Abstract
Background
Adherence to post-polypectomy surveillance guideline recommendations is suboptimal. Surveillance is frequently over- and under-recommend, resulting in strained colonoscopy capacity, potential risks without expected benefits, and missed opportunities for colorectal cancer risk reduction.
Aims
To identify factors associated with adherence to post-polypectomy surveillance guidelines.
Methods
We conducted a three-phase study with a retrospective review of usual care post-polypectomy surveillance recommendations through medical chart abstraction (Phase I), prospective online physician survey (Phase II), and analysis of survey-based and other physician-based predictors of usual care surveillance recommendations (Phase III). Subjects included patients who underwent usual care colonoscopy 2011–2012 (Phases I and III) and gastroenterology (GI) attendings and fellows (Phases II and III). We identified rates of recommendations consistent with guideline adherence, surveillance overuse, and surveillance underuse based on usual care medical chart documentation and physician survey, as well as predictors of physician adherence to guidelines.
Results
We reviewed 640 patient charts for 28 survey respondents. Rates of usual practice recommendations consistent with guideline adherence, surveillance overutilization, and underutilization were 84, 13, and 3 %, respectively. At survey, 82 % of physicians were concerned about missed cancer. Eleven percentage believed that guidelines were not aggressive enough. GI trainees were 2.5 times more likely to issue guideline-adherent recommendations [OR 2.5, 95 % CI (1.5–4.2)]. Disagreement with guideline aggressiveness was independently associated with 40 % lower likelihood of adherence [OR 0.6, 95 % CI (0.4–0.8)].
Conclusions
Belief in the appropriate aggressiveness of guidelines and trainee position, but not fear of missed cancer or guideline knowledge, was associated with adherence to post-polypectomy surveillance guidelines.
Keywords: Polypectomy, Surveillance, Guideline adherence, Practice patterns, Recommendations
Introduction
Adherence to evidence-based guidelines for post-polypectomy colorectal surveillance is suboptimal. Physicians tend to both over-recommend and under-recommend surveillance, and there is discordance between baseline risk and timing of repeat colonoscopy. For example, only 31 % of patients with advanced adenomas were likely to receive timely surveillance colonoscopy after initial adenoma detection in the Prostate, Lung, Colorectal, and Ovarian cancer screening trial [1]. Other research has demonstrated that 35–50 % of gastroenterologists over-recommend surveillance colonoscopy for patients with low-risk polyps [1-4].
Suboptimal post-polypectomy guideline adherence may have significant consequences. Under-recommend surveillance of individuals with high-risk baseline findings (such as piecemeal large adenoma resection) may contribute to the development of advanced neoplasia or even cancer after colonoscopy [3, 5-7]. Over-recommend surveillance of individuals with low-risk baseline findings (such as 1–2 < 1 cm in size adenomas) leads to unnecessary costs and risks of procedure-related complications and may strain capacity for surveillance, diagnostic, and screening colonoscopy [8, 9].
The underlying factors leading to suboptimal post-polypectomy guideline adherence are poorly understood. Suboptimal bowel preparation has been associated with earlier surveillance colonoscopy than recommended by guidelines [10]. Others have hypothesized suboptimal guideline knowledge as a factor, but surveys suggest that despite knowledge of guidelines, physicians ignore guideline recommendations [11, 12]. Unexplored hypothesized factors contributing to guideline non-adherence include fear of missed or interval cancer, distrust of the evidence behind guideline recommendations, imprecise estimation of risk of interval advanced adenoma, medicolegal concerns, and financial gain [10-12]. Understanding physician factors key to guideline non-adherence will inform interventions that may improve adherence and ultimately optimize effectiveness of post-polypectomy surveillance. This knowledge may be especially salient, as current national Medicare quality measures include measurement of the rate of guideline-appropriate post-polypectomy surveillance recommendations [13].
We conducted a study to identify factors that predict physician adherence to post-polypectomy guidelines. Our aims were to (1) evaluate physician knowledge and perception of guidelines, (2) identify factors impacting decisions on surveillance recommendations, (3) characterize actual practice and adherence patterns, and (4) identify factors associated with guideline adherence and inappropriate use of surveillance colonoscopy.
