Abstract
BACKGROUND:
Analgesics that contain codeine are commonly prescribed for postoperative pain, but it is unclear how they compare with nonopioid alternatives. We sought to compare the effectiveness of codeine and nonsteroidal anti-inflammatory drugs (NSAIDs) for adults who underwent outpatient surgery.
METHODS:
We conducted a systematic review and meta-analysis of randomized controlled trials comparing codeine and NSAIDs for postoperative pain in outpatient surgery. We searched MEDLINE and Embase from inception to October 2019 for eligible studies. Our primary outcome was the patient pain score, converted to a standard 10-point intensity scale. Our secondary outcomes were patient-reported global assessments and adverse effects. We used random-effects models and grading of recommendations assessment, development and evaluation (GRADE) to assess the quality of evidence.
RESULTS:
Forty studies, including 102 trial arms and 5116 patients, met inclusion criteria. The studies had low risk of bias and low-to-moderate heterogeneity. Compared with codeine, NSAIDs were associated with better pain scores at 6 hours (weighted mean difference [WMD] 0.93 points, 95% confidence interval [CI] 0.71 to 1.15) and at 12 hours (WMD 0.79, 95% CI 0.38 to 1.19). Stronger NSAID superiority at 6 hours was observed among trials where acetaminophen was coadministered at equivalent doses between groups (WMD 1.18, 95% CI 0.87 to 1.48). NSAIDs were associated with better global assessments at 6 hours (WMD −0.88, 95% CI −1.04 to −0.72) and at 24 hours (WMD −0.67, 95% CI −0.95 to −0.40), and were associated with fewer adverse effects, including bleeding events.
INTERPRETATION:
We found that adult outpatients report better pain scores, better global assessments and fewer adverse effects when their postoperative pain is treated with NSAIDs than with codeine. Clinicians across all specialties can use this information to improve both pain management and opioid stewardship.
Outpatient surgical procedures are now more common than inpatient procedures, given the development of less invasive techniques, the drive for health care efficiency, and improvements in anesthesia and pain management. 1–4 Postoperative pain management after outpatient procedures often includes low-potency or low-dose opioids.5 Codeine use is widespread in this setting and codeine remains the most commonly prescribed opioid in many countries, including Canada.6–9 However, its efficacy is variable, its potency is low and its use is associated with risks of severe adverse effects and misuse.10 Amid the ongoing opioid crisis, management of pain and potential opioid misuse is important across all medical and dental specialties.11
Nonsteroidal anti-inflammatory drugs (NSAIDs) are an alternative to low-potency opioids. The potency, effects and toxicity of NSAIDs depend on the degree to which they inhibit cyclooxygenase 1 and 2 activity. Their main adverse effects are gastrointestinal bleeding, renal impairment and myocardial infarction with long-term use.12–15 Postoperative pain can be effectively managed with NSAIDs, and NSAIDs have been shown to reduce opioid consumption in postoperative patients.16
Given how commonly these medications are used, and the uncertainty in their comparative efficacy and safety, we sought to compare pain and safety outcomes for codeine-based medications and NSAIDs among adults who underwent outpatient surgery through a systematic review and meta-analysis of randomized controlled trials (RCTs).
Methods
We report this meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.17
Search strategy and study selection
With the assistance of a medical librarian, we systematically searched MEDLINE and Embase from inception to Oct. 28, 2019. Our search strategy is described in Appendix 1, eTables 1 and 2, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.201915/tab-related-content.
We included RCTs that compared oral codeine with oral NSAIDs prescribed to adult outpatients having acute postoperative pain. Our primary outcome of interest was efficacy, defined as the level of pain, measured by a validated pain scale. Secondary outcomes included patient-reported post-treatment global assessment score, and safety, measured by reported adverse effects. We included trials in which acetaminophen was coadministered with codeine or NSAIDs. We excluded trials in which intravenous drugs or drugs other than acetaminophen were coadministered. We also excluded trials that used medications that are no longer available because of safety concerns (e.g., zomepirac, indoprofen).
Using a standardized, pilot-tested form, 2 physicians screened titles and abstracts. The same physicians subsequently reviewed full texts of potentially eligible studies, independently and in duplicate, to assess for inclusion. They resolved disagreements with discussion.
Risk of bias assessment
We assessed individual studies’ risk of bias using the Cochrane risk of bias assessment tool, which includes assessment of random sequence generation, allocation concealment, blinding of participants and personnel, outcome assessment, incomplete outcome data, selective reporting and other sources of bias.18 Two authors assessed risk of bias, independently and in duplicate, and resolved disagreements by discussion. For the purposes of subgroup testing based on risk of bias, we dichotomized studies into high or low risk; we considered studies high risk if they had more than 1 bias category rated as high risk.
Data extraction
We developed and piloted a standardized, data extraction form. A single author extracted data, which was then verified independently by a second author. We collected study characteristics, patient characteristics, interventions and outcomes. Our primary outcome was pain, measured on a 10-point scale. If studies reported more than 1 pain score, we preferentially used the patient-reported pain intensity score in the following order: 10-point scale, visual analogue scale, pain intensity difference score or sum of pain intensity difference. In the absence of these options, we used pain relief, mean peak pain relief or total pain relief, in that preferred order. We converted all pain measures to a 10-point scale to facilitate comparison. We collected data on pain scores at 0 to 6 hours, 6 to 12 hours, 12 to 24 hours, 24 to 48 hours, 48 to 72 hours and more than 3 days after treatment. Our secondary outcomes were global assessments, on a 4-point scale, and adverse effects. We extracted all reported outcomes of adverse effects.
When multiarm trials included ineligible treatment arms, we extracted data only on the treatment arms meeting our eligibility criteria. When more than 1 arm within a single trial was eligible for inclusion (e.g., 1 codeine arm and 2 NSAID arms) we halved the “shared” group into 2 groups with halved sample size, then performed 2 direct comparisons, per Cochrane methodology.19 This method was chosen despite its limitations to allow separate trial arm comparisons for subgroup testing. We checked manuscripts by the same authorship for double-counting.
Statistical analysis
We calculated the Cohen κ statistic to evaluate interrater agreement. We converted all continuous outcome measures to a common reference scale, namely a 10-point scale of pain intensity (where lower is better) and a 4-point global assessment scale (where higher is better).20 If pain scores were reported using a scale where higher is better (opposite to the direction of the standard scale), then we inverted the data to maintain a consistent direction that lower is better. When the standard deviation (SD) of each group was not reported, we imputed the SD from the standard error, 95% confidence interval (CI), interquartile range, and p value; if these were not available, we imputed SDs from a similar study, per Cochrane methodology.19
We pooled all continuous outcomes using the weighted mean difference (WMD) and 95% CI, after conversion to the reference scale.20 This was to facilitate ease of clinical interpretation, so that a WMD of 1.0 would be equivalent to a 1-point difference on a 10-point scale for pain and a 1-point difference on a 4-point scale for global assessments. We set the minimal clinically important difference at 1.0 on a 10-point scale for pain, and 0.5 on a 4-point scale for global assessments.21,22 We pooled all binary outcomes using relative risks (RRs) and 95% CIs. We used a DerSimonian–Laird random effects model for all meta-analyses. We performed Grading of Recommendations Assessment, Development, and Evaluation (GRADE) quality and evidence assessments.
