alzet.com/) containing spinal cord CM (SHI, WT, or control media) were implanted subcutaneously and attached to a cannula implanted into the lateral ventricle at the coordinates (relative to Bregma): AP +0.2 mm, ML 0.7 mm, and DV 2.5 mm below the dura. Control media (Neural Basal Media, 21103049, Thermo Fisher Scientific), SHI spCM or WT spCM was delivered for 7 days. Mice received two injections of EdU (50 mg/kg) prior to sacrifice, on day 6 and day 7 (1 hour prior to perfusion).
2.12. Statistics
Data are represented as means ± SEM. Two‐tailed t tests were performed to compare between two groups. One‐way ANOVAs were used to compare multiple groups with Dunnet's post hoc test. Two‐way ANOVAs were used to compare multiple groups with Tukey's post hoc test. Significance is considered P < .05. All graphs and analyses are generated from Excel (Microsoft) or GraphPad Prism 6 (Graph Pad Software, California).
3. RESULTS
3.1. MBP‐mediated inhibition of neural stem cell proliferation and oligodendrogenesis is niche‐dependent
Previous work has shown that spNSC proliferation is inhibited in a dose‐dependent fashion in the presence of exogenous MBP. 12 Furthermore, the absence of MBP leads to increased numbers of spNSCs as seen from shiverer mutant mice (SHI) that are devoid of mature MBP. 18 Here, we asked if brNSCs were similarly affected by MBP. In the first set of experiments, we compared the numbers of primary neurospheres derived from the forebrain and spinal cord of SHI (devoid of MBP) and littermate controls (WT, containing MBP). Primary cultures periventricular regions of the forebrain (lateral ventricles) and spinal cord were plated in the presence of epidermal growth factor (EGF), basic fibroblast growth factors (bFGF), and heparin; the numbers of free‐floating colonies of neural stem and progenitor cells (aka neurospheres) were assayed after 7 days in vitro. We observed a significant 10‐fold increase in the number of spinal cord neurospheres from SHI mice compared to WT controls (Figure 1A); however, there was no difference in the numbers of forebrain‐derived neurospheres between these same groups (Figure 1B). We postulated that this was due to: (a) an intrinsic difference between forebrain and spinal cord‐derived neurospheres in their response to MBP and/or (b) an extrinsic or niche‐dependent difference in how the MBP is processed and/or presented in these two regionally distinct microenvironments.
FIGURE 1.
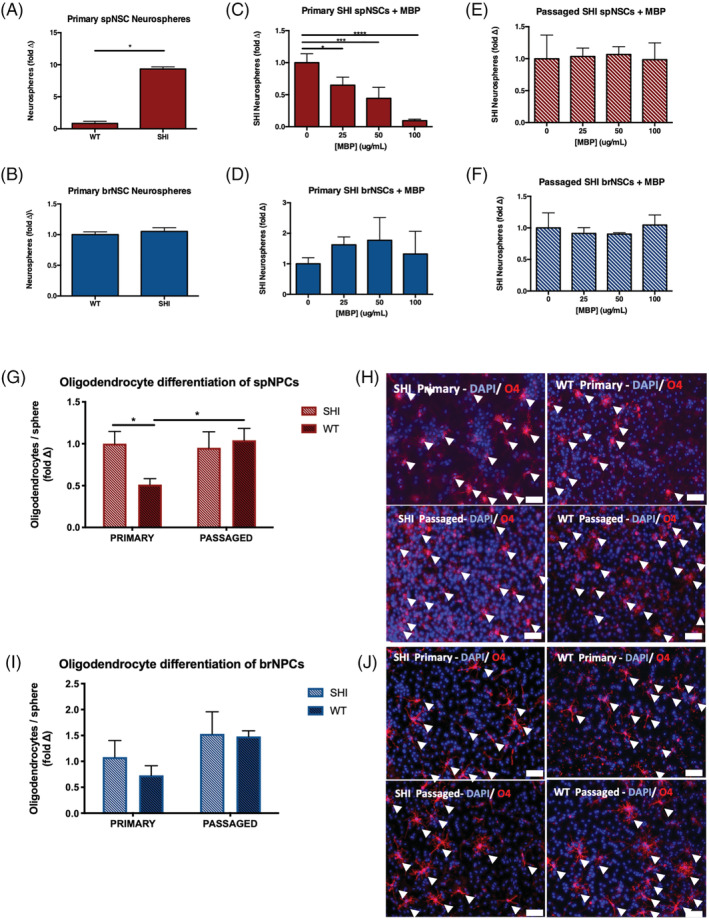
Myelin basic protein (MBP) inhibits neural stem cell proliferation and oligodendrocyte differentiation through its interaction with the spinal cord niche. A, A 10‐fold increase in the number of spinal cord‐derived neurospheres grown from primary culture of SHI mice compared to wild‐type (WT) controls (n = 3 independent experiments; P = .0124). B, No difference in the number of forebrain‐derived neurospheres from SHI and WT control mice. C,D, The numbers of primary SHI neurospheres are reduced in the presence of exogenous MBP in a dose‐dependent manner in the spinal cord—25, 50, and 100 μg/mL—(C) but not the forebrain (D) (n = 3 independent experiments per region). E,F, Passaged SHI neurosphere‐derived cells from spinal cord (E) and forebrain (F) are unaffected by the presence of exogenous MBP—25, 50, and 100 μg/mL (n = 4 independent experiments per region). G, Primary spinal cord neurospheres from WT mice give rise to significantly fewer oligodendrocytes than spinal cord neurospheres from SHI mice (P = .0475). Following passaging, oligodendrogenesis is no longer inhibited from WT‐derived neurospheres (P = .0473); n ≥ 7 neurospheres per group. H, Representative images of differentiated primary and passaged spinal cord‐derived neurospheres from SHI and WT mice; scale bar = 100 μm. Arrowheads indicate DAPI+/O4+ oligodendrocytes. I, Primary and passaged forebrain‐derived neurospheres from WT and SHI mice give rise to similar oligodendrocyte formation; n ≥ 6 neurospheres per group. J, Representative images of differentiated primary and passaged forebrain‐derived neurospheres from SHI and WT mice; scale bar = 100 μm. Arrowheads indicate DAPI+/O4+ oligodendrocytes. Data are represented as means ± SEM. Statistics: A,B, t tests; C‐F, one‐way ANOVAs; G,I, two‐way ANOVAs. *P < .05, ***P < .001, ****P < .0001
To confirm that the increase in neurospheres obtained from the SHI mice spinal cord was due to the lack of myelin and not a difference in the mouse genotype, we performed an experiment to remove myelin from the WT spinal cord and compared neurosphere growth to control WT spinal cord neurospheres (myelin not removed). We used a Percoll Plus density gradient to remove myelin from WT spinal cord tissue and found that myelin‐depleted tissue generated a significant 11‐fold increase in neurospheres compared to control WT spinal cord tissue (Supplemental Figure 1). This is similar to the increase in neurospheres previously observed from SHI spinal cords (Figure 1A).
