Abstract
Rationale
Recent studies have discovered several unique tumor response subgroups outside of response classification by Response Evaluation Criteria for Solid Tumors (RECIST), such as mixed response and oligometastasis. These subtypes have a distinctive property, lesion heterogeneity defined as diversity of tumor growth profiles in RECIST target lesions. Furthermore, many cancer clinical trials have been activated to evaluate various treatment options for heterogeneity-related subgroups (e.g., 29 trials so far listed in clinicaltrials.gov for cancer patients with oligometastasis). Some of the trials have shown survival benefit by tailored treatment strategies. This evidence presents the unmet need to incorporate lesion heterogeneity to improve RECIST response classification.
Method
An approach for Lesion Heterogeneity Classification (LeHeC) was developed using a contemporary statistical approach to assess target lesion variation, characterize patient treatment response, and translate informative evidence to improving treatment strategy. A mixed effect linear model was used to determine lesion heterogeneity. Further analysis was conducted to classify various types of lesion variation and incorporate with RECIST to enhance response classification. A study cohort of 110 target lesions from 36 lung cancer patients was used for evaluation.
Results
Due to small sample size issue, the result was exploratory in nature. By analyzing RECIST target lesion data, the LeHeC approach detected a high prevalence (n = 21; 58%) of lesion heterogeneity. Subgroup classification revealed several informative distinct subsets in a descending order of lesion heterogeneity: mix of progression and regression (n = 7), mix of progression and stability (n = 9), mix of regression and stability (n = 5), and non-heterogeneity (n = 15). Evaluation for association of lesion heterogeneity and RECIST best response classification showed lesion heterogeneity commonly occurred in each response group (stable disease: 16/27; 59%; partial response: 3/5; 60%; progression disease: 2/4; 50%). Survival analysis showed a differential trend of overall survival between heterogeneity and non-heterogeneity in RECIST response groups.
Conclusion
This is the first study to evaluate lesion heterogeneity, an underappreciated metric, for RECIST application in oncology clinical trials. Results indicated lesion heterogeneity is not an uncommon event. The LeHeC approach could enhance RECIST response classification by utilizing granular lesion level discovery of heterogeneity.
Background
Rationale for incorporation of lesion heterogeneity to evaluate treatment responses in oncology clinical trials
As a standard tool to assess treatment efficacy in oncology clinical trials, Response Evaluation Criteria for Solid Tumors (RECIST) has helped advance cancer treatment, such as chemotherapy [1–4], targeted therapy [5–10], immunotherapy [11–17], or combinations of these [14, 15, 18–22]. In parallel, recent studies have discovered several unique tumor response subgroups outside of RECIST response classification, such as mixed response [23–26], oligometastasis [27–30], and pseudo-progression [31–33]. Patients in these subgroups often need special clinical attention to adapt treatment due to different reactions to the drugs of interest. These subtypes have a distinctive property, lesion heterogeneity defined as diversity of tumor growth profiles in RECIST target lesions. Moreover, some types of lesion heterogeneity were found to be associated with improved survival outcome by tailored treatment strategy [25, 27, 29, 34–38] in various cancers. For example, mixed responder to immunotherapy in metastatic melanoma had improved overall survival (OS) by surgical treatment [36]. Similarly, patients with oligometastatic lung or prostate cancer had longer progression-free survival or OS after stereotactic ablative radiation [27, 29, 35].
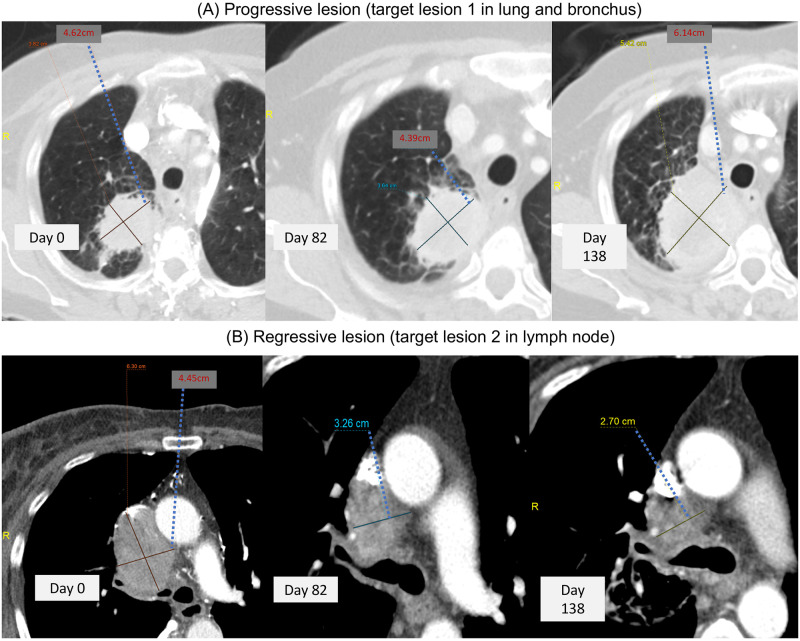
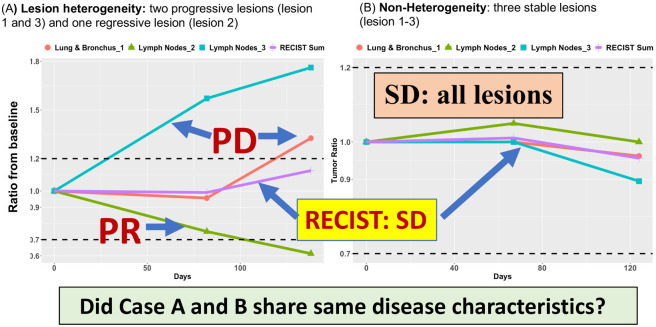
