Abstract
Objectives
To describe incidence, risk factors, and treatment of poststroke epilepsy (PSE) in Germany based on claims data.
Methods
Retrospective analysis of claims data from a German public sickness fund (AOK PLUS). Patients with acute stroke hospitalizations from January 01, 2011 and December 31, 2015 (index hospitalization) were followed for 12–72 months. Outcomes included incidence of PSE (patients with ≥2 seizure claims [during/after index hospitalization], or ≥1 seizure claim after index hospitalization), multivariate Cox‐regression analyses of time to seizure claim and death after index stroke hospitalization discharge, and antiepileptic drug (AED) treatment.
Results
Among 53 883 patients with stroke (mean follow‐up of 829.05 days [median 749]), 6054 (11.24%) had ≥1 seizure claim (mean age 73.95 years, 54.18% female). 2130 (35.18%) patients had a seizure claim during index hospitalization (indicative of acute symptomatic seizures). Estimated incidence of PSE (cases/1000 patient‐years) was 94.49 within 1 year. Risk of seizure claim following hospital discharge was higher in patients with hemorrhagic stroke (hazard ratio [HR] =1.13; p <.001) vs those with cerebral infarction. Seizure claim during index hospitalization was a risk factor for seizure claims after hospital discharge (HR =6.97; p <.001) and early death (HR =1.78; p <.001). In the first year of follow‐up, AEDs were prescribed in 73.75% of patients with seizure claims.
Conclusions
Incidence of PSE was in line with previous studies. Hemorrhagic stroke and seizure claim during index hospitalization were risk factors for seizure claims after hospital discharge. Most patients with seizure claims received AED treatment.
Keywords: anticonvulsants, epilepsy, incidence, risk factors, seizures, stroke
1. INTRODUCTION
Epilepsy is a common neurological disorder caused by multiple factors. 1 , 2 Stroke is one of the most common causes of unprovoked seizures and thus epilepsy in the elderly population. 3 Acute symptomatic seizures (formerly also termed “early seizures”) occur within 7 days after stroke and unprovoked seizures (formerly also termed “late seizures”) thereafter. 4 The occurrence of at least one unprovoked seizure is required for a clinical diagnosis of epilepsy. 5 , 6
Depending on the type of cerebrovascular disease, 8–15% of patients with stroke may develop poststroke epilepsy (PSE), 7 , 8 , 9 which has a negative impact on stroke prognosis and quality of life. 2 Risk factors for unprovoked seizures and epilepsy following stroke may include younger age, greater stroke severity, involvement of middle cerebral artery territory, hemorrhagic stroke, and acute symptomatic seizures. 10 , 11 , 12 , 13
There is no consensus on how to best treat patients with PSE. 14 Representative data for the treatment of PSE in Germany, particularly AED treatment, are limited. The objectives of this study were to describe the incidence and risk factors of PSE in Germany based on claims data, and to describe outpatient treatment of PSE.
2. METHODS
2.1. Overall study design
This retrospective study was based on statutory claims data from AOK PLUS, a German public sickness fund insuring about 3.2 million persons in the states of Saxony and Thuringia. The database contains anonymized patient‐level data including demographics, inpatient care (excluding medication), outpatient care, and outpatient medication. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was consistent with Good Epidemiology Practices and applicable regulatory requirements. Because of the noninterventional nature of the study, which analyzed a retrospective anonymized data set only, no ethical vote was needed.
The study period was from 01 January, 2010 to 31 December, 2016 (Figure 1A). Patients who had at least one hospitalization with a main diagnosis of acute stroke (International Classification of Diseases [ICD]‐10 I60/I61/I62/I63/I64.‐; subarachnoid hemorrhage, intracerebral hemorrhage, other nontraumatic intracranial hemorrhage, cerebral infarction, and stroke not specified as hemorrhage or infarction) were included. The index date was defined as the date of first observed acute stroke hospitalization. To allow for a baseline period of 12 months before and a follow‐up time of at least 12 months after index (a shorter follow‐up was permitted in case of death), patients with acute stroke between 01 January, 2011 and 31 December, 2015 were eligible for the analyses (Figure 1B). Eligible patients had continuous AOK PLUS insurance throughout the follow‐up period, which started at index and lasted until the end of data availability (31 December, 2016) or death. Patients with stroke or an inpatient/outpatient ICD‐10 G40.‐ claim 12 months before the first observed stroke hospitalization were excluded.
FIGURE 1.
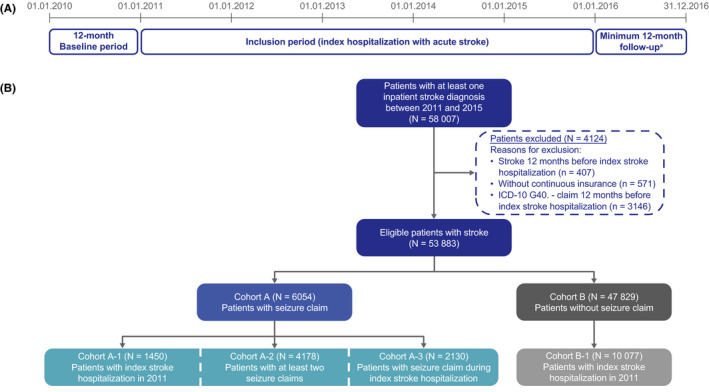
(A) Study design; (B) patient sample. Cohorts A1‐3 are not mutually exclusive. aExcept patients who died in <12 months, who were still included
A seizure claim was defined as at least one ICD‐10 G40.‐ claim during/after index stroke hospitalization. Seizure claims data do not always indicate a clinical diagnosis of epilepsy; for example, patients may have had acute symptomatic seizures following stroke but may not have developed epilepsy. Seizure claims during index hospitalizations were considered indicative of patients with acute symptomatic seizures, whereas claims after index hospitalization (or multiple claims) were considered indicative of unprovoked seizures/PSE. However, there remains a degree of uncertainty as the exact dates when seizures occurred were unknown (acute symptomatic seizures are defined as seizures occurring within 7 days of stroke, and unprovoked seizures are defined as those occurring after 7 days). ICD‐10 R56.8 (other and unspecified convulsions) claims were not analyzed, since this code is not specific to epileptic seizures and would not generally be used for acute symptomatic or unprovoked seizures in Germany.