We used the Cochran χ2 test, I2 and the τ statistic to evaluate statistical heterogeneity. We conducted a priori subgroup analyses to evaluate the presence or absence of acetaminophen cointervention, type of NSAIDs (selective versus nonselective), surgery type and risk of bias. We predicted that acetaminophen would benefit both NSAID and codeine interventions, and we predicted that nonselective NSAIDs would be more effective than selective NSAIDs. If there were at least 10 studies available for meta-analysis, we assessed publication bias using funnel plots and the Egger test.23 We performed statistical analyses using Stata statistical software version ( Version 15.1). All comparisons were 2-tailed using a threshold p ≤ 0.05.
Results
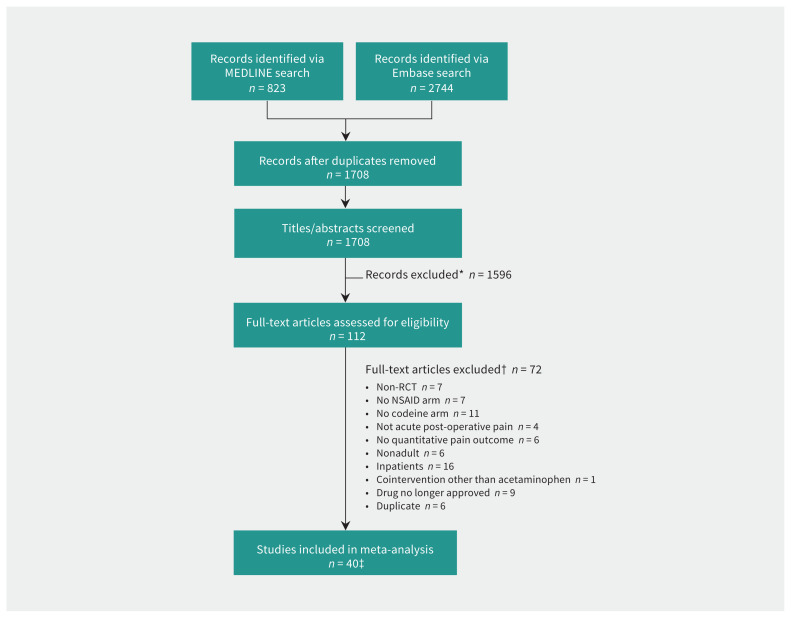
The results of our search strategy are summarized in Figure 1. We found a total of 1708 articles, and did not encounter any instances of double-counting. Screening, review and consensus ultimately resulted in 40 RCTs, 102 treatment arms (40 codeine treatment arms and 62 NSAID treatment arms), and 5116 patients (1872 patients prescribed codeine, and 3243 patients prescribed NSAIDs) being included for meta-analysis (Table 1 and Appendix 1, eTables 3 and 4). Various types and doses of NSAIDs were used. Codeine doses ranged from 15 mg to 90 mg, consistent with the most common outpatient formulations. The quality of studies included in the meta-analysis was high, with generally low risk of bias (Appendix 1, eFigures 1 and 2) and no evidence of publication bias (Appendix 1, eFigures 3, 4 and 5). The overall findings are summarized in the GRADE evidence profiles (Table 2 and Table 3).
Figure 1:
Flow chart for study selection. Note: NSAID = nonsteroidal anti-inflammatory drug, RCT = randomized controlled trial. *Reasons for exclusion: non-RCT, no NSAID arm, no codeine arm, not acute postoperative pain, no quantitative pain outcome measure, nonadult study, inpatient use, cointervention with analgesic other than acetaminophen, drug no longer approved for use in humans and multiple combinations thereof. †See Appendix 1 for full table of exclusions. The same inclusion/exclusion criteria were applied to both stages of review. Full-text review was used when titles and abstracts were ambiguous. An article could be excluded for more than one reason. ‡Cohen κ = 0.80 (95% confidence interval 0.68 to 0.91).
Table 1:
Summary of included studies
| Study | Surgery type | Codeine intervention | NSAID intervention | Maximum follow-up duration | Stated funding sources |
|---|---|---|---|---|---|
| Breivik et al., 199924 | Dental | Acetaminophen/codeine 1000 mg/60 mg, n = 23 |
Diclofenac/acetaminophen 100 mg/1000 mg, n = 24 Diclofenac 100 mg, n = 22 |
8 h | University |
| Chang et al., 200125 | Dental | Acetaminophen/codeine 600 mg/60 mg, n = 180 |
Rofecoxib 50 mg, n = 182 | 24 h | Industry |
| Chen et al., 200926 | Plastic | Acetaminophen/codeine 600 mg/60 mg, n = 17 |
Ibuprofen 400 mg, n = 18 | 4 d | None |
| Comfort et al., 200227 | Dental | Acetaminophen/codeine 500 mg/8 mg, n = 80 |
Diflusinal 250 mg, n = 66 Etodolac 200 mg, n = 80 |
24 h | None |
| Cooper et al., 198228 | Dental | Codeine 60 mg, n = 41 | Ibuprofen 400 mg, n = 38 ASA 650 mg, n = 38 |
4 h | Industry |
| Cooper et al., 198829 | Dental | Acetaminophen/codeine 600 mg/60 mg, n = 31 |
Meclofenamate 100 mg, n = 36 | 6 h | Industry |
| Cooper et al., 199130 | Dental | Acetaminophen/codeine 650/60 mg, n = 39 |
Flurbiprofen 50 mg, n = 42 Flurbiprofen 100 mg, n = 41 |
6 h | None |
| Cooper et al., 199331 | Dental | Codeine 30 mg, n = 37 | Ibuprofen 600 mg, n = 38 Ibuprofen 200 mg, n = 45 |
12 h | Industry |
| Coutinho et al., 197632 | Urologic | Codeine 30 mg, n = 14 | Fenbufen 400 mg, n = 15 Fenbufen 800 mg, n = 16 ASA 600 mg, n = 15 |
5 h | Industry |
| Daniels et al., 2011a33 | Dental | Acetaminophen/codeine 1000 mg/30 mg, n = 113 |
Ibuprofen/acetaminophen 200 mg/500 mg, n = 173 Ibuprofen/acetaminophen 400 mg/1000 mg, n = 168 |
12 h | Industry |
| Daniels et al., 2011b34 | Dental | Acetaminophen/codeine 600 mg/60 mg, n = 62 |
