Abstract
Thyroid hormones exert pleiotropic, essential actions in mammalian, including human, development. These actions depend on provision of thyroid hormones in the circulation but also to a remarkable extent on deiodinase enzymes in target tissues that amplify or deplete the local concentration of the primary active form of the hormone T3 (3,5,3′-triiodothyronine), the high affinity ligand for thyroid hormone receptors. Genetic analyses in mice have revealed key roles for activating (DIO2) and inactivating (DIO3) deiodinases in cell differentiation fates and tissue maturation, ultimately promoting neonatal viability, growth, fertility, brain development, and behavior, as well as metabolic, endocrine, and sensory functions. An emerging paradigm is how the opposing activities of DIO2 and DIO3 are coordinated, providing a dynamic switch that controls the developmental timing of a tissue response, often during neonatal and maturational transitions. A second paradigm is how cell to cell communication within a tissue determines the response to T3. Deiodinases in specific cell types, often strategically located near to blood vessels that convey thyroid hormones into the tissue, can regulate neighboring cell types, suggesting a paracrine-like layer of control of T3 action. We discuss deiodinases as switches for developmental transitions and their potential to influence tissue dysfunction in human thyroid disorders.
Keywords: deiodinase, thyroid hormone, development, neuroendocrine function, sensory system, central nervous system
Thyroid hormones regulate multiple functions in growth and development. Adequate provision of thyroid hormones in the circulation is a prerequisite for these actions as has long been known from the serious impairments that may arise in congenital thyroid disorders, including both hypothyroid and hyperthyroid conditions (1, 2). However, it is increasingly evident that deiodinase enzymes within a given target tissue or cell type regulate the local content of 3,5,3′-triiodothyronine (T3), the primary biologically active form of thyroid hormone, and thereby confer tissue-intrinsic control over the responses. This intrinsic control is dependent on the complement of activating and inactivating deiodinases present in the tissue.
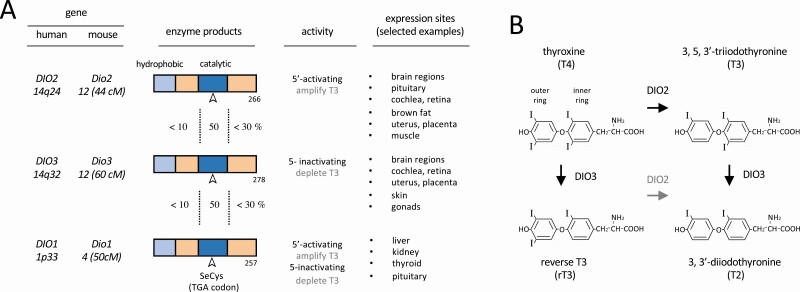
The type 1, 2, and 3 deiodinases (DIO1, DIO2, and DIO3, respectively) are selenoenzymes with related features including an N-terminal transmembrane domain and a catalytic domain incorporating the rare amino acid selenocysteine that is essential for enzymatic function (3, 4) (Fig. 1A). The deiodinases remove an iodine residue from the outer or inner ring of thyroid hormones (Fig. 1B). In development, both the activating DIO2 and the inactivating DIO3 enzymes are critical. DIO2 generates T3 by outer ring deiodination of the more abundant precursor thyroxine (T4), whereas DIO3 depletes T3 sources by inner ring deiodination of both T3 and T4. Owing to their opposing activities and distinct expression patterns, DIO2 and DIO3 are uniquely positioned to control development. Genetic mouse models have been instrumental in demonstrating the biological functions of deiodinases, which add a prereceptor layer of complexity to the control of T3 action (5).
Figure 1.
Main characteristics of deiodinases (DIOs). A, Protein domains of deiodinases with conservation between enzyme domains indicated, type of activity, and selected main sites of expression. B, Structure of thyroid hormones and main biochemical conversions catalyzed by the type 2 deiodinase (DIO2) and type 3 deiodinase (DIO3) enzymes (bold arrows).
Here, we review the developmental roles of deiodinases, based mainly on rodent studies and consider implications for human disorders. We highlight 2 emerging mechanistic concepts: (i) how the coordinated expression of DIO3 and DIO2 provides a dynamic developmental switch, as suggested by early observations 40 years ago in the rat by Kaplan and Yaskoski (6); and (ii) how deiodinases in specific cell types regulate responses in other nearby cell types, indicating paracrine-like control of cellular responses to T3 within a tissue. The insights gained into the developmental roles of deiodinases suggest the potential utility of these enzymes as future therapeutic targets to counter tissue dysfunction in disease.
Deiodinase Switches in Neonatal and Maturational Transitions
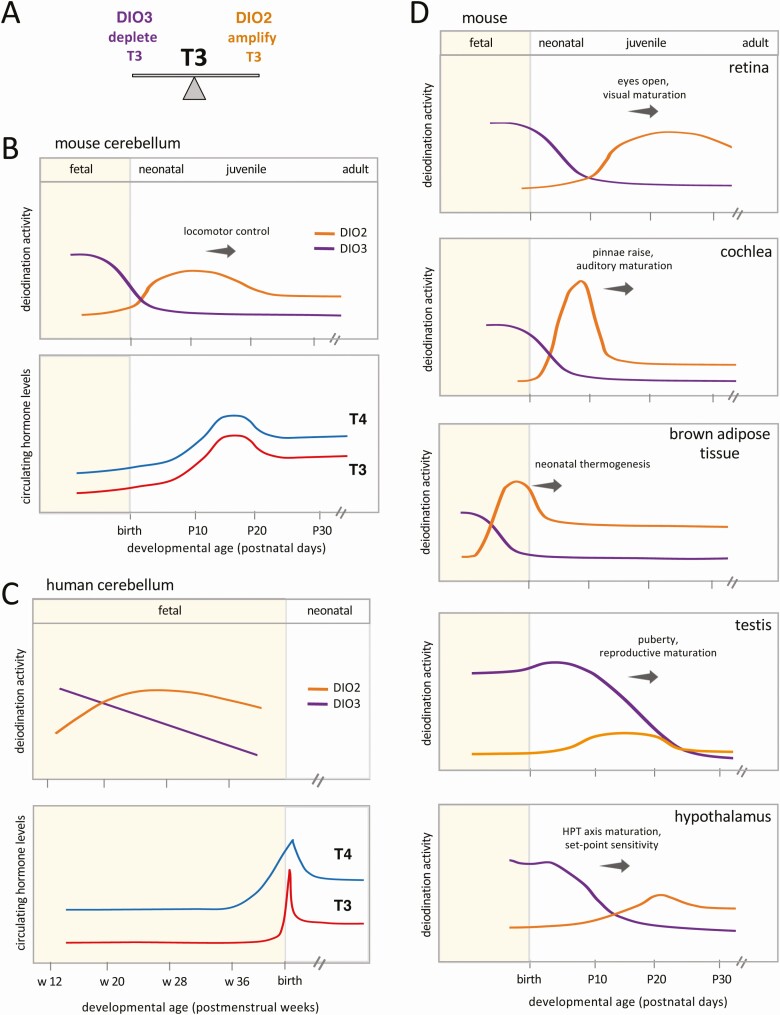
Coordinated, often reciprocal, profiles of DIO3 and DIO2 activities underlie the development of many tissues, providing a dynamic, double control over the T3 content within tissues (Fig. 2A). DIO3 activity generally peaks at fetal and neonatal stages and is also present at a high level in the placenta and uterus during pregnancy (7-9), suggesting that it constrains T3 action at sensitive early stages. DIO2 activity often rises later, as DIO3 activity declines, suggesting a means by which T3 content can be enhanced as tissues mature. The composite profiles of both enzymes indicate a dynamic switch from constraint to amplification of the T3 signal during developmental transitions in many organs.
Figure 2.
Type 3 deiodinase (DIO3) to type 2 deiodinase (DIO2) switches in developmental transitions. A, Opposing activities of DIO3 and DIO2 provide dynamically balanced control of the 3,5,3′-triiodothyronine (T3) signal in a tissue. B and C, DIO3 to DIO2 switches in cerebellar development in mice and humans, and relationship to systemic thyroxine (T4) and T3 levels. (Cerebellar differentiation and the increase in circulating T4 and T3 occur earlier in human than mouse development.) The mouse profile parallels that originally shown for rat (6). The human profile is based on fetal data reported by Kester et al (19). Circulating hormone profiles are adapted from reported data for mice (11) and humans (124, 125). D, DIO3-DIO2 switches are customized in each tissue and often encompass terminal differentiation events and onset of organ function. All deiodinase activity profiles in panels B, C, and D are approximate and based on publications cited in the text.