2.2. Study cohorts
The main cohort (Cohort A) comprised all eligible patients with acute stroke who had at least one seizure claim; Cohort B comprised those without seizure claims (Figure 1B). A subgroup of patients with an index stroke hospitalization between 01 January, 2011 and 31 December, 2011 (follow‐up period of 60–72 months, or until death if sooner), was also analyzed (Cohort A‐1 [with seizure claim] and Cohort B‐1 [without seizure claim]), representing the patients with the longest follow‐up times. A sensitivity analysis was performed in a subgroup of patients with at least two seizure claims (during or after index hospitalization) (Cohort A‐2). Analyses were also performed in patients with at least one seizure claim during index stroke hospitalization (Cohort A‐3).
2.3. Outcomes
We reported the following outcomes: incidence of PSE, patient characteristics, time from stroke to seizure claim, risk factors for seizure claim after index stroke hospitalization, mortality, and risk factors for death after index stroke hospitalization, neuropsychiatric comorbidities at baseline and follow‐up, and treatment of patients with seizure claims.
Incidence of PSE was calculated from the number of eligible stroke patients in the sickness fund (cases per 1000 patient‐years) within the first 90/180/365 days of follow‐up and over the total follow‐up period, and was defined as patients with ≥2 seizure claims (during/after index hospitalization), or ≥1 seizure claim after index hospitalization. Patients who had only one seizure claim during the index hospitalization and no further seizure claims were excluded, since these patients may have had an acute symptomatic seizure but did not develop epilepsy. Reported patient characteristics included age, sex, and Charlson Comorbidity Index (CCI), 15 and description of the index hospitalization (type of stroke [by ICD‐10], length of stay, and complexity of stroke treatment). Stroke treatment complexity was based on German inpatient diagnosis‐related group (DRG) codes, which are based on several parameters including main diagnosis, codiagnoses, procedures, complications, and length of hospital stay. The codes reflect treatment expenditure, which decreases from A to H.
Kaplan‐Meier analysis of time from beginning of the index stroke hospitalization to first seizure claim was used to estimate the risk of seizure claim at specified time points; patients were censored at death and end of observation period. Multivariate Cox‐regression analysis of time from index stroke hospitalization discharge to first post‐index hospitalization seizure claim was performed to identify factors associated with PSE. Similarly, a multivariate Cox‐regression analysis was performed to identify factors associated with early death. Both analyses were performed by stepwise backward elimination of independent variables (p >.1), including sex, age, baseline CCI, seizure claim during index hospitalization (yes/no), number of all‐cause hospitalizations during the baseline period, length of index hospital stay (days), type of stroke, and complexity of stroke treatment. For the analysis of early death, seizure claim during index hospitalization was considered as a time‐dependent variable. For analysis of time to first seizure claim after index hospitalization discharge, patients were censored at death or end of observation. For analysis of time to death after stroke, patients were censored at end of observation.
Treatment outcomes included details of initial seizure claim and AED treatment during the follow‐up period. The first AED regimen recorded in the database was considered to be the first‐line treatment. If the patient had claims for different AEDs on the same day, this was assumed to be combination therapy. Addition of an AED (to monotherapy or combination therapy), withdrawal of an AED (from combination therapy), or switch of one or more AEDs all constituted second‐line treatment.
Statistical tests were performed using Stata 14 statistical software. As this was a descriptive study, no assessment of required sample size was performed. Due to the retrospective nature of the study, all statistical tests and presented P‐values were exploratory; no multiple testing was done. For categorical variables, statistical comparisons to assess differences between groups were conducted using either Chi‐square tests or Fisher's exact test (when the values in any of the cells of a contingency table were below 5). For continuous variables, statistical comparisons were conducted using t tests for variables with a normal distribution in the case of two‐sample comparison groups and one‐way analysis of variance in the case of multisample comparison groups; Wilcoxon rank sum (nonparametric) tests were used for variables with skewed distribution.
3. RESULTS
3.1. Incidence of poststroke epilepsy and characteristics of patients with and without seizure claims
Overall, 58 007 patients in the AOK PLUS database had at least one inpatient stroke diagnosis between 01 January, 2011 and 31 December, 2015, of whom, 53 883 patients were eligible for the analyses, with a mean follow‐up period of 829.05 days (median 749 days, Figure 1B).
Overall, 6054 of 53 883 (11.24%) patients with acute stroke had at least one seizure claim (Cohort A, Figure 1). 826 of 53 883 (1.53%) eligible patients had only one seizure claim (during index hospitalization) and no further seizure claims and were excluded from the analysis of incidence of PSE. The incidence of PSE (cases/1000 patient‐years) was 190.82 within 90 days after stroke, 135.42 within 180 days, 94.49 within 1 year, and 46.97 within the total follow‐up period. Incidences of PSE were similar in both sexes (within total follow‐up period: 45.87 [men], 47.96 [women] cases/1000 patient‐years).