We next performed a dose‐response curve on SHI primary forebrain and spinal cord NSC using exogenous MBP. Our previous work has shown that the physiological concentration of MBP in primary WT spinal cord cultures is ~80 μg/mL. 12 Therefore, we grew primary SHI cultures from forebrain and spinal cord of SHI mice in the presence of exogenous MBP over a range of concentrations (0, 25, 50, and 100 μg/mL). Identical to what was observed in Xu et al, 12 we observed a significant dose‐dependent reduction in the formation of spinal cord neurospheres (Figure 1C). However, the numbers of forebrain‐derived neurospheres were not changed in the presence of the exogenous MBP at these same concentrations (Figure 1D). Thus, this spoke to the fact that at the same concentrations of MBP, spinal cord and forebrain NSCs differ in their ability to proliferate and make neurospheres—compared to MBP void controls. To determine whether the MBP‐mediated inhibition of neurosphere formation was niche‐dependent, pure populations of NPCs (in the absence of niche cells) were derived from twice‐passaged SHI forebrain and spinal cord neurospheres and then grown in the presence of exogenous MBP. Strikingly, we observed a loss of the inhibition of SHI spinal cord neurospheres observed in primary cultures (which include the niche) (Figure 1E) and as predicted, the numbers of forebrain‐derived neurospheres were unchanged in the presence of MBP (Figure 1F). These data support the hypothesis that the interaction between MBP and the spinal cord niche drives the inhibition of spNSC proliferation.
Both SHI and WT neurospheres demonstrate tripotency and interestingly, we observed a significant difference in oligodendrocyte differentiation between SHI and WT neurospheres. As shown in Figure 1G,H, the fold change in the proportion of oligodendrocytes revealed a significant reduction in primary WT spinal cord neurospheres compared to primary SHI spinal cord neurospheres (2.9% ± 0.41% vs 4.9% ± 0.79% O4+ cells/neurosphere, WT vs SHI, respectively). Interestingly, passaged spinal cord neurospheres (grown in the absence of the niche) from WT mice gave rise to similar proportions of oligodendrocytes as passaged SHI and were not significantly different from SHI primary neurospheres (that were never exposed to MBP) (5.1 ± 0.72% vs 5.5% ± 0.85% O4+ cells/neurosphere, WT vs SHI, respectively) (Figure 1G,H). Similar experiments using brain derived neurospheres from WT and SHI mice revealed no significant differences in oligodendrogenesis between primary or passaged neurospheres (Figure 1I,J). Hence, MBP is inhibitory to spNPC‐derived oligodendrogenesis in the presence of the spinal cord niche.
3.2. The spinal cord niche is sufficient to inhibit neurosphere formation and oligodendrocyte differentiation
To further test the hypothesis that the MBP‐mediated inhibition is dependent on the spinal cord niche, we performed a series of coculture experiments with primary forebrain and spinal cord‐derived cells. We predicted that if the niche influenced MBP presentation and subsequently regulated NSC proliferation, then MBP from primary spinal cord cultures would be inhibitory to forebrain‐derived neurosphere formation. We dissected the periventricular region from yellow fluorescent protein (YFP) reporter mice, which express MBP (MBP+), and cocultured these cells with SHI primary forebrain tissue (MBP‐) at plating ratios of 5:5 (YFP: SHI) or 9:1 (YFP: SHI). 15 The use of YFP reporter mice enabled us to distinguish MBP+ neurospheres from MBP‐negative, YFP‐negative SHI neurospheres. Forebrain SHI cells were plated at 1 and 5 cells/μL to serve as controls for the coculture. After 7 days in vitro, the numbers of forebrain SHI neurospheres were quantified (YFP‐negative). We observed a significant reduction in SHI forebrain‐derived neurospheres when plated in the presence of YFP+ spinal cord‐derived cells (28% ± 8% reduction in 5:5 YFP:SHI cultures relative to SHI alone [5 cells/μL] and 80% ± 16% reduction in 1:9 [SHI:YFP] cultures relative to SHI alone [1 cell/μL]) (Figure 2A). Hence, MBP presented in the spinal cord niche inhibited NSC proliferation irrespective of the regional origin of the NSCs (brain or spinal cord). The fact that MBP from primary forebrain cultures was not inhibitory to brNSCs prompted us to make the prediction that MBP in the context of primary forebrain cultures would not be inhibitory. Indeed, in coculture experiments, we observed no change in the numbers of SHI forebrain‐derived neurospheres when plated with YFP (MBP+) forebrain cells compared to SHI forebrain cultures alone (Supplemental Figure 2). Together, these findings support the hypothesis that brNSCs and spNSCs are not intrinsically different and that MBP is sufficient to inhibit their proliferation when presented in the context of the primary spinal cord niche.