DIO3 to DIO2 switches often encompass the maturation of tissue function during transitions from fetal to neonatal life or during later transitions that allow autonomous juvenile and adult life. In the brain, a switch accompanies differentiation and synaptogenesis as circuits are established, for example, in the cerebellum as locomotor control matures (Fig. 2B) (6, 10). In sensory systems, switches in the cochlea and retina encompass the onset of auditory and visual function, respectively (11-13) (Fig. 2D). Switches underlie gonadal and reproductive maturation (14, 15) as well as the neonatal activation of brown adipose tissue and facultative thermogenesis (16, 17). A switch in the hypothalamus accompanies the maturation of the hypothalamic-pituitary control of thyroid gland function (6, 18).
Although human data are limited, evidence supports conserved trends with DIO3 preceding DIO2 activity in fetal brain regions (19) such as the cerebellum (Fig. 2C). Transcriptomic data from human tissues across developmental stages also indicate a progressive switch from DIO3 to DIO2 in the cerebrum, cerebellum, testis, kidney, and heart (20). Another way to assess the roles of deiodinases in human development is to study the differentiation of organoids derived from induced pluripotent stem cells in culture. For example, human retinal organoids display a DIO3 to DIO2 switch at the RNA level (21) resembling the enzyme profiles in rodent retina (12, 22) (see Fig. 2D). In mice, many systems mature at the postnatal or juvenile stages, whereas in humans equivalent events often occur at the fetal or perinatal stages. Thus, deiodinase switches may be expected earlier, in utero, and during the fetal to neonatal transition in human development.
Deiodinase switches are customized in each tissue and can precede or lag the systemic rise in circulating T4 and T3 levels, thereby allowing partly autonomous control over development (see Fig. 2). For example, DIO2 activity rises in the rodent retina in concert with the rise of the systemic hormone level. However, in the cochlea or brown adipose tissue (see Fig. 2D), DIO2 rises before the systemic hormone rises, suggesting an earlier action uncoupled to an extent from circulating hormone levels. Conversely, in the testis, a sustained DIO3 profile suggests prolonged constraint of responses despite the systemic rise of T4 and T3.
At adult stages, the activities of DIO3 and DIO2 display lower, more restricted profiles and are involved in responses to endocrine and environmental challenges as well as responses to injury, infection, tumorigenesis, or other disease states as reviewed elsewhere (23-25).
Developmental Functions of Deiodinases
We illustrate here examples of the versatile developmental roles of the DIO2 and DIO3 enzymes. The DIO1 enzyme lacks known developmental functions although subtle roles cannot be excluded. DIO1 has dual activating and inactivating activities and influences the disposal of iodothyronines and iodine economy but lacks an obvious role in the maintenance of systemic euthyroidism (26, 27). DIO1 deiodinates the outer ring of T4 to generate T3 but also deiodinates the inner ring, especially of sulfated and glucuronidated iodothyronine derivatives, and its preferred substrate is reverse T3 (rT3), a largely inactive metabolite.
The DIO2 and DIO3 enzymes play an important role in many tissues that are not reviewed in detail here, including bone (28, 29), muscle (30, 31), skin (14, 32), heart (33), metabolic systems (34, 35), and innate immune cells (23) (Table 1). Although not the focus of this article, many of these actions may involve a developmental component.
Table 1.
Phenotypes of global and tissue-specific deiodinase deficiencies in mice
| Gene | Phenotypes | References |
|---|---|---|
| Dio2 | HPT axis | |
| Pituitary resistance to thyroid hormone (elevated TSH and T4) | (36-38) | |
| CNS/behavior | ||
| Reduced brain T3 content and altered gene expression | (50, 51) | |
| Increased anxiety-like behavior | (52, 53) | |
| Sensory function | ||
| Deafness | (64) | |
| Other phenotypes | ||
| Impaired BAT thermogenesis | (17, 126) | |
| Increased bone fracture susceptibility | (29) | |
| Skeletal muscle regeneration defect | (127) | |
| Increased susceptibility to obesity | (128) | |
| Altered programming of liver lipid metabolism | (114, 115) | |
| Defective macrophage function | (129) | |
| Dio3 | General phenotypes | |
| perinatal lethality (> 30%, genetic background dependent) | (18) | |
| growth retardation (20%-30% body wt reduction) | (18) | |
| Impaired fertility in both sexes | (15, 18) | |
| HPT axis | ||
| Neonatal thyrotoxicosis | (18) | |
| Adult central hypothyroidism | (18, 130) | |
| (Small thyroid gland, low T4 and T3, unchanged TSH) | (10, 130) | |
| CNS/behavior | ||
| Developmental and adult brain hyperthyroidism | (54, 55) | |
| Cerebellar maldevelopment and motor deficits | (10) | |
| Hyperactivity | (57) | |
| Reduced anxiety- and depression-like behaviors | (57) | |
| Increased aggression and poor maternal behavior | (58) | |
| Sensory function | ||
| Deafness | (63) | |
| Color blindness (achromatopsia) | (13) | |
| Olfactory impairment | (58) | |
| Other phenotypes | ||
| Reduced adiposity and altered energy balance | (59) | |
| Male hypogonadism and reduced testis size | (15) | |
| Muscle regeneration/stem cell defects | (131) | |
| Altered pancreatic and insulin homeostasis | (35) | |
| Keratinocyte developmental defects | (32) | |
| Susceptibility to cardiomyopathy | (33) | |
| Impaired bacterial clearance, neutrophil function defect | (132, 133) | |
| Dio2/Dio3 | Exacerbated deafness | (22) |
| Partial or full rescue of: | ||
| Testis size | (15) | |
| Retinal cone survival | (22) | |
| HPT axis parameters, brain thyroid status | (22, 41) | |
| Dio1 | Elevated rT3, decreased iodide urinary excretion | (27) |
| Dio1/Dio2 | Exacerbated impairment of rT3 clearance Abnormal brain gene expression |
(39, 134) |
Abbreviations: BAT, brown adipose tissue; CNS, central nervous system; Dio1, type 1 deiodinase gene; Dio2, type 2 deiodinase gene; Dio3, type 3 deiodinase gene; HPT, hypothalamic-pituitary-thyroid; rT3, reverse 3,3,5′-triiodothyronine; T3, 3,3,5′-triiodothyronine; T4, thyroxine; TSH, thyrotropin.
Hypothalamic-Pituitary-Thyroid Axis
During development, the thyroid gland, under the control of the hypothalamus and pituitary, secretes calibrated, progressively increasing amounts of T4 and T3 into the circulation, as appropriate for stimulating the maturation of many organs. Also, during development, the hypothalamic-pituitary-thyroid (HPT) axis acquires its own set-point sensitivity for negative feedback control by systemic levels of T4 and T3. The HPT axis may be viewed as a master system under control by deiodination, since other tissues are influenced by the resultant output of circulating T4 and T3.
DIO2 and DIO3 are critical for the development of the function and set point of the HPT axis. Normally, the thyrotropin (TSH) released by the pituitary stimulates the thyroid gland to produce appropriate amounts of T4 and T3. T4 and T3 in turn control their own production via negative feedback that suppresses TSH production in the pituitary. In Dio2–/– mice, the HPT axis fails to mature correctly and displays central resistance to feedback control. Dio2–/– mice display elevated levels of both T4 and TSH, suggesting insensitivity of the pituitary to T4-mediated suppression (36). The elevation of T4 as early as postnatal day 8 is largely recapitulated in mice with pituitary-specific Dio2 deficiency (37), which also show a blunted rise of TSH in response to hypothyroidism (38). Thus, DIO2-mediated deiodination of T4 in the pituitary and possibly hypothalamus contributes to establishing feedback control of the axis. The elevation of T4 and TSH in Dio2–/– mice is slightly exacerbated in Dio2;Dio1 double-knockout mice (39), suggesting that DIO1 cooperates with DIO2 in the HPT axis. Conversely, the elevation of rT3 in Dio1–/– mice is exacerbated in Dio2;Dio1 double knockouts, indicating that DIO2 contributes to the clearance of rT3 in Dio1–/– mice (39).
Dio3 –/– mice exhibit complex alterations in the HPT axis, indicating major functions for DIO3 consistent with its profile in the hypothalamus (see Fig. 2D) (6, 18). In Dio3–/– mice, the thyroid gland is small, the HPT axis does not respond to hypothyroidism, the pituitary response to thyrotropin-releasing hormone (TRH) is blunted, and the thyroid gland response to TSH is impaired (40). In neonatal Dio3–/– mice, serum T3 is elevated and T4 is decreased but this pattern shifts progressively to a pattern of central hypothyroidism in adults, with moderately reduced T3 and T4 and minimally altered TSH (18).
The proposal that coordination of the opposing actions of DIO3 and DIO2 provides balanced control is supported by deletion of Dio2 from Dio3–/– mice, which substantially restores thyroid gland size and serum levels of T4, T3, and TSH at postnatal and adult ages (22). Conversely, the elevated T4 and TSH levels in Dio2–/– mice or double Dio1;Dio2 knockouts, indicative of central resistance to T4, are partly ameliorated in double Dio2;Dio3 knockouts (22) or triple Dio1;Dio2;Dio3 knockouts (41). Future studies may elucidate the critical but complex roles of DIO3 and DIO2 in the HPT axis in the context of specialized cell types both in the hypothalamus and pituitary.
