Abstract
Objectives
To estimate the risk of thrombocytopenia in various cancers and chemotherapy regimens.
Methods
Structured patient‐level data from the Flatiron Health Electronic Health Record database were used to identify adult patients who received chemotherapy for a solid tumor or hematologic malignancy from 2012 to 2017. Three‐month cumulative incidence of thrombocytopenia was assessed based on platelet counts, overall and by grade of thrombocytopenia. Co‐occurrence of anemia, neutropenia, and leukopenia was evaluated.
Results
Of 15,521 patients with solid tumors, 13% had thrombocytopenia within 3 months (platelet count < 100 × 109/L); 4% had grade 3 (25 to < 50 × 109/L), and 2% grade 4 (<25 × 109/L) thrombocytopenia. Of 2537 patients with hematologic malignancies, 28% had any thrombocytopenia, 16% with grade 3, and 12% with grade 4. Among patients with thrombocytopenia, it occurred without another cytopenia in 18% of solid tumors and 7% of hematologic malignancies.
Conclusions
In a large, US‐representative sample of patients undergoing chemotherapy in clinical practice, thrombocytopenia incidence varied across tumor and regimen types. Despite recommendations to alter chemotherapy to avoid severe thrombocytopenia, 4% of patients with solid tumors and 16% with hematologic malignancies experienced grade 3 thrombocytopenia. Prediction and prevention of thrombocytopenia may help oncologists avoid dose modifications and their adverse effects on survival.
Keywords: adverse event, chemotherapy, chemotherapy‐induced thrombocytopenia, CIT, hematologic malignancy, incidence, solid tumor, Thrombocytopenia, toxicity
Summary Statements.
What is the new aspect of your work?
This study provides an updated estimate of thrombocytopenia incidence and severity in a broad cancer population that is representative of cancer patients receiving chemotherapy in the United States.
What is the central finding of your work?
Overall, three‐month cumulative incidence of any thrombocytopenia in this study was 13% for patients with solid tumors and 28% for patients with hematologic malignancies; platinum‐based and gemcitabine‐based chemotherapy regimens were associated with the highest burden of thrombocytopenia. Isolated thrombocytopenia occurred in 15% of those who experienced thrombocytopenia.
What is (or could be) the specific clinical relevance of your work?
Findings add to the body of evidence describing the burden of chemotherapy‐induced thrombocytopenia and highlight the need for therapeutic intervention in this setting to ensure patients continue planned chemotherapy treatment without delay or reduction in dose intensity.
1. INTRODUCTION
Thrombocytopenia, an abnormally low blood platelet count, is a common side effect of myelosuppressive chemotherapy. 1 , 2 , 3 Prior studies estimated that approximately 10% to 38% of patients with a solid tumor and 40% to 68% of patients with a hematologic malignancy experience thrombocytopenia. 3 , 4 , 5 , 6 , 7 The incidence and prevalence of chemotherapy‐induced thrombocytopenia (CIT) vary greatly by type of cancer and chemotherapy regimen, for example, from 16% in head and neck cancer to 68% in hematologic cancers, and from 8% in taxane‐based regimens to 37% in gemcitabine‐based regimens and 82% in carboplatin monotherapy. 2 , 3 , 7 , 8 Gemcitabine‐based and platinum‐based regimens have consistently been associated with the highest risk of thrombocytopenia. 1 , 2 , 3 In solid tumor patients, the highest prevalence of thrombocytopenia was observed in patients with colorectal cancer, followed by non‐small cell lung cancer, and ovarian cancer. 3 Most estimates of CIT incidence have focused on patients with certain solid tumors, used varying definitions of CIT, or relied on claims data—which lack platelet counts—to identify thrombocytopenia. 1 , 2 , 4 , 5 , 7
Currently, there are no standardized guidelines for the prevention or treatment of CIT. To reduce the risk of bleeding or need for transfusions among patients with severe CIT, chemotherapy dose is typically modified, which may decrease relative dose intensity (RDI) and reduce treatment efficacy. 9 , 10 , 11 Patient and institutional factors often affect decisions to adjust chemotherapy. Generally, chemotherapy is administered with caution at platelet counts < 100 × 109/L, and the risk of spontaneous bleeding increases at platelet counts < 10 × 109/L in hematologic malignancy patients 8 and potentially higher platelet thresholds < 20 × 109/L in some solid tumor patients. 12 , 13 When maintaining the chemotherapy dose intensity is critical for response or survival, dose modifications are avoided, and when platelet counts fall below 10 × 109/L or in cases of active bleeding, platelet transfusions are frequently utilized. 8 The most common clinical impact of CIT is on the ability to continue cancer therapy; however, other impacts include hospitalization for active bleeding and platelet transfusion, with its attendant risks. 1 , 14 , 15
Previous literature has shown that reductions in chemotherapy dose intensity can affect overall survival 16 , 17 and that delays in treatment can increase the risk of progression and have a significant financial cost. 11 , 18 Although thrombocytopenia is often one of many toxicities leading to dose modifications, its relative importance is likely to differ by patient population and treatment regimen. For example, in two studies 19 , 20 of patients with renal cell carcinoma, thrombocytopenia was the most common dose‐limiting toxicity, occurring in 24% and 34% of patients, respectively, with a dose modification. In general, studies have shown that patients receiving chemotherapy at a higher RDI have better clinical outcomes than those treated at a lower RDI. 16 , 21 , 22 Specifically, maintaining an RDI ≥ 85% has had a favorable impact on survival, 16 , 23 , 24 highlighting the importance of prevention of chemotherapy toxicities to avoid reduction in RDI. To better understand the full scope and unmet need in CIT management, we assessed the risk of thrombocytopenia among patients with several types of cancer and chemotherapy regimens using a large database of electronic health records from cancer care providers across the United States.
2. METHODS
2.1. Study design, patients, data collection, and analysis
This retrospective cohort study included adult (age ≥18 years) cancer patients in the United States from structured patient‐level data in Flatiron Health's nationwide, longitudinal, demographically, and geographically diverse de‐identified database. The database contains electronic health record (EHR) data from over 280 oncology clinics (~800 sites), including approximately 2.4 million US cancer patients available for analysis. We identified patients who initiated chemotherapy for a solid tumor or hematologic malignancy from 2012 to 2017. All available patient history was leveraged to ensure there was no evidence of non‐cancer causes of thrombocytopenia, and patients who had more than one primary cancer or who had received chemotherapy for another cancer in the year prior to chemotherapy initiation were excluded. Additionally, a sensitivity analysis was performed to examine the number and proportion of patients, by cancer type, who had evidence of thrombocytopenia (platelet count <100 × 109/L) in the 30 days prior to chemotherapy initiation.
