Abstract
The jawbone is a unique structure as it serves multiple functions in mastication. Given the fact that the jawbone is remodeled faster than other skeletal bones, bone cells in the jawbone may respond differently to local and systemic cues to regulate bone remodeling and adaptation. Osteoclasts are bone cells responsible for removing old bone, playing an essential role in bone remodeling. Although bone resorption by osteoclasts is required for dental tissue development, homeostasis and repair, excessive osteoclast activity is associated with oral skeletal diseases such as periodontitis. In addition, antiresorptive medications used to prevent bone homeostasis of tumors can cause osteonecrosis of the jaws that is a major concern to the dentist. Therefore, understanding of the role of osteoclasts in oral homeostasis under physiological and pathological conditions leads to better targeted therapeutic options for skeletal diseases to maintain patients’ oral health. Here, we highlight the unique features of the jawbone compared to the long bone and the involvement of osteoclasts in the jawbone-specific diseases.
Keywords: bone remodeling, jawbone, mechanical stress, osteoclast, osteonecrosis
1 |. INTRODUCTION
The skeletal system consists of over 200 bones to create a framework to support the body, and each bone displays a unique shape and size depending on their anatomical locations. Bones require to be constantly remodeled: old bone is removed and replaced with new bone. Recent studies have suggested that bone cells at differrent skeletal sites display distinct function to maintain skeletal homeostasis.1–4 Particularly, the jawbone differs from other skeletal bones in several aspects. The jawbone is originated from migrating cranial neural crest cells and formed through primarily intramembranous ossification, which differs from the axial and appendicular skeletons that are derived from mesoderm and undergo endochondral ossification. Since the alveolar bone houses teeth, the jawbone is constantly subjected to mechanical loading during mastication. Additionally, some skeletal diseases such as cherubism are only present in the jawbones. Therefore, therapeutic methods used to treat most skeletal diseases may not apply to jawbone diseases.
Bone resorption by osteoclasts is an essential physiological process to maintain bone mass and integrity. However, pathological activation of osteoclast activity leads to serious health conditions such as postmenopausal osteoporosis. Bisphosphonates and denosumab, which target osteoclast and its bone resorption activity, are widely used to treat osteoporosis. One of the adverse side effects of these medications is the development of osteonecrosis, which occurs only in the jawbone. Although the mechanisms of medication-associated osteonecrosis of the jaw (ONJ) remain unknown, it is possible to speculate that osteoclasts in the jawbone respond differently to those mediations or growth factor signals, resulting in the jawbone-specific disease. Therefore, this review aims to understand the distinctive features of the jawbone with a specific focus on the function of osteoclasts, and explore the potential therapeutic strategies for jawbone diseases.
2 |. BIOLOGY OF BONE TISSUE
2.1 |. Bone remodeling
Bone remodeling is a finely coordinated process and is necessary to repair damaged bone and to maintain mineral homeostasis. There are three types of cells that are responsible for proper bone remodeling: (a) the osteoclasts, which degrade bone matrix by secreting hydrochloric acid and proteases; (b) the osteoblasts, which deposit bone matrix and are responsible for its mineralization; and (c) the osteocytes, which are embedded in the bone matrix and act as mechanosensors that respond to changes in mechanical stress of bone. In the adult healthy skeleton, bone resorption is always followed by bone formation to maintain bone mass referred to as coupling.5 Imbalance between bone formation and bone resorption leads to skeletal diseases such as osteoporosis. The activity of bone cells is regulated by a variety of mechanisms such as systemic hormones, local factors, and mechanical stimuli.
2.2 |. Bone development
Bones arise from three distinct embryonic lineages. The somites developed from the paraxial mesoderm generate the axial skeleton and the posterior cranial bones, the lateral plate mesoderm generates the limb skeleton, and the cranial neural crest generates the anterior craniofacial bones including mandibular bones.6–8 Bones are formed through two distinct processes during embryonic development: intramembranous ossification and endochondral ossification.9,10 In the intramembranous ossification, mesenchymal cells directly differentiate into osteoblasts and secrete bone matrix. The flat bones of the skull and face are formed via intramembranous ossification. In the endochondral ossification, mesenchymal cells condense and differentiate into chondrocytes, and form cartilage primordia, which is subsequently replaced by bones. Bones at the base of the skull and long bones of the axial and the appendicular skeleton form via endochondral ossification. The replacement of cartilage with mineralized bone is a complex process. The growth plate, also known as the epiphyseal plate, is the area of growth in the long bone.11 Initially, chondrocytes undergo rapid proliferation that drives the linear growth of the skeletal elements. These proliferating cells in the center of the growth plate eventually exit the cell cycle and differentiate into hypertrophic chondrocytes. Vascular invasion promotes the invasion of osteoblast progenitors, osteoclasts and blood vessel endothelial cells from the inner perichondrium into the hypertrophic cartilage, which leads to degradation of the matrix and cartilage resorption.12
2.3 |. Mandibular development
The mandible is formed through both intramembranous and endochondral ossification (Figure 1). The cranial neural crest cells migrate to the first branchial arch and condense to form Meckel's cartilage which transiently supports the growth of mandible and disappears during development.13,14 The body of the mandible formed lateral to Meckel's cartilage undergoes intramembranous ossification. In contrast, the condylar process and a part of the coronoid process, the mental protuberance, and the mandibular angle are formed via endochondral ossification. These processes are formed after development of the primary cartilage forms, and therefore classified as secondary cartilage.15 The condylar cartilage acts as a growth center of the mandible and greatly contributes to the postnatal mandibular growth. The temporomandibular joint (TMJ) formed between mandibular condyle and the mandibular fossa of the temporal bone plays a pivotal role in jaw movements, which are one of the most complicated movements in the body. Disorders of the TMJ affect numerous individuals, and lead to difficulty in chewing function and chronic facial pain.16
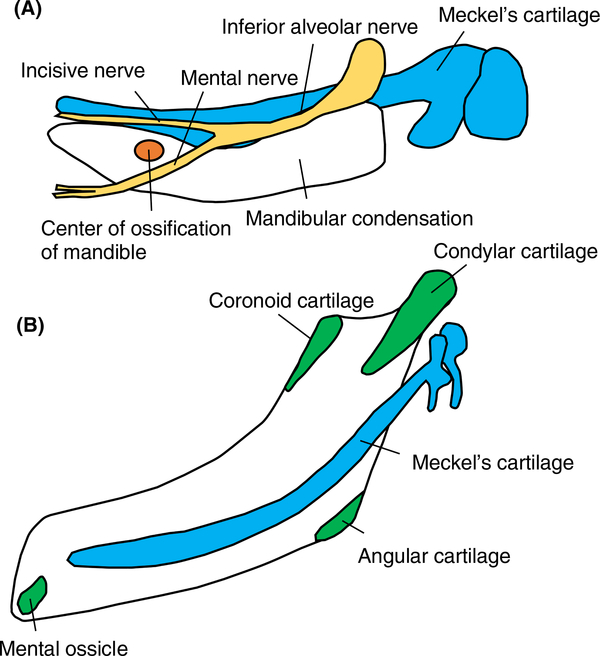
FIGURE 1.
Mandibular development. A, The body of the mandible (white) formed lateral to Meckel's cartilage (blue) undergoes intramembranous ossification. The center of ossification (orange) is lateral to Meckel's cartilage at the bifurcation of the inferior alveolar nerve (yellow). B, The condylar process and a part of the coronoid process, the mental protuberance, and the mandibular angle are formed via endochondral ossification (green). These cartilages are called secondary cartilage
3 |. OSTEOCLAST
3.1 |. Osteoclast function
Osteoclasts are multinucleated cells that form through fusion of mononuclear precursors of hematopoietic origin. Activation and differentiation of osteoclasts are controlled by osteoblast-lineage cells through two essential cytokines: macrophage colony stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL). M-CSF binds to its receptor, c-Fms, on early osteoclast precursors and provides signals required for their survival and proliferation.17,18 Binding of RANKL to its receptor, receptor activator of nuclear factor-κB (RANK), which is expressed on the cell surface of osteoclasts, induces the recruitment of tumor necrosis factor receptor-associated factors (TRAFs). TRAF2, −5, and −6 have been shown to bind to RANK, yet only TRAF6 mutations lead to osteopetrosis due to a loss of osteoclast activity.19,20 TRAF6 binding to RANK activates transcription factors such as nuclear factor-κB (NF-κB), activator protein 1 (AP-1), and nuclear factor-activated T cells c1 (NFATc1), which are required for osteoclast differentiation.21–23 Activation of these transcriptional factors, in turn, regulates osteoclast-specific genes including dendric cell-specific transmembrane protein (DC-STAMP), tartrate-resistant acid phosphatase (TRAP), osteoclast-associated receptor (OSCAR), and cathepsin K, which are critical for exerting osteoclast function.
