INTRODUCTION
The amygdala is a nuclear complex found in the cerebral hemispheres of all vertebrates. It is located in the temporal lobe of primates, and in the caudoventral forebrain of rodents and other mammals that do not have a well-developed temporal lobe. It consists of over a dozen nuclei, each of which exhibit several subdivisions. Each nuclear subdivision is characterized by distinct connections (Pitkänen, 2000). The cortical and medial amygdalar nuclei are distinguished by interconnections with the olfactory system and the hypothalamus. The central nucleus is the only nucleus with extensive projections to the brainstem. The basolateral nuclear complex (BNC), the subject of this chapter, is characterized by extensive interconnections with higher order cortical areas in the prefrontal, temporal, insular, and hippocampal cortices. The size of the BNC increases throughout phylogeny, in concert with the increase in size and differentiation of the neocortices with which it has connections (Janak & Tye, 2015).
Early studies demonstrated that bilateral lesions of the anterior temporal lobes that included the amygdala make monkeys remarkably tame and hypoemotional (Brown & Schafer, 1888; Klüver & Bucy, 1939). These animals exhibited “psychic blindness,” a specific type of visual agnosia characterized by the inability to recognize the emotional or behavioral significance of sensory stimuli. Subsequent studies indicated that lesions limited to the amygdala could produce similar deficits (Aggleton & Passingham, 1981; Weiszkrantz, 1956). Weiskrantz famously stated that the effect of amygdalectomy “is to make it difficult for reinforcing stimuli, whether positive or negative, to become established or recognized as such.” Thus, the amygdala constitutes an essential link between brain regions that process sensory stimuli, such as the cerebral cortex and thalamus, and brain regions responsible for producing emotional and motivational responses, such as the hypothalamus and brainstem. It has thus been called the “sensory gateway to the emotions” (Aggleton & Mishkin, 1986). The BNC is the main target of sensory inputs. It then projects to the central nucleus, bed nucleus of the stria terminalis (BNST) and ventral striatum, which in turn activate the hypothalamus, brainstem, and other regions to generate somatomotor, autonomic, and endocrine components of emotional and motivational behavior.
Circuits involving the BNC are critical for aversive behavior, including fear conditioning, fear extinction, and anxiety (Herry et al., 2010; LeDoux, 2000; Pape & Paré, 2010; Tovote, Fadok, & Lüthi, 2015). In addition, although largely overlooked until recently, the BNC is also important for appetitive conditioning (Janak & Tye, 2015). Understanding the amygdalar circuitry underlying these functions, and how the activity of these circuits may be disrupted in disease, will be critical for developing therapies for neurological and neuropsychiatric disorders involving the amygdala, including anxiety disorders such as posttraumatic stress disorder (PTSD), depression, Alzheimer disease, temporal lobe epilepsy, and drug addiction. Most of the studies on basic systems neuroanatomy of the amygdala were completed by the turn of the century (see reviews by McDonald, 1998; Pitkänen, 2000). Over the last two decades, further details of amygdalar circuitry were clarified at the neuronal and synaptic levels. This review discusses the functional neuroanatomy of the BNC, and emphasizes the roles of individual cell types such as interneuronal subpopulations and projection-specific principal neurons. While the focus is on investigations in rodents, since these species have been heavily studied, the review also provides information on nonhuman primates, since it is anticipated that finer details of these connections in the latter species may more closely resemble those of humans. This information should be useful for designing electrophysiological and behavioral investigations using recently developed optogenetic techniques to selectively activate/inactivate different components of amygdalar circuits in brain slices and conscious behaving animals.
NUCLEI OF THE BASOLATERAL AMYGDALA
The cortical (Co) and medial nuclei (M) are located along the ventral and medial surfaces of the amygdala in most mammals including rodents (Fig. 1A). The central amygdalar nucleus (CM/CL; Fig. 1A) is located dorsolateral to the medial nucleus. The BNC is located deep into the cortical and central nuclei. It consists of three main nuclei, the lateral (LA), basolateral (BL), and basomedial nuclei (BM), arranged from dorsal to ventral, respectively. Each nucleus has several subdivisions [LA: dorsolateral (Ldl), ventrolateral (Lvl), and ventromedial (Lvm) subdivisions; BL: anterior (BLa), posterior (BLp), and ventral (BLv) subdivisions; BM: anterior (Bma), and posterior (BMp) subdivisions of Paxinos & Watson, 1997] (Fig. 1A). Price and coworkers recognized these same nuclei in the rat but used an alternative nomenclature (Price et al., 1987) (Table 1).
FIG. 1.
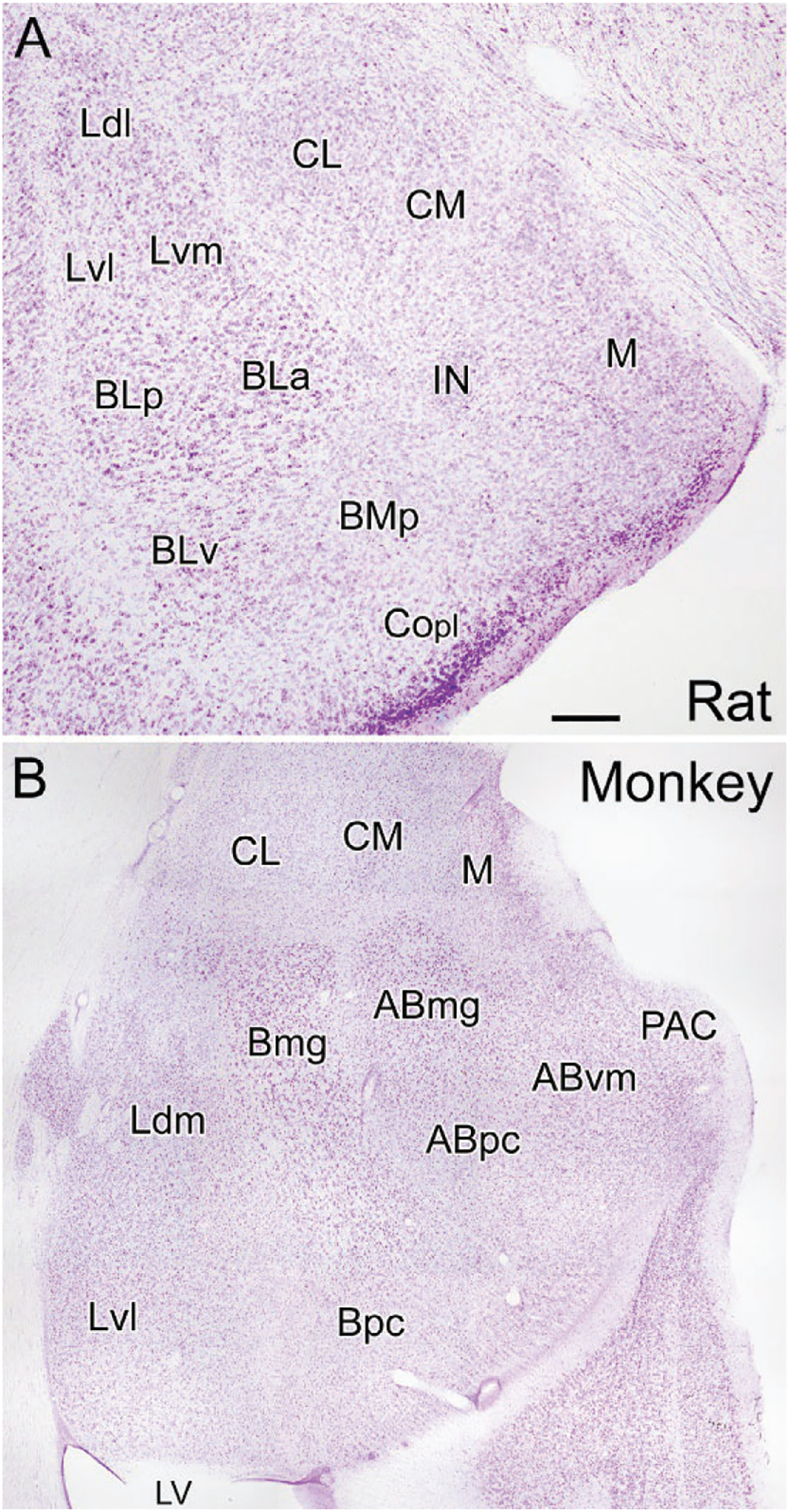
Coronal Nissl-stained sections through the amygdala of the rat (A) and monkey (B). (A) Nuclei of the rat amygdala (nomenclature of Paxinos & Watson, 1997): BLa, anterior basolateral nucleus; BLp, posterior basolateral nucleus; BLv, ventral basolateral nucleus; BMp, posterior basomedial nucleus; CL, lateral central nucleus; CM, medial central nucleus; Copl, posterolateral cortical nucleus; IN, intercalated nucleus; Ldl, dorsolateral lateral nucleus; Lvl, ventrolateral lateral nucleus; Lvm, ventromedial lateral nucleus; M, medial nucleus. (B) Nuclei of the monkey amygdala (nomenclature of Price, Russchen, & Amaral, 1987): ABmg, magnocellular accessory basal nucleus; ABpc, parvicellular accessory basal nucleus; ABvm, ventromedial accessory basal nucleus; Bmg, magnocellular basal nucleus; Bpc, parvicellular basal nucleus; CL, lateral central nucleus; CM, medial central nucleus; Ldm, dorsomedial lateral nucleus; Lvl, ventrolateral lateral nucleus; LV, inferior horn of the lateral ventricle; M, medial nucleus; PAC, periamygdaloid cortex. Scale bar (see A) = 250μm for (A) and 750μm for (B).
TABLE 1.
The main nuclei of the rat amygdala in the atlas by Paxinos and Watson (1997), and the alternative nomenclatures used by Price et al. (1987) in rat and monkey.
| Nuclei in the rat Paxinos and Watson (1997)a |
Nuclei in the rat Price et al. (1987)b |
Nuclei in the monkey Price et al. (1987) |
|---|---|---|
| Amygdalohippocampal area (AHiA) | Amygdalohippocampal area (AHA) | Amygdalohippocampal area (AHA) |
| Anterior basolateral (BLa) | Basal magnocellular (Bmg) | Basal magnocellular (Bmg) |
| Posterior basolateral (BLp) | Basal parvicellular (Bpc) | Basal parvicellular (Bpc) |
| Ventral basolateral (BLv) | Accessory basal, magnocellular (ABmc) | Accessory basal, magnocellular (ABmc) |
| Posterior basomedial (BMp) | Accessory basal, parvicellular (ABpc) | Accessory basal, parvicellular (ABpc) |
| Anterior basomedial (BMa) | Anterior cortical (Coa; deep part) | Anterior cortical (Coa) |
| Lateral central (CL) | Lateral central (CL) | Lateral central (CL) |
| Lateral capsular central (CLC) | Not identified | Not identified |
| Medial central (CM) | Medial central (CM) | Medial central (CM) |
| Anterior cortical (ACo) | Anterior cortical (Coa; superficial part) | Anterior cortical (Coa) |
| Posterolateral cortical (PLCo) | Periamygdaloid cortex (PAC) | Periamygdaloid cortex (PAC) |
| Posteromedial cortical (PMCo) | Posterior cortical (Cop) | Posterior cortical (Cop) |
| Intercalated (I) | Intercalated (I) | Intercalated (I) |
| Lateral (LA) | Lateral (L) | Lateral (L) |
| Dorsolateral lateral (Ldl) | Dorsolateral lateral (Ldl) | Homolog not identified |
| Ventrolateral lateral (Lvl) | Ventrolateral lateral (Lvl) | Homolog not identified |
| Ventromedial lateral (Lvm) | Ventromedial lateral (Lvm) | Homolog not identified |
| Nucleus of the lateral olfactory tract (LOT) | Nucleus of the lateral olfactory tract (NLOT) | Nucleus of the lateral olfactory tract (NLOT) |
| Medial (M) | Medial (M) | Medial (M) |
Central nuclei subdivided according to McDonald (1982a).
Accessory basal nucleus and lateral nucleus subdivided according to Pitkänen (2000).
These same nuclei can also be recognized in primates (Table 1; Fig. 1B), but their spatial relationships to each other are altered by the growth of the temporal lobe in these species (Crosby & Humphrey, 1944; Price et al., 1987). Thus, the expansion of the temporal neocortex along the inferolateral aspect of the hemisphere results in a medial rotation of the BNC in the coronal plane so it lies ventral to the central nucleus, rather than lateral to it as in rodents (Fig. 1B; also see Fig. 8 in Crosby & Humphrey, 1944). Likewise, the forward expansion of the temporal cortex rotates the amygdala in the sagittal plane such that the caudal parts of the BNC are pushed forward beneath the rest of the amygdala so they lie ventral to nuclei that are more rostral in the rodent brain. Thus, for example, in primates, the basal parvicellular nucleus (Bpc; homologous to the posterior subdivision of the basolateral nucleus of rodents, BLp) is located ventral to the basal magnocellular nucleus (Bmg; homologous to the anterior subdivision of the basolateral nucleus of rodents, BLa) rather than caudal to it as in rodents.
NEURONS OF THE BASOLATERAL AMYGDALA
There are two major cell classes in the BNC: pyramidal neurons (PNs) and nonpyramidal neurons (NPNs). Although these cells do not exhibit a laminar organization, their morphology, synaptology, electrophysiology, and pharmacology are remarkably similar to their counterparts in the cerebral cortex (Carlsen & Heimer, 1988; McDonald, 1992a; Rainnie, Asprodini, & Shinnick-Gallagher, 1993; Sah, Faber, Lopez De Armentia, & Power, 2003; Washburn & Moises, 1992a) [see Olucha-Bordonau, Fortes-Marco, Otero-García, Lanuza, and Martinez-García (2015) for a discussion of the developmental and genetic similarities of the BNC and cortex]. PNs have pyramidal or semipyramidal somata, and dendrites with a dense covering of spines (Hall, 1972; McDonald, 1982b; Millhouse & DeOlmos, 1983) (Figs. 2 and 3). One or more of these dendrites is thick and resembles the apical dendrites of cortical PNs, while others are thinner and resemble basal dendrites of cortical PNs. Some BNC PNs have a marked pyramidal morphology, with a clear differentiation of thicker apical dendrites from thinner basal dendrites, whereas others have a semipyramidal or even stellate appearance. Unlike cortical PNs, the apical dendrites of BNC PNs are not oriented in parallel. BNC PNs constitute about 80%–90% of BNC neurons and are the main projection neurons of the BNC (McDonald, 1992b). NPNs in the BNC have aspiny or spine-sparse dendrites and closely resemble NPNs of the cortex (Hall, 1972; McDonald, 1982b; Millhouse & DeOlmos, 1983) (Figs. 2 and 3). Most NPNs are interneurons (INs).
FIG. 2.
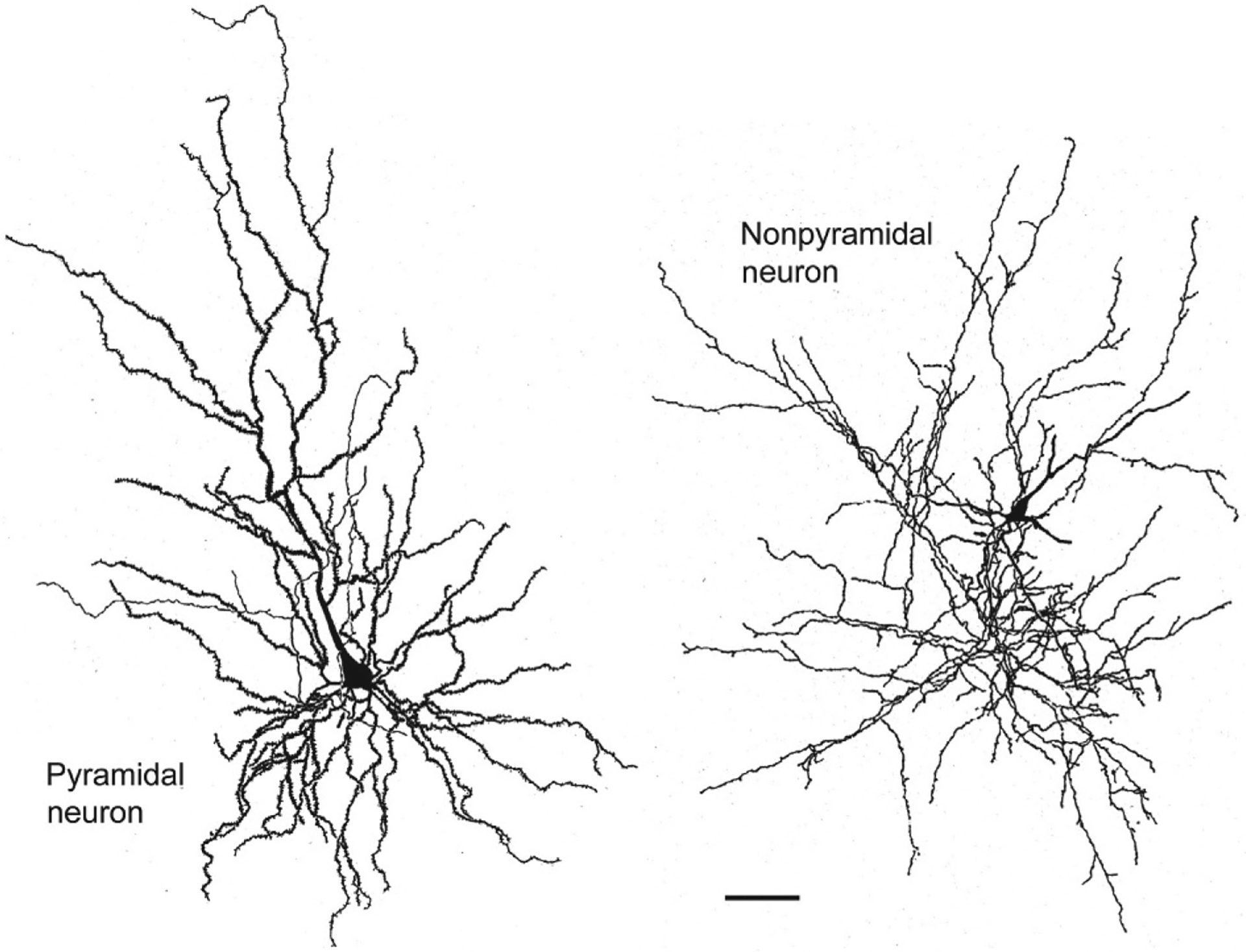
Drawings of a Golgi-stained pyramidal neuron (left) and nonpyramidal neuron (right) in the rat BNC. Note the dense local axonal arborization (thin processes) of the nonpyramidal neuron. Scale bar=50μm. Reproduced with permission from McDonald, A.J. (1982b). Neurons of the lateral and basolateral amygdaloid nuclei: A Golgi study in the rat. The Journal of Comparative Neurology, 212, 293–312.
FIG. 3.
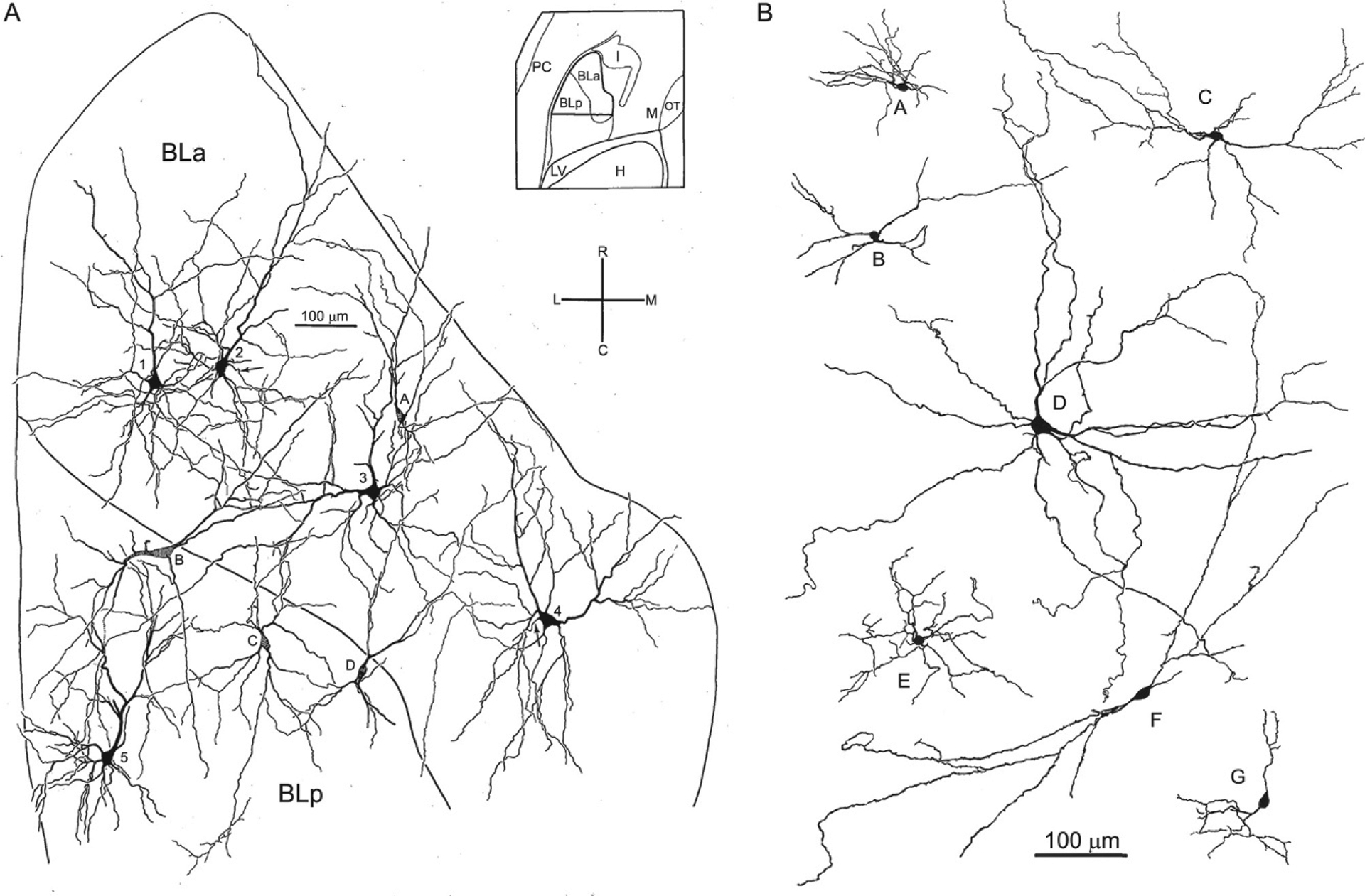
Drawings of Golgi-stained pyramidal neurons and nonpyramidal neurons in the rat BNC. Only the initial portions of the axons were stained in these neurons. (A) Drawing of pyramidal neurons (neurons 1–5) and nonpyramidal neurons (neurons A–D) in BLa and BLp. Spines of pyramidal neurons are not illustrated. Neurons were drawn from a single Golgi-stained horizontal section that was subsequently Nissl-counterstained to reveal subdivisional boundaries (see inset for region depicted). Cross indicates orientation of drawing: R, rostral; C, caudal; L, lateral; M, medial. (B) Drawings of Golgi-stained nonpyramidal neurons in the rat BNC chosen to show their heterogeneous morphology. (A) Reproduced with permission from McDonald, A.J. (1984). Neuronal organization of the lateral and basolateral amygdaloid nuclei in the rat. The Journal of Comparative Neurology, 222, 589–606. (B) Reproduced with permission from McDonald, A.J. (1982b). Neurons of the lateral and basolateral amygdaloid nuclei: A Golgi study in the rat. The Journal of Comparative Neurology, 212, 293–312.
Pyramidal neurons (PNs)
Anatomical and electrophysiological studies have demonstrated that BNC PNs, like cortical PNs, are glutamatergic (Christie, Summers, Stephenson, Cook, & Beart, 1987; Fuller, Russchen, & Price, 1987; McDonald, Mascagni, & Guo, 1996; Sah et al., 2003; Smith & Paré, 1994). Their axons course through the stria terminalis, ventral amygdalofugal pathway, and external capsule to reach targets in the forebrain. In addition to projecting to extra-amygdalar regions, their axons also exhibit a moderately dense local arborization that remains in the nucleus of origin, or takes part in internuclear amygdalar connections (McDonald, Mascagni, Mania, & Rainnie, 2005; Pitkänen, Savander, & LeDoux, 1997; Pitkänen, Savander, Nurminen, & Ylinen, 2003). These local axonal collaterals innervate dendritic spines and dendritic shafts of neighboring BNC PNs, as well as BNC GABAergic NPNs (McDonald, 1982b; McDonald et al., 2005; Paré, Dong, & Gaudreau, 1995; Smith, Paré, & Paré, 2000).
Electron microscopic (EM) studies of the BNC using alpha type II calcium/calmodulin-dependent protein kinase (CaMK) as a marker for pyramidal cells have demonstrated that the synaptic organization of inputs to BLa PNs is similar to that of cortical PNs (McDonald, Muller, & Mascagni, 2002; Muller, Mascagni, & McDonald, 2006). Thus, the somata and axon initial segments of BLa PNs are only targeted by axons forming GABAergic symmetrical synapses. Dendritic shafts of BLa PNs are innervated by axons forming symmetrical (inhibitory or neuromodulatory) synapses and axons forming asymmetrical (excitatory) synapses. The former are more common on proximal dendritic shafts versus distal dendritic shafts (Muller et al., 2006). There are relatively few asymmetrical (excitatory) synapses with proximal dendrites. However, the majority of inputs to spines of BNC PNs are excitatory (70%). Previous EM studies of the BNC indicate that axon terminals forming asymmetrical synapses represent glutamatergic inputs from the cerebral cortex, midline/intralaminar thalamus, and internuclear and intranuclear amygdalar connections arising from BNC PNs, whereas many of the symmetrical synapses onto BNC PNs are formed by several distinct subpopulations of GABAergic interneurons (see below). However, monoaminergic projections from the brainstem and cholinergic projections from the basal forebrain (BF) also form symmetrical synapses in the BNC, primarily with distal dendritic shafts and spines (see below).
The perisomatic region of BNC PNs consists of the soma, axon initial segment (AIS), and proximal dendrites. Recent studies indicate that proximal dendrites can be distinguished from distal dendrites by the expression of the voltage-gated potassium channel subunit Kv2.1, as well as by their robust innervation by GABAergic terminals (Vereczki et al., 2016). PN somata also express Kv2.1. Since the perisomatic region is where sodium-mediated action potentials are generated, the numerous inhibitory GABAergic inputs to this region are important regulators of PN spiking (Freund & Katona, 2007). On the other hand, inhibitory inputs to distal dendrites, which receive extensive excitatory inputs, can regulate dendritic integration and synaptic plasticity, the generation of calcium-dependent action potentials in dendrites, as well as back-propagation of action potentials from somatic to dendritic compartments (Miles, Toth, Gulyas, Hajos, & Freund, 1996; Stuart, Spruston, Sakmann, & Hausser, 1997).
Ensembles of PNs in LA are known to represent memory traces (engrams) in auditory fear conditioning (Josselyn & Frankland, 2018). In this experimental paradigm foot shock (the unconditioned stimulus, US) is paired with a neutral tone (the conditioned stimulus, CS). Subsequent presentations of the CS produce the same fear response (conditioned response, CR) produced by foot shock (e.g., freezing and autonomic changes) (LeDoux, 2000). At the neuronal level fear conditioning involves convergence of nociceptive and auditory inputs onto LA PNs, with subsequent potentiation of excitatory auditory inputs (Rogan, Stäubli, & LeDoux, 1997). Although the majority of PNs receive these convergent inputs, the engram is represented by only 10%–30% of PNs, namely those that exhibit the highest excitability. Similar ensembles of PNs are also known to represent the engram in appetitive conditioning (Josselyn & Frankland, 2018).