In addition, RECIST uses sum of all target lesions as overall tumor burden to determine treatment efficacy based on naïve assumption of lesion homogeneity. Such strategy significantly underestimates individual lesion variability of tumor growth [31, 32, 39, 40]. While many studies attempted to reform RECIST, their methodology still relied on RECIST’s aggregated sum to describe patient treatment response [41–44], including immune-related response criteria for immunotherapy trials (e.g., irRC [45], irRECIST [46], iRECIST [45], or imRECIST [33]). Figs 1 and 2 highlight limits of evaluating lesion heterogeneity by RECIST. Fig 1A and 1B showed CT images of two lesions with completely different response outcome in Case A: progression versus regression, indicating substantial variation of tumor growth among lesions. Fig 2 presented two contrastive cases with one in high degree of lesion heterogeneity (Case A with two progressive lesions and one regressive lesion: Fig 2A) and the other with homogenous lesions (Case B with three stable lesions: Fig 2B). RECIST classifies both cases as stable disease (SD). However, Case A showed opposite response among lesions, indicating potentially different disease and clinical characteristics from Case B. All these evidences (uniqueness of the heterogeneity, associated improvement of clinical outcomes, and limitations of RECIST) present the significant unmet need to incorporate lesion heterogeneity to improve RECIST.
Fig 1. Lesion heterogeneity in CT image for Case A.
(A): Illustrative image data of a progressive lesion for Case A. The lesion was target lesion 1 in lung and bronchus from three time points and demonstrated eventual progression of disease by RECIST criteria. The tumor growth pattern was also displayed in Fig 2A in black color dashed line. (B): Illustrative image data of a regressive lesion for Case A. The lesion was target lesion 2 in lymph node at three timepoints and showed continued decrease in size of lesion consistent with response to treatment. The tumor growth pattern was also displayed in Fig 2B in red color dashed line.
Fig 2.
(A): Case A had three lesions with two in lymph node and one in lung site. In the lymph node site, one lesion had at least 30% reduction (ratio<0.7), but the other lesion progressed with more than 60% increase (ratio>1.6). The lesion in the lung site also increased the tumor size about 30%. Results showed completely different treatment reaction in each lesion with one regressive lesion and one progressive lesion in the lymph node and one progressive lesion in the lung site, indicating significant variation of lesion growth pattern within and between organ sites. However, the RECIST sum averaged these three heterogeneous lesions and classified as SD, suggesting all lesions were under control with no change of tumor growth. (B): In contrast, case B had also three lesions with two in lymph node and one in lung site. All lesion sizes remained relatively stable over time with minor changes from the baseline. The RECIST sum took an average of these three homogeneous lesions and also classified as SD. In comparison of both cases, while they reached the same SD classification, lesion variation to treatment reaction was quite different. Lesions in case B were comparable and did not progress or regress. On the other hand, case A had diverse tumor growth patterns composed of progressive and regressive lesions. While the RECIST sum might well characterize the stable condition in case B, classification of SD in case A seriously distorted lesion variation and could misguide clinical decision.
Methods
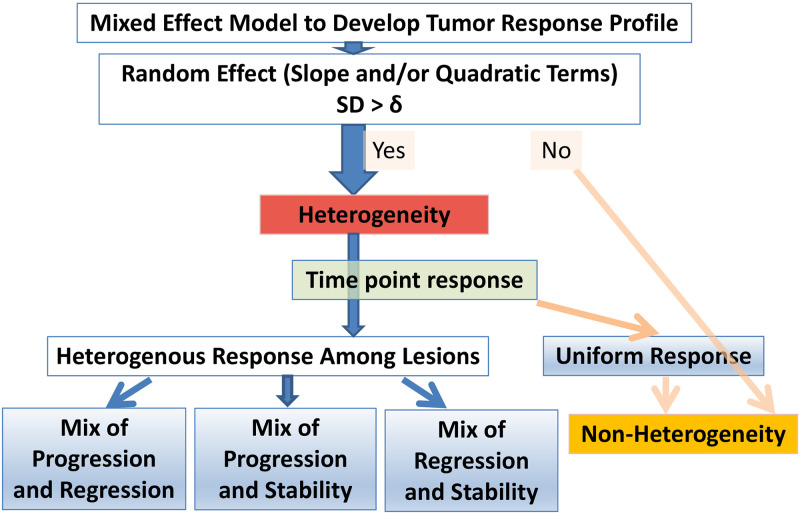
This study takes advantage of target lesion level data, incorporates contemporary statistical method to assess lesion heterogeneity, and provides distinct subgroups to enrich RECIST response classification. Specifically, our approach for Lesion Heterogeneity Classification (LeHeC) utilizes multiple strategies to assess lesion heterogeneity (Fig 3): (1) mixed effect model to characterize tumor growth and determine lesion heterogeneity, (2) modified RECIST time point response to classify heterogeneity-associated subgroups, and (3) mean of squared deviations to evaluate classification performance.
Fig 3. LeHeC algorithm.