3.2 |. Bone resorption
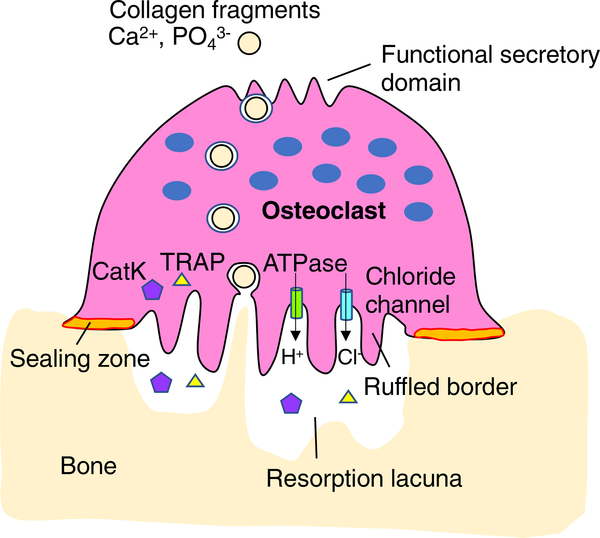
Bone matrix consists of the organic component, type I collagen (>90%), and the inorganic component, primarily hydroxyapatite. Bone resorption by osteoclasts occurs through demineralization of bone matrix by local acidic environment, and then through degradation of the collagen I-rich matrix by secretion of proteases.24 Osteoclasts form unique cytoskeletal structures called the sealing zone and the ruffled border to resorb bone matrix efficiently (Figure 2). When osteoclasts resorb bone matrix, they polarize and form the sealing zone, where the cell attached to the mineralized matrix, and thus bone matrix underneath of the ruffled border localized within the sealing zone is degraded. Osteoclasts demineralize bone matrix by secretion of protons, and degrade the collagen I-rich matrix by secretion of proteases through the ruffled border membrane. Vacuolar H+-adenosine triphosphatase (H+-ATPase) located in the ruffled border membrane secretes protons to acidify the resorption lacuna.25,26 Chloride ions are also transported into the resorption lacuna through a chloride channel, CLCN7, located in the ruffled border membrane to maintain electroneutrality.27–29 The degradation of the organic component of bone is accomplished by a lysosomal protease, cathepsin K.30,31 Patients with mutations in the cathepsin K gene develop pycnodystosis owing to dysfunctional osteoclast activity.32 Pycnodystosis is characterized by dense and brittle bones which are prone to fracture, especially in long bones, jawbones, and clavicle. Moreover, cathepsin K-deficient mice display an osteopetrotic phenotype due to a defect in matrix degradation but not in demineralization.33 The degraded collagen and other protein fragments, calcium and phosphate within the resorption lacuna are then endocytosed and released from the functional secretory domain at the basolateral membrane of the osteoclast.34,35 After completion of resorption, osteoclasts either undergo apoptosis or perform another round of bone resorption.
FIGURE 2.
Bone resorption by osteoclasts. Bone-resorbing osteoclasts form ruffled borders and sealing zones to make the resorption lacuna acidic. Vacuolar H+-ATPase localized to the ruffled border transports protons into the resorption lacuna, while the chloride channel balances the ionic charge by transporting chloride simultaneously. Enzymes such as cathepsin K (CatK) and TRAP are secreted into the resorption lacuna to degrade bone matrix. Matrix degradation products are endocytosed from the ruffled border and released from the functional secretory domain
Bone resorption is controlled by systemic hormones including calcitonin, parathyroid hormone (PTH), vitamin D3 (1α,25(OH)2D3), and estrogen. Calcitonin secreted by the parafollicular cells of the thyroid gland suppresses bone resorption by inhibiting the activity of osteoclasts. The production and secretion of calcitonin is stimulated by an elevated calcium level and results in a reduction in the serum calcium concentration.36 PTH is a peptide hormone which is synthesized in the parathyroid glands. Its main function is to increase the concentration of calcium in the blood plasma. PTH increases osteoclast formation and bone resorption through the regulation of RANKL/OPG expression in osteoblasts.37 Vitamin D3 promotes bone resorption by increasing the number and activity of osteoclasts, at least in part, through increased RANKL expression in osteoblasts.38,39 Estrogen is crucial in the control of osteoclast differentiation and induces osteoclast apoptosis. Therefore, loss of estrogen in women after the menopause results in increased osteoclast formation and survival.40
3.3 |. Anabolic effects: coupling
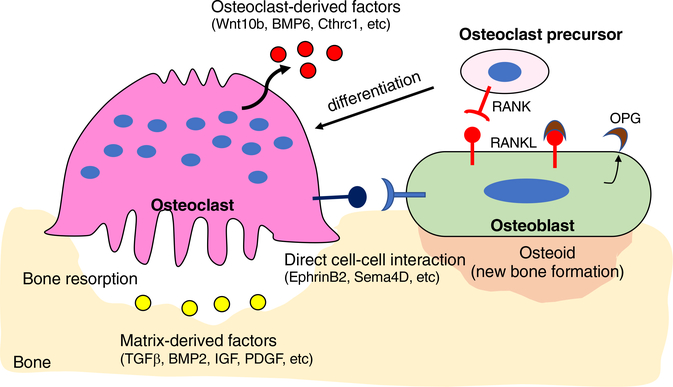
Bone resorption is always followed by an equal amount of new bone formation so that bone mass does not change. It has been long of interest to understand how these two distinct events could be balanced at the same bone surface but at different times. Recent advances in osteoclast studies have suggested that osteoclasts function not only to resorb bone matrix but also to regulate osteoblast-mediated bone formation, thereby maintaining bone mass.5 There are several mechanisms implicated in the coupling process: (a) factors released from the bone matrix during bone resorption, (b) factors secreted from osteoclasts, and (c) direct interaction between osteoclasts and osteoblasts (Figure 3). Due to such critical function of osteoclasts, current antiresorptive therapies such as bisphosphonate have limitations in the treatment of osteoporosis. A critical pharmacological feature of bisphosphonates is to have a high affinity for bone relative to other tissues because they bind to hydroxyapatite crystals, and to inhibit bone resorption either by reducing osteoclast activity or by inducing osteoclast apoptosis.41,42 As such, reduction of osteoclast number at the bone remodeling site leads to reduction of osteoblast activity, and therefore antiresorptive therapies cannot promote bone formation. In contrast to traditional antiresorptive agents, odanacatib, a small-molecule inhibitor of cathepsin K, inhibits bone resorption without reducing osteoclast numbers. Both clinical trials and animal studies of odanacatib showed that it reduces bone resorption with a minimal decrease in bone formation rate,43,44 but unfortunately, further development of odanacatib as a potential treatment to prevent bone loss was stopped by the increased incidence of cardiovascular diseases.45 However, these trials provide new perspectives on drug development: inhibition of osteoclast activity without reducing osteoclast numbers is an effective approach for patients with osteoporosis.
FIGURE 3.
Coupling mechanism between osteoclasts and osteoblasts. Osteoblasts express RANK ligand (RANKL) and osteoprotegerin (OPG). RANKL binds to the RANK receptor, which is expressed on the surface of osteoclast precursors. RANKL binding to RANK receptor leads to differentiation and activation of osteoclasts. OPG acts as a decoy receptor for RANKL, thus preventing the RANK and RANKL interactions. Osteoclasts regulate migration and activity of osteoblasts through several mechanisms: (a) factors released from the bone matrix during bone resorption (yellow), (b) factors secreted from osteoclasts (red), and (c) direct interaction between osteoclasts and osteoblasts (blue)
4 |. OSTEOCLASTS IN THE JAWBONE
4.1 |. Bone remodeling in the jaw
It is well recognized that the jawbone is remodeled faster than the other skeletal bones. For example, the bone formation rate in the alveolar process of the mandible is sixfold higher than the femur.46 Additionally, the jawbone displays smaller mean values of bone mineralization density distribution than the tibia reflecting its higher bone turnover.47 Interestingly, the jawbone exhibits a greater amount of collagen, the lower rate of cross-link maturation, and the lower extent of lysyl hydroxylation that is necessary for the stabilization of collagen.48 These characteristics of extracellular matrix (ECM) suggest a higher bone turnover rate and greater bone flexibility of the jawbone.
Ovariectomized (OVX) animal is the most commonly used experimental animal model for postmenopausal osteoporosis due to estrogen deficiency in women. It is well described that OVX surgery in animals decreases bone mass and alters the microarchitecture at various skeletal sites. Interestingly, alveolar bone in the jaw is less sensitive to estrogen-deficiency-induced osteoporosis than the proximal tibia of the long bone in rats.49–52 These data suggest that each bone exhibits the differential response to estrogen deprivation. Low protein intake has been also associated with decreased bone mass and strength.53,54 Protein undernutrition leads to significant changes in both the proximal tibia and mandibular alveolar process associated with increased bone resorption, but these changes are significantly greater in the case of the tibia,51 suggesting that the mandibular alveolar bone seems to resist more than the long bone to both protein and estrogen deprivation.
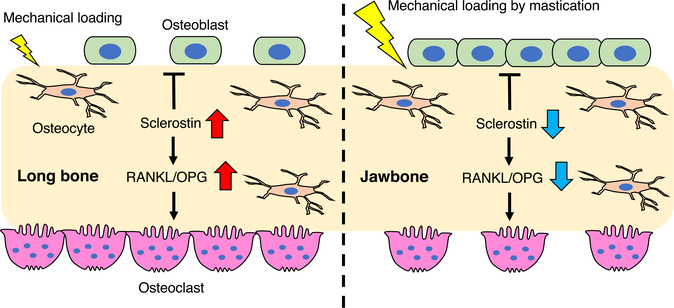
One of the most important features of the jawbone that facilitate bone remodeling may be mechanical stimulus during mastication. Animal experimental studies have shown a functional relationship between the masticatory forces and alveolar bone remodeling.55,56 For example, reducing masticatory forces by a soft diet leads to decreased bone mineral density and alveolar bone volume.57 Importantly, the OVX animals fed a soft diet display significant decreases in alveolar bone volume and bone mineral density, while those fed a normal diet preserve their mandibular alveolar bone architecture.56,58 These data suggest that mechanical loading during mastication may protect the alveolar bone from the detrimental effects observed in other skeletal sites. In the long bone, lack of mechanical stress reduces trabecular bone formation and accelerates bone resorption. Similarly, mice fed a soft diet exhibit a decrease in bone volume associated with increased osteoclast number in the alveolar bone compared to those fed a hard diet.59 Osteocytes, the most abundant cells in bone, are believed to play a key role in regulating adaptive response to mechanical loading. It is reported that sclerostin secreted from the osteocytes inhibits osteoblast differentiation and stimulates osteoclastogenesis in a RANKL-dependent manner.60,61 Experimentally, increased loading reduces sclerostin production in the long bone while reduced loading significantly increases sclerostin production.62 Similarly, increased mechanical loading by a hard diet suppresses sclerostin expression in the mandibular bone.63 These data suggest that constant mechanical loading during mastication inhibits sclerostin production from osteocytes, which may inhibit bone resorption and accelerate new bone formation in the jawbone (Figure 4).
