Abstract
Leishmaniasis is a neglected tropical disease that affects people living in tropical and subtropical areas of the world. There are few therapeutic options for treating this infectious disease, and available drugs induce severe side effects in patients. Different communities have limited access to hospital facilities, as well as classical treatment of leishmaniasis; therefore, they use local natural products as alternative medicines to treat this infectious disease. The present work performed a bibliographic survey worldwide to record plants used by traditional communities to treat leishmaniasis, as well as the uses and peculiarities associated with each plant, which can guide future studies regarding the characterization of new drugs to treat leishmaniasis. A bibliographic survey performed in the PubMed and Scopus databases retrieved 294 articles related to traditional knowledge, medicinal plants and leishmaniasis; however, only 20 were selected based on the traditional use of plants to treat leishmaniasis. Considering such studies, 378 quotes referring to 292 plants (216 species and 76 genera) that have been used to treat leishmaniasis were recorded, which could be grouped into 89 different families. A broad discussion has been presented regarding the most frequent families, including Fabaceae (27 quotes), Araceae (23), Solanaceae and Asteraceae (22 each). Among the available data in the 378 quotes, it was observed that the parts of the plants most frequently used in local medicine were leaves (42.3% of recipes), applied topically (74.6%) and fresh poultices (17.2%). The contribution of Latin America to studies enrolling ethnopharmacological indications to treat leishmaniasis was evident. Of the 292 plants registered, 79 were tested against Leishmania sp. Future studies on leishmanicidal activity could be guided by the 292 plants presented in this study, mainly the five species Carica papaya L. (Caricaceae), Cedrela odorata L. (Meliaceae), Copaifera paupera (Herzog) Dwyer (Fabaceae), Musa × paradisiaca L. (Musaceae), and Nicotiana tabacum L. (Solanaceae), since they are the most frequently cited in articles and by traditional communities.
Keywords: ethnopharmacology, traditional knowledge, natural drugs, leishmaniasis, medicinal plants, neglected disease
Introduction
The use of plants based on existing empirical knowledge, consecrated by continuous use in traditional communities, directs research, saves time and money in pharmacological and phytochemical studies (Mukherjee et al., 2017). The selection of plants for research and production of drugs, based on claims made by traditional communities regarding a given therapeutic effect in humans, can be a valuable shortcut for the discovery of new active molecules (Süntar, 2020) and to provide, from the academic point of view, evidence for the use of plants as medicines.
Some interesting examples of drugs extracted from plants used in traditional knowledge are (i) alpha humulene from Varronia curassavica (Jacq.), which has been used as a topical anti-inflammatory agent (Marques et al., 2019); (ii) quinine, which was purified from Cinchona sp. and has antimalarial activity (Boratyński et al., 2019); (iii) galegine from Galega officinalis L., which was used as a molecular prototype to synthesize the antidiabetic drug metformin (Bailey, 2017); (iv) morphine and codeine, as hypnoanalgesics, both extracted from Papaver somniferum (Stefano et al., 2017); (v) taxol, an antitumour agent extracted from Taxus brevifolia Nutt. (Yang and Horwitz, 2017); (vi) vimblastine, an antineoplastic agent, from Catharanthis roseus (L.) G. Don (Haque et al., 2018); and (vii) digoxin, purified from Digitalis lanata Ehrh. that displays cardiotonic effect (Patocka et al., 2020), among other examples.
Considering that ethnopharmacological studies have guided the characterization of biologically active molecules and drugs for different diseases, it is evident that this science can contribute to the search for active substances to treat neglected diseases, such as leishmaniasis, an infectious disease caused by parasitic protozoa of the genus Leishmania, endemic in tropical and subtropical countries. This neglected infectious disease is transmitted during the blood meal of sandflies of the genera Lutzomyia and Phlebotomus (Francesquini et al., 2014; Courtenay et al., 2017).
Leishmaniasis has a wide variety of clinical manifestations, from cutaneous to visceral forms (Burza et al., 2018). In cutaneous leishmaniasis (CL), the parasite infects phagocytic cells (mainly macrophages) in the skin tissue. This clinical form is characterized by skin lesions that can be single, multiple or diffuse throughout the body (Gabriel et al., 2019). Some patients have lesions in the mucous membranes, mainly in the upper airways; such injuries can occur years after the resolution of skin lesions (Kevric et al., 2015). Visceral leishmaniasis (VL) is a zoonosis of chronic evolution with systemic involvement. In this clinical form, the parasite migrates to the viscera and infects macrophages in the spleen, liver, lymph nodes, and bone marrow. Typical manifestations are chronic fever, weight loss and hepatosplenomegaly, which can lead to patient death if not properly treated (Hermida et al., 2018). These clinical changes progress along with physiological and histological modifications mainly in the spleen, liver, and bone marrow (Faleiro et al., 2014).
According to the World Health Organization, it is estimated that 50,000 to 90,000 new cases of VL and between 600,00 and one million new cases of CL occur annually. The growth in the number of cases in recent decades has been associated with environmental changes, such as deforestation, irrigation schemes, building dams and urbanization (World Health Organization, 2019). Despite these epidemiological data and the fact that there are different species of parasites occurring in 98 countries, the treatment of this important infectious disease has serious limitations and is based on few drugs, such as pentavalent antimonials, amphotericin B and miltefosine (Passero et al., 2018). Additionally, these drugs induce severe side effects in humans, and in some situations, as is the case of liposomal amphotericin B, high costs limit their use in low-income countries. Furthermore, some species of parasites have become resistant to drugs (Ghorbani and Farhoudi, 2017; Ponte-Sucre et al., 2017).
Considering the epidemiology of leishmaniasis, the scarcity of treatment and the severe side effects of drugs currently used it becomes urgent to find new molecules with leishmanicidal activity. The secondary metabolism of plants offers a panel of molecules with important pharmacological activity, and in leishmaniasis, a series of molecules has already been described with leishmanicidal potential (Passero et al., 2014; Jesus et al., 2017). In this regard, it has been observed that some studies have used the information available in published works about traditional knowledge to select plants, purify bioactive molecules and perform in vivo studies; however, only a few works have investigated the natural resources that traditional communities use to treat leishmaniasis and molecules in vitro and in in vivo models.
Thus, this review intends to investigate, through a bibliographic survey, information about medicinal plants indicated by traditional communities that are employed in the treatment of leishmaniasis, as well as their uses and peculiarities, guiding future studies on the characterization of new compounds with leishmanicidal activity.
Bibliographic Survey
To verify the existence of scientific studies about plants used by traditional communities to treat leishmaniasis, a bibliographic survey was carried out. For this purpose, a Boolean search was performed in the Scopus and PubMed databases. It was performed from May to June 2020, and the combination of words was used to expand the possibility of finding data that would meet the expectations of the present study: “(ethnomedicine OR ethnopharmaco* OR ethnobotanic* OR "traditional knowledge") AND (plant OR vegetal) AND (leishmani* OR antileishmani*)”.
The searches in the PubMed and Scopus databases retrieved a total of 238 and 161 articles, respectively. Additionally, it was observed that 105 articles were common to both databases; therefore, a total of 294 articles were analysed herein. The following exclusion criteria were used in this review: 1) review articles; 2) articles that did not clearly mention the genera or species of studied plants; and 3) articles that demonstrated leishmanicidal activity of plants without having carried out an ethnopharmacological study. The following inclusion criteria were used: 1) original articles from any year, referring to any country; 2) articles that contained clear information about the collection of ethnopharmacological data, except for the literature review; and 3) articles in English, Spanish, Portuguese and French. By considering all of these items, 20 articles were selected and analysed.
Plants with identification up to the genus level were included in the present survey, as they represent approximately 20.4% of the total indications. Species indicated with "cf"—whose taxonomic identification could not be confirmed—were also included in the present survey. In addition, all species underwent a review of their correct spelling and current taxonomic classification on the website Plants of the World online: http://www.plantsoftheworldonline.org. The following species: Anthurium muyunense Croat, Trema integerrima (Beurl.) Standl., Inga bourgonii (Aubl.) DC., Meteoridium sp., and Citrus aurantiaca (L.) Swingle, were not found in this website, but data about them were available in the website of TROPICOS: https://www.tropicos.org/home. Species with divergent scientific names in articles and on the website were synonymous, and thus, they were recorded only once. Considering the data found in the selected articles, Tables 1 and 2 and Figures 1 and 2 were included.
TABLE 1.
The 378 plant quotes obtained from the 20 publications, their families, species/genera, vernacular names, traditional recipes, countries (traditional community), traditional uses, and plants tested for leishmaniasis (in vitro).
| Family (Number of quotes and species) * | Species | Vernacular name | Traditional recipe (plant part, route) | Country (traditional community) | Traditional use (emic term) | Tested for leishmaniasis (results) | References |
|---|---|---|---|---|---|---|---|
| Acanthaceae (4 quotes and 4 species) | Fittonia sp. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Hygrophila costata Nees | Chupador | (ae, to) | Colombia (Afro-Colombian and indigenous groups) | p La + | Weniger et al. (2001) | ||
| p Lb - p Li + | |||||||
| a Lp + | |||||||
| Hygrophila sp. | - | (wp, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Sanchezia sp. | (le, to) | ||||||
| Amaranthaceae (6 quotes and 5 species) | Alternanthera sp. | - | (le/st, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Amaranthus caudatus L. | Sangorache | (le) | Ecuador | Cutaneous leishmaniasis | Weigel et al. (1994) | ||
| Chenopodiastrum murale (L.) S.Fuentes, Uotila & Borsch | A’Tra | Fresh-po (ap,to) | Saudi Arabia | - | Ali et al. (2017) | ||
| Dysphania ambrosioides (L.) Mosyakin & Clemants | Paico | (sho) | Peru# | Uta | p Lm IC50>100 μg/ml | Kvist et al. (2006) | |
| (2 quotes) | Paico | (le) | Ecuador | Cutaneous leishmaniasis | - | Weigel et al. (1994) | |
| Iresine diffusa Humb. & Bonpl. ex Willd. | - | (le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Amaryllidaceae (4 quotes and 4 species) | Allium cepa L. | Cebolla Paitena | (le/sta) | Ecuador | Cutaneous leishmaniasis | - | Weigel et al. (1994) |
| Allium sativum L. | Ajo | (cl) | |||||
| Crinum sp. | - | (ro, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Scadoxus multiflorus (Martyn) Raf. | Dem Astefi | po (ro, to) | Ethiopia | ‘Gurtb’ leishmaniasis | Teklehaymanot, (2009) | ||
| Anacardiaceae (5 quotes and 3 species) | Mangifera indica L. (2 quotes) | Mango | (co) | Peru# | Uta | p Lm IC50>100 μg/ml | Kvist et al. (2006) |
| Mã | po (ba, to) | French Guiana (Wayãpi) | Leishmaniasis | - | Odonne et al. (2011a) | ||
| Spondias mombin L. (2 quotes) | Ubos | dec (ba, to/or) | Peru (Chayahuita) | Uta | p La> 100 μg/ml | Estevez et al. (2007) | |
| a La> 100 μg/ml | |||||||
| (co) | Peru# | Uta | p Lm - NA | Kvist et al. (2006) | |||
| Spondias purpurea L. | - | (ba, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Annonaceae (2 quotes and 2 species) | Annona ambotay Aubl. | Iwitay | po (ba, to) | French Guiana (Wayãpi) | Leishmaniasis | - | Odonne et al. (2011a) |
| Cremastosperma longicuspe R.E.Fr. | Maya Sohuit | Pow-po (ba, to) | Peru (Chayahuita) | - | a La> 100 μg/ml | Odonne et al. (2009) | |
| Apocynaceae (9 quotes and 7 species) | Aspidosperma excelsum Benth. | Remo Caspi (De Baja) | (co) | Peru# | Uta | p Lm – NA | Kvist et al. (2006) |
| Aspidosperma rigidum Rusby | Gabetillo | po (st/ba, to) | Bolivia# | Cutaneous leishmaniasis | - | Hajdu and Hohmann, (2012) | |
| Himatanthus articulatus (Vahl) Woodson | Compuhuan | po (ba, to) | Peru (Chayahuita) | - | Odonne et al. (2009) | ||
| Tabernaemontana flavicans Roem. & Schult. | Shinanpi | Pow-po (ba, to) | |||||
| Tabernaemontana sananho Ruiz & Pav. (3 quotes) | Shinambik | Fresh-po (ro, to) | Uta | p La = 9 μg/ml | Estevez et al. (2007) | ||
| a La = 58 μg/ml | |||||||
| - | (ba, to) | Ecuador# | - | - | Gachet et al. (2010) | ||
| Shinanp | pow-po (ba, to) | Peru (Chayahuita) | Odonne et al. (2009) | ||||
| Tabernaemontana siphilitica (L.f.) Leeuwenb | Radie Capiaye | po (lt, to) | French Guiana | Leishmaniasis | Odonne et al. (2011a) | ||
| Tabernaemontana sp. | Lobo sanango | (Ro) | Peru# | Uta | p Lm | Kvist et al. (2006) | |
| IC50 = 15 μg/ml | |||||||
| Araceae (23 quotes and 16 species) | Anthurium muyunense Croat. | Shimpanantë | dec (to) | Peru (Chayahuita) | - | - | Odonne et al. (2009) |
| Anthurium sp. | - | (le, to) | Ecuador# | Gachet et al. (2010) | |||
| Caladium bicolor (Aiton) Vent. | Ahtata’Ta | po, (ro, to) | Peru (Chayahuita) | Uta | p La - ΝΑ | Estevez et al. (2007) | |
| a La IC50>100 μg/ml | |||||||
| Caladium picturatum K.Koch & C.D.Bouché. | Io Ata’ | po, (tu, to) | Ta’Ta’ | a La IC50>100 μg/ml | Odonne et al. (2009) | ||
| Colocasia esculenta (L.) Schott. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Dieffenbachia seguine (Jacq.) Schott | Patiquina, Hoja Blanca | Inf-po (st, to) | Peru | Uta | Vásquez-Ocmín et al. (2018) | ||
| Dieffenbachia williamsii Croat (2 quotes) | Corech | dec-po (wp/le, to) | Peru (Yanesha) | Uta De Agua, Mareñets | a La IC50>100 μg/ml | Valadeau et al. (2009) | |
| dec-po (wp, to) | Cutaneous Leishmaniasis, Wound that Do not heal | - | |||||
| Dieffenbachia sp. (4 quotes) | - | (le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Mata Boro | po (st/ba, to) | Bolivia# | Cutaneous leishmaniasis | Hajdu and Hohmann, (2012) | |||
| Patiquina | (le) | Peru# | Uta | p Lm IC50>100 μg/ml | Kvist et al. (2006) | ||
| Shimpan | dec-po (st, to) | Peru (Chayahuita) | - | - | Odonne et al. (2009) | ||
| Dracontium spruceanum (Schott) G.H.Zhu. | Jergón Sacha, Hierba Del Jergón, | pow-po (tu, to) | Peru | Uta | Vásquez-Ocmín et al. (2018) | ||
| Fer De Lance | |||||||
| Philodendron surinamense (Miq.) Engl. | Huambe | “Is Drunk In Small Quantities Three Times Daily” dec (ro, or) | Peru (Chayahuita) | Uta | p La IC50>100 μg/ml | Estevez et al. (2007) | |
| a La IC50>100 μg/ml | |||||||
| Philodendron sp. (3 quotes) | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| (le, to) | |||||||
| (le, to) | |||||||
| Pistia stratiotes L. | Puto puto | (le) | Peru# | Uta | p Lm - NA | Kvist et al. (2006) | |
| Rhodospatha sp. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Stenospermation sp. (2 quotes) | (le, to) | ||||||
| (le, to) | |||||||
| Thaumatophyllum solimoesense (A.C.Sm.) Sakur., Calazans & Mayo. | Huambe | “Is Drunk In Small Quantities Three Times Daily” dec (ro, or) | Peru (Chayahuita) | p La IC50>100 μg/ml | Estevez et al. (2007) | ||
| a La IC50>100 μg/ml | |||||||
| Xanthosoma sp. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Arecaceae (1 quote and 1 species) | Euterpe oleracea Mart. | Wasey | fresh-po (am/ro, to) | French Guiana (Wayãpi) | Leishmaniasis | - | Odonne et al. (2011a) |
| Aspleniaceae – Pteridophyta (1 quote and 1 species) | Thelypteris sp. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Asteraceae (22 quotes and 20 species) | Achillea arabica Kotschy. | Aldefera | fresh-po (ap, to) | Saudi Arabia | Leishmania | - | Ali et al. (2017) |
| Acmella brachyglossa Cass. | - | (le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Adenostemma brasilianum Cass. | |||||||
| Ageratum conyzoides L. | |||||||
| Baccharis sagittalis (less.) DC. | Charara | (wp/le, to) | Bolivia (Kechua) | Espundia (cutaneous and mucocutaneous leishmaniasis) | p La - NA | Fournet et al. (1994) | |
| p Lb - NA | |||||||
| p Ld -NA | |||||||
| Bidens pilosa L. | - | (se, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Clibadium cf. microcephalum S.F.Blake. | (le/st, to) | ||||||
| Erigeron sp. | |||||||
| Elephantopus mollis Kunth. | (wp, to) | ||||||
| Erigeron bonariensis L. | |||||||
| Eupatorium sp. | |||||||
| Matricaria chamomilla L. | Manzanilla | (fL) | Ecuador | Cutaneous leishmaniasis | Weigel et al. (1994) | ||
| Mikania sp. | - | (le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Munnozia hastifolia (Poepp.) H. Rob. & Brettell. | Huallapnarren | fresh-po (le, to) | Peru (Yanesha) | Uta De Agua, Mareñets | a La IC50 = 14.1 μg/ml | Valadeau et al. (2009) | |
| (2 quotes) | fresh-po (lt, to) | Leishmaniasis | - | Valadeau et al. (2010) | |||