FIGURE 2.
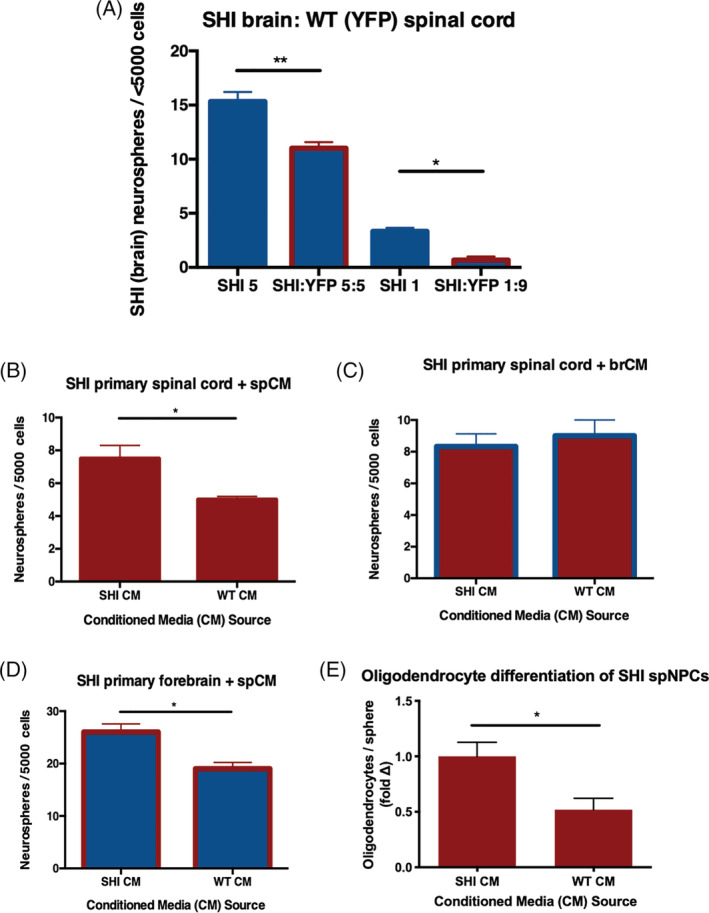
Myelin basic protein (MBP) interacts with the spinal cord niche to release an inhibitory factor that inhibits proliferation and oligodendrocyte differentiation of forebrain and spinal cord neural stem cells (NSCs). A, MBP in primary spinal cord cultures from YFP+ mice leads to reduced numbers of SHI forebrain (YFP‐negative) neurospheres in cocultures (n = 3 independent experiments per condition). B, SHI‐derived spinal cord NSCs cultures generate decreased numbers of neurospheres in the presence of littermate wild‐type (WT) spCM compared to SHI spCM (n = 3 independent experiments). C, SHI‐derived spNSCs form comparable numbers of neurospheres in both SHI brCM and WT brCM (n = 3 independent experiments); blue outline on bars indicates the presence of brCM. D, SHI‐derived brNSCs are similarly impaired in neurosphere formation when exposed to WT spCM compared to SHI spCM (n = 3 independent experiments); red outline on bars indicates the presence of spCM. E, SHI‐derived spinal cord neurospheres generate fewer oligodendrocytes in the presence of littermate WT spCM compared to SHI spCM (P = .0102).; n ≥ 7 neurospheres per group. Data are represented as means ± SEM. Statistics: A, One‐way ANOVA; B‐E, t tests. *P < .05, **P < .01
Since our results suggest that MBP is exerting its inhibition of NSC proliferation through interaction with the spinal cord niche, we sought to determine whether this inhibition was mediated by a released factor. We collected CM from both primary forebrain (brCM) and spinal cord dissections (spCM) from SHI and WT control mice. The WT CM was filtered to remove cellular debris and endogenous MBP (validated using an ELISA, MBP = 9.16 ± 0.82 ng/mL from filtered WT spCM—vs 80 μg/mL from unfiltered spCM 12 ). We found that SHI spNSCs plated in WT spCM resulted in a 33% ± 13% loss of neurosphere formation compared to SHI spCM (Figure 2B). As expected, when CM was derived from forebrain cultures, regardless of the presence of MBP, it did not affect SHI spinal cord neurosphere formation (Figure 2C). These findings suggest that a factor released from spinal cord niche cells, in response to MBP, inhibits neurosphere formation. Importantly, we observed a 26.9% ± 1.6% reduction in forebrain‐derived neurosphere formation with WT spCM (Figure 2D). Hence, these findings reveal that a factor (other than MBP) is released from the primary spinal cord niche in response to MBP which is inhibitory to both spinal cord and forebrain neurosphere formation.