Methods
Overview and Study Setting
We conducted a three-phase study at University of Texas (UT) Southwestern Medical Center and Parkland Memorial Hospital in Dallas, Texas. The first phase was a chart review of actual physician practice patterns of guideline adherence. The second phase included a physician survey assessing guideline knowledge, perception of risk of interval adenomas after polypectomy, and factors motivating surveillance recommendations. The final phase determined predictors of physician adherence to guidelines based on survey responses, physician characteristics, and actual practice patterns. During the study period, attending physicians provided service to the UT Southwestern Gastroenterology (GI) colonoscopy practice. GI trainees operated the Parkland GI colonoscopy practice with attending supervision. At both sites, procedures were scheduled through direct access referrals and clinical visits. The institutional review boards (IRB) at UT Southwestern Medical Center and Parkland Memorial Hospital approved the study (IRB#STU 032012-010).
Phase I: Analysis of Usual Care Post-polypectomy Surveillance Recommendations
The first phase of our study included a retrospective chart review to describe actual practice and adherence patterns of physicians based on patient encounters. For each colonoscopist, we planned to review 25 consecutive unique qualifying patient charts. This sample size was selected to allow evaluation of the association of 8–10 predictors with guideline-adherent practice, assuming a 50 % expected rate of guideline adherence as in prior published studies [1, 2, 4]. We queried our electronic health record and procedure documentation systems to identify patients for each colonoscopist. We included encounters of patients age 40–80 years, meeting the following criteria: (1) diagnostic, screening, or surveillance indication for colonoscopy between July 1, 2011, and April 30, 2012, (2) complete colonoscopy to the cecum, (3) colonoscopy with at least adequate bowel preparation (documented as adequate, good, or excellent), and (4) a post-polypectomy surveillance recommendation based on both colonoscopy and pathology results (if a biopsy was taken) documented in the electronic medical record system. Patients were excluded if the indication for colonoscopy included surveillance for inflammatory bowel disease or any hereditary cancer syndromes, such as hereditary non-polyposis colorectal cancer (HNPCC)/Lynch syndrome, familial adenomatous polyposis (FAP), or Peutz-Jeghers syndrome.
Electronic medical records were reviewed for all qualifying patients to extract demographic information, family history, procedure indication, pertinent findings, pathology, and follow-up recommendation provided to the patient. Surveillance recommendations were provided by attending physicians at UT Southwestern and by GI trainees at Parkland. GI trainee recommendations were available for review by attending physicians, and changed if deemed necessary. We characterized each post-polypectomy surveillance recommendation as either “adherent” or “non-adherent” to 2006 American Cancer Society and United States Multi-Society Task Force on Colorectal Cancer consensus guidelines for surveillance colonoscopy after polypectomy [14, 15]. The 2006 guidelines, rather than the most recent 2012 post-polypectomy guidelines, were used because the 2012 guidelines were not in effect at the time of study conduct. Notably, the 2012 guidelines mainly differ from 2006 in the management of sessile serrated adenomas/polyps (SSA/Ps); in the included sample, no patients had SSA/Ps requiring follow-up recommendations [16]. If a recommendation was determined to be guideline “non-adherent,” it was further characterized as either “early” or “late” with respect to the guideline-recommended follow-up interval. Following the conventions of the 2006 guidelines, hyperplastic polyps or small (<1 cm) tubular adenomas were classified as low-risk polyps, and adenomas ≥1 cm in size, ≥3 small adenomas, any villous features, or high-grade dysplasia were classified as high-risk adenomas (HRAs) [15].
Phase II: Online Survey of Physician Knowledge and Perceptions
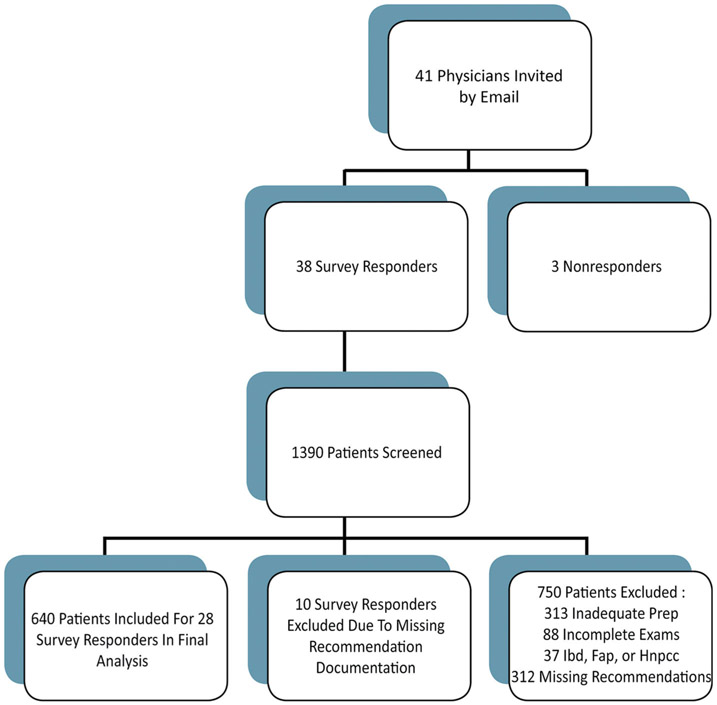
We developed an online survey instrument evaluating physician knowledge, perception of guidelines, estimation of risk of interval adenoma, and factors impacting follow-up recommendations using REDCap© survey software (see Supplementary Table 1 online for the full survey). Study investigators, SG and NP, developed the survey with feedback from several gastroenterologists. Neither study investigators nor gastroenterologists involved in survey development served as survey respondents. Questions were based on validated surveys administered to gastroenterologists and internists in two prior studies [4, 12]. Attending and fellow gastroenterologists performing colonoscopy at UT Southwestern and/or Parkland were invited by email to participate (Fig. 1). Emails were sent twice initially to all candidate participants, and to non-responders (3 physicians out of 41 initially contacted) another two times.