Brain
Thyroid hormones modulate a range of functions in brain development (42-45), many of which may be modified by DIO3 and DIO2 within the brain. In rodents, DIO3 activity declines and DIO2 activity rises in brain regions including the cerebrum and cerebellum during the second postnatal week (6, 14) (see Fig. 2B). This deiodinase switch allows brain tissues to amplify T3 levels during a period that encompasses events such as oligodendrocyte differentiation, myelination, and synaptogenesis and correlates with heightened sensitivity of T3-responsive genes (46, 47).
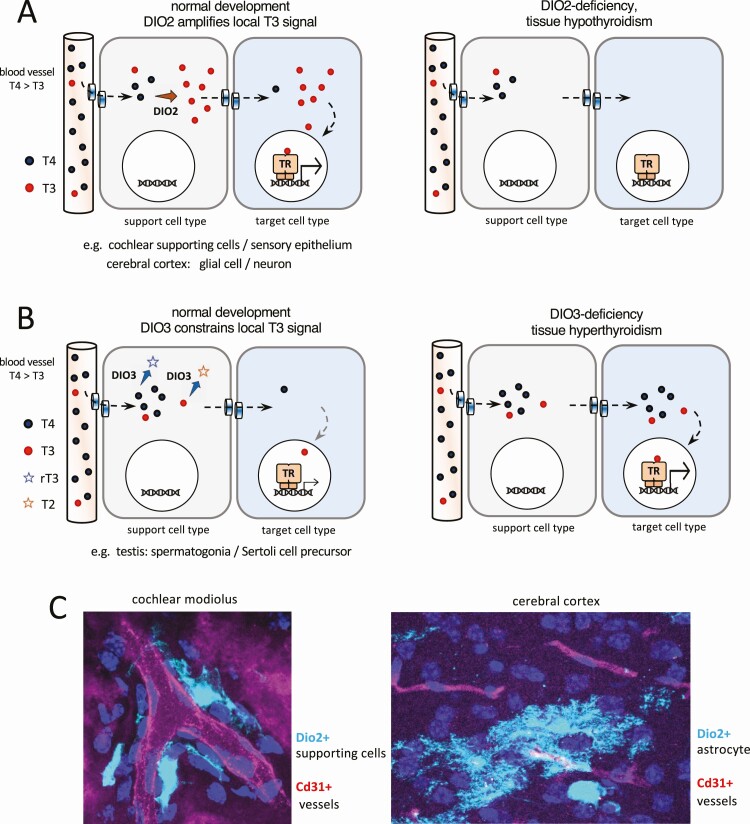
Although neurons are among the main cell types that respond to T3, a remarkable finding was that DIO2 is located predominantly in glial cells in regions such as the cerebral cortex (48, 49), supporting a model whereby separation of T3-producing and T3-responding cell types provides a paracrine-like form of control (Fig. 3A and 3C). Other forms of cellular segregation, for example, in the cochlea and testis, support paracrine-like control of T3 transfer between cell types within tissues (see later sections).
Figure 3.
Conceptual diagrams of paracrine-like control of 3,5,3′-triiodothyronine (T3) action by type 2 deiodinase (DIO2) and type 3 deiodinase (DIO3) within a tissue. A, DIO2+ cell types close to capillaries take up thyroxine (T4) from the circulation, convert T4 to T3, then transfer T3 to neighboring, T3-responsive cell types. Examples are found in the cochlea and cerebrum. B, DIO3+ support cell types deplete T4 and T3, thereby constraining the T3 signal available for other response cell types. An example is found in the testis. Transplasma membrane transport of T4 and T3 by specific transporter proteins is an integral part of the models shown in panels A and B. C, Images of DIO2+ support cells (pale blue) in proximity to blood vessels (red): specifically DIO2+ supporting cells in the cochlea and DIO2+ astrocytes in the cerebral cortex (images are the authors’ own work using a Dio2cre knockin; manuscript in preparation). TR, thyroid hormone receptor in the nucleus of target cells.
The proposal that the requisite T3 content in brain tissues (a euthyroid state) is maintained in part by DIO2 predicts that DIO2 deficiency would cause pronounced neurological deficits comparable to those in hypothyroidism. However, although 2-week-old Dio2–/– mice exhibit low brain T3 content, some neurological functions, as assessed by motor and behavioral tests, are only modestly affected compared to the impact of severe hypothyroidism (50). In addition, changes in T3-dependent gene expression in the cerebrum are milder in Dio2–/– mice than in hypothyroid mice (50, 51), suggesting that compensatory mechanisms partly protect T3-dependent responses in Dio2–/– mice. However, Dio2–/– mice do exhibit increased anxiety and fear memory (52). Astrocyte-specific Dio2 deficiency (53) also results in depression-like behavior and hypothyroid-like changes in gene expression, including neuronal gene expression (53), supporting the proposal that glial-neuronal transfer modifies T3 responses in the brain.
In Dio3–/– mice, the absence of DIO3 together with a transient neonatal excess of circulating T3 results in a thyrotoxic state in the brain that persists into adulthood (54). Abnormalities include increased thickness of the neocortex (55) and premature differentiation of the cerebellum with accelerated regression of the external germinal layer and locomotor defects (10). Dio3–/– mice exhibit increases in T3-dependent expression both of glial and neuronal genes (54-56), in accordance with a complex cellular role of DIO3 and possible control over glial-neuronal transfer of T3.
Dio3 –/– mice exhibit other neuroendocrine and behavioral phenotypes. Oxytocin and arginine-vasopressin functions are altered in neonatal and adult Dio3–/– mice and are associated in both sexes with aggressive behavior, hyperactivity, reduced anxiety-like and depression-like behaviors, and lack of maternal behavior in females (57, 58). These phenotypes are likely developmental in origin, as adult-onset DIO3 deficiency recapitulates only a mild form of hyperactivity (56). Concerning energy balance, Dio3–/– mice are abnormally lean and have alterations in serum leptin and reduced Pomc and increased Agrp expression in the hypothalamus (59), both factors being part of the leptin-melanocortin system that regulates food intake and energy expenditure. Dio3–/– mice also show lengthening of the nocturnal phase of physical activity, suggesting that DIO3 influences hypothalamic and circadian regulation of energy balance and activity (59).
The proposed control by a balance between DIO3 and DIO2 activities is supported by measurements of T3 content in brain tissues. Dio3–/– and Dio2–/– mice exhibit increased and decreased T3 content, respectively, whereas this content is normalized in mice with triple deiodinase deficiency (41).
Cochlea
Thyroid hormones promote auditory development and have a major site of action in the cochlea (60-62). Terminal differentiation of the cochlea depends not only on systemic thyroid hormone levels, but also on the local control of T3 content by deiodinases. In the cochlea, early DIO3 activity is followed by a dramatic rise of DIO2 expression preceding the onset of hearing (see Fig. 2D) (11), and deletion of either enzyme results in deafness (63, 64). Dio3–/– and Dio2–/– mice exhibit opposite cochlear phenotypes of accelerated and delayed differentiation, respectively, illustrating how DIO3 and DIO2 activities safeguard the appropriate time course of development.
Dio2 –/– mice display retarded remodeling of the sensory epithelium, which includes the mechanosensory hair cells, and deformity of the tectorial membrane (64), similar to defects in hypothyroid (65-68) or Thrb (thyroid hormone receptor β)-deficient mice (60, 69). In contrast, Dio3–/– mice exhibit premature remodeling of the sensory epithelium (70) in accord with a protective role for DIO3 at early stages. Excessive T3 given during a transient neonatal phase in wild-type mice also accelerates cochlear differentiation and causes permanent deafness (71).
The vascular network that carries circulating T4 and T3 into the cochlea enters through the central modiolus, then extends laterally through the spiral ligament and stria vascularis. This network is compartmentalized from the sensory tissues in the cochlear duct (72) and implies the need for a cellular route of hormonal transfer from the circulation to internal target tissues. A proposed route involves uptake of circulating T4 from capillaries, conversion of T4 into T3 by DIO2 in intermediary support cell types, then transfer of T3 to internal target cell types (73). The Dio2 gene is expressed in vascularized support tissues in the modiolus and lateral wall rather than internal response tissues (11) and Dio2-positive supporting cells have been identified that extend projections to nearby blood vessels in the cochlea (Fig. 3A and 3C). In contrast, Dio3 expression partly overlaps with internal T3-response tissues (70) suggesting that the T3 content is limited by DIO3 activity in the more immediate vicinity of target cells.
The evidence supports a model whereby the opposing activities of DIO3 and DIO2 modulate the rate of cochlear development and the onset of hearing. However, combined deletion of Dio3 and Dio2 does not restore normal development but instead exacerbates the deafness, suggesting that in the cochlea, DIO3 and DIO2 control complex events perhaps at distinct time points, and possibly in some independent locations, ultimately resulting in an additive phenotype in Dio3;Dio2 double deficiency (22).