The mean (SD) age at index date was 73.95 (12.89) years in patients with at least one seizure claim (Cohort A) and 76.48 (12.41) years in those without (Cohort B, p <.001, Table 1). Cerebral infarction was the most common stroke type at index hospitalization among patients with and without seizure claims (Table 1). Hemorrhagic stroke at index was more common in patients with a seizure claim. The median length of index hospitalization associated with stroke was 12 days in patients with a seizure claim, and 9 days in patients without (Table 1). During the 12‐month baseline period, the most common comorbidities in patients with and without a seizure claim were essential hypertension, disorders of lipoprotein metabolism (and other lipidemias), and type 2 diabetes mellitus (Table S1). Agents acting on the renin‐angiotensin system were the most commonly prescribed non‐AED medications at baseline (Table S1).
TABLE 1.
Baseline patient characteristics and characteristics of index stroke hospitalization.
|
Cohort A: Patients with seizure claim (N = 6054) |
Cohort B: Patients without seizure claim (N = 47 829) |
p‐value |
Cohort A−1: Patients with seizure claim and index hospitalization in 2011 (N = 1450) |
Cohort B−1: Patients without seizure claim and index hospitalization in 2011 (N = 10,077) |
p‐value |
Cohort A−2: Patients with at least two seizure claims (N = 4178) |
Cohort A−3: Patients with seizure claim during index hospitalization (N = 2130) |
|
|---|---|---|---|---|---|---|---|---|
| Age at index date, years | ||||||||
| Mean (SD) | 73.95 (12.89) | 76.48 (12.41) | <.001 | 73.83 (12.41) | 76.83 (11.87) | <.001 | 72.83 (13.09) | 74.69 (13.00) |
| Median (range) | 77 (1–100) | 79 (1–105) | 76 (19–98) | 79 (1–104) | 76 (1–100) | 78 (1–100) | ||
| Sex | ||||||||
| Female (%) | 54.18 | 56.02 | .007 | 57.10 | 58.08 | .480 | 53.14 | 53.57 |
| Charlson Comorbidity Index | ||||||||
| Mean (SD) | 4.09 (3.04) | 4.15 (2.93) | .104 | 4.03 (2.99) | 4.13 (2.87) | .228 | 3.93 (2.98) | 4.13 |
| <3 (%) | 34.77 | 33.01 | 51.52 | 52.56 | 36.43 | 35.59 | ||
| 3–5 (%) | 36.11 | 38.30 | 20.41 | 19.80 | 36.12 | 34.18 | ||
| >5 (%) | 29.12 | 28.69 | 28.07 | 27.65 | 27.45 | 30.23 | ||
| Type of stroke at index hospitalization, % of patients | ||||||||
| Cerebral infarction a | 74.30 | 82.24 | <.001 | 74.97 | 80.83 | <.001 | 73.19 | 66.06 |
| Hemorrhagic b | 22.96 | 13.68 | <.001 | 20.21 | 13.60 | <.001 | 24.34 | 31.74 |
| Unspecified c | 2.74 | 4.09 | <.001 | 4.83 | 5.58 | .241 | 2.47 | 2.21 |
| Complexity of stroke treatment, % of patients (diagnosis‐related groups; B70A = most complex; B70H = least complex) | ||||||||
| B70A | 7.88 | 2.18 | <.001 | 6.48 | 1.94 | <.001 | 7.92 | 15.77 |
| B70B | 15.79 | 17.93 | <.001 | 16.00 | 16.73 | .485 | 16.47 | 7.65 |
| B70C | 7.50 | 6.14 | <.001 | 6.48 | 4.29 | <.001 | 7.56 | 10.52 |
| B70D | 8.28 | 12.75 | <.001 | 9.59 | 10.96 | .116 | 8.28 | 3.94 |
| B70E | 9.60 | 5.73 | <.001 | 9.31 | 6.16 | <.001 | 9.43 | 14.84 |
| B70F | 20.94 | 32.62 | <.001 | 26.00 | 38.29 | <.001 | 20.01 | 11.69 |
| B70G (death <4 days after admission) | 0.23 | 0.68 | <.001 | 0.21 | 0.63 | .059 | 0 | 0.66 |
| B70H (death <4 days after admission) | 0.33 | 1.77 | <.001 | 0.34 | 2.06 | <.001 | 0 | 0.94 |
| DRG code other than B70 | 29.45 | 20.20 | <.001 | 25.59 | 18.95 | <.001 | 30.33 | 33.99 |
| Length of stay, days | ||||||||
| Mean (SD) | 16.11 (14.17) | 11.48 (9.40) | <.001 | 15.52 (14.34) | 11.66 (8.97) | <.001 | 16.68 (14.27) | 16.78 |
| Median (range) | 12 (1–195) | 9 (1–217) | 12 (1–164) | 10 (1–123) | 13 (1–195) | 13 (1–195) | ||
ICD‐10 I63.
ICD‐10 I60.‐/I61.‐/I62.
ICD‐10 I64.‐.
Of the patients with seizure claim (Cohort A, N = 6054), 1450 had their index stroke hospitalization in 2011 (Cohort A‐1), 4178 (69.01%) had at least one further seizure claim (Cohort A‐2), and 2130 had at least one seizure claim during index stroke hospitalization (Cohort A‐3). Patient characteristics (demographics, type of stroke, and length of hospital stay) were generally similar in each of these cohorts (Table 1).
3.2. Time to first epilepsy claim
In all eligible patients with acute stroke (N = 53 883), the Kaplan‐Meier estimated proportion of patients without seizure claims from beginning of the index stroke hospitalization was 94.36% (95% CI: 94.16–94.56) at 90 days, 92.66% (95% CI: 92.42–92.89) at 180 days, 90.38% (95% CI: 90.11–90.65) at 1 year, and 87.79% (95% CI: 87.48–88.10) at 2 years (Figure S1).