FIGURE 4.
Mechanical loading and bone remodeling. Increased mechanical loading reduces sclerostin production from osteocytes. Sclerostin inhibits osteoblast differentiation and stimulates osteoclastogenesis at least in part by upregulation of RANKL/OPG expression. The constant mechanical loading during mastication inhibits sclerostin production from osteocytes, which may decrease bone resorption and accelerate new bone formation in the jawbone
4.2 |. Osteoclast function in the jaw
Several reports support the idea that bone cells in the jawbone behave differently from those in the long bone. For example, bone marrow stromal cells (BMSCs) derived from the jawbone exhibit higher osteogenic potential compared to those derived from other skeletal bones but less ability to differentiate into adipocytes and chondrocytes.1–3 Additionally, bone marrow in the jawbone exhibits a low bone marrow adipose tissue content compared to the long bone.52,64 Furthermore, BMSCs from the jawbone display higher expression of alkaline phosphatase in response to rhBMP-2 compared to those from long bones.65 These findings suggest that BMSCs in the jawbone are more osteogenic and more responsive to osteogenic signals than other skeletal BMSCs.
It is generally accepted that all osteoclasts are alike, but recent studies suggest the heterogeneity of osteoclasts at different bone sites. For example, osteoclasts from the long bone differentiate faster than those from the jawbone in vitro, although there are no difference at the later time point.66 In addition, osteoclasts from the jawbone and the long bone exhibit distinctive cell morphology and response to substrates.67 For example, bone marrow cells from the long bone generate more osteoclasts than those from the jawbone when cultured on the bone slice, while bone marrow cells from the jawbone generate more osteoclasts than those from the long bone when cultured on the dentin slice.67 Since composition of ECM differs between the jawbone and the long bone,48 such differences in ECM can provide an explanation for the differences in osteoclasts and their activity at different skeletal sites. It is reported that TRAP activity is approximately 30-fold higher in calvarial osteoclasts compared to long bone osteoclasts.68 Additionally, osteoclasts from the long bone cultured on plastic express a higher level of TRAP activity than osteoclasts cultured on collagen- or CaP-coated substrates or on bone slices.68 These data further support the idea that characteristic of ECM may influence osteoclast function.
It has been demonstrated that bone marrow cells from the jawbone display increased osteoclast formation and resorption activity comparted to those from the long bone in vitro, while more osteoclasts are observed in the trabecular region of tibia compared to alveolar bone region of the mandible in vivo.4 Given the report that BMSCs from the long bone exhibit higher RANKL/OPG ratio responsive to PTH and Vitamin D3 treatment than those from the jawbone, resulting in increased osteoclastogenesis,4 it is likely that osteoclast activity in the long bone is accelerated by hormonal signals in vivo, while the high activity of osteoclasts in the jawbone may be suppressed by local, systemic, or environmental factors. The distinct characteristics of the jawbone relative to the long bone are summarized in Table 1.
TABLE 1.
Distinct characteristics of the jawbone relative to the long bone
| Jawbone | Long bone | |
|---|---|---|
| Developmental origin | Cranial neural crest cell | Mesoderm |
| Bone development | Intramembranous ossificationa | Endochondral ossification |
| Bone turnover | Fast | Slow |
| ECM | Greater collagen content | Higher amount of mature crosslinks Higher extent of lysyl hydroxylation |
| Estrogen deficiency | Slight decrease in BMD | Significant decrease in BV/TV, BMD |
| Protein undernutrition | Slight decrease in BV/TV | Significant decrease in BV/TV, BMD |
| BMSC | Higher osteogenic potential More responsive to osteogenic factors |
More responsive to PTH and Vitamin D3 |
| Osteoclast | Higher activity in vitro Higher expression of anti-apoptotic genes Internalize more bisphosphonates |
Higher number in vivo |
| Cellular source of RANKL | Dental follicle, osteoblasts, osteocytes, PDL cells, gingival fibroblasts | B cell, T cell, osteoblasts, osteocytes, chondrocytes |
Abbreviations: BMD, bone mineral density; BMSC, bone marrow stromal cells; BV/TV, bone volume/tissue volume; ECM, extracellular matrix; PDL, periodontal ligament; RANKL, receptor activator of nuclear factor-κB ligand.
The condylar process and a part of the coronoid process, the mental protuberance and the mandibular angle are formed via endochondral ossification.
In addition, recent studies suggested that osteoclasts of erythromyeloid progenitor (EMP) origin are required for normal bone development and postnatal bone remodeling in both physiological and pathological settings.69,70 While it remains unknown whether hematopoietic stem cell-derived and EMP-derived osteoclasts display distinct function, it would be possible that distinct origin of osteoclasts may affect the functional difference in osteoclasts at different bony sites.
4.3 |. Osteoclast function in tooth eruption
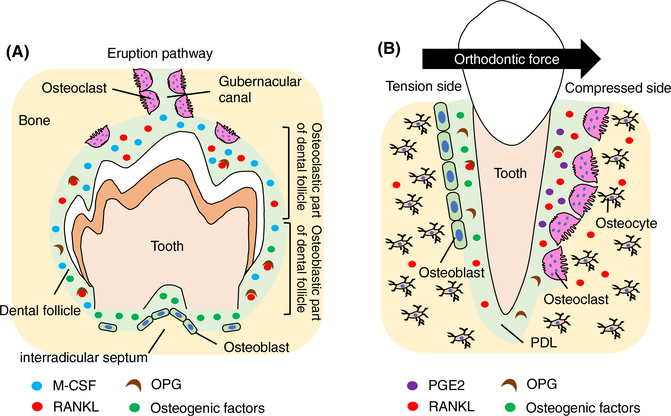
It is interesting that tooth eruption is always affected in diseases such as osteopetrosis known to associate with a lack of osteoclast formation or function.71–73 In contrast, in diseases where osteoclast formation or activity is increased such as osteolysis and Pageťs disease, premature tooth loss or root resorption happens,74 suggesting that bone resorption is required for successful tooth eruption. The alveolar bone develops from the dental follicle during eruption of teeth and its formation is dependent on dental primordia formation.75 In order for the developing tooth to erupt, a gubernacular canal is widened by osteoclast-mediated bone resorption to create the eruption pathway (Figure 5A).76
FIGURE 5.
Tooth eruption and tooth movement. A, The levels of RANKL/OPG and M-CSF in the dental follicle are critical for tooth eruption. Bone resorption by osteoclasts at the coronal half of the dental follicle is required to widen a gubernacular canal to create the eruption pathway. New bone formation by osteoblasts at the base of the bony crypt is important for producing an outward eruption force directed against the erupting tooth. B, During orthodontic tooth movement, osteoclasts resorb bone matrix at the compressed side to create space for tooth movement, while new bone formation occurs at the tension side. RANKL, OPG, and prostaglandin (PG)E2 secreted from periodontal ligament (PDL) cells or osteocytes in response to mechanical stimuli control bone resorption
RANKL/OPG system is also required for tooth eruption as RANKL knockout mice display impaired tooth eruption.71 Rescue of RANKL knockout mice by transgenically expressed RANKL in T and B lymphocytes promotes osteoclast formation in long bones; however, the teeth still did not erupt in the rescued mice.77 These findings suggest that osteoclasts at the different skeletal sites may receive RANKL from the different types of cells. At the molecular level, a major burst of osteoclast formation seen on postnatal day 3 in the rat mandibular first molar as the result of a decrease in OPG expression in the dental follicle.78 At this time, M-CSF is maximally expressed in the dental follicle.79 Although RANKL is expressed in the follicle at day 3, its gene expression is not upregulated at this time,80 suggesting that changes in the levels of OPG and M-CSF in the dental follicle may be critical for alveolar bone resorption and subsequent tooth eruption. In the op/op mice that show an osteopetrotic phenotype due to absence of M-CSF gene, the phenotype in femur resolves with age, but tooth eruption never spontaneously occurs.81,82 Systemic administration of M-CSF can rescue tooth eruption, but only if given at a critical time window between after birth to postnatal day 10,83 suggesting that tooth eruption requires time-limited recruitment of osteoclast precursors and subsequent activation.
In addition to alveolar bone resorption, new bone formation at the base of the bony crypt is important for producing an outward eruption force directed against the erupting tooth. It has been experimentally demonstrated that removal of the coronal half of the dental follicle inhibits alveolar bone resorption, while removal of the basal half of the dental follicle inhibits alveolar bone formation at the base of the crypt, and both cases result in impaired tooth eruption.84 Although RANKL is considered to be a key regulator for tooth eruption, the growing mice that received denosumab, an anti-RANKL antibody, display an osteopetrotic phenotype with normal tooth eruption.85 These mice exhibit a reduced number of osteoclasts with normal number of osteoblasts in the alveolar bone at 8 weeks of age.85 In contrast, bisphosphonate-treated mice display delayed tooth root formation and tooth eruption with a significant reduction in the interradicular septum length. These mice exhibit increased osteoclasts and a significant reduction in osteoblast numbers in the alveolar bone.85 These data suggest that both bone resorption and bone formation are critical for tooth eruption as well as root formation.