Retina
The functions of T3 in retinal development are still being elucidated but include a key role in the cone photoreceptor diversity that allows color vision. Most mammals have cone types for sensitivity to short (S, “blue”) and medium-long (M, “green”) wavelengths of light (74) but in mice lacking thyroid hormone receptor TRβ2 (encoded by the Thrb gene), all cones differentiate as S type cones (75), resulting in a form of color blindness. Related defects occur in hypothyroid rodents (76-79). Human THRB mutations also impair cone functions (80-82) and impair differentiation of medium-long cone-like cells in retinal organoids in culture (21).
DIO3 and DIO2 activity profiles switch during retinal differentiation in rodents (12, 13, 22) (Fig. 2D). DIO3 activity has a protective role as Dio3–/– mice lose cones by T3-induced apoptosis resulting in achromatopsia (13), or color blindness and loss of visual function in daylight conditions. There is a slight loss of rods, the photoreceptors that mediate vision in dim conditions, suggesting that DIO3 also limits T3 availability for rods and possibly other retinal cell types (13). The cone loss is rescued by deletion of TRβ2, demonstrating that cone death results from T3 acting on a specific receptor (13).
The DIO2 peak at juvenile stages suggests that DIO2 amplifies T3 content during retinal maturation. Retinal functions for DIO2 identified to date are subtle. Dio2–/– mice do not display overt defects in cone diversity although rod function is modestly impaired (22). It has been reported that Dio2 expression is under circadian control and modestly influences cone opsin expression in a limited portion of central retina (83). It is possible that more obvious retinal phenotypes in Dio2–/– mice are masked by compensatory mechanisms. A major role for the DIO2 enzyme is unmasked by deletion of Dio2 in Dio3–/– mice, which reverses the cone cell death and restores both M- and S-cone function (22). This combined control of retinal development by DIO3 and DIO2 activities may involve both tissue-intrinsic functions and complex, indirect changes in circulating T4 and T3 levels. The Dio2 gene is expressed in Müller glial cells, which provide support functions for retinal neurons and might modify the T3 supply for photoreceptors (22), raising the possibility of paracrine-like T3 actions in the retina.
Gonads
Hyperthyroidism in rodents causes male hypogonadism associated with low numbers of Sertoli cells, whereas hypothyroidism causes an opposite, hyperplastic outcome in the testis (84). It has been proposed that T3 delineates the time window during which Sertoli cell precursors proliferate. In the testis, DIO3 has activity spanning the fetal and postnatal stages, whereas DIO2 has a modest postnatal peak, suggesting that differentiation depends on sustained protection by DIO3.
Dio3 –/– mice display dramatic testicular defects (15). The adult testes are 25% of normal size with prematurely arrested Sertoli cell proliferation, and these changes are associated with delayed puberty and reduced spermatogenesis. The defects are partially rescued by deletion of the T3 receptor TRα1 or MCT8 plasma membrane transporter (15), demonstrating that the phenotype reflects aberrant T3 signaling. The hypogonadism arises at neonatal stages and is associated with altered gene expression in the testis (85), reduced testosterone levels, and increased expression of gonadotropins follicle-stimulating hormone and luteinizing hormone by the pituitary (15). Spermatogonia-specific inactivation of DIO3 also results in reduced testis size and changes in testicular markers (85), demonstrating that defective clearance of T3 by spermatogonia contributes to the Sertoli cell defects and hypogonadism. The results suggest a variation on the theme of paracrine-like control in which DIO3 activity limits the T3 available for transfer from spermatogonia to Sertoli cells and possibly Leydig or other cell types (15) (Fig. 3B).
The testicular abnormalities in Dio3–/– mice are partially rescued in double-Dio2/Dio3 mutants (15), supporting the proposal that the balance between DIO3 and DIO2 activities determines the appropriate T3 content for the correct differentiation of the tissue.
Deiodinases, an Integral Component of Tissue-Specific Developmental Control
The evidence discussed in the preceding sections indicates that the developmental actions of thyroid hormones in different tissues depend on an intricate combination of endocrine and paracrine-like mechanisms. A corollary of the proposed paracrine-like control by deiodinases within a tissue is a requirement for transfer of T4 and T3 between cell types (see Fig. 3A and 3B). Deiodinases are likely to cooperate closely with specific plasma membrane transporters, such as MCT8, that mediate the cellular uptake and cell-to-cell transfer of T4 and T3 (44, 86-88). For example, Mct8-deficient mice exhibit complex brain dysfunction and behavioral outcomes that may be consistent both with hypothyroid and hyperthyroid states in different brain regions as a result of defects in cell-specific transport of T3 and T4 (89). The potential for cooperation between deiodinases and different plasma membrane transporters in specific cell types presumably confers precision in determining the local availability of ligand for the nuclear receptors that elicit a given cellular transcriptional response.
Type 3 Deiodinase and Cell-Fate Decisions
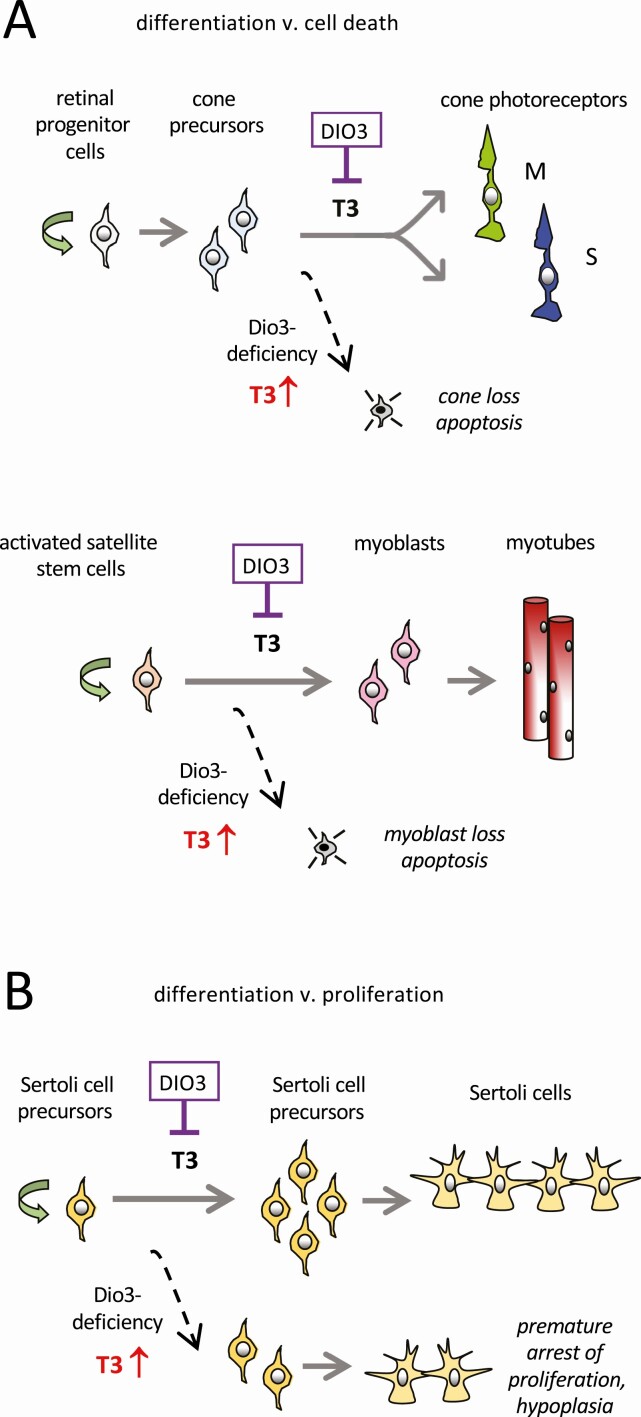
The expression of DIO3 in immature tissues often correlates with developmental windows when progenitor and precursor cell types commit to specific fates: proliferation, cell death, or terminal differentiation into mature cell types. A number of studies have now indicated that DIO3 specifically controls several cell-fate decisions. We highlight here examples of DIO3-dependent cell-fate decisions.
The expression of DIO3 in the immature retina presumably forms a sink that minimizes exposure of cone photoreceptors to T3 thereby preventing T3-induced apoptosis (13) (Fig. 4A). Although some T3 is required for cones to diversify into the M- and S-types required for color vision, these precursors sit on a knife edge between normal differentiation and apoptosis if the T3 signal is excessive. DIO3 crucially constrains the T3 signal to promote the former over latter outcome.
Figure 4.
Control of cell differentiation fates by type 3 deiodinase (DIO3). A, DIO3 critically limits the 3,5,3′-triiodothyronine (T3) signal to allow normal differentiation vs T3-induced cell death. Examples show differentiation of cone photoreceptors in the retina and myoblasts during muscle regeneration. B, For Sertoli cell precursors in the testis, DIO3 limits the T3 signal to allow an appropriate period of proliferation vs premature arrest of proliferation, thereby generating a full complement of Sertoli cells.