Having shown that the neurospheres grown in the presence of MBP within the spinal cord niche were less oligodendrogenic, we next asked whether the presence of spCM was sufficient to inhibit oligodendrocyte formation. We took passaged spinal cord neurospheres from SHI mice and differentiated them in the presence of spCM for 7 days. In the presence of WT spCM, there was a significant 44% ± 13% decrease in the proportion of oligodendrocytes compared to SHI spCM (Figure 2E). Collectively, our results show that the spinal cord niche is sufficient to inhibit NSC proliferation and oligodendrocyte differentiation.
3.3. The polycationic charge of MBP mediates its interaction with the spinal cord niche to release factor(s) inhibitory to neural stem cell proliferation
Studies have shown that MBP and other highly positively charged proteins can embed themselves into the negatively charged cell membrane and can regulate cell signalling. 19 , 20 , 21 To explore whether MBP's high polycationic charge (+19) may play a role in its niche‐dependent activity, we modified the charge and asked if this altered the inhibitory effects of MBP on spNSC behavior in primary spinal cord cultures. We used UTC8, a recombinant form of murine 18.5‐kDa MBP that has pseudo‐citrullinated residues to decrease the net positive charge from +19 to +13, and UTC1, which is a recombinant form of native MBP with a similar +19 net positive charge and serves as our control. 11 As seen in Figure 3A, increasing concentrations of UTC8 was less inhibitory on spinal cord neurosphere formation than the UTC1 controls. This result suggests that the high positive charge and resulting basic nature of MBP plays a role in mediating the inhibition of neurosphere formation.
FIGURE 3.
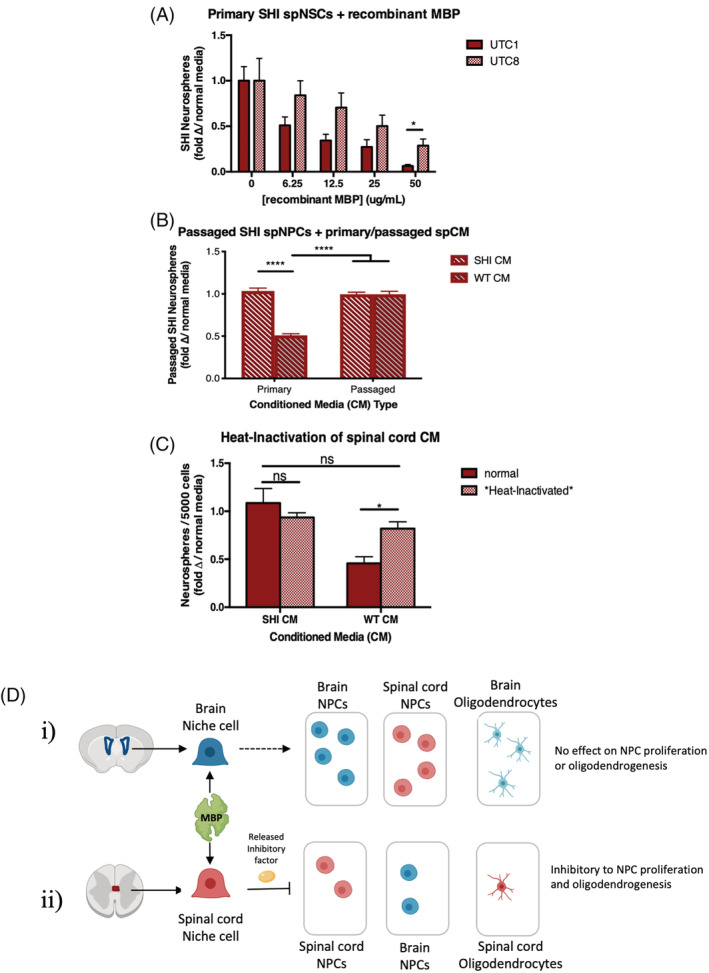
Myelin basic protein (MBP)'s high polycationic charge plays a role in the niche mediated release of a protein that is inhibitory to forebrain and spinal cord neural stem cell (NSC) proliferation and oligodendrogenesis. A, At increasing concentrations of recombinant 18.5‐kDa MBP, the less positively‐charged, pseudo‐deiminated UTC8 isoform has less of an inhibitory effect on primary SHI spinal cord neurosphere formation than the highly positively charged isoform UTC1 (analogous to unmodified 18.5‐kDa MBP) (n = 4 independent experiments). B, Passaged SHI spNPCs are inhibited in their ability to form neurospheres when exposed to primary wild‐type (WT) spCM. Passaged WT spCM (niche removed) has no effect on neurosphere formation compared to passaged and primary SHI spCM. C, Heat‐inactivated WT spCM rescues neural stem cell numbers back to SHI spCM levels. D, Schematic reflecting that MBP's inhibitory effect on NSC proliferation and oligodendrogenesis is mediated by factors released from niche cells. Data are represented as means ± SEM. Statistics: A‐C, Two‐way ANOVAs. *P < .05, ****P < .0001; ns, not significant
Based on our previous observations, we also predicted that NSCs derived from passaged neurospheres (niche removed) would also be inhibited by WT spCM since it contained the inhibitory factor. This would negate the possibility that passaging might select for a subset of NSCs or restricted progenitors that are unresponsive to MBP inhibition (as seen earlier in Figure 1E). Indeed, we found that WT spCM is sufficient to inhibit passaged SHI spinal cord neurosphere formation (Figure 3B) supporting the hypothesis that a spinal cord niche‐dependent factor is released in response to MBP. To test our hypothesis that this inhibition was mediated by a released factor, we performed loss of function experiments using heat inactivation of the CM. We predicted that heat inactivation of WT spCM would result in a rescue in neurosphere formation because of a loss of inhibition. CM was collected and filtered from the spinal cord of WT (WT spCM) and SHI (SHI spCM) mice, and heat‐inactivated or left untreated. The SHI spinal cord primary cultures were exposed to heat‐inactivated or untreated CM and the number of neurospheres was assessed. We found that heat‐inactivated WT spCM was no longer inhibitory to neurosphere formation (Figure 3C). This rescue of neurosphere formation with heat‐inactivated WT spCM supports the hypothesis that an inhibitory, heat‐labile, protein/factor facilitates MBP's indirect inhibition of neurosphere formation. Importantly, heat‐inactivated SHI spCM had no effect on neurosphere formation compared to SHI spCM that was not heat‐inactivated. Together, these findings reveal that MBP is inhibitory to neurosphere formation because of the presence of an inhibitory factor rather than the absence of a permissive factor. This leads us to our current model of MBP's regulation of NSC kinetics via the spinal cord niche (Figure 3D).