| Piptocoma discolor (Kunth) Pruski. | - | (le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Porophyllum ruderale (Jacq.) Cass. | Ebus'A Ina, | pow-po, (le, to) | Bolivia (Takana indians) | Leishmaniasis | p LaIC50 > 100μg/mLp LbIC50 > 100 μg/ml | Arévalo-Lopéz et al. (2018) | |
| Chadhi Ina | |||||||
| Pseudelephantopus spicatus (Juss. ex Aubl.) C.F.Baker. | Huapato, Pato, Cahuario Pacatro | Peru (Chayahuita) | Ta’Ta’ | a La | Odonne et al. (2009) | ||
| (2 quotes) | Wapatu, Cawariu Pacaturu, Patu | IC50 = 27.3 μg/ml | (Odonne et al., 2011b) | ||||
| Taraxacum campylodes G.E.Haglund | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Tessaria integrifolia Ruiz | Cawuara | fresh-po (le, to) | Bolivia (Takana indians) | Leishmaniasis | p La IC50 = 54.2μg/mLp LaeIC50 = 48μg/mLp Lb IC50 = 31.6μg/mLp Lla IC50 = 34.8 μg/ml | Arévalo-Lopéz et al. (2018) | |
| & Pav. | |||||||
| Vernonanthura patens (Kunth) H.Rob. | - | (le/st, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Begoniaceae (1 quote and 1 species) | Begonia sp. | - | (st, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Bignoniaceae (13 quotes and 10 species) | Callichlamys latifolia (rich.) K. Schum. | Kalasapau Poã Ipo Pilã | fresh-po (ba, to) | French Guiana (Wayãpi) | Leishmaniasis | - | Odonne et al. (2011a) |
| Crescentia cujete L. (2 quotes) | Kwi’I | po (ba, to) | |||||
| - | (le, or) | Ecuador# | - | Gachet et al. (2010) | |||
| Fridericia nigrescens (Sandwith) L.G.Lohmann. | Kalasapau Poã Ipo | fresh-po (ba, to) | French Guiana (Wayãpi) | Leishmaniasis | Odonne et al. (2011a) | ||
| Handroanthus impetiginosus (mart. ex DC.) Mattos. | Tahuari | “Boil 200 G of the bark In 1 L Of Water. Wash The Affected Area And Apply As A Compress Until Cicatrization Of The Ulcers” dec-po (ba, to) | Peru | Uta | Vásquez-Ocmín et al. (2018) | ||
| Jacaranda copaia (Aubl.) D.Don. | Charapachpan | dec-po (le, to) | Peru (Yanesha) | Uta De Agua, Mareñets | a La IC50 = 16.5 μg/ml | Valadeau et al. (2009) | |
| (2 quotes) | Leishmaniasis | - | Valadeau et al. (2010) | ||||
| Jacaranda cuspidifolia mart. | Arabisco | (le, to) | Bolivia (Mozetenes, tacanas or Chimanes indians, and other ethnic groups) | Espundia (cutaneous and mucocutaneous leishmaniasis) | p La; p Lb; p Ld | Fournet et al. (1994) | |
| Jacaranda glabra (DC) Bureau & K. Schum. | Chepere Qui | dec-po (ba/le/fr, to) | Bolivia (Takana indians) | Leishmaniasis | p La IC50 = 29.8 μg/ml | Arévalo-Lopéz et al. (2018) | |
| p Lae | |||||||
| IC50 = 45.4 μg/ml | |||||||
| p Lb IC50 = 17.4 μg/ml | |||||||
| p Lla IC50 = 27.5 μg/ml | |||||||
| (2 quotes) | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Mansoa alliacea (Lam.) A.H. Gentry. | Ananan | pow-po (le, to) | Peru (Chayahuita) | Ta’Ta’ | Odonne et al. (2009) | ||
| Mansoa standleyi (Steyerm.) A.H. Gentry. | Ajo sacha (macho) | (ro) | Peru# | Uta | p Lm | Kvist et al. (2006) | |
| IC50 = 18 μg/ml | |||||||
| Mansoa sp. | Ajo Silvestre, De Monte, Sacha, Kofan: Cumpanafema, Palobrea | - | Colombia (Kofan) | Cutaneous leishmaniasis | - | Gutiérrez et al. (2014) | |
| Bixaceae (2 quotes and 1 species) | Bixa orellana L. (2 quotes) | Uluku | fresh-po (se, to) | French Guiana (Wayãpi) | Leishmaniasis | - | Odonne et al. (2011a) |
| Achiote | (le) | Ecuador | Cutaneous leishmaniasis | Weigel et al. (1994) | |||
| Bromeliaceae (1 quote and 1 species) | Billbergia decora Poepp. & Endl. | Nara Shimpanantë | fresh-po (st, to) | Peru (Chayahuita) | - | - | Odonne et al. (2009) |
| Burseraceae (1 quote and 1 species) | Commiphora gileadensis (L.) C.Chr. | Al-Bisham | fresh-po (or, to) | Saudi Arabia | Leishmaniasis | - | Ali et al. (2017) |
| Cactaceae (1 quote and 1 species) | Cereus hexagonus (L.) Mill. | Kau Kau | fresh-po (ba, to) | French Guiana (Wayãpi) | Leishmaniasis | - | Odonne et al. (2011a) |
| Cannabaceae (2 quotes and 2 species) | Trema integerrima (Beurl.) Standl. | - | (le/st, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Trema micrantha (L.) Blume. | Surrumbo, Veraquillo | - | Colombia (Kofan) | Cutaneous leishmaniasis | Gutiérrez et al. (2014) | ||
| Caricaceae (4 quotes and 1 species) | Carica papaya L. (4 quotes) | - | (ba/le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Papaye (Bapaju) (M㘠U) | fresh-po (lt, to) | French Guiana (Wayãpi, Teko) | Leishmaniasis | - | Odonne et al. (2011a) | ||
| Papaypan | Peru (Yanesha) | Uta De Agua, Mareñets | a La IC50 = 11.2 μg/ml | Valadeau et al. (2009) | |||
| Papaya | Leishmaniasis | - | Valadeau et al. (2010) | ||||
| Celastraceae (4 quotes and 3 species) | Maytenus macrocarpa (Ruiz & Pav.) Briq. | Shoshohuasha | pow-po (ba, to) | Peru (Chayahuita) | Ta’Ta’ | - | Odonne et al. (2009) |
| (2 quotes) | Chuchuhuasi, Chuchuhuasha | dec-po (ba, to) | Peru | Uta | Vásquez-Ocmín et al. (2018) | ||
| Maytenus sp. | Chuchuhuasi (Del Bajo) | (co) | Peru# | Uta | p Lm | Kvist et al. (2006) | |
| IC50 = 10–20 μg/ml | |||||||
| Salacia juruana Loes. | Shoshohuasha Nonin | pow-po (ba, to) | Peru (Chayahuita) | Ta’Ta’ | a La | Odonne et al. (2009) | |
| IC50 = 41 μg/ml | |||||||
| Combretaceae (1 quote and 1 species) | cf Combretum sp. | Ipoyu | fresh-po (sa, to) | French Guiana (Teko) | Leishmaniasis | - | Odonne et al. (2011a) |
| Commelinaceae (2 quotes and 2 species) | Dichorisandra hexandra (Aubl.) C.B.Clarke. | - | (le/st/wp, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Dichorisandra sp. | (st, to) | ||||||
| Convolvulaceae (1 quote and 1 species) | Ipomoea sp. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Costaceae (1 quote and 1 species) | Costus sp. | - | Ecuador# | - | - | Gachet et al. (2010) | |
| Crassulaceae (2 quotes and 2 species) | Kalanchoe gastonis-bonnieri Raym.- Hamet & H. Perrier. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Kalanchoe pinnata (Lam.) pers | (le, or/to) | ||||||
| Cucurbitaceae (4 quotes and 3 species) | Cayaponia sp. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Gurania lobata (L.) Pruski. | |||||||
| Gurania sp. (2 quotes) | |||||||
| Hoja Ancha (Kofan, Putumayoc Colombia) | - | Colombia (Kofan) | Cutaneous leishmaniasis | Gutiérrez et al. (2014) | |||
| Cyclanthaceae (1 quote and 1 species) | Cyclanthus sp. | - | - | Colombia (Kofan) | Cutaneous leishmaniasis | - | Gutiérrez et al. (2014) |
| Dilleniaceae (1 quote and 1 species) | Doliocarpus sp. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Equisetaceae (1 quote and 1 species) | Equisetum bogotense Kunth. | - | (st, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Euphorbiaceae (21 quotes and 15 species) | Acalypha alopecuroidea Jacq. | - | (wp, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Acalypha diversifolia Jacq. | Sanquemula | - | Colombia (Kofan) | Cutaneous leishmaniasis | Gutiérrez et al. (2014) | ||
| Acalypha macrostachya Jacq. (2 quotes) | Mareñtsopar | fresh-po (lt, to) | Peru (Yanesha) | Uta De Agua, Mareñets | a La IC50 = 32.9 μg/ml | Valadeau et al. (2009) | |
| Leishmaniasis | - | Valadeau et al. (2010) | |||||
| Croton draconoides Müll.Arg. | Sangre de Grado | fresh-po, (re, to) | Bolivia# | Cutaneous leishmaniasis | Hajdu and Hohmann (2012) | ||
| Croton lechleri Müll.Arg. (2 quotes) | - | (ex, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Sangre de Drago | (re) | Peru# | Uta | p Lm IC50>100 μg/ml | Kvist et al. (2006) | ||
| Croton sp. | - | Colombia (Kofan) | Cutaneous leishmaniasis | - | Gutiérrez et al. (2014) | ||
| Euphorbia ampliphylla Pax. | Adami | resh-po (sa, to) | Ethiopia (Oromo) | Suleman and Alemu (2012) | |||
| Euphorbia heterophylla L. (2 quotes) | T Ate’Ñeñt | fresh-po (st/le, to) | Peru (Yanesha) | Uta De Agua, Mareñets | a La IC50 = 25.6 μg/ml | Valadeau et al. (2009) | |
| fresh-po, (lt, to) | Leishmaniasis | - | Valadeau et al. (2010) | ||||
| Euphorbia sp. | - | (le/st, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Hura crepitans L. (3 quotes) | Catahua | (re) | Peru# | Uta | p Lm IC50>100 μg/ml | Kvist et al. (2006) | |
| Nëquëra | pow-po (ba, to) | Peru (Chayahuita) | Ta’Ta’ | - | Odonne et al. (2009) | ||
| Soliman | (lt, to) | Bolivia (Chimane indians) | Espundia (cutaneous and mucocutaneous leishmaniasis) | p La; p Lb; p Ld | Fournet et al. (1994) | ||
| Jatropha curcas L. (2 quotes) | Shanëquëra | fresh-po (lt, to) | Peru (Chayahuita) | Ta’Ta’ | - | Odonne et al. (2009) | |
| Kalasapau Poã | fresh-po (ba/fr/ro, to) | French Guiana (Wayãpi) | Leishmaniasis | Odonne et al. (2011a) | |||
| Manihot esculenta Crantz. | - | (le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Maprounea guianensis Aubl. | Ka’Asili | po (le, to) | French Guiana (Wayãpi) | Leishmaniasis | Odonne et al. (2011a) | ||
| Sapium ciliatum Hemsl. | Melekene Sili | fresh-po (lt/ba, to) | |||||
| Sapium marmieri Huber. | Tocaï | fresh-po (lt, to) | Peru (Chayahuita) | - | Odonne et al. (2009) | ||
| Fabaceae (27 quotes and 23 species) | Acacia sp. | Wikamallki | (le, to) | Bolivia (Kechua) | Espundia (cutaneous and mucocutaneous leishmaniasis) | p La; p Lb; p Ld | Fournet et al. (1994) |
| Bauhinia tarapotensis Benth. | - | (le/st, or/to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Cajanus cajan (L.) Huth. | (Ba, to) | ||||||
| Campsiandra angustifolia Spruce ex Benth. | Huacapurana | (co) | Peru# | Uta | p Lm IC50>100 μg/ml | Kvist et al. (2006) | |
| Cassia sp. | - | (le/st, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Copaifera officinalis L. | Bálsamo, copaiba | - | Colombia (Kofan) | Cutaneous leishmaniasis | Gutiérrez et al. (2014) | ||
| Copaifera paupera (Herzog) Dwyer. | Nampihuora | fresh-po (sa, to) | Peru (Chayahuita) | Uta | p La IC50>100 μg/ml | Estevez et al. (2007) | |
| a La IC50>100 μg/ml | |||||||
| Copaiba | (re) | Peru# | Uta | p Lm - NA | Kvist et al. (2006) | ||
| Nanpihuara | fresh (re, or/to) | Peru (Chayahuita) | Ta’Ta’ | - | Odonne et al. (2009) | ||
| Copaiba | “Take Five Drops Of Oil (Exsudate) Diluted In A Tablespoon Of Warm Water, On An Empty Stomach, For Seven Days” fresh (sa, or) | Peru | Uta | Vásquez-Ocmín et al. (2018) | |||
| Deguelia chrysophylla (Kleinhoonte) R.A.Camargo & A.M.G.Azevedo. | Imeku | po | French Guiana (Wayãpi) | Odonne et al. (2011a) | |||
| Desmodium axillare (Sw.) DC. | Së’Ë | pow-po (le, to) | Peru (Chayahuita) | Ta’Ta’ | a La | Odonne et al. (2009) | |
| IC50 = 17 μg/ml | |||||||
| Erythrina sp. | Flor De Mayo | (st, to) | Bolivia | Espundia (cutaneous and mucocutaneous leishmaniasis) | p La; p Lb; p Ld | Fournet et al. (1994) | |
| Grona adscendens (Sw.) H.Ohashi & Ohashi. | - | (le/st/wp/fr, or/to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Hydrochorea corymbosa (Rich.) Barneby & J.W.Grimes. | Kalai Pei | po (ba, to) | French Guiana (Teko) | Leishmaniasis | Odonne et al. (2011a) | ||
| Inga bourgonii (Aubl.) DC. | Inga Sisi, Bougouni | French Guiana (Wayãpi, Teko) | |||||
| Inga edulis Mart. (2 quotes) | Inga Wasa | French Guiana (Wayãpi) | |||||
| - | (le, to) | Ecuador# | - | Gachet et al. (2010) | |||
| Inga oerstediana Benth. | (ba/le, to) | ||||||
| Inga sp. | Inga U | po (ba, to) | French Guiana (Mixed Wayãpi/Teko) | Leishmaniasis | Odonne et al. (2011a) | ||
| Lonchocarpus seorsus (J.F. Macbr.) M. Sousa ex D.A. Neill, Klitg. & G.P. Lewis. | - | (ba, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Lupinus tauris Benth. | Tauri | (le) | Ecuador | Cutaneous leishmaniasis | Weigel et al. (1994) | ||
| Mucuna sp. | - | (ba, or/to) | Ecuador# | - | Gachet et al. (2010) | ||
| Myroxylon balsamum (L.) Harms. | (ba, to) | ||||||
| Phaseolus sp. | (le/st, to) | ||||||
| Piptadenia sp. | (le, to) | ||||||
| Senna reticulata (Willd.) H.S.Irwin & Barneby. | Pole | Inf-po (le) | french Guiana (Wayãpi) | Leishmaniasis | Odonne et al. (2011a) | ||
| Gentianaceae (4 quotes and 2 species) | Coutoubea ramosa Aubl. | Mamanwã Puã | fresh-po (le, to) | French Guiana (Teko) | Leishmaniasis | - | Odonne et al. (2011a) |
| Helia alata (Aubl.) Kuntze. (3 quotes) | Puepa’ ∼Tpan | fresh-po (le, to) | Peru (Yanesha) | Leishmaniasis | Valadeau et al. (2010) | ||
| Campanita | dec-po, (le, or) | Peru | Uta | Vásquez-Ocmín et al. (2018)) | |||
| Puepa’T˜Pan | fresh-po (le, to) | Peru (Yanesha) | Uta De Agua, Mareñets | a La IC50 = 37.4 μg/ml | Valadeau et al. (2009) | ||
| Gesneriaceae (2 quotes and 2 species) | Drymonia turrialvae Hanst. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Drymonia sp. | (wp, to) | ||||||
| Haemodoraceae (1 quote and 1 species) | Xiphidium caeruleum Aubl. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Heliconiaceae (1 quote and 1 species) | Heliconia stricta Huber. | Tanan Tancomë | fresh-po (ro, to) | Peru (Chayahuita) | - | - | Odonne et al. (2009) |
| Hypericaceae (2 quotes and 1 species) | Vismia sp. (2 quotes) | Mareñtsorech | fresh-po (lt, to) | Peru (Yanesha) | Leishmaniasis | - | Valadeau et al. (2010) |
| fresh-po, (st, to) | Uta De Agua, Mareñets | a La IC50 = 54.3 μg/ml | Valadeau et al. (2009) | ||||
| Iridaceae (1 quote and 1 species) | Eleutherine bulbosa (Mill.) Urb. | Wasey Laãnga | fresh-po, (bu, to) | French Guiana (Wayãpi) | Leishmaniasis | - | Odonne et al. (2011a) |
| Lamiaceae (9 quotes and 7 species) | Cantinoa mutabilis (rich.) Harley & J.F.B.Pastore. | - | (le/wp, to) | Ecuador# | - | - | Gachet et al. (2010) |
| (2 quotes) | Tapacha Ina | pow-po (le/ro, to) | Bolivia (Takana indians) | Leishmaniasis | p La IC50 = 29.7 μg/ml | Arévalo-Lopéz et al. (2018) | |
| p Lb IC50 = 9.8 μg/ml | |||||||
| Hyptis capitata Jacq. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Hyptis lacustris A.St.-Hil. ex Benth. (2 quotes) | Ollamepan | fresh-po (st/le, to) | Peru (Yanesha) | Uta De Agua, Mareñets | a La | Valadeau et al. (2009) | |
| IC50 = 10 μg/ml | |||||||
| fresh-po (le, to) | Leishmaniasis | - | Valadeau et al. (2010) | ||||
| Mesosphaerum pectinatum (L.) Kuntze. | - | (le/wp, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Minthostachys sp. | (le/fr, to) | ||||||
| Ocimum campechianum Mill. | (le, to) | ||||||
| Salvia sp. | |||||||
| Lecythidaceae (4 quotes and 3 species | Couroupita guianensis Aubl. (2 quotes) | - | (Fr, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Aya huma | (co) | Peru# | Uta | p Lm IC50>100 μg/ml | Kvist et al. (2006) | ||
| Grias neuberthii J.F.Macbr. | - | (se, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Grias peruviana Miers. | Anpi | fresh-po (fr/ba, to) | Peru (Chayahuita) | Ta’Ta’, Huayani | Odonne et al. (2009) | ||
| Loasaceae (1 quote and 1 species) | Klaprothia fasciculata (C. Presl) Poston. | - | (le/st, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Loganiaceae (1 quote and 1 species) | Strychnos sp. | - | (se, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Loranthaceae (1 quote and 1 species) | Struthanthus sp. | - | (wp, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Malpighiaceae (1 quote and 1 species) | Banisteriopsis caapi (Spruce ex Griseb.) Morton. | - | (le/st, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Malvaceae (11 quotes and 11 species) | Abutilon sp. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Ceiba pentandra (L.) Gaertn. | Kumaka | fresh-po (ba, to) | French Guiana (Wayãpi) | Leishmaniasis | - | Odonne et al. (2011a) | |
| Gossypium barbadense L. | Coton Violet | fresh-po (fl/le, to) | French Guiana (Brazilian and mixed Wayãpi/Teko) | ||||
| Gossypium sp. | Jirbi (O) Tit (A) | “The Seed Is Powdered And Pasted With Butter” pow-po (se, to) | Ethiopia (Oromo) | Cutaneous leishmaniasis | Suleman and Alemu (2012) | ||
| Hibiscus rosa-sinensis L. | - | (le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Hibiscus sabdariffa L. | po (le/st, to) | French Guiana (Wayãpi) | Leishmaniasis | Odonne et al. (2011a) | |||
| Hibiscus sp. | (le, to) | Ecuador# | - | Gachet et al. (2010) | |||
| Matisia cordata Bonpl. | (le, to) | Ecuador# | Gachet et al. (2010) | ||||
| Pavonia fruticosa (Mill.) Fawc. and Rendle. | Sëncopi Së’Ë | fresh-po (le, to) | Peru (Chayahuita) | Odonne et al. (2009) | |||
| Sida rhombifolia L. | Escobilla | (le) | Ecuador | Cutaneous leishmaniasis | Weigel et al. (1994) | ||
| Theobroma cacao L. | - | (se, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Marantaceae (2 quotes and 2 species) | Calathea sp. | Tumbaje (Kofan, Putumayoc Colombia) | - | Colombia (Kofan) | Cutaneous leishmaniasis | - | Gutiérrez et al. (2014) |
| Ischnosiphon sp. | - | (le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Melastomataceae (6 quotes and 5 species) | Adelobotrys sp. | - | (wp, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Antherotoma senegambiensis (Guill. & Perr.) Jacq.-Fél. | (le, na) | Ethiopia (Meinit) | Cutaneous leishmaniasis | Giday et al. (2009) | |||
| Clidemia allardii Wurdack. | (le, to) | Ecuador# | - | Gachet et al. (2010) | |||
| Miconia sp. (2 quotes) | |||||||
| Tococa guianensis Aubl. | |||||||
| Meliaceae (5 quotes and 2 species) | Carapa guianensis Aubl. | Yani | fresh-po (ba/se, to) | French Guiana (Wayãpi) | Leishmaniasis | - | Odonne et al. (2011a) |
| Cedrela odorata L. (4 quotes) | Cedro | dec-bath (ba, to) | Peru | Uta | Vásquez-Ocmín et al. (2018) | ||
| - | (ba/le, or/to) | Ecuador# | - | Gachet et al. (2010) | |||
| Cedro | (co) | Peru# | Uta | p Lm | Kvist et al. (2006) | ||
| IC50 = 60 μg/ml | |||||||
| Nonara | pow-po (ba, to) | Peru (Chayahuita) | Ta’Ta’ | - | Odonne et al. (2009) | ||
| Menispermaceae (2 quotes and 1 species) | Curarea tecunarum Barneby and Krukoff. (2 quotes) | Abuta | (st) | Peru# | Uta | p Lm | Kvist et al. (2006) |
| IC50 > 100 μg/ml | |||||||
| Capari Nonirintë | pow-po (ba, to) | Peru (Chayahuita) | Ta’Ta’ | - | Odonne et al. (2009) | ||
| Meteoriaceae (1 quote and 1 species) | Meteoridium sp. | - | (wp, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Metteniusaceae (1 quote and 1 species) | Poraqueiba sericea Tul. | Umarí | (co) | Peru# | Uta | p Lm | Kvist et al. (2006) |
| IC50>100 μg/ml | |||||||
| Moraceae (7 quotes and 6 species) | Artocarpus altilis (Parkinson) Fosberg. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Castilla elastica Cerv. | Caucho Negro | Colombia (Afro-Colombian and indigenous groups) | p La -NA | Weniger et al. (2001) | |||