4.4 |. Tooth movement
Force application to teeth such as mastication and orthodontic treatment induces tooth movement through extensive local remodeling of alveolar bone. During orthodontic tooth movement, bone resorption occurs at the compressed side where osteoclasts occupy lacunae on the periodontal ligament (PDL) side of the alveolar bone, and new bone formation occurs at the tension side where active osteoblasts secrete matrix at the surface of PDL, resulting in changes of tooth position 86 (Figure 5B). Therefore, cells that form PDL are supposed to play a pivotal role in alveolar bone remodeling during orthodontic tooth movement by producing local factors that regulate osteoclast activity. Of these factors, RANKL produced from the PDL cells at the compressed side during orthodontic treatment plays an essential role in tooth movement.87,88 While HVJ-envelope-vector mediated transfer of Rankl gene to the periodontal tissue activates osteoclastogenesis and accelerates tooth movement in rats, transfer of Opg gene suppresses osteoclastogenesis and thus inhibits tooth movement.89,90 Inhibition of RANKL by a neutralizing antibody also reduces orthodontic tooth movement,91 suggesting that the RANKL/RANK/OPG system is a key regulator of orthodontic force-induced osteoclastogenesis and tooth movement. In addition to the cells in PDL, osteocytes in the alveolar bone are involved in tooth movement in response to orthodontic force. For example, osteocyte ablation in mice suppresses osteoclastogenesis in response to mechanical stimuli and inhibits tooth movement.92 Furthermore, osteocyte-specific Rankl knockout mice are resistant to orthodontic tooth movement due to the inhibition of osteoclastogenesis in periodontal tissues.91 Interestingly, osteoblast numbers at the tension side are also reduced in the Rankl knockout mice, suggesting that osteoclast–osteoblast coupling may play a role in orthodontic tooth movement.
Prostaglandin (PG)E2 is considered to be one of the important chemical mediators of bone remodeling and is synthesized by PDL cells in response to mechanical stress.93,94 Clinical studies showed that administration of PGE2 accelerates orthodontic tooth movement without any obvious side effects.95 In addition, local administration of Vitamin D3 or PTH to the experimental tooth movement model increases osteoclastogenesis and accelerates tooth movement.96,97 These studies have shown promise to shorten orthodontic treatment time by increasing osteoclast activity.
5 |. JAWBONE OSTEOCLASTS IN PATHOLOGICAL SITUATIONS
5.1 |. Periodontal disease
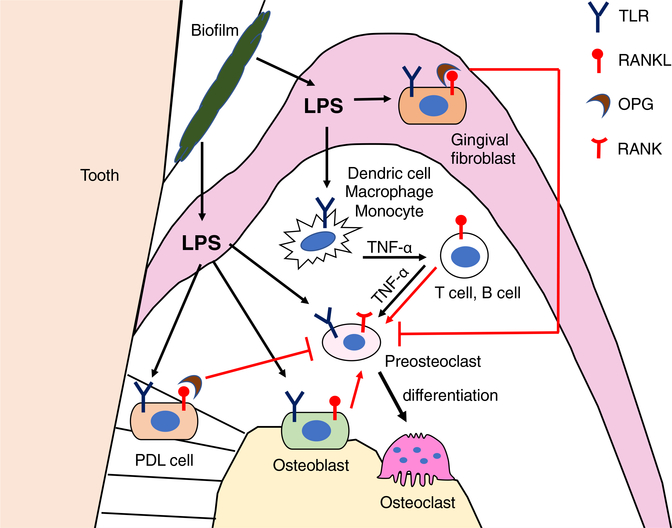
Periodontal disease is one of the most common inflammatory diseases affecting the oral health and possibly affecting other tissues in the body as a risk factor for complication of chronic conditions.98 Aberrant activation of osteoclasts induced by the bacterial products such as lipopolysaccharide (LPS) leads to a pathological bone loss around teeth (Figure 6). RANKL plays a critical role in alveolar bone resorption in periodontal disease. It is reported that upregulation of RANKL expression in gingival tissues is positively correlated with the number of Porphyromonas gingivalis in periodontitis patients.99 In addition, inhibition of RANKL/RANK signal by systemic delivery of OPG reduces alveolar bone loss in the experimental periodontitis animals.100 In chronic periodontal disease, the LPS released from Gram-negative bacteria such as P gingivalis activates Toll-like receptors (TLRs) on monocytes, dendric cells, and macrophages, and promotes proinflammatory cytokine secretion such as tumor necrosis factor α (TNF-α), Interleukin-1 (IL-1), and IL-6.101 These cytokines, in turn, activate RANKL expression in T cells, B cells, and osteoblasts.102,103 LPS also directly interacts with osteoblasts via TLR4 and induces RANKL expression.104,105 RANKL expression in osteoblasts through TLRs is mediated by the extracellular signal-regulated kinase (ERK) and the c-Jun N-terminal kinase.104,105 Similarly, P gingivalis infection induces RANKL expression through activator protein 1 (AP-1) transcription factor in osteoblasts.106 On the other hand, LPS-stimulated human PDL cells or gingival fibroblasts inhibit osteoclast differentiation by producing OPG.107,108 These findings suggest that LPS exerts different biological activities based on the cell types. Interestingly, LPS has a bidirectional role in osteoclastogenesis. For example, when bone marrow cells are stimulated with LPS in the presence of M-CSF and RANKL, LPS inhibits osteoclast formation, whereas LPS stimulates osteoclast formation from those pretreated with RANKL,109 suggesting that RANKL-mediated lineage commitment is required for LPS-induced osteoclastogenesis. However, it has been demonstrated that while OPG does not inhibit LPS-stimulated osteoclastogenesis, inhibition of TNF-α signaling is able to block the enhancing effects of LPS.110 Furthermore, inhibiting IL-1/TNF signaling in experimental periodontitis in the crab-eating macaque, Macaca fascicularis, reduces both inflammatory cell recruitment and osteoclast formation, leading to a decrease in bone loss.111,112 These data suggest that RANKL is required to initiate LPS-induced osteoclastogenesis but enhancing activity of LPS is likely mediated through inflammatory cytokines such as TNF-α but not through RANKL signaling.
FIGURE 6.
Regulation of bone resorption in periodontal disease. Lipopolysaccharide (LPS) originates from bacteria in the oral biofilm. LPS initiates osteoclastogenesis upon binding toll-like receptor (TLR) that is expressed on dendritic cells, macrophages, monocytes, osteoblasts, PDL cells, and gingival fibroblasts. Osteoblasts produce RANKL in response to LPS stimulation. RANKL and TNF-α secretion by B cells and T cells are induced by TNF-α produced by dendritic cells, macrophages and monocytes. Osteoclast differentiation is enhanced either by continuous exposure to RANKL, TNF-α, or both, while OPG secreted form gingival fibroblasts and PDL cells inhibit osteoclast differentiation. The direct interaction of LPS with preosteoclasts through TLR also promotes osteoclast differentiation
5.2 |. Osteonecrosis of the jaw
Osteonecrosis of the jaw is defined as exposed bone in the maxillofacial region for more than 8 weeks in patients receiving an antiresorptive medication without history of radiation therapy to the jaws.113 Antiresorptive agents such as bisphosphonates and denosumab, as well as angiogenesis inhibitors, induce medication-related osteonecrosis of the jaw (MRONJ). Since pathophysiology of ONJ has not been fully understood, definitive treatment strategies have not yet been established. Administration of bisphosphonate or denosumab affects bone turnover by inhibiting osteoclast activity through the whole skeletal system; however, MRONJ occurs only in the jawbone. Dental-related bacterial infection and associated inflammation are recognized as contributing risk factors for ONJ as it typically occurs after tooth extraction with severe periodontal or periapical infections.114,115 Soudia et al investigated alveolar bone healing after extraction of healthy teeth and teeth with experimental periodontitis in rats receiving antiresorptive agents such as zoledronate.116 The zoledronate-treated animals with extractions of healthy teeth exhibited normal mucosal healing and woven bone formation in the sockets, while those had extractions of teeth with periodontitis exhibited impaired healing with visible mucosal defects and inflammatory cell infiltration adjacent to osteonecrotic bone.116 Therefore, timely dental evaluations and subsequent management of patients who are scheduled to receive antiresorptive medications are necessary to reduce the risk of development of ONJ.
As described above, osteoclasts exhibit different characteristic depending on their anatomical location. It has been shown in culture that osteoclast precursors in the jawbone internalize more bisphosphonates than those in the long bone, although the difference in bisphosphonate uptake does not differentially affect osteoclast formation or activity.117 Indeed, the day after administration of zoledronate, the bisphosphonate content in the jawbone is much higher than the ilium, and become more pronounced at subsequent time points.118 Interestingly, expression of the anti-apoptotic genes such as Bcl-2 and Bcl-xL is higher in jawbone osteoclasts than long bone osteoclasts,117 suggesting that osteoclasts in the jawbone might be more resistant to bisphosphonate-induced apoptosis. This marked local difference in the distribution of bisphosphonates may explain why ONJ occurs frequently in the jaw; however, this does not explain why ONJ occurs by other types of medications such as denosumab or angiogenic inhibitors.
5.3 |. TMJ osteoarthritis
Osteoarthritis (OA) is a chronic degenerative disease of synovial joints characterized by progressive articular cartilage deterioration, abnormal subchondral bone remodeling, and synovial inflammation.119 It is reported that inhibitors of osteoclastic bone resorption such as bisphosphonates are able to protect articular cartilage deterioration in the rat OA model,120,121 suggesting that aberrant osteoclast activity in the subchondral bone is one of the pathological reasons for OA development. Temporomandibular joint osteoarthritis (TMJOA) is one of the most common forms of temporomandibular disorder, which frequently associates with pain during functional activities.16 The upregulation of genes involved in osteoclast activity and an increased RANKL/OPG ratio in subchondral bone likely contribute to the increased subchondral bone turnover during the early stage of TMJOA. Particularly, higher levels of inflammatory mediators such as TNF-α, IL-1β, IL-6, and metalloproteinases have been detected in synovial fluid from TMJOA patients.122,123 VEGF has enhancing effects on osteoclast survival and bone resorptive activity,124 and its expression level is upregulated in the synovial tissues and the discs in the patients.125,126 These data suggest that increased osteoclast activity stimulated by inflammatory cytokines, enzymes, or VEGF may be responsible for the progression and regulation of the degenerative changes in the TMJ.