Ibuprofen 600 mg, n = 192 Etoricoxib 90 mg, n = 191 Etoricoxib 120 mg, n = 97 |
24 h | Industry |
| De Los Santos et al., 199835 | General | Acetaminophen/codeine 500 mg/30 mg, n = 67 |
Lysine clonixinate 125 mg, n = 74 | 48 h | None |
| Desjardins et al., 198436 | Dental | Codeine 60 mg, n = 40 | ASA 650 mg, n = 40 | 6 h | Industry |
| Dionne et al., 199437 | Dental | Acetaminophen/codeine 650/60 mg, n= 24 |
Flurbiprofen 50 mg, n = 26 Flurbiprofen 100 mg, n = 22 |
6 h | Industry |
| Forbes et al., 198238 | Dental | Acetaminophen/codeine 600 mg/60 mg, n = 31 |
Diflusinal 500 mg, n = 32 Diflusinal 1000 mg, n = 32 |
12 h | Industry |
| Forbes et al., 198639 | Dental | Codeine 60 mg, n = 44 | Naproxen 550 mg, n = 38 ASA 650 mg, n = 36 |
12 h | Industry |
| Forbes et al., 198940 | Dental | Acetaminophen/codeine 600 mg/60 mg, n = 17 |
Flurbiprofen 100 mg, n = 26 | 12 h | Industry |
| Forbes et al., 1990a41 | Dental | Acetaminophen/codeine 600 mg/60 mg, n = 27 |
Ketorolac 10 mg, n = 37 ASA 650 mg, n = 32 |
6 d | Industry |
| Forbes et al., 1990b42 | Dental | Acetaminophen/codeine 600 mg/60 mg, n = 38 |
Ketorolac 10 mg, n = 31 Ketorolac 20 mg, n = 35 Ibuprofen 400 mg, n = 32 |
6 d | Industry |
| Gatoulis et al., 201243 | Dental | Acetaminophen/codeine 300 mg/30 mg, n = 119 |
ASA 1000 mg, n = 120 | 7 d | Industry |
| Giglio et al., 199044 | Dental | Codeine 60 mg, n = 37 | Meclofenamate 100 mg, n = 41 | 6 h | None |
| Giles et al., 198545 | Dental | Codeine 60 mg, n = 29 | Ibuprofen 400 mg, n = 37 | 3 d | Industry |
| Giles et al., 198646 | Dental | Codeine 15 mg, n = 42 | Ibuprofen 200 mg, n = 37 ASA 600 mg, n = 39 |
7 d | None |
| Habib et al., 199047 | Dental | Acetaminophen/codeine/caffeine 500 mg/8 mg/ 30 mg, n = 25 |
Ibuprofen 400 mg, n = 26 ASA/caffeine 300 mg/30 mg, n = 26 |
2 h | Industry |
| Hersh et al., 199348 | Dental | Codeine 60 mg, n = 30 | Ibuprofen 400 mg, n = 24 | 6 h | Public |
| Indelicato et al., 198649 | Orthopedic | Acetaminophen/codeine 600 mg/60 mg, n = 9 |
Diflusinal 500 mg, n = 11 | 5 d | None |
| Lysell et al., 199250 | Dental | Acetaminophen/codeine 500 mg/30 mg, n = 60 |
Ibuprofen 600 mg, n = 60 | 6 d | None |
| Malmstrom et al., 200451 | Dental | Acetaminophen/codeine 600 mg/60 mg, n = 50 |
Naproxen 550 mg, n = 51 Etoricoxib 120 mg, n = 50 |
10 d | Industry |
| Malmstrom et al., 200552 | Dental | Acetaminophen/codeine 600 mg/60 mg, n = 50 |
Etoricoxib 120 mg, n = 100 | 24 h | Industry |
| Mehlisch et al., 198453 | Dental | Codeine 90 mg, n = 27 | Ketoprofen 25 mg, n = 24 Ketoprofen 50 mg, n = 27 Ketoprofen 100 mg, n = 27 |
6 h | Industry |
| Mitchell et al., 200854 | General | Acetaminophen/codeine/caffeine 300 mg/30 mg/15 mg, n = 71 | Ibuprofen/acetaminophen 400 mg/325 mg, n = 69 | 7 d | Public and unrestricted industry grant |
| Mitchell et al., 201255 | Plastic | Acetaminophen/codeine/caffeine 600 mg/60 mg/30 mg, n = 70 | Ibuprofen/acetaminophen 400 mg/650 mg, n = 71 | 7 d | Public |
| Ottinger et al., 199056 | Orthopedic | Acetaminophen/codeine 300 mg/30 mg, n = 42 |
Flurbiprofen 50 mg, n = 41 | 4 d | Industry |
| Raeder et al., 200157 | General | Acetaminophen/codeine 800 mg/60 mg, n = 53 |
Ibuprofen 800 mg, n = 51 | 3 d | Industry |
| Scoren et al., 198758 | Dental | Acetaminophen/codeine 300 mg/30 mg, n = 30 |
Naproxen 275 mg, n = 47 | 7 d | Industry |
| Sniezek et al., 201159 | Otolaryngology | Acetaminophen/codeine 325 mg/30 mg, n = 70 |
Ibuprofen/acetaminophen 400 mg/1000 mg, n = 68 | 12 h | None |
| Soulier et al., 199760 | Orthopedic | Acetaminophen/codeine 300 mg/30 mg, n = 24 |
Flurbiprofen 50 mg, n = 29 | 4 d | Industry |
| Sunshine et al., 198661 | Dental | Acetaminophen/codeine 650 mg/60 mg, n = 31 |
Flurbiprofen 50 mg, n = 31 Flurbiprofen 100 mg, n = 29 |
6 h | Industry |
| Vargas Busquets et al., 199862 | Plastic | Acetaminophen/codeine 600 mg/60 mg, n = 48 |
Naproxen 550 mg, n = 43 | 6 h | None |
| Wittenberg et al., 198463 | Orthopedic | Acetaminophen/codeine 300 mg/30 mg, n = 31 |
Ibuprofen 400 mg, n = 34 | 4 h | Industry |
Note: ASA = acetylsalicylic acid, NSAID = nonsteroidal anti-inflammatory drug.