Fig. 1.
Flow diagram of study population. Inflammatory bowel disease (IBD), familial adenomatous polyposis (FAP), hereditary non-polyposis colorectal cancer (HNPCC)
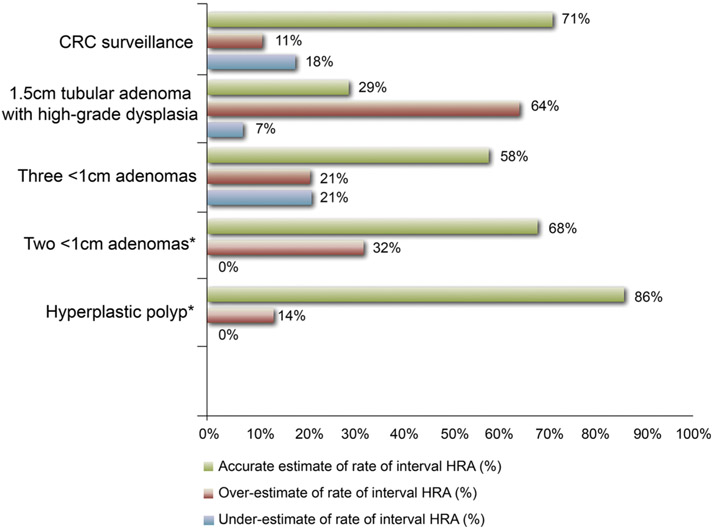
The 31-item survey included demographic information such as age, gender, years of experience, and board certification. Physicians were given six clinical vignettes and asked to provide appropriate follow-up for repeat colonoscopy based on 2006 polyp surveillance guidelines [14, 15]. Respondents were also asked to provide estimates of 5-year risk of metachronous advanced adenoma based on each polypectomy scenario. Scenarios included surveillance of the following findings: hyperplastic polyps, small <1 cm tubular adenomas, three <1 cm tubular adenomas, piecemeal resection of an advanced adenoma, large (>1 cm) tubulovillous adenoma with high-grade dysplasia, and resected colorectal cancer. Since 2006 guidelines did not cover recommendations for SSA/Ps, scenarios for SSA/Ps follow-up were not included in the survey. Respondent estimates of risk of metachronous advanced adenoma on follow-up were adjudicated as accurate, overestimate, or underestimate based on prior observations [17-20] (Fig. 2).
Fig. 2.
Accuracy of physician estimates of rate of interval high-risk adenoma (HRA) at 5-year follow-up based on polypectomy scenarios. Estimates were determined as accurate estimate, overestimate, and underestimate of rate of interval HRA based on data from prior observations. Reference standards for rate of interval HRA based on initial findings were as follows: hyperplastic polyp: 3 %; small tubular adenomas: 6 %; multiple small adenomas: 16 %; advanced adenoma (villous component): 16 %; resected colorectal cancer: 15–35 %. *No underestimation was observed for rate of interval HRA for baseline findings of two small (<1 cm) adenomas or hyperplastic polyps. Colorectal cancer (CRC)
The survey also included 13 Likert-scale statements highlighting the influence of physician, system, and patient factors when determining follow-up recommendations. Physician-level factors included awareness and agreement with current guidelines, medicolegal action and reimbursement concerns, fear of missed cancers, and beliefs about the aggressiveness of current guidelines. System-level factors incorporated into the survey were the changing nature of guidelines, use of clinical guideline reminders, and scheduling availability. Patient-level factors included impact of comorbid conditions, insurance and compliance concerns, and the quality of bowel preparation. Description of the survey questions and actual survey statements are available in Supplementary material (Supplementary Table 1).