Mechanical overloading of the TMJ is implicated in TMJOA development.127 Several non-invasive models mimic the functional overloading of the TMJ by steady mouth opening, creating OA-like lesions including degradation of the articular cartilage and upregulation of inflammatory cytokines production in the TMJ.128,129 Interestingly, reducing dietary loading suppresses the progression of TMJ degradation in association with an increase in OPG/RANKL ratio in the cartilage and subchondral bone, resulting in a decrease in osteoclast activity.130 These findings suggest that although moderate loadings are beneficial for joint cartilage, excessive mechanical loading is likely to influence the onset and progression of TMJOA.
5.4 |. Rare genetic disease: cherubism
Cherubism is a skeletal dysplasia characterized by excessive bone resorption and accumulation of fibro-osseous lesions limited to the jawbone.131 These lesions show TRAP-positive multinucleated osteoclast-like giant cells in a background of oval to spindle-shaped mononuclear cells. Cherubism is a rare autosomal dominant disorder caused by mutation of the SH3BP2 gene.132 Animal studies have shown a possibility that cherubism is characterized as an inflammatory bone disease. Although the fact that SH3BP2 is required for antigen receptor-mediated activation of B cells,133 Ueki et al demonstrated that cherubism lesions develop independently of B- or T-cell involvement in mice.134 Indeed, cherubism is associated with a high level of TNF-α that is likely responsible for the lesion: hyperactive macrophages secrete a high level of TNF-α that drives systemic inflammation and stimulates secretion of RANKL and M-CSF from stromal cells, resulting in enhanced osteoclastic bone resorption.134 Giant cell lesions, previously referred to as giant cell granulomas, are benign tumors of the jaws. Similar to cherubism, multinucleated osteoclast-like giant cells have been identified in the central giant cell lesion (CGCL).135 However, studies have, for the most part, failed to identify cherubism-associated SH3BP2 mutations in sporadic CGCL cases.136
Calcitonin has been reported to be beneficial in the treatment of CGCL and cherubism by inhibiting osteoclast formation and bone resorption137,138; however, several clinical cases have shown that cal citonin treatment is less effective in rapidly growing lesions.139,140 Based on findings in the cherubism mice, TNF-α plays a critical role in disease pathogenesis, and removal of TNF-α prevents the development of fibro-osseous lesions.134 However, children with cherubism who received TNF-α blocking therapy showed no significant improvement.141,142 Recently, denosumab appears to be effective in reducing bone turnover and bone pain in patients with CGCL and cherubism.143,144 The effect of denosumab on bone turnover is rapidly reversible after discontinuation of the drug, representing a key difference from bisphosphonates that have a long half-life. There are case reports of increased lesion growth after denosumab discontinuation in patients with giant cell tumor, suggesting that long-term therapy may be required to maintain therapeutic efficacy of denosumab.
Previous report suggests that cherubism may be associated with impaired osteoblast function.145 Thus, pharmacologic intervention to suppress osteoclastic bone resorption and at the same time to enhance new bone formation may improve outcomes in those patients. As of yet, a key question remains unanswered: why these fibro-osseous lesions only appear in the jawbone. Addressing this question may help better understand the pathophysiology of jawbone diseases and ultimately lead to new therapeutic strategies.
6 |. SUMMARY
Each bone exhibits differential ability of the marrow environment to support osteoclastogenesis and the differential response to local, systemic, or environmental factors. The jawbone seems to be more resistant to estrogen depletion, at least in part, due to constant mechanical loading by mastication. Although RANKL/RANK/OPG system is required for osteoclastogenesis in each skeletal bone under physiological and pathological conditions, it remains unclear why some bone diseases appear only in the jawbone. The differences in developmental origin, bone formation process, mechanical stimuli, oral/periodontal infection, and inflammation are likely contributing to the development of jawbone-specific diseases. It is also unknown whether osteoporosis treatments have the same beneficial effect on oral health as they do on other bones in the skeleton. Most research regarding bone remodeling to date has been carried out in the appendicular or axial skeleton, and thus investigating multiple skeletal sites including the jawbone is necessary to develop a variable treatment and maintain oral health for patients with various skeletal diseases.
ACKNOWLEDGMENTS
We thank Dr Junro Yamashita, Vesa Kaartinen, and Megan Weivoda for long-term collaboration. We thank Drs Honghao Zhang, Hiroki Ueharu, Jingwen Yang, and Haichun Pan for critical reading of the manuscript. We apologize to colleagues whose work we could not discuss due to the space limitations. This study was supported by the National Institute of Dental and Craniofacial Research (R01DE020843 to YM).
Funding information
National Institute of Dental and Craniofacial Research, Grant/Award Number: R01DE020843
Footnotes
DISCLOSURES
All authors declare no conflicts of interest.
REFERENCES
- 1.Matsubara T, Suardita K, Ishii M, Sugiyama M, Igarashi A, Oda R, et al. Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 2005;20(3):399–409. [DOI] [PubMed] [Google Scholar]
- 2.Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38(6):758–68. [DOI] [PubMed] [Google Scholar]
- 3.Aghaloo TL, Chaichanasakul T, Bezouglaia O, Kang B, Franco R, Dry SM, et al. Osteogenic potential of mandibular vs. long-bone marrow stromal cells. J Dent Res. 2010;89(11):1293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaichanasakul T, Kang B, Bezouglaia O, Aghaloo TL, Tetradis S. Diverse osteoclastogenesis of bone marrow from mandible versus long bone. J Periodontol. 2014;85(6):829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11(2):76–81. [DOI] [PubMed] [Google Scholar]
- 6.Tam PP, Trainor PA. Specification and segmentation of the paraxial mesoderm. Anat Embryol (Berl). 1994;189(4):275–305. [DOI] [PubMed] [Google Scholar]
- 7.Cohn MJ, Tickle C. Limbs: a model for pattern formation within the vertebrate body plan. Trends Genet. 1996;12(7):253–7. [DOI] [PubMed] [Google Scholar]
- 8.Bronner-Fraser M. Neural crest cell formation and migration in the developing embryo. FASEB J. 1994;8(10):699–706. [DOI] [PubMed] [Google Scholar]
- 9.Erlebacher A, Filvaroff EH, Gitelman SE, Derynck R. Toward a molecular understanding of skeletal development. Cell. 1995;80(3):371–8. [DOI] [PubMed] [Google Scholar]
- 10.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–6. [DOI] [PubMed] [Google Scholar]
- 11.Hunziker EB. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsc Res Tech. 1994;28(6):505–19. [DOI] [PubMed] [Google Scholar]
- 12.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93(3):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116(2):297–307. [DOI] [PubMed] [Google Scholar]
- 14.Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch DH, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127(8):1671–9. [DOI] [PubMed] [Google Scholar]
- 15.Hall BK. Immobilization and cartilage transformation into bone in the embryonic chick. Anat Rec. 1972;173(4):391–403. [DOI] [PubMed] [Google Scholar]
- 16.Scrivani SJ, Keith DA, Kaban LB. Temporomandibular disorders. N Engl J Med. 2008;359(25):2693–705. [DOI] [PubMed] [Google Scholar]
- 17.Felix R, Cecchini MG, Hofstetter W, Elford PR, Stutzer A, Fleisch H. Impairment of macrophage colony-sttimulating factor production and lack of resident bone marrow marcophages in the osteopetrotic op/op mouse. J Bone Miner Res. 1990;5(7):781–9 [DOI] [PubMed] [Google Scholar]
- 18.Udagawa N, Takahashi N, Akatsu T, Tanak a, Saaki T, Nishihara T, et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A 1990;87(18):7260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev 1999;13(8):1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, et al. Segregation of-AingF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001;20(6):1271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing L, Bushnell TP, Carlson L, Tai Z, Tondravi M, Siebenlist U, et al. NF-kappaB p50 and p52 expression is not required for RANK-expressing osteoclast progenitor formation but is essential for RANK- and cytokine-mediated osteoclastogenesis. J Bone Miner Res 2002;17(7):1200–10. [DOI] [PubMed] [Google Scholar]
- 22.Grigoriadis A, Wang Z, Cecchini M, Hofstetter W, Felix R, Fleisch H, et al. c-Fos: a key regulat- phageosteoclast-macrophage lineage determination and bone remodeling. Science. 1994;66(5184):443–8. [DOI] [PubMed] [Google Scholar]