3.4. Exposure of the MBP‐exposed spinal cord niche is sufficient to inhibit proliferation of NPCs along the neuraxis
Our findings indicate that MBP‐exposed spCM contains an inhibitory factor that regulates NSC behavior in vitro. Next, we asked if this would also be true in vivo. We predicted that following SCI, we would observe reduced NPC proliferation in WT mice compared to SHI mice, because of the presence of MBP. To test our hypothesis, we performed a minimal SCI, 16 , 17 which is known to cause MBP to be released into the spinal cord parenchyma, in SHI and WT mice. Mice were injected with the thymidine analogue EdU to label proliferating cells, on day 5 post‐injury (Figure 4A). The numbers of EdU+ cells in the periventricular region of the central canal of the spinal cord was assessed. We observed a 2.3 ± 0.4‐fold increase in the numbers of EdU+ cells in the injured SHI periventricular region, compared to injured WT mice (Figure 4B,C). Under baseline conditions, there was no difference in periventricular proliferation in WT vs SHI mice (Supplemental Figure 3). Hence, the presence of MBP in the spinal cord niche was sufficient to inhibit injury‐induced proliferation of NPCs in the spinal cord in vivo.
FIGURE 4.
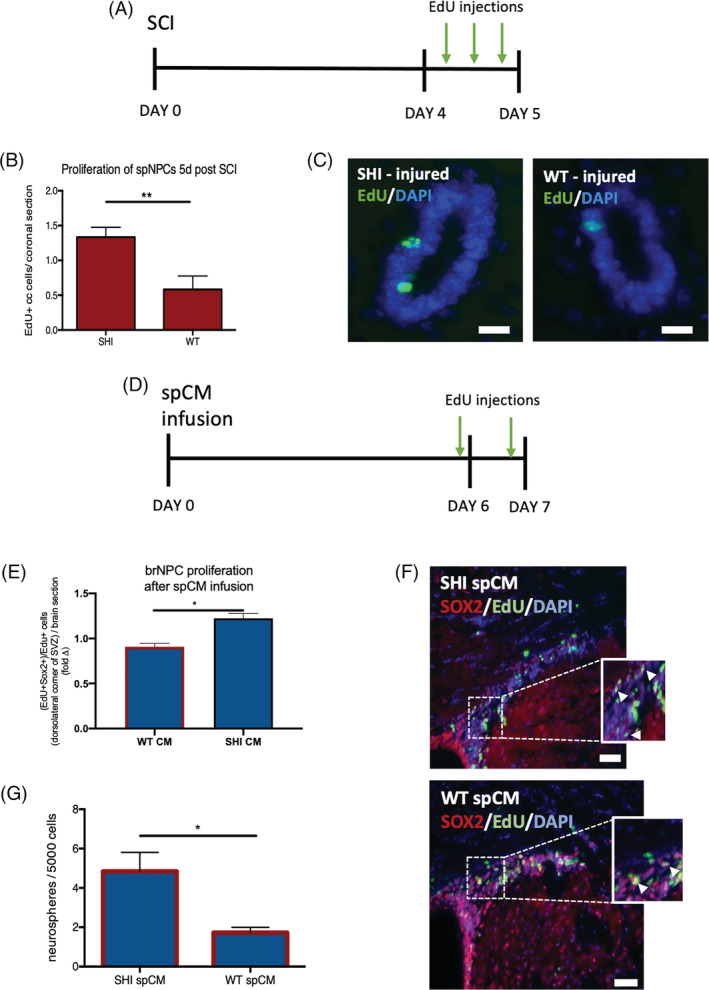
Infusion of spinal cord‐derived conditioned media from wild‐type (WT) cultures inhibits neural precursor cell proliferation. A, Experimental paradigm; SCI, spinal cord injury, arrows = EdU injections. B, The numbers of EdU+ cells in the periventricular region of the spinal cord in WT mice is significantly reduced compared to SHI mice (n = 3 mice per group). C, Representative image of EdU+ cells (green) in spinal cord periventricular region at 5 days post SCI; scale bar = 20 μm. D, Experimental paradigm for the spCM intraventricular infusion. E, The fold change in SHI CM vs WT CM are shown relative to the Control media infused brains. There is a significant decrease in Sox2+/EdU+ NPC proliferation in the presence of WT spCM in the dorsolateral corner of the LV (P = .0152). Red outline indicates the presence of CM; n = 3 mice per group. F Representative images of Sox2+/EdU+ cells from SHI‐derived and WT‐derived spCM infused brains; scale bar = 100 μm. G, Significantly fewer neurospheres are formed from forebrain of mice that received WT spCM infusion (n = 3 mice/condition). Red outline represents the presence of spCM. Data are represented as means ± SEM. Statistics: B,E, t tests; G, one‐way ANOVA. *P < .05, **P < .01
In the next series of experiments, we asked if in vivo infusion of CM from the MBP‐exposed spinal cord (WT spCM) would lead to reduced proliferation in the subventricular zone (SVZ) where NPCs reside in the forebrain. We observed 41% ± 14% fewer proliferating (EdU+) cells in the dorsolateral corner of the SVZ in mice that received WT CM compared to SHI CM infused mice (Supplemental Figure 4). To determine the identity of the EdU+ cells accounting for the change in proliferation, we performed immunohistochemistry for Sox2 (to label NPCs) and calculated the proportion of EdU+Sox2+ cells over the total number of EdU+ cells. Consistent with our prediction, the proportion of proliferating NPCs in the dorsolateral corner of the SVZ was significantly decreased between WT CM infused and SHI CM infused mice (0.91 ± 0.07 vs 1.22 ± 0.09 relative to control media infused mice, Sox2+EdU+ cells; Figure 4E,F). In a second series of mice, an identical infusion paradigm was performed followed by the neurosphere assay on day 7. Mice that received infusions of WT CM had a 64% ± 4.5% decrease in forebrain neurospheres compared to mice that received SHI CM (Figure 4G). Taken together, these findings demonstrate that the spinal cord niche‐derived inhibitory factor impacts neural stem and progenitor cells along the neuraxis.
4. DISCUSSION