Phase III: Correlation of Survey-Based Candidate Predictors of Post-polypectomy Recommendations with Usual Practice
Our primary outcome was the physician-specific rate of guideline adherence. Secondary outcomes were rate of early and late physician recommendations for surveillance relative to guideline-based practice. Physician-specific guideline adherence was determined as the frequency of adherent recommendations among all recommendations provided. Similarly, rates of early and late recommendations were calculated using the frequency of early and late recommendations among all recommendations provided, respectively.
Survey responses, as well as physician characteristics, were considered as candidate predictors of guideline adherence. The Likert-scale responses were collected as ordinal variables. Physician-specific variables included demographic information such as trainee position, years of experience, and gender.
Data comprising physician demographics, survey responses, and procedure findings with recommendations were summarized using frequencies and percentages. Univariate and multivariate generalized linear mixed models were adjusted for random cluster effect to adjust for within physician clustering of guideline-adherent practice. For multivariate analyses, variables were entered using the backward selection method. A p value < 0.05 was considered significant for all statistical comparisons. Data analysis was performed using StataMP version 11.0 software (StataCorp, College Station, TX) and SAS 9.2 (SAS Institute Inc, Cary, NC).
Results
Phase I: Assessment of Usual Care Post-polypectomy Recommendations
Of 41 colonoscopists invited to participate in the study, survey responses and actual practice data from 28 (68 %) were included in our final analysis. These 28 colonoscopists had 640 patients meeting inclusion criteria (Fig. 1). Among the 640 patients included, 45 % were male. Sixty-five patients (10 %) had documented first-degree relatives with colorectal cancer. Indications for colonoscopy included both screening and diagnostic colonoscopies (see Supplementary Table 2). Of 640 patients, 291 (45 %) were documented as having normal colonoscopies. Low-risk polyps were found among 298 patients (47 %). High-risk adenomas were found in 51 (8 %) patients.
The average physician-specific rate of guideline-appropriate follow-up in actual practice was 85 %. Physician-specific rates of guideline adherence ranged from 40 to 100 %. The average physician-specific rate of underutilization of surveillance was 3 %, ranging from 0 to 12 %. A total of 19 of 640 patients (3 %) were issued late follow-up recommendations. Among the 19 receiving late recommendations, 8 had a baseline HRA. The average physician-specific rate of overutilization of surveillance was 13 %, ranging from 0 to 30 %.
Of 51 patients with HRA, six patients (12 %) received surveillance recommendations earlier than advised by guideline recommendations. Forty-five of 298 (15 %) patients with low-risk polyps received early surveillance recommendations. More than 90 % of early recommendations were issued for normal findings and low-risk polyps (Table 1).
Table 1.
Usual care physician practice patterns of guideline adherence on timing of repeat colonoscopy based on colonoscopy findings
| Baseline risk group N = 640 | Guideline-adherent recommendation1 (%) | Early recommendation2 (%) | Late recommendation3 (%) |
|---|---|---|---|
| All findings | 537 (83.9) | 84 (13.1) | 19 (3) |
| Normal | 253 (86.9) | 33 (11.3) | 5 (1.7) |
| Low-risk polypsa | 247 (82.9) | 45 (15.1) | 6 (2) |
| High-risk adenomasb | 37 (72.5) | 6 (11.8) | 8 (15.7) |
Low-risk polyps: hyperplastic polyps or 1–2 small (<1 cm) tubular adenomas
High-risk adenomas: adenomas size ≥1 cm, ≥3 small adenomas, any villous features, or high-grade dysplasia
Guideline-adherent recommendations based on 2006 American Cancer Society (ACS) and US Multi-Society Task Force (USMSTF) on Colorectal Cancer consensus guidelines for surveillance colonoscopy after polypectomy
Early recommendations defined as recommendations offered at a shorter interval than recommended by the 2006 ACS and USMSTF on Colorectal Cancer consensus guidelines for surveillance colonoscopy after polypectomy
Late recommendations defined as recommendations offered at a longer interval than recommended by 2006 ACS and USMSTF on Colorectal Cancer consensus guidelines for surveillance colonoscopy after
Phase II: Survey of Physician Knowledge and Perceptions Regarding Guidelines
Of 41 colonoscopists invited to participate in the study, survey responses from 28 physicians were included in our final analysis. Reasons for exclusion included survey non-completion (n = 3) and no electronic medical record documentation of post-polypectomy follow-up (n = 10) (Fig. 1). We focused this analysis on the 28 survey respondents with electronic medical record documentation of post-polypectomy follow-up (survey responses for the 10 individuals without electronic medical documentation of post-polypectomy follow-up were qualitatively similar to the included physicians). Twelve attending physicians and sixteen trainee physicians were included in the survey and chart review. Nearly all physicians (96 %) were younger than 55 years of age. Ninety-six percentage of all survey respondents had less than 20 years of experience (Supplementary Table 3).