- 23.Aliprantis AO, Ueki Y, Sulyanto R, Park A, Sigrist KS, Sharma SM, et al. NFATc1 in mice represses osteoprots-itogenesisng osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008;18(11):3775–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rucci N, Teti A. The, "love-hate" relationship between osteoclasts and bone matrix. Matrix Bio 2016;52–54:176–90. [DOI] [PubMed] [Google Scholar]
- 25.Blair HC, Teitelbaum SL, Ghiselli R, Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989;45(4920):855–7. [DOI] [PubMed] [Google Scholar]
- 26.Li YP, Chen W, Liang Y, Li E, Stashenko P. Atpi6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat Gene. 1999;23(4):447–51. [DOI] [PubMed] [Google Scholar]
- 27.Schlesinger PH, Blair HC, Teitelbaum SL, Edwards JC. Characterization of the osteocla-urideffled border chlorride channel and its role in bone resorption. J Biol Chem. 1997;272(30):18636–43. [DOI] [PubMed] [Google Scholar]
- 28.Kornak U, Kasper D, Bösl MR, Kaiser E, Schweizer M, Schulz A, et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104(2):205–15. [DOI] [PubMed] [Google Scholar]
- 29.Lange PF, Wartosch L, Jentsch TJ, Fuhrmann JC. ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature. 2006;440(7081):220–3. [DOI] [PubMed] [Google Scholar]
- 30.Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S, et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem. 1996;271(21):12511–6. [DOI] [PubMed] [Google Scholar]
- 31.Gowen M, Lazner F, Dodds R, Kapadia R, Feild J, Tavaria M, et al. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res. 1999;14(10):1654–63. [DOI] [PubMed] [Google Scholar]
- 32.Gelb BD, Shi GP, Chapman HA, Desnick RJ. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science. 1996;273(5279):1236–8. [DOI] [PubMed] [Google Scholar]
- 33.Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, et al. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci U S A. 1998;95(23):13453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nesbitt SA, Horton MA. Trafficking of matrix collagens through bone-resorbing osteoclasts. Science. 1997;276(5310):266–9. [DOI] [PubMed] [Google Scholar]
- 35.Salo J, Lehenkari P, Mulari M, Metsikko K, Vaananen HK. Removal of osteoclast bone resorption products by transcytosis. Science. 1997;276(5310):270–3. [DOI] [PubMed] [Google Scholar]
- 36.Zaidi M, Inzerillo AM, Moonga BS, Bevis PJ, Huang CL. Forty years of calcitonin–where are we now? A tribute to the work of Iain Macintyre. FRS. Bone. 2002;30(5):655–63. [DOI] [PubMed] [Google Scholar]
- 37.Fu Q, Jilka RL, Manolagas SC, O'Brien CA. Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J Biol Chem. 2002;277(50):48868–75. [DOI] [PubMed] [Google Scholar]
- 38.Suda T, Takahashi F, Takahashi N. Bone effects of vitamin D – Discrepancies between in vivo and in vitro studies. Arch Biochem Biophys. 2012;523(1):22–9. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto Y, Yoshizawa T, Fukuda T, Shirode-Fukuda Y, Yu T, Sekine K, et al. Vitamin D receptor in osteoblasts is a negative regulator of bone mass control. Endocrinology. 2013;154(3):1008–20. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130(5):811–23. [DOI] [PubMed] [Google Scholar]
- 41.Roelofs AJ, Stewart CA, Sun S, Blazewska KM, Kashemirov BA, McKenna CE, et al. Influence of bone affinity on the skeletal distribution of fluorescently labeled bisphosphonates in vivo. J Bone Miner Res. 2012;27(4):835–47. [DOI] [PubMed] [Google Scholar]
- 42.Frith JC, Monkkonen J, Blackburn GM, Russell RG, Rogers MJ. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5'-(beta, gamma-dichloro-methylene) triphosphate, by mammalian cells in vitro. J Bone Miner Res. 1997;12(9):1358–67. [DOI] [PubMed] [Google Scholar]
- 43.Duong LT, Crawford R, Scott K, Winkelmann CT, Wu G, Szczerba P, et al. Odanacatib, effects of 16-month treatment and discontinuation of therapy on bone mass, turnover and strength in the ovariectomized rabbit model of osteopenia. Bone. 2016;93:86–96. [DOI] [PubMed] [Google Scholar]
- 44.Rizzoli R, Benhamou CL, Halse J, Miller PD, Reid IR, Rodriguez Portales JA, et al. Continuous treatment with odanacatib for up to 8 years in postmenopausal women with low bone mineral density: a phase 2 study. Osteoporos Int. 2016;27(6):2099–107. [DOI] [PubMed] [Google Scholar]
- 45.Merck A. Merck &Co. drops osteoporosis drug odanacatib. Nat Rev Drug Discov. 2016;15(10):669. [DOI] [PubMed] [Google Scholar]
- 46.Huja SS, Fernandez SA, Hill KJ, Li Y. Remodeling dynamics in the alveolar process in skeletally mature dogs. Anat Rec A Discov Mol Cell Evol Biol. 2006;288(12):1243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hesse B, Langer M, Varga P, Pacureanu A, Dong P, Schrof S, et al. Alterations of mass density and 3D osteocyte lacunar properties in bisphosphonate-related osteonecrotic human jaw bone, a synchrotron microCT study. PLoS One. 2014;9(2):e88481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuura T, Tokutomi K, Sasaki M, Katafuchi M, Mizumachi E, Sato H. Distinct characteristics of mandibular bone collagen relative to long bone collagen: relevance to clinical dentistry. Biomed Res Int. 2014;2014:769414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moriya Y, Ito K, Murai S. Effects of experimental osteoporosis on alveolar bone loss in rats. J Oral Sci. 1998;40(4):171–5. [DOI] [PubMed] [Google Scholar]
- 50.Goldberg S, Grynpas MD, Glogauer M. Heterogeneity of osteoclast activity and bone turnover in different skeletal sites. Arch Oral Biol. 2016;71:134–43. [DOI] [PubMed] [Google Scholar]
- 51.Mavropoulos A, Rizzoli R, Ammann P. Different responsiveness of alveolar and tibial bone to bone loss stimuli. J Bone Miner Res. 2007;22(3):403–10. [DOI] [PubMed] [Google Scholar]
- 52.Coutel X, Delattre J, Marchandise P, Falgayrac G, Béhal H, Kerckhofs G, et al. Mandibular bone is protected against microarchitectural alterations and bone marrow adipose conversion in ovariectomized rats. Bone. 2019;127:343–52. [DOI] [PubMed] [Google Scholar]
- 53.Ammann P, Bourrin S, Bonjour JP, Meyer JM, Rizzoli R. Protein undernutrition-induced bone loss is associated with decreased IGF-I levels and estrogen deficiency. J Bone Miner Res. 2000;15(4):683–90. [DOI] [PubMed] [Google Scholar]
- 54.Bourrin S, Toromanoff A, Ammann P, Bonjour JP, Rizzoli R. Dietary protein deficiency induces osteoporosis in aged male rats. J Bone Miner Res. 2000;15(8):1555–63. [DOI] [PubMed] [Google Scholar]
- 55.Mavropoulos A, Odman A, Ammann P, Kiliaridis S. Rehabilitation of masticatory function improves the alveolar bone architecture of the mandible in adult rats. Bone. 2010;47(3):687–92. [DOI] [PubMed] [Google Scholar]
- 56.Patullo IM, Takayama L, Patullo RF, Jorgetti V, Pereira RM. Influence of ovariectomy and masticatory hypofunction on man-r dibular bone remodeling. Oral Dis. 2009;15(8):580–6. [DOI] [PubMed] [Google Scholar]
- 57.Mavropoulos A, Kiliaridis S, Bresin A, Ammann P. Effect of different ent masticatory functional and mechanical demands on the structural adaptation of the mandibular alveolar bone in young growing rats. Bone. 2004;35(1):191–7. [DOI] [PubMed] [Google Scholar]
- 58.Mavropoulos A, Kiliaridis S, Rizzoli R, Ammann P. Normal mas-y ticatory function partially protects the rat mandibular bone from estrogen-deficiency induced osteoporosis. J Biomech. 2014;47(11):2666–71. [DOI] [PubMed] [Google Scholar]
- 59.Fujita Y, Maki K. Association of feeding behavior with jaw bone metabolism and tongue pressure. Jpn Dent Sci Rev. 2018;54(4):174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;6(10):e25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283(9):5866–75. [DOI] [PubMed] [Google Scholar]
- 63.Inoue M, Ono T, Kameo Y, Sasaki F, Ono T, Adachi T, et al. Forceful mastication activates osteocytes and builds a stout jawbone. Sci Rep. 2019;9(1):4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coutel X, Olejnik C, Marchandise P, Delattre J, Béhal H, Kerckhofs G, et al. A novel microCT method for bone and marrow adipose tissue alignment identifies key differences between mandible and tibia in rats. Calcif Tissue Int. 2018;103(2):189–97. [DOI] [PubMed] [Google Scholar]
- 65.Osyczka AM, Damek-Poprawa M, Wojtowicz A, Akintoye SO. Age and skeletal sites affect BMP-2 responsiveness of human bone marrow stromal cells. Connect Tissue Res. 2009;50(4):270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Souza Faloni AP, Schoenmaker T, Azari A, Katchburian E, Cerri PS, de Vries TJ, et al. Jaw and long bone marrows have a different osteoclastogenic potential. Calcif Tissue Int. 2011;88(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Azari A, Schoenmaker T, de Souza Faloni AP, Everts V, de Vries TJ. Jaw and long bone marrow derived osteoclasts differ in shape and their response to bone and dentin. Biochem Biophys Res Commun. 2011;409:205–10. [DOI] [PubMed] [Google Scholar]