Patients were included if they had a diagnosis of a primary solid tumor or hematologic malignancy and subsequently initiated chemotherapy. Cancer types included bladder, breast, colorectal, head and neck, lung, melanoma, ovarian, pancreatic, prostate, uterine, Hodgkin lymphoma, multiple myeloma, non‐Hodgkin lymphoma, and a composite of all other hematologic malignancies. Chemotherapy regimens were defined using a hierarchical structure of highest associated risk of thrombocytopenia, based on review of the literature. 1 , 2 , 3 Gemcitabine‐based regimens included all regimens that contained gemcitabine, followed by platinum‐based regimens (that did not include gemcitabine), anthracycline‐based, taxane‐based, and all other regimens. For example, a gemcitabine + cisplatin regimen would be categorized as gemcitabine‐based.
2.2. Outcomes
The primary outcome of interest was the cumulative incidence of thrombocytopenia following chemotherapy, defined by the first platelet count below 100 × 109/L. Patients were followed until the first evidence of any thrombocytopenia and until each grade of thrombocytopenia. Grades were defined as follows, based on platelet thresholds clinically relevant for chemotherapy‐induced thrombocytopenia; grade 1: 75 to <100 × 109/L; grade 2: 50 to <75 × 109/L; grade 3: 25 to <50 × 109/L; and grade 4: <25 × 109/L. Grades 2 through 4 follow Common Terminology Criteria for Adverse Events (CTCAE) V5.0, and CTCAE grade 1 includes all platelet counts lower limit of normal (LLN) to 75 × 109/L (redefined as 75 to <100 to avoid overlapping categories in this analysis). 25 Follow‐up for each outcome started at chemotherapy initiation and ended at the first of the following: occurrence of thrombocytopenia, >45 days without chemotherapy, loss to follow‐up (defined as >30 days without a clinical encounter), receipt of romiplostim, receipt of eltrombopag, death, or end of study period (31 December 2017). Three‐month cumulative incidence (%) of thrombocytopenia following initiation of chemotherapy was estimated overall and for each grade of thrombocytopenia, stratified by cancer type, chemotherapy regimen, and patient age and sex, treating death as a competing risk. Median time to first evidence of thrombocytopenia was also estimated among thrombocytopenic patients by select cancer type, chemotherapy regimen, and by sex and age group (dichotomized at 65 years). Only the most common solid tumor types and hematologic malignancy are presented by time to thrombocytopenia.
Among patients with thrombocytopenia, we evaluated the co‐occurrence of anemia, neutropenia, and leukopenia. Only patients with laboratory values for hemoglobin, neutrophil count, and leukocyte count on the same day (±3 days) as first evidence of thrombocytopenia were included. Since elderly cancer patients can experience clinical symptoms of anemia at higher hemoglobin levels than anemic patients without cancer, a broad criterion of recorded hemoglobin <12 g/dL (<LLN for women) was used to define anemia. 26 Similarly, neutropenia and leukopenia were defined according to the National Cancer Institute classification of grade 1 events and higher, as an absolute neutrophil count <2 × 109/L for neutropenia, 27 , 28 and as leukocytes <4 × 109/L for leukopenia. 29 Pancytopenia was defined as having thrombocytopenia, anemia, and leukopenia; isolated thrombocytopenia was defined as thrombocytopenia with no evidence of anemia, neutropenia, or leukopenia. Frequencies and proportions were used to describe the co‐occurrence of these hematologic abnormalities at first evidence of thrombocytopenia.
3. RESULTS
There were 15 521 patients with solid tumors and 2537 patients with a hematologic malignancy who initiated chemotherapy from 2012 to 2017. Of patients with solid tumors, 57% were female and 59% were ≥65 years; 42% of those with hematologic malignancies were female and 55% were ≥65 years old. The most common treatments were platinum‐based chemotherapies (45% of patients), with gemcitabine‐, anthracycline‐, and taxane‐based regimens each used in approximately 10% of patients (Table 1).
TABLE 1.
Descriptive characteristics of patients receiving myelosuppressive chemotherapy for primary solid tumor or hematologic malignancy
|
Overall (N = 18 058) |
All solid tumors (N = 15 521) |
All hematologic malignancies (N = 2537) |
|
|---|---|---|---|
| Characteristic | n (%) | n (%) | n (%) |
| Overall | |||
| Sex | |||
| Female | 9899 (54.8) | 8823 (56.8) | 1076 (42.4) |
| Male | 8158 (45.2) | 6697 (43.1) | 1461 (57.6) |
| Age group | |||
| < 65 years | 7530 (41.7) | 6399 (41.2) | 1131 (44.6) |
| ≥ 65 years | 10 528 (58.3) | 9122 (58.8) | 1406 (55.4) |
| Year of chemotherapy initiation | |||
| 2012‐2013 | 5573 (30.9) | 4843 (31.2) | 730 (28.8) |
| 2014‐2015 | 6693 (37.1) | 5793 (37.3) | 900 (35.5) |
| 2016‐2017 | 5792 (32.1) | 4885 (31.5) | 907 (35.8) |
| Chemotherapy regimen | |||
| Gemcitabine‐based | 1662 (9.2) | 1631 (10.5) | 31 (1.2) |
| Platinum‐based | 8051 (44.6) | 8040 (51.8) | 11 (0.4) |
| Anthracycline‐based | 1972 (10.9) | 939 (6.0) | 1033 (40.7) |
| Taxane‐based | 2116 (11.7) | 2115 (13.6) | 1 (0.0) |
| Other | 4257 (23.6) | 2796 (18.0) | 1461 (57.6) |
Three‐month cumulative incidence of thrombocytopenia in patients with solid tumors was 12.8% (95% confidence interval [CI]: 12.3‐13.4) and in patients with hematologic malignancies was 28.2% (95% CI: 26.5‐30.0). For patients with solid tumors, incidence of thrombocytopenia did not differ by sex or age group. Younger patients in the hematologic malignancy group (<65 years) had a higher 3‐month incidence of thrombocytopenia than those ≥65 years (31.7% vs. 25.4%) (Table 2). Results of the sensitivity analysis revealed that thrombocytopenia occurred in the 30 days prior to chemotherapy initiation in 21% of hematologic cancers and it ranged from 2% (CRC) to 6% (prostate cancer) in solid tumor patients (Table S1). Patients with solid tumors who received gemcitabine‐ and platinum‐based regimens had a higher incidence of thrombocytopenia (14.8% and 13.5%, respectively) than those who received anthracycline‐ or taxane‐based regimens (9.3% and 6.5%, respectively). Rankings were similar but the incidence of thrombocytopenia was higher for patients with hematologic malignancies: 31.0%, 37.8%, and 22.5% for those who received gemcitabine‐, platinum‐, and anthracycline‐based regimens, respectively (Table 2). Most patients with hematologic cancers, however, received anthracycline‐based or other chemotherapy regimens. Specific regimens were difficult to define accurately in the data, but the most common “other” agents administered to these patients included cyclophosphamide, vincristine, etoposide, bleomycin, and bendamustine.