A similar paradigm is observed for muscle satellite cells, which differentiate into the myoblasts that ultimately form muscle fibers (see Fig. 4A) (90). During muscle regeneration in injury models, satellite stem cells are prone to T3-induced apoptosis so that DIO3 activity is critical in safeguarding normal differentiation as these cells form myoblasts (91).
In the testis, DIO3 activity is thought to control whether Sertoli precursor cells continue to proliferate or exit from the cell cycle and enter terminal differentiation (Fig. 4B). In Dio3–/– mice, unconstrained T3 signals lead to premature arrest of proliferation, resulting in a reduced pool of Sertoli cell precursors and consequently fewer mature Sertoli cells, leading to testicular hypoplasia (15, 85). Sertoli cell sensitivity is controlled at least in part by DIO3 in adjacent spermatogonia, as discussed earlier.
Together these findings suggest that the timing, duration, and level of T3 exposure as determined in part by DIO3 are critical factors in determining cell-fate decisions. We suggest that further studies will reveal additional controls by DIO3 over specific cell lineages in a wider range of tissues. Better definition of DIO3 and DIO2 functions at this cellular level awaits improved methods for identifying the cell types that express each enzyme. This has been difficult because deiodinases are expressed transiently and often at low levels in restricted cell populations.
Deiodinases and Human Development
Inactivating mutations of human DIO2 and DIO3 are currently unknown but evidence from mouse models predicts that such mutations might be associated with a range of syndromic features (see Table 1). We discuss here other known forms of genetic changes that may influence DIO2 and DIO3 function.
Type 2 Deiodinase
Approximately 30% of human populations carry a single-nucleotide variation that substitutes alanine for threonine at position 92 of the DIO2 protein (92). This T92A variant modestly reduces T3 generation from T4 in cultured cells or when introduced into mice (93). Studies in mice carrying the human variant suggest that T92A alters proteostasis associated with binding of chaperones that traffic and recycle the protein between the Golgi and endoplasmic reticulum (93). In hypothyroid patients who also carry T92A, thyroid status and neurological outcomes improve when treated with a combination of T4 and T3, instead of T4 alone (94), suggesting impaired T4 to T3 conversion in T92A carriers. This hypomorphic variant has been associated with impaired response of the HPT axis to TRH (95) and an increased incidence of metabolic and neurological disorders (96-98).
Type 3 Deiodinase
DIO3 is an imprinted gene in the imprinted DLK1 to DIO3 cluster on chromosome 14 in humans (chromosome 12 in mice) and may contribute to abnormalities in human uniparental disomy of chromosome 14 (UPD14) (99). DIO3 is preferentially expressed from the paternally inherited allele (100-102). Inheritance of 2 copies of maternal or paternal DIO3 in UPD14 patients results in a double dosage of the underexpressed maternal or overexpressed paternal allele, respectively. Reduced DIO3 activity is expected with maternal UPD14 (Temple syndrome), characterized by neonatal failure to thrive, growth retardation, reduced head circumference, hydrocephalus, mild mental impairment, and early puberty (103-105). Conversely, DIO3 overexpression is expected with paternal UPD14 (Kagami-Ogata syndrome), characterized by cranial dysmorphism, neurological impairment, and rib cage abnormalities (105). Aberrant dosage of other genes in the DLK1 to DIO3 cluster presumably contributes to the pathophysiology of both UPD14 syndromes.
Type 1 Deiodinase
Two families have been identified that carry heterozygous DIO1 mutations (106). The mutations change amino acids 94 and 201 and decrease enzymatic activity in vitro largely due to lower substrate affinity. As in Dio1+/– mice (or more obviously in Dio1–/– mice) (27), the human carriers exhibit impaired metabolism of iodothyronines with elevated rT3 and higher rT3/T3 ratios presumably due to reduced clearance of rT3 by DIO1. As reported to date, the patients display otherwise mild symptoms.
Other Genes
As selenoproteins, all 3 deiodinases require specialized factors, including SECISBP2, for incorporation of the rare amino acid selenocysteine during protein translation (107). Human mutations that interfere with this incorporation impair deiodinases and other selenoproteins and are associated with abnormal metabolism of thyroid hormones (108, 109), hearing loss, azoospermia, skin photosensitivity, as well as growth and metabolic impairment (110, 111). Inactivation of SECISBP2 in humans or mice results in elevated T4 and rT3 and decreased T3, indicative of impaired deiodination (112, 113). Many of the human symptoms, for example, in the HPT axis, hearing and growth, potentially indicate the involvement of all 3 deiodinases in multiple human tissues.
Future Challenges
The expression of deiodinases in most, if not all, tissues in mammalian development suggests that additional functions remain to be discovered, and that these functions may profoundly influence tissue maturation. The overall phenotype of Dio3–/– mice (see Table 1) is the most severe of any deiodinase deficiency, suggesting that new DIO3 functions remain to be elucidated, including key life-sustaining functions at perinatal stages. Although DIO2 tends to be associated with later maturation, a novel early role was reported in perinatal programming of liver function in mice (114). Hepatocyte-specific Dio2 deficiency was associated with altered lipid metabolism, rendering the liver resistant to high-fat diet– or alcohol-induced steatosis (114, 115).
Apart from overt mutations in deiodinase genes, further studies may investigate whether persistent environmental pollutants that act as endocrine-disrupting chemicals might disturb deiodinase activities. Environmental chemicals may produce relatively modest changes in circulating T4 and T3 but more severe end-tissue defects (116, 117), for example, in brain function (118) or hearing (119), suggesting possible interference with tissue determinants of thyroid hormone action including deiodinases.
Finally, knowledge of the developmental roles of deiodinases in determining cell death or survival may offer opportunities for exploiting these enzymes as therapeutic targets in a range of pathological conditions. For example, deletion of Dio2 recovers cone photoreceptor survival in Dio3–/– mice (22) but, remarkably, also improves cone survival in other forms of retinal degeneration caused by defects unrelated to T3 signaling (120). The possibility that suppressing T3 signaling in the diseased retina helps photoreceptor survival has been supported by interventions in the eye in mouse models of Leber congenital amaurosis by overexpression of DIO3 via adenoviral vectors or by application of iopanoic acid, an inhibitor of deiodinase activity (121). There is much scope to explore targeting of deiodinases in different tissues as a way to ameliorate disease outcomes. Accessible tissues, such as the eye, may be amenable to direct intervention, bypassing some of the concerns that arise with systemic treatments. The use of novel inhibitors of deiodinase activity (122, 123) or gene therapy may allow therapeutic approaches based on local interference with T3 signaling.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health (NIH; grant Nos. DK095908, MH096050, and DE028732 to A.H. and M.E.M.) and by the intramural research program at the NIH National Institute of Diabetes and Digestive and Kidney Diseases (to L.N. and D.F.).