Among patients with a seizure claim (Cohort A, N = 6054), 2130 (35.18%) had their first claim during index hospitalization and 3924 (64.82%) after discharge. 2384 of 6054 (39.38%) patients had their first seizure claim within 1 year of discharge and 1540 (25.44%) thereafter. In patients with a seizure claim during index hospitalization (Cohort A‐3), 61.55% (1311 of 2130) had a second claim following discharge. Of the patients who had their first seizure claim following hospital discharge, 1250 of 3924 (31.86%) received their first claim from a general practitioner, and 542 of 3924 (13.81%) from a neurologist.
In patients with index stroke hospitalization in 2011 (N = 11 527), 1450 had seizure claims (Cohort A‐1). In these patients, 425 (29.31%) had their first claim during index hospitalization and 1025 (70.69%) after discharge. The Kaplan‐Meier estimated proportion of patients without seizure claims was 94.55% (95% CI: 94.11–94.97) at 90 days, 92.53% (95% CI: 92.00–93.03) at 180 days, 90.15% (95% CI: 89.53–90.73) at 1 year, and 87.53% (95% CI: 86.83–88.20) at 2 years (Figure S2).
3.3. Risk factors for poststroke epilepsy claims
Multivariate Cox‐regression analysis in all patients with stroke (N = 53 883) showed that hemorrhagic stroke (HR = 1.13, 95% CI: 1.06–1.21; p <.001) was associated with a higher risk of seizure claim following hospital discharge than cerebral infarction (Table 2). Furthermore, a seizure claim during index hospitalization was a significant risk factor for a seizure claim after discharge (HR = 6.97; 95% CI: 6.53–7.43; p <.001). Age (HR = 0.99; 95% CI: 0.99–0.99; p <.001) and length of index hospitalization (HR = 1.02; 95% CI: 1.02–1.02; p <.001) were also associated with risk of seizure claim after hospital discharge. Sex, CCI, number of all‐cause hospitalizations during baseline, and stroke treatment complexity were not significantly associated with risk of seizure claims after index hospitalization.
TABLE 2.
Multivariate Cox‐regression analysis of seizure claim after index stroke hospitalization: all patients with acute stroke (N = 53 883)
| Variables | Hazard ratio (95% CI) | p‐value |
|---|---|---|
| Age at stroke a | 0.99 (0.99–0.99) | <.001 |
| Sex b | ||
| Female | 1.06 (1.00–1.12) | .058 |
| Type of stroke c | ||
| Hemorrhage d (ICD−10 Code: ICD−10 I60.‐/I61.‐/I62.‐.) | 1.13 (1.06–1.21) | <.001 |
| Not specified as hemorrhage or infarction (ICD−10 code: I64.‐.) | 0.88 (0.75–1.04) | .146 |
| Length of index hospitalization a | 1.02 (1.02–1.02) | <.001 |
| Seizure claim during index hospitalization e | 6.97 (6.53–7.43) | <.001 |
Patients were censored at death or end of observation. Number of patients with at least one seizure claim after index hospitalization: 5228.
CCI, number of all‐cause hospitalizations during baseline, and complexity of stroke treatment (based on DRG codes) did not show significant impact on time to first seizure claim after index hospitalization and are therefore not included in the table.
Abbreviations: CCI, Charlson Comorbidity Index; ICD‐10, International classification of diseases, 10th revision.
Analyzed as a continuous variable.
Reference: male.
Reference: cerebral infarction (ICD‐10 code: I63.‐).
Subarachnoid, intracerebral or nontraumatic intracranial.
Reference: no seizure claim during index hospitalization (patients may have had a seizure claim after index hospitalization).
3.4. Mortality following stroke
During follow‐up, mortality rate (deaths/1000 patient‐years) was 187.0 in patients with seizure claim (Cohort A, N = 6054), and 204.6 in patients without (Cohort B, N = 47 829). The highest mortality rate was observed in patients with a seizure claim during index stroke hospitalization (Cohort A‐3, N = 2130; 318.9 deaths/1000 patient‐years). In patients with index hospitalization in 2011, mortality rate was 168.6 in patients with seizure claims (Cohort A‐1) and 185.5 in patients without (Cohort B‐1).
Multivariate Cox‐regression analysis in all patients with stroke (N = 53 883) showed that patients with hemorrhagic stroke had a significantly higher risk of early death after index hospitalization (HR = 1.72; 95% CI: 1.66–1.78; p <.001) compared with those who had cerebral infarction (Table 3). Stroke not specified as hemorrhage or infarction was also associated with increased risk of mortality compared with cerebral infarction (HR = 1.26; 95% CI: 1.19–1.34; p <.001). Patients with a seizure claim during index hospitalization had an increased risk of death after hospital discharge in comparison with patients who had no seizure claims during index hospitalization (HR = 1.78; 95% CI: 1.68–1.89; p <.001). Age (HR = 1.07, 95% CI: 1.07–1.07), CCI at baseline (HR = 1.07, 95% CI: 1.06–1.07), length of index hospitalization (HR = 0.99, 95% CI: 0.99–0.99), and number of all‐cause hospitalizations (HR = 1.09, 95% CI: 1.08–1.10) during baseline were also associated with risk of death (p <.001 for all). Female sex was associated with decreased risk of death (HR = 0.97, 95% CI: 0.95–1.00; p =.047). Stroke treatment complexity was not significantly associated with risk of death.
TABLE 3.