Mixed effect model for assessment of lesion heterogeneity
Statistically, a mixed effect regression model of lesion growths for a patient conducts two types of analysis: fixed effect and random effect. The fixed effect depicts overall tumor growth (mean function), similar to the RECIST sum metric. In contrast, the random effect component describes individual lesion’s deviation from the overall pattern and uses standard deviation (SD*) to summarize the deviation for growth parameters (e.g., linear slope or quadratic curve). Intuitively, when lesions grow diversely, the corresponding SD* will become large as shown in S1 Fig in S1 File. Therefore, SD* becomes a natural indicator of lesion heterogeneity. Strategically, lesion size from CT image is standardized and converted into ratio using the 1st CT scan data as the baseline in each lesion. The logarithm of ratio is then used for analysis in the mixed effect model. The derived SD*s from the random components in linear slope or quadratic term are used to determine lesion heterogeneity (S1 Fig in S1 File). If a patient has the largest estimated SD*s above a threshold, δ, lesion heterogeneity is claimed initially.
Modified RECIST time point response for subgroup classification
Once patients with lesion heterogeneity are identified (i.e., SD*>δ), they will be further classified based on characteristics of lesion variation using a modified RECIST response classification. Specifically, the modified RECIST response classification defines time point response as follows (S2 Fig in S1 File): (a) progression for a ratio > 1.2 (equivalent to 20% increase), (b) regression for a ratio <0.7 (equivalent to 30% reduction), and (c) stability for a ratio between 0.7 and 1.2. Each lesion thus has a set of time point responses to describe the degree of change in tumor size (e.g., stability in CT scan 1 and 2 and progression in CT scan 3). This strategy could address slow tumor progression issue [31] encountered in RECIST. Four heterogeneity-associated subgroups are defined based on comparison of time point responses among all lesions within a patient: (1) mix of progression and regression if lesions experience at least one event of progression and one event of regression. Mixed response and pseudo-progression are in this category because mixed response by definition is combination of progressive and regressive lesions while pseudo-progression has early progression event with follow-up of regression event due to treatment effect (e.g., immunotherapy); (2) mix of progression and stability if lesions experience at least one event of progression and one event of stability, and without regression event. Oligometastasis belongs to this category because of its unique property with majority of stable lesions and few progressive lesions; (3) mix of regression and stability if lesions develop at least one event of regression and one event of stability, and without progression event; and (4) non-heterogeneity if all lesions had stability event. Patients with SD*<δ are also considered as non-heterogeneity.
Mean of squared deviations (MSD) for classification performance
A set of MSD metrics are used to evaluate classification performance for the LeHeC approach in three aspects, deviation from RECIST sum, outlier detection, and model goodness of fit.
Deviation from RECIST sum (S3 Fig in S1 File): When lesion growth patterns within a patient are largely different from the RECIST sum, degree of lesion heterogeneity will be high accordingly. Thus, deviation from the RECIST sum could be a natural metric to evaluate classification performance for lesion heterogeneity. Our strategy is to compare log ratio of each lesion to the one in the RECIST sum. Difference between lesions and the RECIST sum will be squared and averaged, denoted as MSD(RECIST), to assess magnitude of lesion heterogeneity. We expect the largest MSD(RECIST) in patients with a mix of progressive and regressive lesions, followed by the subgroup with a mix of progressive and stable lesions, the subgroup with mixed lesions of regression and stability, and the non-heterogeneity subgroup in a descending order of MSD(RECIST). We also evaluate MSD in the mixed effect model by measuring deviation away from the mean function and denoted as MSD(model). We consider that the mean function of the mixed effect model is able to resemble the RECIST sum and expect MSD(model) to be comparable to MSD(RECIST).
Detection of outlier lesion (S4 Fig in S1 File): Another metric to examine lesion heterogeneity is number of lesions with substantial deviation from the model mean function (i.e., frequency of outlier lesions). A lesion level of mean squared deviations, MSD(model_lesion), is used to determine outlier lesion. It compares log ratio between a lesion and the model mean function at each time point. The difference is squared and averaged, then defined as MSD(model_lesion). A large value of MSD(model_lesion) indicates the lesion has a different tumor growth pattern and will be considered as an outlier lesion if it exceeds a cutoff, γ.
Model goodness of fit (S5 Fig in S1 File): To ensure the model fits well within the lesion level data in the heterogeneity-associated subgroups, a quasi R2 is used to measure goodness of fit and defined as where MSD(lesion) is calculated by the mean of squared differences across each time between predicted lesion curves and the observed lesion curves in a patient. A value of close to 1 indicates good fit of data while a near 0 value implies poor prediction.
Statistical analysis
Evaluation of lesion heterogeneity was performed by two-sample t test, one-way analysis of variance (ANOVA), and Dunnett test to compare the heterogeneity group or subgroups to the non-heterogeneity group. Survival curve was generated by Kaplan-Meier (KM) method. The log-rank test was then used to evaluate survival difference due to heterogeneity in each RECIST response group.