TABLE 2.
Three‐month cumulative incidence of thrombocytopenia following chemotherapy by cancer type and patient characteristics
|
Overall (N = 18 058) |
All solid tumors (N = 15 521) |
All hematologic malignancies (N = 2537) |
|
|---|---|---|---|
| Characteristic | % (95% CI) | % (95% CI) | % (95% CI) |
| Overall | 15.1 (14.6 −15.6) | 12.8 (12.3‐13.4) | 28.2 (26.5‐30.0) |
| Sex | |||
| Female (N = 9899) | 14.2 (13.5‐14.9) | 12.7 (11.9‐13.5) | 25.8 (23.4‐28.4) |
| Male (N = 8158) | 16.0 (15.4‐16.7) | 13.0 (12.3‐13.8) | 29.9 (27.7‐32.3) |
| Age group | |||
| < 65 years (N = 7530) | 15.6 (14.9‐16.4) | 12.7 (11.8‐13.5) | 31.7 (29.4‐34.2) |
| ≥ 65 years (N = 10 528) | 14.7 (14.2‐15.2) | 13.0 (12.3‐13.6) | 25.4 (23.4‐27.6) |
| Year of chemotherapy initiation | |||
| 2012‐2013 (N = 5573) | 15.2 (14.4‐16.1) | 13.1 (12.1‐14.2) | 27.4 (24.7‐30.4) |
| 2014‐2015 (N = 6693) | 15.3 (14.7‐16.0) | 13.4 (12.5‐14.3) | 27.9 (25.1‐31.0) |
| 2016‐2017 (N = 5792) | 14.7 (13.9‐15.6) | 11.9 (11.1‐12.9) | 29.1 (26.6‐31.9) |
| Chemotherapy regimen | |||
| Gemcitabine (N = 1662) | 15.8 (14.3‐17.4) | 14.8 (13.2‐16.6) | 31.0 (19.8‐48.5) |
| Platinum (N = 8051) | 13.6 (13.0‐14.2) | 13.5 (12.8‐14.2) | 37.8 (21.7‐65.7) |
| Anthracycline (N = 1972) | 16.4 (15.0‐18.0) | 9.3 (7.6‐11.4) | 22.5 (20.5‐24.7) |
| Taxane (N = 2116) | 6.6 (5.7‐7.7) | 6.5 (5.5‐7.7) | 0.0 (0.0‐0.0) |
| Other (N = 4257) | 21.2 (20.1‐22.2) | 15.5 (14.3‐16.9) | 32.1 (30.0‐34.3) |
Abbreviations: CI, confidence interval; N/A, not applicable.
Overall, 15.1% of patients had any‐grade thrombocytopenia within 3 months following chemotherapy initiation with fewer experiencing grade 3 (5.8%) or grade 4 (3.3%) thrombocytopenia (Figure 1; Table S2). Among patients with solid tumors, 12.8% had evidence of thrombocytopenia, with 6.4%, 4.2%, and 1.9% exhibiting grades 2, 3, and 4 thrombocytopenia, respectively (Figure 1; Table S2). Three‐month risk of grades 3 and 4 thrombocytopenia was greatest in melanoma patients (13.3% and 5.0%, respectively); among common tumor types, such as lung and colorectal, risk was ≤5% (Figure 1; Table S2). The incidence of any thrombocytopenia ranged from 9.6% (breast) to 21.4% (melanoma). Incidence appeared highest in melanoma patients, though the confidence intervals were wide due to the small sample size (N = 58) (Figure 2). Of the most common solid tumor types, the incidence of thrombocytopenia was highest in lung cancer (14.3%); followed by colorectal (13.5%), pancreatic (12.9%), and breast (9.6%) cancers (Figure 2). Each of the other tumor types were present in less than 10% of the patient population and three‐month incidence of thrombocytopenia ranged from 10.7% to 14.7% (Figure 2).
FIGURE 1.
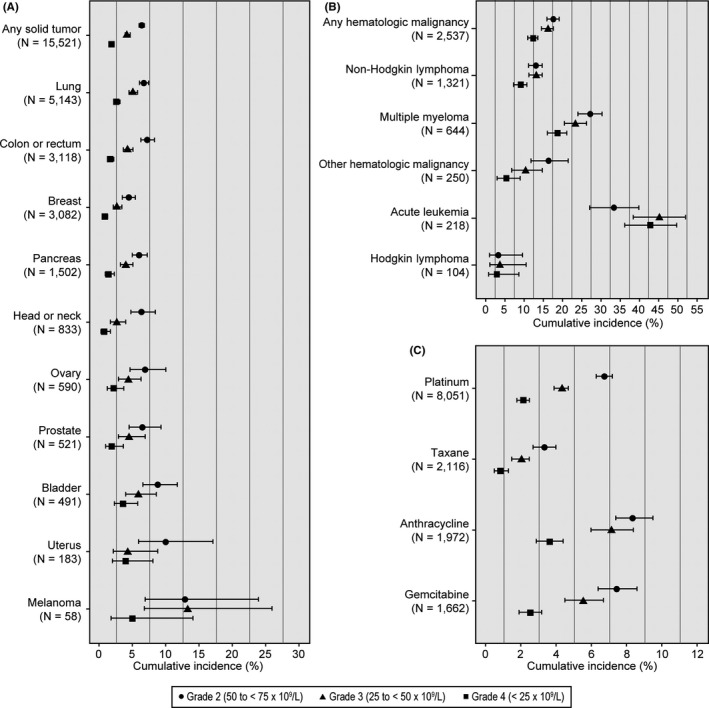
Three‐month cumulative incidence and 95% confidence intervals of thrombocytopenia, by grade of severity, for solid tumor (A), hematologic malignancy (B), and chemotherapy regimen categories (C). For results in tabular format, see Table S2
FIGURE 2.