Multivariate Cox‐regression analysis of death after index stroke hospitalization: all patients with acute stroke (N = 53 883)
| Variables | Hazard ratio (95% CI) | p‐value |
|---|---|---|
| Age at stroke a | 1.07 (1.07–1.07) | <.001 |
| Sex b | ||
| Female | 0.97 (0.95–1.00) | .047 |
| Type of stroke c | ||
| Hemorrhage d (ICD−10 Code: ICD−10 I60.‐/I61.‐/I62.‐.) | 1.72 (1.66–1.78) | <.001 |
| Not specified as hemorrhage or infarction (ICD−10 code: I64.‐.) | 1.26 (1.19–1.34) | <.001 |
| CCI at baseline a | 1.07 (1.06–1.07) | <.001 |
| Length of index hospitalization a | 0.99 (0.99–0.99) | <.001 |
| Seizure claim during index hospitalization (included as a time‐dependent variable) e | 1.78 (1.68–1.89) | <.001 |
| Number of all‐cause hospitalizations during baseline a | 1.09 (1.08–1.10) | <.001 |
Patients were censored at end of observation. Number of patient deaths: 24 257.
Complexity of stroke treatment (based on DRG codes) did not show significant impact on time to first seizure claim after index hospitalization and are therefore not included in the table.
CCI, Charlson Comorbidity Index; ICD‐10, International classification of diseases, 10th revision.
Analyzed as a continuous variable.
Reference: male.
Reference: cerebral infarction (ICD‐10 code: I63.‐).
Subarachnoid, intracerebral or nontraumatic intracranial.
Reference: no seizure claim during index hospitalization (patients may have had a seizure claim after index hospitalization).
3.5. Neuropsychiatric comorbidities
The most common neuropsychiatric comorbidities during the 12‐month baseline period in patients who later had seizure claims (Cohort A), as well as those who did not (Cohort B), were anxiety, dissociative and somatoform disorders, and depression (Table 4). During 12‐months of follow‐up, depression (p <.001) was more common in patients with than without seizure claims (Table 4).
TABLE 4.
Neuropsychiatric comorbidities at baseline and follow‐up: all patients with acute stroke
| Comorbidity, n (%) | 12 months baseline | 12 months follow‐up | ||
|---|---|---|---|---|
|
Cohort A: Patients with seizure claim (N = 6054) |
Cohort B: Patients without seizure claim (N = 47 829) |
Cohort A: Patients with seizure claim (N = 6054) |
Cohort B: Patients without seizure claim (N = 47 829) |
|
| Anxiety, dissociative, and somatoform disorders | 1098 (18.14) | 8186 (17.12) | 1198 (19.79) | 8708 (18.21) |
| Depression | 1044 (17.24) | 7731 (16.16) | 1877 (31.00) | 10 893 (22.77) |
| Personality disorder | 67 (1.11) | 344 (0.72) | 67 (1.11) | 344 (0.72) |
| Schizophrenia | 60 (0.99) | 379 (0.79) | 77 (1.27) | 449 (0.94) |
| Psychoses | 58 (0.96) | 296 (0.62) | 90 (1.49) | 346 (0.72) |
| Bipolar disorder | 40 (0.66) | 214 (0.45) | 53 (0.88) | 240 (0.50) |
| Delusional disorders | 29 (0.48) | 189 (0.40) | 39 (0.64) | 212 (0.44) |
| Adjustment / disturbance of conduct disorder | 28 (0.46) | 205 (0.43) | 39 (0.64) | 235 (0.49) |
| Other psychiatric disorder | 6 (0.10) | 52 (0.11) | 15 (0.25) | 89 (0.19) |
3.6. Outpatient AED treatment
In the first year of follow‐up, 4465 of 6054 (73.75%) patients with a seizure claim (Cohort A) received AED treatment; no AED treatment was recorded in the remaining 1589 (26.25%) patients of whom 1052 (66.21%) died during follow‐up. The most commonly prescribed AEDs (prescribed in ≥5% of patients) were levetiracetam, valproic acid, gabapentin, lamotrigine, and pregabalin (Figure 2A). Most patients (73.73% [3292 of 4465]) who received AED treatment in the first year of follow‐up remained on first‐line therapy. Of 1173 patients with a second‐line treatment, 711 (60.61%) remained on that therapy. In the second year of follow‐up, 3024 (64.57%) surviving patients (N = 4683; patients who did not die before the start of the second observational year) received AED treatment (Figure 2B); 2292 (48.94%) patients had more than two AED prescriptions (Figure 2C). In patients with at least one seizure claim during index hospitalization (Cohort A‐3), 1278 of 2130 (60.00%) received AED treatment in the first year of follow‐up. In the second year of follow‐up, 612 (49.80%) patients surviving into the second observational year (N = 1229) had more than two AED prescriptions.
FIGURE 2.