Table 2:
GRADE evidence profile for pain and global assessment scores, codeine compared with NSAIDs
| No. of trials* | No. of patients | Outcome time horizon | Serious risk of bias† | Serious inconsistency (I2, τ) | Serious indirectness | Serious imprecision | Publication bias detected‡ | Treatment effect, points, WMD (95% CI) | Overall quality |
|---|---|---|---|---|---|---|---|---|---|
| Pain§ | |||||||||
| 31 (54 comparisons) | 4436 | ≤ 6 h | No | No (33.9%, 0.43) | No | No | No (symmetric, p = 0.57) | 0.93 (0.71 to 1.15) | High |
| 10 (19 comparisons) | 1660 | ≤ 12 h | No | No (62.7%, 0.68) | No | No | No (symmetric, p = 0.31) | 0.79 (0.38 to 1.19) | High |
| 8 (9 comparisons) | 888 | ≤ 24 h | No | No (20.8%, 0.25) | No | No¶ | No | 0.16 (−0.20 to 0.52) | High |
| 6 | 552 | ≤ 48 h | No | Yes (88.7%, 1.42) | No | Yes** | No | 0.60 (−0.74 to 1.93) | Low |
| 6 | 485 | ≤ 72 h | No | No (0%, 0.00) | No | No¶ | No | 0.07 (−0.26 to 0.39) | High |
| 5 | 305 | 4 to 7 d | No | No (54.8%, 0.52) | No | No¶ | No | 0.03 (−0.59 to 0.65) | High |
| Global assessment§ | |||||||||
| 12 (21 comparisons) | 1452 | ≤ 6 h | No | No (0%, 0.00) | No | No | No (symmetric, p = 0.69) | −0.88 (−1.04 to −0.72) | High |
| 7 (13 comparisons) | 1043 | ≤ 12 h | No | No (54.2%, 0.38) | No | No | Yes (asymmetric, p = 0.02) | −0.48 (−0.78 to −0.19) | Moderate |
| 4 (7 comparisons) | 947 | ≤ 24 h | No | No (60.7%, 0.29) | No | No | No | −0.67 (−0.95 to −0.40) | High |
| 4 (5 comparisons) | 343 | 2 to 7 d | No | No (12.2%, 0.13) | No | No | No | −0.32 (−0.63 to −0.02) | High |
Note: CI = confidence interval, GRADE = grading of recommendations, assessment, development and evaluations, NSAIDs = nonsteroidal anti-inflammatory drugs, WMD = weighted mean difference.
We used comparison-level data for multiarm trials.
We did not rate down for risk of bias, as we did not detect any significant difference between low and high risk of bias.
Publication bias detected using funnel plots if there were at least 10 studies available for meta-analysis. The Egger test measures the symmetry of funnel plots.
Pain was measured on a 10-point scale (positive WMD favours NSAIDs) and global asessments were measured on a 4-point scale (negative WMD favours NSAIDs).
We did not rate down for imprecision, as the 95% CI is still narrow (i.e., the clinical decisions will not change based on the boundaries of 95% CI), although the 95% CI crosses null effect line.
We rated down for serious imprecision, as the 95% CI includes both benefit and harm, and the clinical decisions will change based on the boundaries of 95%CI.
Table 3:
GRADE evidence profile for adverse effect outcomes, codeine compared with NSAIDs
| No. of trials* | No. of patients | Outcome time horizon | Serious risk of bias | Serious inconsistency (I2, τ) | Serious indirectness | Serious imprecision | Publication bias detected† | RR (95% CI) | Overall quality |
|---|---|---|---|---|---|---|---|---|---|
| Nausea | |||||||||
| 27 | 3780 | 2 h to 8 d | No‡ | Yes (80.7%, 0.90) | No | No | No (symmetric, p = 0.05) | 1.98 (1.22 to 3.20) | Moderate |
| Vomiting | |||||||||
| 11 | 2282 | 5 h to 8 d | No‡ | Yes (76.2%, 0.97) | No | No | No (symmetric, p = 0.14) | 3.45 (1.51 to 7.90) | Moderate |
| Dizziness | |||||||||
| 17 | 2896 | 4 h to 8 d | No‡ | No (44.5%, 0.68) | No | No | No (symmetric, p = 0.54) | 2.49 (1.43 to 4.33) | High |
| Drowsiness | |||||||||
| 15 | 2052 | 2 h to 8 d | No‡ | No (40.4%, 0.56) | No | Yes§ | No, (symmetric, p = 0.20) | 1.53 (0.94 to 2.49) | Moderate |
| Headache | |||||||||
| 23 | 3547 | 2 h to 8 d | No‡ | No (0%, 0.00) | No | No | No (symmetric, p = 0.18) | 1.77 (1.32 to 2.36) | High |
| Bleeding/hematoma | |||||||||
| 8 | 895 | 12 h to 7 d | No | No (0%, 0.00) | No | Yes§ | No | 1.66 (0.50 to 1.20) | Moderate |
| Any adverse effect | |||||||||
| 23 | 3246 | 2 h to 8 d | No‡ | No (35.5%, 0.17) | No | No | No (symmetric, p = 0.08) | 1.47 (1.28 to 1.68) | High |
Note: CI = confidence interval, GRADE = Grading of recommendations, assessment, development and evaluations, NSAIDs: nonsteroidal anti-inflammatory drugs, RR = Relative risk
We used comparison-level data for multiarm trials.
Publication bias detected using funnel plots if there were at least 10 studies available for meta-analysis. The Egger test measures the symmetry of funnel plots.
We did not rate down for risk of bias, as we did not detect significant difference between low versus high risk of bias.
We rated down for serious imprecision as the 95% CI includes both benefit and harm; and the clinical decisions will change based on the boundaries of 95% CI.
Pain
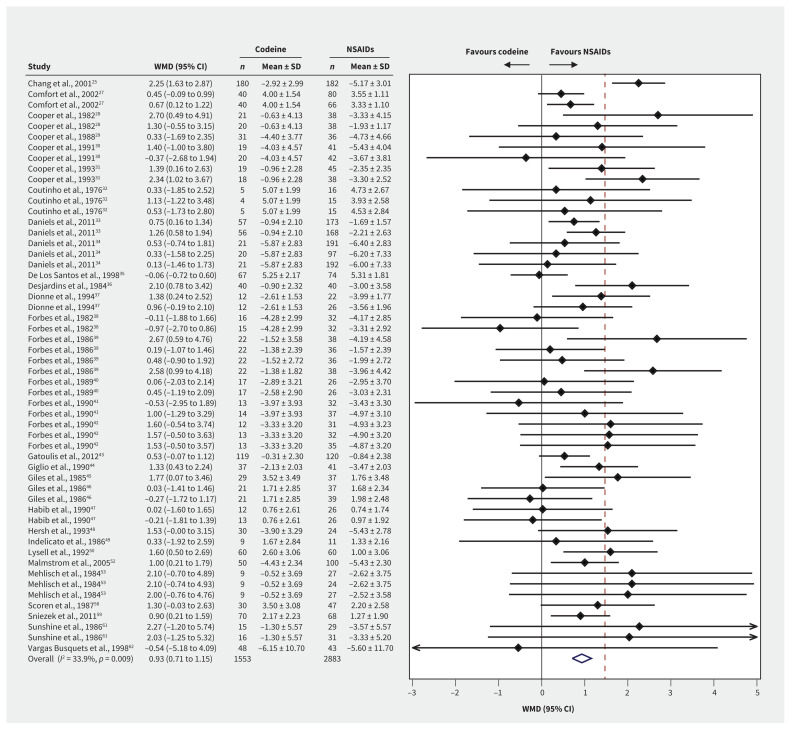
Pain at ≤ 6 hours after treatment was reported for 4436 patients from 54 trial arm comparisons (Figure 2). High-quality evidence showed that patients who received NSAIDs had lower pain scores than those who received codeine, with a WMD of 0.93 points on a 10-point scale (95% CI 0.71 to 1.15, p = 0.009, I2 = 33.9%). When comparing studies that either did not coadminister acetaminophen or used it with both NSAID and codeine groups, we found that patients using NSAIDs had even lower pain scores (WMD 1.18 points, 95% CI 0.87 to 1.48, test of interaction p = 0.05). NSAIDs had weaker superiority when acetaminophen was coadministered only with the codeine group (WMD 0.73 points, 95% CI 0.43 to 1.03). We did not detect any other subgroup effects (Appendix 1, eTable 5).