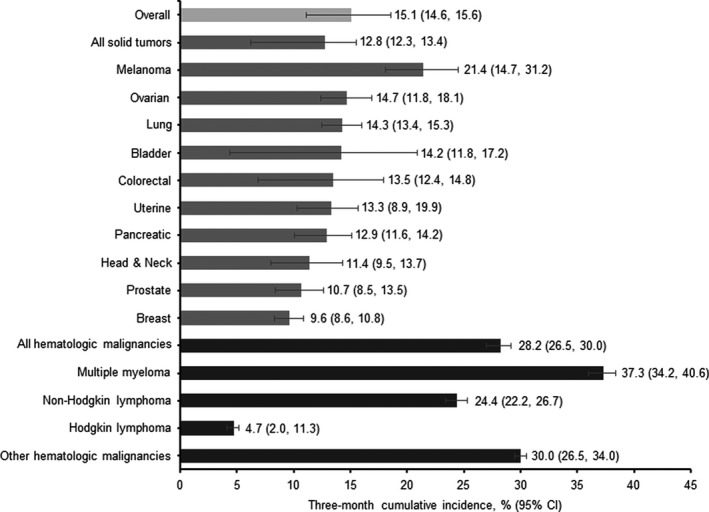
Three‐month cumulative incidence (%) of thrombocytopenia following chemotherapy by cancer type. CI, confidence interval
Among patients with hematologic malignancies, 28.2% had evidence of thrombocytopenia within 3 months following chemotherapy initiation, with 16.3% and 12.4% exhibiting grade 3 and grade 4 thrombocytopenia, respectively (Figure 1; Table S2). Of the hematologic malignancies examined, incidence was highest in patients with multiple myeloma (37.3%), followed by those with non‐Hodgkin lymphoma (24.4%). Patients with other hematologic malignancies (N = 468) included those with leukemia (218/468 [47%] with acute leukemia) or myelodysplastic syndromes and had a 30.0% incidence of thrombocytopenia within 3 months of chemotherapy initiation. Incidences of grades 2, 3, and 4 thrombocytopenia were highest in patients with multiple myeloma at 27.4%, 23.5%, and 18.8%, respectively. Grade 3 and 4 thrombocytopenia occurred in 16.3% and 14.3% of patients with other hematologic malignancies (Figure 1; Table S2).
For most chemotherapy regimens, median time to first evidence of thrombocytopenia was approximately 1‐2 weeks following initiation of chemotherapy (Table 3). However, for platinum‐based regimens, median time to first thrombocytopenia was longer than two weeks in most tumor types: in breast cancer and NHL patients, median times were 5.9 and 5.4 weeks, respectively. In CRC and ovarian cancer patients on platinum‐based regimens, median times were 2.7 and 2.6 weeks, respectively. Median time to first evidence of thrombocytopenia by sex, race, or age was similar across tumor types; however, except for CRC, there was a shorter time to thrombocytopenia in older versus younger patients.
TABLE 3.
Median (IQR) time (in weeks) to first evidence of any thrombocytopenia following chemotherapy initiation
| Characteristic |
Lung N = 774 |
CRC N = 437 |
Breast N = 270 |
Ovarian N = 83 |
NHL N = 333 |
|---|---|---|---|---|---|
| Overall | 1.3 (1.0‐2.9) | 2.0 (1.6‐3.1) | 2.0 (1.0‐7.0) | 2.3 (1.0‐8.0) | 1.1 (0.9‐4.0) |
| Chemotherapy Regimen | |||||
| Gemcitabine | 1.1 (1.0‐5.0) | 0.0 (0.0‐0.0) | 1.1 (1.0‐2.3) | 1.9 (1.0‐7.0) | 1.4 (1.0‐2.6) |
| Platinum | 1.3 (1.0‐2.9) | 2.7 (1.4‐11.4) | 5.9 (1.0‐11.9) | 2.6 (1.1‐8.4) | 5.4 (0.4‐6.0) |
| Anthracycline | 0.9 (0.9‐0.9) | 0.0 (0.0‐0.0) | 2.9 (1.0‐7.0) | 0.0 (0.0‐0.0) | 1.1 (1.0‐5.7) |
| Taxane | 1.0 (1.0‐2.7) | 1.0 (1.0‐1.0) | 1.0 (0.7‐2.0) | 1.9 (0.7‐3.0) | 0.0 (0.0‐0.0) |
| Other | 1.3 (1.0‐2.3) | 2.0 (1.9‐2.1) | 1.0 (0.1‐6.3) | 1.7 (0.6‐2.0) | 1.4 (0.6‐2.9) |
| Sex | |||||
| Female | 1.6 (1.0‐4.0) | 2.0 (1.9‐2.7) | 2.0 (1.0‐7.0) | 2.3 (1.0‐8.0) | 1.1 (1.0‐4.0) |
| Male | 1.1 (1.0‐2.1) | 2.0 (1.4‐6.0) | N/A | N/A | 1.1 (0.9‐4.1) |
| Age | |||||
| < 65 years | 1.6 (1.0‐5.6) | 2.0 (1.6‐6.1) | 2.8 (1.0‐8.9) | 3.0 (1.0‐7.0) | 1.9 (1.0‐4.4) |
| ≥ 65 years | 1.3 (1.0‐2.0) | 2.0 (1.6‐2.4) | 1.1 (1.0‐6.9) | 2.0 (1.1‐8.3) | 1.0 (0.9‐2.6) |
Abbreviations: CRC, colorectal cancer; IQR, interquartile range; N/A, not applicable; NHL, non‐Hodgkin lymphoma.