Nonpyramidal neurons (NPNs)
Like their counterparts in the cortex, NPNs in the BNC are morphologically heterogenous and have dendrites that are aspiny or spine-sparse (Figs. 2 and 3). Most NPNs have smaller somata than PNs, and have 2–6 primary dendrites that branch sparingly. The axons of NPNs arise from the soma or a primary dendrite and give rise to varicose collateral branches that form a moderate to dense local axonal arborization (Fig. 2). As in the cortex, immunohistochemical studies of the BNC of all species, including rodents (Carlsen, 1988; McDonald, 1985) and primates (McDonald & Augustine, 1993; Pitkänen & Amaral, 1994), indicate that the great majority of NPNs are GABAergic. However, similar to the cortex (Houser, Crawford, Salvaterra, & Vaughn, 1985; von Engelhardt, Eliava, Meyer, Rozov, & Monyer, 2007), some studies have reported the existence of a small number of small choline acetyltransferase positive (ChAT+) bipolar interneurons in the BNC (Carlsen & Heimer, 1986; Nitecka & Frotscher, 1989). It has not been determined whether these putative cholinergic BNC NPNs also express GABA. Bipolar cholinergic NPNs in the cortex are not GABAergic, but other multipolar cholinergic NPNs are GABAergic (von Engelhardt et al., 2007).
Immunohistochemical studies in the rat suggest that the BNC contains at least four distinct subpopulations of NPNs that can be distinguished on the basis of their calcium-binding protein and peptide contents (Fig. 4). These subpopulations are: (1) parvalbumin+/calbindin+ (PV+/CB+) neurons, (2) somatostatin+/calbindin+ (SOM+/CB+) neurons, (3) large multipolar cholecystokinin+ (CCK+) neurons that are often calbindin+, and (4) small bipolar and bitufted interneurons that exhibit extensive colocalization of calretinin (CR), CCK, and vasoactive intestinal peptide (VIP) (Kemppainen & Pitkänen, 2000; Mascagni & McDonald, 2003; McDonald & Betette, 2001; McDonald & Mascagni, 2001a, 2002). In addition, a subpopulation of SOM+ neurons, but not other NPN subpopulations, expresses neuropeptide Y (NPY; Fig. 4) (McDonald, 1989). Interestingly, NPN subpopulations in the rat frontal cortex closely resemble those in the BNC, including the two types of CCK+ neurons (Kubota, Hattori, & Yui, 1994; Kubota & Kawaguchi, 1997). In the rat BLa, the approximate contribution of each subpopulation to the total GABAergic population is: (1) PV+ neurons=41%; (2) SOM+ neurons=17%; (3) type L large CCK+ neurons=7%; and (4) the small bipolar/bitufted (CR+/VIP+/CCK+) neuronal family=35% (Mascagni & McDonald, 2003). In the rat LA, the percentages are roughly the same with the exception of the PV+ neurons, which constitute only about 20% of GABA neurons. However, studies in the rat and mouse using single-cell RT-PCR to classify BNC NPNs based on the expression of mRNAs for calcium-binding proteins and peptides are not congruent with the immunohistochemical findings (Sosulina, Graebenitz, & Pape, 2010; Sosulina, Meis, Seifert, Steinhauser, & Pape, 2006). This suggests that mRNAs for some of these neurochemicals are not translated into protein, or are translated at levels below the level of immunohistochemical detection, in certain NPN subpopulations. The ability to identify specific NPN subpopulations using cell type-specific markers has permitted analysis of their connections, electrophysiological properties, and behavioral significance (for reviews see Ehrlich et al., 2009; Spampanato, Polepalli, & Sah, 2011; Capogna, 2014; Krabbe, Gründemann, & Lüthi, 2018). Similar NPN subpopulations appear to be present in the primate BNC, but there is far less colocalization of PV with CB in NPNs in human and nonhuman primates (Amaral, Avendaño, & Benoit, 1989; Mascagni, Muly, Rainnie, & McDonald, 2009; McDonald, Mascagni, & Augustine, 1995; Pantazopoulos, Lange, Hassinger, & Berretta, 2006; Schwartzberg, Unger, Weindl, & Lange, 1990).
FIG. 4.
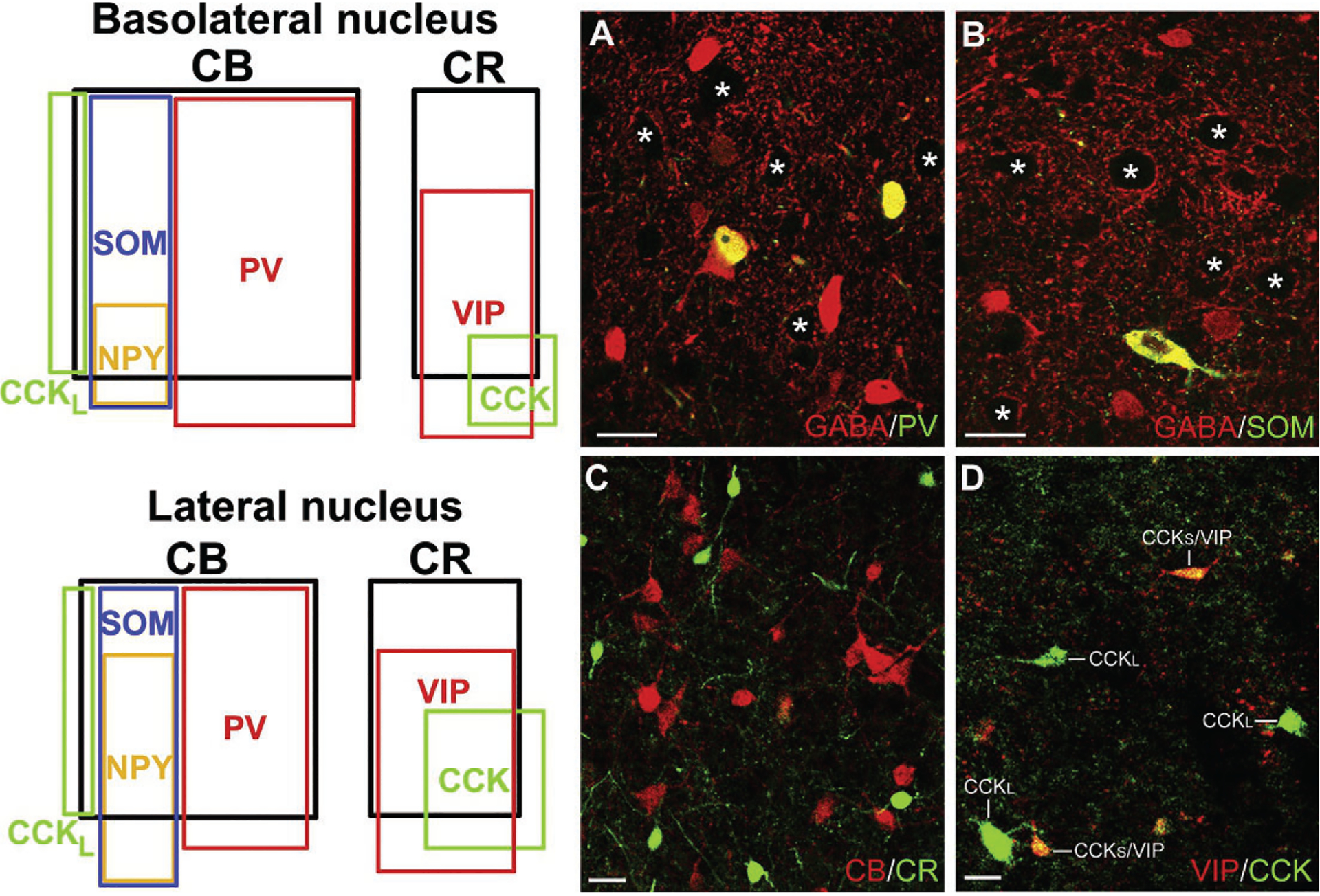
Left: Venn diagrams showing the overlap and relative proportions of nonpyramidal neuronal populations containing calcium-binding proteins and neuropeptides in the rat BL and LA. The relative sizes of the rectangles representing these subpopulations are depicted in relation to the total population of GABA+ neurons in each nucleus. Thus, since CB+ neurons, which are almost entirely GABAergic, comprise 55% of GABA+ neurons in the BLa and 41% of the GABA+ neurons in the lateral nucleus, the rectangle representing the relative size of these subpopulations is proportionately smaller in the latter nucleus. Note that there are two separate subpopulations of CCK+ interneurons in both nuclei. The subpopulation exhibiting partial overlap with CB are large CCK+ neurons (CCKL), whereas the subpopulation exhibiting partial overlap with CR and VIP are small CCK+ neurons. Right: Photomicrographs of representative NPN subpopulations in the rat. (A) Colocalization of PV (green) and GABA (red) in BL NPNs (yellow indicates colocalization). (B) Colocalization of SOM (green) and GABA (red) in a LA NPN (yellow indicates colocalization). (C) Lack of colocalization of CR (green) with CB (red) in BL NPNs. (D) Colocalization of VIP (red) with CCK (green) in small CCK+ NPNs (CCKS), but not in large CCK+ NPNs (CCKL), in the BL. Asterisks in A and B indicate several of the GABA-negative PNs in these fields. Scale bars=25μm. (A) and (C) Reproduced with permission from McDonald A.J., & Mascagni, F. (2001a). Colocalization of calcium-binding proteins and gamma-aminobutyric acid in neurons of the rat basolateral amygdala. Neuroscience, 105, 681–693. (B) Reproduced with permission from McDonald, A.J., & Mascagni, F. (2002). Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Research, 943, 237–244. (D) Reproduced with permission from Mascagni, F., & McDonald, A.J. (2003). Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Research, 976, 171–184.
Parvalbumin NPNs
PV NPNs are the predominant NPN subpopulation in the BNC (McDonald & Mascagni, 2001a). With the exception of one type of PV NPN in the lateral nucleus that projects to the adjacent amygdalostriatal transition area, all are interneurons (INs) with locally arborizing axons (Bienvenu, Busti, Magill, Ferraguti, & Capogna, 2012; Vereczki et al., 2016). As in cortical structures there appear to be three major types of PV INs in both the rodent and the primate BNC: (1) axo-axonic cells (AACs) whose axons only target the axon initial segments of PNs, (2) basket cells whose axons mainly target the somata and proximal dendrites of PNs, and (3) dendrite-targeting cells whose axons mainly target distal dendrites and spines of PNs (rodents: McDonald & Betette, 2001; Rainnie, Mania, Mascagni, & McDonald, 2006; Muller et al., 2006; Bienvenu et al., 2012; Vereczki et al., 2016) (human and nonhuman primates: Pitkänen & Amaral, 1993a; Sorvari, Miettinin, Soininen, & Pitkänen, 1996). In rodents, all PV+ basket cells (PVBCs) also express CB, whereas PV+ AACs do not (Andrási et al., 2017; Bienvenu et al., 2012). In primates, neither PVBCs nor AACs express CB (Pitkänen & Amaral, 1993b; Sorvari, Soininen, Paljärvi, Karkola, & Pitkänen, 1995), which may explain the lower percentages of PV+/CB+ INs in primates compared to rodents (Mascagni et al., 2009). PV and CB have different calcium binding rates and affinities (Schwaller, Meyer, & Schiffmann, 2002) and could therefore differentially modulate presynaptic short-term plasticity in INs (Blatow, Caputi, Burnashev, Monyer, & Rozov, 2003; Vreugdenhil, Jefferys, Celio, & Schwaller, 2003).
AACs in the BNC were first described in Golgi studies in opossum and rat (McDonald, 1982b; McDonald & Culberson, 1981). Axonal collaterals of Golgi-stained AACs branch to form a local axonal arborization that is 300–400μm in diameter. As in the cortex, axon terminals of these collaterals are clustered to form axonal “cartridges” that contact axon initial segments (AISs) of PNs (Fig. 5A). Cartridges formed by individual Golgi-stained AACs consist of a variable number of axon terminals (4–15) and contact variable lengths of the AIS (10–30μm). Although the great majority of axon terminals formed by AACs are PV+, some are not (Fig. 5B) (Muller et al., 2006). This suggests that other cell types, perhaps belonging to other IN subpopulations in the BNC, also form synapses with PN AISs. Likewise, some PV − negative peptidergic INs in the neocortex form synapses with the AISs of cortical PNs (Gonchar, Turney, Price, & Burkhalter, 2002; Lewis & Lund, 1990).
FIG. 5.
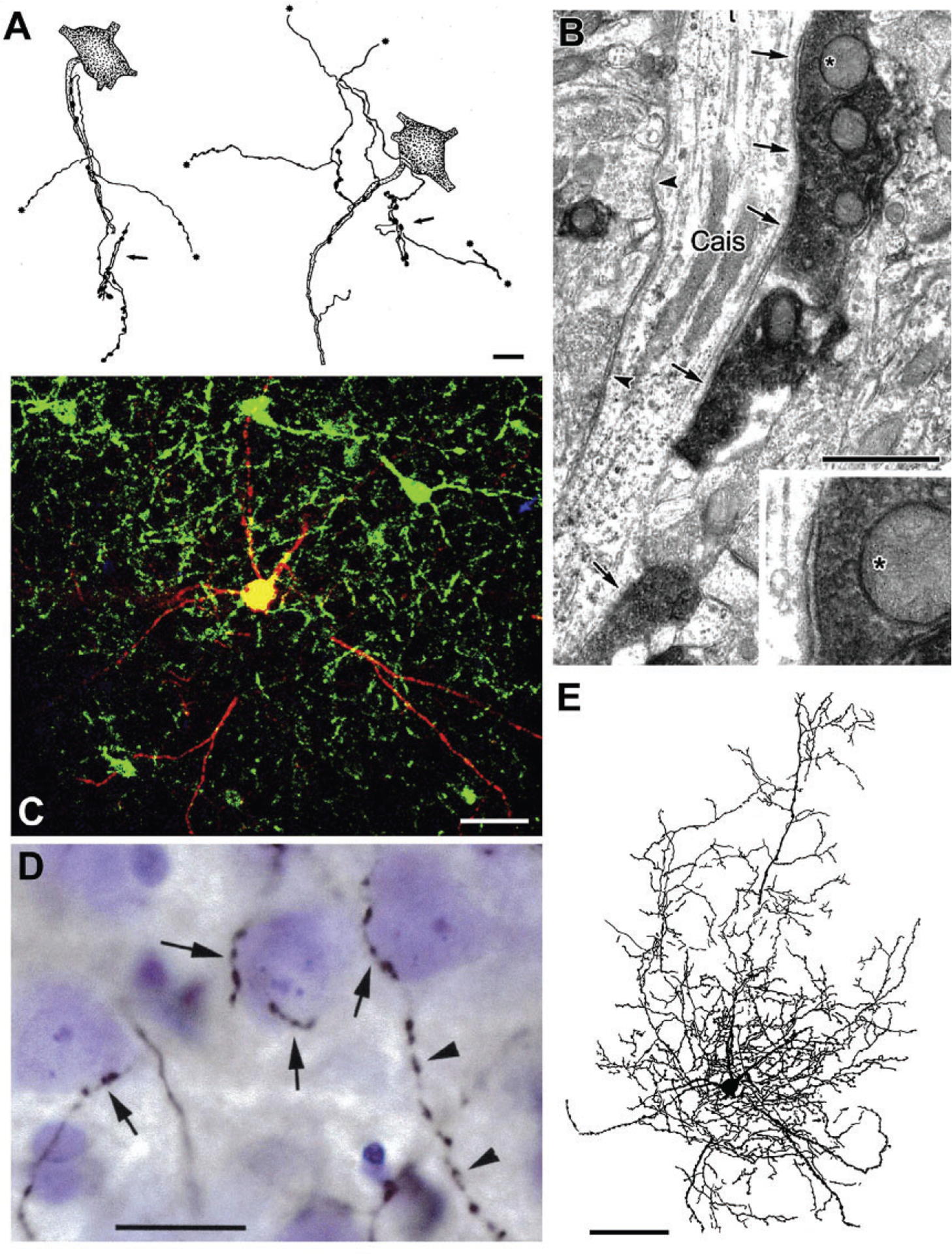
Axo-axonic cells (AACs) and basket cells in the rat BLa. (A) Drawings of two AAC axons whose axonal cartridges form multiple contacts with AISs of PNs (stippled). Asterisks indicate points at which collaterals continue but are not drawn. Each axon also forms additional axonal cartridges whose targets are not stained (arrows). (B) A CaMK+ axon initial segment of a PN (Cais), exhibiting particulate labeling, receives symmetrical synaptic inputs from densely stained PV+ axon terminals (arrows) and unstained PV− negative terminals (arrowheads). The upper PV+ terminal (asterisk) is shown magnified in the inset. (C) Merged confocal image of a biocytin-filled burst-firing PVBC. Biocytin is red. PV+ neurons are green. Yellow indicates double labeling (i.e., biocytin-filled structures that are PV+). (D) Photomontage of a Nissl-stained section showing a portion of the axonal arborization of the neuron shown in (C), after processing for the avidin-biotin peroxidase technique. Its biocytin-filled axonal collaterals (black) form multiple contacts with the somata of three presumptive PNs (arrows). The axon of this PV+ cell contacted the somata of over 100 surrounding Nissl-stained cells. The cell on the right receives additional axonal contacts along the proximal portion of a downwardly projecting process. This axon then continues its downward course (arrowheads) forming a series of varicosities that do not contact Nissl-stained structures. (E) Drawing of the burst-firing PVBC shown in (C) and (D). Only the dendrites and axonal branches in the 75-μm-thick section containing the cell body are drawn. Additional axonal and dendritic branches extended into two adjacent 75-μm-thick sections. Scale bars=10μm in (A), 1μm in (B), 50μm in (C), 20μm in (D), 100μm in (E). (A) Reproduced with permission from McDonald, A.J. (1982b). Neurons of the lateral and basolateral amygdaloid nuclei: A Golgi study in the rat. The Journal of Comparative Neurology, 212, 293–312. (B) Reproduced with permission from Muller JF, Mascagni F, McDonald AJ (2006) Pyramidal cells of the rat basolateral amygdala: Synaptology and innervation by parvalbumin-immunoreactive interneurons. The Journal of Comparative Neurology, 494, 635–650. (C)–(E) Reproduced with permission from Rainnie DG, Mania I, Mascagni F, McDonald AJ (2006) Physiological and morphological characterization of parvalbumin-containing interneurons of the rat basolateral amygdala. The Journal of Comparative Neurology, 498, 142–161.
Hajos and coworkers have conducted quantitative analyses of the innervation of individual PN AISs by AACs in the mouse BL (Vereczki et al., 2016; Veres, Nagy, Vereczki, Andrási, & Hájos, 2014). It was found that each AAC formed an average of eight contacts with each AIS targeted, and that the average total number of AAC synapses formed with each AIS was 52. AACs typically innervated about 20% of the PNs within their axonal arborization (perhaps as many as 600–650 PNs), and each AIS was innervated by an average of 6–7 AACs. Although some PV+ basket cells (PVBCs) innervated the first 10μm of the PN axon (i.e., axon hillock), the density of AAC inputs to AISs was highest between 20μm and 40μm from the soma, where the threshold for action potential generation was lowest, and where 10–12 AAC synapses, originating from 2 to 3 AACs could block PN firing (Veres et al., 2014).
PVBCs exhibiting pericellular baskets of PV+ axon terminals encapsulating PN somata are very common in the BNC of all species, including humans (McDonald & Betette, 2001; Pitkänen & Amaral, 1993a, 1993b; Sorvari, Miettinin, et al., 1996). However, EM studies revealed that only about half of the synapses with PN somata are PV+, indicating that other neuronal populations also innervate these somata (Muller et al., 2006). Recent studies have shown that another BNC IN basket cell subpopulation consists of CCK+ INs (Vereczki et al., 2016; Veres, Nagy, & Hájos, 2017). Hajos and coworkers recently conducted quantitative analyses of the innervation of the perisomatic region of PNs by individual PVBCs in the mouse BL (Vereczki et al., 2016; Veres et al., 2017). It was found that each PVBC formed an average of six contacts with the perisomatic region of each PN targeted and that the average total number of synapses formed by PVBCs with the perisomatic region of each PN was 93. Moreover, PVBCs typically innervated about 10% of the PNs within their axonal arborization (perhaps as many as 800–900 PNs), and each PN was innervated by an average of 15–16 PVBCs. In the mouse BL, it has been found that both PVBCs and CCKBCs (see below) provided roughly equal regulation of PN firing (Veres et al., 2017). Although on average only one-third of the synapses provided by both types of BCs are formed with the perisomatic compartment of PNs, the number of synapses with this compartment determined the inhibitory efficacy on PN firing (Veres et al., 2017).
A study of the targets of individual PVBCs in the rat BL surprisingly demonstrated that the majority of the axon terminals of these cells do not contact somata, suggesting that dendrites are also a major target of PVBCs (Rainnie et al., 2006). Likewise, a study of individual PVBCs in the mouse BL has shown that 40% of PVBC terminals synapse with dendritic shafts, especially the proximal dendrites of PNs (Vereczki et al., 2016) as well as with more distal dendrites. PVBCs actually form a continuum, with each individual cell forming varying proportions of perisomatic (somata and proximal dendrites) versus distal dendritic synapses with PNs (Veres et al., 2017). Some of the PV INs studied by Hajos’ group only targeted dendrites, and provided no inputs to the perisomatic compartment of PNs, although these cells were also considered to be part of the PVBC continuum. A small number of CB INs in the LA that only targeted thin distal CaMK+ dendrites of PNs were described in the rat (Bienvenu et al., 2012). These neurons exhibited very light PV immunoreactivity, were SOM− negative, and displayed a firing pattern in response to noxious stimuli that was distinct from those of PVBCs and AACs. EM studies have demonstrated that about half of the terminals forming symmetrical synapses with dendritic shafts and spines of BL PNs are PV+ (Muller et al., 2006). Thus, in addition to the regulation of somatic signal integration and PN spiking via innervation of the perisomatic region of PNs, synapses of PVBCs with dendrites allow these cells to influence the effects of excitatory dendritic inputs involved in synaptic plasticity.
Rainnie et al. (2006) found that PV INs in the rat BL exhibited burst-firing or stutter-firing in response to transient depolarizing current injection. Both subpopulations of PV INs included PVBCs (Fig. 5C–E). PV+ stutter-firing neurons fired high-frequency spike trains that were interrupted by short periods of quiescence. PV+ burst-firing neurons fired repetitive bursts of high-frequency discharges, followed by a regular pattern of firing. The same patterns of high-frequency firing are also seen in cortical PV INs (Buhl et al., 1994; Kawaguchi, 1995; Pawelzik, Hughes, & Thomson, 2002) where they appear to be due to the expression of Kv3.1b and Kv3.2 potassium channel subunits (Toledo-Rodriguez et al., 2004). Not surprisingly, it was found that about 95% of PV INs in the BNC were Kv3.1b+ and 75% were Kv3.2+ (McDonald & Mascagni, 2006). Moreover, Kv3.1b+ pericellular baskets and axonal cartridges resembling those formed by PVBCs and PVAACs, respectively, were also observed in the BL. Studies in mice reported that PV INs in the BL exhibit four different firing patterns (Sosulina et al., 2010; Woodruff & Sah, 2007a). Woodruff and Sah suggested that their accommodating neurons may correspond to the burst-firing neurons mentioned by Rainnie et al. (2006), whereas their fast spiking, delayed firing, and stutter firing may correspond to the stutter-firing neurons mentioned by Rainnie and coworkers. Although PV INs appear to be the main IN subtype in the BNC exhibiting high-frequency firing, there is evidence that some VIP INs and SOM INs are fast-firing neurons (Rainnie et al., 2006; Rhomberg et al., 2018; Sosulina et al., 2010).
EM studies have shown that the BL contains a network of PV INs interconnected by chemical and electrical synapses (Muller, Mascagni, & McDonald, 2005). Twenty percent of chemical synapses onto PV INs were formed by PV+ axons. Electrical synapses mediated by gap junctions were mainly seen between dendrites, but also between axon terminals. Interestingly, some of the axon terminals interconnected by gap junctions formed synapses with PN somata and were from separate PVBCs (Muller et al., 2005). The application of a combination of optogenetics, electrophysiology, and high-resolution microscopy allowed Andrási et al. (2017) to demonstrate that PVBCs and CCKBCs form separate networks that are interconnected by chemical synapses and gap junctions. However, both types of basket cells synapse with PV AACs. Electrophysiological studies of PV INs have confirmed the anatomical findings concerning PV IN networks in the BL (Woodruff & Sah, 2007a). Moreover, it was found that electrical coupling preferentially occurs between pairs of INs that have the same firing pattern, and that these IN pairs were also more likely to be interconnected by GABAergic chemical synapses. Likewise, electrical synapses interconnect INs of the same class in the neocortex (Hestrin & Galarreta, 2005). In addition to their innervation of PV INs, PV INs also innervate SOM INs in the BNC. Interestingly, it has been shown that conditioned stimuli (CS) activate PV INs during fear conditioning, which in turn inhibit SOM INs, resulting in a disinhibition of PN dendrites which facilitates excitatory synaptic plasticity (Wolff et al., 2014).
Because PV INs make up a large percentage of the GABAergic INs in the BNC, their synaptic interactions with PNs are extremely important in determining neuronal activity in this region. Excitatory inputs from the cortex and the thalamus mainly target the spines and distal dendrites of PNs. In addition to projections to extrinsic regions, axons of BNC PNs also provide a dense innervation of the dendritic spines of numerous surrounding PNs (Smith et al., 2000) as well as a robust innervation of many neighboring PV INs (McDonald et al., 2005; Moryś et al., 1999; Smith et al., 2000). As in the cortex (Geiger et al., 1995), excitatory drive from PNs onto PV INs in the BNC exhibits rapid kinetics that appears to be mediated primarily by GluA1 AMPA receptors (Mahanty & Sah, 1998; McDonald, 1996b). As described above, the BNC, like the cortex, contains networks of PV INs interconnected by gap junctions and GABAergic synapses (Muller et al., 2005). As in the cortex (Freund, 2003; Whittington & Traub, 2003; Whittington, Traub, & Jefferys, 1995), these networks of PV INs in the BNC appear to be important for the generation of theta and gamma rhythms. In response to PN excitation, PVBCs and AACs provide robust perisomatic feedback inhibition of PNs. The extensive divergence and convergence of the axons of BNC PVBCs and AACs synapsing with the perisomatic domains of numerous surrounding PNs can entrain the firing of these cells. Rebound firing of inhibited PNs results in the reactivation of the PV IN network (Paré, Dong, et al., 1995; Woodruff & Sah, 2007b). The high speed and temporal precision of these reciprocal PN-PV interconnections is critical for the generation of rhythmic oscillations in the BNC (Ryan et al., 2012). This mechanism is consistent with electrophysiological studies in the BNC which have demonstrated counterphase firing of PNs and fast-firing (presumptive PV+) INs during delta and theta rhythms (Paré & Gaudreau, 1996), similar to the hippocampus (Freund, 2003; Freund & Buzsaki, 1996). Prominent theta and gamma oscillations occur in the BNC during emotional arousal (Oya, Kawasaki, Howard 3rd, & Adolphs, 2002; Paré & Collins, 2000; Paré, Collins, & Pelletier, 2002; Seidenbecher, Laxmi, Stork, & Pape, 2003). These network rhythms, as well as intrinsic oscillations in BNC PNs (Pape, Paré, & Driesang, 1998), are critical for synaptic plasticity associated with the formation and retrieval of emotional memories (Bocchio, Nabavi, & Capogna, 2017; Pape & Paré, 2010; Paré et al., 2002). In contrast, fear extinction is associated with a decrease in the theta rhythm (Lesting et al., 2011).