Guideline Knowledge
Up to 80 % of survey responses to our post-polypectomy clinical scenarios were guideline appropriate. Ninety-three percentage of respondents chose correct surveillance recommendations for hyperplastic polyps and low-risk adenomas (Table 2). Seven percent chose late recommendations for HRA, and 18 % chose late recommendations for multiple small adenomas. Eleven of 28 (39 %) respondents chose late follow-up for piecemeal resection of an advanced adenoma. Almost half of respondents (46 %) chose early recommendations for surveillance of high-risk adenoma (HRA).
Table 2.
Survey-based physician responses of guideline-adherent, early, and late surveillance recommendations based on six post-polypectomy scenarios
| Case scenario | Guideline-adherent recommendation1 (%) |
Early recommendation2 (%) |
Late recommendation3 (%) |
|---|---|---|---|
| Hyperplastic polyp | 26 (92.9) | 2 (7.1) | 0 (0) |
| Two <1 cm adenomas | 26 (92.9) | 2 (7.1) | 0 (0) |
| Three <1 cm adenomas | 23 (82.1) | 0 (0) | 5 (17.9) |
| 1.5-cm tubular adenoma with high-grade dysplasia | 13 (46.4) | 13 (46.4) | 2 (7.1) |
| CRC surveillance | 27 (96.4) | 0 (0) | 1 (3.6) |
| Piecemeal resection of tubulovillous adenoma | 17 (60.7) | 0 (0) | 11 (39.3) |
Guideline-adherent recommendations based on 2006 American Cancer Society (ACS) and US Multi-Society Task Force (USMSTF) on Colorectal Cancer consensus guidelines for surveillance colonoscopy after polypectomy
Early recommendations defined as recommendations offered at a shorter interval than recommended by the 2006 ACS and USMSTF on Colorectal Cancer consensus guidelines for surveillance colonoscopy after polypectomy
Late recommendations defined as recommendations offered at a longer interval than recommended by 2006 ACS and USMSTF on Colorectal Cancer consensus guidelines for surveillance colonoscopy after polypectomy
Knowledge of Risk-Stratified Rates of Incident Advanced Adenoma
Up to 68 % of physicians accurately estimated rate of interval HRA for patients with low-risk adenomas. Six of 28 (21 %) respondents underestimated interval rate of HRA for multiple (>3) small adenomas. Five of 28 (18 %) respondents underestimated interval rate of HRA after curative resection of colorectal cancer (CRC). Eighteen of 28 respondents (64 %) overestimated the interval rate of HRA for high-risk patients with a large tubular adenoma with high-grade dysplasia (Fig. 2).
Factors Influencing Surveillance Recommendations
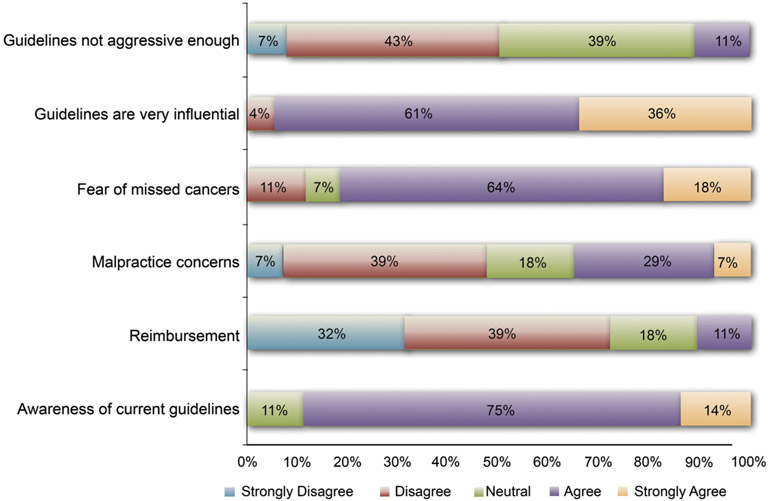
When asked about factors impacting recommendations of surveillance colonoscopy, 82 % of physicians were concerned about fear of missed or interval cancer. Malpractice and reimbursement opportunities had less bearing on physician recommendations (Fig. 3). Nearly all (96 %) respondents agreed that guidelines were very influential in their current clinical practice. Only 11 % of respondents felt that guidelines were not aggressive enough.