- 68.Ferez-Amodio S, Jansen DC, Schoenmaker T, Vogels IMC, Reinheckel T, Hayman AR, et al. Calvarial osteoclasts express a higher level of tartrate-resistant acid phosphatase than long bone osteoclasts and activation does not depend on cathepsin K or L activity. Calcif Tissue Int. 2006;79(4):245–54. [DOI] [PubMed] [Google Scholar]
- 69.Jacome-Galarza CE, Percin GI, Muller JT, Mass E, Lazarov T, Eitler J, et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature. 2019;568(7753):541–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yahara Y, Barrientos T, Tang YJ, Puviindran V, Nadesan P, Zhang H, et al. Erythromyeloid progenitors give rise to a population of osteoclasts that contribute to bone homeostasis and repair. Nat Cell Biol. 2020;22(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kong Y-Y, Yoshida H, Sarosi I, Tan H-L, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–23. [DOI] [PubMed] [Google Scholar]
- 72.Van Wesenbeeck L, Odgren PR, MacKay CA, D'Angelo M, Safadi FF, Popoff SN, et al. The osteopetrotic mutation toothless (tl) is a loss-of-function frameshift mutation in the rat Csf1 gene: Evidence of a crucial role for CSF-1 in osteoclastogenesis and endochondral ossification. Proc Natl Acad Sci U S A. 2002;99(22):14303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alfaqeeh S, Oralova V, Foxworthy M, Matalova E, Grigoriadis AE, Tucker AS. Root and eruption defects in c-fos mice are driven by loss of osteoclasts. J Dent Res. 2015;94(12):1724–31. [DOI] [PubMed] [Google Scholar]
- 74.Nakatsuka K, Nishizawa Y, Ralston SH. Phenotypic characterization of early onset Pageťs disease of bone caused by a 27-bp duplication in the TNFRSF11A gene. J Bone Miner Res. 2003;18(8):1381–5. [DOI] [PubMed] [Google Scholar]
- 75.Cho MI, Garant PR. Development and general structure of the periodontium. Periodontol. 2000;2000(24):9–27. [DOI] [PubMed] [Google Scholar]
- 76.Cahill DR. Histological changes in the bony crypt and gubernacular canal of erupting permanent premolars during deciduous premolar exfoliation in beagles. J Dent Res. 1974;53(4):786–91. [DOI] [PubMed] [Google Scholar]
- 77.Odgren PR, Kim N, MacKay CA, Mason-Savas A, Choi Y, Marks SC Jr. The role of RANKL (TRANCE/TNFSF11), a tumor necrosis factor family member, in skeletal development: effects of gene knockout and transgenic rescue. Connect Tissue Res. 2003;44(Suppl 1):264–71. [PubMed] [Google Scholar]
- 78.Wise GE, Lumpkin SJ, Huang H, Zhang Q. Osteoprotegerin and osteoclast differentiation factor in tooth eruption. J Dent Res. 2000;79(12):1937–42. [DOI] [PubMed] [Google Scholar]
- 79.Wise GE, Lin F, Zhao L. Transcription and translation of CSF-1 in the dental follicle. J Dent Res. 1995;74(9):1551–7. [DOI] [PubMed] [Google Scholar]
- 80.Liu D, Yao S, Pan F, Wise GE. Chronology and regulation of gene expression of RANKL in the rat dental follicle. Eur J Oral Sci. 2005;113(5):404–9. [DOI] [PubMed] [Google Scholar]
- 81.Begg SK, Radley JM, Pollard JW, Chisholm OT, Stanley ER, Bertoncello I. Delayed hematopoietic development in osteopetrotic (op/op) mice. J Exp Med. 1993;177(1):237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ida-Yonemochi H, Noda T, Shimokawa H, Saku T. Disturbed tooth eruption in osteopetrotic (op/op) mice: histopathogenesis of tooth malformation and odontomas. J Oral Pathol Med. 2002;31(6):361–73. [DOI] [PubMed] [Google Scholar]
- 83.Wiktor-Jedrzejczak W, Urbanowska E, Aukerman SL, Pollard JW, Stanley ER, Ralph P, et al. Correction by CSF-1 of defects in the osteopetrotic op/op mouse suggests local, developmental, and humoral requirements for this growth factor. Exp Hematol. 1991;19(10):1049–54. [PubMed] [Google Scholar]
- 84.Marks SC Jr, Cahill DR. Regional control by the dental follicle of alterations in alveolar bone metabolism during tooth eruption. J Oral Pathol. 1987;16(4):164–9. [DOI] [PubMed] [Google Scholar]
- 85.Isawa M, Karakawa A, Sakai N, Nishina S, Kuritani M, Chatani M, et al. Biological Effects of Anti-RANKL antibody and zoledronic acid on growth and tooth eruption in growing mice. Sci Rep. 2019;9(1):19895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vavidovitch Z Bone metabolism associated with tooth eruption and orthodontic tooth movement. J Periodontol. 1979;50(4 Spec No):22–9. [DOI] [PubMed] [Google Scholar]
- 87.Shiotani A, Shibasaki Y, Sasaki T. Localization of receptor activator of NFkappaB ligand, RANKL, in periodontal tissues during experimental movement of rat molars. J Electron Microsc (Tokyo). 2001;50(4):365–9. [DOI] [PubMed] [Google Scholar]
- 88.Kim T, Handa A, Iida J, Yoshida S. RANKL expression in rat periodontal ligament subjected to a continuous orthodontic force. Arch Oral Biol. 2007;52(3):244–50. [DOI] [PubMed] [Google Scholar]
- 89.Kanzaki H, Chiba M, Arai K, Takahashi I, Haruyama N, Nishimura M, et al. Local RANKL gene transfer to the periodontal tissue accelerates orthodontic tooth movement. Gene Ther. 2006;13(8):678–85. [DOI] [PubMed] [Google Scholar]
- 90.Kanzaki H, Chiba M, Takahashi I, Haruyama N, Nishimura M, Mitani H. Local OPG gene transfer to periodontal tissue inhibits orthodontic tooth movement. J Dent Res. 2004;83(12):920–5. [DOI] [PubMed] [Google Scholar]
- 91.Shoji-Matsunaga A, Ono T, Hayashi M, Takayanagi H, Moriyama K, Nakashima T. Osteocyte regulation of orthodontic force-mediated tooth movement via RANKL expression. Sci Rep. 2017;7(1):8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsumoto T, Iimura T, Ogura K, Moriyama K, Yamaguchi A. The role of osteocytes in bone resorption during orthodontic tooth movement. J Dent Res. 2013;92(4):340–5. [DOI] [PubMed] [Google Scholar]
- 93.Saito M, Saito S, Ngan PW, Shanfeld J, Davidovitch Z. Interleukin 1 beta and prostaglandin E are involved in the response of periodontal cells to mechanical stress in vivo and in vitro. Am J Orthod Dentofacial Orthop. 1991;99(3):226–40. [DOI] [PubMed] [Google Scholar]
- 94.Yamaguchi M, Shimizu N, Goseki T, Shibata Y, Takiguchi H, Iwasawa T, et al. Effect of different magnitudes of tension force on prostaglandin E2 production by human periodontal ligament cells. Arch Oral Biol. 1994;39(10):877–84. [DOI] [PubMed] [Google Scholar]
- 95.Yamasaki K, Shibata Y, Imai S, Tani Y, Shibasaki Y, Fukuhara T. Clinical application of prostaglandin E1 (PGE1) upon orthodontic tooth movement. Am J Orthod. 1984;85(6):508–18. [DOI] [PubMed] [Google Scholar]
- 96.Takano-Yamamoto T, Kawakami M, Yamashiro T. Effect of age on the rate of tooth movement in combination with local use of 1,25(OH)2D3 and mechanical force in the rat. J Dent Res. 1992;71(8):1487–92. [DOI] [PubMed] [Google Scholar]
- 97.Soma S, Matsumoto S, Higuchi Y, Takano-Yamamoto T, Yamashita K, Kurisu K, et al. Local and chronic application of PTH accelerates tooth movement in rats. J Dent Res. 2000;79(9):1717–24. [DOI] [PubMed] [Google Scholar]
- 98.arcia RI, Henshaw MM, Krall EA. Relationship between periodontal disease and systemic health. Periodontology. 2000;2001(25):21–36. [DOI] [PubMed] [Google Scholar]
- 99.Wara-aswapati N, Surarit R, Chayasadom A, Boch JA, Pitiphat W. RANKL upregulation associated with periodontitis and Porphyromonas gingivalis. J Periodontol. 2007;78(6):1062–9. [DOI] [PubMed] [Google Scholar]
- 100.Jin Q, Cirelli JA, Park CH, Sugai JV, Taba M, Kostenuik PJ, et al. RANKL inhibition through osteoprotegerin blocks bone loss in expperimental periodontitis. J Periodontol. 2007;78(7):1300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diaz-Zuniga J, Monasterio G, Alvarez C, Melgar-Rodriguez S, Benitez A, Ciuchi P, et al. Variability of the dendritic cell response triggered by different serotypes of Aggregatibacter actinomycetemcomitans or Porphyromonas gingivalis is toll-like receptor 2 (TLR2) or TLR4 dependent. J Periodontol. 2015;86(1):108–19. [DOI] [PubMed] [Google Scholar]
- 102.Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169(3):987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999;25(3):255–9. [DOI] [PubMed] [Google Scholar]
- 104.Kikuchi T, Matsuguchi T, Tsuboi N, Mitani A, Tanaka S, Matsuoka M, et al. Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoblasts via Toll-like receptors. J Immunol. 2001;166(5):3574–9. [DOI] [PubMed] [Google Scholar]
- 105.Tang Y, Sun F, Li X, Zhou Y, Yin S, Zhou X. Porphyromonas endodontalis lipopolysaccharides induce RANKL by mouse osteoblast in a way different from that of Escherichia coli lipopolysaccharide. J Endod. 2011;37(12):1653–8. [DOI] [PubMed] [Google Scholar]