Another characteristic linking PV INs to the formation and extinction of emotional memories is their ensheathment by perineuronal nets (PNNs). PNNs are specialized condensations of extracellular matrix that ensheath particular neuronal subpopulations in the brain and the spinal cord. PNNs regulate synaptic plasticity, including the encoding of fear memories by the amygdala (Gogolla, Caroni, Lüthi, & Herry, 2009). Numerous studies in rodents and human/nonhuman primates have shown that PV INs are the main cell type ensheathed by PNNs in the BNC (Härtig, Brückner, Brauer, Schmidt, & Bigl, 1995; McDonald, Hamilton, & Barnstable, 2018; Pantazopoulos et al., 2006). The formation of PNNs in the BLa during postnatal days 16–21 protects fear memories from erasure during extinction training (Gogolla et al., 2009). Conditioned fear memories formed before this critical postnatal period can be erased by extinction training whereas those formed after PNNs are formed cannot be erased. Injections of chondroitinase to deplete PNNs in the basolateral amygdala restored the juvenile condition in which extinguished fear memories cannot be restored by spontaneous recovery or context-dependent renewal training (Gogolla et al., 2009). Although it is known that amygdalar PV INs are critical for inhibitory and disinhibitory influences during conditioning and extinction (Trouche, Sasaki, Tu, & Reijmers, 2013; Wolff et al., 2014), the exact mechanism by which PNNs surrounding these interneurons prevent the erasure of memories has yet to be determined.
Recent studies have shown that there are anatomical and functional differences in PV INs in the LA versus the BL (Lucas, Jegarl, Morishita, & Clem, 2016). PV INs in LA, but not BL, receive potent excitation from auditory portions of the thalamus which results in feedforward inhibition of LA PNs. Fear conditioning reduces this excitatory drive onto PV INs and also reduces GABA release from PV INs, both of which serve to disinhibit LA PNs and promote acquisition of fear learning (Lucas et al., 2016).
Somatostatin NPNs
Immunohistochemical studies in the 1980s found that GABAergic SOM+ NPNs in the rat BNC were distinct from CCK+ and VIP+ GABAergic neurons, and that many co-expressed neuropeptide Y (NPY) (McDonald, 1989; McDonald & Pearson, 1989). In fact, virtually all NPY+ NPNs were SOM+ (Fig. 4). Later investigations revealed that most SOM+ NPNs express CB, but not calretinin (CR), and were distinct from two other subpopulations of CB+ NPNs that express PV or CCK (McDonald & Mascagni, 2002) (Fig. 4). Similar SOM+/NPY+ NPNs have been described in the monkey BNC (McDonald et al., 1995). Recent immunohistochemical studies have indicated that SOM and NPY NPNs are also the main BNC subpopulations expressing nitric oxide synthase (NOS; Wang, Liu, Wang, Gao, & Zhan, 2017). Earlier studies in rat and monkey used NADPH-diaphorase histochemistry to identify NOS containing neurons, but did not attempt colocalization with SOM (McDonald, Payne, & Mascagni, 1993; Pitkänen & Amaral, 1991). Although many of the SOM+ and SOM+/NPY+ axons in the BNC arise from BNC NPNs, it is noteworthy that a small percentage of SOM+ and SOM+/NPY+ neurons in the entorhinal cortex (ERC), amygdalopiriform transition area, amygdalostriatal transition area, BNST, and amygdalar intercalated nuclei have projections to the BNC (Leitermann, Rostkowski, & Urban, 2016; McDonald & Zaric, 2015b).
EM studies have shown that SOM+ axon terminals mainly innervate the distal dendritic compartment of PNs (78% of SOM+ terminals), with inputs to spines (45% of SOM+ terminals) outnumbering inputs to dendritic shafts (33% of SOM+ terminals) (Muller, Mascagni, & McDonald, 2007a; Wolff et al., 2014). These inputs were often in close proximity to excitatory inputs forming asymmetrical synapses. The close proximity of SOM+ terminals to these excitatory terminals suggests that an important function of SOM+ inhibitory NPNs may be to shunt excitatory inputs to the distal dendritic domain of BNC PNs, thus regulating synaptic plasticity involved in emotional learning. At least 15% of SOM+ terminals innervate NPNs including PV+, VIP+, and other SOM+ NPNs. Although most SOM+ NPNs appear to be INs, a small percentage of SOM+ and SOM+/NPY+ NPNs in the BNC are projection neurons that innervate the ERC or basal forebrain (McDonald, Mascagni, & Zaric, 2012; McDonald & Zaric, 2015a).
Somatostatin and GABA released from SOM+ GABAergic INs in the BNC may act synergistically to affect amygdalar LTP. Somatostatin is released from axon terminals during high-frequency activity and acts via several G protein-coupled receptors (SST1-SST5; Moller, Stidsen, Hartmann, & Holst, 2003). Somatostatin inhibits high-voltage-activated calcium currents and dampens excitability in BNC PNs via SST2 receptors (Meis, Sosulina, Schulz, Hollt, & Pape, 2005; Viana & Hille, 1996). A similar action of somatostatin was seen in the hippocampus, where this mechanism was shown to block the induction of LTP in this region (Baratta, Lamp, & Tallent, 2002). There is selective loss of SOM+ interneurons in the BNC in animal models of temporal lobe epilepsy (TLE) (Tuunanen, Halonen, & Pitkänen, 1996, 1997). These findings suggest that the loss of the dendritic hyperpolarization produced by the release of both somatostatin and GABA by these SOM neurons may produce hyperexcitability in the amygdala in TLE. These findings are in agreement with computational modeling studies which suggest that loss of dendritic inhibition can drive ictogenesis (Wendling, Bartolomei, Bellanger, & Chauvel, 2002).
Many SOM+ INs co-express NPY. NPY hyperpolarizes and dampens the excitability of BNC PNs via postsynaptic Y1 receptors (Giesbrecht, Mackay, Silveira, Urban, & Colmers, 2010; Sosulina, Schwesig, Seifert, & Pape, 2008). These mechanisms may underlie the decrease in anxiety-like behavior associated with NPY injections into the BNC (Sajdyk, Vandergriff, & Gehlert, 1999). Long-term stress resilience produced by NPY injections into the BNC appears to involve modulation of synaptic plasticity of excitatory inputs to PN distal dendrites by SOM/NPY inputs (Leitermann, Sajdyk, & Urban, 2012; Sajdyk et al., 2008; Silveira Villarroel et al., 2018). Moreover, selective ablation of BNC neurons expressing the NK-1 substance P receptor by the toxin SSP-saporin increases anxiety-like behaviors (Truitt, Johnson, Dietrich, Fitz, & Shekhar, 2009); about 40%–50% of these NK-1+ neurons are SOM+/NPY+ NPNs (including virtually all NPY+ NPNs) (Levita, Mania, & Rainnie, 2003; Truitt et al., 2009). Importantly, the loss of NPY+ NPNs in the BNC was correlated with anxiety-like behavior and reflects the loss of BNC NPY+ NPNs rather than NPY+ inputs from regions outside of the BNC (Truitt et al., 2009).
The great majority of SOM/NPY INs in the BNC are medium-sized neurons with 3–4 primary dendrites (McDonald, 1989; McDonald et al., 1995). However, one subpopulation of SOM/NPY INs are neurogliaform cells (NGFCs) with small somata, many primary dendrites (as many as 10–12), and dense dendritic and axonal arborizations that are confined to the region near the cell (Mańko, Bienvenu, Dalezios, & Capogna, 2012). Most axon terminals of these cells form nonsynaptic appositions with dendrites, rather than synaptic junctions, suggesting that GABA is released into the extracellular space and reaches receptors via volume transmission. These cells evoked a slow IPSC in PNs that was most likely due to a low concentration of GABA as a result of spillover from the nonsynaptic terminals (Mańko et al., 2012). The anatomy, neurochemistry, and electrophysiology of BNC NGFCs closely resemble those of the neocortex and hippocampus (Armstrong, Krook-Magnuson, & Soltesz, 2012; Overstreet-Wadiche & McBain, 2015).
BNC NGFCs were originally observed in Golgi preparations in a variety of species (Kamal & Tőmböl, 1975; McDonald, 1982b; McDonald & Culberson, 1981; Tőmböl & Szafranska-Kosmal, 1972). Dendrites and axons of Golgi-stained BNC NGFCs make frequent intimate contacts with dendritic shafts and spines of PNs (McDonald, 1982b; McDonald & Culberson, 1981). The dendrodendritic contacts could represent gap junctions since NGFCs are connected with other NGFCs and non-NGFCs via gap junctions in the cortex (Simon, Oláh, Molnár, Szabadics, & Tamás, 2005).
CCK, VIP, and CR NPNs
CCK+ NPNs are GABAergic INs whose axons form dense intranuclear arborizations (McDonald & Pearson, 1989; Vereczki et al., 2016). As in the rat frontal cortex (Kubota & Kawaguchi, 1997) there are two major subpopulations of CCK INs in the rat BNC that can be identified on the basis of morphological differences. Large CCK INs (CCKL) are multipolar, bipolar, or bitufted neurons with thick dendrites and somata that average 15–20μm in diameter, whereas small CCK INs (CCKS) are bipolar or bitufted neurons with thin dendrites and somata that average 10μm in diameter (Mascagni & McDonald, 2003). There is evidence that these CCK+ subpopulations exhibit distinctive electrophysiological properties (Jasnow, Ressler, Hammack, Chhatwal, & Rainnie, 2009).
In colchicine-injected rats, where colchicine interrupts axonal transport of CCK due to microtubule disruption, light CCK immunoreactivity (CCK-ir) is also seen in PNs in the BLa, but not in other BNC nuclei (Mascagni & McDonald, 2003). More intense CCK-ir was observed in the initial portion of the axons of these PNs, suggesting that CCK, or a CCK-like peptide, is normally transported to axon terminals for synaptic release. These findings are consistent with in situ hybridization studies which have found that the BLa contains a large number of PNs with moderate levels of CCK mRNA (Ingram, Krause, Baldino, Skeen, & Lewis, 1989; Marsicano & Lutz, 1999), similar to the cortex (Ingram et al., 1989).
CCKL INs in the BNC are basket cells, and most also express either calbindin (CB) or type 3 vesicular glutamate transporter protein (VGLUT3), but rarely both (Mascagni & McDonald, 2003; Omiya et al., 2015; Rovira-Esteban et al., 2017; Yoshida et al., 2011). Like PV+ basket cells, CCKL basket cells innervate both somata and dendrites of BNC PNs, with each individual cell forming varying proportions of perisomatic versus distal dendritic synapses with PNs (Veres et al., 2017). CCKL INs also express high levels of type 1 cannabinoid receptors (CB1Rs) (Katona et al., 2001; McDonald & Mascagni, 2001b).
Hajos and coworkers recently conducted quantitative analyses of the innervation of the perisomatic region of PNs by individual CCK+/CB1R+ basket cells (CCK/CB1RBCs) in the mouse BLa at the light microscopic level (Vereczki et al., 2016; Veres et al., 2017). It was found that each CCK/CB1RBC formed an average of four contacts with the perisomatic region of each PN targeted, compared to six contacts for PVBCs. Like PVBCs, CCK/CB1RBCs typically innervated about 10% of the PNs within their axonal arborization (perhaps as many as 700–800 PNs for CCK/CB1RBCs), and each PN was innervated by an average of 15–16 CCK/CB1RBCs.
EM studies have shown that in the BLa, but not the lateral nucleus, terminals of CCK/CB1RBCs invaginate into PN somata when forming symmetrical axosomatic synapses (Katona et al., 2001; Omiya et al., 2015; Yoshida et al., 2011). These synapses exhibit the molecular machinery required for endocannabinoid signaling. Thus, the somatic region apposed to these CCK+ axon terminals expresses diacylglyerol lipase (DGL), the enzyme that synthesizes the endocannabinoid 2-arachidonoylglycerol (2-AG), whereas the CCK+ axon terminals express CB1Rs and monoacylglyerol lipase (MGL; the enzyme that degrades 2-AG) (Omiya et al., 2015; Yoshida et al., 2011). There is evidence that GABA and CCK released at axosomatic synapses activate GABAAα1-containing and CCK-2 receptors, respectively (Omiya et al., 2015). In contrast to GABA, the activation of CCK-2 receptors at these synapses increases neuronal excitability through membrane depolarization (Meis, Munsch, Sosulina, & Pape, 2007). As at other cannabinergic synapses, depolarization of postsynaptic PNs synthesizes and releases endocannabinoids which suppress GABA release from presynaptic CCK+ terminals by activating presynaptic CB1Rs in a retrograde manner (Katona et al., 2001; Yoshida et al., 2011).
Many of the small CCKS INs in the rat BNC also express VIP and/or calretinin (CR), but not CB (Fig. 4) (Mascagni & McDonald, 2003). Although almost all CCK INs that express high levels of somatic CB1Rs are CCKL INs in the rat, about 10% of CCKS INs express low somatic levels of this receptor (Mascagni & McDonald, 2003). These findings are in agreement with in situ hybridization studies showing that some BNC INs that express VIP mRNA also have low levels of CB1R mRNA (Omiya et al., 2015) and immunohistochemical studies that have demonstrated that some VIP+ axon terminals in mouse are CB1R+ (18% in LA, 3% in BL) and some contact PN somata (Rhomberg et al., 2018). These findings suggest that a subset of CCK+ basket cells in the BNC are of the CCLS variety. Although there have been no detailed studies of CCK INs or VIP INs in the primate BNC, the morphology of most CR+ INs in the monkey (McDonald, 1994) and human BNC (Sorvari, Soininen, & Pitkänen, 1996) closely resemble those seen in the rat, and these neurons have been shown to be GABAergic in the monkey (Mascagni et al., 2009).
The innervation of PNs and INs by VIP+ terminals in the rat BLa was analyzed using a dual-labeling EM technique to label VIP INs and CB INs (i.e., most non-VIP INs); unlabeled neurons were considered presumptive PNs since they did not express either of the two major IN markers (Muller, Mascagni, & McDonald, 2003). The main targets of VIP+ terminals, via symmetrical synapses, were the somata and proximal dendrites of PNs (23%), the PN distal dendritic compartment (47%), and the CB+ distal dendritic compartment (16%). Thus, VIP INs, in addition to PV and CCKL INs, provide a significant innervation of the PN perisomatic compartment, which, in part, explains why 33% of GABAergic synapses onto the somata and proximal dendrites of PNs are not PV+ or CB1R+ (Vereczki et al., 2016). Interestingly, some of the VIP+ terminals innervating PN somata formed invaginating synapses (Muller et al., 2003), typical of CCK+/CB1R+ terminals (see above).
VIP+ INs form twice as many synapses with INs (either VIP+ or CB+) as are formed by CB terminals (Muller et al., 2003), suggesting that a subpopulation of VIP or VIP/CR INs may selectively innervate INs, as in the hippocampus (Freund & Buzsaki, 1996). In the hippocampus, these VIP+ and CR+ INs specialized to innervate other INs are CCK-negative and CB1R-negative. Recent studies found that about 60%–65% of VIP+/CB1R-negative boutons in the mouse BNC innervate dendrites of GABAergic INs, and that all of the major subpopulations of CB INs in the mouse BNC (PV, SOM, NPY, CCK) receive inputs from these VIP INs (Krabbe et al., 2016; Rhomberg et al., 2018). Likewise, CR+ terminals innervate CB+ somata and dendrites in the human LA (Sorvari et al., 1998). This inhibition of INs results in a disinhibition of PNs.
One important role of CCK and endocannabinoids in the BNC is the regulation of anxiety and fear expression/extinction (Chhatwal & Ressler, 2007). The anxiogenic properties of CCK appear to be due to its excitation of PNs via CCK-2 receptors (CCK2Rs; Meis et al., 2007). However, CCK also excites fast-firing GABAergic BNC INs, which are most likely PV INs based on their firing pattern (see above) (Chung & Moore, 2009a). These electrophysiological results are consistent with immunohistochemical studies demonstrating expression of CCK2Rs in PNs, as well as in PV+ and CR+ INs (Bowers & Ressler, 2015). The activation of PV+ INs may be critical for the ability of CCK to generate oscillatory activity in the BNC (Chung & Moore, 2009b). It is well established that CB1R activation in the BNC is essential for fear extinction (Marsicano et al., 2002), and studies by Ressler and coworkers have shown that interactions of CCK and endocannabinoids are essential for fear expression and extinction (Bowers & Ressler, 2015; Chhatwal et al., 2009). These investigations by Ressler’s group indicate that endocannabinoids released from the activated PNs and INs suppress CCK release from presynaptic CCK terminals by activating CB1Rs, which in turn decreases the normal activation of these neurons via CCK2Rs. This weakening of PN excitation, and possible disruption of oscillatory activity due to weakening of PV IN excitation, appears to dampen fear behavior and enhance fear extinction (Bowers & Ressler, 2015).
EXTRINSIC CONNECTIONS OF THE BASOLATERAL AMYGDALA
Sensory inputs from the cerebral cortex
In all mammals the cortical areas with the most robust sensory projections to the amygdala are higher order sensory association areas. Sensory information reaches these higher order cortical areas by a series of cortico-cortical projections (“cascades”) that originate from the primary sensory cortex of each modality. The neuroanatomy of these sensory cascades was elucidated in the 1980s and 1990s using tract-tracing techniques and was reviewed by this author in the late 1990s (McDonald, 1998). Although the cascades appear to be similar in all mammals, the following account of these cortical pathways to the amygdala will focus on the rat, since most studies have been done in this species, and monkey, since the anatomy in this species presumably more closely approximates that seen in humans (for additional details see McDonald, 1998).
Visual and auditory cortical projections
Visual and auditory stimuli are the main modalities used as conditioned stimuli in fear conditioning. The primary and secondary visual cortices of the rat (Oc1 and Oc2, respectively) have no direct projections to the BNC, but Oc2 does project to Te2 (a tertiary visual area) (Fig. 6A) (McDonald, 1998). Te2, which is important for discriminating complex visual stimuli in the rat (Kolb, 1990), projects mainly to the middle and caudal portions of LA, and adjacent portions of Bmg (i.e., BLa) (Mascagni, McDonald, & Coleman, 1993; Shi & Cassell, 1997).
FIG. 6.
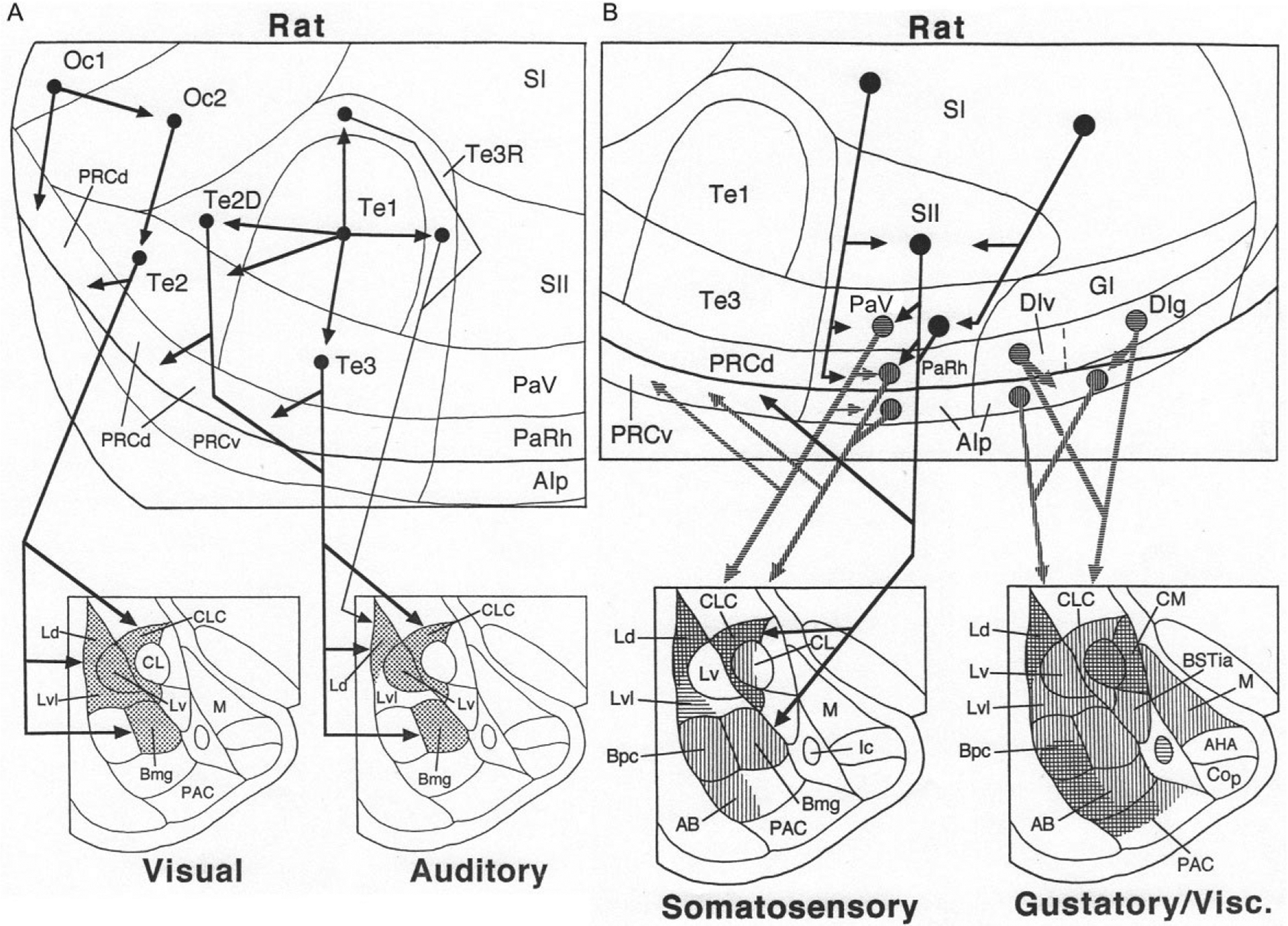
(A) Cortical cascades (above) and cortico-amygdalar projections (below) associated with transmission of visual and auditory information to the rat amygdala. (B) Cortical cascades (above) and cortico-amygdalar projections (below) associated with transmission of somatosensory and gustatory/visceral information to the rat amygdala. Vertical and horizontal hatching is used to distinguish the targets of distinct cortical regions (somatosensory: horizontal hatching shows targets of area PaV; vertical hatching shows targets of areas PaRH and the caudal AIp) (gustatory/visceral: horizontal hatching shows targets of areas DIv and DIg; vertical hatching shows targets of the rostral AIp). Reproduced with modification from McDonald, A.J. (1998). Cortical pathways to the mammalian amygdala. Progress in Neurobiology, 55, 257–332.
The primary auditory cortex in the rat (Te1) has little or no direct projection to the BNC, but does project to secondary auditory areas surrounding it (auditory belt cortices: Te3, Te2D, and Te3R) (Fig. 6A). The projections of the auditory belt cortices to the BNC mainly terminate in the LA and Bmg (BLa) (Mascagni et al., 1993; Romanski & LeDoux, 1993; Shi & Cassell, 1997). The LA and BL send projections back to the occipitotemporal region in the rat, but these projections do not extend dorsally beyond the perirhinal cortices, and so do not innervate secondary visual and auditory areas in Te2 or Te3 (Pitkänen, 2000).
In the monkey, there are no projections from primary or secondary visual areas of the occipital lobe to the amygdala. The ventral visual stream, concerned with object recognition (Ungerleider & Mishkin, 1982), originates in V4 and cascades down the inferior temporal cortex involving successively higher order visual areas TEO, TEp, and TEa (Fig. 7A). The latter three areas have projections to the BNC that mainly target the dorsal portions of LA and Bmg (Amaral, Price, Pitkanen, & Carmichael, 1992; McDonald, 1998). There are, however, projections of the BNC back to the TEa, TEp, and TEO that arise mainly in the basal and accessory basal nuclei, and generally exhibit a diminishing rostral to caudal gradient. In contrast, the projection from the dorsal part of Bmg, which receives a robust input from the inferior temporal lobe, has additional projections to primary, secondary, and tertiary (V4) visual areas in the occipital lobe (Amaral & Price, 1984; Gattass, Galkin, Desimone, & Ungerleider, 2014).
FIG. 7.
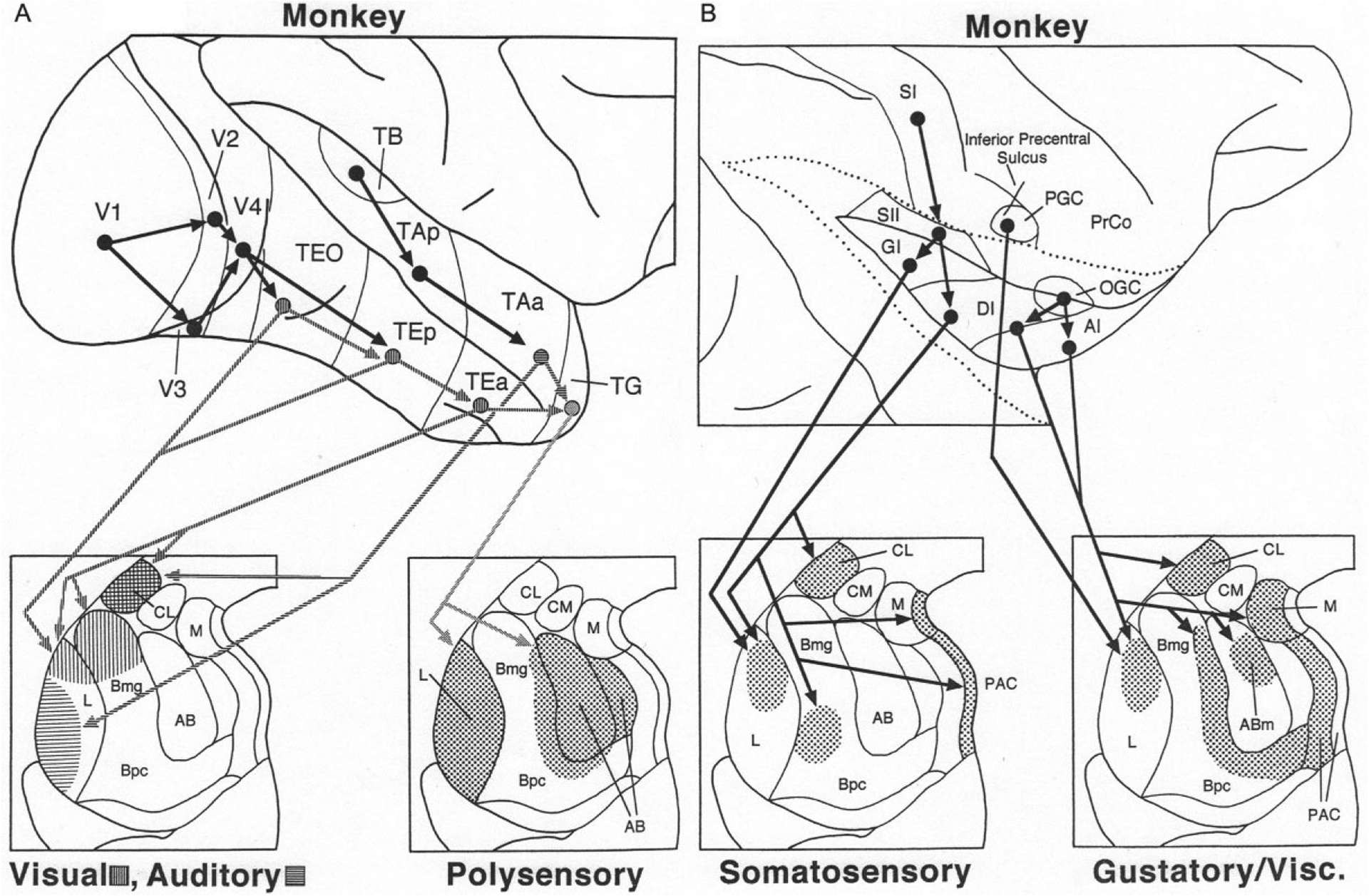
(A) Cortical cascades (above) and cortico-amygdalar projections (below) associated with transmission of visual, auditory, and polysensory information from the temporal lobe to the monkey amygdala. Hatching is used to distinguish the targets of visual areas (horizontal hatching) versus auditory areas (vertical hatching). (B) Cortical cascades (above) and cortico-amygdalar projections (below) associated with transmission of somatosensory and gustatory/visceral information from the parietal and insular lobes to the monkey amygdala. The insula has been exposed by separating the apposed edges of the opercular cortices (dotted lines). See text for details. Reproduced with modification from McDonald, A.J. (1998). Cortical pathways to the mammalian amygdala. Progress in Neurobiology, 55, 257–332.