Study cohort
This retrospective study was derived from a research protocol (MCC20369 approved by Scientific Review Committee and IRB exempt) at the Moffitt Cancer Center and Research Institute. Data used for the study was from 36 late stage lung cancer patients receiving immunotherapy at the institute between October 2015 and July 2018. The demographic characteristics of patients were presented in Table 1. Distribution of RECIST best overall response was 5 partial responses (PR), 27 stable diseases (SD), and 4 progression diseases (PD). A total of 110 target lesions from the 36 patients were collected. Tumor measurements of these lesions were calculated per RECIST from CT scans for data analysis. Distribution of CT scans were 50% with 3 scans (n = 18), and 50% with 4 scans or more (n = 18). For lesion frequency per patient, there were 33% patients with 2 lesions (n = 12), 33% patients with 3 lesions (n = 12), 28% patients with 4 lesions (n = 10), and 6% patients with 5 lesions (n = 2). Data and R code were provided in S1 File.
Table 1. Demographic characteristics of 36 patients with stage 4 NSCLC.
| Baseline and clinical variable | N (%) | |
|---|---|---|
| Gender | Female | 15 (42%) |
| Male | 21 (58%) | |
| Race/Ethnicity | Non-Hispanic White | 28 (78%) |
| Hispanic | 4 (11%) | |
| Black | 2 (6%) | |
| Asian | 1 (3%) | |
| Unknown | 1 (3%) | |
| Smoking history | Current smoker | 2 (6%) |
| Previous smoker | 11 (31%) | |
| Never | 4 (11%) | |
| Unknown | 19 (53%) | |
| Best overall response | PR | 5 (14%) |
| SD | 27 (75%) | |
| PD | 4 (11%) | |
| OS | Dead | 28 (78%) |
| Alive | 8 (22%) | |
| Median (range) | ||
| Follow-up time | 10.41 months (2.01–36.26) | |
| Age | 64 (38–80) | |
Results
LeHeC classification associated with lesion heterogeneity
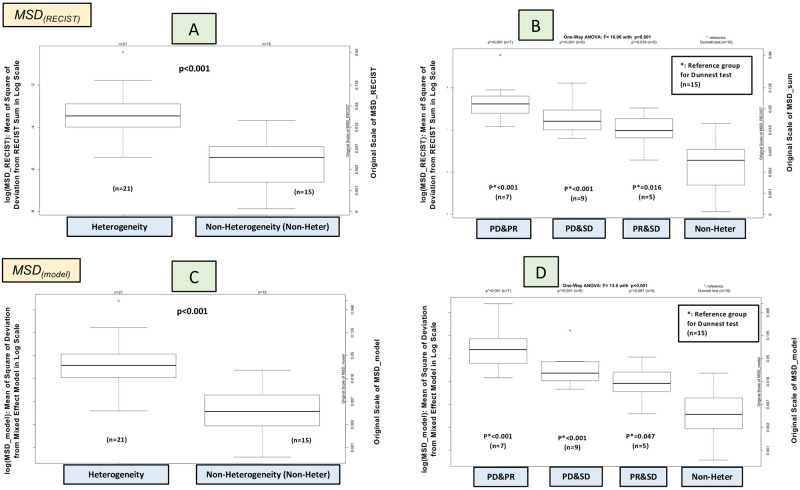
The LeHeC approach identified 21 patients (58%) with heterogeneous lesions and 15 patients (42%) with non-heterogeneity based on a threshold δ = 0.15 for SD*. Determination of the threshold was based on empirical evaluation of change in slope. which occurred at 0.15 (S6A Fig in S1 File). The lesion heterogeneity-associated group showed a significantly higher MSD(RECIST) (p<0.001 by t-test; Fig 4A). Further subgroup analysis unveiled a decreasing trend of median of log(MSD(RECIST)) with the highest in the mix of progression and regression (n = 7; 19%), followed by the mix of progression and stability (n = 9; 25%), then by the mix of regression and stability (n = 5; 14%), and lastly by the non-heterogeneity (n = 15; 42%). One-way ANOVA and Dunnett test showed an overall group difference (p<0.001; Fig 4B) and subgroup difference between the first three subsets and the non-heterogeneity group (p<0.05; Fig 4B). Analysis of MSD(model) for the mixed effect model also yielded comparable results in evaluation of lesion heterogeneity (Fig 4C and 4D and S6 Fig in S1 File). The order of lesion heterogeneity was further assured by analysis of outlier lesion based on a cutoff γ = 0.045 derived from the 80th percentile MSD(model_lesion) among the 110 lesions. The approach identified most outlier lesions in the mix of progression and regression (12 lesions), 7 outliers in the mix of progression and stability, and 3 outliers in the mix of regression and stability, and no outlier lesions detected in the non-heterogeneity group (S7 Fig in S1 File). The random effect model had 71% of >0.8 (S8 Fig in S1 File: percentile of 25th, 50th, 75th: 0.79, 0.90, 0.94). The high value indicates the predicted lesion curves by the random effect model were able to depict the observed lesion curves. Distribution of was comparable with a median value>0.8 among the 3 heterogeneity-associated subgroups (p = 0.91 by one-way ANOVA). All these results showed that the classification was able to differentiate lesion heterogeneity.
Fig 4. Comparison of MSD among lesion heterogeneous subgroups.
PD&PR: mix of progression and regression; PD&SD: mix of regression and stability; PR&SD: mix of regression and stability; Non-Heter: non-heterogeneity.
Enrichment of RECIST lesion-heterogeneity response classification
The 4 subgroups were detailed in each RECIST response category. Overall, lesion heterogeneity commonly occurred in each RECIST response group (Table 2).