Fig. 3.
Survey responses to motivators of physician recommendations on timing of repeat colonoscopy. Data are based on 28 survey responders included in the final analysis
Phase III: Predictors of Guideline-Based Post-polypectomy Surveillance Recommendations
Based on our univariate analysis, several factors were associated with guideline adherence. Guideline knowledge was associated with a 1.5-fold greater likelihood of adherence. Trainee status was associated with a 2.5-fold greater likelihood of guideline adherence (Table 3). Physicians with uncertainty in the aggressiveness of guidelines were 30 % less likely to practice guideline adherence (Table 3). Physicians who found guidelines influential in their practice were 1.8-fold more likely to offer guideline-concordant recommendations (Table 3). Fear of missed cancer, malpractice concerns, and overestimation of interval HRA were not associated with guideline-discordant behavior or overutilization of surveillance colonoscopy.
Table 3.
Survey-measured physician factors associated with guideline-adherent practice and overutilization of surveillance colonoscopy based on univariate analysis
| Survey-measured physician factor | Guideline-adherent versus guideline-non- adherent recommendation |
Guideline-adherent versus early recommendation |
||
|---|---|---|---|---|
| OR, CI | p value | OR, CI | p value | |
| Guideline knowledge | 1.5 (1.2–2.0) | 0.003 | 1.5 (1.1–2.1) | 0.02 |
| Trainee status | 2.1 (1.1–3.7) | 0.02 | 2.7 (1.3–5.5) | 0.005 |
| Belief that guidelines are not appropriately aggressive | 0.7 (0.5–1.0) | 0.04 | 0.7 (0.4–1.1) | 0.12 |
| Influenced by guidelines in clinical practice | 1.8 (1.2–2.7) | 0.005 | 1.9 (1.2–3.1) | 0.01 |
| Fear of missed cancer | 0.7 (0.5–1.1) | 0.10 | 0.6 (0.4–1.0) | 0.05 |
| Increasing concern about malpractice | 0.8 (0.6–1.1) | 0.26 | 0.8 (0.6–1.1) | 0.15 |
| Inaccurate estimation of risk of interval HRA | 1.1 (0.9–1.4) | 0.41 | 1.0 (0.8–1.4) | 0.77 |
Factors associated with underutilization were not identified given small number of late recommendations (n = 19)
We identified two factors as independent predictors of guideline adherence in our multivariate analyses. GI trainee status was independently associated with a 2.5-fold greater likelihood of guideline adherence [OR 2.5,95 % CI (1.5–4.2), p = 0.0005]. The absolute rate of guideline adherence among GI trainees was 88 %, compared to 78 % for attending physicians. Additionally, disagreement with the aggressiveness of guidelines was independently associated with 40 % lower likelihood of adherence [OR 0.6, 95 % CI (0.4–0.8), p = 0.001]. The absolute rate of guideline adherence for those physicians who believe that guidelines were not aggressive enough was 67 %, compared to 88 % for those physicians who believed guidelines were appropriately aggressive.
Discussion
Physician adherence to post-colonoscopy surveillance guidelines is suboptimal, but determinants of non-adherence have not been investigated extensively. In this study, we examined the association of several factors with guideline-appropriate practice among 28 gastroenterologists. Using a detailed survey and comprehensive chart review, we were able to describe physician knowledge and perceptions of guidelines and uniquely correlate these with actual practice patterns. We found that GI trainee physicians were 2.5-times more likely to adhere to guidelines. We also determined that physicians who disagree with the aggressiveness of guidelines were 40 % less likely to practice guideline adherence and more likely to recommend overuse of surveillance colonoscopy. Although many physicians were concerned about missed or interval cancer, these factors were not associated with guideline-adherent practice. Inaccurate estimates of interval HRA were also observed. However, physicians with a tendency toward these inaccurate estimates were not more likely to issue guideline-non-adherent recommendations in practice. Additionally, neither medicolegal concerns nor motivation for reimbursement were found to factor into post-polypectomy recommendations [4, 12, 21].