The superior temporal gyrus in the monkey contains a hierarchically organized auditory cascade that is similar to the visual cascade in the inferior temporal lobe (Fig. 7A). The anterior part of area TA (TAa) has projections to the BNC that target the ventrolateral portion of LA, thus avoiding the dorsal visual portion of the nucleus (Fig. 7A) (Amaral et al., 1992; McDonald, 1998). Projections back to the superior temporal gyrus are very light (Amaral & Price, 1984). Area TAa and visual area TEa have projections to the polymodal temporal polar cortex (TG) which in turn projects to the entire LA and AB (Amaral et al., 1992; McDonald, 1998). Another polymodal area in the depths of the superior temporal sulcus (STS) separating the superior and inferior temporal gyri has a robust projection to the lateral LA, and moderate projections to the Bmg and AB (Amaral, Insausti, & Cowan, 1983). PET studies in humans have demonstrated increased activity in both the amygdala and STS cortex in response to expressive body movements (Bonda, Petrides, Ostry, & Evans, 1996).
Somatosensory and gustatory/visceral cortical projections
The BNC receives somatosensory and gustatory/visceral inputs from the posterior and anterior insular cortices, respectively, in both rat and monkey (Figs. 6B and 7B). There is no direct projection from the primary somatosensory cortex (SI) to the BNC in the rat (Fig. 6B). However, there is a hierarchically organized somatosensory cascade that originates in SI, and involves successive projections to the secondary somatosensory cortex (SII), parietal ventral area (PaV), parietal rhinal area (PaRh), and adjacent posterior agranular insular cortex (AIp) (McDonald, 1998; Shi & Cassell, 1998a). The latter three areas have significant projections to the BNC. PaV mainly targets the lateral part of LA, whereas PaRh/AIp mainly targets Bmg (BLa), Bpc (BLp), and the dorsal part of LA (Ld) (Fig. 6B) (McDonald, 1998; Shi & Cassell, 1998a). The LA and basal nuclei send projections back to the posterior insular region in the rat, but these projections do not extend dorsally beyond AIp, and so do not innervate PaV or PaRh (Pitkänen, 2000).
In the rat, the BNC receives gustatory and general visceral inputs from the anterior insular cortices (Fig. 6B). Within the BNC, the primary gustatory cortex (DIg) and primary visceral (DIv) cortices mainly project to the dorsal part of LA, Bpc (BLp), and the adjacent portion of AB (BM) (McDonald, 1998; Shi & Cassell, 1998b; Yasui, Breder, Saper, & Cechetto, 1991). The primary gustatory and primary visceral cortices also project to adjacent portions of AIp, which in turn has widespread projections to the BNC. The LA and BL send projections back to the anterior insular region in the rat, but these projections do not extend dorsally beyond AIp, and so do not innervate the primary gustatory and visceral cortices (Pitkänen, 2000).
As in the rat, there is a somatosensory cascade in the monkey which traverses the posterior insular region (Fig. 7B). SI projects to SII, which in turn projects to the granular and dysgranular insular cortices (GI and DI). The latter two regions project to the dorsomedial LA and central portions of the basal nucleus of the BNC (Friedman, Murray, O’Neill, & Mishkin, 1986; Mufson, Mesulam, & Pandya, 1981). In regard to gustatory/visceral inputs to the monkey BNC, the precentral gustatory cortex (PGC) has direct projections to the dorsomedial LA (Van Hoesen, 1981), whereas the opercular-insular gustatory cortex (OGC) has indirect projections to BNC that are relayed via the dysgranular insular cortex (DI) and agranular insular cortex (AI). A portion of the AI contains the primary visceral cortex (Carmichael & Price, 1995). DI and AI have projections to the dorsomedial LA, medial portions of the Bmg and Bpc, and AB (Mufson et al., 1981; Turner, Mishkin, & Knapp, 1980). The BNC projections back to the monkey insula have widespread origins; in general projections exhibit a diminishing gradient from rostral (AI) to caudal (GI) (Amaral & Price, 1984).
Olfactory projections
There are no direct projections from the olfactory bulb to the BNC in either rodents or primates. In both species olfactory projections are confined to the superficial amygdalar nuclei (e.g., cortical and medial). Projections from the main olfactory bulb (MOB) in the rat mainly target the piriform cortex and medially adjacent portions of the cortical nuclei, whereas the accessory olfactory bulb (AOB), which receives pheromonal information from the vomeronasal organ, mainly projects to the medial and posteromedial cortical nuclei (Scalia & Winans, 1975). The AOB is difficult to identify in humans and old world monkeys, but is present in new world monkeys (Wysocki, 1979). The projections of the MOB in primates mainly target the cortical nucleus and periamygdaloid cortex (PAC, posterolateral cortical nucleus of rats) (McDonald, 1998). In both rats and monkeys olfactory information from the piriform cortex, cortical nucleus, and medial nucleus is transmitted to various BNC nuclei, especially the basomedial nucleus. In rodents, but not in primates, the lateral nucleus receives robust inputs from the cortical nuclei (McDonald, 1998; Pitkänen, 2000). The BNC has projections back to the amygdalar olfactory nuclei as well as portions of the piriform cortex (Majak, Rönkkö, Kemppainen, & Pitkänen, 2004; Pitkänen, 2000).
Connections with the hippocampal region
The term hippocampal region (HR), as used in this review, consists of the hippocampal formation (dentate gyrus [DG], cornu ammonis fields CA1-CA3, and subiculum [Sub]), as well as the adjacent parahippocampal cortices, including the ERC, perirhinal cortex (PRh), parasubiculum, and presubiculum (Fig. 8B). The HR consists of polymodal association areas that integrate highly processed sensory information from all sensory modalities into complex configurational representations such as context. In all mammals, sensory information is transmitted through the HR by a series of connections that includes the so-called trisynaptic circuit of the hippocampus (PRh→ERC→DG→CA3→CA1→SUB→ERC→PRh→ association cortices) (Witter, Groenewegen, Lopes da Silva, & Lohman, 1989). These structures and circuits of the HR are critical for memory formation and constitute the medial temporal lobe memory system (MTLMS; Squire & Zola-Morgan, 1991). The amygdala has extensive, complex interconnections with the MTLMS that are important for emotional memory.
FIG. 8.
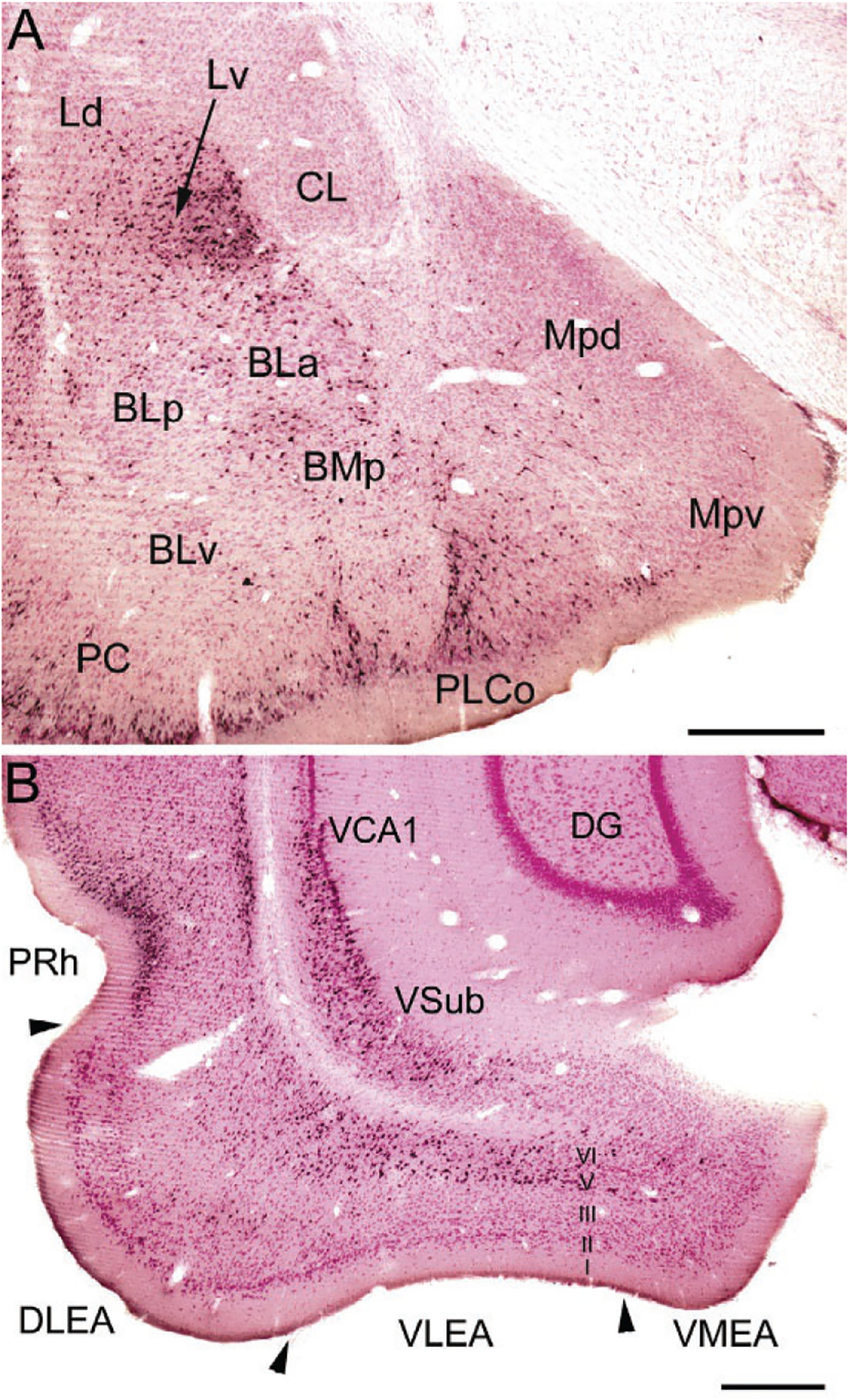
(A) Photomicrograph of retrogradely labeled neurons in the amygdala (black) at the bregma −3.1 level in a rat that received an injection of Fluorogold (FG) into the dorsolateral entorhinal area (immunoperoxidase technique with pink pyronin Y Nissl counterstain). The nomenclature used to denote the amygdalar nuclei is that used in the atlas by Paxinos and Watson (1997) (see Table 1). Additional abbreviations: Mpd, posterodorsal medial nucleus; Mpv, posteroventral medial nucleus; PC, piriform cortex. (B) Photomicrograph showing the locations of FG+ retrogradely-labeled neurons (black) in the ventral hippocampus and parahippocampal area at the bregma −6.3 level in a rat that received a large injection of FG into the BNC that involved both LA and BL (immunoperoxidase technique with pink pyronin Y Nissl counterstain). Note dense retrograde labeling in the perirhinal cortex (PRh), the deep layers (layers V and VI) of the dorsolateral and ventrolateral ERC (DLEA and VLEA, respectively), and the ventral subiculum (VSub) and adjacent ventral part of CA1 (VCA1), but not in the dentate gyrus (DG). Scale bars=500μm. (A) and (B) Reproduced with permission from McDonald, A.J., Zaric, V. (2015a). GABAergic somatostatin-immunoreactive neurons in the amygdala project to the entorhinal cortex. Neuroscience, 290, 227–242.
Interconnections between the amygdala and the HR in the rat and monkey have been discussed in detail in previous reviews (e.g., Amaral et al., 1992; McDonald, 1998; McDonald & Mott, 2017; Petrovich, Canteras, & Swanson, 2001; Pitkänen, 2000; Pitkänen, Pikkarainen, Nurminen, & Ylinen, 2000; Price et al., 1987; Witter et al., 1989). There are no direct interconnections of the amygdala with the dorsal (septal) half of the hippocampal formation; these regions interact with the amygdala via relays in the parahippocampal cortices and other brain regions (McDonald & Mott, 2017). The interconnections in the rat mainly involve the parahippocampal cortices and the ventral (temporal) half of the hippocampal formation, with the exception of the dentate gyrus. This ventral portion of the rodent hippocampus, which corresponds to the anterior part of the primate hippocampus, is mainly involved in stress, emotion, and affect, whereas the dorsal hippocampus, which corresponds to the posterior part of the primate hippocampus, performs primarily cognitive functions (Fanselow & Dong, 2010; Strange, Witter, Lein, & Moser, 2014). The connections of the BNC and the HR in the rat are described below. The basic organization of interconnections in primates is similar, but the relative contributions of individual BNC nuclei appears to differ (Amaral et al., 1992).
The ventral subiculum (VSub) has widespread projections to the amygdala that target all three nuclei of the BNC (Fig. 8B; McDonald, 1998). The ventral part of CA1 (VCA1), located adjacent to the VSub, has projections to the BNC that are lighter than those of VSub. EM studies have shown that the projections from the VSub/VCA1 to the BNC mainly form asymmetrical (excitatory) synapses with spines of PNs and thin distal dendrites, but dendrites of GABAergic INs are also targeted (Bazelot et al., 2015; Müller et al., 2012). Neurobehavioral studies have shown that contextual information received by the BNC from the VSub/VCA1, either directly or indirectly via the prelimbic area of the medial prefrontal cortex (mPFC), mediates the contextual control of fear after extinction (Orsini, Kim, Knapska, & Maren, 2011).
The lateral ERC and PRh have widespread and topographically organized projections to all three nuclei of the BNC (McDonald, 1998; McDonald & Mascagni, 1997). Most of the projections of the ERC to the amygdala arise from layers 5 and 6 (Fig. 8B). These deep layers of the ERC do not have significant projections to the hippocampal formation, but instead receive afferents from the hippocampus (Amaral & Witter, 1995). These inputs form asymmetrical (excitatory) synapses that target PN spines, and to a lesser extent dendritic shafts, in the BNC (Smith et al., 2000). Only 1% of the projections from the PRh target dendritic shafts of PV+ INs, but 10% target dendritic shafts of other INs, half of which are CB+ (Smith et al., 2000; Unal, Paré, Smith, & Paré, 2014). Since SOM+ INs are the main subpopulation of CB+/PV− negative INs in the BNC, it is presumed that SOM+ neurons are one of the main IN subpopulations providing feedforward inhibition of PNs related to PRh inputs.
The projections from the BNC to the HR largely reciprocate the projections of the HR to the BNC (McDonald & Mott, 2017). Although CA3 and CA2 do not have projections to the BNC, the temporal parts of CA3 and CA2 receive projections from the BNC (Petrovich et al., 2001; Pikkarainen, Rönkkö, Savander, Insausti, & Pitkänen, 1999). The VSub and VCA1 receive inputs from all three BNC nuclei (Petrovich et al., 2001). Optogenetic activation of BNC inputs to the ventral hippocampus has been shown to increase anxiety-related behaviors (Felix-Ortiz et al., 2013). The ERC and PRh also receive widespread projections from all three of the BNC nuclei, especially LA and BMp (Fig. 8A), that mainly target layers 1–3 (Petrovich et al., 2001). EM studies have shown that the projections from the BNC to VSub and PRh form asymmetrical (excitatory) synapses with dendritic spines of PNs and thin dendrites (French, Hailstone, & Totterdell, 2003; Smith & Paré, 1994).
Both the BNC and the HR exhibit epochs of rhythmic, synchronized firing of large populations of neurons. These rhythmic oscillations, which emerge from the intrinsic properties of constituent neurons as well as network properties, are often synchronized in the BNC and HR because of their interconnections (Pape & Paré, 2010). These synchronous oscillations create recurring “time windows” in which synaptic interactions between these structures, including synaptic plasticity, is facilitated (Paré et al., 2002). These oscillations are critical for synaptic plasticity associated with the formation and retrieval of emotional memories (Bocchio et al., 2017; Pape & Paré, 2010; Paré et al., 2002).
Glutamatergic PNs are the main neuronal type associated with projections from the HR to the BNC, and vice versa (Brothers & Finch, 1985; Finch et al., 1986; Lang & Paré, 1998; Maren & Fanselow, 1995). However, there is a small number of GABAergic long-range nonpyramidal projection neurons (LRNP neurons) in the HR that project to the BNC, and vice versa (Ino et al., 1990; Köhler, Smialowska, Eriksson, Chanpalay, & Davies, 1986; Müller et al., 2012). Recent studies in the author’s lab have demonstrated that SOM+, NPY+, and SOM+/NPY+ neurons in the ERC, but not in the hippocampal formation, project to the rat BNC, and similar neurons in the BNC have projections back to the ERC (McDonald & Zaric, 2015a, 2015b). In the BNC these neurons constitute 2% of all projection neurons. It is also of interest that all major portions of the MTLMS are interconnected by small numbers of LRNP neurons, in addition to the numerous PNs, including many LRNP neurons that are SOM+ (see Jinno, 2009, for a review). These interconnections have been termed the temporal lobe GABAergic “supernetwork” (Buzsáki & Chrobak, 1995). The finding of reciprocal interconnections between the BNC and the HR involving GABAergic LRNP neurons indicates that this temporal lobe GABAergic supernetwork includes the BNC. In addition, for both the HR and the BNC, interconnections of these regions with the basal forebrain also involve GABAergic neurons, thus extending the supernetwork (Fig. 9). Determination of the functional significance of these BNC-HR LRNP neuronal interconnections will require knowledge of the postsynaptic targets of these neurons. Either PNs (Jinno et al., 2007; Pinto, Fuentes, & Paré, 2006) or NPNs (Melzer et al., 2012; Van Haeften, Wouterlood, Jorritsma-Byham, & Witter, 1997) have been identified as targets of LRNP neurons in different HR interconnections.
FIG. 9.
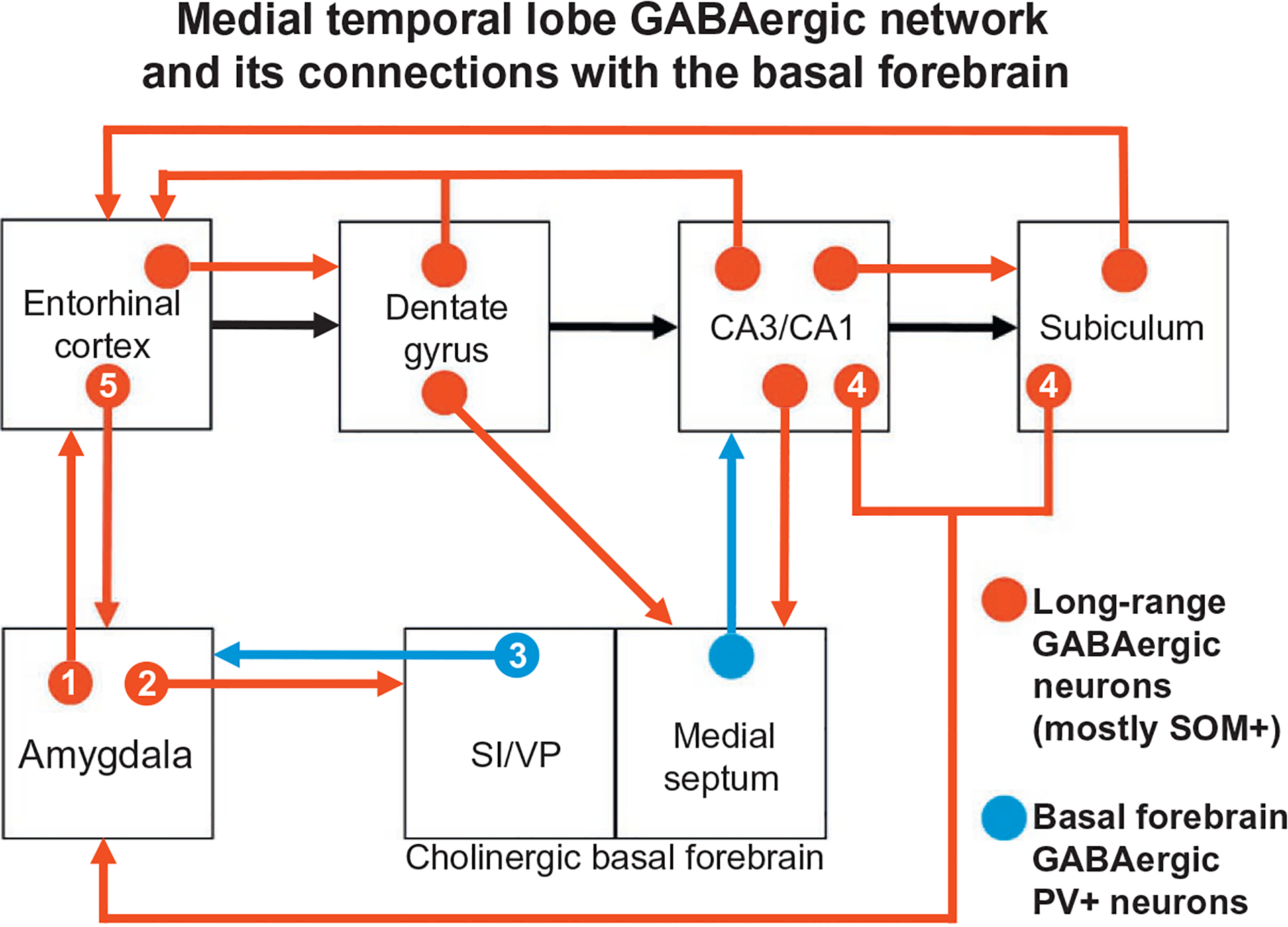
Schematic diagram illustrating interconnections between the BNC, hippocampal/parahippocampal areas, and basal forebrain that are mediated in part by GABAergic projection neurons, most of which also express SOM (shown in red). The series of glutamatergic projections starting at the entorhinal cortex and extending through the hippocampal formation, including the “trisynaptic circuit,” are indicated by black arrows (all other glutamatergic projections are not shown). GABAergic projections from the basal forebrain to the amygdala (from the substantia innominata and ventral pallidal regions; SI/VP) and hippocampus (from the medial septum) are shown in blue; these neurons also express PV. Numbers in the connections of the BNC refer to the following studies: (1) McDonald and Zaric (2015a); (2) McDonald et al. (2012); (3) Mascagni and McDonald (2009) and McDonald, Muller, and Mascagni (2011); (4) Müller et al. (2012); (5) McDonald and Zaric (2015b). See reviews by Jinno (2009) and Caputi, Melzer, Michael, and Monyer (2013) for the studies demonstrating the connections that are not designated by numbers. Reproduced with modification from McDonald, A.J., Zaric, V. (2015b). Extrinsic origins of the somatostatin and neuropeptide Y innervation of the rat basolateral amygdala. Neuroscience, 294, 82–100.
Prefrontal cortical connections
The prefrontal cortex (PFC) in the rat includes the mPFC, orbital PFC (oPFC), and lateral PFC (lPFC). The projections of the rat mPFC and lPFC to the BNC as revealed by anterograde tract tracing are shown in Fig. 10A (McDonald et al., 1996). Ventral portions of the mPFC (infralimbic area, IL) and lPFC (ventral agranular insular area, AIv) mainly project to BM (BA) and LA (Lv), whereas dorsal portions of the mPFC (prelimbic and anterior cingulate areas, PL and AC) and lPFC (dorsal agranular insular area, AId) mainly project to BL (Bmg). Projections to the BNC from the oPFC mainly originate from its medial portion, and target LA, BL, and BM (Hoover & Vertes, 2011). Projections from IL, PL, AC, and AIv to the BNC mainly originate from superficial layers (layers 2 and 3) of the PFC (Hurley, Herbert, Moga, & Saper, 1991; Ottersen, 1982). EM studies have shown that PL projections to the BL target PN spines (93%) and thin dendritic shafts (7%) (Brinley-Reed, Mascagni, & McDonald, 1995; Smith et al., 2000).
FIG. 10.
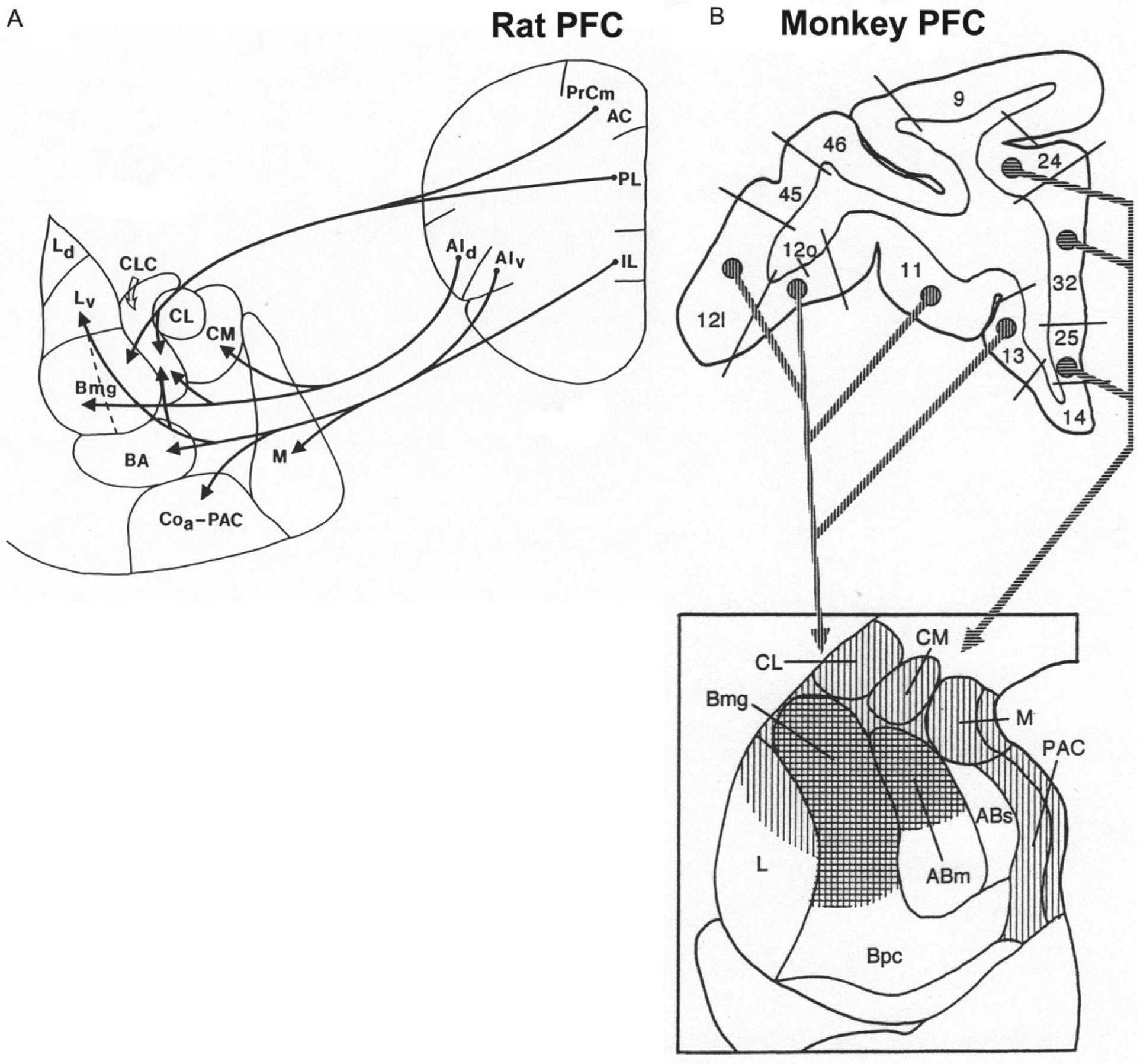
(A) Main projections from the PFC to the amygdala in the rat. (B) Main projections from the PFC to the amygdala in the monkey (coronal sections). Vertical hatching indicates projections from the orbital PFC, whereas horizontal hatching indicates projections from the medial PFC. (A) Reproduced with modification from McDonald, A.J., Mascagni, F., & Guo, L. (1996). Projections of the medial and lateral prefrontal cortices to the amygdala: A Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience, 71, 55–75. (B) Reproduced with modification from McDonald, A.J. (1998). Cortical pathways to the mammalian amygdala. Progress in Neurobiology, 55, 257–332.