Glossary
Abbreviations
- Dio1
type 1 deiodinase gene
- Dio2
type 2 deiodinase gene
- Dio3
type 3 deiodinase gene
- DIO1
type 1 deiodinase enzyme
- DIO2
type 2 deiodinase enzyme
- DIO3
type 3 deiodinase enzyme
- HPT axis
hypothalamic-pituitary-thyroid axis;
- M opsin
medium-long wavelength opsin;
- rT3
reverse 3,3′,5′-triiodothyronine;
- S opsin
short wavelength opsin;
- T3
3,5,3′-triiodothyronine;
- T4
thyroxine;
- THRB
thyroid hormone receptor β;
- TRH
thyrotropin-releasing hormone;
- TSH
thyrotropin;
- UPD14
uniparental disomy of chromosome 14
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Osler W. Sporadic Cretinism in America. Transactions; Congress of American Physicians and Surgeons. 1897;4:169-206. [Google Scholar]
- 2. Polak M, Legac I, Vuillard E, Guibourdenche J, Castanet M, Luton D. Congenital hyperthyroidism: the fetus as a patient. Horm Res. 2006;65(5):235-242. [DOI] [PubMed] [Google Scholar]
- 3. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23(1):38-89. [DOI] [PubMed] [Google Scholar]
- 4. Galton VA, Larsen PR, Berry MJ. The deiodinases: their identification and cloning of their genes. Endocrinology. 2021;162(3):bqab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bianco AC, Dumitrescu A, Gereben B, et al. Paradigms of dynamic control of thyroid hormone signaling. Endocr Rev. 2019;40(4):1000-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaplan MM, Yaskoski KA. Maturational patterns of iodothyronine phenolic and tyrosyl ring deiodinase activities in rat cerebrum, cerebellum, and hypothalamus. J Clin Invest. 1981;67(4):1208-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roti E, Fang SL, Braverman LE, Emerson CH. Rat placenta is an active site of inner ring deiodination of thyroxine and 3,3′,5-triiodothyronine. Endocrinology. 1982;110(1):34-37. [DOI] [PubMed] [Google Scholar]
- 8. Galton VA, Martinez E, Hernandez A, St Germain EA, Bates JM, St Germain DL. Pregnant rat uterus expresses high levels of the type 3 iodothyronine deiodinase. J Clin Invest. 1999;103(7):979-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang SA, Dorfman DM, Genest DR, Salvatore D, Larsen PR. Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab. 2003;88(3):1384-1388. [DOI] [PubMed] [Google Scholar]
- 10. Peeters RP, Hernandez A, Ng L, et al. Cerebellar abnormalities in mice lacking type 3 deiodinase and partial reversal of phenotype by deletion of thyroid hormone receptor α1. Endocrinology. 2013;154(1):550-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campos-Barros A, Amma LL, Faris JS, Shailam R, Kelley MW, Forrest D. Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proc Natl Acad Sci U S A. 2000;97(3):1287-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ientile R, Macaione S, Russo P, Pugliese G, Di Giorgio RM. Phenolic and tyrosyl ring deiodination in thyroxine from rat retina during postnatal development. Eur J Biochem. 1984;142(1):15-19. [DOI] [PubMed] [Google Scholar]
- 13. Ng L, Lyubarsky A, Nikonov SS, et al. Type 3 deiodinase, a thyroid-hormone-inactivating enzyme, controls survival and maturation of cone photoreceptors. J Neurosci. 2010;30(9):3347-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bates JM, St Germain DL, Galton VA. Expression profiles of the three iodothyronine deiodinases, D1, D2, and D3, in the developing rat. Endocrinology. 1999;140(2):844-851. [DOI] [PubMed] [Google Scholar]
- 15. Martinez ME, Karaczyn A, Stohn JP, et al. The type 3 deiodinase is a critical determinant of appropriate thyroid hormone action in the developing testis. Endocrinology. 2016;157(3):1276-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Obregón MJ, Ruiz de Oña C, Hernandez A, Calvo R, Escobar del Rey F, Morreale de Escobar G. Thyroid hormones and 5′-deiodinase in rat brown adipose tissue during fetal life. Am J Physiol. 1989;257(5 Pt 1):E625-E631. [DOI] [PubMed] [Google Scholar]
- 17. Hall JA, Ribich S, Christoffolete MA, et al. Absence of thyroid hormone activation during development underlies a permanent defect in adaptive thermogenesis. Endocrinology. 2010;151(9):4573-4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116(2):476-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kester MH, Martinez de Mena R, Obregon MJ, et al. Iodothyronine levels in the human developing brain: major regulatory roles of iodothyronine deiodinases in different areas. J Clin Endocrinol Metab. 2004;89(7):3117-3128. [DOI] [PubMed] [Google Scholar]
- 20. Cardoso-Moreira M, Halbert J, Valloton D, et al. Gene expression across mammalian organ development. Nature. 2019;571(7766):505-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eldred KC, Hadyniak SE, Hussey KA, et al. Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science. 2018;362(6411):eaau6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ng L, Liu H, St Germain DL, Hernandez A, Forrest D. Deletion of the thyroid hormone-activating type 2 deiodinase rescues cone photoreceptor degeneration but not deafness in mice lacking type 3 deiodinase. Endocrinology. 2017;158(6):1999-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van der Spek AH, Fliers E, Boelen A. Thyroid hormone and deiodination in innate immune cells. Endocrinology. 2021;162(1):bqaa200. [DOI] [PubMed] [Google Scholar]
- 24. Nappi A, De Stefano MA, Dentice M, Salvatore D. Deiodinases and cancer. Endocrinology. 2021;162(4):bqab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Russo SC, Salas-Lucia F, Bianco AC. Deiodinases and the metabolic code for thyroid hormone action. Endocrinology. Published online March 15, 2021. doi:10.1210/endocr/bqab059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Streckfuss F, Hamann I, Schomburg L, et al. Hepatic deiodinase activity is dispensable for the maintenance of normal circulating thyroid hormone levels in mice. Biochem Biophys Res Commun. 2005;337(2):739-745. [DOI] [PubMed] [Google Scholar]
- 27. Schneider MJ, Fiering SN, Thai B, et al. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147(1):580-589. [DOI] [PubMed] [Google Scholar]
- 28. Williams AJ, Robson H, Kester MHA, et al. Iodothyronine deiodinase enzyme activities in bone. Bone. 2008;43(1-3):126-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duncan Bassett JH, Boyde A, Howell PGT, et al. Optimal bone strength and mineralization requires the type 2 iodothyronine deiodinase in osteoblasts. Proc Natl Acad Sci U S A. 2010;107(16):7604-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. An X, Ogawa-Wong A, Carmody C, et al. A type 2 deiodinase-dependent increase in Vegfa mediates myoblast-endothelial cell crosstalk during skeletal muscle regeneration. Thyroid. 2021;31(1):115-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dentice M, Marsili A, Ambrosio R, et al. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J Clin Invest. 2010;120(11):4021-4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mancino G, Sibilio A, Luongo C, et al. The thyroid hormone inactivator enzyme, type 3 deiodinase, is essential for coordination of keratinocyte growth and differentiation. Thyroid. 2020;30(7):1066-1078. [DOI] [PubMed] [Google Scholar]
- 33. Ueta CB, Oskouei BN, Olivares EL, et al. Absence of myocardial thyroid hormone inactivating deiodinase results in restrictive cardiomyopathy in mice. Mol Endocrinol. 2012;26(5):809-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Medina MC, Fonesca TL, Molina J, et al. Maternal inheritance of an inactive type III deiodinase gene allele affects mouse pancreatic β-cells and disrupts glucose homeostasis. Endocrinology. 2014;155(8):3160-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Medina MC, Molina J, Gadea Y, et al. The thyroid hormone-inactivating type III deiodinase is expressed in mouse and human β-cells and its targeted inactivation impairs insulin secretion. Endocrinology. 2011;152(10):3717-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol. 2001;15(12):2137-2148. [DOI] [PubMed] [Google Scholar]
- 37. Fonseca TL, Correa-Medina M, Campos MP, et al. Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J Clin Invest. 2013;123(4):1492-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luongo C, Martin C, Vella K, et al. The selective loss of the type 2 iodothyronine deiodinase in mouse thyrotrophs increases basal TSH but blunts the thyrotropin response to hypothyroidism. Endocrinology. 2015;156(2):745-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Galton VA, Schneider MJ, Clark AS, St Germain DL. Life without thyroxine to 3,5,3′-triiodothyronine conversion: studies in mice devoid of the 5′-deiodinases. Endocrinology. 2009;150(6):2957-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hernandez A, Martinez ME, Liao XH, et al. Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology. 2007;148(12):5680-5687. [DOI] [PubMed] [Google Scholar]
- 41. Galton VA, de Waard E, Parlow AF, St Germain DL, Hernandez A. Life without the iodothyronine deiodinases. Endocrinology. 2014;155(10):4081-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Itoh Y, Esaki T, Kaneshige M, et al. Brain glucose utilization in mice with a targeted mutation in the thyroid hormone α or β receptor gene. Proc Natl Acad Sci U S A. 2001;98(17):9913-9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bernal J. Thyroid hormones and brain development. Vitam Horm. 2005;71:95-122. [DOI] [PubMed] [Google Scholar]
- 44. Bernal J, Guadaño-Ferraz A, Morte B. Thyroid hormone transporters—functions and clinical implications. Nat Rev Endocrinol. 2015;11(7):406-–417.. [DOI] [PubMed] [Google Scholar]
- 45. Legrand J. Effects of thyroid hormones on central nervous system development. In: Yanai J, ed. Neurobehavioral Teratology. Elsevier; 1984:331-363. [Google Scholar]
- 46. Bárez-López S, Obregon MJ, Bernal J, Guadaño-Ferraz A. Thyroid hormone economy in the perinatal mouse brain: implications for cerebral cortex development. Cereb Cortex. 2018;28(5):1783-1793. [DOI] [PubMed] [Google Scholar]