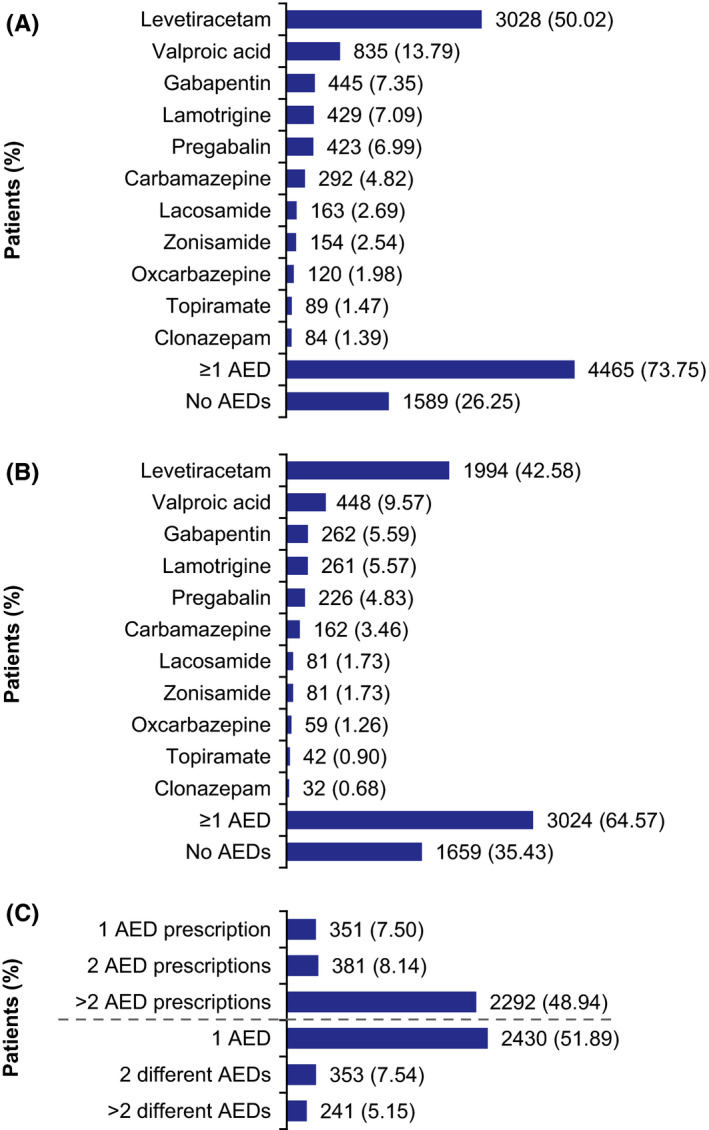
Most common outpatient AED prescriptions during follow‐up: all patients with acute stroke and seizure claim (Cohort A, N = 6054). (A) During first year of follow‐up (AEDs prescribed in ≥1% of patients); (B) During second year of follow‐up (AEDs prescribed in ≥0.5% of patients);a (C) Number of prescriptions and AEDs during the second year of follow‐up.a Two or more AED prescriptions are indicative of continuous AED treatment. For continuous treatment in Germany, the medical prescription refill interval is typically 3 months. aAnalysis was based on a subsample of patients who did not die before start of second observational year after stroke (N = 4683). AED, antiepileptic drug
4. DISCUSSION
The results of this large retrospective claims database analysis with a median follow‐up of nearly 2.5 years showed that a substantial proportion of patients (11.24%) with acute stroke and no previous epilepsy diagnosis had at least one seizure claim during/after stroke hospitalization. A seizure claim does not necessarily indicate an epilepsy diagnosis, as patients may have had an acute symptomatic seizure following stroke without developing epilepsy. Of 6054 patients with a seizure claim (Cohort A), 69.01% had an additional seizure claim (Cohort A‐2), indicating that these patients had developed epilepsy. The incidence of epilepsy following hospitalization for stroke (defined as patients with ≥2 seizure claims [during/after index hospitalization], or ≥1 seizure claim after index hospitalization) was 94.49 cases/1000 patient‐years within 1 year and 46.97 cases/1000 patient‐years over the total follow‐up period.
A similar analysis of administrative claims using data from the United States 7 found that 8–15% of patients with stroke may develop PSE, depending on the type of stroke. Analyses based on clinical data have shown comparable results. 16 , 17 , 18 A prospective multicenter study in 1897 patients with stroke (and no previous epilepsy) found that 8.9% of patients had at least one poststroke seizure (either acute symptomatic or unprovoked). 16 The incidence of poststroke seizures was likely lower because of a shorter mean follow‐up time (9 months vs approximately 31 months in the current study). In a study of 421 patients with a mean observation period of 30 months, incidence of seizures after a cerebrovascular event (>80% stroke) was 11.6%. 17 In a prospective German study of 1020 patients with stroke, 8.2% developed PSE within 2 years. 18 A retrospective study of 240 patients with a mean follow‐up time of 868 days (median: 1062 days) found that 13 patients (5.4%) developed PSE (11 were diagnosed with epilepsy and two had patient records indicative of epilepsy). 19 Incidence of PSE was estimated to be 23 cases/1000 patient‐years. However, only patients with ischemic stroke were included in that study, which may have contributed to the lower incidence of epilepsy. 19
In our analysis, among all patients with a seizure claim, 35.18% received their first claim during index stroke hospitalization (Cohort A‐3) and 64.82% after hospital discharge. In all eligible patients with stroke, the Kaplan‐Meier estimated proportion of patients without seizure claims from beginning of the index stroke hospitalization was 90.38% at 1 year and 87.79% at 2 years. A seizure claim during index hospitalization was a risk factor for further claims, as demonstrated by Cox regression analysis and the high proportion of patients in Cohort A‐3 who had seizure claims after hospital discharge (61.55%). Previous studies have indicated that seizures occurring shortly after stroke may be a risk factor for developing epilepsy. 10 , 12 A study in 192 patients with stroke found that patients with acute symptomatic seizures had a 10‐year recurrence risk for an unprovoked seizure of 33.0%; patients with unprovoked seizures had a 71.5% recurrence risk. 5 Since acute symptomatic seizures occur within 7 days of stroke, 4 this may reflect pathophysiological changes in the first few days after stroke which are not apparent weeks later. 20 It should be noted that the median length of hospital stay in the current study was more than 7 days; therefore, seizures occurring during stroke‐associated hospitalizations were not necessarily acute symptomatic seizures.