Within 3 days of a thrombocytopenic platelet count, other cytopenias co‐occurred in most patients; however, 15.2% of all thrombocytopenia occurred without another cytopenia (17.7% of solid tumor patients and 7.4% of hematologic malignancy patients; Figure 3; Table S3). Anemia was the most common hematologic abnormality at first evidence of thrombocytopenia (78.7% of thrombocytopenia cases) and often occurred without the co‐occurrence of neutropenia or leukopenia (48.7%). Anemia and neutropenia occurred with thrombocytopenia in 19.1% of patients, followed by anemia and leukopenia in 6.4% of patients. Approximately, 93% of patients with hematologic malignancies had at least one other accompanying blood disorder at the time of thrombocytopenia. Thrombocytopenia without other cytopenia (isolated thrombocytopenia) occurred in 15.2% of thrombocytopenia cases overall and was a more common occurrence for patients with solid tumors (17.7%) than those with hematologic malignancies (7.4%). In contrast, pancytopenia occurred in 11% of patients overall, and more commonly in those with hematologic malignancies (21%) than solid tumors (8%) .
FIGURE 3.
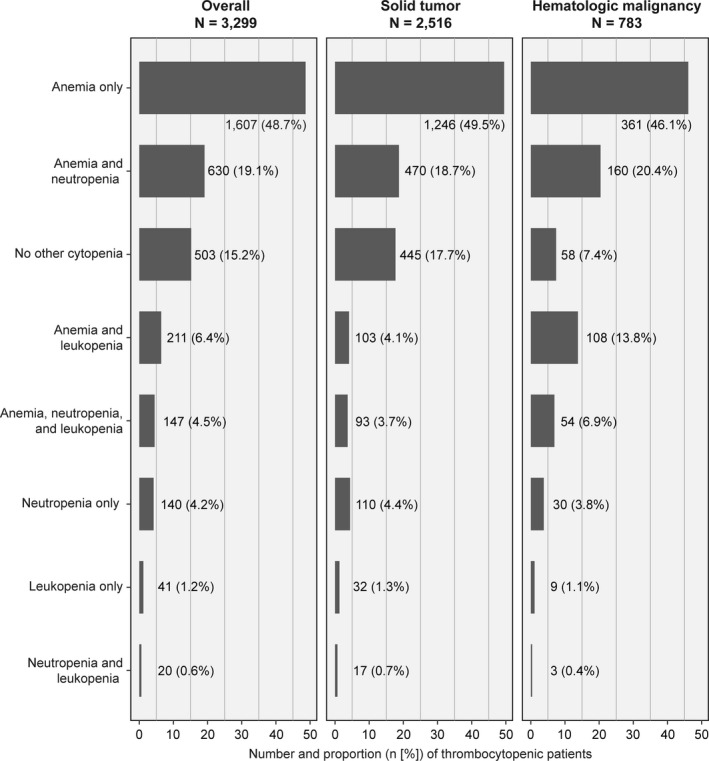
Percent of thrombocytopenic patients with co‐occurring cytopenia (among patients with complete lab values at first evidence of thrombocytopenia). For results in tabular format, see Table S3
4. DISCUSSION
A substantial proportion of patients experienced thrombocytopenia after chemotherapy initiation in this representative sample of US patients with cancer being treated in routine clinical practice. Overall, three‐month incidence of any thrombocytopenia in this study was 13% for solid tumors and 28% for hematologic malignancies, and the first instance of thrombocytopenia generally occurred within 2 weeks of chemotherapy initiation. Platinum‐based and gemcitabine‐based chemotherapy regimens were associated with the highest burden of thrombocytopenia. The incidence was between 13% and 15% for most tumor types (lung, colorectal, pancreas, ovary, and bladder); approximately 10% for breast and prostate; and highest for melanoma (21%). Thrombocytopenia was far more common in multiple myeloma (37%) and non‐Hodgkin lymphoma (24%) than in solid tumors. Thrombocytopenia occurred without other cytopenias in 15% of those who experienced thrombocytopenia, while anemia was the most common co‐occurring hematologic abnormality (79%), followed by neutropenia (28%), and leukopenia (13%).
The overall three‐month incidence of thrombocytopenia in this study is consistent with previously reported estimates, which range from 10% to 68%. 1 , 2 , 3 , 7 , 11 In a retrospective hospital‐based study in the Netherlands from 2004‐2006, overall thrombocytopenia prevalence, defined as a platelet count <100 × 109/L, was 22% in patients with solid tumors. 2 Another study of patients with solid tumors receiving chemotherapy in the UK used a threshold of <75 × 109/L to indicate clinically‐significant thrombocytopenia and estimated that 10% of patients had CIT. 1 As expected, a higher incidence of thrombocytopenia was seen among patients with hematologic cancers in the current study, and it is important to note that many patients had low platelet counts prior to starting chemotherapy. Multiple myeloma, acute leukemia, and other hematologic malignancies often cause low blood counts. In a study by Braunlin et al, 27% of multiple myeloma patients received bone marrow transplant during follow‐up with 2.4% of them receiving two or more transplants. 30
In the most recent, large observational study of CIT risk and consequences in the United States using the MarketScan and PharMetrics claims databases, overall incidence of CIT was estimated at 9.7%. 7 Incidence estimates in several common tumor types (colorectal, lung, and breast) were slightly higher in the current study and were much higher for non‐Hodgkin lymphoma (24.4%) than the recent analysis of US claims data (9.8%). 7 Importantly, the definitions of thrombocytopenia differed between the claims‐based study and the current analysis. The claims‐based study 7 defined CIT using diagnostic and procedural codes (platelet counts were unavailable), while our study used a platelet threshold of <100 × 109/L. Additionally, the claims‐based study excluded patients with any history of thrombocytopenia in the year prior to chemotherapy initiation from their estimates, while we excluded only patients with secondary non‐cancer causes of thrombocytopenia prior to chemotherapy. This may explain some of the differences in incidence of CIT among NHL patients particularly, as a sensitivity analysis revealed that 13% of NHL patients in the current study had thrombocytopenia (platelets <100 × 109/L) in the 30 days prior to chemotherapy initiation (Table S1).