Our study addresses several gaps in the current medical literature. We were able to provide insight into determinants of guideline-concordant practice and overutilization of surveillance colonoscopy. We found that knowledge of guidelines is partly associated with adherence, but knowledge alone does not predict guideline-adherent behavior. This concept expands on similar findings by Saini et al. [12] where 76 % of GIs who knew the guidelines still disagreed with and ignored guideline recommendations. Our GI trainees were more likely to recommend guideline-appropriate follow-up than attending gastroenterologists. We speculate that perhaps trainees have more recent exposure to the evidence behind guidelines and therefore have more confidence in the guidelines. We captured beliefs and knowledge of physicians at a large academic center through an extensive survey. Prior studies have proposed that fear of missed cancer, medicolegal concerns, or reimbursement opportunities may impact physicians’ practice [4, 11, 12, 21]. The majority of our respondents were indeed concerned about missed or interval cancer. However, many respondents were not influenced by medicolegal concerns and financial incentives. Interestingly, survey-based attitudes toward these factors did not predict guideline-inappropriate practice or overutilization of surveillance colonoscopy. In our analysis, the major predictor of guideline-inappropriate practice was the belief that guidelines are not aggressive enough. This distrust in the evidence behind guidelines was paralleled in a recent questionnaire among physicians who deviate from guideline recommendations [11]. This finding suggests that future interventions to improve guideline adherence need to address misconceptions regarding risk of recurrent neoplasia potentially contributing to the belief that guidelines are insufficiently aggressive.
We determined actual patterns of adherence based on the detailed review of patient charts. Prior studies focus on findings from self-reported practice; however, we were able to minimize recall bias and describe actual practice among our colonoscopists. In our survey, 40 % of physicians incorrectly provided late recommendations for follow-up of a patient with piecemeal resection of an advanced adenoma. A recent study observed that incomplete resection of polyps is quite frequent, especially for large polyps removed piecemeal [5]. Longer-interval follow-up for these patients may contribute to the development of interval cancer [22]. We also observed overutilization of surveillance colonoscopy largely for low-risk polyps similar to prior studies [1-4]. This pattern exposes patients to unnecessary harms and costs. Additionally, inappropriate use of surveillance colonoscopy for low-risk individuals diverts attention away from high-risk individuals who benefit most from surveillance. Indeed, lower adenoma detection rates have been observed for those receiving early follow-up colonoscopy compared to those with guideline-appropriate follow-up [9]. Providing recommendations that are guideline-based may optimize appropriate resource allocation.
Our study has some potential limitations. Underutilization of surveillance colonoscopy was only observed at a rate of 3 %. Therefore, our study lacked power to identify predictors of underutilization of surveillance colonoscopy, considering that only 51 patients were observed to have HRA. Our study population was drawn from gastroenterologists in academic practice and GI fellows. Thus, findings may not be representative of the general population of all colonoscopists. Also, survey data are only as good as the accuracy and honesty of responses received—our results may still not represent all physician concerns or beliefs. During our retrospective chart review, we found 22 % of charts were missing follow-up recommendations; we are unable to provide data on why these recommendations were missing. The time frame of the study allowed for collection of provider recommendations, but not patient response to the guidelines (e.g., completion of 3-year surveillance when recommended); this area will be the subject of future research. These limitations may be balanced by our unique approach of directly correlating survey responses with actual post-colonoscopy practice and including a wide range of factors hypothesized to be associated with guideline adherence.
In conclusion, by correlating survey-measured knowledge and attitudes regarding post-colonoscopy guidelines with actual surveillance recommendations, we were able to explore the importance of several hypothesized causes of guideline non-adherence. Trainee physicians were more likely to practice guideline-based surveillance. Physicians who disagree with the aggressiveness of guidelines were 40 % less likely to issue guideline-based recommendations and more likely to overuse surveillance colonoscopy. The apparent unimportance of factors such as guideline knowledge and medicolegal concerns suggests that there may be few physician-specific opportunities for optimizing practice. Thus, we speculate that interventions such as use of guideline-appropriate post-polypectomy surveillance recommendations as a Medicare quality measure, tying colonoscopy reimbursement to guideline-appropriate practice, or developing and implementing decision support into generation of post-polypectomy follow-up recommendations may be required to minimize both underuse and overuse of surveillance colonoscopy. Overall, further study is needed to identify key factors that influence guideline-appropriate practice and interventions that can ensure efficient, timely use of surveillance colonoscopy. Valuable avenues to pursue might include expanding surveys of practicing endoscopists or even examining whether system changes (such as centralized decision support for delivering follow-up recommendations) might improve our understanding and approach toward ensuring guideline-appropriate follow-up after colonoscopy.