Our results showed that hemorrhagic stroke at index was more common in patients with a subsequent seizure claim than in patients without. Furthermore, Cox regression analysis showed that patients with hemorrhagic stroke and seizure claim during index hospitalization were at higher risk of seizure claim after index hospitalization discharge. This is in line with previous studies. 11 , 13 , 16 , 17 Prospective studies in 1200 participants with ischemic stroke in Switzerland 12 and 1020 patients from Germany 18 demonstrated that severity of stroke is a predictor of unprovoked seizures/PSE. In the current study, a direct assessment of stroke severity (such as the National Institutes of Health Stroke Scale) was not possible based on the available claims data. However, treatment complexity (based on DRG codes) had no significant impact on risk for seizure claim after index stroke hospitalization. Data on additional characteristics, such as location of lesion, were not available in the AOK Plus database.
We observed the highest mortality rate (318.9 deaths/1000 patient‐years) in the subgroup of patients with seizure claims during index hospitalization (Cohort A‐3). The mortality rate following stroke was 187.0 deaths/1000 patient‐years in patients with seizure claims (Cohort A) and 204.6 in patients without (Cohort B). The lower mortality in patients with seizure claims may be due to survival bias, since patients need to survive for some time to develop PSE. We, therefore, performed a Cox regression analysis in which seizure claim during index hospitalization was included as a time‐dependent variable to prevent survival bias. Results showed that a seizure claim during index stroke hospitalization was associated with a 1.78‐fold higher risk of early death. These results are in agreement with previous research; a retrospective study in the United States 5 found higher 30‐day mortality in patients with first acute symptomatic seizures (N = 262) compared with patients with a first unprovoked seizure (N = 148). Similarly, a study using data from Swedish national registries found that patients with PSE had an increased risk of death; however, the definition for PSE included ICD‐10 G41 (status epilepticus) and R56.8 (other and unspecified convulsions) codes, in addition to the G40 (epilepsy) code used in this study. 21
Analysis of neuropsychiatric comorbidities showed that the incidence of depression during 12‐month follow‐up was slightly higher in patients with than without seizure claims, in line with data from previous studies. 22 , 23 , 24 Based on the literature, depression may be both a consequence of stroke and a marker for PSE. 22 , 23 , 24 Such relationships with epilepsy have been found for other neuropsychiatric comorbidities including anxiety and psychosis. 22 , 24
The European Stroke Organization guidelines for the management of poststroke seizures weakly recommend immediate AED treatment after an unprovoked seizure. 25 In the absence of evidence from randomized controlled trials, this recommendation was based on an observational study which demonstrated a high risk of seizure recurrence following the first unprovoked seizure. 5 In our study, the majority of patients with seizure claims received outpatient AED treatment, and remained on their first‐line therapy. Treatment patterns were relatively stable across the first and second year of follow‐up, and nearly half of patients received more than two AED prescriptions in the second year, indicating continuous treatment. A similar pattern was observed in patients with seizure claim during index hospitalization (Cohort A‐3); since the majority had a further claim after hospital discharge, many of these patients had likely developed epilepsy and therefore required AED treatment. A large Swedish study in patients initiating AED treatment between 2005 and 2010 found that carbamazepine was the most commonly prescribed AED, 26 whereas the most commonly prescribed AED in our study was levetiracetam, similar to a smaller Swedish study 19 using data from 2015. Nonenzyme‐inducing AEDs such as levetiracetam are generally preferred for the treatment of patients with PSE, in order to minimize the potential for drug‐drug interactions and exacerbation of vascular risk. 27 Few trials have assessed the efficacy and safety of AEDs for the treatment of PSE. 14 The limited available data have not shown any differences in efficacy between levetiracetam, lamotrigine, or controlled‐release carbamazepine, though levetiracetam and lamotrigine appeared to have a better safety profile than controlled‐release carbamazepine. 14
Our study has several limitations. All data were based on inpatient and outpatient claims from an administrative claims database; no clinical data were available for confirmation of epilepsy diagnoses. As mentioned previously, the exact dates when seizures occurred were unknown. We were, therefore, unable to confirm whether patients had acute symptomatic seizures (within 7 days of stroke) or unprovoked seizures (more than 7 days after stroke). We analyzed ICD‐10 G40.‐ claims only. It is possible that a small number of relevant claims were made using the ICD‐10 R56.8 code, though ICD‐10 G40.‐ would generally be used for acute symptomatic or unprovoked seizures in Germany. Furthermore, clinical characteristics that may contribute to risk of seizures, such as stroke severity, could not be assessed. Finally, these data were collected from a German database, and may not be generalizable to other countries. Despite these limitations, this study provides long‐term data for a large number of patients with stroke and seizure claims, with a substantial number of patients followed up for at least 60 months.
Overall, our results support previous findings that PSE occurs in a substantial proportion of patients with stroke. Hemorrhagic stroke and seizure claim during index hospitalization were risk factors for seizure claims after hospital discharge. In line with guideline recommendations, most patients with seizure claims were treated with AEDs, despite a lack of evidence from clinical trials in this patient population.
CONFLICT OF INTEREST
F. Hardtstock had no disclosures. N. Foskett, P. Gille, L. Joeres, M. Molzan, and J. C. Wilson are employees of UCB Pharma. T. Wilke has received honoraria from several pharmaceutical/consultancy companies, including AbbVie, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, LEO Pharma, Merck, Novo Nordisk, and Pharmerit. M. Holtkamp received speaker's honoraria and/or consultancy fees from Arvelle, Bial, Desitin, Eisai, GW Pharmaceuticals, UCB Pharma, and Zogenix.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by UCB Pharma. The authors thank Fabian Dorff for reviewing the data and report. Publication management was provided by Fabien Debailleul, PhD (UCB Pharma, Brussels, Belgium) and Vincent Laporte, PhD (UCB Pharma, Brussels, Belgium). Writing assistance was provided by Jayna Patel, PhD (Evidence Scientific Solutions, Horsham, UK), and was funded by UCB Pharma.