Herein, we have shown that primary forebrain and spinal cord tissue differ in their response to exogenously presented MBP. Neurosphere formation from spNSCs is inhibited whereas those from brNSCs are unaffected at the same concentration of exogenous MBP. We have demonstrated that spNSCs behave similar to brNSCs and are no longer inhibited by MBP when cultured in the presence of MBP in the absence of their niche, thus indicating that MBP does not provide a direct inhibitory effect. Furthermore, we have shown that following exposure to MBP, a heat‐labile factor from the spinal cord niche, but not the forebrain niche, is sufficient to alter NSC behavior along the neuraxis. Based on our studies, we cannot rule out the possibility that a combination of more than one factor (membrane‐bound or secreted) is responsible for the inhibitory effect on neural precursor proliferation however, we have demonstrated that a soluble, heat‐labile factor in the CM (after MBP exposure to the primary spinal cord niche) is sufficient to account for the observed inhibition. We have further demonstrated that following SCI, and in response to intraventricular infusion of MBP‐exposed spinal cord niche CM (spCM), the NSPC response is consistent with predictions, demonstrating reduced proliferation. Finally, our findings indicate that the presence of MBP can lead to reduced oligodendrogenesis in the spinal cord derived NPC differentiation assay. Together, these findings have potential implications for disease and injury models.
MBP is classified as an IDP meaning that its conformation in solution is dependent on the composition of the microenvironment. 13 Indeed, IDPs have been shown to serve multifunctional regulatory roles in aspects such as signal transduction, adhesion, and cell cycle regulation—owing to their molecular flexibility which enables them to adopt any local conformations needed to bind to different targets. 19 , 20 , 21 In support of this, our findings suggest that MBP plays a signaling role in the spinal cord niche, but not the forebrain, which results in the release of a factor that regulates the behavior of NSC populations along the neuraxis. This function is different from its intracellular role of bringing together cytosolic leaflets of oligodendrocytes to create the compact myelin sheath. Further studies are warranted to determine what factors in the extracellular space might be contributing to this signaling role of MBP, as well as what cells in the spinal cord niche are responsive to this adaptative conformation that leads to released factors that regulate NSC kinetics. This would also have implications in designing therapeutic interventions to treat spinal cord injuries as we consider modifying the microenvironment at the site of injury to remove MBP‐mediated inhibition of NSCs as a means to promote recovery. For example, herein we have demonstrated that MBP's high net positive charge plays a role in its niche interactions, and thus a possible therapeutic target could be neutralizing this positive charge to prevent the downstream cascade leading to NSC inhibition.
The finding that oligodendrogenesis is regulated by the presence of MBP in the spinal cord has important implications for neural repair. It is well established in the literature that oligodendrocyte formation following SCI is critical for neural repair and functional recovery. 22 , 23 Studies have shown that after SCI, spinal cord NPCs have a limited ability to differentiate into oligodendrocytes because of a modified expression of growth factors that are necessary for oligodendrocyte maintenance and growth. Additionally, Salewski et al have shown that mature remyelinating cells are necessary for functional recovery following SCI. 24 Our results have demonstrated that we are able to regulate the number of oligodendrocyte precursors with the presence of MBP. Therefore, the effects seen on mature oligodendrocytes after SCI may, in part, result from the effects of MBP on oligodendrocyte precursors.