Figure 2:
Forest plots of the weighted mean differences (WMD) and 95% confidence intervals (CIs) of pain scores at ≤ 6 hours among postoperative patients who were prescribed codeine or nonsteroidal anti-inflammatory drugs (NSAIDs). Note: SD = standard deviation.
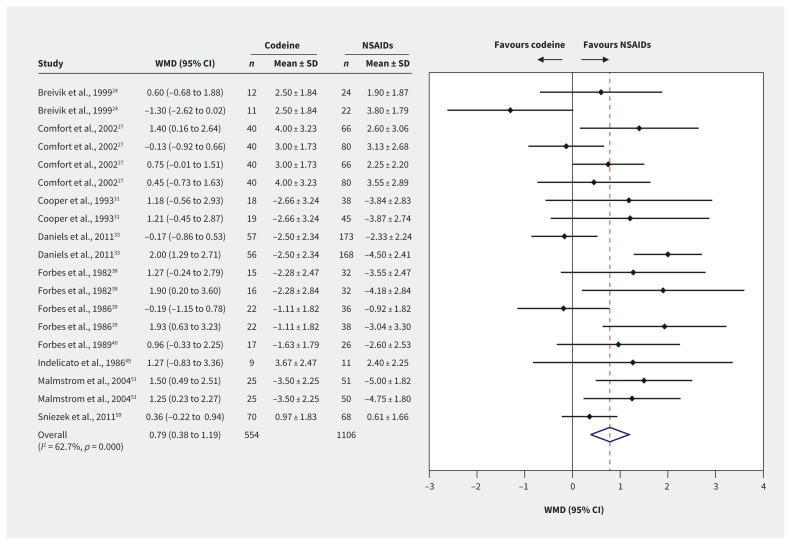
Pain at ≤ 12 hours after treatment was reported for 1660 patients from 19 trial arm comparisons (Figure 3). High-quality evidence showed that patients who received NSAIDs had lower pain scores than those who received codeine, with a WMD 0.79 points on a 10-point scale (95% CI 0.38 to 1.19, p < 0.001, I2 = 62.7%). We did not find any subgroup effects. We found smaller differences between treatments at longer outcome horizons, with no subgroup effects.
Figure 3:
Forest plots of the weighted mean differences (WMD) and 95% confidence intervals (CIs) of pain scores at ≤ 12 hours among postoperative patients who were prescribed codeine or nonsteroidal anti-inflammatory drugs (NSAIDs). Note: SD = standard deviation.
Secondary outcomes
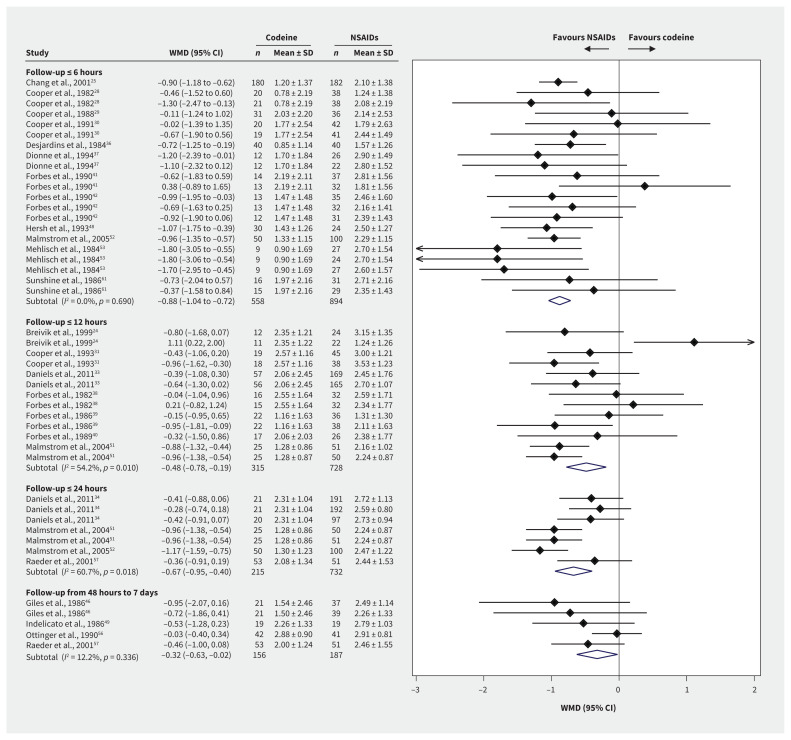
Global assessments showed statistically significant superiority of NSAIDs over codeine at all time measurements (Figure 4). Global assessment scores at ≤ 6 hours were available for 1452 patients and 21 trial arm comparisons. The WMD on a 4-point scale was −0.88 points at ≤ 6 hours (95% CI −1.04 to −0.72), −0.48 points at ≤ 12 hours (95% CI −0.78 to −0.19) and −0.67 points at ≤ 24 hours (95% CI −0.95 to −0.40). The minimal clinically important difference threshold of 0.5 was surpassed at 6 hours and 24 hours, according to high-quality evidence, indicating clinical importance. Between 2 and 7 days, the WMD was −0.32 points (95% CI −0.63 to −0.02), based on the smaller number of studies that reported this time horizon. Subgroup testing showed no interactions (Appendix 1, eTable 6).
Figure 4:
Forest plots of the weighted mean differences (WMD) and 95% confidence intervals (CIs) of global assessment scores by time horizon among postoperative patients who were prescribed codeine or nonsteroidal anti-inflammatory drugs (NSAIDs). Note: SD = standard deviation.