Few observational studies have assessed thrombocytopenia by CTCAE grade in relation to cancer type and chemotherapy regimen. The risk of bleeding and the number of platelet transfusions increase with increasing grade of CIT. 1 , 8 , 11 However, grade 1 or 2 CIT may identify patients at risk of experiencing more severe CIT and its sequelae, as chemotherapy is often modified before grade 3 or 4 CIT is observed. 8 In a large US study of outpatient oncology clinics, 3 grade 2, 3, and 4 thrombocytopenia occurred in 1.1%, 0.9%, and 0.7% of all cancers, respectively. A study of Dutch patients found that grades 2, 3, and 4 thrombocytopenia occurred in 5.0%, 3.6%, and 3.3% of patients. 2 Estimates in our study were slightly higher overall, with 8% experiencing grade 2, 6% with grade 3, and 3% with grade 4 thrombocytopenia. Though incidence of grade 3‐4 thrombocytopenia is low, early detection of low‐grade thrombocytopenia could identify patients at higher risk of future complications for early intervention.
Like previous reports, 3 gemcitabine‐based regimens were associated with the highest risk of thrombocytopenia in patients with solid tumors (15%), followed by platinum‐based regimens (14%). One study of patients seen in outpatient oncology clinics in the United States found that the prevalence was lowest (8%) for those on taxane‐based regimens, followed by anthracycline‐based regimens (17%) and platinum‐based regimens (31%), and it was highest for those on gemcitabine‐based regimens (37%). 3 The US claims‐based study also found the highest association of thrombocytopenia with gemcitabine (14%), followed by carboplatin (13%), and oxaliplatin (11%). 7 In the single‐center study in the UK, CIT occurred most often in gemcitabine/cisplatin regimens, followed by gemcitabine/carboplatin regimens, and cisplatin/etoposide regimens. 1 Another single‐center study of Dutch patients noted that the most common combinations of chemotherapy associated with thrombocytopenia included gemcitabine/carboplatin, gemcitabine/cisplatin, and paclitaxel/carboplatin. 2 By design of the current study, if patients were exposed to more than one class of chemotherapy agent, they were assigned to a single chemotherapy class in hierarchical order of hematotoxicity. For example, a gemcitabine/carboplatin regimen was only counted as a gemcitabine‐based and not a platinum‐based chemotherapy. This likely resulted in an underestimation of thrombocytopenia incidence in some classes of chemotherapy agents.
Several therapies, including thrombopoietin receptor agonists (TPO‐RAs), are effective in correcting platelet counts and have been approved for treating other conditions of thrombocytopenia. 31 , 32 A recent study of romiplostim to treat patients with solid tumors who have CIT found romiplostim to be safe and effective at rapidly boosting platelet counts in most patients, thereby allowing chemotherapy to be given at full dose and on schedule. 9 In a phase II randomized trial of patients with solid tumors given romiplostim versus untreated observation for CIT, 85% (44/52) of romiplostim‐treated patients achieved platelet correction, allowing chemotherapy to resume with romiplostim maintenance. 33 Two other randomized, controlled trials are also underway (NCT03362177 and NCT03937154). Further, in a phase II randomized, placebo‐controlled study of patients with advanced solid tumors receiving gemcitabine‐based chemotherapy, eltrombopag treatment shortened the time to platelet recovery and reduced dose delays/reductions due to thrombocytopenia. 34 No significant adverse events have been associated with the use of TPO‐RAs to treat CIT. 33 , 35
Responses to CIT therapy will likely depend on several patient and disease characteristics. A recent multicenter study 35 showed three settings where CIT was refractory to romiplostim treatment: (1) bone marrow invasion, (2) prior pelvic irradiation, and (3) prior temozolomide. 9 Additionally, the phase II romiplostim trial found that 100% of patients with liver involvement from their cancer responded to romiplostim. 27 Therefore, the clinical profile of a thrombocytopenic cancer patient who may benefit from a therapeutic intervention with TPO mimetics, would likely be a patient without bone metastases, without prior pelvic radiation, and who has had hepatic involvement from cancer.
Cancer patients with low platelets and full or relative preservation of white blood cells and hemoglobin, that is, isolated CIT, are also most likely to benefit from TPO‐RA therapy. Thus, the key to identifying appropriate therapeutic intervention is to determine which patients have CIT without concurrent neutropenia, anemia, or other factors that could contribute to chemotherapy delays/dose reductions. While thrombocytopenia was usually accompanied by at least one other cytopenia, 15.2% of thrombocytopenia cases occurred without other cytopenias. Results were similar to those reported from a single‐center study of solid tumor patients: overall thrombocytopenia was estimated at 22% (compared to 13% in the current study), while isolated thrombocytopenia occurred in 6%, 2 compared to 3% in the current study if you calculate the proportion out of all solid tumor patients who experienced thrombocytopenia alone (445/15 521). In a large study of US outpatient oncology patients, 55% of patients with thrombocytopenia also experienced anemia. 3 Within our sample, anemia was the most common co‐occurring cytopenia, with 79% of patients experiencing it at first evidence of thrombocytopenia. Although guidelines for management of chemotherapy‐related anemia and neutropenia have been established, 36 guidance for the prevention of thrombocytopenia has not yet been established. However, it is worth noting that NCCN guidelines published during the COVID‐19 global pandemic, due to limited blood supply products, recommended use of thrombopoietin mimetics such as romiplostim as such: “for patients with severe thrombocytopenia post‐cancer chemotherapy (solid tumor; eg, Soff et al 33 ), start for platelet count < 30‐50K and then discontinue for platelet count > 75‐100K.” 37 Identifying patients at risk for dose modifications due to isolated thrombocytopenia may allow for preventive therapy and maintenance of scheduled chemotherapy.
Limitations of this study and those inherent to observational studies should be acknowledged. Although this is a large sample relevant to the cancer patient population, it may not represent cancer patients who do not have access to such clinics (where economic and social barriers could differ and affect access to diagnosis and management) or those being treated outside of this specialty setting. Academic centers are largely underrepresented in Flatiron, as most contributing clinics are community‐based. Additionally, diagnoses or platelet draws that occur outside of the oncology clinic setting are likely to be under‐recorded in the structured Flatiron data. Therefore, thrombocytopenia occurring in hospitalized patients may be underreported in this analysis. Patients who had thrombocytopenia prior to initiating chemotherapy were not excluded, because this study sought to characterize the overall burden of thrombocytopenia in cancer patients receiving chemotherapy; thus, we could not assume whether the thrombocytopenia was due to the myelosuppressive chemotherapy or the underlying disease itself. Clinical trial patients may be exposed to different agents than those described in the current study and it is unknown what CIT incidence would be in these new investigational agents. Incidence of thrombocytopenia in cancer patients receiving immunotherapy is also unknown and should be explored in future studies.