Projections from the rat BNC to the PFC have been studied using tract-tracing techniques (Hoover & Vertes, 2007; Krettek & Price, 1977; Petrovich, Risold, & Swanson, 1996; Reppucci & Petrovich, 2016). These investigations revealed that the IL receives inputs from LA, BLa, BLp, and BMp. The PL receives inputs from LA, BLa, and BLp. The AC receives inputs mainly from BLa and BLp. The oPFC and AId receive inputs from BLa. These BNC inputs in the rat mainly target layers 5, 2, and the deep part of layer 1 (Krettek & Price, 1977). It is of interest that layer 2 projects to layer 5, which in turn gives rise to projections to subcortical brain regions (Krettek & Price, 1977). A comparison of the inputs and outputs of IL revealed that there are reciprocal connections of IL with LA and BM, but BL has projections to IL that are not significantly reciprocated. The results of a recent comprehensive study using both anterograde and retrograde tract tracing to analyze BNC-PFC interconnections in the mouse are largely consistent with the findings described above for the rat, although there are some apparent differences (Mátyás, Lee, Shin, & Acsády, 2014).
In the monkey, the PFC projections to the BNC originate from the orbital PFC (areas 11–13) and from the infralimbic (area 25), prelimbic (area 32), and anterior cingulate (area 24) areas of the medial PFC (Aggleton, Burton, & Passingham, 1980) (Fig. 10B). The medial PFC targets the dorsal portions of the Bmg and AB, whereas the orbital PFC targets these same areas, but has additional projections to the dorsal part of the LA (Carmichael & Price, 1995; Van Hoesen, 1981). Projections from these BNC nuclei back to the monkey PFC largely reciprocate the corticoa-mygdalar projections (Aggleton, Wright, Rosene, & Saunders, 2015; Amaral & Price, 1984; Barbas & De Olmos, 1990; Carmichael & Price, 1995; Cho, Ernst, & Fudge, 2013; Ghashghaei & Barbas, 2002).
Two distinct subpopulations of BLa PNs that project to the mPFC can be distinguished in the mouse based on differences in function and connections. “Fear neurons” increase their firing rate in response to a CS during fear conditioning and reduce their firing following extinction, whereas “extinction neurons” increase their firing rate in response to a CS during extinction (Herry et al., 2008). These two types of PNs are intermingled in the BLa. Subsequent studies found that extinction neurons in the BLa mainly project to the IL of the mPFC as well as to the central amygdalar nucleus, whereas fear neurons project mainly to the PL, and have no projection to the central nucleus (Senn et al., 2014). The silencing of fear neurons with extinction appears to be due to remodeling of inhibitory synaptic inputs to these PNs from PV+ perisomatic INs (Trouche et al., 2013). Thus, after extinction there is an increase in PV+ perisomatic terminals contacting silenced fear neurons, but not contacting fear neurons that remained active, suggesting increased inhibition of silenced fear neurons. Furthermore, there was no change in the number of CCK+ perisomatic terminals contacting fear neurons, but an upregulation of CB1Rs in CCK+ terminals contacting fear neurons that were still active after extinction. This suggests that increased suppression of GABA release by CB1Rs contributes to the firing of these fear neurons after extinction (Trouche et al., 2013).
PL-projecting PNs (PL-Ps, presumptive fear neurons) and IL-projecting PNs (IL-Ps; presumptive extinction neurons) in the mouse BL are contacted by equal numbers of CCK+/CB1R+ axon terminals, but the levels of the endocannabinoid synthesizing enzyme DGL are much greater in IL-Ps (Vogel, Krabbe, Gründemann, Wamsteeker Cusulin, & Lüthi, 2016). During fear extinction when IL-Ps are activated, the higher levels of 2-AG produced by DGL in IL-Ps suppresses GABA release, thereby further activating IL-P activity through disinhibition. This further enhances the contrast in activity between fear and extinction neurons during extinction (Vogel et al., 2016).
Thalamic connections
The BNC has connections with thalamic sensory nuclei, thalamic midline/intralaminar nuclei, and the mediodorsal thalamic nucleus. The posterior thalamic region, which consists of several nuclei that are located medial to the ventral and dorsal divisions of the medial geniculate nucleus (MGNv and MGNd) including the medial division of the MGN (MGNm), suprageniculate nucleus, and posterior intralaminar nuclei, projects to LA and BM (LeDoux, Farb, & Ruggiero, 1990; Turner & Herkenham, 1991). These thalamic nuclei receive inputs from the inferior colliculus and spinothalamic tracts and can therefore transmit auditory and somatosensory (including nociceptive) information to the BNC (LeDoux, Ruggiero, Forest, Stornetta, & Reis, 1987). The main targets of auditory thalamic inputs to the LA are spines of PNs (LeDoux, Farb, & Milner, 1991). Interestingly, many neurons in the posterior thalamic region project to both the LA and the auditory association cortices that project to the LA (Doron & Ledoux, 2000). Lesions of the lateral nucleus disrupt auditory fear conditioning (Campeau & Davis, 1995a; LeDoux et al., 1990). Lesions of the MGNm or auditory association cortices do not disrupt auditory fear conditioning when a simple tone is paired with footshock, but combined lesions of the MGNm and cortex do block conditioning (Romanski & LeDoux, 1992). Thus, transmission of an auditory stimulus to LA is necessary for fear conditioning, but either thalamo-amygdalar (via the posterior thalamus) or thalamo-cortico-amygdalar pathways (via MGNv) are sufficient for fear learning.
There is evidence that the rat LA receives visual information from the lateral posterior (LP) thalamic nucleus (Doron & Ledoux, 1999; Linke, De Lima, Schwegler, & Pape, 1999). Likewise, there is a homologous projection from the medial pulvinar to the monkey LA (Aggleton et al., 1980; Jones & Burton, 1976). Lesions of LA in the rat block fear conditioning to a simple visual stimulus (Sananes & Davis, 1992). Large lesions of the primary and secondary visual cortices do not block fear conditioning to visual stimuli (LeDoux, Romanski, & Xagoraris, 1989; Rosen et al., 1992), suggesting that visual information must reach the amygdala by some extra-geniculate route. However, lesions of Te2/PRC, the cortical targets of LP, do not block visual fear conditioning whereas lesions of Te3/PRC do (Campeau & Davis, 1995b; Rosen et al., 1992).
In the rat, the parvicellular part of the ventral posteromedial nucleus (VPMpc) transmits gustatory/visceral information to the same part of LA that is the target of gustatory/visceral cortical inputs (Turner & Herkenham, 1991; Yasui, Itoh, Sugimoto, Kaneko, & Mizuno, 1987). Thus, in general, thalamic nuclei relaying visual, auditory, somatosensory, and gustatory/visceral information to the amygdala tend to target the same BNC regions that receive inputs from the cortices associated with these modalities. However, the BNC does not project back to any of these sensory thalamic nuclei.
The BNC also has connections with the “limbic thalamus” (Vertes, Linley, & Hoover, 2015). The limbic thalamus consists of several midline nuclei and two other nuclei that are close to the midline, the centromedial (CM) and mediodorsal (MD) nuclei. The main midline nuclei, from dorsal to ventral, are the paraventricular (PVT), paratenial (PT), rhomboid (RH), and reuniens (RE). Unlike sensory thalamic nuclei, the limbic thalamic nuclei have extensive interconnections with various portions of the limbic system and are mainly involved in cognitive and affective functions (Vertes et al., 2015). The central and medial amygdalar nuclei have reciprocal connections with the limbic nuclei, whereas, with the exception of MD, the BNC receives inputs but does not send projections to these nuclei (Pitkänen, 2000). The rat BNC receives remarkably robust projections from CM that mainly target BLa, dense projections from PVT that target BLa and BLp, moderate projections from RH that target BLp, moderate projections from PT that target the medial parts of BL and LA, and light projections from RE that target BLp (Turner & Herkenham, 1991; Vertes & Hoover, 2008; Vertes, Hoover, Do Valle, Sherman, & Rodriguez, 2006; Vertes, Hoover, & Rodriguez, 2012). Similarly, the monkey BNC also receives inputs from the midline and intralaminar nuclei (Mehler, 1980). The main targets of midline thalamic inputs to the BNC are spines of PNs (Carlsen & Heimer, 1988).
MD, whose cortical targets traditionally define the PFC, has a distinct relationship with the amygdala. Unlike other thalamic nuclei with amygdalar connections, there are very few neurons in the MD that project to the amygdala (Kuroda & Price, 1991; Mátyás et al., 2014), but there are many neurons in the amygdala that project to the medial part of MD (MDm). The latter neurons are diffusely scattered at low density throughout all amygdalar nuclei, including the BNC, and these neurons are part of an array of similar neurons scattered throughout the ventral forebrain in both rat and monkey (Price & Slotnick, 1983; Russchen, Amaral, & Price, 1987). Interestingly, these neurons in all regions, including the BNC, are large polymorphic NPNs. In the BNC they are distinct from the PNs that project to the PFC in both the rat and monkey (McDonald, 1987; Timbie & Barbas, 2015). The axon terminals of these BNC NPNs form asymmetrical synapses in the MDm (Kuroda & Price, 1991) and express VGLUT2 in both rodents and primates, suggesting that they are glutamatergic (Mátyás et al., 2014; Timbie & Barbas, 2015). However, there is other evidence which suggests that they are not glutamatergic (McDonald, 1996a; Ray, Russchen, Fuller, & Price, 1992).
Hypothalamic connections
The rat BNC has connections, both inputs and outputs, with many hypothalamic nuclei, but most are light (Pitkänen, 2000). However, BLp, BMa, and BMp do have significant projections to the lateral hypothalamus (Reppucci & Petrovich, 2016) and BMp has a robust projection to the ventromedial nucleus (VMN; Krettek & Price, 1978; Petrovich et al., 1996). The VMN, posterior nucleus, and perifornical area mainly project to LA (Canteras, Simerly, & Swanson, 1994; Risold, Canteras, & Swanson, 1994; Vertes, Crane, Colom, & Bland, 1995). It is of interest that the main amygdalar target of the VMN (i.e., LA), and the main source of amygdalar inputs to the VMN (i.e., BM) are strongly interconnected (see below). This suggests that this triangular amygdalar network in the rat is involved, in part, with hypothalamic mechanisms associated with VMN, including regulation of reproductive, agonistic, and ingestive behaviors (Swanson, 2000). In the monkey, the main targets of the VMN in the BNC are the parvicellular portions of the basal and accessory basal nuclei (Amaral, Veazey, & Cowan, 1982).
Intra-amygdalar connections
There are extensive internuclear, interdivisional, and intradivisional interconnections in the rat amygdala (for details see Pitkänen et al., 1997). There is a cascade of reciprocal interconnections involving LA, BL, BM (AB of Pitkänen), cortical nucleus (PAC of Pitkänen), and medial nucleus (Fig. 11). Similar connectivity appears to be present in primates but there are many more fibers directed from the lateral portions of the BNC to more superficial amygdalar nuclei than in the other direction (Aggleton, 1985; Amaral et al., 1992; Pitkänen & Amaral, 1998). Virtually all of these interconnections among BNC nuclei involve PNs that form asymmetrical (excitatory) synapses with PN dendritic spines and thin dendrites (Paré, Smith, & Paré, 1995; Smith et al., 2000; Smith & Paré, 1994; Stefanacci et al., 1992). However, Stefanacci et al. (1992) reported that 10% of axon terminals of projections from the LA to BL were symmetrical (to somata, dendrites, and spines), suggesting involvement of some INs in these connections. In regard to sensory inputs, these intra-amygdalar connections permit olfactory information from the cortical, medial, and BM nuclei to be integrated with nonolfactory information from the LA and the BL. In a more general manner, it allows representations of sensory stimuli to be distributed to various nuclei in parallel, and modified by the unique functional systems providing inputs to each nucleus (Pitkänen et al., 1997). Virtually all amygdalar nuclei, including all of the BNC nuclei, have projections to the central amygdalar nucleus (CeA), which provides outputs to subcortical regions involved in various components of fear behavior (Davis, 1992; LeDoux, Iwata, Cicchetti, & Reis, 1988). The CeA does not have projections back to the BNC.
FIG. 11.
Reciprocal intra-amygdalar connections of the lateral, basal, accessory basal, PAC, and medial nucleus. Projections to the central nucleus (Ce) are not illustrated. Reproduced with modification from Pitkänen, A., Savander, V., LeDoux, J.E. (1997). Organization of intra-amygdaloid circuitries in the rat: An emerging framework for understanding functions of the amygdala. Trends in Neurosciences, 20, 517–523.
The BNC also has interconnections with the intercalated nuclei (ICNs) of the amygdala. These nuclei are clusters of small spiny GABAergic neurons that surround LA and BL. The lateral ICNs, located near the external capsule, provide feedforward inhibition to BL PNs from cortical regions (Marowsky, Yanagawa, Obata, & Vogt, 2005). The medial ICNs, located between the BL and the CeA, have reciprocal connections with BL PNs, and project to the central nucleus (Asede, Bosch, Luthi, Ferraguti, & Ehrlich, 2015; Marowsky et al., 2005). The projections to BL provide feedforward inhibition of BL PNs for cortical and thalamic inputs to the medial ICNs, whereas the feedforward inhibitory projections to the CeA are important for fear extinction (Asede et al., 2015; Likhtik, Popa, Apergis-Schoute, Fidacaro, & Paré, 2008; Marowsky et al., 2005). There are also subpopulations of large neurons along the edges of the ICNs that innervate the BNC. One subpopulation is GABAergic and innervates BL INs (Bienvenu et al., 2015), whereas the other subpopulation is SOM+/NPY+ (McDonald & Zaric, 2015b).
Monoaminergic inputs
Monoaminergic inputs (serotonin [5-HT], norepinephrine [NE], and dopamine [DA]) as well as cholinergic inputs arise from subcortical nuclei and are released into the BNC during particular brain states (e.g., during stress and certain aspects of sleep). Despite the fact that these transmitters are often co-released in response to stress, there are very few investigations that have studied interactions among these transmitters (Briand, Gritton, Howe, Young, & Sarter, 2007). The main targets of all of these neuromodulatory inputs are distal dendrites and spines of PNs, where they can regulate synaptic plasticity involved in emotional learning. However, these neuromodulatory inputs also differentially target IN subpopulations. The BNC has no direct projections to monoaminergic brainstem nuclei. However, it can activate these nuclei via projections to the CeA, which has robust inputs to monoaminergic nuclei that project to the BNC, including the dorsal raphe, locus ceruleus, and ventral tegmental area (Pitkänen, 2000).
Serotoninergic inputs
The amygdala of rats and monkeys receives a robust serotonergic innervation from the dorsal raphe nucleus that targets all amygdalar nuclei, especially the BNC (Fig. 12, upper left) (Bauman & Amaral, 2005; Fallon & Ciofi, 1992; Muller et al., 2007b; Sadikot & Parent, 1990). 5-HT release is increased in the BNC during behavioral arousal and stress (Kawahara, Yoshida, Yokoo, Nishi, & Tanaka, 1993; Rueter & Jacobs, 1996), which plays a critical role in anxiety and depression. The fact that chronic administration of selective serotonin reuptake inhibitors (SSRIs) to depressed patients normalizes the activity of the BNC and leads to clinical improvement along a time course that is consistent with the delayed onset of clinical efficaciousness of SSRIs strongly suggests that one of the main sites of the antidepressant actions of this drug class is the BNC (Drevets, Bogers, & Raichle, 2002; Sheline et al., 2001).
FIG. 12.
Innervation of the rostral BNC by 5-HT+ serotonergic axons (upper left), NET+ (norepinephrine transporter) noradrenergic axons (upper right), VAChT+ (vesicular acetylcholine transporter) cholinergic axons (lower left), and TH+ (tyrosine hydroxylase) dopaminergic axons (lower right). For each transmitter, the left photomicrograph (A) is at low power (scale bars=200μm for 5-HT, 100 μm for NET, 150μm for VAChT, and 200μm for TH) and the right photomicrograph (B) is at high power (scale bars=20μm for 5-HT, 10μm for NET, 10μm for VAChT, and 20μm for TH). Upper left: Reproduced with permission from Muller, J.F., Mascagni, F., & McDonald, A.J. (2007b). Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. The Journal of Comparative Neurology, 505, 314–335. Upper right: Reproduced with permission from Zhang, J., Muller, J.F., McDonald, A.J. (2013). Noradrenergic innervation of pyramidal cells in the rat basolateral amygdala. Neuroscience, 228, 395–408. Lower left: Reproduced with permission from Muller, J.F., Mascagni, F., & McDonald, A.J. (2011). Cholinergic innervation of pyramidal cells and parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. The Journal of Comparative Neurology, 519, 790–805. Lower right: Reproduced with permission from Muller, J.F., Mascagni, F., & McDonald, A.J. (2009). Dopaminergic innervation of pyramidal cells in the rat basolateral amygdala. Brain Structure & Function, 213, 275–288.
The main targets of 5-HT+ terminals in the BNC are spines and distal dendrites of PNs, but other targets include PV, VIP, SOM, NPY, and CR INs (Bonn, Schmitt, Lesch, Van Bockstaele, & Asan, 2013; Muller et al., 2007b). The great majority of synapses are symmetrical. Stutzmann and LeDoux (1999) have reported that the main action of 5-HT in the BNC was to reduce pyramidal cell firing by activating GABAergic interneurons. This is consistent with the expression of excitatory 5-HT receptors (5-HT2Rs and 5-HT3Rs) in BNC INs. Thus, many PV and SOM INs in the BNC are 5-HT2AR+ (McDonald & Mascagni, 2007), and many NPY INs express 5-HT2CR mRNA (Bonn et al., 2013), consistent with findings that the main action of 5-HT is a direct activation of about 75% of INs via 5-HT2Rs (Rainnie, 1999). Large CCK INs (CCKLs) are the main BNC IN subpopulation that is 5-HT3AR+ (Mascagni & McDonald, 2007). This is in agreement with the coexpression of mRNAs for 5-HT3ARs and CB1Rs in the BNC (Morales, Wang, Diaz-Ruiz, & Jho, 2004), since CCKLs are the main INs that express CB1Rs (Katona et al., 2001; McDonald & Mascagni, 2001b).
Noradrenergic inputs
In both rats and monkeys the BNC receives a dense noradrenergic innervation from the locus coeruleus (Fig. 12, upper right) (Asan, 1998; Sadikot & Parent, 1990; Zhang et al., 2013), which is heavily involved in stress and stress-related pathologies (Sved, Cano, Passerin, & Rabin, 2002). Stressful stimuli such as foot shock induces NE release in the rat BNC (Galvez, Mesches, & McGaugh, 1996; Quirarte, Galvez, Roozendaal, & McGaugh, 1998), and it is well documented that the NE system in the human and rat BNC is involved in memory modulation by stressful events (Cahill, Babinsky, Markowitsch, & McGaugh, 1995; McGaugh, 2004).
There have been several EM studies of the noradrenergic innervation of the rat BNC (Asan, 1998; Farb, Chang, & LeDoux, 2010; Zhang et al., 2013). All of these studies found that the synaptic incidence of NE terminals is low. A serial section analysis of NE terminals found that only 46% of NE terminals formed synapses, mostly symmetrical (Zhang et al., 2013). Using the same techniques, this same group demonstrated that other neuromodulators in the BNC have a much higher synaptic incidence (serotonin: 76%; dopamine: 78%; acetylcholine: 76%) (Muller et al., 2007b, 2009, 2011). These percentages provide low estimates of synaptic incidence since symmetrical synapses are difficult to recognize when the plane of section is not orthogonal to the synapse. Nevertheless, these comparisons suggest that the NE system in the BNC is unique in its greater reliance on nonsynaptic release of transmitter into the extracellular space to modulate neuronal activity (i.e., “volume transmission”). In regard to synaptic contacts, 85% of NE axon terminals synapse with CaMK+ PNs, primary with distal dendrites and spines, and 15% with CaMK-negative INs (Zhang et al., 2013). These findings were consistent with previous single-labeling EM studies which reported that most contacts were with thin dendritic shafts and spines (Asan, 1998; Farb et al., 2010).
Dopaminergic inputs
The BNC is one of the main targets of the mesolimbic DA system (Fig. 12, lower right) (Fallon & Ciofi, 1992). These projections, which originate in the ventral tegmental area and substantia nigra, play a major role in the generation of both appetitive and aversive behaviors by the amygdala. Release of DA during stress is much higher in the BNC than in other targets of the mesolimbic DA system (Coco, Kuhn, Ely, & Kilts, 1992; Inglis & Moghaddam, 1999) and is critical for fear conditioning (Rosenkranz & Grace, 2003). It is also involved in drug addiction since the administration of a dopamine D1 antagonist into the BLa profoundly attenuates conditioned-cue reinstatement of cocaine seeking (Berglind, Case, Parker, Fuchs, & See, 2006).
Of note, 90% of DA axon terminals synapse with CaMK+ PNs, primarily on distal dendrites and spines, and 10% with CaMK-negative INs (Muller et al., 2009). Most synapses are symmetrical. These findings are consistent with a previous single-labeling EM study which reported that most contacts were with thin dendritic shafts and spines (Asan, 1997). Dopamine D1 receptors co-distribute with NMDA (N-methyl-D-aspartate) receptors in BNC PNs, including in dendritic spines, and can modulate synaptically evoked NMDA currents, suggesting a role in modulating synaptic plasticity involved in fear conditioning (Pickel, Colago, Mania, Molosh, & Rainnie, 2006). PV INs are the main IN subpopulation targeted by DA inputs (Brinley-Reed & McDonald, 1999; Pinard, Muller, Mascagni, & McDonald, 2008). The soma of some PV INs are innervated by up to 20 DA terminals, and their dendrites also receive a dense innervation. These findings are in agreement with studies which have shown that DA activates fast-spiking (presumptive PV) INs in the BNC (Bissière, Humeau, & Lüthi, 2003; Kröner, Rosenkranz, Grace, & Barrionuevo, 2005; Rosenkranz & Grace, 1999). In addition, DA activation of INs via D1 receptors induces rhythmic oscillations in the BLC, perhaps mediated by networks of PV INs that synchronize the outputs of PNs (Lorétan, Bissière, & Lüthi, 2004). Synchronized oscillations of BNC neurons are associated with emotional arousal and the consolidation and retrieval of fear memories (see section on PV INs above).
Opioids
Opioids are also important modulators of BNC activity (see chapter by Wilson and McDonald in this volume).
Basal forebrain connections
The BNC in rats, as well as in human and nonhuman primates, receives an especially dense cholinergic (ACh—acetylcholine) innervation from the ventral pallidum and substantia innominata of the basal forebrain (BF) that mainly targets the BL nucleus of the rat and basal nucleus of primates (Fig. 12, lower left) (Amaral & Bassett, 1989; Carlsen & Heimer, 1986; Carlsen, Zaborszky, & Heimer, 1985; Kordower, Bartus, Marciano, & Gash, 1989). The cholinergic innervation of the BNC is reduced by up to 40% in Alzheimer disease (Emre, Heckers, Mash, Geula, & Mesulam, 1993), and impairments of emotional event memory in Alzheimer patients are correlated with the extent of amygdalar degeneration (Mori et al., 1999). Cholinergic afferents to the rat BL nucleus are primary mediators of neuromodulation involved in memory consolidation of emotionally arousing experiences (McGaugh, 2004), and have also been implicated in fear conditioning and extinction (Boccia, Blake, Baratti, & McGaugh, 2009; Jiang et al., 2016; Vazdarjanova & McGaugh, 1999) and drug seeking behavior (See, McLaughlin, & Fuchs, 2003).
EM studies have shown that the main targets of ACh inputs to the rat BL are distal dendrites and spines of PNs via symmetrical synapses (Carlsen & Heimer, 1986; Muller et al., 2011; Nitecka & Frotscher, 1989). These inputs increase the excitability of PNs via both nicotinic receptors (Klein & Yakel, 2006) and muscarinic receptors (Power & Sah, 2008; Washburn & Moises, 1992b; Yajeya et al., 1997). About 10% of ACh inputs to the BL target INs, and most of these are PV+ (Muller et al., 2011), consistent with the reports that both muscarinic (Washburn & Moises, 1992b; Yajeya et al., 1997) and nicotinic (Zhu, Stewart, McIntosh, & Weight, 2005) agonists increase the excitability of fast-spiking (presumptive PV+) INs in BL. Moreover, preliminary studies indicate that muscarine depolarizes fast-firing PV+ INs via M3Rs, generating theta frequency oscillations in the rat BL (Liu, McDonald, & Mott, 2014).
The rat BL has extremely high levels of muscarinic M1 and M2 receptors (M1Rs and M2Rs) (McDonald & Mascagni, 2010, 2011). M1R immunoreactivity (M1R-ir) is mainly expressed in PNs, where it is present in somata, dendrites, and spines (McDonald & Mascagni, 2010; Muller, Mascagni, & McDonald, 2013). M2R-ir is also mainly expressed in PNs, where it is found in dendrites and spines, but not in somata, except in the Golgi complex (Fajardo-Serrano, Liu, Mott, & McDonald, 2017; McDonald & Mascagni, 2011; Muller, Mascagni, Zaric, Mott, & McDonald, 2016). A small number of SOM and NPY NPNs in the LA, BLp, and BM are also M2R+ (McDonald & Mascagni, 2011). EM studies demonstrated that some M2R+ dendrites in the BNC have spines, suggesting that they belong to PNs, whereas others belong to INs based on morphology or PV-ir (Fajardo-Serrano et al., 2017; Muller et al., 2016). M1R-ir and M2R-ir are also seen in axon terminals, most of which form asymmetrical synapses. The main targets of M1R+ and M2R+ terminals forming asymmetrical synapses are dendritic spines, whereas the main targets of M1R+ and M2R+ terminals forming symmetrical synapses are somata and dendritic shafts (Fajardo-Serrano et al., 2017; Muller et al., 2013, 2016). About one-quarter of PV+ axon terminals are M2R+, which is in agreement with electrophysiological studies demonstrating M2R-mediated suppression of GABA release (Fajardo-Serrano et al., 2017). The presence of M2R-ir in cholinergic terminals supports a possible role as an autoreceptor (Muller et al., 2016).