- 47. Richard S, Guyot R, Rey-Millet M, et al. A pivotal genetic program controlled by thyroid hormone during the maturation of GABAergic neurons. iScience. 2020;23(3):100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guadaño-Ferraz A, Obregón MJ, St Germain DL, Bernal J. The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc Natl Acad Sci U S A. 1997;94(19):10391-10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guadaño-Ferraz A, Escámez MJ, Rausell E, Bernal J. Expression of type 2 iodothyronine deiodinase in hypothyroid rat brain indicates an important role of thyroid hormone in the development of specific primary sensory systems. J Neurosci. 1999;19(9):3430-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Galton VA, Wood ET, St. Germain EA, et al. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology. 2007;148(7):3080-3088. [DOI] [PubMed] [Google Scholar]
- 51. Morte B, Ceballos A, Diez D, et al. Thyroid hormone-regulated mouse cerebral cortex genes are differentially dependent on the source of the hormone: a study in monocarboxylate transporter-8- and deiodinase-2-deficient mice. Endocrinology. 2010;151(5):2381-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bárez-López S, Montero-Pedrazuela A, Bosch-García D, Venero C, Guadaño-Ferraz A. Increased anxiety and fear memory in adult mice lacking type 2 deiodinase. Psychoneuroendocrinology. 2017;84:51-60. [DOI] [PubMed] [Google Scholar]
- 53. Bocco BM, Werneck-de-Castro JP, Oliveira KC, et al. Type 2 deiodinase disruption in astrocytes results in anxiety-depressive-like behavior in male mice. Endocrinology. 2016;157(9):3682-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hernandez A, Morte B, Belinchón MM, Ceballos A, Bernal J. Critical role of types 2 and 3 deiodinases in the negative regulation of gene expression by T3in the mouse cerebral cortex. Endocrinology. 2012;153(6):2919-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hernandez A, Quignodon L, Martinez ME, Flamant F, St Germain DL. Type 3 deiodinase deficiency causes spatial and temporal alterations in brain T3 signaling that are dissociated from serum thyroid hormone levels. Endocrinology. 2010;151(11):5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stohn JP, Martinez ME, St Germain DL, Hernandez A. Adult onset of type 3 deiodinase deficiency in mice alters brain gene expression and increases locomotor activity. Psychoneuroendocrinology. 2019;110:104439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stohn JP, Martinez ME, Hernandez A. Decreased anxiety- and depression-like behaviors and hyperactivity in a type 3 deiodinase-deficient mouse showing brain thyrotoxicosis and peripheral hypothyroidism. Psychoneuroendocrinology. 2016;74:46-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stohn JP, Martinez ME, Zafer M, López-Espíndola D, Keyes LM, Hernandez A. Increased aggression and lack of maternal behavior in Dio3-deficient mice are associated with abnormalities in oxytocin and vasopressin systems. Genes Brain Behav. 2018;17(1):23-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu Z, Martinez ME, St Germain DL, Hernandez A. Type 3 deiodinase role on central thyroid hormone action affects the leptin-melanocortin system and circadian activity. Endocrinology. 2017;158(2):419-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ng L, Kelley MW, Forrest D. Making sense with thyroid hormone—the role of T3 in auditory development. Nat Rev Endocrinol. 2013;9(5):296-307. [DOI] [PubMed] [Google Scholar]
- 61. Lichtenberger-Geslin L, Dos Santos S, Hassani Y, Ecosse E, Van Den Abbeele T, Léger J. Factors associated with hearing impairment in patients with congenital hypothyroidism treated since the neonatal period: a national population-based study. J Clin Endocrinol Metab. 2013;98(9):3644-3652. [DOI] [PubMed] [Google Scholar]
- 62. Sohmer H, Freeman S. Functional development of auditory sensitivity in the fetus and neonate. J Basic Clin Physiol Pharmacol. 1995;6(2):95-108. [DOI] [PubMed] [Google Scholar]
- 63. Ng L, Hernandez A, He W, et al. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology. 2009;150(4):1952-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ng L, Goodyear RJ, Woods CA, et al. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci U S A. 2004;101(10):3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Deol MS. The role of thyroxine in the differentiation of the organ of Corti. Acta Otolaryngol. 1976;81(5-6):429-435. [DOI] [PubMed] [Google Scholar]
- 66. Johnson KR, Marden CC, Ward-Bailey P, Gagnon LH, Bronson RT, Donahue LR. Congenital hypothyroidism, dwarfism, and hearing impairment caused by a missense mutation in the mouse dual oxidase 2 gene, Duox2. Mol Endocrinol. 2007;21(7):1593-1602. [DOI] [PubMed] [Google Scholar]
- 67. Mustapha M, Fang Q, Gong TW, et al. Deafness and permanently reduced potassium channel gene expression and function in hypothyroid Pit1dw mutants. J Neurosci. 2009;29(4):1212-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Knipper M, Zinn C, Maier H, et al. Thyroid hormone deficiency before the onset of hearing causes irreversible damage to peripheral and central auditory systems. J Neurophysiol. 2000;83(5):3101-3112. [DOI] [PubMed] [Google Scholar]
- 69. Rusch A, Ng L, Goodyear R, et al. Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J Neurosci. 2001;21(24):9792-9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ng L, Hernandez A, He W, et al. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology. 2009;150(4):1952-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Peeters RP, Ng L, Ma M, Forrest D. The timecourse of apoptotic cell death during postnatal remodeling of the mouse cochlea and its premature onset by triiodothyronine (T3). Mol Cell Endocrinol. 2015;407:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Axelsson A. Comparative anatomy of cochlear blood vessels. Am J Otolaryngol. 1988;9(6):278-290. [DOI] [PubMed] [Google Scholar]
- 73. Sharlin DS, Visser TJ, Forrest D. Developmental and cell-specific expression of thyroid hormone transporters in the mouse cochlea. Endocrinology. 2011;152(12):5053-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nathans J. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron. 1999;24(2):299-312. [DOI] [PubMed] [Google Scholar]
- 75. Ng L, Hurley JB, Dierks B, et al. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001;27(1):94-98. [DOI] [PubMed] [Google Scholar]
- 76. Glaschke A, Weiland J, Del Turco D, Steiner M, Peichl L, Glösmann M. Thyroid hormone controls cone opsin expression in the retina of adult rodents. J Neurosci. 2011;31(13):4844-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pessôa CN, Santiago LA, Santiago DA, et al. Thyroid hormone action is required for normal cone opsin expression during mouse retinal development. Invest Ophthalmol Vis Sci. 2008;49(5):2039-2045. [DOI] [PubMed] [Google Scholar]
- 78. Lu A, Ng L, Ma M, et al. Retarded developmental expression and patterning of retinal cone opsins in hypothyroid mice. Endocrinology. 2009;150(3):1536-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Boyes WK, Degn L, George BJ, Gilbert ME. Moderate perinatal thyroid hormone insufficiency alters visual system function in adult rats. Neurotoxicology. 2018;67:73-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Weiss AH, Kelly JP, Bisset D, Deeb SS. Reduced L- and M- and increased S-cone functions in an infant with thyroid hormone resistance due to mutations in the THRβ2 gene. Ophthalmic Genet. 2012;33(4):187-195. [DOI] [PubMed] [Google Scholar]
- 81. Campi I, Cammarata G, Bianchi Marzoli S, et al. Retinal photoreceptor functions are compromised in patients with resistance to thyroid hormone syndrome (RTHβ). J Clin Endocrinol Metab. 2017;102(7):2620-2627. [DOI] [PubMed] [Google Scholar]
- 82. Newell FW, Diddie KR. Typical monochromacy, congenital deafness, and resistance to intracellular action of thyroid hormone (author’s transl) [article in German]. Klin Monbl Augenheilkd. 1977;171(5):731-734. [PubMed] [Google Scholar]
- 83. Sawant OB, Horton AM, Zucaro OF, et al. The circadian clock gene Bmal1 controls thyroid hormone-mediated spectral identity and cone photoreceptor function. Cell Rep. 2017;21(3):692-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Holsberger DR, Cooke PS. Understanding the role of thyroid hormone in Sertoli cell development: a mechanistic hypothesis. Cell Tissue Res. 2005;322(1):133-140. [DOI] [PubMed] [Google Scholar]
- 85. Martinez ME, Lary CW, Karaczyn AA, Griswold MD, Hernandez A. Spermatogonial type 3 deiodinase regulates thyroid hormone target genes in developing testicular somatic cells. Endocrinology. 2019;160(12):2929-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mayerl S, Müller J, Bauer R, et al. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest. 2014;124(5):1987-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Schweizer U, Köhrle J. Function of thyroid hormone transporters in the central nervous system. Biochim Biophys Acta. 2013;1830(7):3965-3973. [DOI] [PubMed] [Google Scholar]
- 88. Groeneweg S, van Geest FS, Peeters RP, Heuer H, Visser WE. Thyroid hormone transporters. Endocrine Rev. 2020;41(2):146-201. [DOI] [PubMed] [Google Scholar]