| p Lb -NA | |||||||
| p Li - NA | |||||||
| a Lp -NA | |||||||
| Dorstenia foetida Schweinf | Om -Lakef | bath (to) | Saudi Arabia | - | Ali et al. (2017) | ||
| Ficus dendrocida Kunth. | Matapalo | (as) | Ecuador | Cutaneous leishmaniasis | Weigel et al. (1994) | ||
| Ficus insipida Willd. (2 quotes) | Ojé | (re) | Peru# | Uta | p Lm | Kvist et al. (2006) | |
| IC50 > 100 μg/ml | |||||||
| Ojé, Doctor Ojé | fresh-po (lt, to) | Peru | Uta | - | Vásquez-Ocmín et al. (2018) | ||
| Ficus sp. | Matapalo | (lt, to) | Bolivia | Espundia (cutaneous and mucocutaneous leishmaniasis) | p La; p Lb; p Ld | Fournet et al. (1994) | |
| Musaceae (5 quotes and 2 species) | Musa acuminata Colla. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Musa × paradisiaca L. (4 quotes) | Pako | po (to) | French Guiana (Wayãpi) | Leishmaniasis | Odonne et al. (2011a) | ||
| - | (fr, to) | Ecuador# | - | Gachet et al. (2010) | |||
| Plátano | (sa/fr) | Ecuador | Cutaneous leishmaniasis | Weigel et al. (1994) | |||
| Pantapi | pow-po (fr, to) | Peru (Chayahuita) | Ta’Ta’ | Odonne et al. (2009) | |||
| Myristicaceae (3 quotes and 3 species) | Otoba novogranatensis Moldenke. | Otobo | (re, to) | Colombia (Afro-Colombian and indigenous groups) | - | p La + | Weniger et al. (2001) |
| Otoba parvifolia (Markgr.) A.H.Gentry. | fresh-po (re, to) | p Lb + p Li + a Lp + | |||||
| Virola surinamensis (Rol. ex Rottb.) Warb. | Cumala Colorada | “Boil 5 G Of The Bark In One Liter Of Water. Drink One Cup Every Morning For Three Days” dec (ba, or) | Peru | Uta | - | Vásquez-Ocmín et al. (2018) | |
| Myrtaceae (4 quotes and 3 species) | Myrtus communis L. | Al-A’S | fresh-po (le, to) | Saudi Arabia | - | - | Ali et al. (2017) |
| Psidium acutangulum DC. | Alali (Goyave Saut) | fresh-po (ba, to) | French Guiana (Wayãpi) | Leishmaniasis | Odonne et al. (2011a) | ||
| Psidium guajava L. (2 quotes) | - | (ba/le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Guayaba | - | Ecuador | Cutaneous leishmaniasis | Weigel et al. (1994) | |||
| Olacaceae (2 quotes and 1 species) | Minquartia guianensis Aubl. (2 quotes) | Huacapú | (co) | Peru# | Uta | p Lm | Kvist et al. (2006) |
| IC50 < 10 μg/ml | |||||||
| - | (ba/le, to) | Ecuador# | - | - | Gachet et al. (2010) | ||
| Oleaceae (1 quote and 1 species) | Olea europaea L. | Al-aotem | inf-po (st, to) | Saudi Arabia | - | - | Ali et al. (2017) |
| Onagraceae (1 quote and 1 species) | Ludwigia sp. | - | (le/st, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Oxalidaceae (1 quote and 1 species) | Oxalis sp. | ‘Sebastian’ | (le, to) | Bolivia | Espundia (cutaneous and mucocutaneous leishmaniasis) | p La; p Lb; p Ld | Fournet et al. (1994) |
| Papaveraceae (1 quote and 1 species) | Bocconia integrifolia Bonpl. | Palo Amarillo/Amakari | (le/lt/st, to) | Bolivia (Kechua) | Espundia (cutaneous and mucocutaneous leishmaniasis) | p La; p Lb; p Ld | Fournet et al. (1994) |
| Peraceae (2 quotes and 1 species) | Pera benensis Rusby. (2 quotes) | Apaïñiki | (st/ro/ba, to) | Bolivia (Chimane indians) | Espundia (cutaneous and mucocutaneous leishmaniasis) | p La; p Lb; p Ld | Fournet et al. (1994) |
| fresh-po (st, to) | Espundia | L.sp | Fournet et al. (1992a) | ||||
| + | |||||||
| Polypodiaceae (2 quotes and 2 species) | Campyloneurum angustifolium Fée. | Calaguaça | (ss) | Ecuador | Cutaneous leishmaniasis | - | Weigel et al. (1994) |
| Phlebodium decumanum (Willd.) J. Sm. | Coto Chupe | (rh) | Peru# | Uta | p Lm | Kvist et al. (2006) | |
| IC50 > 100 μg/ml | |||||||
| Phyllanthaceae (1 quote and 1 species) | Phyllanthus attenuatus Miq. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Phytolaccaceae (1 quote and 1 species) | Phytolacca dodecandra L'Hér. | Endode (O,A) | “The Root Is Powdered And Pasted With Butter” pow-po (ro, to) | Ethiopia (Oromo) | Cutaneous leishmaniasis | - | Suleman and Alemu, (2012) |
| Picramniaceae (1 quote and 1 species) | Picramnia sp. | - | - | Colombia (Kofan) | Cutaneous leishmaniasis | - | Gutiérrez et al. (2014) |
| Pinaceae (1 quote and 1 species) | Pinus sp. | Piñón | (ss) | Ecuador | Cutaneous leishmaniasis | - | Weigel et al. (1994) |
| Piperaceae (13 quotes and 10 species) | Piper aduncum L. | Matico Chico | (le, to) | Bolivia | Espundia (cutaneous and mucocutaneous leishmaniasis) | p La; p Lb; p Ld | Fournet et al. (1994) |
| Piper barbatum Kunth. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Piper consanguineum (Kunth) Steud. | Matico | (le) | Ecuador | Cutaneous leishmaniasis | Weigel et al. (1994) | ||
| Piper hispidum Sw. (2 quotes) | Atukan | fresh-po (le, to) | Peru (Chayahuita) | Uta | p La = 69 μg/ml | Estevez et al. (2007) | |
| a La = 5 μg/ml | |||||||
| - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) | ||
| Piper loretoanum Trel. | Atocan | pow-po (le, to) | Peru (Chayahuita) | a La = 13.6 μg/ml | Odonne et al. (2009) | ||
| Piper mediocre CDC. | - | ||||||
| Piper musteum Trel. | - | (le, to) | Ecuador# | Gachet et al. (2010) | |||
| Piper peltatum L. (2 quotes | Sipu-sipu | (to) | Bolivia (Kechua) | Espundia (cutaneous and mucocutaneous leishmaniasis) | p La; p Lb; p Ld | Fournet et al. (1994) | |
| - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) | ||
| Piper umbellatum L. | Amintë Huëron | fresh-po (le, to) | Peru (Chayahuita) | Ta’Ta’ | Odonne et al. (2009) | ||
| Piper sp. (2 quotes) | - | (le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Atocan | pow-po (le, to) | Peru (Chayahuita) | Odonne et al. (2009) | ||||
| Ta’Ta’ | |||||||
| Plantaginaceae (3 quotes and 3 species) | Conobea scoparioides (Cham. & Schltdl.) Benth | Hierba De Sapo | (ae, to) | Colombia (Afro-Colombian and indigenous groups) | - | p La + | Weniger et al. (2001) |
| p Lb + | |||||||
| p Li + | |||||||
| a Lp + | |||||||
| Plantago major L. | Llantén | (le) | Ecuador | Cutaneous leishmaniasis | - | Weigel et al. (1994) | |
| Scoparia dulcis L. | - | (le/wp, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Poaceae (3 quotes and 3 species) | Panicum trichoides Sw | Lapakunga | - | Peru# | Uta or Chagas | p Lm - NA | Kvist et al. (2006) |
| Pharus sp. | - | (le, in) | Ecuador# | - | - | Gachet et al. (2010) | |
| Zea mays L. | (fl/le/fr, to) | ||||||
| Polygonaceae (3 quotes and 3 species) | Rumex nepalensis Spreng. | Tult | fresh-po (ro/le, to) | Ethiopia | ‘Gurtb’ leishmaniasis | - | Teklehaymanot (2009) |
| Rumex pulcher L. | - | (le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Triplaris weigeltiana (Rchb.) Kuntze. | Tangarana | (co) | Peru# | Uta or Chagas | p Lm | Kvist et al. (2006) | |
| IC50 > 100 μg/ml | |||||||
| Portulacaceae (1 quote and 1 species) | Portulaca pilosa L. | Tui | po (ae, to) | French Guiana (Wayãpi) | Leishmaniasis | - | Odonne et al. (2011a) |
| Primulaceae (1 quote and 1 species) | Clavija weberbaueri Mez. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Pteridaceae (1 quote and 1 species) | Pityrogramma calomelanos (L.) Link. | Seseronapan | inf-bath (le, to) | Peru (Yanesha) | Uta De Agua, Mareñets | a La | Valadeau et al. (2009) |
| IC50 = 88 μg/ml | |||||||
| Rhamnaceae (1 quote and 1 species) | Gouania lupuloides (L.) Urb. | - | (ba, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Rosaceae (1 quote and 1 species) | Prunus sp. | - | (le/st, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Rubiaceae (20 quotes and 15 species) | Calycophyllum multiflorum Griseb. (2 quotes) | Capirona | fresh-po (ba, to) | Peru | Uta | Vásquez-Ocmín et al. (2018) | |
| Quëmanan | pow-po (ba, to) | Peru (Chayahuita) | Ta’Ta’ | a La | Odonne et al. (2009) | ||
| IC50 > 100 μg/ml | |||||||
| Calycophyllum spruceanum (Benth.) Hook.f. ex K.Schum. | Capirona | (co) | Peru# | Uta | p Lm - NA | Kvist et al. (2006) | |
| Capirona decorticans Spruce (2 quotes) | inf-bath (ba, to) | Peru | Uta | - | Vásquez-Ocmín et al. (2018) | ||
| Yoquinan | Peru (Chayahuita) | Ta’Ta’ | Odonne et al. (2009) | ||||
| Llukina | “The Bark Is Boiled And Watery Preparation Is Drunk Twice Dauly Until Cicatrisation” dec (ba, or) | Uta | p La - NA | Estevez et al. (2007) | |||
| a La IC50>100 μg/ml | |||||||
| Coussarea sp. | - | (ba, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Genipa americana L. | Isa | fresh-po (fr, to) | Peru (Chayahuita) | Ta’Ta’ | Odonne et al. (2009) | ||
| Hamelia sp. | - | (le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Kutchubaea cf. oocarpa (Standl.) C.H.Perss. | Guayabochi | po (st/ba, to) | Bolivia# | Cutaneous leishmaniasis | Hajdu and Hohmann, (2012) | ||
| Ladenbergia sp. | Quina, Miraña, Guayabate, Resbalomono, Sicomue (Col.) | - | Colombia (Kofan) | Cutaneous leishmaniasis | Gutiérrez et al. (2014) | ||
| Palicourea sp. | - | ||||||
| Psychotria sp. (4 quotes) | (le, to) | Ecuador# | - | Gachet et al. (2010) | |||
| (le/st, to) | |||||||
| (le, to) | |||||||
| Beso Rojo | - | Colombia (Kofan) | Cutaneous leishmaniasis | Gutiérrez et al. (2014) | |||
| - | |||||||
| Rudgea bremekampiana Steyerm. | (le, to) | Ecuador# | - | Gachet et al. (2010) | |||
| Rudgea loretensis Standl. | Niahuënara | fresh-po (ba, to) | Peru (Chayahuita) | a La | Odonne et al. (2009) | ||
| IC50 = 34–39.6 μg/ml | |||||||
| Spermacoce laevis Lam. | - | (le/st, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Uncaria guianensis (Aubl.) J.F.Gmel. | Ochara | (or/to) | Peru (Chayahuita) | Ta’Ta’ | Odonne et al. (2009) | ||
| Uncaria tomentosa (Willd. ex Schult.) DC. | - | Cutaneous leishmaniasis | |||||
| Rutaceae (12 quotes and 7 species) | Angostura longiflora (K.Krause) Kallunki. | Evanta | (le/st/ro, to) | Chimane indians (Bolivia) | Espundia (cutaneous and mucocutaneous leishmaniasis) | p La; p Lb; p Ld | Fournet et al. (1994) |
| Citrus aurantiaca (L.) Swingle. | Limón | - | Ecuador | Cutaneous leishmaniasis | - | Weigel et al. (1994) | |
| Citrus × aurantiifolia (Christm.) Swingle | Nimo | fresh-po (fr/ba, to/in) | Peru (Chayahuita) | Ta’Ta’, Huayani | Odonne et al. (2009) | ||
| (2 quotes) | Citron Vert | inf-po (fr, to) | French Guiana (Wayãpi) | Leishmaniasis | Odonne et al. (2011a) | ||
| Citrus × aurantium L. (3 quote) | Naranja | - | Ecuador | Cutaneous leishmaniasis | Weigel et al. (1994) | ||
| Toronja | (ro) | Peru# | Uta | p Lm | Kvist et al., (2006) | ||
| IC50 = 95 μg/ml | |||||||
| Mandarina | - | Ecuador | Cutaneous leishmaniasis | - | Weigel et al. (1994) | ||
| Citrus × limon (L.) Osbeck. | Lìmón | (ro) | Peru# | Uta | p Lm | Kvist et al. (2006) | |
| IC50 = 70 μg/ml | |||||||
| Citrus sp. (2 quotes) | - | (se, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| (fr, to) | |||||||
| Ruta graveolens L. (2 quotes) | (le/fr, to) | ||||||
| Ruda | (le) | Ecuador | Cutaneous leishmaniasis | Weigel et al. (1994) | |||
| Sapindaceae (1 quote and 1 species) | Dodonaea viscosa Jacq. | Shath | po (le, to) | Saudi Arabia | Leishmaniasis | - | Ali et al. (2017) |
| Sapotaceae (7 quotes and 6 species) | Chrysophyllum prieurii ADC. | Cotoquinilla | fresh-po (le, to) | Peru | Uta | - | Vásquez-Ocmín et al. (2018) |
| Chrysophyllum sp. | - | (se, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Manilkara sp. | Baytakini | inf-bath, lt, to | French Guiana (Teko) | Leishmaniasis | Odonne et al. (2011a) | ||
| Pouteria caimito (Ruiz & Pav.) Radlk. | Caimito | (le) | Peru# | Uta | p Lm | Kvist et al. (2006) | |
| IC50 > 100 μg/ml | |||||||
| (2 quotes) | Guëpa | fresh-po (le, to) | Peru (Chayahuita) | - | - | Odonne et al. (2009) | |
| Pouteria guianensis Aubl. | Caimito | Peru | Uta | Vásquez-Ocmín et al. (2018) | |||
| Pouteria torta subsp. tuberculata (Sleumer) T.D.Penn. | - | (le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Siparunaceae (3 quotes and 1 species) | Siparuna sp. (3 quotes) | Huaya Muuktuna | “The Woody Stem Grated Ad Boiled. This Preparation Is Drunk Three Times A Day For 8 Days” dec (st, or) | Peru (Chayahuita) | Uta | p La | Estevez et al. (2007) |
| IC50 = 30 μg/ml | |||||||
| a La IC50>100 μg/ml | |||||||
| Huayan Motonan | fresh-po (le, to) | - | - | Odonne et al. (2009) | |||
| Smilacaceae (4 quotes and 2 species) | Smilax salicifolia Griseb. | Sankarin | “Roots Are Boild, And This Preparation Is Drunk Many Times A Day, Until Symptoms Disappear” dec (ro, or) | Peru (Chayahuita) | - | p La - NA | Estevez et al. (2007) |
| a La IC50>100 μg/ml | |||||||
| Smilax sp. (3 quotes) | - | (wp, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Zarzaparilla | (ro) | Peru# | Uta or Chagas | p Lm | Kvist et al. (2006) | ||
| IC50 > 100 μg/ml | |||||||
| (le) | Ecuador | Cutaneous leishmaniasis | - | Weigel et al. (1994) | |||
| Solanaceae (22 quotes and 14 species) | Brugmansia sp. (2 quotes) | - | (le/fl, to) | Ecuador# | - | - | Gachet et al. (2010) |
| (le, to) | |||||||
| Brunfelsia grandiflora D.Don. (3 quotes) | Ohuinishqui | pow-po (le, to/in) | Peru (Chayahuita) | Ta’Ta’, Huayani | Odonne et al. (2009) | ||
| - | (le, to) | Ecuador# | - | Gachet et al. (2010) | |||
| Chiric Sanango | (ro) | Peru# | Uta | p Lm | Kvist et al. (2006) | ||
| IC50 = 53 μg/ml | |||||||
| Capsicum sp. (2 quotes) | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| No’Ca | fresh-po, (le/fr, to) | Peru (Chayahuita) | Ta’Ta’, Huayani | a La | Odonne et al. (2009) | ||
| IC50 = 28 μg/ml | |||||||
| Cestrum lindenii Dunal. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Cestrum sp. | - | Colombia (Kofan) | Cutaneous leishmaniasis | - | Gutiérrez et al. (2014) | ||
| Markea sp. | (le, to) | Ecuador# | - | Gachet et al. (2010) | |||
| Nicotiana tabacum L. (3 quotes) | (le, to) | ||||||
| Pinchi | pow-po (le, to/in) | Peru (Chayahuita) | Ta’Ta’, Huayani | Odonne et al. (2009) | |||
| Tabaco | fresh-po (le fermented, to) | French Guiana | Leishmaniasis | Odonne et al. (2011a) | |||
| Solanum americanum Mill. (2 quotes) | - | (wp, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Yerba Mora (Mortino) | (fr/le) | Ecuador | Cutaneous leishmaniasis | Weigel et al. (1994) | |||
| Solanum crinitum Lam. | Y˜ U ãsisi | po (ba, to) | French Guiana (Wayãpi) | Leishmaniasis | Odonne et al. (2011a) | ||
| Solanum incanum L. | Al-hadak | po | Saudi Arabia | Ali et al. (2017) | |||
| Solanum mammosum L. | - | (le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Solanum subinerme Jacq. | Y˜ U Sõwú | po (ba, to) | French Guiana (Wayãpi) | Leishmaniasis | Odonne et al. (2011a) | ||
| Solanum sp. (2 quotes) | - | (le, to) | Ecuador# | - | Gachet et al. (2010) | ||
| (fr, to) | |||||||
| Witheringia solanacea L'Hér. | (le/st/wp, to) | ||||||
| Talinaceae (1 quote and 1 species) | Talinum paniculatum (Jacq.) Gaertn. | Yoro Qui’Sha | fresh-po (ro, to) | Peru (Chayahuita) | Ta’Ta’ | a La IC50>100 μg/ml | Odonne et al. (2009) |
| Thurniaceae (1 quote and 1 species) | Thurnia sphaerocephala (Rudge) Hook.f. | Kwayiti | (fr, to) | French Guiana (Teko) | Leishmaniasis | - | Odonne et al. (2011a) |
| Ulmaceae (1 quote and 1 species) | Ampelocera edentula Kuhlm. | Sou’Sou’ | (st/ro, to) | Bolivia (Chimane indians) | Espundia (cutaneous and mucocutaneous leishmaniasis) | p La; p Lb; p Ld | Fournet et al. (1994) |
| Urticaceae (3 quotes and 3 species) | Cecropia obtusa Trécul. | Ama’I | fresh-po (ba, to) | French guiana (wayãpi) | Leishmaniasis | - | Odonne et al. (2011a) |
| Urera laciniata Wedd. | - | (le/st, to) | Ecuador# | - | Gachet et al. (2010) | ||
| Urtica dioica L. | (le, to) | ||||||
| Verbenaceae (8 quotes and 6 species) | Duranta sp. | - | (le, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Lantana camara L. | Gachet et al. (2010) | ||||||
| Lantana trifolia L. | Yahua’Tan Huëron | pow-po (le, to) | Peru (Chayahuita) | Odonne et al. (2009) | |||
| Lantana sp. (3 quotes) | T Epeshpan | inf-po (le, to) | Peru (Yanesha) | Uta De Agua, Mareñets | a La | Valadeau et al. (2009) | |
| IC50 = 10 μg/ml | |||||||
| Leishmaniasis | - | Valadeau et al. (2010) | |||||
| - | (le/st, to) | Ecuador# | - | Gachet et al. (2010) | |||
| Verbena litoralis Kunth. | (le/st/wp, to) | ||||||
| Verbena microphylla Kunth. | Berbena/Verbena | (le) | Ecuador | Cutaneous leishmaniasis | - | Weigel et al. (1994) | |
| Viburnaceae (1 quote and 1 species) | Sambucus nigra L. | - | (le/st, to) | Ecuador# | - | - | Gachet et al. (2010) |
| Violaceae (1 quote and 1 species) | Leonia sp. | (le, or/to) | Ecuador# | - | - | Gachet et al. (2010) | |
| Zamiaceae (3 quotes and 3 species) | Zamia amazonum D.W.Stev. | Oreja De Perro | fresh-po (ro, or/to) | Peru (Chayahuita) | Uta | p La>100 μg/ml | Estevez et al. (2007) |
| a La = 81 μg/ml | |||||||
| Zamia poeppigiana Mart. & Eichler. | Ukuapampe | fresh-po (st, to) | p La>100 μg/ml | ||||
| a La = 33 μg/ml | |||||||
| Zamia sp. | Ocohua Panp | fresh-po (ba, to) | - | - | Odonne et al. (2009) | ||
| Zingiberaceae (2 quotes and 1 species) | Zingiber officinale Roscoe. (2 quotes) | Natio | fresh-po (rh, to/in) | Peru (Chayahuita) | Ta’Ta’, Huayani | - | Odonne et al. (2009) |
| - | (wp, to) | Ecuador# | - | Gachet et al. (2010) |
Traditional recipe: Decoction—dec; Decoction used as a poultice - dec-po; Decoction used as a bath—dec-bath; Fresh plant used as a poultice—fresh-po; Infusion—inf; Infusion used as a bath—inf-bath; Infusion used as a poultice—inf -po; Poultice—po; Powder plant used as a poultice—pow-po. Plant Part: Aerial Part—ae; Apical meristem—am; Bark—ba; Bulb—bu; Cloves—cl; Cortex—co; Exudate—ex; Flower—fl; Fruit—fr; Leaf -le; Látex—lt; Oleogum Resin—or; Resin - re; Rhizome—rh; Root—ro; Sap—sa; Seed—se; Shoot—sho; Stalk—sta; Stem—st; Tuber—tu; Whole Plant -wp. Route: Inhalation—in; Nasal—na; Oral—or; Topical—to. Countries and Communities: Ecuador# - Kichwa of Amazonia, Kichwa of the Andes, Chachi, Mestizo, Afroecuadorian, Awa, Épera; Bolivia# - Guarasug'we indigenous and Chiquitano mestizos; Peru# - Mestizo, Chayahuito, Cocama, Quechua, Ticuna. ppromastigote; aamastigote; La—L. amazonensis; Lae—L. aethiopica; Lb—L. braziliensis Lp—L. panamensis; L. donovani; Lm—L. major; Li—L.infantum; IC50—Inhibitory concentration 50%; NA—Not active.