Patients who received NSAIDs reported significantly fewer total adverse effects than those who received codeine. Moreover, fewer patients in the NSAID group reported nausea (10.4% v. 20.6%, WMD 10.2%, 95% CI 2.3% to 23.0%), vomiting (5.3% v. 18.8%, WMD 13.0%, 95% CI 2.7% to 36.6%), dizziness (3.4% v. 8.4%, WMD 5.0%, 95% CI 1.5% to 11.2%), drowsiness (5.6% v. 8.6%, WMD 3.0%, 95% CI −0.3% to 8.4%), and headache (4.5% v. 8.0%, WMD 3.5%, 95% CI 1.5% to 6.2%). Fewer patients in the NSAID groups reported any adverse effect than in the codeine group (28.9% v. 42.4%, WMD 13.6%, 95% CI 8.1% to 19.6%). Eight trials (895 patients) specifically reported bleeding or hematoma as a separate outcome measure. These bleeding events were less frequent in the NSAID arms, but no statistically significant difference was observed in the event rate between the 2 treatments (0.9% v. 1.5%, WMD 0.6%, 95% CI −0.4% to 4.0%).
Interpretation
We found high-quality evidence that outpatient postoperative adults taking NSAIDs reported less pain at 6 and 12 hours than those taking codeine in a meta-analysis of RCTs. The mean effect size for this superiority was below the minimal clinically important difference threshold of 1.0; however, the 95% CI included the threshold and did not include zero. This showed that many patients experience a clinically important benefit with NSAIDs over codeine. Furthermore, the risk of an inferior clinical effect from NSAIDs was statistically negligible.64,65
We also observed that when NSAIDs and codeine were coadministered with equivalent doses of acetaminophen, NSAID superiority was above the threshold for a minimal clinically important difference. This shows that, with or without acetaminophen coadministration, NSAIDs delivered a clinically superior analgesic effect over codeine. We observed comparative NSAID analgesic efficacy with various NSAID types, selective and nonselective NSAIDs, with various surgical procedures and at various time horizons between 6 hours and 7 days. Although a variety of pain scores were used by investigators, these were converted easily to a 10-point scale, allowing reference calculations and ease of clinical interpretation. We had suspected that the pain scores and results might be influenced by the presence or absence of acetaminophen coadministration, type of NSAID, type of surgery and risk of bias. However, we found no evidence of any statistically significant interaction effects contradicting our main results.
We evaluated patient global assessments and found moderate-to high-quality GRADE evidence that NSAIDs were statistically and clinically superior to codeine at all time points. The magnitude of difference on a 4-point scale was about 1 point at 6 hours, 0.5 points at 12 hours and 0.7 points at 24 hours. Global assessments of pain intervention can be interpreted clinically as a composite patient-reported outcome that incorporates patients’ experiences of analgesia efficacy, tolerability and adverse effects. We also found moderate-to high-quality GRADE evidence of fewer adverse effects from NSAIDs than from codeine, with significantly lower rates of any adverse effects, and of the most commonly reported adverse effects (i.e., nausea, vomiting, dizziness, headache). One common hesitation with the use of NSAIDs in a postoperative setting is the potential increased risk of bleeding, but we found moderate-quality evidence to the contrary. We found more bleeding or hematoma events with codeine treatment, although the event rates between the 2 treatments were not statistically different.
Codeine remains the most commonly prescribed opioid in Canada, despite its many shortcomings.6–9,66 Codeine is a prodrug that requires metabolism to morphine by the CYP2D6 enzyme for analgesic effect. Overall, about 10% of ingested codeine is metabolized to morphine; however, there is substantial individual-level variability in CYP2D6 enzyme expression, ranging from nonmetabolizing people without any morphine conversion to ultra-rapid metabolizers with extremely high morphine conversion.67–69 Consequently, routine codeine dosages can result in a spectrum of effects ranging from no analgesia to life-threatening levels of circulating morphine. These unique pharmacogenetic features have raised a number of safety concerns, particularly with respiratory depression.69,70 This is in addition to codeine’s many common adverse effects, including nausea, vomiting, constipation, urinary retention and sedation, all of which reduce compliance and increase rescue medication usage.71 Our findings are consistent with codeine’s known disadvantages. We suspect that the anti-inflammatory mechanism of action of NSAIDs are better suited to the acute pain of postoperative patients.16
These findings are of general importance to any clinician performing painful medical procedures. The various trials in our meta-analysis evaluated a range of procedures, different NSAID types and various degrees of acetaminophen coadministration. The low-to-moderate heterogeneity, combined with the consistent statistically significant findings, allows for generalizable results with broad clinical application. In all surgery types, subgroups and outcome time points, NSAIDs were equal or superior to codeine for postoperative pain, with higher global assessments and fewer adverse effects.
Limitations
We found low-to-moderate heterogeneity in our analysis because of the variety of interventions and dosages. However, NSAIDs showed consistent equivalence or superiority, but there may be some dosages of codeine or NSAID for which our findings do not apply. The overall results may not generalize to all types of patients. Many of the trials analyzed came from the dental literature; however, our findings remained consistent when excluding dental studies from analysis (data not shown).
Conclusion
In our meta-analysis of RCTs, we found that patients randomized to NSAIDs following outpatient surgical procedures reported better pain scores, better global assessment scores, fewer adverse effects and no difference in bleeding events, compared with those receiving codeine. These findings strengthen existing evidence and are broadly generalizable to patients across surgical disciplines. Further studies should assess the comparative effectiveness of other nonopioid analgesics, and test these findings in other populations and settings.
Acknowledgements
The authors thank Toni Tidy and Christian Sirko (Department of Anesthesia, McMaster University), and Natalie Wainwright (Department of Anesthesiology and Pain Medicine, University of Toronto), for their assistance with data checking and organization.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work, and the acquisition, analysis, and interpretation of data. All of the authors drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Data sharing: All data are presented in the article and appendices. No additional data are available.