Table 2. Frequency of the heterogeneity associated subgroups in each RECIST response category.
| RECIST (Best Response) | Mix of Progression and Regression | Mix of Progression and Stability | Mix of Regression and Stability | Non-Heterogeneity |
|---|---|---|---|---|
| Stable Disease (SD) | 5 | 8 | 3 | 11 |
| Partial Response (PR) | 2 | 0 | 1 | 2 |
| Progressive Disease (PD) | 0 | 1 | 1 | 2 |
RECIST SD (n = 27)
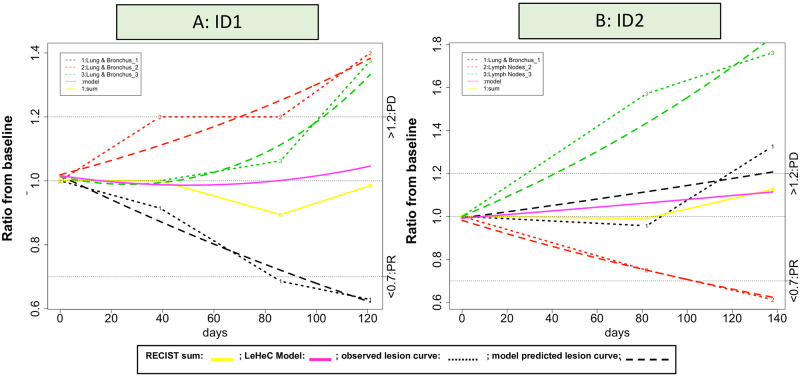
This group had 16 cases (59%) with lesion heterogeneity. Specifically, five were classified as a mix of progression and regression. Two cases had a clear diverse pattern with more than 30% reduction in one lesion and over 20% increase in other lesions over time (Fig 5: ID 1–2). The other three cases showed a sporadic mixed response defined as some stable lesions with temporary regression at some time points or some lesions experienced regression to progression over time (S9 Fig in S1 File: ID S9 1–3). Eight cases showed a mix of progression and stability with three subtle patterns (S10 Fig in S1 File). One had most stable lesions with a near flat growth rate, but had only one or few progressive lesions with a high growth rate (n = 3; S10A Fig in S1 File: ID S10A 1–3). Such pattern is relevant to oligometastasis. Another pattern had most progressive lesions and few stable lesions (n = 1; S10B Fig in S1 File; ID S10B-1). The other pattern was a balanced mix of progressive and stable lesions (n = 4; S10B Fig; ID S10B 2, 4–6). Three cases showed a mix of regression and stability (S11 Fig in S1 File; ID S11A2 and B1-2). Their regressive lesions had either tumor reduction over time or temporary regression. Eleven cases did not show lesion heterogeneity (S12 Fig in S1 File). They had similar response profiles among lesions either in stable condition (n = 8; S12A Fig; ID S12A 1–7, 9) or consistent growth curve in an increasing, decreasing, or irregular trend (n = 3; S12B Fig in S1 File; ID S12B 1–3).
Fig 5. Mixed response.
RECIST PR (n = 5)
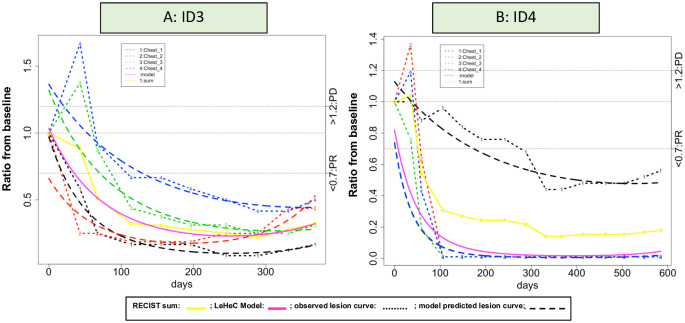
Two had a mix of progression and regression (Fig 6). The progression part was caused by pseudo-progressive lesions with initial significant increase and later substantial reduction of tumor size. One case with a mix of regression and stability had two lesions with initial regressive trend, but later split into one stable lesion and one regressive lesion (S11B Fig in S1 File; ID S11B3). The two cases with non-heterogeneity had either with homogenous regressive lesions or a pattern with initial decline and later growth (S12B Fig in S1 File; ID S12B 5–6). Notably, the three heterogeneity cases had a longer duration of treatment over 300 days, compared to the two cases without heterogeneity (<120 days).
Fig 6. Mix of pseudo-progressive and regressive lesions.
(A): Case ID 3 had 4 chest lesions with two chest lesions (lesion 3-4) experienced early progression and then developed response with a ratio below 0.7 after day 100. The other two chest lesions had a ratio below 0.7 after the 1st CT scan. (B): Case ID 4 had a similar pattern where an initial ratio >1.2 and then disappeared after 100 days in two upper lobe nodule lesions (lesion 2 and 4). The two lower lobe nodule lesions had tumor reduction at least 30% with one after day 100 and the other one after day 300.
RECIST PD (n = 4)
PD was determined due to early new lesion (n = 3) or unequivocal increase of non-target lesion (n = 1). One had a mix of progression and stability (S10B Fig in S1 File; ID S10B3), one had a mix of regression and stability (S11A Fig in S1 File; ID S11A1), and two had non-heterogeneity (S12 Fig in S1 File; ID S12 A8 and B4).