Despite these limitations, this study had many strengths. Data used in this analysis are structured EHR data from oncology clinics, where cancer diagnosis and recorded chemotherapy administrations are highly likely to be accurate, and longitudinal platelet counts are well‐captured. Therefore, results of this study are generalizable to cancer patients receiving chemotherapy treatment in US outpatient oncology clinics. Analyses of databases derived from Flatiron have shown a reasonable degree of completeness for most EHR structured information, and overall, the characteristics of populations included in this database are comparable with other large population‐based cancer registries in the US. 38 Thus, the potential for misclassification in terms of cancer type and/or receipt of chemotherapy in this data source is very low. A major advantage of utilizing data from Flatiron Health is the comprehensive capture of laboratory tests. Because eligible patients were those actively receiving chemotherapy, it is expected that these patients were actively monitored, including platelet draws, on a regular basis. Further, clinics contributing data to Flatiron are geographically diverse, and the large size of the underlying population provides a diverse patient demographic.
5. CONCLUSION
Given the substantial incidence of thrombocytopenia across many common tumor types and chemotherapy regimens, future research should elucidate the contribution of CIT to chemotherapy dose modifications, receipt of platelet transfusions, and bleeding‐related episodes and how these events ultimately contribute to cancer outcomes, such as a reduction in overall survival. Ongoing trials of CIT interventional agents hold promise for avoiding adverse outcomes of CIT while allowing patients to continue planned chemotherapy treatment.
CONFLICT OF INTEREST
JS, CN, AM, and JP are Amgen employees and have stock ownership in Amgen Inc; GS is and has been a clinical investigator for Amgen and trials.
AUTHOR CONTRIBUTIONS
JS contributed study concept and design, analysis, and interpretation of data, and drafting of manuscript; CN contributed study concept and design, interpretation of data, and critical revision of manuscript for important intellectual content; AM contributed study concept and design, analysis, and interpretation of data, drafting and critical revision of manuscript for important intellectual content; JP contributed study concept and design, interpretation of data, and critical revision of manuscript for important intellectual content; and GS contributed study concept and design, interpretation of data, and critical revision of manuscript for important intellectual content. All authors have read and approved the final draft of the manuscript.
Supporting information
Table S1‐S3
ACKNOWLEDGEMENTS
This study was funded by Amgen, Inc
Shaw JL, Nielson CM, Park JK, Marongiu A, Soff GA. The incidence of thrombocytopenia in adult patients receiving chemotherapy for solid tumors or hematologic malignancies. Eur J Haematol. 2021;106:662–672. 10.1111/ejh.13595
DATA AVAILABILITY STATEMENT
The data that support the findings of this study have been originated by Flatiron Health, Inc These de‐identified data may be made available upon request, and are subject to a license agreement with Flatiron Health; interested researchers should contact DataAccess@flatiron.com to determine licensing terms.
REFERENCES
- 1. Hitron A, Steinke D, Sutphin S, Lawson A, Talbert J, Adams V. Incidence and risk factors of clinically significant chemotherapy‐induced thrombocytopenia in patients with solid tumors. J Oncol Pharm Pract. 2011;17(4):312‐319. [DOI] [PubMed] [Google Scholar]
- 2. ten Berg MJ, van den Bemt PMLA, Shantakumar S, et al. Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy results from a retrospective hospital‐based cohort. Drug Saf. 2011;34(12):1151‐1160. [DOI] [PubMed] [Google Scholar]
- 3. Wu Y, Aravind S, Ranganathan G, Martin A, Nalysnyk L. Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: a descriptive study of a large outpatient oncology practice database, 2000–2007. Clin Ther. 2009;31(Pt 2):2416‐2432. [DOI] [PubMed] [Google Scholar]
- 4. Goldberg GL, Gibbon DG, Smith HO, DeVictoria C, Runowicz CD, Burns ER. Clinical impact of chemotherapy‐induced thrombocytopenia in patients with gynecologic cancer. J Clin Oncol. 1994;12(11):2317‐2320. [DOI] [PubMed] [Google Scholar]
- 5. Kantarjian H, Giles F, List A, et al. The incidence and impact of thrombocytopenia in myelodysplastic syndromes. Cancer. 2007;109(9):1705‐1714. [DOI] [PubMed] [Google Scholar]
- 6. Liou SY, Stephens JM, Carpiuc KT, Feng W, Botteman MF, Hay JW. Economic burden of haematological adverse effects in cancer patients: a systematic review. Clin Drug Investig. 2007;27(6):381‐396. [DOI] [PubMed] [Google Scholar]
- 7. Weycker D, Hatfield M, Grossman A, et al. Risk and consequences of chemotherapy‐induced thrombocytopenia in US clinical practice. BMC Cancer. 2019;19(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology (Williston Park). 2015;29(4):282‐294. [PubMed] [Google Scholar]
- 9. Al‐Samkari H, Marshall AL, Goodarzi K, Kuter DJ. The use of romiplostim in treating chemotherapy‐induced thrombocytopenia in patients with solid tumors. Haematologica. 2018;103(4):e169‐e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Denduluri N, Patt DA, Wang Y, et al. Dose delays, dose reductions, and relative dose intensity in patients with cancer who received adjuvant or neoadjuvant chemotherapy in community oncology practices. J Natl Compr Canc Netw. 2015;13(11):1383‐1393. [DOI] [PubMed] [Google Scholar]
- 11. Elting LS, Rubenstein EB, Martin CG, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy‐induced thrombocytopenia. J Clin Oncol. 2001;19(4):1137‐1146. [DOI] [PubMed] [Google Scholar]
- 12. Larsen JB, Hojbjerg JA, Hvas AM. The Role of Platelets in Cancer‐Related Bleeding Risk: A Systematic Review. Semin Thromb Hemost. 2020;46(3):328‐341. [DOI] [PubMed] [Google Scholar]