In addition to cholinergic neurons, there are also BF noncholinergic neurons that project to the BNC. Approximately 5% of amygdalopetal BF neurons in primates (Kordower et al., 1989) and 20%–25% of these neurons in rodents (Carlsen et al., 1985; Záborszky, Heimer, Eckenstein, & Leranth, 1986) are noncholinergic. About 10% of amygdalopetal BF neurons in the rat are PV+ GABAergic neurons (Fig. 9) (Mascagni & McDonald, 2009). The axon terminals of these BF GABAergic inputs, which mainly target the BL, are much larger than those of ACh neurons projecting to the BL, and provide a particularly robust innervation of BL PV INs (McDonald et al., 2011). Unlike the innervation of the hippocampus by BF GABAergic neurons, which only target INs (Freund & Buzsaki, 1996), there is also a moderate innervation of BL PNs by GABAergic BF neurons (McDonald et al., 2011). These data are in agreement with a study that observed short-latency inhibition of both PNs and presumptive INs in BL upon stimulation of the BF (Mello, Tan, & Finch, 1992). It is possible that both cholinergic and GABAergic inputs may contribute to the generation of theta rhythms in the BL as in the hippocampus (Freund & Buzsaki, 1996). The finding that ensembles of putative BF GABAergic neurons in the BF encode the motivational salience and hedonic valence of sensory stimuli (Lin & Nicolelis, 2008), and that neurons encoding these features are also found in the BNC (Belova, Paton, Morrison, & Salzman, 2007), suggests that interconnections of GABAergic BF neurons with the BL are important for emotional learning, attention, and arousal.
The BNC of the rat and monkey also have projections back to the BF (Grove, 1988; Russchen, Amaral, & Price, 1985). There are projections from presumptive PNs that innervate cholinergic neurons via asymmetrical synapses (Jolkkonen, Miettinen, Pikkarainen, & Pitkänen, 2002; Záborszky, Léránth, & Heimer, 1984). There are also a small number of SOM and NPY NPNs in the BL and BM that project to the BF (Fig. 9) (McDonald et al., 2012).
BNC projections to the striatum and central extended amygdala
Like the cortex, the cortex-like BNC has projections to the striatum, but does not receive inputs from this region. The CeA, which is located adjacent to caudomedial portions of the striatum, contains GABAergic medium spiny projection neurons that are virtually identical to those of the striatum (McDonald, 1982a). Columns of neurons extend anteriorly from the central nucleus, through the stria terminalis and ventral amygdalofugal pathway, and merge with the lateral part of the bed nucleus of the stria terminalis (BNSTL), which is caudally adjacent to the nucleus accumbens (NAc). The CeA, BNSTL, and the columns of neurons between them constitute the “central extended amygdala” (Alheid & Heimer, 1988). Similar to the CeA, the BNSTL contains GABAergic medium spiny projection neurons that are virtually identical to those of the striatum (McDonald, 1983). Like the striatum, the striatal-like CeA and BNSTL receive projections from the BNC, but do not project back to it. However, a study using monosynaptic rabies tracing found that the striatum, CeA, and BNSTL can indirectly influence BNC activity by synapsing with amygdalopetal BF cholinergic neurons (Gielow & Zaborszky, 2017).
BNC projections to the striatum
Anterograde and retrograde tract-tracing studies in the rat indicate that projections to the striatum, including the olfactory tubercle, nucleus accumbens (NAc), and caudatoputamen (CP), mainly originate in BL, with lesser contributions from adjacent portions of LA and BM (Kelley, Domesick, & Nauta, 1982; Kita & Kitai, 1990; Krettek & Price, 1978; McDonald, 1991a; Russchen, Bakst, Amaral, & Price, 1985; Russchen & Price, 1984). These studies demonstrate that BL projects to all portions of the striatum, with the exception of the rostro-dorsolateral part of the CP, which receives projections from the sensory/motor cortex (Kelley et al., 1982). Most studies have demonstrated a reversed sagittal topography, such that the rostral pole of BL projects most strongly to the caudal CP, moderately to the rostroventral CP and adjacent core of the NAc, and not at all to the shell of the NAc, whereas the latter only receives projections from the caudal BL. There is also evidence for a medial to lateral topography (McDonald, 1991a). EM studies have shown that BNC projections to the striatum, which arise from BNC PNs, form asymmetrical (excitatory) synapses mainly with spines of medium spiny neurons (Kita & Kitai, 1990).
There are triangular relationships involving the BNC, cortex, and striatum. Thus, subregions of the BNC that target specific cortical areas also innervate the same striatal regions targeted by these same cortical areas, and many PNs in the BL that project to the medial and lateral PFC also send collaterals to their striatal targets (McDonald, 1991a, 1991b). It is of interest that amygdalocortical relationships with the striatum are reminiscent of corticocortical relationships with the striatum. Thus, cortical areas that are interconnected have overlap in their projections to the striatum (Yeterian & Van Hoesen, 1978). There is also evidence that midline thalamic nuclei are part of this organization, including the organization of thalamic, cortical, and BNC projections to distinct striatal compartments (e.g., cell clusters, patch, and matrix). Thus, the prelimbic (PL) area, BLp (which projects to PL), and the paraventricular thalamic nucleus (PVT, which projects to both BLp and PL) all target the NAc. BLp and PL target the same compartments in the NAc shell (cell clusters) and core (patches), whereas BLp and PVT projections to the NAc overlap in the core (patches) but are complementary in the shell (Wright & Groenewegen, 1995). Moreover, different BNC nuclei have projections to distinct compartments in the ventral striatum (Wright, Beijer, & Groenewegen, 1996). The functional significance of these compartmentalized striatal projections is that different compartments receive distinct inputs and have projections to distinct autonomic and somatomotor nuclei in the brainstem.
As in the rat, there is a substantial projection of the monkey BNC to the striatum from the Bmg (BLa of rat) and Bpc (BLp of rat) (Cho et al., 2013; Fudge, Kunishio, Walsh, Richard, & Haber, 2002; Russchen, Bakst, et al., 1985). The projection from the ABmg (BM of rat) to the striatum is significantly more robust in the monkey compared to the rat. The monkey Bmg has a much stronger projection to the caudate nucleus than the Bpc or ABmg. Similar to the rat, amygdalar projections in the monkey exhibit triangular relationships with different cortical areas and have a patchy distribution in relation to distinct striatal compartments (Cho et al., 2013; Fudge et al., 2002; Fudge, Breitbart, & McClain, 2004; Russchen, Amaral, & Price, 1985; Russchen, Bakst, et al., 1985).
BNC projections to the central amygdalar nucleus
The CeA of the rat has four subdivisions arranged from medial to lateral: the medial (CM), intermediate (CI), lateral (CL), and lateral capsular (CLC) (McDonald, 1982a; Barbier, Fellmann, & Risold, 2018). The BNC nuclei project to all subdivisions with the exception of CI, but the projections to CL are light (Pitkänen, 2000). The projections of LA are confined to the CLC (Pitkänen et al., 1995). BL projects mainly to CM and CLC, and the projections from BLp are much more robust than those from BLa (Savander, Go, LeDoux, & Pitkänen, 1995). Both subdivisions of BM have strong projections to CM and CLC (Petrovich et al., 1996; Savander, Go, Ledoux, & Pitkänen, 1996). EM studies in cat indicate that BNC projections to the CeA mainly target spines and distal dendrites of spiny projection neurons (Paré, Smith, et al., 1995; Smith & Paré, 1994).
The monkey LA has no significant projection to either of the two main subdivisions of the central nucleus (CM, medial subdivision; CL, lateral subdivision) (Fudge & Tucker, 2009). However, the Bmg, ABmg, and ABpc have strong projections to CM and light projections to CL, whereas Bpc has robust projections to both CM and CL.
BNC projections to the BNST
The lateral subdivision of the BNST (BNSTL), which contains neurons that are very similar to those of the CeA, is the rostral component of the central extended amygdala. Injections of retrograde tracers into the BNSTL produce patterns of retrograde labeling in the amygdala that are very similar to those seen with injections into CeA (McDonald, 1991a). Thus, in addition to labeling in the CeA, there are numerous retrogradely labeled neurons in BMa and the caudal BLp, fewer in BMp, and almost none in LA and BLa with BNSTL injections. These results are consistent with anterograde tract-tracing studies (Dong, Petrovich, & Swanson, 2001).
It is of interest that the retrograde labeling in the rat BL seen with BNSTL injections (i.e., caudal BLp) is virtually identical to that seen with injections into the rostrally adjacent NAc shell (McDonald, 1991a). Likewise, in the monkey the same areas in the BNC that project to the lateral BNST (Bpc and ABmg) also project to the rostrally adjacent NAc shell (deCampo & Fudge, 2013). This suggests that the BSTL, and also the CeA, could be considered as highly modified caudomedial extensions of the striatum, and thus comprise parts of an effector macrostructure that exhibits regional specializations for the production of different types of responses. Since the BNC projections to this continuum are topographically organized, different BNC domains could activate distinct portions to produce different responses.
Functions of BNC projections to different components of the NAc/CeA/BNSTL complex
Most neurobehavioral studies indicate that BNC projections to the NAc are involved in reward-related behavior (Ambroggi, Ishikawa, Fields, & Nicola, 2008; Cador, Robbins, & Everitt, 1989; Stuber et al., 2011), whereas projections to the CeA (Davis, 1992; LeDoux, 2000) and BNSTL (Gungor & Paré, 2016; Walker, Miles, & Davis, 2009) are involved in fear and anxiety-like behavior, respectively. These findings are consistent with evidence that there are PNs in the BNC that encode positive or negative valences in both rodents (Beyeler et al., 2016; Namburi et al., 2015; Uwano, Nishijo, Ono, & Tamura, 1995) and primates (Belova et al., 2007; Paton, Belova, Morrison, & Salzman, 2006). Using optogenetics in mouse, it was found that photostimulation of BLa/LA PNs or their axon terminals projecting to NAc is associated with positive reinforcement whereas photostimulation of PNs or their axon terminals projecting to CM is associated with negative reinforcement (Beyeler et al., 2016; Namburi et al., 2015). The PNs projecting to NAc or CM are intermingled in the BLa and LA suggesting that they could interact with each other. However, the results of another recent study suggest that positive valence PNs are located in BLp, whereas negative valence PNs are located in BLa (Kim, Pignatelli, Xu, Itohara, & Tonegawa, 2016). In addition, contrary to the findings of Namburi, Beyeler, and coworkers, Kim et al. (2016) found that positive valence PNs, but not negative valence PNs, project to CeA, and that many negative valence PNs project to NAc. While these results contradict most behavioral studies, there is evidence that the CeA is involved in appetitive behavior, in addition to its well established role in aversive behavior (Kim, Zhang, Muralidhar, LeBlanc, & Tonegawa, 2017; Knapska et al., 2006; Parkinson, Robbins, & Everitt, 2000). The BNSTL is also involved in modulating emotional behavior. Thus, optogenetic studies indicate that the activation of the dorsolateral nucleus of the BNSTL, which receives projections from BLa, produces anxiolysis, whereas the activation of the oval nucleus of the BNSTL, which does not receive inputs from BLa, produces anxiety-like behavior (Kim et al., 2013).
CONCLUDING REMARKS
The functional neuroanatomy of the BNC is exceedingly complex. Each nucleus has connections with more than a dozen distinct cortical and subcortical brain regions. Moreover, discrete cell types within each nucleus may play different functional roles. Thus, individual nuclei may contain PNs with distinct functions and projections (e.g., fear neurons vs. extinction neurons in BL) and INs that innervate distinct PN domains (e.g., PV and CCK basket cells vs. SOM dendrite-targeting INs in the BNC). Knowledge of brain connections obtained from neuroanatomical studies has always guided subsequent electrophysiological and behavioral studies. Prior to 2010 most behavioral studies were at the nuclear level of resolution (e.g., lesion studies attempted to confine damage to individual amygdalar nuclei), whereas neuroanatomical and electrophysiological investigations were providing details of circuitry at the neuronal and synaptic levels of resolution. However, with the advent of new tools such as optogenetics, viral vectors, transgenic mice, and Designer Receptors Exclusively Activated by Designer Drugs (DREADD)-based chemogenetic tools, it is now possible to understand the behavioral circuity of the amygdala at the neuronal and synaptic levels. These studies permit the selective activation/inactivation of different components of amygdalar circuits in brain slices and conscious behaving animals. Most of these investigations are being conducted in rodents, especially mice, but as this review has demonstrated, the basic connections of BNC nuclei and neurons is very similar in rodents and primates. This offers hope that the information about functional amygdalar circuits obtained in rodents will also apply to primates, including humans, thus providing novel therapeutic interventions for diseases involving the amygdala, including anxiety disorders such as posttraumatic stress disorder (PTSD), depression, Alzheimer disease, temporal lobe epilepsy, and drug addiction. Of particular interest are the neuromodulatory systems; the expression of distinct monoaminergic and cholinergic receptor subtypes in discrete BNC neuronal populations, especially distinct IN subpopulations, suggests that the activity of the BNC may be subtly modulated to treat neurological and neuropsychiatric diseases.
Acknowledgments
The author is grateful for the support of the NIH over the last 35 years, currently NIH R01MH104638 to AJ McDonald and DD Mott. The author thanks Drs. Francesco Ferraguti (Medical University of Innsbruck) and David Mott (University of South Carolina School of Medicine) for helpful comments on an earlier version of this manuscript, and Dr. Asla Pitkänen (University of Kuopio) for permission to use a modified version of her illustration for Fig. 11.
References
- Aggleton JP (1985). A description of intra-amygdaloid connections in old world monkeys. Experimental Brain Research, 57, 390–399. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Burton MJ, & Passingham RE (1980). Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta). Brain Research, 190, 347–368. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, & Mishkin M (1986). The amygdala: Sensory gateway to the emotions. In Plutchik R, & Kellerman H (Eds.), Vol. 3. Emotion: Theory, research and experience (pp. 281–299). New York: Academic Press. [Google Scholar]
- Aggleton JP, & Passingham RE (1981). Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta). Journal of Comparative and Physiological Psychology, 95, 961–977. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Wright NF, Rosene DL, & Saunders RC (2015). Complementary patterns of direct amygdala and hippocampal projections to the macaque prefrontal cortex. Cerebral Cortex, 25, 4351–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, & Heimer L (1988). New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience, 27, 1–39. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Avendaño C, & Benoit R (1989). Distribution of somatostatin-like immunoreactivity in the monkey amygdala. The Journal of Comparative Neurology, 284, 294–313. [DOI] [PubMed] [Google Scholar]
- Amaral DG, & Bassett JL (1989). Cholinergic innervation of the monkey amygdala: An immunohistochemical analysis with antisera to choline acetyltransferase. The Journal of Comparative Neurology, 281, 337–361. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R, & Cowan WM (1983). Evidence for a direct projection from the superior temporal gyrus to the entorhinal cortex in the monkey. Brain Research, 275, 263–277. [DOI] [PubMed] [Google Scholar]
- Amaral DG, & Price JL (1984). Amygdalocortical projections in the monkey (Macaca f’ascicularis). The Journal of Comparative Neurology, 230, 465–496. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, & Carmichael ST (1992). Anatomical organization of the primate amygdala. In Aggleton JP (Ed.), The amygdala. Neurobiological aspects of emotion, memory, and mental dysfunction (pp. 1–66). New York: Wiley-Liss. [Google Scholar]
- Amaral DG, Veazey RB, & Cowan WM (1982). Some observations on hypothalamo-amygdaloid connections in the monkey. Brain Research, 252, 13–27. [DOI] [PubMed] [Google Scholar]
- Amaral DG, & Witter MP (1995). Hippocampal formation. In Paxinos G (Ed.), The rat nervous system (2nd ed., pp. 443–494). San Diego: Academic Press. [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, & Nicola SM (2008). Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron, 59, 648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrási T, Veres JM, Rovira-Esteban L, Kozma R, Vikór A, Gregori E, & Hájos N (2017). Differential excitatory control of 2 parallel basket cell networks in amygdala microcircuits. PLoS Biology, 15, e2001421. 10.1371/journal.pbio.2001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C, Krook-Magnuson E, & Soltesz I (2012). Neurogliaform and ivy cells: A major family of nNOS expressing GABAergic neurons. Frontiers in Neural Circuits, 6, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asan E (1997). Ultrastructural features of tyrosine-hydroxylase-immunoreactive afferents and their targets in the rat amygdala. Cell and Tissue Research, 288, 449–469. [DOI] [PubMed] [Google Scholar]
- Asan E (1998). The catecholaminergic innervation of the rat amygdala. Advances in Anatomy, Embryology and Cell Biology, 142, 1–118. [DOI] [PubMed] [Google Scholar]
- Asede D, Bosch D, Luthi A, Ferraguti F, & Ehrlich I (2015). Sensory inputs to intercalated cells provide fear-learning modulated inhibition to the basolateral amygdala. Neuron, 86, 541–554. [DOI] [PubMed] [Google Scholar]
- Baratta MV, Lamp T, & Tallent MK (2002). Somatostatin depresses long-term potentiation and Ca2+ signaling in mouse dentate gyrus. Journal of Neurophysiology, 88, 3078–3086. [DOI] [PubMed] [Google Scholar]
- Barbas H, & De Olmos J (1990). Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. The Journal of Comparative Neurology, 300, 549–571. [DOI] [PubMed] [Google Scholar]
- Barbier M, Fellmann D, & Risold PY (2018). Characterization of McDonald’s intermediate part of the Central nucleus of the amygdala in the rat. The Journal of Comparative Neurology, 526, 2165–2186. [DOI] [PubMed] [Google Scholar]
- Bauman MD, & Amaral DG (2005). The distribution of serotonergic fibers in the macaque monkey amygdala: An immunohistochemical study using antisera to 5-hydroxytryptamine. Neuroscience, 136, 193–203. [DOI] [PubMed] [Google Scholar]
- Bazelot M, Bocchio M, Kasugai Y, Fischer D, Dodson PD, Ferraguti F, & Capogna M (2015). Hippocampal theta input to the amygdala shapes feedforward inhibition to gate heterosynaptic plasticity. Neuron, 87, 1290–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, & Salzman CD (2007). Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron, 55, 970–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, Case JM, Parker MP, Fuchs RA, & See RE (2006). Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience, 137, 699–706. [DOI] [PubMed] [Google Scholar]
- Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GF, … Tye KM (2016). Divergent routing of positive and negative information from the amygdala during memory retrieval. Neuron, 90, 348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienvenu TC, Busti D, Magill PJ, Ferraguti F, & Capogna M (2012). Cell-type-specific recruitment of amygdala interneurons to hippocampal theta rhythm and noxious stimuli in vivo. Neuron, 74, 1059–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienvenu TC, Busti D, Micklem BR, Mansouri M, Magill PJ, Ferraguti F, & Capogna M (2015). Large intercalated neurons of amygdala relay noxious sensory information. The Journal of Neuroscience, 35, 2044–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissière S, Humeau Y, & Lüthi A (2003). Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nature Neuroscience, 6, 587–592. [DOI] [PubMed] [Google Scholar]
- Blatow M, Caputi A, Burnashev N, Monyer H, & Rozov A (2003). Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron, 38, 79–88. [DOI] [PubMed] [Google Scholar]
- Bocchio M, Nabavi S, & Capogna M (2017). Synaptic plasticity, engrams, and network oscillations in amygdala circuits for storage and retrieval of emotional memories. Neuron, 94, 731–743. [DOI] [PubMed] [Google Scholar]
- Boccia MM, Blake MG, Baratti CM, & McGaugh JL (2009). Involvement of the basolateral amygdala in muscarinic cholinergic modulation of extinction memory consolidation. Neurobiology of Learning and Memory, 91, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda E, Petrides M, Ostry D, & Evans A (1996). Specific involvement of human parietal systems and the amygdala in the perception of biological motion. The Journal of Neuroscience, 16, 3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn M, Schmitt A, Lesch KP, Van Bockstaele EJ, & Asan E (2013). Serotonergic innervation and serotonin receptor expression of NPY-producing neurons in the rat lateral and basolateral amygdaloid nuclei. Brain Structure & Function, 218, 421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers ME, & Ressler KJ (2015). Interaction between the cholecystokinin and endogenous cannabinoid systems in cued fear expression and extinction retention. Neuropsychopharmacology, 40, 688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Gritton H, Howe WM, Young DA, & Sarter M (2007). Modulators in concert for cognition: Modulator interactions in the prefrontal cortex. Progress in Neurobiology, 83, 69–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley-Reed M, Mascagni F, & McDonald AJ (1995). Synaptology of prefrontal cortical projections to the basolateral amygdala: An electron microscopic study in the rat. Neuroscience Letters, 202, 45–48. [DOI] [PubMed] [Google Scholar]
- Brinley-Reed M, & McDonald AJ (1999). Evidence that dopaminergic axons provide a dense innervation of specific neuronal subpopulations in the rat basolateral amygdala. Brain Research, 850, 127–135. [DOI] [PubMed] [Google Scholar]
- Brothers LA, & Finch DM (1985). Physiological evidence for an excitatory pathway from entorhinal cortex to amygdala in the rat. Brain Research, 359, 10–20. [DOI] [PubMed] [Google Scholar]
- Brown S, & Schafer A (1888). An investigation into the functions of the occipital and temporal lobes of the monkey’s brain. Philosophical Transactions of the Royal Society of London B, 179, 303–327. [Google Scholar]
- Buhl EH, Han ZS, Lörinczi Z, Stezhka VV, Karnup SV, & Somogyi P (1994). Physiological properties of anatomically identified axo-axonic cells in the rat hippocampus. Journal of Neurophysiology, 71, 1289–1307. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, & Chrobak JJ (1995). Temporal structure in spatially organized neuronal ensembles: A role for interneuronal networks. Current Opinion in Neurobiology, 5, 504–510. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, & Everitt BJ (1989). Involvement of the amygdala in stimulus-reward associations: Interaction with the ventral striatum. Neuroscience, 30, 77–86. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowitsch HJ, & McGaugh JL (1995). The amygdala and emotional memory. Nature, 377, 295–296. [DOI] [PubMed] [Google Scholar]
- Campeau S, & Davis M (1995a). Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. The Journal of Neuroscience, 15, 2301–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, & Davis M (1995b). Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. The Journal of Neuroscience, 15, 2312–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, & Swanson LW (1994). Organization of projections from the ventromedial nucleus of the hypothalamus: A Phaseolus vulgaris-leucoagglutinin study in the rat. The Journal of Comparative Neurology, 348, 41–79. [DOI] [PubMed] [Google Scholar]
- Capogna M (2014). GABAergic cell type diversity in the basolateral amygdala. Current Opinion in Neurobiology, 26, 110–116. [DOI] [PubMed] [Google Scholar]
- Caputi A, Melzer S, Michael M, & Monyer H (2013). The long and short of GABAergic neurons. Current Opinion in Neurobiology, 23, 179–186. [DOI] [PubMed] [Google Scholar]
- Carlsen J (1988). Immunocytochemical localization of glutamate decarboxylase in the rat basolateral amygdaloid nucleus, with special reference to GABAergic innervation of amygdalostriatal projection neurons. The Journal of Comparative Neurology, 273, 513–526. [DOI] [PubMed] [Google Scholar]
- Carlsen J, & Heimer L (1986). A correlated light and electron microscopic immunocytochemical study of cholinergic terminals and neurons in the rat amygdaloid body with special emphasis on the basolateral amygdaloid nucleus. The Journal of Comparative Neurology, 244, 121–136. [DOI] [PubMed] [Google Scholar]
- Carlsen J, & Heimer L (1988). The basolateral amygdaloid complex as a cortical-like structure. Brain Research, 441, 377–380. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Zaborszky L, & Heimer L (1985). Cholinergic projections from the basal forebrain to the basolateral amygdaloid complex: A combined retrograde fluorescent and immunohistochemical study. The Journal of Comparative Neurology, 234, 155–167. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, & Price JL (1995). Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. The Journal of Comparative Neurology, 363, 615–641. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Gutman AR, Maguschak KA, Bowser ME, Yang Y, Davis M, & Ressler KJ (2009). Functional interactions between endocannabinoid and CCK neurotransmitter systems may be critical for extinction learning. Neuropsychopharmacology, 34, 509–521. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, & Ressler KJ (2007). Modulation of fear and anxiety by the endogenous cannabinoid system. CNS Spectrums, 12, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YT, Ernst M, & Fudge JL (2013). Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. Neuroscience, 33, 14017–14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ, Summers RJ, Stephenson JA, Cook CJ, & Beart PM (1987). Excitatory amino acid projections to the nucleus accumbens septi in the rat: A retrograde transport study utilizing D[3H]aspartate and [3H]GABA. Neuroscience, 22, 425–439. [DOI] [PubMed] [Google Scholar]
- Chung L, & Moore SD (2009a). Cholecystokinin excites interneurons in rat basolateral amygdala. Journal of Neurophysiology, 102, 272–284. [DOI] [PubMed] [Google Scholar]
- Chung L, & Moore SD (2009b). Neuropeptides modulate compound postsynaptic potentials in basolateral amygdala. Neuroscience, 164, 1389–1397. [DOI] [PubMed] [Google Scholar]
- Coco ML, Kuhn CM, Ely TD, & Kilts CD (1992). Selective activation of mesoamygdaloid dopamine neurons by conditioned stress: Attenuation by diazepam. Brain Research, 590, 39–47. [DOI] [PubMed] [Google Scholar]
- Crosby EC, & Humphrey T (1944). Studies of the vertebrate telencephalon. III. The amygdaloid complex in the shrew (Blarina brevicada). The Journal of Comparative Neurology, 81, 285–305. [Google Scholar]
- Davis M (1992). The role of the amygdala in fear and anxiety. Annual Review of Neuroscience, 15, 353–375. [DOI] [PubMed] [Google Scholar]
- deCampo DM, & Fudge JL (2013). Amygdala projections to the lateral bed nucleus of the stria terminalis in the macaque: Comparison with ventral striatal afferents. The Journal of Comparative Neurology, 521, 3191–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, & Swanson LW (2001). Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Research. Brain Research Reviews, 38, 192–246. [DOI] [PubMed] [Google Scholar]
- Doron NN, & Ledoux JE (1999). Organization of projections to the lateral amygdala from auditory and visual areas of the thalamus in the rat. The Journal of Comparative Neurology, 412, 383–409. [PubMed] [Google Scholar]
- Doron NN, & Ledoux JE (2000). Cells in the posterior thalamus project to both amygdala and temporal cortex: A quantitative retrograde double-labeling study in the rat. The Journal of Comparative Neurology, 425, 257–274. [PubMed] [Google Scholar]
- Drevets WC, Bogers W, & Raichle ME (2002). Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. European Neuropsychopharmacology, 12, 527–544. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, & Lüthi A (2009). Amygdala inhibitory circuits and the control of fear memory. Neuron, 62, 757–771. [DOI] [PubMed] [Google Scholar]
- Emre M, Heckers S, Mash DC, Geula C, & Mesulam MM (1993). Cholinergic innervation of the amygdaloid complex in the human brain and its alterations in old age and Alzheimer’s disease. The Journal of Comparative Neurology, 336, 117–134. [DOI] [PubMed] [Google Scholar]
- Fajardo-Serrano A, Liu L, Mott DD, & McDonald AJ (2017). Evidence for M2 muscarinic receptor modulation of axon terminals and dendrites in the rodent basolateral amygdala: An ultrastructural and electrophysiological analysis. Neuroscience, 357, 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, & Ciofi P (1992). Distribution of monoamines within the amygdala. In Aggleton JP (Ed.), The amygdala. Neurobiological aspects of emotion, memory, and mental dysfunction (pp. 97–114). New York: Wiley-Liss. [Google Scholar]
- Fanselow MS, & Dong HW (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron, 65, 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb CR, Chang W, & LeDoux JE (2010). Ultrastructural characterization of noradrenergic axons and Beta-adrenergic receptors in the lateral nucleus of the amygdala. Frontiers in Behavioral Neuroscience, 4, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, & Tye KM (2013). BLA to vHPC inputs modulate anxiety-related behaviors. Neuron, 79, 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch DM, Wong EE, Derian EL, Chen XH, Nowlin-Finch NL, & Brothers LA (1986). Neurophysiology of limbic system pathways in the rat: Projections from the amygdala to the entorhinal cortex. Brain Research, 370, 273–284. [DOI] [PubMed] [Google Scholar]
- French SJ, Hailstone JC, & Totterdell S (2003). Basolateral amygdala efferents to the ventral subiculum preferentially innervate pyramidal cell dendritic spines. Brain Research, 981, 160–167. [DOI] [PubMed] [Google Scholar]
- Freund TF (2003). Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends in Neurosciences, 26, 489–495. [DOI] [PubMed] [Google Scholar]
- Freund TF, & Buzsaki G (1996). Interneurons of the hippocampus. Hippocampus, 6, 347–470. [DOI] [PubMed] [Google Scholar]