Survival analysis
Survival analysis was conducted in each RECIST response group (PR, SD, and PD). Results (Fig 7) showed the heterogeneity group with a longer OS in patients with PR and SD, but a shorter OS in patients with PD. Specifically, for PR (n = 4), the median OS was not reached in the heterogeneity subgroup (n = 3) and 9 months in the non-heterogeneity subgroup (n = 2) (p = 0.039). The SD group also had a similar trend with a median OS of 12 and 6 months in the heterogeneity (n = 16) and non-heterogeneity (n = 11) subgroups, respectively (p = 0.11). The PD showed a different pattern with a median OS of 9 and 17 months in the heterogeneity (n = 2) and non-heterogeneity (n = 2) subgroups, respectively (p = 0.09).
Fig 7. Survival analysis.
*: median OS; m: month.
Discussion
This is the first study to utilize target lesion data to assess lesion heterogeneity. The results support evidence of high prevalence of lesion heterogeneity in cancer patients (~20% mixed response [24, 36, 47], 20–50% oligometastasis [48], and 5–36% pseudo-progression [39, 49]). Moreover, analysis by the LeHeC approach showed granular lesion level discovery of heterogeneity to enrich RECIST response classification.
Methodology-wise, the LeHeC approach first employs the mixed effect model to depict tumor growth patterns and takes advantage of random effect components to quantify lesion heterogeneity. The modified RECIST time point response is then applied to classify four distinct subgroups with potential clinical application. Moreover, a set of diagnosis tools using various forms of MSD is deployed to examine classification performance. Specifically, the LeHeC approach identified more than 50% patients with heterogeneous lesions by assessing variation of lesion growth curve parameters through SD*. Lesion deviation from the RECIST sum and the mixed effect mean function (MSD(RECIST) and MSD(model)) validated the classification result by showing a significantly larger deviation in patients with lesion heterogeneity. Subgroup classification by the modified RECIST time point response further formed 4 informative subsets in a descending order of lesion heterogeneity: mix of progression and regression, mix of progression and stability, mix of regression and stability, and non-heterogeneity. The pattern was also supported by lesion deviation from MSD(RECIST) and MSD(model), as well as analysis of outlier lesion detection. Moreover, 71% cases had >0.8 supporting a high degree of model goodness of fit by the mixed effect model in predicting observed lesion curves.
From clinical prospective, significant efforts have been made in clinical trials to determine treatment effect. However, utilization of target lesion data has been suboptimal by the RECIST sum metric, especially in abundance of lesion heterogeneity as shown in this study and literature [24, 36, 39, 47–49]. Subgroup classification by the LeHeC approach revealed the 4 distinct heterogeneity-associated subgroups. The heterogeneity-associated subgroups did not show association with RECIST best response groups (p = 0.63; Table 2). Instead, lesion heterogeneity was pervasive (50–60%) in each response group (SD: 16/27; 59%; PR: 3/5; 60%; PD: 2/4; 50%).
Specifically, for patients with SD, they experienced all the 4 heterogeneity-associate subtypes, indicating attention needed for lesion heterogeneity. In particular, the mix of progression and regression identified two mixed responders with some progressive lesions and some regressive lesions (Fig 5). The RECIST took the sum, consequentially cancelled out both opposite treatment effects, and presented it as SD. The LeHeC approach realized the issue and incorporated such heterogeneity information into RECIST so clinicians could have better knowledge to determine if “lesionalized” tailored treatment strategy is needed. In fact, several studies [25, 50, 51] noticed mixed response and reported clinical benefit in some mixed responders using personalized treatment. The mix of progression and stability also identified a unique subtype, combination of a few progressive lesions with majority of stable lesions, which resembles oligometastasis. Since oligometastasis is clinically actionable (e.g., stereotactic radiation, cryoablation, and minimally invasive surgery; 29 oligometastasis-associated trials listed in clinicaltrials.gov so far), this subtype may deserve attention for potential tailored treatment strategy. Patients with PR had two cases with pseudo-progressive lesions and regressive lesions in the mix of progression and regression. Interestingly, they had a longer duration of treatment over 12 months. While a study [52] showed the survival benefit of pseudo-progression over progression in patients with PD, the hypothesis of a clinical benefit by pseudo-progressive lesions over regressive lesions may be worth the investigation.
Moreover, survival analysis showed some interesting results: heterogeneity group had a longer OS in patients with PR and SD, but a shorter OS in patients with PD. The last part was consistent with literature considering heterogeneity as poor prognosis, such as mixed response [24]. In contrast, the first part was a new discovery that heterogeneity improves OS for non-PD patients, with p<0.05 for PR patients. It also showed an interaction pattern of lesion heterogeneity and RECIST classification in predicting OS, meaning heterogeneity affected OS differently among RECIST response groups. Nevertheless, due to the small sample size issue, the results were preliminary.
The study has several limitations. The major concern is the small sample size from a single institution which has limited various analysis. One issue is to restrict the study into exploratory nature without confirmatory results. However, even with this constraint, the study was able to support lesion heterogeneity as a common event and revealed distinct heterogeneity subtypes to tune up RECIST classification. Another issue is lack of power for survival analysis to test if the new discovery heterogeneity-associated subtypes could predict OS (prognostic biomarker) and/or treatment (predictive biomarker). Even so with this small sample size, the study showed a differential OS trend of heterogeneity versus non-heterogeneity from PR, SD, to PD (Fig 7). The other limitation is small frequency of heterogeneity subtypes. It raises uncertainty whether some subtypes of lesion heterogeneity were due to randomness. Future multiple large retrospective studies across multiple institutes and prospective randomized interventional trials are needed to address these concerns and draw definitive conclusion.