In the above citations we counted each species and genus as a single citation, for example, in the case of the genus Gurania sp. although it was cited two times in the articles, it was accounted as one species, because it was not possible to classify Gurania sp. as one or two species. Additionally, it is not possible to know if these two quotes of the genus Gurania in this table belong to Gurania lobate (L.) Pruski, because taxonomic elements are lacking in the published articles. Thus, the 292 plants here presented refer to 216 species (identified until species level) and 76 genera (the ones counted only once).
TABLE 2.
In vivo activity of medicinal plants. Families, plant species, clinical form of leishmaniasis, parasite species, extract or purified molecules employed in experimental treatment, doses, route of administration, scheme of treatment and efficacy of the treatments in experimental leishmaniasis.
| Family | Plant species | Clinical form and parasite species | Treatment | Route of administration | Efficacy | Ref |
|---|---|---|---|---|---|---|
| Amaranthaceae | Dysphania ambrosioides (L.) Mosyakin & Clemants | CL – L.a | Essential oil (30 mg/kg) | Intraperitoneal, once a day for 15 days | Reduced by ∼68% the number of parasites | Monzote et al. (2006) |
| Leaves - hydroalcoholic crude extract (5 mg/kg) | Intralesional, 5 injection at every 4 days | Intralesional: Reduced parasitism by ∼66, 95, 66% in the skin, lymph nodes and spleen | Patrício et al. (2008) | |||
| Oral, once a day for 15 days | Oral: No effect | |||||
| Amaryllidaceae | Allium sativum L. | CL—L.m | Fresh garlic bulb—aqueous extract (20 mg/kg) | Intraperitoneal, daily for 15 days | Reduced by ∼ 65% the size of cutaneous lesions | Ghazanfari et al. (2000) |
| Fresh and dried garlic bulb—aqueous extract (20 mg/kg) | Intraperitoneal, daily for 15 days | Dried extract—inhibited lesion progression and parasite multiplication | Gamboa-León et al. (2007) | |||
| Fresh extract—No effect | ||||||
| CL—L.m | Fresh garlic bulb—methanolic extract (20 mg/kg) | Oral and intraperitoneal, daily for 4 weeks | CL—oral and intraperitoneal treatment reduced by ∼90 and 80% the size of skin lesion, respectively | Wabwoba et al. (2010) | ||
| VL—L.d | VL—oral and intraperitoneal treatments reduced by ∼65 and 55% the number of splenic parasites, respectively | |||||
| Apocynaceae | Pentalinon andrieuxii (Müll.Arg.) B.F.Hansen & Wunderlin | CL—L.me | Root hexanic extract (10 μg) | Topical; once a day for 6 weeks | Reduced in 2 times the number of parasites in the skin | Lezama-Dávila et al. (2014) |
| VL—L.d | Liposome-encapsulated pentalinonsterol (2.5 mg/kg) | Intravenous | Reduction of 64, 83 and 57% of parasites in the liver, spleen and bone marrow, respectively | Gupta et al. (2015) | ||
| Tabernaemontana divaricata (L.) R.Br. ex Roem. & Schult. | VL—L.d | Voacamine (2.5—5 mg/kg) | Intraperitoneal; twice a week for three weeks | Hepatic parasitism | Chowdhury et al. (2017) | |
| 2.5 and 5 mg/kg: decreased in ∼3 and 30 times the tissue parasitism, respectively | ||||||
| Splenic parasitism | ||||||
| 2.5 and 5 mg/kg: decreased in ∼5 and 15 times the tissue parasitism, respectively | ||||||
| Asteraceae | Munnozia maronii (André) H.Rob | CL—L.a | Dehydrozaluzanin C (100 mg/kg) | Once a day for 14 days | Reduced the severity of cutaneous lesions | Fournet et al. (1993b) |
| Bignoniaceae | Handroanthus serratifolius (Vahl) S.O.Grose | CL—L.a | Lapachol (25 mg/kg) | Oral; once a day for 10 days | CL—reduction of ∼24.5 times the number of parasites | Araújo et al. (2019) |
| VL—L.i | VL—reduction of ∼4.6 and 5.3 the number of parasites in the spleen and liver, respectively | |||||
| Euphorbiaceae | Croton caudatus Geiseler | LV—L.d | Leaves - semi purified fraction (1.25; 2.5; 3.75; and 5 mg/kg) | Oral; five consecutive days | Hepatic parasitism | Dey et al. (2015) |
| 2.5, 3.75 and 5 mg/kg reduced the parasitism by ∼ 40, 60, and 65%, respectively | ||||||
| Splenic parasitism | ||||||
| 1.25; 2.5, 3.75, and 5 mg/kg reduced the parasitism by 36.2, 51.7, 66.71 and 69.12%, respectively | ||||||
| Fabaceae | Pleurolobus gangeticus (L.) J.St.-Hil. ex H.Ohashi & K.Ohashi | VL—L.d | Whole plant - ethanolic extract; hexane; n-butanol and aqueous fractions (100 mg/day) | Oral route, once a day for 5 consecutive days | Animals treated with n-butanol fraction reduced the number of splenic parasites by 46.7% | Singh et al. (2005) |
| Copaifera martii Hayne | CL—L.a | Copaiba oil (100 mg/kg) | Subcutaneous; oral; topical; oral + topical; for 4 weeks | Oral, oral plus topical treatments decreased the lesion size by ∼ 4 times | dos Santos et al. (2011) | |
| Piperaceae | Piper rusbyi C. DC. | CL—L.a | Flavokavain B (1–5 mg/kg) | Subcutaneous, alternative days for 28 days | Animals treated with 5 mg/kg displayed reduction in the size of lesions by 32.2% | Flores et al. (2007) |
| Piper pseudoarboreum Yunck | CL—L.a | (E)-piplartine | Intralesional, once a day for 4 days | Reduction of skin lesions and visceralization by 35 and 55%, respectively | Ticona et al. (2020) | |
| (25 mg/kg) | ||||||
| Rutaceae | Angostura longiflora (K.Krause) Kallunki | CL—L.a | Root and stem bark- total alkaloid extract (50 mg/kg) | Oral, twice daily for 15 days | Root extract: Oral and intralesional treatments reduced the parasite load by 95 and 96%, respectively | Fournet et al. (1996) |
| Intralesional, five times at intervals of 5 days | Stem extract: Intralesional and oral treatments decreased the parasite loads by 99 and 49%, respectively | |||||
| CL—L.b | Bark - total alkaloid extract (12.5mg/animal) | Intraperitoneal, once a day until the week 14 | Reduced in ∼10 times the number of parasites | Calla-Magariños et al. (2013) | ||
| Solanaceae | Solanum lycocarpum A.St.-Hil | CL—L.me | Solamargine plus solasonine (10 μg) | Topical, once a day for 6 weeks | Reduced in 3 times the number of parasites | Lezama-Dávila et al. (2016) |
| Solanum havanense Jacq., Solanum myriacanthum Dunal, Solanum nudum Humb. & Bonpl. ex Dunal, Solanum seaforthianum Andrews | CL—L.a | Leaves—hydroalcoholic extracts (30 mg/kg) | Intralesional, every 4 days, 5 doses | Reduction of parasites in animals treated with | Cos et al. (2018) | |
| S. havanense (93.6%), S. nudum (80%) S. myriacanthum (56.8%) and S. seaforthianum (49.9%) | ||||||
| Urticaceae | Urtica thunbergiana Siebold & Zucc. | CL—L.m | Plant aqueous extract (150; 200, and 250 mg/kg) | Intramuscular and intralesional, three times/week for 30 days | All treatments inhibited lesion development and suppressed parasite dissemination, with special activity to the intralesional treatment | Badirzadeh et al. (2020) |
CL—Cutaneous leishmaniasis; VL—visceral leishmaniasis; L.a—Leishmania (Leishmania) amazonensis; L.d—Leishmania (Leishmania) donovani; L.i—Leishmania (Leishmania) infantum; L.m—Leishmania (Leishmania) major; L.me—Leishmania (Leishmania) mexicana; L.b—Leishmania (Viannia) braziliensis.
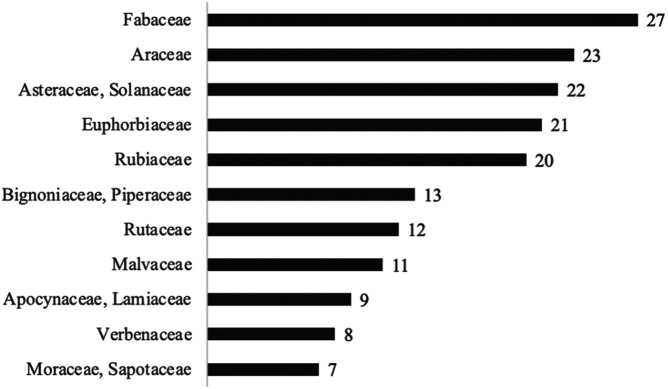
FIGURE 1.
Frequency of the most cited families referring to the 378 quotes of species extracted from the 20 articles, only those species quoted at least 7 times.
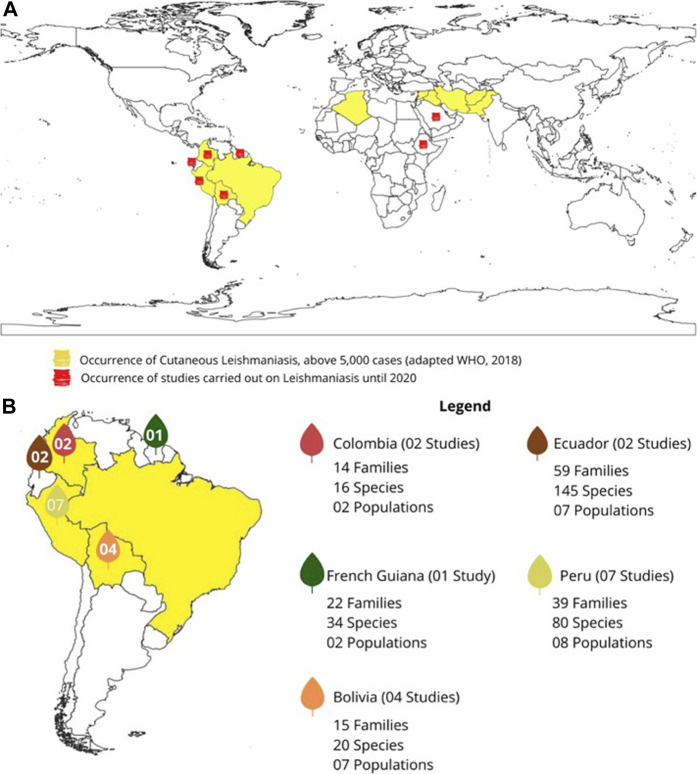
FIGURE 2.
(A) World map of the occurrence of cutaneous leishmaniasis and the countries where they have had studies on leishmaniasis. (B) Highlights of studies on leishmaniasis carried out in South America.
Table 1 summarizes the findings observed in the ethnopharmacological surveys and contains the following data: family, scientific and vernacular names, traditional recipe (part of plant used and route), country (traditional community involved in the knowledge), traditional use (emic term, the one used by the communities), and whether the study included laboratory assays to determine the efficacy of plant extracts on Leishmania sp.
The map (Figure 2) was prepared using the software QGIS (available at www.qgis.org) using a collection of spatial data from the Brazilian Institute of Geography and Statistics (available at https://mapas.ibge.gov.br/bases-e-referencial/bases-cartograficas/digital meshes) and using the geographic coordinates reference system "sirgas 200" (Geocentric Reference System for the Americas).
Plants Recommended for the Treatment of leishmaniasis by traditional Communities Worldwide
Plants (species, families and vernacular names)
From the 20 selected articles, 378 quotes were obtained referring to 292 plants indicated by several traditional communities around the world to treat leishmaniasis. These plants belong to 89 taxonomic families (Table 1). To record the number of plants, each species and genus was counted as a single citation; for example, in the case of the genus Gurania sp. Although it was cited two times in the articles, it was considered one species because it was not possible to classify Gurania sp. as one or two species. Additionally, it is not possible to know if these two examples of the genus Gurania belong to Gurania lobate (L.) Pruski—as illustrated in Table 1 - because taxonomic elements were not available in the articles. Thus, the 292 plants presented herein refer to 216 species (identified until the species level) and 76 genera (the ones counted only once) (Table 1). Only 74% of the plants available in the articles could be identified to the species level, pointing out the need for more adequate ethnopharmacology methods during fieldwork.
Considering those 378 plant quotes, the most frequent families used by traditional communities were Fabaceae (27 quotes); Araceae (23); Asteraceae and Solanaceae (22 each), Euphorbiaceae (21) and Rubiaceae (20) (Figure 1).
Moreover, 207 out 292 plants had their vernacular names (Table 1) described in the publications. The absence of these data makes ethnopharmacological analysis precarious, since recording the vernacular name of a certain plant can provide valuable information about its potential pharmacological effects. An example discussed by us in a previous work is the plant caprankohirehô (Euphorbiaceae), which has been used by the Brazilian Krahô Indians as a tranquilizer, and the literal translation of caprankohireho is the ‘leaf of turtle spine’. This translation describes the pharmacological effect of this plant—which induces ‘slowness’ (Rodrigues and Barnes, 2013). This and many other examples demonstrate that the careful recording of vernacular names of plants during ethnopharmacological studies is extremely relevant to increase the probability of finding bioactive molecules according to the knowledge of traditional communities.
In addition, from the 216 plants described up to the species level, only 29 were present in at least two articles; six out 29 species were described in three articles: Brunfelsia grandiflora D. Don (Solanaceae), Capirona decorticans Spruce (Rubiaceae), Chelonanthus alatus (Aubl.) Pulle (Gentianaceae), Hura crepitans L. (Euphorbiaceae), Nicotiana tabacum L. (Solanaceae), Tabernaemontana sananho Ruiz & Pav. (Apocynaceae), while the following four species were cited in four articles: Carica papaya L. (Caricaceae), Cedrela odorata L. (Meliaceae), Copaifera paupera (Herzog) Dwyer (Fabaceae), and Musa × paradisiaca L. (Musaceae) (Table 1).
In Table 1, it was also observed that most of the species were cited by traditional communities from only one country, 26 species were cited by at least two countries. Three of them belonged to the traditional communities of Peru, Ecuador, and French Guiana simultaneously: Carica papaya L. (Caricaceae), Musa × paradisiaca L. (Musaceae), and Nicotiana tabacum L. (Solanaceae).
Recipes (parts of the plants used, method of preparation, route of administration)
As registered in Table 1, not all ethnopharmacological studies gave information on the parts of the plants used, the form of preparation, route of administration, dose, and/or duration of the treatment. Considering the 378 quotes, only 138 (36.5%) specified the recipes, 351 (92.9%) mentioned the plant parts used in the recipe, and 300 (79.4%) detailed the routes of administration of the recipes. The absence of these data offers two possible justifications. The first possible explanation may be the lack of adequate methods during ethnopharmacological fieldwork; although this may be less likely, such works may reflect the lack of knowledge of these data on the part of the communities under study. The absence of these data can impact further studies on phytochemistry and pharmacology and, as a consequence, the discovery of new bioactive molecules of medicinal plants. On the other hand, several ethnopharmacological studies described in great detail the recipes used in the treatment of leishmaniasis. An example is the study conducted by Vásquez-Ocmín and collaborators (Vásquez-Ocmín et al., 2018), which described the use of the plant Virola surinamensis (Rol. ex Rottb.) Warb. (Myristicaceae), whose popular name is Cumala Colorada (Table 1). The bark was used as described by the interviewee during the field work “… Boiled 5 g of the bark in 1 L of water. Drink one cup every morning for three days … ”. In other words, all necessary information was offered in detail, except for possible contraindications and adverse events of the plant.
Among the available data in the 378 quotes, it was observed that the parts of the plants most frequently used in local medicine were leaves (42.3% of recipes), followed by bark (15%), stems (11.6%), and roots (5.6%). On the other hand, the fruits, aerial parts, flowers, oleoresins, seeds, tubers, whole plants, stalks, shoots, saps, resin, rhizomes, apical meristems, bulbs, cloves, exudates and latex were used at minor frequencies. The most suitable route of administration for plants was the topical route (74.6% of the recipes), followed by the oral route (5%) and inhalation/nasal route (1.3%); for a large number of plants, no route of administration was indicated (20.6%).
In addition, as shown in Table 1, 17.2% of the methods used to prepare the recipes refer to fresh poltices (lotion juice in natura, crushed, crude parts, paste) applied on the affected area, named fresh-po in Table 1, followed by pow-po (6.3%), which are powered plants that are also applied on the wounds. Finally, with minor frequencies, other methods were mentioned, such as decoction and infusion that can be ingested and/or used to wash the affected area. In these last cases, they were presented in Table 1 as inf-po (infusion used as a poltice) and dec-po (decoction used as a poltice).
In the selected studies, a predominance of leaves (42.3%) used topically (74.6%) for the treatment of leishmaniasis was observed. Several studies, including those carried out by some members of our team, point out the use of leaves and the topical route in traditional treatments for leishmaniasis. Thus, the quilombolas in the Pantanal from Poconé, Brazil, use a decoction-type tea with the leaf/bark of mangava-brava—Lafoensia pacari A. St.-Hil. (Lythraceae) to be ingested twice a day; the juice from the leaves of mastruz, Dysphania ambrosioides (L.) Mosyakin & Clemants (Amaranthaceae), is used as a compress to treat leishmaniasis; finally, the river dwellers from Amazon, Brazil, use the bark of mango, Mangifera indica L. (Anacardiaceae), as a compress directly on the cutaneous lesions (Rodrigues, 2006).
Knowledge of traditional communities in the world
The analysed works showed that traditional communities spread across seven countries use plants for the treatment of leishmaniasis. The majority of these communities are located in Latin America. Ecuador is the most representative of the range of plants indicated in the treatment of leishmaniasis (59 botanical families; 145 plant species; seven traditional communities; two articles), followed by Peru (39; 80; 8; 7), French Guiana (22; 34; 2; 1), Bolivia (15; 20; 7; 4) and Colombia (14; 16; 2; 2). In addition to these countries, studies developed in Saudi Arabia (8; 8; 1; 1) and Ethiopia (6; 6; 2; 3) (Figure 2) also highlighted the use of medicinal plants in the treatment of leishmaniasis.