- Freund TF, & Katona I (2007). Perisomatic inhibition. Neuron, 56, 33–42. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Murray EA, O’Neill JB, & Mishkin M (1986). Cortical connections of the somatosensory fields of the lateral sulcus of macaques: Evidence for a corticolimbic pathway for touch. The Journal of Comparative Neurology, 252, 323–347. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, & McClain C (2004). Amygdaloid inputs define a caudal component of the ventral striatum in primates. The Journal of Comparative Neurology, 476, 330–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Kunishio K, Walsh P, Richard C, & Haber SN (2002). Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience, 110, 257–275. [DOI] [PubMed] [Google Scholar]
- Fudge JL, & Tucker T (2009). Amygdala projections to central amygdaloid nucleus subdivisions and transition zones in the primate. Neuroscience, 159, 819–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller TA, Russchen FT, & Price JL (1987). Sources of presumptive glutamatergic/aspartergic afferents to the rat ventral striatopallidal region. The Journal of Comparative Neurology, 258, 317–338. [DOI] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, & McGaugh JL (1996). Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiology of Learning and Memory, 66, 253–257. [DOI] [PubMed] [Google Scholar]
- Gattass R, Galkin TW, Desimone R, & Ungerleider LG (2014). Subcortical connections of area V4 in the macaque. The Journal of Comparative Neurology, 522, 1941–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, & Monyer H (1995). Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron, 15, 193–204. [DOI] [PubMed] [Google Scholar]
- Gielow MR, & Zaborszky L (2017). The input-output relationship of the cholinergic basal forebrain. Cell Reports, 18, 1817–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, & Barbas H (2002). Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience, 115, 1261–1279. [DOI] [PubMed] [Google Scholar]
- Giesbrecht CJ, Mackay JP, Silveira HB, Urban JH, & Colmers WF (2010). Countervailing modulation of Ih by neuropeptide Y and corticotrophin-releasing factor in basolateral amygdala as a possible mechanism for their effects on stress-related behaviors. The Journal of Neuroscience, 30, 16970–16982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Lüthi A, & Herry C (2009). Perineuronal nets protect fear memories from erasure. Science, 325, 1258–1261. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Turney S, Price JL, & Burkhalter A (2002). Axo-axonic synapses formed by somatostatin-expressing GABAergic neurons in rat and monkey visual cortex. The Journal of Comparative Neurology, 443, 1–14. [DOI] [PubMed] [Google Scholar]
- Grove EA (1988). Neural associations of the substantia innominata in the rat: Afferent connections. The Journal of Comparative Neurology, 277, 315–346. [DOI] [PubMed] [Google Scholar]
- Gungor NZ, & Paré D (2016). Functional heterogeneity in the bed nucleus of the stria terminalis. The Journal of Neuroscience, 36, 8038–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E (1972). The amygdala of the cat: A Golgi study. Zeitschrift für Zellforschung, 134, 439–458. [DOI] [PubMed] [Google Scholar]
- Härtig W, Brückner G, Brauer K, Schmidt C, & Bigl V (1995). Allocation of perineuronal nets and parvalbumin-, calbindin-D28k- and glutamic acid decarboxylase-immunoreactivity in the amygdala of the rhesus monkey. Brain Research, 698, 265–269. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, & Lüthi A (2008). Switching on and off fear by distinct neuronal circuits. Nature, 454, 600–606. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, & Lüthi A (2010). Neuronal circuits of fear extinction. The European Journal of Neuroscience, 31, 599–612. [DOI] [PubMed] [Google Scholar]
- Hestrin S, & Galarreta M (2005). Electrical synapses define networks of neocortical GABAergic neurons. Trends in Neurosciences, 28, 304–309. [DOI] [PubMed] [Google Scholar]
- Hoover WB, & Vertes RP (2007). Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure & Function, 212, 149–179. [DOI] [PubMed] [Google Scholar]
- Hoover WB, & Vertes RP (2011). Projections of the medial orbital and ventral orbital cortex in the rat. The Journal of Comparative Neurology, 519, 3766–3801. [DOI] [PubMed] [Google Scholar]
- Houser CR, Crawford GD, Salvaterra PM, & Vaughn JE (1985). Immunocytochemical localization of choline acetyltransferase in rat cerebral cortex: A study of cholinergic neurons and synapses. The Journal of Comparative Neurology, 234, 17–34. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, & Saper CB (1991). Efferent projections of the infralimbic cortex of the rat. The Journal of Comparative Neurology, 308, 249–276. [DOI] [PubMed] [Google Scholar]
- Inglis FM, & Moghaddam B (1999). Dopaminergic innervation of the amygdala is highly responsive to stress. Journal of Neurochemistry, 72, 1088–1094. [DOI] [PubMed] [Google Scholar]
- Ingram SM, Krause RG, Baldino F, Skeen LC, & Lewis ME (1989). Neuronal localization of cholecystokinin mRNA in the rat brain using in situ hybridization histochemistry. The Journal of Comparative Neurology, 287, 260–272. [DOI] [PubMed] [Google Scholar]
- Ino T, Matsuzaki S, Shinonaga Y, Ohishi H, Ogawa-Meguro R, & Mizuno N (1990). Direct projections of non-pyramidal neurons of Ammon’s horn to the amygdala and the entorhinal cortex. Neuroscience Letters, 115, 161–166. [DOI] [PubMed] [Google Scholar]
- Janak PH, & Tye KM (2015). From circuits to behaviour in the amygdala. Nature, 517, 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Ressler KJ, Hammack SE, Chhatwal JP, & Rainnie DG (2009). Distinct subtypes of cholecystokinin (CCK)-containing interneurons of the basolateral amygdala identified using a CCK promoter-specific lentivirus. Journal of Neurophysiology, 101, 1494–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Kundu S, Lederman JD, López-Hernández GY, Ballinger EC, Wang S, … Role LW (2016). Cholinergic signaling controls conditioned fear behaviors and enhances plasticity of cortical-amygdala circuits. Neuron, 90, 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S (2009). Structural organization of long-range GABAergic projection system of the hippocampus. Frontiers in Neuroanatomy, 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S, Klausberger T, Marton LF, Dalezios Y, Roberts JD, Fuentealba P, … Somogyi P (2007). Neuronal diversity in GABAergic long-range projections from the hippocampus. The Journal of Neuroscience, 27, 8790–8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolkkonen E, Miettinen R, Pikkarainen M, & Pitkänen A (2002). Projections from the amygdaloid complex to the magnocellular cholinergic basal forebrain in rat. Neuroscience, 111, 133–149. [DOI] [PubMed] [Google Scholar]
- Jones EG, & Burton H (1976). A projection from the medial pulvinar to the amygdala in primates. Brain Research, 104, 142–147. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, & Frankland PW (2018). Memory allocation: Mechanisms and function. Annual Review of Neuroscience, 41, 389–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal AM, & Tőmböl T (1975). Golgi studies on the amygdaloid nuclei of the cat. Journal für Hirnforschung, 16, 175–201. [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, & Freund TF (2001). Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. The Journal of Neuroscience, 21, 9506–9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y (1995). Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. The Journal of Neuroscience, 15, 2638–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H, Yoshida M, Yokoo H, Nishi M, & Tanaka M (1993). Psychological stress increases serotonin release in the rat amygdala and prefrontal cortex assessed by in vivo microdialysis. Neuroscience Letters, 162, 81–84. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, & Nauta WJ (1982). The amygdalostriatal projection in the rat—An anatomical study by anterograde and retrograde tracing methods. Neuroscience, 7, 615–630. [DOI] [PubMed] [Google Scholar]
- Kemppainen S, & Pitkänen A (2000). Distribution of parvalbumin, calretinin, and calbindin-D (28k) immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. The Journal of Comparative Neurology, 426, 441–467. [DOI] [PubMed] [Google Scholar]
- Kim J, Pignatelli M, Xu S, Itohara S, & Tonegawa S (2016). Antagonistic negative and positive neurons of the basolateral amygdala. Nature Neuroscience, 19, 1636–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zhang X, Muralidhar S, LeBlanc SA, & Tonegawa S (2017). Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron, 93, 1464–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, … Deisseroth K (2013). Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature, 496, 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, & Kitai ST (1990). Amygdaloid projections to the frontal cortex and the striatum in the rat. The Journal of Comparative Neurology, 298, 40–49. [DOI] [PubMed] [Google Scholar]
- Klein RC, & Yakel JL (2006). Functional somato-dendritic alpha7-containing nicotinic acetylcholine receptors in the rat basolateral amygdala complex. The Journal of Physiology, 576, 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüver H, & Bucy PC (1939). Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology and Psychiatry, 42, 979–1000. [DOI] [PubMed] [Google Scholar]
- Knapska E, Walasek G, Nikolaev E, Neuhäusser-Wespy F, Lipp HP, Kaczmarek L, & Werka T (2006). Differential involvement of the central amygdala in appetitive versus aversive learning. Learning & Memory, 13, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C, Smialowska M, Eriksson LG, Chanpalay V, & Davies S (1986). Origin of the neuropeptide Y innervation of the rat retrohippocampal region. Neuroscience Letters, 65, 287–292. [DOI] [PubMed] [Google Scholar]
- Kolb B (1990). Posterior parietal and temporal association cortex. In Kolb B, & Tees RB (Eds.), The cerebral cortex of the rat (pp. 459–471). Cambridge: MIT Press. [Google Scholar]
- Kordower JH, Bartus RT, Marciano FF, & Gash DM (1989). Telencephalic cholinergic system of the New World monkey (Cebus apella): Morphological and cytoarchitectonic assessment and analysis of the projection to the amygdala. The Journal of Comparative Neurology, 279, 528–545. [DOI] [PubMed] [Google Scholar]
- Krabbe S, Gründemann J, & Lüthi A (2018). Amygdala inhibitory circuits regulate associative fear conditioning. Biological Psychiatry, 83, 800–809. [DOI] [PubMed] [Google Scholar]
- Krabbe S, Gründemann J, Paradiso E, Markovic M, Ferraguti F, & Lüthi A (2016). Inhibitory amygdala microcircuits for aversive learning. In 10th FENS forum of neurocience. 2–6 July, Copenhagen, Denmark, index number 1675. [Google Scholar]
- Krettek JE, & Price JL (1977). Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. The Journal of Comparative Neurology, 172, 681–722. [DOI] [PubMed] [Google Scholar]
- Krettek JE, & Price JL (1978). Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. The Journal of Comparative Neurology, 178, 225–254. [DOI] [PubMed] [Google Scholar]
- Kröner S, Rosenkranz JA, Grace AA, & Barrionuevo G (2005). Dopamine modulates excitability of basolateral amygdala neurons in vitro. Journal of Neurophysiology, 93, 1598–1610. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Hattori R, & Yui Y (1994). Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Research, 649, 159–173. [DOI] [PubMed] [Google Scholar]
- Kubota Y, & Kawaguchi Y (1997). Two distinct subgroups of cholecystokinin-immunoreactive cortical interneurons. Brain Research, 752, 175–183. [DOI] [PubMed] [Google Scholar]
- Kuroda M, & Price JL (1991). Synaptic organization of projections from basal forebrain structures to the mediodorsal thalamic nucleus of the rat. The Journal of Comparative Neurology, 303, 513–533. [DOI] [PubMed] [Google Scholar]
- Lang EJ, & Paré D (1998). Synaptic responsiveness of interneurons of the cat lateral amygdaloid nucleus. Neuroscience, 83, 877–889. [DOI] [PubMed] [Google Scholar]
- LeDoux J (2000). The amygdala and emotion: A view through fear. In Aggleton JP (Ed.), The amygdala (2nd ed., pp. 289–310). Oxford: Oxford University Press. [Google Scholar]
- LeDoux JE, Farb C, & Ruggiero DA (1990). Topographic organization of neurons in the acoustic thalamus that project to the amygdala. The Journal of Neuroscience, 10, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, & Milner TA (1991). Ultrastructure and synaptic associations of auditory thalamo-amygdala projections in the rat. Experimental Brain Research, 85, 577–586. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, & Reis DJ (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of Neuroscience, 8, 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Romanski L, & Xagoraris A (1989). Indelibility of subcortical emotional memories. Journal of Cognitive Neuroscience, 1, 238–243. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Forest R, Stornetta R, & Reis DJ (1987). Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. The Journal of Comparative Neurology, 264, 123–146. [DOI] [PubMed] [Google Scholar]
- Leitermann RJ, Rostkowski AB, & Urban JH (2016). Neuropeptide Y input to the rat basolateral amygdala complex and modulation by conditioned fear. The Journal of Comparative Neurology, 524, 2418–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitermann RJ, Sajdyk TJ, & Urban JH (2012). Cell-specific expression of calcineurin immunoreactivity within the rat basolateral amygdala complex and colocalization with the neuropeptide Y Y1 receptor. Journal of Chemical Neuroanatomy, 45, 50–56. [DOI] [PubMed] [Google Scholar]
- Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, & Pape HC (2011). Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS One, 6, e21714. 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Mania I, & Rainnie DG (2003). Subtypes of substance P receptor immunoreactive interneurons in the rat basolateral amygdala. Brain Research, 981, 41–51. [DOI] [PubMed] [Google Scholar]
- Lewis DA, & Lund JS (1990). Heterogeneity of chandelier neurons in monkey neocortex: Corticotropin-releasing factor- and parvalbumin-immunoreactive populations. The Journal of Comparative Neurology, 293, 599–615. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, & Paré D (2008). Amygdala intercalated neurons are required for expression of fear extinction. Nature, 454, 642–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, & Nicolelis MA (2008). Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron, 59, 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke R, De Lima AD, Schwegler H, & Pape HC (1999). Direct synaptic connections of axons from superior colliculus with identified thalamo-amygdaloid projection neurons in the rat: Possible substrates of a subcortical visual pathway to the amygdala. The Journal of Comparative Neurology, 403, 158–170. [PubMed] [Google Scholar]
- Liu L, McDonald AJ, & Mott DD (2014). Generation of synchronized inhibition in BLa pyramidal cells by activation of mAChRs. Program No. 747.22, 2014 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, [Online]. [Google Scholar]
- Lorétan K, Bissière S, & Lüthi A (2004). Dopaminergic modulation of spontaneous inhibitory network activity in the lateral amygdala. Neuropharmacology, 47, 631–639. [DOI] [PubMed] [Google Scholar]
- Lucas EK, Jegarl AM, Morishita H, & Clem RL (2016). Multimodal and site-specific plasticity of amygdala parvalbumin interneurons after fear learning. Neuron, 91, 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty NK, & Sah P (1998). Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature, 394, 683–687. [DOI] [PubMed] [Google Scholar]
- Majak K, Rönkkö S, Kemppainen S, & Pitkänen A (2004). Projections from the amygdaloid complex to the piriform cortex: A PHA-L study in the rat. The Journal of Comparative Neurology, 476, 414–428. [DOI] [PubMed] [Google Scholar]
- Mańko M, Bienvenu TC, Dalezios Y, & Capogna M (2012). Neurogliaform cells of amygdala: A source of slow phasic inhibition in the basolateral complex. The Journal of Physiology, 590, 5611–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, & Fanselow MS (1995). Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. The Journal of Neuroscience, 15, 7548–7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, & Vogt KE (2005). A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron, 48, 1025–1037. [DOI] [PubMed] [Google Scholar]
- Marsicano G, & Lutz B (1999). Expression of the cannabinoid receptor CB1 in district neuronal subpopulations in the adult mouse forebrain. The European Journal of Neuroscience, 11, 4213–4225. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, … Lutz B (2002). The endogenous cannabinoid system controls extinction of aversive memories. Nature, 418, 530–534. [DOI] [PubMed] [Google Scholar]
- Mascagni F, & McDonald AJ (2003). Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Research, 976, 171–184. [DOI] [PubMed] [Google Scholar]
- Mascagni F, & McDonald AJ (2007). A novel subpopulation of 5-HT3A receptor immunoreactive interneurons in the rat basolateral amygdala. Neuroscience, 144, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascagni F, & McDonald AJ (2009). Parvalbumin-immunoreactive neurons and GABAergic neurons of the basal forebrain project to the rat basolateral amygdala. Neuroscience, 160, 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ, & Coleman JR (1993). Cortico-amygdaloid and cortico-cortical projections of the rat temporal cortex: A Phaseolus vulgaris leucoagglutinin study. Neuroscience, 57, 697–715. [DOI] [PubMed] [Google Scholar]
- Mascagni F, Muly EC, Rainnie DG, & McDonald AJ (2009). Immunohistochemical characterization of parvalbumin-containing interneurons in the monkey basolateral amygdala. Neuroscience, 158, 1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mátyás F, Lee J, Shin HS, & Acsády L (2014). The fear circuit of the mouse forebrain: Connections between the mediodorsal thalamus, frontal cortices and basolateral amygdala. The European Journal of Neuroscience, 39, 1810–1823. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1982a). Cytoarchitecture of the central amygdaloid nucleus of the rat. The Journal of Comparative Neurology, 208, 401–418. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1982b). Neurons of the lateral and basolateral amygdaloid nuclei: A Golgi study in the rat. The Journal of Comparative Neurology, 212, 293–312. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1983). Neurons of the bed nucleus of the stria terminalis: A Golgi study in the rat. Brain Research Bulletin, 10, 11–120. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1985). Immunohistochemical identification of gamma-aminobutyric acid containing neurons in the rat basolateral amygdala. Neuroscience Letters, 53, 203–207. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1987). Organization of amygdaloid projections to the mediodorsal thalamus and prefrontal cortex: A fluorescence retrograde transport study in the rat. The Journal of Comparative Neurology, 262, 46–58. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1989). Coexistence of somatostatin with neuropeptide Y, but not with cholecystokinin or vasoactive intestinal peptide, in neurons of the rat amygdala. Brain Research, 500, 37–45. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1991a). Topographical organization of amygdaloid projections tothe caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience, 14, 15–33. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1991b). Organization of amygdaloid projections to the prefrontal cortex and associated rostral striatum in the rat. Neuroscience, 44, 1–14. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1992a). Cell types and intrinsic connections of the amygdala. In Aggleton JP (Ed.), The amygdala. Neurobiological aspects of emotion, memory, and mental dysfunction (pp. 67–96). New York: Wiley-Liss. [Google Scholar]
- McDonald AJ (1992b). Projection neurons of the basolateral amygdala: A correlative Golgi and retrograde tract tracing study. Brain Research Bulletin, 28, 179–185. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1994). Calretinin immunoreactive neurons in the basolateral amygdala of the rat and monkey. Brain Research, 667, 238–242. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1996a). Glutamate and aspartate immunoreactive neurons of the rat basolateral amygdala: Colocalization of excitatory amino acids and projections to the limbic circuit. The Journal of Comparative Neurology, 365, 367–379. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1996b). Localization of AMPA glutamate receptor subunits in subpopulations of nonpyramidal neurons in the rat basolateral amygdala. Neuroscience Letters, 208, 175–178. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1998). Cortical pathways to the mammalian amygdala. Progress in Neurobiology, 55, 257–332. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, & Augustine JR (1993). Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience, 52, 281–294. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, & Betette R (2001). Parvalbumin containing neurons in the rat basolateral amygdala: Morphology and colocalization of calbindin D-28k. Neuroscience, 102, 413–425. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, & Culberson JL (1981). Neurons of the basolateral amygdala: A Golgi study in the opossum. Amer. Journal of Anatomy, 162, 327–342. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Hamilton PG, & Barnstable CJ (2018). Perineuronal nets labeled by monoclonal antibody VC1.1 ensheath interneurons expressing parvalbumin and calbindin in the rat amygdala. Brain Structure & Function, 223, 1133–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, & Mascagni F (1997). Projections of the lateral entorhinal cortex to the amygdala: A Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience, 77, 445–459. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, & Mascagni F (2001a). Colocalization of calcium-binding proteins and gamma-aminobutyric acid in neurons of the rat basolateral amygdala. Neuroscience, 105, 681–693. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, & Mascagni F (2001b). Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: High concentrations in a subpopulation of cholecystokinin containing interneurons. Neuroscience, 107, 641–652. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, & Mascagni F (2002). Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Research, 943, 237–244. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, & Mascagni F (2006). Differential expression of Kv3.1b and Kv3.2 potassium channel subunits in interneurons of the basolateral amygdala. Neuroscience, 138, 537–547. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, & Mascagni F (2007). Neuronal localization of 5-HT type 2A receptor immunoreactivity in the rat basolateral amygdala. Neuroscience, 146, 306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, & Mascagni F (2010). Neuronal localization of m1 muscarinic receptor immunoreactivity in the rat basolateral amygdala. Brain Structure & Function, 215, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, & Mascagni F (2011). Neuronal localization of m2 muscarinic receptor immunoreactivity in the rat amygdala. Neuroscience, 196, 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, & Augustine JR (1995). Neuropeptide Y and somatostatin-like immunoreactivity in neurons of the monkey amygdala. Neuroscience, 66, 959–982. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, & Guo L (1996). Projections of the medial and lateral prefrontal cortices to the amygdala: A Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience, 71, 55–75. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Mania I, & Rainnie DG (2005). Evidence for a perisomatic innervation of parvalbumin-containing interneurons by individual pyramidal cells in the basolateral amygdala. Brain Research, 1035, 32–40. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, & Zaric V (2012). Subpopulations of somatostatin-immunoreactive non-pyramidal neurons in the amygdala and adjacent external capsule project to the basal forebrain: Evidence for the existence of GABAergic projection neurons in the cortical nuclei and basolateral nuclear complex. Frontiers in Neural Circuits, 6, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, & Mott DD (2017). Functional neuroanatomy of amygdalohippocampal interconnections and their role in learning and memory. Journal of Neuroscience Research, 95, 797–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, & Mascagni F (2002). GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. The Journal of Comparative Neurology, 446, 199–218. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, & Mascagni F (2011). Postsynaptic targets of GABAergic basal forebrain projections to the basolateral amygdala. Neuroscience, 183, 144–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Payne DR, & Mascagni F (1993). Identification of putative nitric oxide producing neurons in the rat amygdala using NADPH-diaphorase histochemistry. Neuroscience, 52, 97–106. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, & Pearson JC (1989). Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basolateral amygdala. Neuroscience Letters, 100, 53–58. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, & Zaric V (2015a). GABAergic somatostatin-immunoreactive neurons in the amygdala project to the entorhinal cortex. Neuroscience, 290, 227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, & Zaric V (2015b). Extrinsic origins of the somatostatin and neuropeptide Y innervation of the rat basolateral amygdala. Neuroscience, 294, 82–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience, 27, 1–28. [DOI] [PubMed] [Google Scholar]
- Mehler WR (1980). Subcortical afferent connections of the amygdala in the monkey. The Journal of Comparative Neurology, 190, 733–762. [DOI] [PubMed] [Google Scholar]
- Meis S, Munsch T, Sosulina L, & Pape HC (2007). Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to cholecystokinin are mediated by a transient receptor potential-like current. Molecular and Cellular Neurosciences, 35, 356–367. [DOI] [PubMed] [Google Scholar]
- Meis S, Sosulina L, Schulz S, Hollt V, & Pape HC (2005). Mechanisms of somatostatin-evoked responses in neurons of the rat lateral amygdala. The European Journal of Neuroscience, 21, 755–762. [DOI] [PubMed] [Google Scholar]
- Mello LE, Tan AM, & Finch DM (1992). Convergence of projections from the rat hippocampal formation, medial geniculate and basal forebrain onto single amygdaloid neurons: An in vivo extra- and intracellular electrophysiological study. Brain Research, 587, 24–40. [DOI] [PubMed] [Google Scholar]
- Melzer S, Michael M, Caputi A, Eliava M, Fuchs EC, Whittington MA, & Monyer H (2012). Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science, 335, 1506–1510. [DOI] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, & Freund TF (1996). Differences between somatic and dendritic inhibition in the hippocampus. Neuron, 16, 815–823. [DOI] [PubMed] [Google Scholar]
- Millhouse OE, & DeOlmos J (1983). Neuronal configurations in lateral and basolateral amygdala. Neuroscience, 10, 1269–1300. [DOI] [PubMed] [Google Scholar]
- Moller LN, Stidsen CE, Hartmann B, & Holst JJ (2003). Somatostatin receptors. Biochimica et Biophysica Acta, 1616, 1–84. [DOI] [PubMed] [Google Scholar]
- Morales M, Wang SD, Diaz-Ruiz O, & Jho DH (2004). Cannabinoid CB1 receptor and serotonin 3 receptor subunit A (5-HT3A) are co-expressed in GABA neurons in the rat telencephalon. The Journal of Comparative Neurology, 468, 205–216. [DOI] [PubMed] [Google Scholar]
- Mori E, Ikeda M, Hirono N, Kitagaki H, Imamura T, & Shimomura T (1999). Amygdalar volume and emotional memory in Alzheimer’s disease. The American Journal of Psychiatry, 156, 216–222. [DOI] [PubMed] [Google Scholar]
- Moryś J, Berdel B, Kowiański P, Majak K, Tarnawski M, & Wisniewski HM (1999). Relationship of calcium-binding protein containing neurons and projection neurons in the rat basolateral amygdala. Neuroscience Letters, 259, 91–94. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM, & Pandya DN (1981). Insular connections with the amygdala in the rhesus monkey. Neuroscience, 6, 1231–1248. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, & McDonald AJ (2003). Synaptic interconnections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. The Journal of Comparative Neurology, 456, 217–236. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, & McDonald AJ (2005). Coupled networks of parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. The Journal of Neuroscience, 25, 7366–7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, & McDonald AJ (2006). Pyramidal cells of the rat basolateral amygdala: Synaptology and innervation by parvalbumin-immunoreactive interneurons. The Journal of Comparative Neurology, 494, 635–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, & McDonald AJ (2007a). Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. The Journal of Comparative Neurology, 500, 513–529. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, & McDonald AJ (2007b). Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. The Journal of Comparative Neurology, 505, 314–335. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, & McDonald AJ (2009). Dopaminergic innervation of pyramidal cells in the rat basolateral amygdala. Brain Structure & Function, 213, 275–288. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, & McDonald AJ (2011). Cholinergic innervation of pyramidal cells and parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. The Journal of Comparative Neurology, 519, 790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, & McDonald AJ (2013). Muscarinic cholinergic receptor M1 in the rat basolateral amygdala: Ultrastructural localization and synaptic relationships to cholinergic axons. The Journal of Comparative Neurology, 521, 1743–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, Zaric V, Mott DD, & McDonald AJ (2016). Localization of the m2 muscarinic cholinergic receptor in dendrites, cholinergic terminals and non-cholinergic terminals in the basolateral amygdala: An ultrastructural analysis. The Journal of Comparative Neurology, 524, 2400–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Faber-Zuschratter H, Yanagawa Y, Stork O, Schwegler H, & Linke R (2012). Synaptology of ventral CA1 and subiculum projections to the basomedial nucleus of the amygdala in the mouse: Relation to GABAergic interneurons. Brain Structure & Function, 217, 5–17. [DOI] [PubMed] [Google Scholar]
- Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, … Tye KM (2015). A circuit mechanism for differentiating positive and negative associations. Nature, 520, 675–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitecka L, & Frotscher M (1989). Organization and synaptic interconnections of GABAergic and cholinergic elements in the rat amygdaloid nuclei: Single and double immunolabeling studies. The Journal of Comparative Neurology, 279, 470–488. [DOI] [PubMed] [Google Scholar]
- Olucha-Bordonau FE, Fortes-Marco L, Otero-García M, Lanuza E, & Martinez-García F (2015). In Paxinos G (Ed.), The rat nervous system (4th ed., pp. 441–490). San Diego: Academic Press. [Google Scholar]