In summary, this study raises awareness of the heterogeneity issue, quantitatively assesses lesion variation, and utilizes the heterogeneity information to tune up RECIST response classification. These contributions are valuable since this research field is still at infancy stage. Moreover, the concept of lesion heterogeneity fits naturally into principle of precision medicine by the potential application of lesion-guided treatment strategy. Thus, incorporation of this component could enhance RECIST assessment of treatment efficacy in granular lesion level. Moreover, while our current research is limited to the patients experienced with PD, or the ones with completion of treatment, the LeHeC approach has potential to guide treatment management in two scenarios: (a) SD case: during clinical trial, patient will continue treatment if overall tumor burden shows SD at current tumor measurement (i.e., CT scan). However, the SD could mean a result of either homogenous stable lesions or a mix of progressive and regressive lesions. The LeHeC approach could help differentiate both patterns. Such information could help clinicians determine if adaptive treatment strategy is needed for the latter case before it reaches PD or completion of treatment, (b) mix of pseudo-progressive and regressive lesions: Two cases were observed with more than one year of treatment duration. While this observation is limited due to small sample size, it prompts the question of whether such lesion heterogeneity has a longer response duration than the one with homogenous regressive lesions. If the hypothesis is true, the LeHeC approach could help early detect such a mix of pseudo-progressive and regressive lesions so clinicians could adjust treatment strategy accordingly.
Supporting information
(PDF)
Acknowledgments
We thank Mrs. Jennifer E. Dortch for editorial assistance.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
Alberto Chiappori serves in Advisory Board or Speaker Bureau or has research support from Bristol-Myers Squibb, Novartis, Merck, AstraZeneca, Genetech, Celgene, Amgen, Jazz, and Pfizer. Ben Creelan is an advisor or consultant with E.R. Squibb & Sons LLC, AbbVie Inc, GlaxoSmithKline plc, Celgene Corp, Gilead Sciences Inc., Achilles plc.; Xilio Therapeutics; KSQ Therapeutics. He is on the Speaker’s Bureau for Hoffman-LaRoche AG, E.R. Squibb & Sons LLC, AstraZeneca LLC, Takeda Pharmaceutical Company Ltd, Foundation Medicine Inc.; has a Contracted/Support Research Grant from Prometheus Inc., Iovance Biotherapeutics Inc., Adaptive Biotechnologies Corp. Andreas Saltos has travel reimbursement or research support from Novartis, Daiichi Sankyo, Mersana, and Prime Oncology. Eric Haura is a consultant for Ellipses, Janssen, and Amgen. Jhanelle E. Gray serves in Advisory Board or consultant, or has research support from AstraZeneca, Blueprint Medicines, Boehringer Ingelheim, Bristol-Myers Squibb, EMD Serono - Merck KGaA, Genetech, G 1 Therapeutics, Inivata, Merck, Novartis, Pfizer, and Ludwig Institute of Cancer Research. Other authors declare that there is no conflict of interest.
References
- 1.Cohen SM, Lippard SJ. Cisplatin: from DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol. 2001;67:93–130. doi: 10.1016/s0079-6603(01)67026-0 . [DOI] [PubMed] [Google Scholar]
- 2.Chen DT, Hsu YL, Fulp WJ, Coppola D, Haura EB, Yeatman TJ, et al. Prognostic and predictive value of a malignancy-risk gene signature in early-stage non-small cell lung cancer. J Natl Cancer Inst. 2011;103(24):1859–70. Epub 2011/12/14. doi: 10.1093/jnci/djr420 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramalingam S, Belani CP. Paclitaxel for non-small cell lung cancer. Expert Opin Pharmacother. 2004;5(8):1771–80. doi: 10.1517/14656566.5.8.1771 . [DOI] [PubMed] [Google Scholar]
- 4.Shukuya T, Ishiwata T, Hara M, Muraki K, Shibayama R, Koyama R, et al. Carboplatin plus weekly paclitaxel treatment in non-small cell lung cancer patients with interstitial lung disease. Anticancer research. 2010;30(10):4357–61. . [PubMed] [Google Scholar]
- 5.Kim DW, Mehra R, Tan DSW, Felip E, Chow LQM, Camidge DR, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17(4):452–63. doi: 10.1016/S1470-2045(15)00614-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felip E, Crino L, Kim DW, Spigel DR, Nishio M, Mok T, et al. 141PD: Whole body and intracranial efficacy of ceritinib in patients (pts) with crizotinib (CRZ) pretreated, ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) and baseline brain metastases (BM): Results from ASCEND-1 and ASCEND-2 trials. J Thorac Oncol. 2016;11(4 Suppl):S118–9. doi: 10.1016/S1556-0864(16)30251-9 . [DOI] [Google Scholar]
- 7.Shaw AT, Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–97. doi: 10.1056/NEJMoa1311107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazieres J, Zalcman G, Crino L, Biondani P, Barlesi F, Filleron T, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33(9):992–9. doi: 10.1200/JCO.2014.58.3302 . [DOI] [PubMed] [Google Scholar]