Our findings support the hypothesis that regionally distinct NSCs along the neuraxis are not intrinsically different, but rather their behavior is dictated by their respective environments. A compelling example of niche regulated behavior of NPCs comes from studies showing that when NPCs from the aneurogenic rat spinal cord were transplanted into the SVZ of rat pups, a known neurogenic niche of NSCs, the spinal cord‐derived cells migrated to distinct regions of the brain (ie, olfactory bulb, frontal cortex, and occipital cortex), and differentiated into neurons that were morphologically and functionally similar to host neurons. 25 Additionally, NSCs from the neurogenic SVZ of the adult human brain have been shown to exclusively give rise to glial lineages when transplanted into the adult rat spinal cord. 26 This observation is consistent with our findings that regionally distinct NSC behavior, proliferation, and oligodendrogenesis are dictated by the environment.
Our results demonstrating CNS niche‐dependent effects of a single molecule have also been shown with the drug metformin, which has different effects on NSC kinetics (such as proliferation and differentiation) depending on the niche. 27 Specifically, metformin's effects on NSCs are sex and age‐dependent. Moreover, nonresponsive NPCs cultured with metformin‐exposed niche cells leads to altered NPC kinetics. The presence of sex hormones is thought to underlie this differential responsiveness. 27 The findings are comparable to the niche‐dependent behavior in response to MBP whereby similar effects on brain and spinal cord NSCs can be elicited, but only when MBP is presented in the context of the spinal cord niche.
5. CONCLUSION
Neural stem cells are a promising therapeutic for neural regeneration through activation of endogenous precursors or cell transplantation. To harness their potential, it is important to understand the factors and environmental conditions which influence their behavior. Here, we have shown that regionally distinct environments, in response to the same protein (MBP), can differentially regulate NPC kinetics and cell fate. Delineating the mechanism in which spinal cord MBP mediates its inhibitory effects will aid in the optimization of treatment strategies to repair the injured CNS.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
N.L.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript; C.B.: collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript; R.S.: collection and assembly of data and final approval of the manuscript; V.V.B., G.H.: provision of study material, data analysis, final approval of the manuscript; W.X.: conception and design, collection and assembly of data, data analysis and interpretation; C.M.D.: conception and design, financial support, administrative support, data analysis and interpretation, manuscript writing, final approval of the manuscript.
Supporting information
Supplemental Figure 1 The removal of myelin from primary WT spinal cord cultures results in an increase in neurosphere formation. The use of Percoll Plus reagent to remove myelin from primary WT spinal cord cultures results in an 11‐fold increase in neurospheres compared to normal WT spinal cord controls. ****P ≤ 0.0001
Supplemental Figure 2 No change in the number of SHI forebrain neurospheres was found when plated with YFP+ forebrain cells (MBP+) compared to SHI forebrain cultures alone
Supplemental Figure 3 Mice received 6 injections of EdU over 3 days and were perfused on day 3. The numbers of EdU+/DAPI+ cells in the periventricular region are similar in both WT and SHI mice. Scale bar = 20 μm
Supplemental Figure 4 Mice were infused with spCM for 7 days and received EdU injections on days 6 and 7 prior to sacrifice. Significantly fewer proliferating EdU+/DAPI+ cells are found in the dorsolateral corner of the SVZ in WT spCM infused mice compared to SHI spCM infused mice. Scale bar = 100 μm. CC = corpus callosum, LV = Lateral ventricle
ACKNOWLEDGMENTS
This work was funded by CIHR (CMM) and the Krembil Foundation (CMM); Nishanth Lakshman is the recipient of the Fredrick Banting and Charles Best Canada Graduate Scholarship ‐ Doctoral Award (CGS‐D) and WX is the recipient of the Carlton Marguerite Smith Medical Research Fellowship (U of Toronto). The support of NSERC (Discovery Grant to GH) is also acknowledged.
Lakshman N, Bourget C, Siu R, et al. Niche‐dependent inhibition of neural stem cell proliferation and oligodendrogenesis is mediated by the presence of myelin basic protein. Stem Cells. 2021;39:776–786. 10.1002/stem.3344
Funding information Canadian Institutes of Health Research; Krembil Foundation (CMM)
Contributor Information
Nishanth Lakshman, Email: nishanth.lakshman@mail.utoronto.ca.