Brazil and Colombia are countries with a high occurrence of cases of cutaneous leishmaniasis, above five thousand. However, the data collected show few or no published studies involving the use of traditional knowledge for the treatment of this infectious disease, with only two studies found in Colombia and none in Brazil. Although during this review it was not possible to obtain Brazilian studies focusing on “ethnopharmacology x leishmaniasis”, some studies within the scope of ethnopharmacology have offered information on the use of natural resources for the treatment of leishmaniasis (França et al., 1996; Rodrigues, 2006; Santos et al., 2019), but they were not included in this review, as they were not found during the Boolean search.
Figure 2 (a) highlights in yellow the endemic countries that had more than five thousand cases of cutaneous leishmaniasis until 2018 (World Health Organization, 2019). In part (b) of Figure 2, emphasis was given to the numbers of botanical families and species, articles, and traditional communities that contributed to ethnopharmacological research in each of the countries of Latin America, since these were the most expressive when considering the data on traditional knowledge vs. leishmaniasis.
The data on the traditional communities that participated in the studies analyzed herein exhibited the relevant contribution of traditional knowledge from South America in the treatment of leishmaniasis, and this is correlated with the continent that displays the highest number of cases of cutaneous leishmaniasis in the world, suggesting that in some areas, medical services are not available, and people need to use alternative medicines. Figure 2 shows the amount of data associated with the traditional treatment of leishmaniasis generated by traditional communities in countries with a high incidence of leishmaniasis. Of all countries with cases of cutaneous leishmaniasis, only 40% also presented ethnopharmacological studies on the disease. Among them, the country that presented the most studies was Peru (7 studies), followed by Bolivia (4). Both are low-income countries, with deficiencies in their economic and educational systems. The main traditional communities cited among the analyzed articles belong to the following ethnic groups from Ecuador: Kichwa of Amazonia, Kichwa of the Andes, Chachi, Mestizo, Afroecuadorian, Awa and Épera (contributing 38.3% of the citations of plants to treat leishmaniasis), followed by Peruvian ethnic groups Chayahuita (22.7%), Wayãpi of French Guiana (7.6%) and Yanesha of Peru (5.5%). In addition, 12.9% of the citations did not mention the community that provided traditional knowledge, and some of the authors referred to them as local people or ethnic groups. In relation to the total number of studies analyzed, two out seven countries (Ethiopia and Saudi Arabia) had no record of the occurrence of cutaneous leishmaniasis above 5,000 cases. According to World Health Organization (2019), both Ethiopia and Saudi Arabia had a record of 100–999 cases of cutaneous leishmaniasis.
It is important to note that leishmaniasis exhibits different clinical forms that can be recognized and named in different ways depending on the specificity of each country and ethnic group. In ethnopharmacological studies, the correlation between the emic terms (the ones used by the traditional communities) and their corresponding etic terms (the ones used in biomedicine) may provide insights to guide further pharmacological studies since they are the bases for suggesting the potential bioactivity of these resources (Pagani et al., 2017). Approximately half of the articles present records of emic terms to leishmaniasis, such as “Gurtb”, in Ethiopia (Teklehaymanot, 2009); “Espundia” for the Chimane Indians, in Bolivia (Fournet et al., 1992b, 1994); “Ta’Ta’ ”, for the Chayahuitas in Peru (Odonne et al., 2009); “Uta” and “Uta De Agua” for some communities in Peru, such as Chayahuitas or Yaneshas (Estevez et al., 2007; Valadeau et al., 2009; Vásquez-Ocmín et al., 2018).
Plants tested for leishmaniasis
From the 292 plants registered, 79 described in nine of the twenty selected articles were tested against Leishmania sp. Among the Leishmania species investigated in these studies, L. (L.) amazonensis predominated, followed by L. (L.) major and L. (V.) braziliensis. The results of the tests with some of these plants are available in more than one publication, including the resins and saps of Copaifera paupera (Herzog) Dwyer and the bark and cortex of Spondias mombin L. (Kvist et al., 2006; Estevez et al., 2007), the latex and resin of Hura crepitans L. (Fournet et al., 1994; Kvist et al., 2006), the stem bark and root bark of Pera benensis Rusby (Fournet et al., 1992a, 1994), and the leaves of Pseudelephantopus spicatus (B. Juss. ex Aubl.) Rohr ex C.F. Baker (Odonne et al., 2009; 2011b). Below, descriptions of the in vitro activity of extracts or purified molecules from the plants used in traditional communities will be provided.
Estevez and colleagues (Estevez et al., 2007) investigated the leishmanicidal activity of nineteen plants indicated by the Chayahuite community to treat cutaneous leishmaniasis. Among them, only the ethanolic extracts produced with the leaves of Piper hispidum Sw. and P. strigosum Trel (Piperaceae) showed expressive activity against intracellular forms of L. (L.) amazonensis.
Odonne and collaborators (Odonne et al., 2009) observed that different plants have been used by the Chayahuites in the treatment of leishmaniasis, probably because they live in an endemic area of the disease and have limited access to medical centers. The leishmanicidal activities of ethanolic extracts produced with the selected plants were evaluated in axenic amastigote forms of L. (L.) amazonensis. Ethanolic extracts produced with the aerial parts of Desmodium axillare, Pseudoelephantopus spicatus and Piper loreteanum were the most active extracts at eliminating amastigote forms (IC50 between 13.6 and 27 μg/ml). Ethanolic extracts produced with the bark and/or leaves of Rudgea loretensis Standl and Salacia juruana Loes showed moderate leishmanicidal activity (IC50 between 34 and 41 μg/ml). In addition, all these plants were clearly indicated to treat leishmaniasis. On the other hand, it was also demonstrated that ethanolic extracts produced with plants that have not been used to treat leishmaniasis showed significant leishmanicidal activity (IC50 between 10 and 15.7 μg/ml), as is the case for ethanolic extracts produced with the leaves, roots and aerial parts of Piper sanguineispicum Trel., Cybianthus anthuriophyllus Pipoly, (Myrsinaceae), Clibadium sylvestre (Aubl.) Baill. (Asteraceae), respectively.
Further studies characterize the major components in the ethanolic extract produced with the leaves of Pseudoelephantopus spicatus. The purified molecules 1) 8,13-diacetyl-piptocarfol, 2) 8-acetyl-13-O-ethyl-piptocarfol [also isolated from other species: Vernonia mollissima (D. Donex Hook. & Arn.), Eirmocephala megaphylla (Hieron.) H. Rob., Chrysolaena verbascifolia, Lepidaploa remotiflora, and Vernonia scorpioides] and 3) ursolic acid (Odonne et al., 2011b) were assayed on axenic amastigote forms of L. (L.) amazonensis. Molecules 1 (IC50 = 0.2 μM) and 2 (IC50 = 0.37 μM) showed leishmanicidal activity (in vitro) comparable with amphotericin B (IC50 = 0.41 μM), which is used in the treatment of human leishmanial infections. Molecule 3 also eliminated amastigote forms with high activity (IC50 = 0.99 μM). Although leishmanicidal action has been observed, the authors considered that the second compound originated from the chemical reaction resulting from the extraction of the ethanolic extract and not from the plant in natura. This work corroborated the leishmanicidal effects observed during traditional treatment (Odonne et al., 2009; 2011b); in addition, it showed for the first time the production and accumulation of such classes of secondary metabolites in P. spicatus and supported further preclinical works with molecule 3 in the context of cutaneous and visceral leishmaniasis (Jesus et al., 2020; de Jesus et al., 2021), which in fact reinforces the occurrence of important bioactive molecules in plants traditionally used to treat leishmaniasis.
In the community of Buena Vista, Bolivia, thirty-eight plants have been used to treat skin problems, and eight of them were recommended by Tacana medicine for the treatment of leishmaniasis (Arévalo-Lopéz et al., 2018). Extracts were produced with all these plants, and the leishmanicidal activity assayed on promastigote forms of L. (L.) amazonensis and L. (V.) braziliensis. It was observed that 42.1% of them were inactive and 23.7% highly active, and the leishmanicidal activity of 34.2% of them was dependent on the part of plant used to produce the extracts. With respect to the plants that were specifically indicated to treat leishmaniasis, extracts produced with the leaves of Hyptis mutabilis (Laminaceae) and the bark of Jacaranda glabra (Bignoniaceae) and Tessaria integrifolia (Asteraceae) were active on L. (L.) amazonensis and L. (V.) braziliensis. Further studies showed that fractions purified from the crude ethanolic extracts of J. glabra and T. integrifolia were also active toward promastigote forms of L. (L.) amazonensis, L. (L.) aethiopica, L. (V.) braziliensis and L. (V.) lainsoni. Although extracts and fractions produced with these plants displayed multispecies action, it was noted that the selective indexes of these natural medicines were low when compared with amphotericin B. On the other hand, it is relevant to observe that in the field, the production of these natural medicines is completely different from those produced in the laboratory, and it can account for the extraction of cytotoxic molecules. Furthermore, this study showed the leishmanicidal activity of five species of Tacana medicinal plants for the first time, showing the relevance of ethnopharmacology to characterize leishmanicidal molecules.
An ethanopharmacological study conducted among Chimane Indians from Amazonian Bolivia showed that stem bark Pera benensis Rusby has been used to treat cutaneous leishmaniasis caused by L. (V.) braziliensis. In the laboratory, it was verified that chloroform extracts containing quinones were active on promastigote forms of L. (V.) braziliensis (Fournet et al., 1992a). Further fractionation of the extract led to the identification of plumbagin, 3,3′-biplumbagin, 8-8′-biplumbagin and lupeol. Promastigote forms of L. (L.) amazonensis, L. (V.) braziliensis and L. (L.) donovani were eliminated when incubated with plumbagin; 3,3′-biplumbagin; 8-8′-biplumbagin; and intracellular amastigotes of L. (L.) amazonensis were highly sensitive to plumbagin and 3,3′-biplumbagin, which were able to eliminate 100 and 85% of intracellular parasites at 50 μg/ml.
Subsequently, an ethnopharmacological study conducted in Bolivia among settlers and Chimane Indians recorded that 14 plants were used to treat leishmaniasis as a topical poultice. Ten plants were indicated by the colonists and four by the indigenous people (Fournet et al., 1994). Extracts were prepared with different plant parts using petroleum ether, chloroform, and ethyl acetate of ethanol 50%; additionally, alkaloidal and quinoic fractions were also produced. Extracts were tested in vitro against L. (L.) amazonensis, L. (V.) braziliensis, and L. (L.) donovani; and from 10 plants indicated by the colonists, only Bocconia integrifolia Bonpl. and B. pearcei (Papaveraceae) were active. However, according to Plants of the World online, these plants are currently classified as synonyms, and the accepted name is Bocconia integrifolia Bonpl. Considering the four plants indicated by the Chimane Indians, extracts produced with the leaves, stem bark or root bark of the following three species were active on Leishmania sp.: Galipea longiflora K. Krause, Ampelocera edentula Kulm. and Pera benensis Rusby. In previous studies, it was demonstrated that 4-hydroxy-1-tetralone from A. edentula, three naphthoquinones from P. benensis, and quinoline alkaloids from G. longiflora displayed leishmanicidal activity (Fournet et al., 1989; 1992a; 1992b; 1993a). These studies reinforce that medicinal plants indicated by the Chimane Indians are potentially more effective than those indicated by the group of colonists, and extracts, fractions or purified molecules may be used as prototype drugs to treat human leishmaniasis according to the traditional knowledge of native people from Colombia.
An ethnopharmacological survey performed in northeastern Peru recorded 289 uses of plants for the treatment of leishmaniasis (Kvist et al., 2006). Twenty-eight plants were selected, and ethanolic extracts were produced and tested toward promastigote forms of L. (L.) major. It was observed that crude ethanolic extracts produced with the cortex of Maytenus sp., Minquartia guianensis, Aspidosperma rigidum, with the roots of Mansoa standleyi, Rauwolfia sp., Tabernaemontana sp., with the bulb of Curcuma longa and with the resin of Copaifera pauperi displayed significant IC50 values against promastigote forms (between 10 and 20 μg/ml). In addition, 62 citations of the genus Maytenus were recorded in the treatment of leishmaniasis, suggesting that in addition to the high bioactivity of this plant on L. (L.) major (IC50 < 10 μg/ml), it has been used by different people living in traditional communities.
In the Yanesha community, Peru, ninety-four plants have been used to treat symptoms related to malaria and cutaneous leishmaniasis. In this community, twelve plants have been employed in the treatment of leishmaniasis (Valadeau et al., 2009); however, only eleven plants were tested in a laboratory context. In this case, ethanolic extracts of the plant parts were produced and assayed in axenic amastigote forms of L. (L.) amazonensis. Among plants used by the Yanesha group, ethanolic extracts produced from the leaves of Carica papaya L. (Caricaceae), Hyptis lacustris A. St.-Hil. ex Benth. (Lamiaceae) and Lantana sp. (Verbenaceae) were highly active plants for the elimination of parasites (IC50 = 10 μg/ml). However, it is important to note that the community uses the latex of C. papaya, the exudate of the bark or leaves from the stem from Hyptis lacustris and finally uses concentrated infusion of Lantana sp. This was the first study to record the leishmanicidal activity of latex from papaya (C. papaya). In addition, it was found that the treatment widely used in the fight against leishmaniasis by the community consists of the application of whitish latex, recently dripped from Acalypha macrostachya Jacq. (Euphorbiaceae) in the entire affected area for three consecutive days. This recipe is used for both cutaneous and mucocutaneous leishmaniasis. On the other hand, other plants used as traditional medicinal, such as Vismia sp. (Clusiaceae) and Pityrogramma calomelanos (L.) Link (Pteridaceae) showed low/moderate activity in the laboratory, possibly because the authors were unable to legitimately reproduce the mode of use, that is, testing the latex recently extracted from these plants, as indicated by the healers in the Yanesha community. On the other hand, some plants not employed in the traditional treatment of leishmaniasis also displayed significant leishmanicidal activity, as is the case for hydroalcoholic extracts produced with the leaves of Cestrum racemosum Ruiz & Pav. (IC50 = 9.8 μg/ml), Piper dennisii Trel. (IC50 = 10 μg/ml) and with the rhizome of Hedychium coronarium J. König (IC50 = 10 μg/ml), Renealmia alpinia (Rottb.) Maas (IC50 = 9 μg/ml) and Renealmia thyrsoidea (Ruiz & Pavon) Poepp. & Endl. (IC50 = 10 μg/ml).
In Colombia, an ethnopharmacological survey was carried out among Afro-Colombians and indigenous people to record plants traditionally used to treat malaria, Chagas disease and leishmaniasis. Based on ethnopharmacological and chemotaxonomy, the antiprotozoal activity of methylene chloride and methanolic extracts produced with 44 plants were analyzed. Among these plants, five have been used to treat leishmaniasis (Weniger et al., 2001). In this case, it was verified that the aerial parts of Conobea scoparioides (Scrophulariaceae) and Hygrophila guianensis (Acanthaceae), the bark exudate of Otoba novogranatensis and O. parviflora (Myristicaceae), and Castilla elastica (Moraceae) have been used as traditional medicines to treat leishmaniasis. In vitro experiments showed that methylene chloride extract produced with the leaves of C. scoparioides was highly active at eliminating promastigote forms of L. (L.) amazonensis, L. (L.) infantum and L. (V.) braziliensis; additionally, macrophages infected with L. (V.) panamensis and incubated with this extract for 96 h eliminated 50% of parasites at 6.7 μg/ml. Methylene chloride and methanolic extracts produced with the fruits of O. novagranatensis were also active against the same species, and on amastigote forms, both eliminated intracellular L. (V.) panamensis (IC50 = 6.5 and 10.6 μg/ml, respectively). Apolar and polar extracts produced with the leaves of this plant also killed promastigote forms; however, they displayed only low or moderate activity on intracellular amastigotes (IC50 = 177 and >40 μg/ml, respectively); similar findings were observed with the apolar extract produced with the bark of O. parvifolia. Although some extracts displayed moderate or low activity on amastigote forms, once more, it becomes important to highlight the fundamental differences in the production of the natural medicines used by healers in communities and the way that researchers produce extracts in laboratories and use them in biological systems, which obviously minimizes the complexity of human physiology and the interactions between molecules, cells and parasites.
Table 1 summarizes the leishmanicidal activity of plants described above, displaying the 50% inhibitory concentrations (IC50) if available, parasite species and form (amastigote or promastigote) used and described in the selected articles.
Contributions of some botanical families and species in the experimental treatment of leishmaniasis
In the present review, it was verified that at least 292 plants may be employed in the traditional treatment of leishmaniasis in different communities around the world, and it was verified that some families of plants have been widely used by communities, such as Apocynaceae, Araceae, Bignoniaceae, Asteraceae, Euphorbiaceae, Lamiaceae, Fabaceae, Malvaceae, Piperaceae, Rubiaceae, Rutaceae, Solanaceae and Verbenaceae. Below are described mainly in vivo studies about the efficacy of extracts and/or purified molecules from the botanical families used by traditional communities. Furthermore, details about the treatment, route of administration, parasite species, clinical form and efficacy of treatment are shown in Table 2.
Plants from the Apocynaceae family are rich in bioactive secondary metabolites (Siddiqui et al., 1986; Arambewela and Ranatunge, 1991; Muruganandam et al., 2000; Bhaskar and Natarajan, 2015; Kaunda and Zhang, 2017), and such molecules may have activity on tissue amastigote forms. In this regard, it was found that the genus Tabernaemontana has been cited several times in different communities as healing symptoms related to leishmaniasis, but few scientific advances have been made with this genus. Despite few works about the species traditionally used, it has been verified that the leishmanicidal effect of molecules purified from a related species, T. catharinensis A. DC., may be linked to the immunomodulatory activity of this genus (Soares et al., 2007). In addition, it was verified that the leishmanicidal molecule voacamine, an indole alkaloid purified from T. divaricata (L.) R.Br. ex Roem. & Schult, altered the mitochondria, kinetoplast and nucleus of L. (L.) amazonensis and L. (L.) donovani promastigotes, and such morphological changes correlated with the relaxation activity of topoisomerase IB. Additionally, it was verified that BALB/c mice infected with wild-type or drug-resistant L. donovani treated with 2.5 and 5 mg/kg voacamine by the intraperitoneal route twice a week for three weeks displayed fewer parasites in the spleen and liver than the untreated control (Chowdhury et al., 2017), reinforcing that this genus contains important classes of antileishmanial molecules. Although these species were not cited by traditional communities, it is possible that plants belonging to the same genus share similar compounds. Hexanic extract produced with the roots of the less cited species from this family, Pentalinon andrieuxii (Müll.Arg.) B.F.Hansen & Wunderlin, was active on promastigote forms of L. (L.) mexicana in vitro (Lezama-Dávila et al., 2007) and BALB/c mice infected with L. (L.) mexicana treated with 10 μg of this extract by the topical route, once a day for six weeks, presented fewer parasites in the skin; in addition, treated animals produced high levels of IL-12 cytokine along with the expression of the costimulatory molecules CD40, CD80, and CD86 (Lezama-Dávila et al., 2014), suggesting that, at least in part, the leishmanicidal activity in vivo may be associated with stimulation of innate immune cells. Further studies led to the identification of sterols from the roots of this plant that were active on intracellular amastigote forms of L. (L.) mexicana with an IC50 between 0.03 and 14.5 μM (Pan et al., 2012), and the sterol pentalinonsterol encapsulated in liposomes, given by the intravenous route at 2.5 mg/kg, significantly reduced the number of viable parasites in the liver, spleen and bone marrow of BALB/c mice infected with L. (L.) donovani; additionally, this molecule activated the Th1 immune response in treated animals (Gupta et al., 2015). The genus Aspidospermum has been cited as a source of natural medicine against leishmaniasis, and bioactive alkaloids purified from different species of this genus may be responsible for the efficacy of plants observed in traditional communities (Tanaka et al., 2007; Reina et al., 2014); however, studies involving experimental models of leishmaniasis (in vivo) have not been performed thus far.
Different species of plants from the family Bignoniaceae were cited 13 times to treat symptoms associated with leishmaniasis in communities. Among these plants, it was demonstrated that the naphthoquinone lapachol, purified from Handroanthus serratifolius (Vahl) S.O.Grose, was active (in vitro) on amastigote forms of L. (L.) amazonensis (Costa et al., 2017), and the possible mechanism of action of this molecule involves programmed cell death (Araújo et al., 2019). In addition to the in vitro studies, it was demonstrated that lapachol, given orally for 10 days, decreased the number of amastigote forms of L. (L.) amazonensis in experimental cutaneous leishmaniasis, and a significant reduction in splenic and hepatic parasites was observed in visceral leishmaniasis caused by L. (L.) infantum (Araújo et al., 2019). In the same way, it was verified that Jacaranda species have also been traditionally used to treat leishmaniasis; however, only in vitro studies were carried out (Passero et al., 2007).