References
- 1.Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report 2009;January 28:1–25. [PubMed] [Google Scholar]
- 2.Hollenbeck BK, Dunn RL, Suskind AM, et al. Ambulatory surgery centers and outpatient procedure use among Medicare beneficiaries. Med Care 2014;52: 926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawal N. Analgesia for day-case surgery. Br J Anaesth 2001;87:73–87. [DOI] [PubMed] [Google Scholar]
- 4.Analysis in brief: trends in acute inpatient hospitalizations and day surgery visits in Canada, 1995–1996 to 2005–2006. Ottawa: Canadian Institute for Health Information; 2007. [Google Scholar]
- 5.Tawfic QA, Faris AS. Acute pain service: past, present and future. Pain Manag 2015;5:47–58. [DOI] [PubMed] [Google Scholar]
- 6.Biskup M, Dzioba A, Sowerby LJ, et al. Opioid prescribing practices following elective surgery in otolaryngology — head & neck surgery J Otolaryngol Head Neck Surg 2019;48:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ladha KS, Neuman MD, Broms G, et al. Opioid prescribing after surgery in the United States, Canada, and Sweden. JAMA Netw Open 2019;2:e1910734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suda KJ, Durkin MJ, Calip GS, et al. Comparison of opioid prescribing by dentists in the United States and England. JAMA Netw Open 2019;2:e194303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opioid prescribing in Canada: How are practices changing? Ottawa: Canadian Institute for Health Information; 2019. [Google Scholar]
- 10.Toms L, Derry S, Moore RA, et al. Single dose oral paracetamol (acetaminophen) with codeine for postoperative pain in adults. Cochrane Database Syst Rev 2009; (1):CD001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laniado N, Hersh EV, Badner VM, et al. Prescribing analgesics for postoperative dental pain. Compend Contin Educ Dent 2020;41:466–73, quiz 74. [PubMed] [Google Scholar]
- 12.Theken KN. Variability in analgesic response to non-steroidal anti-inflammatory drugs. Prostaglandins Other Lipid Mediat 2018;139:63–70. [DOI] [PubMed] [Google Scholar]
- 13.Moore N, Duong M, Gulmez SE, et al. Pharmacoepidemiology of non-steroidal anti-inflammatory drugs. Therapie 2019;74:271–7. [DOI] [PubMed] [Google Scholar]
- 14.Lee A, Cooper MG, Craig JC, et al. Effects of nonsteroidal anti-inflammatory drugs on postoperative renal function in adults with normal renal function. Cochrane Database Syst Rev 2007;(2):CD002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 2011;342:c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maund E, McDaid C, Rice S, et al. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. Br J Anaesth 2011; 106:292–7. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. [updated March 2011]. Oxford (UK): The Cochrane Collaboration; 2011. [Google Scholar]
- 20.Thorlund K, Walter SD, Johnston BC, et al. Pooling health-related quality of life outcomes in meta-analysis-a tutorial and review of methods for enhancing interpretability. Res Synth Methods 2011;2:188–203. [DOI] [PubMed] [Google Scholar]
- 21.Salaffi F, Stancati A, Silvestri CA, et al. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004;8:283–91. [DOI] [PubMed] [Google Scholar]
- 22.Todd KH, Funk KG, Funk JP, et al. Clinical significance of reported changes in pain severity. Ann Emerg Med 1996;27:485–9. [DOI] [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breivik EK, Barkvoll P, Skovlund E. Combining diclofenac with acetaminophen or acetaminophen-codeine after oral surgery: a randomized, double-blind single-dose study. Clin Pharmacol Ther 1999;66:625–35. [DOI] [PubMed] [Google Scholar]
- 25.Chang DJ, Fricke JR, Bird SR, et al. Rofecoxib versus codeine/acetaminophen in postoperative dental pain: a double-blind, randomized, placebo-and active comparator-controlled clinical trial. Clin Ther 2001;23:1446–55. [DOI] [PubMed] [Google Scholar]
- 26.Chen T, Adamson PA. Comparison of ibuprofen and acetaminophen with codeine following cosmetic facial surgery. J Otolaryngol Head Neck Surg 2009;38: 580–6. [PubMed] [Google Scholar]
- 27.Comfort MB, Tse AS, Tsang AC, et al. A study of the comparative efficacy of three common analgesics in the control of pain after third molar surgery under local anaesthesia. Aust Dent J 2002;47:327–30. [DOI] [PubMed] [Google Scholar]
- 28.Cooper SA, Engel J, Ladov M, et al. Analgesic efficacy of an ibuprofen-codeine combination. Pharmacotherapy 1982;2:162–7. [DOI] [PubMed] [Google Scholar]
- 29.Cooper SA, Firestein A, Cohn P. Double-blind comparison of meclofenamate sodium with acetaminophen, acetaminophen with codeine and placebo for relief of postsurgical dental pain. J Clin Dent 1988;1:31–4. [PubMed] [Google Scholar]
- 30.Cooper SA, Kupperman A. The analgesic efficacy of flurbiprofen compared to acetaminophen with codeine. J Clin Dent 1991;2:70–4. [PubMed] [Google Scholar]
- 31.Cooper SA, Quinn PD, MacAfee K, et al. Ibuprofen controlled-release formulation. A clinical trial in dental impaction pain. Oral Surg Oral Med Oral Pathol 1993;75:677–83. [DOI] [PubMed] [Google Scholar]
- 32.Coutinho A, Bonelli J, de Carvalho PC. A double-blind comparative study of the analgesic effects of fenbufen, codeine, aspirin, propoxyphene and placebo. Curr Ther Res Clin Exp 1976;19:58–65. [PubMed] [Google Scholar]
- 33.Daniels SE, Goulder MA, Aspley S, et al. A randomised, five-parallel-group, placebo-controlled trial comparing the efficacy and tolerability of analgesic combinations including a novel single-tablet combination of ibuprofen/paracetamol for postoperative dental pain. Pain 2011;152:632–42. [DOI] [PubMed] [Google Scholar]
- 34.Daniels SE, Bandy DP, Christensen SE, et al. Evaluation of the dose range of etoricoxib in an acute pain setting using the postoperative dental pain model. Clin J Pain 2011;27:1–8. [DOI] [PubMed] [Google Scholar]
- 35.de los Santos AR, Di Girolamo G, Martí ML. Efficacy and tolerance of lysine clonixinate versus paracetamol/codeine following inguinal hernioplasty. Int J Tissue React 1998;20:71–81. [PubMed] [Google Scholar]
- 36.Desjardins PJ, Cooper SA, Gallegos TL, et al. The relative analgesic efficacy of propiram fumarate, codeine, aspirin, and placebo in post-impaction dental pain. J Clin Pharmacol 1984;24:35–42. [DOI] [PubMed] [Google Scholar]
- 37.Dionne RA, Snyder J, Hargreaves KM. Analgesic efficacy of flurbiprofen in comparison with acetaminophen, acetaminophen plus codeine, and placebo after impacted third molar removal. J Oral Maxillofac Surg 1994;52:919–24, discussion 25–6. [DOI] [PubMed] [Google Scholar]
- 38.Forbes JA, Beaver WT, White EH, et al. Diflunisal. A new oral analgesic with an unusually long duration of action. JAMA 1982;248:2139–42. [DOI] [PubMed] [Google Scholar]