- 13. Rioux‐Massé B, Laroche V, Bowman RJ, et al. The influence of bleeding on trigger changes for platelet transfusion in patients with chemotherapy‐induced thrombocytopenia. Transfusion. 2013;53(2):306‐314. [DOI] [PubMed] [Google Scholar]
- 14. Goodnough LT, DiPersio JF. Issues in the management of cancer‐related thrombocytopenia. Oncology (Williston Park). 2002;16(11):1558‐1567; discussion 1570, 1572–1574. [PubMed] [Google Scholar]
- 15. Schiffer CA, Bohlke K, Delaney M, et al. Platelet transfusion for patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2018;36(3):283‐299. [DOI] [PubMed] [Google Scholar]
- 16. Havrilesky LJ, Reiner M, Morrow PK, Watson H, Crawford J. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol. 2015;93(3):203‐210. [DOI] [PubMed] [Google Scholar]
- 17. Crawford J, Denduluri N, Patt D, et al. Relative dose intensity of first‐line chemotherapy and overall survival in patients with advanced non‐small‐cell lung cancer. Support Care Cancer. 2020;28(2):925‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagel CI, Backes FJ, Hade EM, et al. Effect of chemotherapy delays and dose reductions on progression free and overall survival in the treatment of epithelial ovarian cancer. Gynecol Oncol. 2012;124(2):221‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwamoto K, Ishihara H, Takagi T, et al. Evaluation of relative dose intensity during the early phase of first‐line sunitinib treatment using a 2‐week‐on/1‐week‐off regimen for metastatic renal cell carcinoma. Med Oncol. 2018;35(6):78. [DOI] [PubMed] [Google Scholar]
- 20. Ishiyama R, Ishihara H, Kondo T, et al. Negative effect of immediate sunitinib interruption on survival in patients with metastatic renal cell carcinoma. Vivo. 2019;33(6):2153‐2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bosly A, Bron D, Van Hoof A, et al. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B‐cell lymphoma patients treated with CHOP. Ann Hematol. 2008;87(4):277‐283. [DOI] [PubMed] [Google Scholar]
- 22. Chirivella I, Bermejo B, Insa A, et al. Optimal delivery of anthracycline‐based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat. 2009;114(3):479‐484. [DOI] [PubMed] [Google Scholar]
- 23. Hanna RK, Poniewierski MS, Laskey RA, et al. Predictors of reduced relative dose intensity and its relationship to mortality in women receiving multi‐agent chemotherapy for epithelial ovarian cancer. Gynecol Oncol. 2013;129(1):74‐80. [DOI] [PubMed] [Google Scholar]
- 24. Loibl S, Skacel T, Nekljudova V, et al. Evaluating the impact of Relative Total Dose Intensity (RTDI) on patients' short and long‐term outcome in taxane‐ and anthracycline‐based chemotherapy of metastatic breast cancer‐ a pooled analysis. BMC Cancer. 2011;11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. National Institutes of Health, National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. In. November 27, 2017 ed: U.S. Department of Health and Human Services. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed 21 December 2020 [Google Scholar]
- 26. Groopman JE, Itri LM. Chemotherapy‐Induced Anemia in Adults: Incidence and Treatment. JNCI. 1999;91(19):1616‐1634. [DOI] [PubMed] [Google Scholar]
- 27. Wang L, Hai‐Gang L, Zhong‐Sheng X, Jian‐Ming W, Lv J. Prognostic significance of erythropoietin and erythropoietin receptor in gastric adenocarcinoma. World J Gastroenterol. 2011;17(34):3933‐3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muturi‐Kioi V, Lewis D, Launay O, et al. Neutropenia as an Adverse Event following Vaccination: Results from Randomized Clinical Trials in Healthy Adults and Systematic Review. PLoS One. 2016;11(8):e0157385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu W, Zhang CC, Li K. Prognostic value of chemotherapy‐induced leukopenia in small‐cell lung cancer. Cancer Biol Med. 2013;10(2):92‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Braunlin M, Belani R, Buchanan J, Wheeling JT, Kim C. Trends in the multiple myeloma treatment landscape: a United States analysis of OSCER electronic health record data 2011–2019. Blood. 2019;134(Suppl 1):4722. [Google Scholar]
- 31. Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237‐2247. [DOI] [PubMed] [Google Scholar]
- 32. Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double‐blind randomised controlled trial. Lancet. 2008;371(9610):395‐403. [DOI] [PubMed] [Google Scholar]
- 33. Soff GA, Miao Y, Bendheim G, et al. Romiplostim treatment of chemotherapy‐induced thrombocytopenia. J Clin Oncol. 2019;37(31):2892‐2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Winer ES, Safran H, Karaszewska B, et al. Eltrombopag for thrombocytopenia in patients with advanced solid tumors receiving gemcitabine‐based chemotherapy: a randomized, placebo‐controlled phase 2 study. Int J Hematol. 2017;106(6):765‐776. [DOI] [PubMed] [Google Scholar]
- 35. Al‐Samkari H, Parnes AD, Goodarzi K, Weitzman JI, Connors JM, Kuter DJ. A multicenter study of romiplostim for chemotherapy‐induced thrombocytopenia in solid tumors and hematologic malignancies. Haematologica; 2020. 10.3324/haematol.2020.251900 [Early view] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Becker PS, Griffiths EA, Alwan LM, et al. NCCN clinical practice guidelines in oncology (NCCN Guidelines): hematopoietic growth factors, version 2.2020. J Natl Compr Canc Netw. 2020;18(2). [Google Scholar]
- 37. NCCN . NCCN Hematopoietic Growth Factors: Short‐Term Recommendations Specific to Issues with COVID‐19 (SARS‐CoV‐2); 2020 March 28, 2020. https://pubmed.ncbi.nlm.nih.gov/31910384/. Accessed 21 December 2020
- 38. Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of population characteristics in real‐world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. https://www.medrxiv.org/content/10.1101/2020.03.16.20037143v2. Accessed 21 December 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3
Data Availability Statement
The data that support the findings of this study have been originated by Flatiron Health, Inc These de‐identified data may be made available upon request, and are subject to a license agreement with Flatiron Health; interested researchers should contact DataAccess@flatiron.com to determine licensing terms.