With respect to the family Asteraceae, 22 citations of plants that have been used in the context of skin diseases by traditional communities were observed. However, few works have been developed thus far with the most frequently cited genera. The genus Munnozia, cited as a healing agent, was studied with respect to leishmanicidal and tryponocidal activities. In this regard, the petroleum ether extract produced with the leaves of Munnozia maronii (André) H.Rob and the isolated compound dehydrozaluzanin C showed in vitro activity against L. (L.) amazonensis; additionally, it was demonstrated that dehydrozaluzanin C, given once a day for 14 days at 100 mg/kg, reduced the severity of cutaneous lesions in the experimental model of cutaneous leishmaniasis caused by L. (L.) amazonensis (Fournet et al., 1993b). Sesquiterpene lactones have also been isolated from Pseudelephantopus spicatus (Juss. ex Aubl.) C.F.Baker), a species used by traditional communities, and the leishmanicidal activity of such molecules (IC50 = 0.2–0.99 μM) was similar to the activity of amphotericin B (IC50 = 0.41 μM), a second-line drug used in the treatment of patients with leishmaniasis (Odonne et al., 2011b). The thiophene derivative 5-methyl-2,2':5′,2″-terthiophene purified from Porophyllum ruderale (Jacq.) Cass. was also active on axenic amastigote forms of L. (L.) amazonensis (Takahashi et al., 2011), and such activity was associated with physiological and morphological alterations in parasite mitochondria (Takahashi et al., 2013). Despite these interesting in vitro data described with plants from the Asteraceae family that have been used by traditional communities, it was observed that experiments confirming the efficacy in vivo of molecules purified from plants used in traditional medicine are missing; however, in vitro data obtained with bioactive molecules suggest that plants produce and accumulate leishmanicidal compounds.
In the present review, 21 citations related to the traditional uses of plants from the Euphorbiaceae family were observed. Among them, the genus Croton has been used to treat skin diseases, and the medicinal activity can be related to the molecule linalool present in the essential oil of Croton cajucara Benth., which displayed a strong leishmanicidal potential against amastigote forms of L. (L.) amazonensis (IC50 = 8.7 ng/ml) and immunomodulatory effects on peritoneal macrophages that, once treated, were able to produce elevated amounts of nitric oxide, an important microbicidal molecule (Rosado et al., 2003). In addition, other compounds, such as 7-hydroxycalamenene, trans-dehydrocrotonin, trans-crotonin, and acetylaleuritolic acid, from C. cajucara Benth. also inhibited the proliferation of intracellular amastigote forms of L. (L.) amazonensis or L (L.) chagasi (Rosado et al., 2003; Rodrigues et al., 2013; Lima et al., 2015). Despite these phytochemical studies revealing the molecular diversity of the Croton genus as well as the leishmanicidal potential of molecules, only one study showed that a fraction purified from the hexanic extract from the leaves of C. caudatus Geiseler, given by oral route for five consecutive days at 5 mg/kg, reduced the number of viable parasites by 65 and 69% in the spleen and liver of experimental animals infected with L. (L.) donovani, respectively (Dey et al., 2015), and this therapeutic activity was associated with the restoration of IFN-γ levels in CD4 T lymphocytes. In addition to Croton species, several molecules purified from Euphorbia genus showed leishmanicidal activity in vitro on intracellular amastigotes, such as piceatannol, simiarenol, 1-hexacosanol, β-sitosterol, and β-sitosterol-3-O-glucoside (Duarte et al., 2008; Amin et al., 2017). Tannin- and saponin-rich fractions from the root of E. wallichii Hook.f. eliminated extra and intracellular forms of L. tropica with similar activities as the standard treatment; additionally, these fractions permeabilized the parasite’s cell membrane and triggered apoptosis in L. tropica (Ahmad et al., 2019), but to the best of our knowledge, no in vivo studies were performed with all of these purified molecules.
Traditional communities have used plants from the Fabaceae family to treat symptoms related to leishmaniasis. The genera Copaifera, Desmodium, Lonchocarpus, and Senna have been cited and recorded in different studies. The copaiba oil extracted from different species of Copaifera showed activity against promastigote and amastigote forms of L. (L.) amazonensis (Santos et al., 2008); additionally, it was observed that BALB/c mice infected with L. (L.) amazonensis and treated with the essential oil of Copaifera martii Hayne at 100 mg/kg by the oral, subcutaneous and topical routes displayed smaller skin lesions than untreated BALB/c mice (dos Santos et al., 2011). Further studies suggested that pinifolic and kaurenoic acids (Dos Santos et al., 2011) or β-caryophyllene may be responsible, at least in part, for the in vitro and in vivo activities observed in such studies. The species Desmodium adscendens and D. axillare have also been used as traditional remedies. Although scientific records about the leishmanicidal activity of such species do not exist, studies have already shown that the n-butanol fraction produced with whole Pleurolobus gangeticus (L.) J.St.-Hil. ex H.Ohashi & K.Ohashi plants given orally once a day for five consecutive days inhibited the multiplication of amastigote forms in the spleen of experimental animals with visceral leishmaniasis caused by L. (L.) donovani (Singh et al., 2005); on the other hand, the ethanolic extract and hexanic and aqueous fractions displayed moderate and weak leishmanicidal activity in vivo. Furthermore, the therapeutic activity of D. gangeticum may be associated with the occurrence of glycolipids, aminoglucosyl glycerolipids and cerebrosides in extracts (Mishra et al., 2005). Similarly, Senna reticulata is used by traditional communities, but pharmacological studies with respect to leishmanicidal activity have been performed only with S. spectabilis (DC.) H.S.Irwin & Barneby, and its activity was related to the presence of alkaloids (Melo et al., 2014), which can possibly interact with leishmanial arginase (Lacerda et al., 2018), inducing cell death; however, no proof of concept exists concerning the in vivo properties of such molecules.
Plants from the Piperaceae family have also been used by traditional communities, and there are many works addressing advances with the genus Piper. These works describe the molecular diversity of the genus as well as the leishmanicidal activity of the purified molecules. In this regard, it was observed that chalcones, phenolic compounds, lignans, and terpenes, among other molecules, display leishmanicidal properties (Torres-Santos et al., 1999; Hermoso et al., 2003; Cabanillas et al., 2010; Vendrametto et al., 2010; Garcia et al., 2013; Dal Picolo et al., 2014; Capello et al., 2015; Ceole et al., 2017). Additionally, it was observed that the possible cellular targets of such molecules were the mitochondria and plasma membrane of Leishmania sp. (Misra et al., 2009; de Oliveira et al., 2012), in addition, these molecules can stimulate immune responses, facilitating the destruction of intracellular parasites (Chouhan et al., 2015). Despite knowledge about the molecular diversity of the Piper genus and the bioactivity of such molecules on Leishmania sp., only a few works have shown the in vivo relevance of this genus. Chalcone flavokavain B purified from the leaves of Piper rusbyi C. DC. given by the subcutaneous route to BALB/c mice infected with L. (L.) amazonensis at 5 mg/kg was able to reduce the size of lesions by 32% (Flores et al., 2007), and (E)-piplartine isolated from the leaves of Piper pseudoarboreum Yunck, given once a day for 4 days by the intralesional route at 25 mg/kg, reduced the size of cutaneous lesions by 35% and inhibited the visceralization of L. (L.) amazonensis in BALB/c mice (Ticona et al., 2020).
Plants from the Solanaceae family have been cited by traditional communities to treat symptoms related to leishmaniasis; however, only a few scientific advances have been made with plants of this family. Recently, it was demonstrated that hydroalcoholic extracts produced with the leaves of Solanum havanense Jacq., S. myriacanthum Dunal, S. nudum Humb. & Bonpl. ex Dunal, and S. seaforthianum Andrews showed high selective indexes on L. (L.) amazonensis (in vitro) and in experimental leishmaniasis caused by L. (L.) amazonensis, it was observed that the hydroalcoholic extract produced with S. havanense, given every 4 days (5 doses) by the intralesional route at 30 mg/kg, decreased the number of parasites by 93.6%. Hydroalcoholic extracts produced with the leaves of S. nudum, S. myriacanthum and S. seaforthianum reduced the number of amastigotes in the skin of experimental animals by 80, 56.8 and 49.9%, respectively (Cos et al., 2018). In addition, it was demonstrated that the combination of the alkaloids solamargine and solasonine purified from S. lycocarpum A.St.-Hil. topically applied at 10 μg in the skin of C57BL/6 mice infected with L. (L.) mexicana reduced the size of cutaneous lesions and the number of tissue parasites (Lezama-Dávila et al., 2016), emphasizing the presence of potent bioactive molecules in the family Solanaceae.
Plants from the families Rubiaceae and Rutaceae have been used by traditional communities in the treatment of leishmaniasis; however, few works have characterized and tested the bioactive molecules of these plants (Muhammad et al., 2003; Quintin et al., 2009). Despite this, studies have shown that quinolines and alkaloids from Angostura longiflora (K.Krause) Kallunki (Rutaceae) exhibit leishmanicidal activity (in vitro), and in vivo, it was demonstrated that quinolic alkaloids from the bark or root of this plant given by oral or intralesional routes to experimental animals infected with L. (L.) amazonensis or L. (V.) braziliensis controlled the experimental infection, reducing the number of parasites in the skin (Fournet et al., 1996; Calla-Magariños et al., 2013); additionally, these studies suggested that animals treated by the intraperitoneal route displayed a significant reduction in parasites.
Some families were less cited by healers in communities; however, interesting results have been observed in the scientific literature, as is the case for Dysphania ambrosioides (Amaranthaceae) (L.) Mosyakin & Clemants. This plant has been used by a rural population in a coastal area of Bahia state, Brazil, in cases of cutaneous leishmaniasis (França et al., 1996). Experimentally, it was verified that the essential oil given by the intraperitoneal route once a day for 15 days at 30 mg/kg reduced the number of amastigote forms in the skin of BALB/c mice by 68% (Monzote et al., 2006). In addition, it was demonstrated that hydroalcoholic extract produced with the leaves of this plant given by the intralesional route reduced the number of amastigote forms of L. (L.) amazonensis in the skin, lymph nodes and spleen of BALB/c mice. However, the treatment given by the oral route did not alter the course of infection. The essential oil of D. ambrosioides (L.) Mosyakin & Clemants given by the oral route also reduced the number of amastigote forms in experimental cutaneous leishmaniasis caused by L. (L.) amazonensis (Patrício et al., 2008). Furthermore, it was demonstrated that the essential oil of this plant and its components can affect the mitochondria of parasites (Monzote et al., 2006, 2007; Pastor et al., 2015). Allium sativum L. (Amaryllidaceae), garlic, was cited only once as a traditional remedy for the treatment of leishmaniasis; however, advances concerning leishmanicidal activity in vitro and in vivo have been demonstrated. In the experimental model of cutaneous leishmaniasis caused by L. (L.) major, it was demonstrated that aqueous extract produced with dried bulbs of garlic, given by intraperitoneal route daily for 15 days at 20 mg/kg, inhibited the progression of cutaneous lesions as well as parasite multiplication. However, it was demonstrated that aqueous extract produced with fresh bulbs given at the same dose and route was inactive (Gamboa-León et al., 2007), but interestingly, it was verified that the aqueous extract produced with fresh bulbs of garlic collected in Hamadan (Iran), given by the intraperitoneal route at 20 mg/kg daily for 15 days to BALB/c mice infected with L. (L.) major, was able to reduce the size of lesions by 65% (Ghazanfari et al., 2000). These data suggest that the origin of garlic may impact the pharmacological activity of this plant. Methanolic extract produced with fresh bulbs and given daily by oral or intraperitoneal routes for 4 weeks also inhibited the size of cutaneous lesions in experimental animals infected with L. (L.) major by approximately 90 and 80%, respectively; and in experimental visceral leishmaniasis caused by L. (L.) donovani, the same treatment reduced the rate of parasitism in the spleen by 65 and 55% when it was given by oral or intraperitoneal routes, respectively (Wabwoba et al., 2010). Furthermore, the efficacy of A. sativum L. in leishmaniasis may be associated with the immunomodulatory activity of molecules produced by this plant (Ghazanfari et al., 2000; Gamboa-León et al., 2007). Unfortunately, no biomolecules were purified and assayed in vivo in an attempt to produce a standardized medicine.
Maytenus sp. (Celastraceae) has also been cited as a natural medicine used in leishmaniasis. It has been demonstrated that different species have leishmanicidal activity, and such activity can be mainly related to terpenes and sesquiterpenes synthesized by this genus (Alvarenga et al., 2008). Although only in vitro studies have been carried out so far, the most important finding is related to the potential of molecules against multidrug resistant parasites (Pérez-Victoria et al., 1999; Kennedy et al., 2001, 2011). The plant Juniperus excelsa M. Bieb (Cupressaceae) was cited only once by traditional communities, and few studies have been conducted on this species. The first published work showed that different extracts of the aforementioned species were able to eliminate L. major promastigotes (Moein et al., 2017). A further triple-blind randomized controlled clinical trial showed that 82% of patients with cutaneous leishmaniasis treated with a topical formulation produced with the leaves of J. excelsa M. Bieb hydroalcoholic extract plus cryotherapy healed the cutaneous lesions compared to the placebo control; additionally, they healed the lesions shorter than placebo control (Parvizi et al., 2017), suggesting that this plant species has bioactive molecules that can be further explored to develop new leishmanicidal drugs.
In this study, Curcuma longa L. (Zingiberaceae) was cited as a natural remedy for leishmaniasis only once. However, the leishmanicidal activity of curcumins has been recorded since 2000 (Rasmussen et al., 2000; Saleheen et al., 2002), and further works demonstrated that synthetic derivatives also present high activity at eliminating extra- and intracellular parasites (Gomes et al., 2002; Chauhan et al., 2018; Teles et al., 2019), and such activity may be related to programmed cell death in L. donovani (Chauhan et al., 2018). Despite these advances, in vivo studies with Curcuma longa L. or curcumin are scarce in the literature.
In addition, it was verified that the species Urtica dioica L. (Urticaceae) was cited only once, and just one work was published characterizing the leishmanicidal activity of this plant. In this regard, BALB/c mice infected with L. major and treated with the aqueous extract of E. dioica L. at 150, 200 or 250 mg/kg by intralesional or intramuscular routes three times per week for 30 days significantly decreased the size of cutaneous lesions and suppressed the dissemination of parasites to the spleen; furthermore, the in vivo activity was related to the reduction of arginase levels (Badirzadeh et al., 2020). This enzyme is able to inhibit nitric oxide production, and therefore, low levels of this circulating enzyme may be essential to achieve cure in leishmaniasis.
Details about families, plant species, clinical form of leishmaniasis, parasite species, extract or purified molecules employed in the treatment, doses, route of administration, scheme of treatment and efficacy of the treatments in experimental leishmaniasis are shown in Table 2.
Limitations
In the present review, it was observed that only 20 articles addressed the traditional treatment of leishmaniasis using medicinal plants. Despite the few articles published to date, a substantial diversity of plants (89 plant families referring to 292 plants) has been cited by 29 traditional communities from different nationalities, which in fact supports the local treatment of symptoms related to leishmaniasis. On the other hand, this potential is far from reflecting reality, and there is still considerable work from an ethnopharmacological point of view to be conducted, which will certainly expand our knowledge about medicinal plants with antileishmanial properties. In this review, the authors emphasize that future ethnopharmacological studies must follow methodological rigor, consistent with the data to be collected. This should be carefully considered because in this review, several limits were found in terms of analysis due to the unavailability of some ethnopharmacological data in the articles consulted. As examples, only 74% of the plants were identified to the species level, 36.5% specified the recipes, 20.6% detailed the route of administration, and only 55.5% mentioned the vernacular names of the plants. Furthermore, 12.9% of the articles did not mention the community that provided traditional knowledge, and some of the authors referred to them as local people or ethnic groups. This is a critical point in the field of ethnopharmacology, as it weakens the right to intellectual property of the traditional communities involved. Furthermore, it was observed that practically no article mentioned the contraindications and possible adverse reactions to these plants, although it is well known that traditional communities often obtain this knowledge from their therapeutic practices. These specific data would be relevant in the case of the development of drugs to treat leishmaniasis, since it is necessary to find drugs with fewer adverse reactions in comparison with those currently in use.
In addition, although a plethora of plants have been described in the traditional treatment of leishmaniasis, only a few works were capable of describing them from a chemical or pharmacological point of view. Furthermore, only a minority of them analysed, in experimental models of cutaneous or visceral leishmaniasis, the efficacy of such plants or purified molecules. Finally, it would be promising to perform bioprospective studies on such plants, since in fieldwork, researchers have already observed their curative properties, which in fact could shorten the time of development of an effective medicine.
Future Perspectives and Priorities
This review opens up a huge range of research possibilities in the field of leishmaniasis from a chemical and pharmacological point of view. Table 1 presents 292 plants (216 species and 76 genera) to be investigated as extracts and/or as drugs aimed at developing antileishmanial medicines. Some of these possible “hint plants” are presented in Contributions of Some Botanical Families and Species in the Experimental Treatment of Leishmaniasis. The botanical families and genera that had a higher frequency of citations during this survey are presented and compared with data from other studies in this section.
In addition, the species most frequently mentioned in articles and by the traditional communities in certain countries were highlighted throughout the text. In this context, four species are noteworthy since they were mentioned in four articles: Carica papaya L. (Caricaceae), Cedrela odorata L. (Meliaceae), Copaifera paupera (Herzog) Dwyer (Fabaceae), and Musa × paradisiaca L. (Musaceae), while Nicotiana tabacum L. (Solanaceae), Carica papaya L. (Caricaceae), and Musa × paradisiaca L. (Musaceae) were cited simultaneously by traditional communities from Peru, Ecuador, and French Guiana. Thus, these last two species are among the most cited in articles and by traditional communities.
On the other hand, it becomes important to note that the majority of articles dealing with extracts or purified molecules from plants with ethnopharmacology relevance presented only an inhibitory concentration of 50% against promastigote and/or amastigote forms. Although such data shed light on this scenario, articles should investigate the leishmanicidal properties of plant extracts or molecules against the intracellular amastigote form, which is the form of the parasite that persists and causes disease in the host. Furthermore, it was observed that preclinical studies with medicinal plants traditionally used to treat leishmaniasis are surprisingly rare, but they should be encouraged, since the proof of concept—that a given plant has therapeutic activity in humans—was already provided by healers, and in these specific cases, scientists should standardize mandatory steps related to phytochemistry, pharmacology and parasitology to produce effective medicines.
Finally, this review suggests that future investigations should be guided but not limited to the five species cited above, expanding the chance of discovering new medicines for this disease since, according to the survey presented herein, few or no studies have been performed with plants traditionally used to treat leishmaniasis.
Acknowledgments
The authors thank the scientists for the important work they do and for sharing information and their knowledge; also, to traditional communities that contribute immensely to scientific research by sharing their legitimate and precious knowledge about nature and its particularities.