Supplementary Material
Acknowledgments
The project described was supported in part by Merit Review Award number 1 I01 HX001574-01A1 (Gupta, PI) from the United States Department of Veterans Affairs Health Services Research & Development Service of the VA Office of Research and Development. The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs. Support was also provided in part by the NCI-funded consortium Population-Based Research Optimizing Screening through Personalized Regiments (PROSPR) through NIH/NCI Grant U54CA163308-01 (Singal, CO-I). We thank Drs. Kenneth R. McQuaid, Robert H. Lee, and John E. Pandolfino for reviewing and providing feedback on the physician survey.
Footnotes
Conflict of interest The authors have no conflicts of interest to disclose.
Electronic supplementary material The online version of this article (doi:10.1007/s10620-015-3685-x) contains supplementary material, which is available to authorized users
References
- 1.Schoen RE, Pinsky PF, Weissfeld JL, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laiyemo AO, Pinsky PF, Marcus PM, et al. Utilization and yield of surveillance colonoscopy in the continued follow-up study of the polyp prevention trial. Clin Gastroenterol Hepatol. 2009;7:562–567; quiz 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Heijningen EM, Lansdorp-Vogelaar I, Steyerberg EW, et al. Adherence to surveillance guidelines after removal of colorectal adenomas: a large, community-based study. Gut. 2015. doi: 10.1136/gutjnl-2013-306453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mysliwiec PA, Brown ML, Klabunde CN, et al. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141:264–271. [DOI] [PubMed] [Google Scholar]
- 5.Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74 e1–80 e1. [DOI] [PubMed] [Google Scholar]
- 6.Pabby A, Schoen RE, Weissfeld JL, et al. Analysis of colorectal cancer occurrence during surveillance colonoscopy in the dietary Polyp Prevention Trial. Gastrointest Endosc. 2005;61:385–391. [DOI] [PubMed] [Google Scholar]
- 7.Farrar WD, Sawhney MS, Nelson DB, et al. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol. 2006;4:1259–1264. [DOI] [PubMed] [Google Scholar]
- 8.Ransohoff DF. Economic impact of surveillance. Gastrointest Endosc. 1999;49:S67–S71. [DOI] [PubMed] [Google Scholar]
- 9.Sint Nicolaas J, de Jonge V, van Baalen O, et al. Optimal resource allocation in colonoscopy: timing of follow-up colonoscopies in relation to adenoma detection rates. Endoscopy. 2013;45:545–552. [DOI] [PubMed] [Google Scholar]
- 10.Menees SB, Elliott E, Govani S, et al. Adherence to recommended intervals for surveillance colonoscopy in average-risk patients with 1 to 2 small (<1 cm) polyps on screening colonoscopy. Gastrointest Endosc. 2014;79:551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah TU, Voils CI, McNeil R, et al. Understanding gastroenterologist adherence to polyp surveillance guidelines. Am J Gastroenterol. 2012;107:1283–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saini SD, Nayak RS, Kuhn L, et al. Why don’t gastroenterologists follow colon polyp surveillance guidelines? Results of a national survey. J Clin Gastroenterol. 2009;43:554–558. [DOI] [PubMed] [Google Scholar]
- 13.CMS. CMS proposes hospital outpatient and ambulatory surgical centers policy and payment changes for 2014 Fact Sheets. 2013, July 8 ed, 2013. [Google Scholar]
- 14.Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2006;130:1865–1871. [DOI] [PubMed] [Google Scholar]
- 15.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56:143–159; quiz 184–185. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–1085. [DOI] [PubMed] [Google Scholar]
- 18.Laiyemo AO, Murphy G, Albert PS, et al. Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years. Ann Intern Med. 2008;148:419–426. [DOI] [PubMed] [Google Scholar]
- 19.Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinsky PF, Schoen RE, Weissfeld JL, et al. The yield of surveillance colonoscopy by adenoma history and time to examination. Clin Gastroenterol Hepatol. 2009;7:86–92. [DOI] [PubMed] [Google Scholar]
- 21.Ransohoff DF, Yankaskas B, Gizlice Z, et al. Recommendations for post-polypectomy surveillance in community practice. Dig Dis Sci. 2011;56:2623–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.le Clercq CM, Bouwens MW, Rondagh EJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014;63:957–963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.