- 89. Wirth EK, Roth S, Blechschmidt C, et al. Neuronal 3′,3,5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in Mct8, the neuronal T3 transporter mutated in Allan-Herndon-Dudley syndrome. J Neurosci. 2009;29(30):9439-9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dentice M, Marsili A, Zavacki A, Larsen PR, Salvatore D. The deiodinases and the control of intracellular thyroid hormone signaling during cellular differentiation. Biochim Biophys Acta. 2013;1830(7):3937-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ambrosio R, Damiano V, Sibilio A, et al. Epigenetic control of type 2 and 3 deiodinases in myogenesis: role of lysine-specific demethylase enzyme and FoxO3. Nucleic Acids Res. 2013;41(6):3551-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. McAninch EA, Bianco AC. New insights into the variable effectiveness of levothyroxine monotherapy for hypothyroidism. Lancet Diabetes Endocrinol. 2015;3(10):756-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jo S, Fonseca TL, Bocco BMLC, et al. Type 2 deiodinase polymorphism causes ER stress and hypothyroidism in the brain. J Clin Invest. 2019;129(1):230-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Panicker V, Saravanan P, Vaidya B, et al. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009;94(5):1623-1629. [DOI] [PubMed] [Google Scholar]
- 95. Butler PW, Smith SM, Linderman JD, et al. The Thr92Ala 5′ type 2 deiodinase gene polymorphism is associated with a delayed triiodothyronine secretion in response to the thyrotropin-releasing hormone-stimulation test: a pharmacogenomic study. Thyroid. 2010;20(12):1407-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mentuccia D, Proietti-Pannunzi L, Tanner K, et al. Association between a novel variant of the human type 2 deiodinase gene Thr92Ala and insulin resistance: evidence of interaction with the Trp64Arg variant of the β-3-adrenergic receptor. Diabetes. 2002;51(3):880-883. [DOI] [PubMed] [Google Scholar]
- 97. McAninch EA, Rajan KB, Evans DA, et al. A common DIO2 polymorphism and Alzheimer disease dementia in African and European Americans. J Clin Endocrinol Metab. 2018;103(5):1818-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bianco AC, Kim BS. Pathophysiological relevance of deiodinase polymorphism. Curr Opin Endocrinol Diabetes Obes. 2018;25(5):341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hernandez A, Park JP, Lyon GJ, Mohandas TK, St. Germain DL. Localization of the type 3 iodothyronine deiodinase (DIO3) gene to human chromosome 14q32 and mouse chromosome 12F1. Genomics. 1998;53(1):119-121. [DOI] [PubMed] [Google Scholar]
- 100. Tsai CE, Lin SP, Ito M, Takagi N, Takada S, Ferguson-Smith AC. Genomic imprinting contributes to thyroid hormone metabolism in the mouse embryo. Curr Biol. 2002;12(14):1221-1226. [DOI] [PubMed] [Google Scholar]
- 101. Hernandez A, Fiering S, Martinez E, Galton VA, St Germain D. The gene locus encoding iodothyronine deiodinase type 3 (Dio3) is imprinted in the fetus and expresses antisense transcripts. Endocrinology. 2002;143(11):4483-4486. [DOI] [PubMed] [Google Scholar]
- 102. Martinez ME, Cox DF, Youth BP, Hernandez A. Genomic imprinting of DIO3, a candidate gene for the syndrome associated with human uniparental disomy of chromosome 14. Eur J Hum Genet. 2016;24(11):1617-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Temple IK, Cockwell A, Hassold T, Pettay D, Jacobs P. Maternal uniparental disomy for chromosome 14. J Med Genet. 1991;28(8):511-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Buiting K, Kanber D, Martín-Subero JI, et al. Clinical features of maternal uniparental disomy 14 in patients with an epimutation and a deletion of the imprinted DLK1/GTL2 gene cluster. Hum Mutat. 2008;29(9):1141-1146. [DOI] [PubMed] [Google Scholar]
- 105. Kagami M, Matsuoka K, Nagai T, et al. Paternal uniparental disomy 14 and related disorders: placental gene expression analyses and histological examinations. Epigenetics. 2012;7(10):1142-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. França MM, German A, Fernandes GW, et al. Human type 1 iodothyronine deiodinase (DIO1) mutations cause abnormal thyroid hormone metabolism. Thyroid. 2021;31(2):202-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Labunskyy VM, Hatfield DL, Gladyshev VN. Selenoproteins: molecular pathways and physiological roles. Physiol Rev. 2014;94(3):739-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dumitrescu AM, Liao XH, Abdullah MS, et al. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet. 2005;37(11):1247-1252. [DOI] [PubMed] [Google Scholar]
- 109. Fu J, Korwutthikulrangsri M, Gönç EN, et al. Clinical and molecular analysis in 2 families with novel compound heterozygous SBP2 (SECISBP2) mutations. J Clin Endocrinol Metab. 2020;105(3):e6-e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Schoenmakers E, Chatterjee K. Human disorders affecting the selenocysteine incorporation pathway cause systemic selenoprotein deficiency. Antioxid Redox Signal. 2020;33(7):481-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Köhrle J. Selenium in endocrinology—selenoprotein-related diseases, population studies, and epidemiological evidence. Endocrinology. 2021;162(2):bqaa228. [DOI] [PubMed] [Google Scholar]
- 112. Fujisawa H, Korwutthikulrangsri M, Fu J, Liao XH, Dumitrescu AM. Role of the thyroid gland in expression of the thyroid phenotype of Sbp2-deficient mice. Endocrinology. 2020;161(5):bqz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fu J, Fujisawa H, Follman B, Liao XH, Dumitrescu AM. Thyroid Hormone metabolism defects in a mouse model of SBP2 deficiency. Endocrinology. 2017;158(12):4317-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Fonseca TL, Fernandes GW, McAninch EA, et al. Perinatal deiodinase 2 expression in hepatocytes defines epigenetic susceptibility to liver steatosis and obesity. Proc Natl Acad Sci U S A. 2015;112(45):14018-14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fonseca TL, Fernandes GW, Bocco BMLC, et al. Hepatic inactivation of the type 2 deiodinase confers resistance to alcoholic liver steatosis. Alcohol Clin Exp Res. 2019;43(7):1376-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bansal R, Tighe D, Danai A, et al. Polybrominated diphenyl ether (DE-71) interferes with thyroid hormone action independent of effects on circulating levels of thyroid hormone in male rats. Endocrinology. 2014;155(10):4104-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ramhøj L, Hass U, Gilbert ME, et al. Evaluating thyroid hormone disruption: investigations of long-term neurodevelopmental effects in rats after perinatal exposure to perfluorohexane sulfonate (PFHxS). Sci Rep. 2020;10(1):2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. O’Shaughnessy KL, Wood CR, Ford RL, et al. Thyroid hormone disruption in the fetal and neonatal rat: predictive hormone measures and bioindicators of hormone action in the developing cortex. Toxicol Sci. 2018;166(1):163-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Crofton KM. Developmental disruption of thyroid hormone: correlations with hearing dysfunction in rats. Risk Anal. 2004;24(6):1665-1671. [DOI] [PubMed] [Google Scholar]
- 120. Yang F, Ma H, Butler MR, Ding XQ. Deficiency of type 2 iodothyronine deiodinase reduces necroptosis activity and oxidative stress responses in retinas of Leber congenital amaurosis model mice. FASEB J. 2018;32(11):fj201800484RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yang F, Ma H, Belcher J, et al. Targeting iodothyronine deiodinases locally in the retina is a therapeutic strategy for retinal degeneration. FASEB J. 2016;30(12):4313-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Renko K, Hoefig CS, Dupuy C, et al. A nonradioactive DEHAL assay for testing substrates, inhibitors, and monitoring endogenous activity. Endocrinology. 2016;157(12):4516-4525. [DOI] [PubMed] [Google Scholar]
- 123. Renko K, Schäche S, Hoefig CS, et al. An improved nonradioactive screening method identifies genistein and xanthohumol as potent inhibitors of iodothyronine deiodinases. Thyroid. 2015;25(8):962-968. [DOI] [PubMed] [Google Scholar]
- 124. LaFranchi SH. Thyroid physiology and screening in preterm infants. UpToDate. 2020;2020:1-24. [Google Scholar]
- 125. Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med. 1994;331(16):1072-1078. [DOI] [PubMed] [Google Scholar]
- 126. de Jesus LA, Carvalho SD, Ribeiro MO, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108(9):1379-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Marsili A, Tang D, Harney JW, et al. Type II iodothyronine deiodinase provides intracellular 3,5,3′-triiodothyronine to normal and regenerating mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2011;301(5):E818-E824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Marsili A, Aguayo-Mazzucato C, Chen T, et al. Mice with a targeted deletion of the type 2 deiodinase are insulin resistant and susceptible to diet induced obesity. PloS One. 2011;6(6):e20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. van der Spek AH, Surovtseva OV, Jim KK, et al. Regulation of intracellular triiodothyronine is essential for optimal macrophage function. Endocrinology. 2018;159(5):2241-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hernandez A, Martinez ME, Liao XH, et al. Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology. 2007;148(12):5680-5687. [DOI] [PubMed] [Google Scholar]
- 131. Dentice M, Ambrosio R, Damiano V, et al. Intracellular inactivation of thyroid hormone is a survival mechanism for muscle stem cell proliferation and lineage progression. Cell Metab. 2014;20(6):1038-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Boelen A, Kwakkel J, Wieland CW, St Germain DL, Fliers E, Hernandez A. Impaired bacterial clearance in type 3 deiodinase-deficient mice infected with Streptococcus pneumoniae. Endocrinology. 2009;150(4):1984-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. van der Spek AH, Jim KK, Karaczyn A, et al. The thyroid hormone inactivating type 3 deiodinase is essential for optimal neutrophil function: observations from three species. Endocrinology. 2018;159(2):826-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Liao XH, Di Cosmo C, Dumitrescu AM, et al. Distinct roles of deiodinases on the phenotype of Mct8 defect: a comparison of eight different mouse genotypes. Endocrinology. 2011;152(3):1180-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.