Author Contributions
Conceptualization and Supervision: LP and ER. Data acquisition: EB, TS, TP, JJ, LP, and ER. Data curation: EB, LP, and ER. Formal analysis: EB, LP, and ER. Software: TS and TP. Writing: EB, LP, and ER. Writing, review; editing: EB, TS, TP, JJ, LP, and ER.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahmad B., Islam A., Khan A., Khan M. A., Ul Haq I., Jafri L., et al. (2019). Comprehensive Investigations on Anti-leishmanial Potentials of Euphorbia Wallichii Root Extract and its Effects on Membrane Permeability and Apoptosis. Comp. Immunol. Microbiol. Infect. Dis. 64, 138–145. 10.1016/j.cimid.2019.03.007 [DOI] [PubMed] [Google Scholar]
- Alvarenga N., Canela N., Gómez R., Yaluff G., Maldonado M. (2008). Leishmanicidal Activity of Maytenus Illicifolia Roots. Fitoterapia 79, 381–383. 10.1016/j.fitote.2008.03.001 [DOI] [PubMed] [Google Scholar]
- Amin E., Moawad A., Hassan H. (2017). Biologically-guided Isolation of Leishmanicidal Secondary Metabolites from Euphorbia peplus L. Saudi Pharm. J. 25, 236–240. 10.1016/j.jsps.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arambewela L. S. R., Ranatunge T. (1991). Indole Alkaloids from Tabernaemontana Divaricata. Phytochemistry 30, 1740–1741. 10.1016/0031-9422(91)84254-P [DOI] [Google Scholar]
- Araújo I. A. C., de Paula R. C., Alves C. L., Faria K. F., Oliveira M. M. d., Mendes G. G., et al. (2019). Efficacy of Lapachol on Treatment of Cutaneous and Visceral Leishmaniasis. Exp. Parasitol. 199, 67–73. 10.1016/j.exppara.2019.02.013 [DOI] [PubMed] [Google Scholar]
- Arévalo-Lopéz D., Nina N., Ticona J. C., Limachi I., Salamanca E., Udaeta E., et al. (2018). Leishmanicidal and Cytotoxic Activity from Plants Used in Tacana Traditional Medicine (Bolivia). J. Ethnopharmacology 216, 120–133. 10.1016/j.jep.2018.01.023 [DOI] [PubMed] [Google Scholar]
- Awadh Ali N. A., Al Sokari S. S., Gushash A., Anwar S., Al-Karani K., Al-Khulaidi A. (2017). Ethnopharmacological Survey of Medicinal Plants in Albaha Region, Saudi Arabia. Pharmacognosy Res. 9, 401, 407. 10.4103/pr.pr_11_17, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badirzadeh A., Heidari-Kharaji M., Fallah-Omrani V., Dabiri H., Araghi A., Salimi Chirani A. (2020). Antileishmanial Activity of Urtica Dioica Extract against Zoonotic Cutaneous Leishmaniasis. Plos Negl. Trop. Dis., 14. e0007843. 10.1371/journal.pntd.0007843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. J. (2017). Metformin: Historical Overview. Diabetologia 60, 1566–1576. 10.1007/s00125-017-4318-z [DOI] [PubMed] [Google Scholar]
- Bhaskar V. H., Natarajan B. (2015). Analgesic, Anti-inflammatory and Antipyretic Activities of Pergularia Daemia and Carissa Carandas. DARU. J. Pharm. Sci. 17, 168–174. [Google Scholar]
- Boratyński P. J., Zielińska-Błajet M., Skarżewski J. (2019). Cinchona Alkaloids-Derivatives and Applications, Alkaloids Chem. Biol., 82, 29–145. 10.1016/bs.alkal.2018.11.001 [DOI] [PubMed] [Google Scholar]
- Burza S., Croft S. L., Boelaert M. (2018). Leishmaniasis. The Lancet 392, 951–970. 10.1016/S0140-6736(18)31204-2 [DOI] [PubMed] [Google Scholar]
- Cabanillas B. J., Le Lamer A.-C., Castillo D., Arevalo J., Rojas R., Odonne G., et al. (2010). Caffeic Acid Esters and Lignans fromPiper Sanguineispicum. J. Nat. Prod. 73, 1884–1890. 10.1021/np1005357 [DOI] [PubMed] [Google Scholar]
- Calla-Magariños J., Quispe T., Giménez A., Freysdottir J., Troye-Blomberg M., Fernández C. (2013). Quinolinic Alkaloids from Galipea Longiflora KrauseSuppress Production of Proinflammatory Cytokinesin Vitroand Control Inflammationin vivouponLeishmaniaInfection in Mice. Scand. J. Immunol. 77, 30–38. 10.1111/sji.12010 [DOI] [PubMed] [Google Scholar]
- Capello T. M., Martins E. G. A., Farias De. C. F., Figueiredo C. R., Matsuo A. L., et al. (2015). Chemical Composition and In Vitro Cytotoxic and Antileishmanial Activities of Extract and Essential Oil from Leaves of Piper Cernuum. Nat. Prod. Commun. 10. [PubMed] [Google Scholar]
- Ceole L. F., Cardoso M. D. G., Soares M. J. (2017). Nerolidol, the Main Constituent of Piper Aduncum Essential Oil, Has Anti-leishmania Braziliensis Activity. Parasitology 144, 1179–1190. 10.1017/S0031182017000452 [DOI] [PubMed] [Google Scholar]
- Chauhan I. S., Rao G. S., Shankar J., Chauhan L. K. S., Kapadia G. J., Singh N. (2018). Chemoprevention of Leishmaniasis: In - Vitro Antiparasitic Activity of Dibenzalacetone, a Synthetic Curcumin Analog Leads to Apoptotic Cell Death in Leishmania Donovani. Parasitol. Int. 67, 627–636. 10.1016/j.parint.2018.06.004 [DOI] [PubMed] [Google Scholar]
- Chouhan G., Islamuddin M., Want M. Y., Ozbak H. A., Hemeg H. A., Sahal D., et al. (2015). Leishmanicidal Activity of Piper Nigrum Bioactive Fractions Is Interceded via Apoptosis In Vitro and Substantiated by Th1 Immunostimulatory Potential In Vivo . Front. Microbiol. 6, 1368. 10.3389/fmicb.2015.01368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S. R., Kumar A., Godinho J. L. P., De Macedo Silva S. T., Zuma A. A., Saha S., et al. (2017). Voacamine Alters Leishmania Ultrastructure and Kills Parasite by Poisoning Unusual Bi-subunit Topoisomerase IB. Biochem. Pharmacol. 138, 19–30. 10.1016/j.bcp.2017.05.002 [DOI] [PubMed] [Google Scholar]
- Cos P., Janssens J., Piñón A., Cuesta-Rubio O., Yglesias-Rivera A., Díaz-García A., et al. (2018). Efficacy of Four Solanum Spp. Extracts in an Animal Model of Cutaneous Leishmaniasis. Medicines, 5, 49. 10.3390/medicines5020049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E. V. S., Brígido H. P. C., Silva J. V. d. S. e., Coelho-Ferreira M. R., Brandão G. C., Dolabela M. F. (2017). Antileishmanial Activity ofHandroanthus serratifolius(Vahl) S. Grose (Bignoniaceae). Evidence-Based Complement. Altern. Med. 2017, 1, 6. 10.1155/2017/8074275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay O., Peters N. C., Rogers M. E., Bern C. (2017). Combining Epidemiology with Basic Biology of Sand Flies, Parasites, and Hosts to Inform Leishmaniasis Transmission Dynamics and Control. Plos Pathog. 13, e1006571. 10.1371/journal.ppat.1006571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Picolo C. R., Bezerra M. P., Gomes K. S., Passero L. F. D., Laurenti M. D., Martins E. G. A., et al. (2014). Antileishmanial Activity Evaluation of Adunchalcone, a New Prenylated Dihydrochalcone from Piper Aduncum L. Fitoterapia 97, 28–33. 10.1016/j.fitote.2014.05.009 [DOI] [PubMed] [Google Scholar]
- de Jesus J. A., Laurenti M. D., Antonangelo L., Faria C. S., Lago J. H. G., Passero L. F. D., et al. (2021). Related Pentacyclic Triterpenes Have Immunomodulatory Activity in Chronic Experimental Visceral Leishmaniasis. J. Immunol. Res. 2021, 1–15. 10.1155/2021/6671287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira A., Mesquita J. T., Tempone A. G., Lago J. H. G., Guimarães E. F., Kato M. J. (2012). Leishmanicidal Activity of an Alkenylphenol from Piper Malacophyllum Is Related to Plasma Membrane Disruption. Exp. Parasitol. 132, 383–387. 10.1016/j.exppara.2012.08.019 [DOI] [PubMed] [Google Scholar]
- Dey S., Mukherjee D., Chakraborty S., Mallick S., Dutta A., Ghosh J., et al. (2015). Protective Effect of Croton Caudatus Geisel Leaf Extract against Experimental Visceral Leishmaniasis Induces Proinflammatory Cytokines In Vitro and In Vivo . Exp. Parasitol. 151-152, 84–95. 10.1016/j.exppara.2015.01.012 [DOI] [PubMed] [Google Scholar]
- Dos Santos A. O., Costa M. A., Ueda-Nakamura T., Dias-Filho B. P., da Veiga-Júnior V. F., de Souza Lima M. M., et al. (2011). Leishmania Amazonensis: Effects of Oral Treatment with Copaiba Oil in Mice. Exp. Parasitol. 129, 145–151. 10.1016/j.exppara.2011.06.016 [DOI] [PubMed] [Google Scholar]
- Duarte N., Kayser O., Abreu P., Ferreira M.-J. U. (2008). Antileishmanial Activity of Piceatannol Isolated fromEuphorbia Lagascae Seeds. Phytother. Res. 22, 455–457. 10.1002/ptr.2334 [DOI] [PubMed] [Google Scholar]
- Estevez Y., Castillo D., Pisango M. T., Arevalo J., Rojas R., Alban J., et al. (2007). Evaluation of the Leishmanicidal Activity of Plants Used by Peruvian Chayahuita Ethnic Group. J. Ethnopharmacology 114, 254–259. 10.1016/j.jep.2007.08.007 [DOI] [PubMed] [Google Scholar]
- Faleiro R. J., Kumar R., Hafner L. M., Engwerda C. R. (2014). Immune Regulation during Chronic Visceral Leishmaniasis. Plos Negl. Trop. Dis. 8, e2914. 10.1371/journal.pntd.0002914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores N., Cabrera G., Jiménez I., Piñero J., Giménez A., Bourdy G., et al. (2007). Leishmanicidal Constituents from the Leaves of Piper Rusbyi. Planta Med. 73, 206–211. 10.1055/s-2007-967123 [DOI] [PubMed] [Google Scholar]
- Fournet A., Barrios A. A., Muñoz V., Hocquemiller R., Cavé A. (1992b). Effect of Natural Naphthoquinones in BALB/c Mice Infected with Leishmania Amazonensis and L. Venezuelensis. Trop. Med. Parasitol. 43, 219–222. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1293723. [PubMed] [Google Scholar]
- Fournet A., Angelo A., Muñoz V., Roblot F., Hocquemiller R., Cavé A. (1992a). Biological and Chemical Studies of Pera Benensis, a Bolivian Plant Used in Folk Medicine as a Treatment of Cutaneous Leishmaniasis. J. Ethnopharmacology 37, 159–164. 10.1016/0378-8741(92)90074-2 [DOI] [PubMed] [Google Scholar]
- Fournet A., Barrios A. A., Munoz V., Hocquemiller R., Cave A., Bruneton J. (1993a). 2-substituted Quinoline Alkaloids as Potential Antileishmanial Drugs. Antimicrob. Agents Chemother. 37, 859–863. 10.1128/AAC.37.4.859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournet A., Barrios A. A., Muñoz V. (1994). Leishmanicidal and Trypanocidal Activities of Bolivian Medicinal Plants. J. Ethnopharmacology 41, 19–37.Available at: http://www.ncbi.nlm.nih.gov/pubmed/8170156. 10.1016/0378-8741(94)90054-x [DOI] [PubMed] [Google Scholar]
- Fournet A., Ferreira M. E., Rojas De Arias A., Torres De Ortiz S., Fuentes S., Nakayama H., et al. (1996). In Vivo efficacy of Oral and Intralesional Administration of 2-substituted Quinolines in Experimental Treatment of New World Cutaneous Leishmaniasis Caused by Leishmania Amazonensis. Antimicrob. Agents Chemother. 40, 2447–2451. 10.1128/AAC.40.11.2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournet A., Muñoz V., Roblot F., Hocquemiller R., Cavé A., Gantier J.-C. (1993b). Antiprotozoal Activity of Dehydrozaluzanin C, a Sesquiterpene Lactone Isolated fromMunnozia Maronii (Asteraceae). Phytother. Res. 7, 111–115. 10.1002/ptr.2650070203 [DOI] [Google Scholar]
- Fournet A., Vagneur B., Richomme P., Bruneton J. (1989). Aryl-2 et alkyl-2 quinoléines nouvelles isolées d'une Rutacée bolivienne: Galipealongiflora. Can. J. Chem. 67, 2116–2118. 10.1139/v89-329 [DOI] [Google Scholar]
- Francesquini F. C., Silveira F. T., Passero L. F. D., Tomokane T. Y., Carvalho A. K., Corbett C. E. P., et al. (2014). Salivary Gland Homogenates from Wild-Caught Sand fliesLutzomyia flaviscutellataandLutzomyia (Psychodopygus) Complexusshowed Inhibitory Effects onLeishmania (Leishmania) amazonensisandLeishmania (Viannia) Braziliensisinfection in BALB/c Mice. Int. J. Exp. Path. 95, 418–426. 10.1111/iep.12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- França F., Lago E. L., Marsden P. D. (1996). Plants Used in the Treatment of Leishmanial Ulcers Due to Leishmania (Viannia) Braziliensis in an Endemic Area of Bahia, Brazil. Rev. Soc. Bras. Med. Trop. 29, 229–232. 10.1590/S0037-86821996000300002 [DOI] [PubMed] [Google Scholar]
- Gabriel Á., Valério-Bolas A., Palma-Marques J., Mourata-Gonçalves P., Ruas P., Dias-Guerreiro T., et al. (2019). Cutaneous Leishmaniasis: The Complexity of Host's Effective Immune Response against a Polymorphic Parasitic Disease. J. Immunol. Res. 2019, 1–16. 10.1155/2019/2603730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet M. S., Lecaro J. S., Kaiser M., Brun R., Navarrete H., Muñoz R. A., et al. (2010). Assessment of Anti-protozoal Activity of Plants Traditionally Used in Ecuador in the Treatment of Leishmaniasis. J. Ethnopharmacology 128, 184–197. 10.1016/j.jep.2010.01.007 [DOI] [PubMed] [Google Scholar]
- Gamboa-León M. R., Aranda-González I., Mut-Martín M., García-Miss M. R., Dumonteil E. (2007). In Vivo and In Vitro Control of Leishmania Mexicana Due to Garlic-Induced NO Production. Scand. J. Immunol. 66, 508–514. 10.1111/j.1365-3083.2007.02000.x [DOI] [PubMed] [Google Scholar]
- Garcia F. P., Lazarin-Bidóia D., Ueda-Nakamura T., Silva S. d. O., Nakamura C. V. (2013). Eupomatenoid-5 Isolated from Leaves ofPiper regnelliiInduces Apoptosis inLeishmania Amazonensis. Evidence-Based Complement. Altern. Med. 2013, 1, 11. 10.1155/2013/940531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfari T., Hassan Z. M., Ebtekar M., Ahmadiani A., Naderi G., Azar A. (2000). Garlic Induces a Shift in Cytokine Pattern in Leishmania Major-Infected BALB/c Mice. Scand. J. Immunol. 52, 491–495. 10.1046/j.1365-3083.2000.00803.x [DOI] [PubMed] [Google Scholar]
- Ghorbani M., Farhoudi R. (2017). Leishmaniasis in Humans: Drug or Vaccine Therapy?, Dddt Vol. 12, 25–40. 10.2147/DDDT.S146521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giday M., Asfaw Z., Woldu Z. (2009). Medicinal Plants of the Meinit Ethnic Group of Ethiopia: An Ethnobotanical Study. J. Ethnopharmacol. 124, 513–521. 10.1016/j.jep.2009.05.009 [DOI] [PubMed] [Google Scholar]
- Gomes Dde. C., Alegrio L. V., de Lima M. E., Leon L. L., Araújo C. A. (2002). Synthetic Derivatives of Curcumin and Their Activity against Leishmania Amazonensis. Arzneimittelforschung. 52, 120–124. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11878200 [PubMed] [Google Scholar]
- Gupta G., Peine K. J., Abdelhamid D., Snider H., Shelton A. B., Rao L., et al. (2015). A Novel Sterol Isolated from a Plant Used by Mayan Traditional Healers Is Effective in Treatment of Visceral Leishmaniasis Caused byLeishmania Donovani. ACS Infect. Dis. 1, 497–506. 10.1021/acsinfecdis.5b00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J., Afl O., Elizabeth M., Arteaga J. (2014). Wild Medicinal Plants Used by Colombian Kofan Indians to Treat Cutaneous Leishmaniasise. Rev. Cuba. Plantas Med. 19, 407–420. [Google Scholar]
- Hajdu Z., Hohmann J. (2012). An Ethnopharmacological Survey of the Traditional Medicine Utilized in the Community of Porvenir, Bajo Paraguá Indian Reservation, Bolivia. J. Ethnopharmacology 139, 838–857. 10.1016/j.jep.2011.12.029 [DOI] [PubMed] [Google Scholar]
- Haque A., Rahman M. A., Faizi M. S. H., Khan M. S. (2018). Next Generation Antineoplastic Agents: A Review on Structurally Modified Vinblastine (VBL) Analogues. Cmc 25, 1650–1662. 10.2174/0929867324666170502123639 [DOI] [PubMed] [Google Scholar]
- Hermida. M. d. E.-R., De Melo C. V. B., Lima I. d. S., Oliveira G. G. d. S., Dos-Santos W. L. C. (2018). Histological Disorganization of Spleen Compartments and Severe Visceral Leishmaniasis. Front. Cel. Infect. Microbiol., 8. 10.3389/fcimb.2018.00394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoso A., Jiménez I. A., Mamani Z. A., Bazzocchi I. L., Piñero J. E., Ravelo A. G., et al. (2003). Antileishmanial Activities of Dihydrochalcones from Piper Elongatum and Synthetic Related Compounds. Structural Requirements for Activity. Bioorg. Med. Chem. 11, 3975–3980. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12927858. 10.1016/s0968-0896(03)00406-1 [DOI] [PubMed] [Google Scholar]
- Jesus J. A., Fragoso da Silva T. N., Yamamoto E. S., G. Lago J. H., Dalastra Laurenti M., Passero L. F. D. (2020). Ursolic Acid Potentializes Conventional Therapy in Experimental Leishmaniasis. Pathogens, 9, 855. 10.3390/pathogens9100855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesus J. A., Fragoso T. N., Yamamoto E. S., Laurenti M. D., Silva M. S., Ferreira A. F., et al. (2017). Therapeutic Effect of Ursolic Acid in Experimental Visceral Leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 7, 1–11. 10.1016/j.ijpddr.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaunda J. S., Zhang Y.-J. (2017). The Genus Carissa: An Ethnopharmacological, Phytochemical and Pharmacological Review. Nat. Prod. Bioprospect. 7, 181–199. 10.1007/s13659-017-0123-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. L., Cortés-Selva F., Pérez-Victoria J. M., Jiménez I. A., González A. G., Muñoz O. M., et al. (2001). Chemosensitization of a Multidrug-ResistantLeishmania tropicaLine by New Sesquiterpenes fromMaytenus magellanicaandMaytenus Chubutensis. J. Med. Chem. 44, 4668–4676. 10.1021/jm010970c [DOI] [PubMed] [Google Scholar]
- Kennedy M. L., Llanos G. G., Castanys S., Gamarro F., Bazzocchi I. L., Jiménez I. A. (2011). Terpenoids from Maytenus Species and Assessment of Their Reversal Activity against a Multidrug-Resistant Leishmania Tropica Line. Chem. Biodiversity 8, 2291–2298. 10.1002/cbdv.201000356 [DOI] [PubMed] [Google Scholar]
- Kevric I., Cappel M. A., Keeling J. H. (2015). New World and Old World Leishmania Infections. Dermatol. Clin. 33, 579–593. 10.1016/j.det.2015.03.018 [DOI] [PubMed] [Google Scholar]
- Kvist L. P., Christensen S. B., Rasmussen H. B., Mejia K., Gonzalez A. (2006). Identification and Evaluation of Peruvian Plants Used to Treat Malaria and Leishmaniasis. J. Ethnopharmacology 106, 390–402. 10.1016/j.jep.2006.01.020 [DOI] [PubMed] [Google Scholar]
- Lacerda R. B. M., Freitas T. R., Martins M. M., Teixeira T. L., da Silva C. V., Candido P. A., et al. (2018). Isolation, Leishmanicidal Evaluation and Molecular Docking Simulations of Piperidine Alkaloids from Senna Spectabilis. Bioorg. Med. Chem. 26, 5816–5823. 10.1016/j.bmc.2018.10.032 [DOI] [PubMed] [Google Scholar]