- 106.Okahashi N, Inaba H, Nakagawa I, Yamamura T, Kuboniwa M, Nakayama K, et al. Porphyromonas gingivalis induces receptor activator of NF-kappaB ligand expression in osteoblasts through the activator protein 1 pathway. Infect Immun. 2004;72(3):1706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wada N, Maeda H, Yoshimine Y, Akamine A. Lipopolysaccharide stimulates expression of osteoprotegerin and receptor activator of NF-kappa B ligand in periodontal ligament fibroblasts through the induction of interleukin-1 beta and tumor necrosis factor-alpha. Bone. 2004;35(3):629–35. [DOI] [PubMed] [Google Scholar]
- 108.Nagasawa T, Kobayashi H, Kiji M, Aramaki M, Mahanonda R, Kojima T, et al. LPS-stimulated human gingival fibroblasts inhibit the differentiation of monocytes into osteoclasts through the production of osteoprotegerin. Clin Exp Immunol. 2002;130(2):338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu J, Wang S, Zhang P, Said-Al-Naief N, Michalek SM, Feng X. Molecular mechanism of the bifunctional role of lipopolysaccharide in osteoclastogenesis. J Biol Chem. 2009;284(18):12512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zou W, Bar-Shavit Z. Dual modulation of osteoclast differentiation by lipopolysaccharide. J Bone Miner Res. 2002;17(7):1211–8. [DOI] [PubMed] [Google Scholar]
- 111.Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160(1):403–9. [PubMed] [Google Scholar]
- 112.Graves DT, Delima AJ, Assuma R, Amar S, Oates T, Cochran D. Interleukin-1 and tumor necrosis factor antagonists inhibit the progression of inflammatory cell infiltration toward alveolar bone in experimental periodontitis. J Periodontol. 1998;69(12):1419–25. [DOI] [PubMed] [Google Scholar]
- 113.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–56. [DOI] [PubMed] [Google Scholar]
- 114.Filleul O, Crompot E, Saussez S. Bisphosphonate-induced osteonecrosis of the jaw: a review of 2,400 patient cases. J Cancer Res Clin Oncol. 2010;136(8):1117–24. [DOI] [PubMed] [Google Scholar]
- 115.Dodson TB. The frequency of medication-related osteonecrosis of the jaw and its associated risk factors. Oral Maxillofac Surg Clin North Am. 2015;27(4):509–16. [DOI] [PubMed] [Google Scholar]
- 116.Soundia A, Hadaya D, Esfandi N, Gkouveris I, Christensen R, Dry SM, et al. Zoledronate impairs socket healing after extraction of teeth with experimental periodontitis. J Dent Res. 2018;97(3):312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vermeer JAF, Jansen IDC, Marthi M, Coxon FP, McKenna CE, Sun S, et al. Jaw bone marrow-derived osteoclast precursors internalize more bisphosphonate than long-bone marrow precursors. Bone. 2013;57(1):242–51. [DOI] [PubMed] [Google Scholar]
- 118.Su J, Feng M, Han W, Zhao H. The effects of bisphosphonate on the remodeling of different irregular bones in mice. J Oral Pathol Med. 2015;44(8):638–48. [DOI] [PubMed] [Google Scholar]
- 119.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hayami T, Pickarski M, Wesolowski GA, Mclane J, Bone A, Destefano J, et al. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50(4):1193–206. [DOI] [PubMed] [Google Scholar]
- 121.Zhu S, Chen K, Lan Y, Zhang N, Jiang R, Hu J. Alendronate protects against articular cartilage erosion by inhibiting subchondral bone loss in ovariectomized rats. Bone. 2013;53(2):340–9. [DOI] [PubMed] [Google Scholar]
- 122.Kaneyama K, Segami N, Sun W, Sato J, Fujimura K. Analysis of tumor necrosis factor-alpha, interleukin-6, interleukin-1beta, soluble tumor necrosis factor receptors I and II, interleukin-6 soluble receptor, interleukin-1 soluble receptor type II, interleukin-1 receptor antagonist, and protein in the synovial fluid of patients with temporomandibular joint disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99(3):276–84. [DOI] [PubMed] [Google Scholar]
- 123.Kanyama M, Kuboki T, Kojima S, Fujisawa T, Hattori T, Takigawa M, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids of patients with temporomandibular joint osteoarthritis. J Orofac Pain. 2000;14(1):20–30. [PubMed] [Google Scholar]
- 124.Nakagawa M, Kaneda T, Arakawa T, Morita S, Sato T, Yomada T, et al. Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts. FEBS Lett. 2000;473(2):161–4. [DOI] [PubMed] [Google Scholar]
- 125.Sato J, Segami N, Yoshitake Y, Nishikawa K. Correlations of the expression of fibroblast growth factor-2, vascular endothelial growth factor, and their receptors with angiogenesis in synovial tissues from patients with internal derangement of the temporomandibular joint. J Dent Res. 2003;82(4):272–7. [DOI] [PubMed] [Google Scholar]
- 126.Leonardi R, Lo Muzio L, Bernasconi G, Caltabiano C, Piacentini C, Caltabiano M. Expression of vascular endothelial growth factor in human dysfunctional temporomandibular joint discs. Arch Oral Biol. 2003;48(3):185–92. [DOI] [PubMed] [Google Scholar]
- 127.Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87(4):296–307. [DOI] [PubMed] [Google Scholar]
- 128.Ikeda Y, Yonemitsu I, Takei M, Shibata S, Ono T. Mechanical loading leads to osteoarthritis-like changes in the hypofunctional temporomandibular joint in rats. Arch Oral Biol. 2014;59(12):1368–76. [DOI] [PubMed] [Google Scholar]
- 129.Kartha S, Zhou T, Granquist EJ, Winkelstein BA. Development of a rat model of mechanically induced tunable pain and associated temporomandibular joint responses. J Oral Maxillofac Surg. 2016;74:54.e1–54.e10. [DOI] [PubMed] [Google Scholar]
- 130.Liu Y-D, Liao L-F, Zhang H-Y, Lu L, Jiao K, Zhang M, et al. Reducing dietary loading decreases mouse temporomandibular joint degradation induced by anterior crossbite prosthesis. Osteoarthritis Cartilage. 2014;22(2):302–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Papadaki ME, Lietman SA, Levine MA, Olsen BR, Kaban LB, Reichenberger EJ. Cherubism: best clinical practice. Orphanet J Rare Dis. 2012;7(Suppl 1):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ueki Y, Tiziani V, Santanna C, Fukai N, Maulik C, Garfinkle J, et al. Mutations in the gene encoding c-Abl-binding protein SH3BP2 cause cherubism. Nat Genet. 2001;28(2):125–6. [DOI] [PubMed] [Google Scholar]
- 133.de la Fuente MA, Kumar L, Lu B, Geha RS. 3BP2 deficiency impairs the response of B cells, but not T cells, to antigen receptor ligation. Mol Cell Biol. 2006;26(14):5214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ueki Y, Lin C-Y, Senoo M, Ebihara T, Agata N, Onji M, et al. Increased myeloid cell responses to M-CSF and RANKL cause bone loss and inflammation in SH3BP2 "cherubism" mice. Cell. 2007;128(1):71–83. [DOI] [PubMed] [Google Scholar]
- 135.Valentine JC, Nelson BL. Central giant cell lesion. Head Neck Pathol. 2011;5(4):385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Idowu BD, Thomas G, Frow R, Diss TC, Flanagan AM. Mutations in SH3BP2, the cherubism gene, were not detected in central or peripheral giant cell tumours of the jaw. Br J Oral Maxillofac Surg. 2008;46(3):229–30. [DOI] [PubMed] [Google Scholar]
- 137.Borges HO, Machado RA, Vidor MM, Beltrao RG, Heitz C, Filho MS. Calcitonin: a non-invasive giant cells therapy. Int J Pediatr Otorhinolaryngol. 2008;72(7):959–63. [DOI] [PubMed] [Google Scholar]
- 138.Southgate J, Sarma U, Townend JV, Barron J, Flanagan AM. Study of the cell biology and biochemistry of cherubism. J Clin Pathol. 1998;51(11):831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lannon DA, Earley MJ. Cherubism and its charlatans. Br J Plast Surg. 2001;54(8):708–11. [DOI] [PubMed] [Google Scholar]
- 140.da Silva Sampieri MB, Yaedu RY, Santos PS, Goncales ES, Santa'ana E, Consolaro A, et al. Central giant cell granuloma: treatment with calcitonin, triamcinolone acetonide, and a cystic finding 3 years and 6 months after the primary treatment. Oral Maxillofac Surg. 2013;17(3):229–34. [DOI] [PubMed] [Google Scholar]
- 141.Pagnini I, Simonini G, Mortilla M, Giani T, Pascoli L, Cimaz R. Ineffectiveness of tumor necrosis factor-alpha inhibition in association with bisphosphonates for the treatment of cherubism. Clin Exp Rheumatol. 2011;29(1):147. [PubMed] [Google Scholar]
- 142.Hero M, Suomalainen A, Hagström J, Stoor P, Kontio R, Alapulli H, et al. Anti-tumor necrosis factor treatment in cherubism–clinical, radiological and histological findings in two children. Bone. 2013;52(1):347–53. [DOI] [PubMed] [Google Scholar]
- 143.Bredell M, Rordorf T, Kroiss S, Rucker M, Zweifel DF, Rostetter C. Denosumab as a treatment alternative for central giant cell granuloma: a long-term retrospective cohort study. J Oral Maxillofac Surg. 2018;76(4):775–84. [DOI] [PubMed] [Google Scholar]
- 144.Upfill-Brown A, Bukata S, Bernthal NM, Felsenfeld AL, Nelson SD, Singh A, et al. Use of denosumab in children with osteoclast bone dysplasias: report of three cases. JBMR Plus. 2019;3(10):e10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Levaot N, Simoncic PD, Dimitriou ID, Scotter A, La Rose J, Ng AHM, et al. 3BP2-deficient mice are osteoporotic with impaired osteoblast and osteoclast functions. J Clin Invest. 2011;121(8):3244–57. [DOI] [PMC free article] [PubMed] [Google Scholar]