- Omiya Y, Uchigashima M, Konno K, Yamasaki M, Miyazaki T, Yoshida T, … Watanabe M (2015). VGluT3-expressing CCK-positive basket cells construct invaginating synapses enriched with endocannabinoid signaling proteins in particular cortical and cortex-like amygdaloid regions of mouse brains. The Journal of Neuroscience, 35, 4215–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, & Maren S (2011). Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. The Journal of Neuroscience, 31, 17269–17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP (1982). Connections of the amygdala of the rat. IV. Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. The Journal of Comparative Neurology, 205, 30–48. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche L, & McBain CJ (2015). Neurogliaform cells in cortical circuits. Nature Reviews Neuroscience, 16, 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya H, Kawasaki H, Howard MA 3rd, & Adolphs R (2002). Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. The Journal of Neuroscience, 22, 9502–9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulos H, Lange N, Hassinger L, & Berretta S (2006). Subpopulations of neurons expressing parvalbumin in the human amygdala. The Journal of Comparative Neurology, 496, 706–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, & Paré D (2010). Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiological Reviews, 90, 419–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Paré D, & Driesang RB (1998). Two types of intrinsic oscillations in neurons of the lateral and basolateral nuclei of the amygdala. Journal of Neurophysiology, 79, 205–216. [DOI] [PubMed] [Google Scholar]
- Paré D, & Collins DR (2000). Neuronal correlates of fear in the lateral amygdala: Multiple extracellular recordings in conscious cats. The Journal of Neuroscience, 20, 2701–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Collins DR, & Pelletier JG (2002). Amygdala oscillations and the consolidation of emotional memories. Trends in Cognitive Sciences, 6, 306–314. [DOI] [PubMed] [Google Scholar]
- Paré D, Dong J, & Gaudreau H (1995). Amygdalo-entorhinal relations and their reflection in the hippocampal formation: Generation of sharp sleep potentials. The Journal of Neuroscience, 15, 2482–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, & Gaudreau H (1996). Projection cells and interneurons of the lateral and basolateral amygdala: Distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. The Journal of Neuroscience, 16, 3334–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Smith Y, & Paré J (1995). Intra-amygdaloid projections of the basolateral and basomedial nuclei in the cat: Phaseolus vulgaris-leucoagglutinin anterograde tracing at the light and electron microscopic level. Neuroscience, 69, 567–583. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, & Everitt BJ (2000). Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. The European Journal of Neuroscience, 12, 405–413. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, & Salzman CD (2006). The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature, 439, 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelzik H, Hughes DI, & Thomson AM (2002). Physiological and morphological diversity of immunocytochemically defined parvalbumin- and cholecystokinin-positive interneurones in CA1 of the adult rat hippocampus. Journal of Comparative Neurology, 443, 346–367. [DOI] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (1997). The rat brain in stereotaxic coordinates. New York: Academic Press. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, & Swanson LW (2001). Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Research Brain Research Reviews, 38, 247–289. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Risold PY, & Swanson LW (1996). Organization of projections from the basomedial nucleus of the amygdala: A PHAL study in the rat. The Journal of Comparative Neurology, 374, 387–420. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Colago EE, Mania I, Molosh AI, & Rainnie DG (2006). Dopamine D1 receptors co-distribute with N-methyl-D-aspartic acid type-1 subunits and modulate synaptically-evoked N-methyl-D-aspartic acid currents in rat basolateral amygdala. Neuroscience, 142, 671–690. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Rönkkö S, Savander V, Insausti R, & Pitkänen A (1999). Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. The Journal of Comparative Neurology, 403, 229–260. [PubMed] [Google Scholar]
- Pinard CR, Muller JF, Mascagni F, & McDonald AJ (2008). Dopaminergic innervation of interneurons in the rat basolateral amygdala. Neuroscience, 157, 850–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Fuentes C, & Paré D (2006). Feedforward inhibition regulates perirhinal transmission of neocortical inputs to the entorhinal cortex: Ultrastructural study in guinea pigs. The Journal of Comparative Neurology, 495, 722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A (2000). Connectivity of the rat amygdala. In Aggleton JP (Ed.), The amygdala (2nd ed., pp. 31–116). Oxford: Oxford University Press. [Google Scholar]
- Pitkänen A, & Amaral DG (1991). Distribution of reduced nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d) cells and fibers in the monkey amygdaloid complex. The Journal of Comparative Neurology, 313, 326–348. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, & Amaral DG (1993a). Distribution of parvalbumin-immunoreactive cells and fibers in the monkey temporal lobe: The amygdaloid complex. Journal of Comparative Neurology, 331, 14–36. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, & Amaral DG (1993b). Distribution of calbindin-D28k immunoreactivity in the monkey temporal lobe: The amygdaloid complex. The Journal of Comparative Neurology, 331, 199–224. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, & Amaral DG (1994). The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: An immunohistochemical and in situ hybridization study. The Journal of Neuroscience, 14, 2200–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, & Amaral DG (1998). Organization of the intrinsic connections of the monkey amygdaloid complex: Projections originating in the lateral nucleus. The Journal of Comparative Neurology, 398, 431–458. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, & Ylinen A (2000). Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. 911, 369–391. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander M, Nurminen N, & Ylinen A (2003). Intrinsic synaptic circuitry of the amygdala. Annals of the New York Academy of Sciences, 985, 34–49. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, & LeDoux JE (1997). Organization of intra-amygdaloid circuitries in the rat: An emerging framework for understanding functions of the amygdala. Trends in Neurosciences, 20, 517–523. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Stefanacci L, Farb CR, Go GG, LeDoux JE, & Amaral DG (1995). Intrinsic connections of the rat amygdaloid complex: Projections originating in the lateral nucleus. The Journal of Comparative Neurology, 356, 288–310. [DOI] [PubMed] [Google Scholar]
- Power JM, & Sah P (2008). Competition between calcium-activated K+ channels determines cholinergic action on firing properties of basolateral amygdala projection neurons. The Journal of Neuroscience, 28, 3209–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Russchen FT, & Amaral DG (1987). The limbic region. II. The amygdaloid complex. In Bjorklund A, Hokfelt T, & Swanson LW (Eds.), Vol. 5. Handbook of chemical neuroanatomy (pp. 279–388). Amsterdam: Elsevier Science Publishers. [Google Scholar]
- Price JL, & Slotnick BM (1983). Dual olfactory representation in the rat thalamus: An anatomical and electrophysiological study. The Journal of Comparative Neurology, 215, 63–77. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, & McGaugh JL (1998). Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Research, 808, 134–140. [DOI] [PubMed] [Google Scholar]
- Rainnie DG (1999). Serotonergic modulation of neurotransmission in the rat basolateral amygdala. Journal of Neurophysiology, 82, 69–85. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, & Shinnick-Gallagher P (1993). Intracellular recordings from morphologically identified neurons of the basolateral amygdala. Journal of Neurophysiology, 69, 1350–1361. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Mania I, Mascagni F, & McDonald AJ (2006). Physiological and morphological characterization of parvalbumin-containing interneurons of the rat basolateral amygdala. The Journal of Comparative Neurology, 498, 142–161. [DOI] [PubMed] [Google Scholar]
- Ray JP, Russchen FT, Fuller TA, & Price JL (1992). Sources of presumptive glutamatergic/aspartatergic afferents to the mediodorsal nucleus of the thalamus in the rat. The Journal of Comparative Neurology, 320, 435–456. [DOI] [PubMed] [Google Scholar]
- Reppucci CJ, & Petrovich GD (2016). Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: A single and double retrograde tracing study in rats. Brain Structure & Function, 221, 2937–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhomberg T, Rovira-Esteban L, Vikór A, Paradiso E, Kremser C, Nagy-Pál P, … Hájos N (2018). VIP-immunoreactive interneurons within circuits of the mouse basolateral amygdala. The Journal of Neuroscience, 38, 6983–7003. 10.1523/JNEUROSCI.2063-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold PY, Canteras NS, & Swanson LW (1994). Organization of projections from the anterior hypothalamic nucleus: A Phaseolus vulgaris-leucoagglutinin study in the rat. The Journal of Comparative Neurology, 348, 1–40. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Stäubli UV, & LeDoux JE (1997). Fear conditioning induces associative long-term potentiation in the amygdala. Nature, 390(6660), 604–607. [DOI] [PubMed] [Google Scholar]
- Romanski LM, & LeDoux JE (1992). Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. The Journal of Neuroscience, 12, 4501–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, & LeDoux JE (1993). Information cascade from primary auditory cortex to the amygdala: Corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cerebral Cortex, 3, 515–532. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Hitchcock JM, Miserendino MJ, Falls WA, Campeau S, & Davis M (1992). Lesions of the perirhinal cortex but not of the frontal, medial prefrontal, visual, or insular cortex block fear-potentiated startle using a visual conditioned stimulus. The Journal of Neuroscience, 12, 4624–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, & Grace AA (1999). Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. The Journal of Neuroscience, 19, 11027–11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, & Grace AA (2003). Affective conditioning in the basolateral amygdala of anesthetized rats is modulated by dopamine and prefrontal cortical inputs. Annals of the New York Academy of Sciences, 985, 488–491. [DOI] [PubMed] [Google Scholar]
- Rovira-Esteban L, Péterfi Z, Vikór A, Máté Z, Szabó G, & Hájos N (2017). Morphological and physiological properties of CCK/CB1R-expressing interneurons in the basal amygdala. Brain Structure & Function, 222, 3543–3565. [DOI] [PubMed] [Google Scholar]
- Rueter LE, & Jacobs BL (1996). A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Research, 739, 57–69. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Amaral DG, & Price JL (1985). The afferent connections of the substantia innominata in the monkey, Macaca fascicularis. The Journal of Comp Neurology, 242, 1–27. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Amaral DG, & Price JL (1987). The afferent input to the magnocellular division of the mediodorsal thalamic nucleus in the monkey, Macaca fascicularis. The Journal of Comparative Neurology, 256, 175–210. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Bakst I, Amaral DG, & Price JL (1985). The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Research, 329, 241–257. [DOI] [PubMed] [Google Scholar]
- Russchen FT, & Price JL (1984). Amygdalostriatal projections in the rat. Topographical organization and fiber morphology shown using the lectin PHA-L as an anterograde tracer. Neuroscience Letters, 47, 15–22. [DOI] [PubMed] [Google Scholar]
- Ryan SJ, Ehrlich DE, Jasnow AM, Daftary S, Madsen TE, & Rainnie DG (2012). Spike-timing precision and neuronal synchrony are enhanced by an interaction between synaptic inhibition and membrane oscillations in the amygdala. PLoS One, 7, e35320. 10.1371/journal.pone.0035320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikot AF, & Parent A (1990). The monoaminergic innervation of the amygdala in the squirrel monkey: An immunohistochemical study. Neuroscience, 36, 431–447. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, & Power J (2003). The amygdaloid complex: Anatomy and physiology. Physiological Reviews, 83, 803–834. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Johnson PL, Leitermann R, Fitz SD, Dietrich A, Morin M, … Shekhar A (2008). Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. The Journal of Neuroscience, 28, 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Vandergriff MG, & Gehlert DR (1999). Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. European Journal of Pharmacology, 368, 143–147. [DOI] [PubMed] [Google Scholar]
- Sananes CB, & Davis M (1992). N-methyl-D-aspartate lesions of the lateral and basolateral nuclei of the amygdala block fear-potentiated startle and shock sensitization of startle. Behavioral Neuroscience, 106, 72–80. [DOI] [PubMed] [Google Scholar]
- Savander V, Go CG, LeDoux JE, & Pitkänen A (1995). Intrinsic connections of the rat amygdaloid complex: Projections originating in the basal nucleus. The Journal of Comparative Neurology, 361, 345–368. [DOI] [PubMed] [Google Scholar]
- Savander V, Go CG, Ledoux JE, & Pitkänen A (1996). Intrinsic connections of the rat amygdaloid complex: Projections originating in the accessory basal nucleus. The Journal of Comparative Neurology, 374, 291–313. [DOI] [PubMed] [Google Scholar]
- Scalia F, & Winans SS (1975). The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. The Journal of Comparative Neurology, 161, 31–55. [DOI] [PubMed] [Google Scholar]
- Schwaller B, Meyer M, & Schiffmann S (2002). ‘New’ functions for ‘old’ proteins: The role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum, 1, 241–258. [DOI] [PubMed] [Google Scholar]
- Schwartzberg M, Unger J, Weindl A, & Lange W (1990). Distribution of neuropeptide Y in the prosencephalon of man and cotton-head tamarin (Saguinus oedipus): Colocalization with somatostatin in neurons of striatum and amygdala. Anatomy and Embryology (Berlin), 181, 157–166. [DOI] [PubMed] [Google Scholar]
- See RE, McLaughlin J, & Fuchs RA (2003). Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine-stimulus association in a model of relapse to cocaine-seeking behavior in rats. Neuroscience, 117, 477–483. [DOI] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, & Pape HC (2003). Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science, 301, 846–850. [DOI] [PubMed] [Google Scholar]
- Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Gründemann J, … Lüthi A (2014). Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron, 81, 428–437. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, & Mintun MA (2001). Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biological Psychiatry, 50, 651–658. [DOI] [PubMed] [Google Scholar]
- Shi CJ, & Cassell MD (1998a). Cascade projections from somatosensory cortex to the rat basolateral amygdala via the parietal insular cortex. The Journal of Comparative Neurology, 399, 469–491. [DOI] [PubMed] [Google Scholar]
- Shi CJ, & Cassell MD (1998b). Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. The Journal of Comparative Neurology, 399, 440–468. [DOI] [PubMed] [Google Scholar]
- Shi C-J, & Cassell MD (1997). Cortical, thalamic and amygdaloid projections of rat temporal cortex. The Journal of Comparative Neurology, 382, 153–175. [PubMed] [Google Scholar]
- Silveira Villarroel H, Bompolaki M, Mackay JP, Miranda Tapia AP, Michaelson SD, Leitermann RJ, … Colmers WF (2018). NPY Induces stress resilience via downregulation of Ih in principal neurons of rat basolateral amygdala. The Journal of Neuroscience, 38, 4505–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A, Oláh S, Molnár G, Szabadics J, & Tamás G (2005). Gap-junctional coupling between neurogliaform cells and various interneuron types in the neocortex. The Journal of Neuroscience, 5, 6278–6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, & Paré D (1994). Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with postembedding GABA and glutamate immunocytochemistry. The Journal of Comparative Neurology, 342, 232–248. [DOI] [PubMed] [Google Scholar]
- Smith Y, Paré JF, & Paré D (2000). Differential innervation of parvalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. The Journal of Comparative Neurology, 416, 496–508. [PubMed] [Google Scholar]
- Sorvari H, Miettinen R, Soininen H, Paljärvi L, Karkola K, & Pitkänen A (1998). Calretinin-immunoreactive terminals make synapses on calbindin D28k-immunoreactive neurons in the lateral nucleus of the human amygdala. Brain Research, 783, 355–358. [DOI] [PubMed] [Google Scholar]
- Sorvari H, Miettinin R, Soininen H, & Pitkänen A (1996). Parvalbumin-immunoreactive neurons make inhibitory synapses on pyramidal cells in the human amygdala: A light and electron microscopic study. Neuroscience Letters, 217, 93–96. [PubMed] [Google Scholar]
- Sorvari H, Soininen H, Paljärvi L, Karkola K, & Pitkänen A (1995). Distribution of parvalbumin-immunoreactive cells and fibers in the human amygdaloid complex. The Journal of Comparative Neurology, 360, 185–212. [DOI] [PubMed] [Google Scholar]
- Sorvari H, Soininen H, & Pitkänen A (1996). Calretinin-immunoreactive cells and fibers in the human amygdaloid complex. The Journal of Comparative Neurology, 369, 188–208. [DOI] [PubMed] [Google Scholar]
- Sosulina L, Graebenitz S, & Pape HC (2010). GABAergic interneurons in the mouse lateral amygdala: A classification study. Journal of Neurophysiology, 104, 617–626. [DOI] [PubMed] [Google Scholar]
- Sosulina L, Meis S, Seifert G, Steinhauser C, & Pape HC (2006). Classification of projection neurons and interneurons in the rat lateral amygdala based upon cluster analysis. Molecular and Cellular Neurosciences, 33, 57–67. [DOI] [PubMed] [Google Scholar]
- Sosulina L, Schwesig G, Seifert G, & Pape HC (2008). Neuropeptide Y activates a G-protein-coupled inwardly rectifying potassium current and dampens excitability in the lateral amygdala. Molecular and Cellular Neurosciences, 39, 491–498. [DOI] [PubMed] [Google Scholar]
- Spampanato J, Polepalli J, & Sah P (2011). Interneurons in the basolateral amygdala. Neuropharmacology, 60, 765–773. [DOI] [PubMed] [Google Scholar]
- Squire LR, & Zola-Morgan S (1991). The medial temporal lobe memory system. Science, 253, 1380–1386. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Farb CR, Pitkänen A, Go G, LeDoux JE, & Amaral DG (1992). Projections from the lateral nucleus to the basal nucleus of the amygdala: A light and electron microscopic PHA-L study in the rat. Journal of Comparative Neurology, 323, 586–601. [DOI] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, & Moser EI (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews. Neuroscience, 15, 655–669. [DOI] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Sakmann B, & Hausser M (1997). Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends in Neurosciences, 120, 125–131. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, … Bonci A (2011). Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature, 475, 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE, & LeDoux JE (1999). GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: A mechanism for modulation of sensory inputs related to fear conditioning. The Journal of Neuroscience, 19, RC8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sved AF, Cano G, Passerin AM, & Rabin BS (2002). The locus coeruleus, Barrington’s nucleus, and neural circuits of stress. Physiology & Behavior, 77, 737–742. [DOI] [PubMed] [Google Scholar]
- Swanson LW (2000). Cerebral hemisphere regulation of motivated behavior. Brain Research, 886, 113–164. [DOI] [PubMed] [Google Scholar]
- Timbie C, & Barbas H (2015). Pathways for emotions: Specializations in the amygdalar, mediodorsal thalamic, and posterior orbitofrontal network. The Journal of Neuroscience, 35, 11976–11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Blumenfeld B, Wu C, Luo J, Attali B, Goodman P, & Markram H (2004). Correlation maps allow neuronal electrical properties to be predicted from single-cell gene expression profiles in rat neocortex. Cerebral Cortex, 14, 1310–13127. [DOI] [PubMed] [Google Scholar]
- Tőmböl T, & Szafranska-Kosmal A (1972). A Golgi study of the amygdaloid complex in the cat. Acta Neurobiologiae Experimentalis, 32, 835–848. [PubMed] [Google Scholar]
- Tovote P, Fadok JP, & Lüthi A (2015). Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience, 16, 317–331. [DOI] [PubMed] [Google Scholar]
- Trouche S, Sasaki JM, Tu T, & Reijmers LG (2013). Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron, 80, 1054–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt WA, Johnson PL, Dietrich AD, Fitz SD, & Shekhar A (2009). Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience, 160, 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BH, & Herkenham M (1991). Thalamoamygdaloid projections in the rat: A test of the amygdala’s role in sensory processing. The Journal of Comparative Neurology, 313, 295–325. [DOI] [PubMed] [Google Scholar]
- Turner BH, Mishkin M, & Knapp M (1980). Organization of the amygdalopetal projections from modality-specific cortical association areas in the monkey. The Journal of Comparative Neurology, 191, 515–543. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Halonen T, & Pitkänen A (1996). Status epilepticus causes selective regional damage and loss of GABAergic neurons in the rat amygdaloid complex. The European Journal of Neuroscience, 8, 2711–2725. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Halonen T, & Pitkänen A (1997). Decrease in somatostatin-immunoreactive neurons in the rat amygdaloid complex in a kindling model of temporal lobe epilepsy. Epilepsy Research, 26, 315–327. [DOI] [PubMed] [Google Scholar]
- Unal G, Paré JF, Smith Y, & Paré D (2014). Cortical inputs innervate calbindin-immunoreactive interneurons of the rat basolateral amygdaloid complex. The Journal of Comparative Neurology, 522, 1915–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, & Mishkin M (1982). Two cortical visual systems. In Ingle DJ, Goodale MA, & Mansfeld RJW (Eds.), Analysis of visual behavior (pp. 549–586). Cambridge: MIT Press. [Google Scholar]
- Uwano T, Nishijo H, Ono T, & Tamura R (1995). Neuronal responsiveness to various sensory stimuli, and associative learning in the rat amygdala. Neuroscience, 68, 339–361. [DOI] [PubMed] [Google Scholar]
- Van Haeften T, Wouterlood FG, Jorritsma-Byham B, & Witter MP (1997). GABAergic presubicular projections to the medial entorhinal cortex of the rat. The Journal of Neuroscience, 17, 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoesen GW (1981). The differential distribution, diversity and sprouting of cortical projections to the amygdala in the rhesus monkey. In Ben-Ari Y (Ed.), The amygdaloid complex (pp. 77–90). Amsterdam: Elsevier. [Google Scholar]
- Vazdarjanova A, & McGaugh JL (1999). Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning. The Journal of Neuroscience, 19, 6615–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereczki VK, Veres JM, Müller K, Nagy GA, Rácz B, Barsy B, & Hájos N (2016). Synaptic organization of perisomatic GABAergic inputs onto the principal cells of the mouse basolateral amygdala. Frontiers in Neuroanatomy, 10, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veres JM, Nagy GA, & Hájos N (2017). Perisomatic GABAergic synapses of basket cells effectively control principal neuron activity in amygdala networks. Elife, 6. 10.7554/eLife.20721, pii: e20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veres JM, Nagy GA, Vereczki VK, Andrási T, & Hájos N (2014). Strategically positioned inhibitory synapses of axo-axonic cells potently control principal neuron spiking in the basolateral amygdala. The Journal of Neuroscience, 34, 16194–161206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Crane AM, Colom LV, & Bland BH (1995). Ascending projections of the posterior nucleus of the hypothalamus: PHA-L analysis in the rat. The Journal of Comparative Neurology, 359, 90–116. [DOI] [PubMed] [Google Scholar]
- Vertes RP, & Hoover WB (2008). Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. Journal of Comparative Neurology, 508, 212–237. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, Sherman A, & Rodriguez JJ (2006). Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. Journal of Comparative Neurology, 499, 768–796. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, & Rodriguez JJ (2012). Projections of the central medial nucleus of the thalamus in the rat: Node in cortical, striatal and limbic forebrain circuitry. Neuroscience, 219, 120–136. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, & Hoover WB (2015). Limbic circuitry of the midline thalamus. Neuroscience and Biobehavioral Reviews, 54, 89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F, & Hille B (1996). Modulation of high voltage-activated calcium channels by somatostatin in acutely isolated rat amygdaloid neurons. The Journal of Neuroscience, 16, 6000–6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel E, Krabbe S, Gründemann J, Wamsteeker Cusulin JI, & Lüthi A (2016). Projection-specific dynamic regulation of inhibition in amygdala micro-circuits. Neuron, 91, 644–651. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Eliava M, Meyer AH, Rozov A, & Monyer H (2007). Functional characterization of intrinsic cholinergicinterneurons in the cortex. The Journal of Neuroscience, 27, 5633–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil M, Jefferys JG, Celio MR, & Schwaller B (2003). Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. Journal of Neurophysiology, 89, 1414–1422. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, & Davis M (2009). Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 33, 1291–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu C, Wang X, Gao F, & Zhan RZ (2017). Density and neurochemical profiles of neuronal nitric oxide synthase-expressing interneuron in the mouse basolateral amygdala. Brain Research, 1663, 106–113. [DOI] [PubMed] [Google Scholar]
- Washburn MS, & Moises HC (1992a). Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. The Journal of Neuroscience, 12, 4066–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, & Moises HC (1992b). Muscarinic responses of rat basolateral amygdaloid neurons recorded in vitro. The Journal of Physiology, 449, 121–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiszkrantz L (1956). Behavioral changes associated with ablation of the amygdaloid complex in monkeys. Journal of Comparative and Physiological Psychology, 49, 381–391. [DOI] [PubMed] [Google Scholar]
- Wendling F, Bartolomei F, Bellanger JJ, & Chauvel P (2002). Epileptic fast activity can be explained by a model of impaired GABAergic dendritic inhibition. The European Journal of Neuroscience, 15, 1499–1508. [DOI] [PubMed] [Google Scholar]
- Whittington MA, & Traub RD (2003). Interneuron diversity series: Inhibitory interneurons and network oscillations in vitro. Trends in Neurosciences, 26, 676–682. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, & Jefferys JG (1995). Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature, 373, 612–615. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, & Lohman AH (1989). Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Progress in Neurobiology, 33, 161–253. [DOI] [PubMed] [Google Scholar]
- Wolff SB, Gründemann J, Tovote P, Krabbe S, Jacobson GA, Müller C, … Lüthi A (2014). Amygdala interneuron subtypes control fear learning through disinhibition. Nature, 509, 453–458. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, & Sah P (2007a). Networks of parvalbumin-positive interneurons in the basolateral amygdala. The Journal of Neuroscience, 27, 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, & Sah P (2007b). Inhibition and synchronization of basal amygdala principal neuron spiking by parvalbumin-positive interneurons. Journal of Neurophysiology, 98, 2956–29561. [DOI] [PubMed] [Google Scholar]
- Wright CI, Beijer AV, & Groenewegen HJ (1996). Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. The Journal of Neuroscience, 16, 1877–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, & Groenewegen HJ (1995). Patterns of convergence and segregation in the medial nucleus accumbens of the rat: Relationships of prefrontal cortical, midline thalamic, and basal amygdaloid afferents. The Journal of Comparative Neurology, 361, 383–403. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ (1979). Neurobehavioral evidence for the involvement of the vomeronasal system in mammalian reproduction. Neuroscience and Biobehavioral Reviews, 3, 301–341. [DOI] [PubMed] [Google Scholar]
- Yajeya J, de la Fuente Juan A, Merchan MA, Riolobos AS, Heredia M, & Criado JM (1997). Cholinergic responses of morphologically and electrophysiologically characterized neurons of the basolateral complex in rat amygdala slices. Neuroscience, 78, 731–743. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, & Cechetto DF (1991). Autonomic responses and efferent pathways from the insular cortex in the rat. The Journal of Comparative Neurology, 303, 355–374. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Itoh K, Sugimoto T, Kaneko T, & Mizuno N (1987). Thalamocortical and thalamo-amygdaloid projections from the parvicellular division of the posteromedial ventral nucleus in the cat. The Journal of Comparative Neurology, 257, 253–268. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, & Van Hoesen GW (1978). Cortico-striate projections in the rhesus monkey: The organization of certain cortico-caudate connections. Brain Research, 139, 43–63. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Uchigashima M, Yamasaki M, Katona I, Yamazaki M, Sakimura K, … Watanabe M (2011). Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proceedings of the National Academy of Sciences of the United States of America, 108, 3059–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Záborszky L, Heimer L, Eckenstein F, & Leranth C (1986). GABAergic input to cholinergic forebrain neurons: An ultrastructural study using retrograde tracing of HRP and double immunolabeling. The Journal of Comparative Neurology, 250, 282–295. [DOI] [PubMed] [Google Scholar]
- Záborszky L, Léránth C, & Heimer L (1984). Ultrastructural evidence of amygdalofugal axons terminating on cholinergic cells of the rostral forebrain. Neuroscience Letters, 52, 219–225. [DOI] [PubMed] [Google Scholar]
- Zhang J, Muller JF, & McDonald AJ (2013). Noradrenergic innervation of pyramidal cells in the rat basolateral amygdala. Neuroscience, 228, 395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Stewart RR, McIntosh JM, & Weight FF (2005). Activation of nicotinic acetylcholine receptors increases the frequency of spontaneous GABAergic IPSCs in rat basolateral amygdala neurons. Journal of Neurophysiology, 94, 3081–3091. [DOI] [PubMed] [Google Scholar]