DATA AVAILABILITY STATEMENT
Data from noninterventional studies are outside of UCB Pharma's data sharing policy and are unavailable for sharing.
REFERENCES
- 1. Ngugi AK, Kariuki SM, Bottomley C, et al. Incidence of epilepsy: a systematic review and meta‐analysis. Neurology. 2011;77(10):1005‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao Y, Li X, Zhang K, et al. The progress of epilepsy after stroke. Curr Neuropharmacol. 2018;16(1):71‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olafsson E, Ludvigsson P, Gudmundsson G, et al. Incidence of unprovoked seizures and epilepsy in Iceland and assessment of the epilepsy syndrome classification: a prospective study. Lancet Neurol. 2005;4(10):627‐634. [DOI] [PubMed] [Google Scholar]
- 4. Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51(4):671‐675. [DOI] [PubMed] [Google Scholar]
- 5. Hesdorffer DC, Benn EK, Cascino GD, et al. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia. 2009;50(5):1102‐1108. [DOI] [PubMed] [Google Scholar]
- 6. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475‐482. [DOI] [PubMed] [Google Scholar]
- 7. Merkler AE, Gialdini G, Lerario MP, et al. Population‐based assessment of the long‐term risk of seizures in survivors of stroke. Stroke. 2018;49(6):1319‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graham NS, Crichton S, Koutroumanidis M, et al. Incidence and associations of poststroke epilepsy: the prospective South London Stroke Register. Stroke. 2013;44(3):605‐611. [DOI] [PubMed] [Google Scholar]
- 9. Lahti AM, Saloheimo P, Huhtakangas J, et al. Poststroke epilepsy in long‐term survivors of primary intracerebral hemorrhage. Neurology. 2017;88(23):2169‐2175. [DOI] [PubMed] [Google Scholar]
- 10. So EL, Annegers JF, Hauser WA, et al. Population‐based study of seizure disorders after cerebral infarction. Neurology. 1996;46(2):350‐355. [DOI] [PubMed] [Google Scholar]
- 11. Serafini A, Gigli GL, Gregoraci G, et al. Are early seizures predictive of epilepsy after a stroke? Results of a population‐based study. Neuroepidemiology. 2015;45(1):50‐58. [DOI] [PubMed] [Google Scholar]
- 12. Galovic M, Döhler N, Erdélyi‐Canavese B, et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): a multivariable prediction model development and validation study. Lancet Neurol. 2018;17(2):143‐152. [DOI] [PubMed] [Google Scholar]
- 13. Arntz R, Rutten‐Jacobs L, Maaijwee N, et al. Post‐stroke epilepsy in young adults: a long‐term follow‐up study. PLoS One. 2013;8(2):e55498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brigo F, Lattanzi S, Zelano J, et al. Randomized controlled trials of antiepileptic drugs for the treatment of post‐stroke seizures: a systematic review with network meta‐analysis. Seizure. 2018;61:57‐62. [DOI] [PubMed] [Google Scholar]
- 15. Roffman CE, Buchanan J, Allison GT. Charlson comorbidities index. J Physiother. 2016;62(3):171. [DOI] [PubMed] [Google Scholar]
- 16. Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol. 2000;57(11):1617‐1622. [DOI] [PubMed] [Google Scholar]
- 17. Conrad J, Pawlowski M, Dogan M, et al. Seizures after cerebrovascular events: risk factors and clinical features. Seizure. 2013;22(4):275‐282. [DOI] [PubMed] [Google Scholar]
- 18. Jungehulsing GJ, Heuschmann PU, Holtkamp M, et al. Incidence and predictors of post‐stroke epilepsy. Acta Neurol Scand. 2013;127(6):427‐430. [DOI] [PubMed] [Google Scholar]
- 19. Hassani M, Cooray G, Sveinsson O, et al. Post‐stroke epilepsy in an ischemic stroke cohort—Incidence and diagnosis. Acta Neurol Scand. 2020;141(2):141–147. [DOI] [PubMed] [Google Scholar]
- 20. Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391‐397. [DOI] [PubMed] [Google Scholar]
- 21. Zelano J, Redfors P, Asberg S, et al. Association between poststroke epilepsy and death: a nationwide cohort study. Eur Stroke J. 2016;1(4):272‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hesdorffer DC, Ishihara L, Mynepalli L, et al. Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Ann Neurol. 2012;72(2):184‐191. [DOI] [PubMed] [Google Scholar]
- 23. Wu SH, Ma L, Sun ZY, et al. Analysis of depression and anxiety in patients with post‐stroke epilepsy. Int J Clin Exp Med. 2017;10(4):6994‐6999. [Google Scholar]
- 24. Kanner AM, Ribot R, Mazarati A. Bidirectional relations among common psychiatric and neurologic comorbidities and epilepsy: do they have an impact on the course of the seizure disorder? Epilepsia Open. 2018;3(Suppl 2):210‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holtkamp M, Beghi E, Benninger F, et al. European Stroke Organisation guidelines for the management of post‐stroke seizures and epilepsy. Eur Stroke J. 2017;2(2):103‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Larsson D, Asberg S, Kumlien E, et al. Retention rate of first antiepileptic drug in poststroke epilepsy: a nationwide study. Seizure. 2019;64:29‐33. [DOI] [PubMed] [Google Scholar]
- 27. Zelano J. Poststroke epilepsy: update and future directions. Ther Adv Neurol Disord. 2016;9(5):424‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data from noninterventional studies are outside of UCB Pharma's data sharing policy and are unavailable for sharing.