- 39.Forbes JA, Keller CK, Smith JW, et al. Analgesic effect of naproxen sodium, codeine, a naproxen-codeine combination and aspirin on the postoperative pain of oral surgery. Pharmacotherapy 1986;6:211–8. [DOI] [PubMed] [Google Scholar]
- 40.Forbes JA, Butterworth GA, Burchfield WH, et al. Evaluation of flurbiprofen, acetaminophen, an acetaminophen-codeine combination, and placebo in postoperative oral surgery pain. Pharmacotherapy 1989;9:322–30. [DOI] [PubMed] [Google Scholar]
- 41.Forbes JA, Butterworth GA, Burchfield WH, et al. Evaluation of ketorolac, aspirin, and an acetaminophen-codeine combination in postoperative oral surgery pain. Pharmacotherapy 1990;10:77S–93S. [PubMed] [Google Scholar]
- 42.Forbes JA, Kehm CJ, Grodin CD, et al. Evaluation of ketorolac, ibuprofen, acetaminophen, and an acetaminophen-codeine combination in postoperative oral surgery pain. Pharmacotherapy 1990;10:94S–105S. [PubMed] [Google Scholar]
- 43.Gatoulis SC, Voelker M, Fisher M. Assessment of the efficacy and safety profiles of aspirin and acetaminophen with codeine: results from 2 randomized, controlled trials in individuals with tension-type headache and postoperative dental pain. Clin Ther 2012;34:138–48. [DOI] [PubMed] [Google Scholar]
- 44.Giglio JA, Laskin DM. Double-blind comparison of meclofenamate sodium plus codeine, meclofenamate sodium, codeine, and placebo for relief of pain following surgical removal of third molars. J Oral Maxillofac Surg 1990;48:785–90. [DOI] [PubMed] [Google Scholar]
- 45.Giles AD, Pickvance N. Combination analgesia following oral surgery: a double-blind comparison of ibuprofen, codeine phosphate and two combination ratios. Clin Trials J 1985;22:300–11. [Google Scholar]
- 46.Giles AD, Hill CM, Shepherd JP, et al. A single dose assessment of an ibuprofen/codeine combination in postoperative dental pain. Int J Oral Maxillofac Surg 1986;15:727–32. [DOI] [PubMed] [Google Scholar]
- 47.Habib S, Matthews RW, Scully C, et al. A study of the comparative efficacy of four common analgesics in the control of postsurgical dental pain. Oral Surg Oral Med Oral Pathol 1990;70:559–63. [DOI] [PubMed] [Google Scholar]
- 48.Hersh EV, Ochs H, Quinn P, et al. Narcotic receptor blockade and its effect on the analgesic response to placebo and ibuprofen after oral surgery. Oral Surg Oral Med Oral Pathol 1993;75:539–46. [DOI] [PubMed] [Google Scholar]
- 49.Indelicato PA. Efficacy of diflunisal versus acetaminophen with codeine in controlling mild to moderate pain after arthroscopy. Clin Ther 1986;8:164–9. [PubMed] [Google Scholar]
- 50.Lysell L, Anzén B. Pain control after third molar surgery — a comparative study of ibuprofen (Ibumetin) and a paracetamol/codeine combination (Citodon). Swed Dent J 1992;16:151–60. [PubMed] [Google Scholar]
- 51.Malmstrom K, Kotey P, Coughlin H, et al. A randomized, double-blind, parallel-group study comparing the analgesic effect of etoricoxib to placebo, naproxen sodium, and acetaminophen with codeine using the dental impaction pain model. Clin J Pain 2004;20:147–55. [DOI] [PubMed] [Google Scholar]
- 52.Malmstrom K, Ang J, Fricke JR, et al. The analgesic effect of etoricoxib relative to that of cetaminophen analgesics: a randomized, controlled single-dose study in acute dental impaction pain. Curr Med Res Opin 2005;21:141–9. [DOI] [PubMed] [Google Scholar]
- 53.Mehlisch D, Frakes L, Cavaliere MB, et al. Double-blind parallel comparison of single oral doses of ketoprofen, codeine, and placebo in patients with moderate to severe dental pain. J Clin Pharmacol 1984;24:486–92. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell A, van Zanten SV, Inglis K, et al. A randomized controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine after outpatient general surgery. J Am Coll Surg 2008;206:472–9. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell A, McCrea P, Inglis K, et al. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol 2012;19:3792–800. [DOI] [PubMed] [Google Scholar]
- 56.Ottinger ML, Kinney KW, Black JR, et al. Comparison of flurbiprofen and acetaminophen with codeine in postoperative foot pain. J Am Podiatr Med Assoc 1990;80:266–70. [DOI] [PubMed] [Google Scholar]
- 57.Raeder JC, Steine S, Vatsgar TT. Oral ibuprofen versus paracetamol plus codeine for analgesia after ambulatory surgery. Anesth Analg 2001;92:1470–2. [DOI] [PubMed] [Google Scholar]
- 58.Scoren R, Rhodes P, Schwarz M, et al. Pain following periodontal surgery: treatment with a nonnarcotic analgesic compared with two codeine combinations. Curr Ther Res Clin Exp 1987;42:463–71. [Google Scholar]
- 59.Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg 2011;37:1007–13. [DOI] [PubMed] [Google Scholar]
- 60.Soulier SM, Page JC, Larsen LC, et al. The efficacy of ANSAID (flurbiprofen) as an analgesic in foot surgery. J Foot Ankle Surg 1997;36:414–7, discussion 66. [DOI] [PubMed] [Google Scholar]
- 61.Sunshine A, Marrero I, Olson N, et al. Comparative study of flurbiprofen, zomepirac sodium, acetaminophen plus codeine, and acetaminophen for the relief of postsurgical dental pain. Am J Med 1986;80(3A):50–4. [DOI] [PubMed] [Google Scholar]
- 62.Vargas Busquets M, Keoshian L, Kelleher R, et al. Naproxen sodium versus acetaminophen-codeine for pain following plastic surgery. Curr Ther Res Clin Exp 1988;43:311–6. [Google Scholar]
- 63.Wittenberg M, Kinney KW, Black JR. Comparison of ibuprofen and acetaminophen-codeine in postoperative foot pain. J Am Podiatry Assoc 1984;74:233–7. [DOI] [PubMed] [Google Scholar]
- 64.Guyatt GH, Juniper EF, Walter SD, et al. Interpreting treatment effects in randomised trials. BMJ 1998;316:690–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol 2011;64:1283–93. [DOI] [PubMed] [Google Scholar]
- 66.De Conno F, Ripamonti C, Brunelli C. Opioid purchases and expenditure in nine western European countries: ‘Are we killing off morphine?’ Palliat Med 2005;19: 179–84. [DOI] [PubMed] [Google Scholar]
- 67.Mattia C, Coluzzi F. A look inside the association codeine-paracetamol: clinical pharmacology supports analgesic efficacy. Eur Rev Med Pharmacol Sci 2015;19: 507–16. [PubMed] [Google Scholar]
- 68.Fortenberry M, Crowder J, So TY. The use of codeine and tramadol in the pediatric population — What is the verdict now? J Pediatr Health Care 2019;33: 117–23. [DOI] [PubMed] [Google Scholar]
- 69.Livingstone MJ, Groenewald CB, Rabbitts JA, et al. Codeine use among children in the United States: a nationally representative study from 1996 to 2013. Paediatr Anaesth 2017;27:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poonai N, Zhu R. Analgesia for children in acute pain in the post-codeine era. Curr Pediatr Rev 2018;14:34–40. [DOI] [PubMed] [Google Scholar]
- 71.Paul JE, Buckley N, McLean RF, et al. Hamilton acute pain service safety study: using root cause analysis to reduce the incidence of adverse events. Anesthesiology 2014;120:97–109. [DOI] [PubMed] [Google Scholar]