- Lezama-Dávila C. M., Isaac-Márquez A. P., Zamora-Crescencio P., Úc-Encalada M. d. R., Justiniano-Apolinar S. Y., del Angel-Robles L., et al. (2007). Leishmanicidal Activity of Pentalinon Andrieuxii. Fitoterapia 78, 255–257. 10.1016/j.fitote.2006.12.005 [DOI] [PubMed] [Google Scholar]
- Lezama-Dávila C. M., McChesney J. D., Bastos J. K., Miranda M. A., Tiossi R. F., da Costa J. d. C., et al. (2016). A New Antileishmanial Preparation of Combined Solamargine and Solasonine Heals Cutaneous Leishmaniasis through Different Immunochemical Pathways. Antimicrob. Agents Chemother. 60, 2732–2738. 10.1128/AAC.02804-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezama-Dávila C. M., Pan L., Isaac-Márquez A. P., Terrazas C., Oghumu S., Isaac-Márquez R., et al. (2014). Pentalinon Andrieuxii Root Extract Is Effective in the Topical Treatment of Cutaneous Leishmaniasis Caused by Leishmania Mexicana. Phytother. Res. 28, 909–916. 10.1002/ptr.5079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima G. S., Castro-Pinto D. B., Machado G. C., Maciel M. A. M., Echevarria A. (2015). Antileishmanial Activity and Trypanothione Reductase Effects of Terpenes from the Amazonian Species Croton Cajucara Benth (Euphorbiaceae). Phytomedicine 22, 1133–1137. 10.1016/j.phymed.2015.08.012 [DOI] [PubMed] [Google Scholar]
- Marques A. P. S., Bonfim F. P. G., Dantas W. F. C., Puppi R. J., Marques M. O. M. (2019). Chemical Composition of Essential Oil from Varronia Curassavica Jacq. Accessions in Different Seasons of the Year. Ind. Crops Prod. 140, 111656. 10.1016/j.indcrop.2019.111656 [DOI] [Google Scholar]
- Melo G. M. A., Silva M. C. R., Guimarães T. P., Pinheiro K. M., da Matta C. B. B., de Queiroz A. C., et al. (2014). Leishmanicidal Activity of the Crude Extract, Fractions and Major Piperidine Alkaloids from the Flowers of Senna Spectabilis. Phytomedicine 21, 277–281. 10.1016/j.phymed.2013.09.024 [DOI] [PubMed] [Google Scholar]
- Mishra P. K., Singh N., Ahmad G., Dube A., Maurya R. (2005). Glycolipids and Other Constituents from Desmodium gangeticum with Antileishmanial and Immunomodulatory Activities. Bioorg. Med. Chem. Lett. 15, 4543–4546. 10.1016/j.bmcl.2005.07.020 [DOI] [PubMed] [Google Scholar]
- Misra P., Kumar A., Khare P., Gupta S., Kumar N., Dube A. (2009). Pro-apoptotic Effect of the Landrace Bangla Mahoba of Piper Betle on Leishmania Donovani May Be Due to the High Content of Eugenol. J. Med. Microbiol. 58, 1058–1066. 10.1099/jmm.0.009290-0 [DOI] [PubMed] [Google Scholar]
- Moein M., Hatam G., Taghavi-Moghadam R., Zarshenas M. M. (2017). Antileishmanial Activities of Greek Juniper (Juniperus Excelsa M.Bieb.) against Leishmania Major Promastigotes. J. Evid. Based. Complement. Altern. Med. 22, 31–36. 10.1177/2156587215623435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzote L., Montalvo A. M., Almanonni S., Scull R., Miranda M., Abreu J. (2006). Activity of the Essential Oil from Chenopodium Ambrosioides Grown in Cuba against Leishmania Amazonensis. Chemotherapy 52, 130–136. 10.1159/000092858 [DOI] [PubMed] [Google Scholar]
- Monzote L., Montalvo A. M., Scull R., Miranda M., Abreu J. (2007). Activity, Toxicity and Analysis of Resistance of Essential Oil from Chenopodium Ambrosioides after Intraperitoneal, Oral and Intralesional Administration in BALB/c Mice Infected with Leishmania Amazonensis: A Preliminary Study. Biomed. Pharmacother. 61, 148–153. 10.1016/j.biopha.2006.12.001 [DOI] [PubMed] [Google Scholar]
- Muhammad I., Dunbar D. C., Khan S. I., Tekwani B. L., Bedir E., Takamatsu S., et al. (2003). Antiparasitic Alkaloids fromPsychotria Klugii. J. Nat. Prod. 66, 962–967. 10.1021/np030086k [DOI] [PubMed] [Google Scholar]
- Mukherjee P. K., Harwansh R. K., Bahadur S., Banerjee S., Kar A., Chanda J., et al. (2017). Development of Ayurveda - Tradition to Trend. J. Ethnopharmacology 197, 10–24. 10.1016/j.jep.2016.09.024 [DOI] [PubMed] [Google Scholar]
- Muruganandam A., Bhattacharya S., Ghosal S. (2000). Indole and Flavanoid Constituents of Wrightia Tinctoria, W. Tomentosa and W. Coccinea. Indian J. Chem. 39B, 125–131. [Google Scholar]
- Odonne G., Berger F., Stien D., Grenand P., Bourdy G. (2011a). Treatment of Leishmaniasis in the Oyapock basin (French Guiana): A K.A.P. Survey and Analysis of the Evolution of Phytotherapy Knowledge Amongst Wayãpi Indians. J. Ethnopharmacology 137, 1228–1239. 10.1016/j.jep.2011.07.044 [DOI] [PubMed] [Google Scholar]
- Odonne G., Bourdy G., Castillo D., Estevez Y., Lancha-Tangoa A., Alban-Castillo J., et al. (2009). Ta'ta', Huayani: Perception of Leishmaniasis and Evaluation of Medicinal Plants Used by the Chayahuita in Peru. Part II. J. Ethnopharmacology 126, 149–158. 10.1016/j.jep.2009.07.015 [DOI] [PubMed] [Google Scholar]
- Odonne G., Herbette G., Eparvier V., Bourdy G., Rojas R., Sauvain M., et al. (2011b). Antileishmanial Sesquiterpene Lactones from Pseudelephantopus Spicatus, a Traditional Remedy from the Chayahuita Amerindians (Peru). Part III. J. Ethnopharmacology 137, 875–879. 10.1016/j.jep.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Pagani E., Santos J. d. F. L., Rodrigues E. (2017). Culture-Bound Syndromes of a Brazilian Amazon Riverine Population: Tentative Correspondence between Traditional and Conventional Medicine Terms and Possible Ethnopharmacological Implications. J. Ethnopharmacology 203, 80–89. 10.1016/j.jep.2017.03.024 [DOI] [PubMed] [Google Scholar]
- Pan L., Lezama-Davila C. M., Isaac-Marquez A. P., Calomeni E. P., Fuchs J. R., Satoskar A. R., et al. (2012). Sterols with Antileishmanial Activity Isolated from the Roots of Pentalinon Andrieuxii. Phytochemistry 82, 128–135. 10.1016/j.phytochem.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi M. M., Handjani F., Moein M., Hatam G., Nimrouzi M., Hassanzadeh J., et al. (2017). Efficacy of Cryotherapy Plus Topical Juniperus Excelsa M. Bieb Cream versus Cryotherapy Plus Placebo in the Treatment of Old World Cutaneous Leishmaniasis: A Triple-Blind Randomized Controlled Clinical Trial. Plos Negl. Trop. Dis. 11. e0005957. 10.1371/journal.pntd.0005957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passero L. F. D., Castro A. A., Tomokane T. Y., Kato M. J., Paulinetti T. F., Corbett C. E. P., et al. (2007). Anti-leishmania Activity of Semi-purified Fraction of Jacaranda Puberula Leaves. Parasitol. Res. 101, 677–680. 10.1007/s00436-007-0530-y [DOI] [PubMed] [Google Scholar]
- Passero L. F. D., Cruz L. A., Santos-Gomes G., Rodrigues E., Laurenti M. D., Lago J. H. G. (2018). Conventional versus Natural Alternative Treatments for Leishmaniasis :a Review. Curr. Top. Med. Chem. 10.2174/1568026618666181002114448. [DOI] [PubMed] [Google Scholar]
- Passero L. F. D., Laurenti M. D., Santos-Gomes G., Campos B. L. S., Sartorelli P., Lago J. H. G. (2014). Plants Used in Traditional Medicine: Extracts and Secondary Metabolites Exhibiting Antileishmanial Activity. Curr. Clin. Pharmacol. 9. [DOI] [PubMed] [Google Scholar]
- Pastor J., García M., Steinbauer S., Setzer W. N., Scull R., Gille L., et al. (2015). Combinations of Ascaridole, Carvacrol, and Caryophyllene Oxide against Leishmania. Acta Tropica 145, 31–38. 10.1016/j.actatropica.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Patocka J., Nepovimova E., Wu W., Kuca K. (2020). Digoxin: Pharmacology and Toxicology-A Review. Environ. Toxicol. Pharmacol. 79, 103400. 10.1016/j.etap.2020.103400 [DOI] [PubMed] [Google Scholar]
- Patrício F. J., Costa G. C., Pereira P. V. S., Aragão-Filho W. C., Sousa S. M., Frazão J. B., et al. (2008). Efficacy of the Intralesional Treatment with Chenopodium Ambrosioides in the Murine Infection by Leishmania Amazonensis. J. Ethnopharmacology 115, 313–319. 10.1016/j.jep.2007.10.009 [DOI] [PubMed] [Google Scholar]
- Pérez-Victoria J. M., Tincusi B. M., Jiménez I. A., Bazzocchi I. L., Gupta M. P., Castanys S., et al. (1999). New Natural Sesquiterpenes as Modulators of Daunomycin Resistance in a Multidrug-ResistantLeishmaniatropicaLine‖,⊥. J. Med. Chem. 42, 4388–4393. 10.1021/jm991066b [DOI] [PubMed] [Google Scholar]
- Ponte-Sucre A., Gamarro F., Dujardin J.-C., Barrett M. P., López-Vélez R., García-Hernández R., et al. (2017). Drug Resistance and Treatment Failure in Leishmaniasis: A 21st century challenge. Plos Negl. Trop. Dis. 11. e0006052. 10.1371/journal.pntd.0006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin J., Desrivot J., Thoret S., Menez P. L., Cresteil T., Lewin G. (2009). Synthesis and Biological Evaluation of a Series of Tangeretin-Derived Chalcones. Bioorg. Med. Chem. Lett. 19, 167–169. 10.1016/j.bmcl.2008.10.126 [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Christensen S., Kvist L., Karazmi A. (2000). A Simple and Efficient Separation of the Curcumins, the Antiprotozoal Constituents of Curcuma Longa. Planta Med. 66, 396–398. 10.1055/s-2000-8533 [DOI] [PubMed] [Google Scholar]
- Reina M., Ruiz-Mesia L., Ruiz-Mesia W., Sosa-Amay F. E., Arevalo-Encinas L., González-Coloma A., et al. (2014). Antiparasitic Indole Alkaloids from Aspidosperma Desmanthum and A. Spruceanum from the Peruvian Amazonia. Nat. Prod. Commun. 9, 1075–1080. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25233577 10.1177/1934578x1400900805 [DOI] [PubMed] [Google Scholar]
- Reina M., Ruiz-Mesia W., Ruiz-Mesia L., Martínez-Díaz R., González-Coloma A. (2011). Indole Alkaloids from Aspidosperma Rigidum and A. Schultesii and Their Antiparasitic Effects. Z. Naturforsch. C 66, 0225–0234. 10.1515/znc-2011-5-605 [DOI] [PubMed] [Google Scholar]
- Rodrigues E., Barnes J. (2013). Pharmacovigilance of Herbal Medicines. Drug Saf. 36, 1–12. 10.1007/s40264-012-0005-7 [DOI] [PubMed] [Google Scholar]
- Rodrigues E. (2006). Plants and Animals Utilized as Medicines in the Jaú National Park (JNP), Brazilian Amazon. Phytother. Res. 20, 378–391. 10.1002/ptr.1866 [DOI] [PubMed] [Google Scholar]
- Rodrigues I. A., Azevedo M. M. B., Chaves F. C. M., Bizzo H. R., Corte-Real S., Alviano D. S., et al. (2013). In Vitro cytocidal Effects of the Essential Oil from Croton Cajucara (Red Sacaca) and its Major Constituent 7- Hydroxycalamenene against Leishmania Chagasi. BMC Complement. Altern. Med. 13, 249. 10.1186/1472-6882-13-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M. d. S. S., Mendonça-Filho R. R., Bizzo H. R., Rodrigues I. d. A., Soares R. M. A., Souto-Padrón T., et al. (2003). Antileishmanial Activity of a Linalool-Rich Essential Oil from Croton Cajucara. Aac 47, 1895–1901. 10.1128/AAC.47.6.1895-1901.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleheen D., Ali S. A., Ashfaq K., Siddiqui A. A., Agha A., Yasinzai M. M. (2002). Latent Activity of Curcumin against Leishmaniasis In Vitro . Biol. Pharm. Bull. 25, 386–389. 10.1248/bpb.25.386 [DOI] [PubMed] [Google Scholar]
- Santos A. O. d., Izumi E., Ueda-Nakamura T., Dias-Filho B. P., Veiga-Júnior V. F. d., Nakamura C. V. (2013). Antileishmanial Activity of Diterpene Acids in Copaiba Oil. Mem. Inst. Oswaldo Cruz 108, 59–64. 10.1590/s0074-02762013000100010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A. O., Ueda-Nakamura T., Dias Filho B. P., Veiga Junior V. F., Pinto A. C., Nakamura C. V. (2008). Effect of Brazilian Copaiba Oils on Leishmania Amazonensis. J. Ethnopharmacology 120, 204–208. 10.1016/j.jep.2008.08.007 [DOI] [PubMed] [Google Scholar]
- Santos B. M., Bezerra-Souza A., Aragaki S., Rodrigues E., Umehara E., Ghilardi Lago J. H., et al. (2019). Ethnopharmacology Study of Plants from Atlantic Forest with Leishmanicidal Activity. Evidence-Based Complement. Altern. Med. 2019, 1–8. 10.1155/2019/8780914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui S., Hafeez F., Begum S., Siddiqui B. S. (1986). Isolation and Structure of Two Cardiac Glycosides from the Leaves of Nerium Oleander. Phytochemistry 26, 237–241. 10.1016/S0031-9422(00)81519-8 [DOI] [Google Scholar]
- Singh N., Mishra P. K., Kapil A., Arya K. R., Maurya R., Dube A. (2005). Efficacy of Desmodium gangeticum Extract and its Fractions against Experimental Visceral Leishmaniasis. J. Ethnopharmacology 98, 83–88. 10.1016/j.jep.2004.12.032 [DOI] [PubMed] [Google Scholar]
- Soares D. C., Pereira C. G., Meireles M. Â. A., Saraiva E. M. (2007). Leishmanicidal Activity of a Supercritical Fluid Fraction Obtained from Tabernaemontana Catharinensis. Parasitol. Int. 56, 135–139. 10.1016/j.parint.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Stefano G. B., Pilonis N., Ptacek R., Kream R. M. (2017). Reciprocal Evolution of Opiate Science from Medical and Cultural Perspectives. Med. Sci. Monit. 23, 2890–2896. 10.12659/MSM.905167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleman S., Alemu T. (2012). A Survey on Utilization of Ethnomedicinal Plants in Nekemte Town, East Wellega (Oromia), Ethiopia. J. Herbs, Spices Med. Plants 18, 34–57. 10.1080/10496475.2011.645188 [DOI] [Google Scholar]
- Süntar I. (2020). Importance of Ethnopharmacological Studies in Drug Discovery: Role of Medicinal Plants. Phytochem. Rev. 19, 1199–1209. 10.1007/s11101-019-09629-9 [DOI] [Google Scholar]
- Takahashi H., Britta E., Longhini R., Ueda-Nakamura T., Palazzo de Mello J., Nakamura C. (2013). Antileishmanial Activity of 5-Methyl-2,2′ : 5′,2″-terthiophene Isolated from Porophyllum Ruderale Is Related to Mitochondrial Dysfunction in Leishmania Amazonensis. Planta Med. 79, 330–333. 10.1055/s-0032-1328258 [DOI] [PubMed] [Google Scholar]
- Takahashi H. T., Novello C. R., Ueda-Nakamura T., Filho B. P. D., Palazzo de Mello J. C., Nakamura C. V. (2011). Thiophene Derivatives with Antileishmanial Activity Isolated from Aerial Parts of Porophyllum Ruderale (Jacq.) Cass. Molecules 16, 3469–3478. 10.3390/molecules16053469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J. C. A., da Silva C. C., Ferreira I. C. P., Machado G. M. C., Leon L. L., de Oliveira A. J. B. (2007). Antileishmanial Activity of Indole Alkaloids from Aspidosperma Ramiflorum. Phytomedicine 14, 377–380. 10.1016/j.phymed.2006.09.002 [DOI] [PubMed] [Google Scholar]
- Teklehaymanot T. (2009). Ethnobotanical Study of Knowledge and Medicinal Plants Use by the People in Dek Island in Ethiopia. J. Ethnopharmacology 124, 69–78. 10.1016/j.jep.2009.04.005 [DOI] [PubMed] [Google Scholar]
- Teles A. M., Rosa T. D. d. S., Mouchrek A. N., Abreu-Silva A. L., Calabrese K. d. S., Almeida-Souza F., et al. (2019). Cinnamomum Zeylanicum, Origanum Vulgare, and Curcuma Longa Essential Oils: Chemical Composition, Antimicrobial and Antileishmanial Activity. Evidence-Based Complement. Altern. Med. 2019, 1–12. 10.1155/2019/2421695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticona J. C., Bilbao-Ramos P., Flores N., Dea-Ayuela M. A., Bolás-Fernández F., Jiménez I. A., et al. (2020). (E)-Piplartine Isolated from Piper Pseudoarboreum, a Lead Compound against Leishmaniasis. Foods 9, 1250. 10.3390/foods9091250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Santos E. C., Moreira D. L., Kaplan M. A. C., Meirelles M. N., Rossi-Bergmann B. (1999). Selective Effect of 2′,6′-Dihydroxy-4′-Methoxychalcone Isolated from Piper Aduncum on Leishmania Amazonensis. Antimicrob. Agents Chemother. 43, 1234–1241. 10.1128/AAC.43.5.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadeau C., Pabon A., Deharo E., Albán-Castillo J., Estevez Y., Lores F. A., et al. (2009). Medicinal Plants from the Yanesha (Peru): Evaluation of the Leishmanicidal and Antimalarial Activity of Selected Extracts. J. Ethnopharmacol. 123, 413–422. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19514108. 10.1016/j.jep.2009.03.041 [DOI] [PubMed] [Google Scholar]
- Valadeau C., Castillo J. A., Sauvain M., Lores A. F., Bourdy G. (2010). The Rainbow Hurts My Skin: Medicinal Concepts and Plants Uses Among the Yanesha (Amuesha), an Amazonian Peruvian Ethnic Group. J. Ethnopharmacology 127, 175–192. 10.1016/j.jep.2009.09.024 [DOI] [PubMed] [Google Scholar]
- Vásquez-Ocmín P., Cojean S., Rengifo E., Suyyagh-Albouz S., Amasifuen Guerra C. A., Pomel S., et al. (2018). Antiprotozoal Activity of Medicinal Plants Used by Iquitos-Nauta Road Communities in Loreto (Peru). J. Ethnopharmacology 210, 372–385. 10.1016/j.jep.2017.08.039 [DOI] [PubMed] [Google Scholar]
- Vendrametto M. C., Santos A. O. d., Nakamura C. V., Filho B. P. D., Cortez D. A. G., Ueda-Nakamura T. (2010). Evaluation of Antileishmanial Activity of Eupomatenoid-5, a Compound Isolated from Leaves of Piper Regnellii Var. Pallescens. Parasitol. Int. 59, 154–158. 10.1016/j.parint.2009.12.009 [DOI] [PubMed] [Google Scholar]
- Wabwoba B. W., Anjili C. O., Ngeiywa M. M., Ngure P. K., Kigondu E. M., Ingonga J., et al. (2010). Experimental Chemotherapy with Allium Sativum (Liliaceae) Methanolic Extract in Rodents Infected with Leishmania Major and Leishmania Donovani. J. Vector Borne Dis. 47, 160–167. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20834086. [PubMed] [Google Scholar]
- Weigel M. M., Armijos R. X., Racines R. J., Zurita C., Izurieta R., Herrera E., et al. (1994). Cutaneous Leishmaniasis in Subtropical Ecuador: Popular Perceptions, Knowledge, and Treatment. Bull. Pan Am. Health Organ. 28, 142–155. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8069334. [PubMed] [Google Scholar]
- Weniger B., Robledo S., Arango G. J., Deharo E., Aragón R., Muñoz V., et al. (2001). Antiprotozoal Activities of Colombian Plants. J. Ethnopharmacology 78, 193–200. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11694364. 10.1016/s0378-8741(01)00346-4 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2019). Leishmaniasis. World Health Organization. [Google Scholar]
- Yang C.-P., Horwitz S. (2017). Taxol: The First Microtubule Stabilizing Agent. Ijms 18, 1733. 10.3390/ijms18081733 [DOI] [PMC free article] [PubMed] [Google Scholar]