Cindi M. Morshead, Email: cindi.morshead@utoronto.ca.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Rao MS. Multipotent and restricted precursors in the central nervous system. Anat Rec. 1999;257(4):137‐148. [DOI] [PubMed] [Google Scholar]
- 2. Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433‐1438. [DOI] [PubMed] [Google Scholar]
- 3. Weiss S, Dunne C, Hewson J, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16(23):7599‐7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morshead CM, van der Kooy D. Postmitotic death is the fate of constitutively proliferating cells in the subependymal layer of the adult mouse brain. J Neurosci. 1992;12(1):249‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol. 2006;498(4):525‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fuentealba LC, Obernier K, Alvarez‐Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10(6):698‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mirzadeh Z, Merkle FT, Soriano‐Navarro M, Garcia‐Verdugo JM, Alvarez‐Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamilton LK, Truong MKV, Bednarczyk MR, Aumont A, Fernandes KJL. Cellular organization of the central canal ependymal zone, a niche of latent neural stem cells in the adult mammalian spinal cord. Neuroscience. 2009;164(3):1044‐1056. [DOI] [PubMed] [Google Scholar]
- 9. Hugnot JP, Franzen R. The spinal cord ependymal region: a stem cell niche in the caudal central nervous system. Front Biosci (Landmark Ed). 2011;16:1044‐1059. [DOI] [PubMed] [Google Scholar]
- 10. Osman AM, Rodhe J, Shen X, Dominguez CA, Joseph B, Blomgren K. The secretome of microglia regulate neural stem cell function. Neuroscience. 2019;405:92‐102. [DOI] [PubMed] [Google Scholar]
- 11. Harauz G, Boggs JM. Myelin management by the 18.5‐kDa and 21.5‐kDa classic myelin basic protein isoforms. J Neurochem. 2013;125(3):334‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu W, Sachewsky N, Azimi A, Hung M, Gappasov A, Morshead CM. Myelin basic protein regulates primitive and definitive neural stem cell proliferation from the adult spinal cord. Stem Cells. 2017;35(2):485‐496. [DOI] [PubMed] [Google Scholar]
- 13. Vassall KA, Bamm VV, Harauz G. MyelStones: the executive roles of myelin basic protein in myelin assembly and destabilization in multiple sclerosis. Biochem J. 2015;472(1):17‐32. [DOI] [PubMed] [Google Scholar]
- 14. Mirzadeh Z, Merkle FT, Soriano‐Navarro M, Garcia‐Verdugo JM, Alvarez‐Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coles‐Takabe BLK, Brain I, Purpura KA, et al. Don't look: growing clonal versus nonclonal neural stem cell colonies. Stem Cells. 2008;26(11):2938‐2944. [DOI] [PubMed] [Google Scholar]
- 16. Lakshman N, Xu W, Morshead CM. A neurosphere assay to evaluate endogenous neural stem cell activation in a mouse model of minimal spinal cord injury. J Vis Exp. 2018;139. https://www.jove.com/t/57727/a-neurosphere-assay-to-evaluate-endogenous-neural-stem-cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131(1):177‐187. [DOI] [PubMed] [Google Scholar]
- 18. Chernoff GF. Shiverer: an autosomal recessive mutant mouse with myelin deficiency. J Hered. 1981;72(2):128. [DOI] [PubMed] [Google Scholar]
- 19. Tompa P, Szász C, Buday L. Structural disorder throws new light on moonlighting. Trends Biochem Sci. 2005;30(9):484‐489. [DOI] [PubMed] [Google Scholar]
- 20. Tompa P. The interplay between structure and function in intrinsically unstructured proteins. FEBS Lett. 2005;579(15):3346‐3354. [DOI] [PubMed] [Google Scholar]
- 21. Benmerah A, Scott M, Poupon V, Marullo S. Nuclear functions for plasma membrane‐associated proteins? Traffic. 2003;4(8):503‐511. [DOI] [PubMed] [Google Scholar]
- 22. Duncan ID, Brower A, Kondo Y, Curlee JF, Schultz RD. Extensive remyelination of the CNS leads to functional recovery. Proc Natl Acad Sci USA. 2009;106(16):6832‐6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barnabé‐Heider F, Göritz C, Sabelström H, et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7(4):470‐482. [DOI] [PubMed] [Google Scholar]
- 24. Salewski RP, Mitchell RA, Li L, et al. Transplantation of induced pluripotent stem cell‐derived neural stem cells mediate functional recovery following thoracic spinal cord injury through remyelination of axons. Stem Cells Translational Medicine. 2015;4(7):743‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang H, Mujtaba T, Venkatraman G, Wu YY, Rao MS, Luskin MB. Region‐specific differentiation of neural tube‐derived neuronal restricted progenitor cells after heterotopic transplantation. Proc Natl Acad Sci USA. 2000;97(24):13366‐13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akiyama Y, Honmou O, Kato T, Uede T, Hashi K, Kocsis JD. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001;167(1):27‐39. [DOI] [PubMed] [Google Scholar]
- 27. Ruddy RM, Adams KV, Morshead CM. Age‐ and sex‐dependent effects of metformin on neural precursor cells and cognitive recovery in a model of neonatal stroke. Sci Adv. 2019;5(9):eaax1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 The removal of myelin from primary WT spinal cord cultures results in an increase in neurosphere formation. The use of Percoll Plus reagent to remove myelin from primary WT spinal cord cultures results in an 11‐fold increase in neurospheres compared to normal WT spinal cord controls. ****P ≤ 0.0001
Supplemental Figure 2 No change in the number of SHI forebrain neurospheres was found when plated with YFP+ forebrain cells (MBP+) compared to SHI forebrain cultures alone
Supplemental Figure 3 Mice received 6 injections of EdU over 3 days and were perfused on day 3. The numbers of EdU+/DAPI+ cells in the periventricular region are similar in both WT and SHI mice. Scale bar = 20 μm
Supplemental Figure 4 Mice were infused with spCM for 7 days and received EdU injections on days 6 and 7 prior to sacrifice. Significantly fewer proliferating EdU+/DAPI+ cells are found in the dorsolateral corner of the SVZ in WT spCM infused mice compared to SHI spCM infused mice. Scale bar = 100 μm. CC = corpus callosum, LV = Lateral ventricle
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