- 9.Muller IB, de Langen AJ, Giovannetti E, Peters GJ. Anaplastic lymphoma kinase inhibition in metastatic non-small cell lung cancer: clinical impact of alectinib. OncoTargets and therapy. 2017;10:4535–41. doi: 10.2147/OTT.S109493 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antoniu SA. Crizotinib for EML4-ALK positive lung adenocarcinoma: a hope for the advanced disease? Evaluation of Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363(18):1693–703. Expert Opin Ther Targets. 2011;15(3):351–3. doi: 10.1517/14728222.2011.550880 . [DOI] [PubMed] [Google Scholar]
- 11.Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924–33. doi: 10.1200/JCO.2017.74.3062 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. New England Journal of Medicine. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 13.Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. New England Journal of Medicine. 2016;375(19):1856–67. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J, et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J Clin Oncol. 2017:JCO2017737379. doi: 10.1200/JCO.2017.73.7379 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–33. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 17.Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Durable Complete Response After Discontinuation of Pembrolizumab in Patients With Metastatic Melanoma. J Clin Oncol. 2017:JCO2017756270. doi: 10.1200/JCO.2017.75.6270 . [DOI] [PubMed] [Google Scholar]
- 18.Dy GK, Bogner PN, Tan W, Demmy TL, Farooq A, Chen HB, et al. Phase II Study of Perioperative Chemotherapy with Cisplatin and Pemetrexed in Non-Small-Cell Lung Cancer. Journal of Thoracic Oncology. 2014;9(2):222–30. doi: 10.1097/JTO.0000000000000062 [DOI] [PubMed] [Google Scholar]
- 19.Takano M, Kouta H, Ikeda Y, Sasaki N, Kudoh K, Ikeda S, et al. Combination chemotherapy with temsirolimus and trabectedin for recurrent clear cell carcinoma of the ovary: A phase II single arm clinical trial. Journal of Clinical Oncology. 2014;32(15). [Google Scholar]
- 20.Hohenberger P, Bauer S, Gruenwald V, Hartmann JT, Jaeger E, Ottmann OG, et al. Multicenter, single-arm, two-stage phase II trial of everolimus (RAD001) with imatinib in imatinib-resistant patients (pts) with advanced GIST. Journal of Clinical Oncology. 2010;28(15). doi: 10.1200/jco.2010.28.15_suppl.10048 [DOI] [Google Scholar]
- 21.Khuri FR, Owonikoko TK, Subramanian J, Sica G, Behera M, Saba NF, et al. Everolimus, an mTOR inhibitor, in combination with docetaxel for second- or third-line therapy of advanced-stage non-small cell lung cancer: A phase II study. Journal of Clinical Oncology. 2011;29(15). doi: 10.1200/jco.2011.29.15_suppl.e13601 [DOI] [Google Scholar]
- 22.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018:JCO2017769901. doi: 10.1200/JCO.2017.76.9901 . [DOI] [PubMed] [Google Scholar]
- 23.Chen ZY, Zhong WZ, Zhang XC, Su J, Yang XN, Chen ZH, et al. EGFR mutation heterogeneity and the mixed response to EGFR tyrosine kinase inhibitors of lung adenocarcinomas. The oncologist. 2012;17(7):978–85. Epub 2012/06/08. doi: 10.1634/theoncologist.2011-0385 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong ZY, Zhai HR, Hou QY, Su J, Liu SY, Yan HH, et al. Mixed Responses to Systemic Therapy Revealed Potential Genetic Heterogeneity and Poor Survival in Patients with Non-Small Cell Lung Cancer. The oncologist. 2017;22(1):61–9. Epub 2017/01/28. doi: 10.1634/theoncologist.2016-0150 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinno Y, Goto Y, Sato J, Morita R, Matsumoto Y, Murakami S, et al. Mixed response to osimertinib and the beneficial effects of additional local therapy. Thorac Cancer. 2019;10(4):738–43. Epub 2019/02/09. doi: 10.1111/1759-7714.12991 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabatabai R, Natale R. Immunotherapy and Mixed Radiographic Response in Non-Small Cell Lung Cancer. J Cancer Clin. 2018;1(1). [Google Scholar]
- 27.Gomez DR, Tang C, Zhang J, Blumenschein GR Jr., Hernandez M, Lee JJ, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol. 2019;37(18):1558–65. Epub 2019/05/09. doi: 10.1200/JCO.19.00201 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8–10. Epub 1995/01/01. doi: 10.1200/JCO.1995.13.1.8 . [DOI] [PubMed] [Google Scholar]
- 29.Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018;4(1):e173501. Epub 2017/10/04. doi: 10.1001/jamaoncol.2017.3501 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimada Y. Accelerating the development of therapeutic strategy for oligometastasis. J Thorac Dis. 2019;11(12):5670–3. Epub 2020/02/08. doi: 10.21037/jtd.2019.12.42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishino M. Tumor Response Assessment for Precision Cancer Therapy: Response Evaluation Criteria in Solid Tumors and Beyond. Am Soc Clin Oncol Educ Book. 2018;38:1019–29. Epub 2018/09/21. doi: 10.1200/EDBK_201441 . [DOI] [PubMed] [Google Scholar]
- 32.Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017;14(11):655–68. Epub 2017/06/28. doi: 10.1038/nrclinonc.2017.88 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodi FS, Ballinger M, Lyons B, Soria JC, Nishino M, Tabernero J, et al. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J Clin Oncol. 2018;36(9):850–8. Epub 2018/01/18. doi: 10.1200/JCO.2017.75.1644 . [DOI] [PubMed] [Google Scholar]
- 34.Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol. 2018;36(5):446–53. Epub 2017/12/15. doi: 10.1200/JCO.2017.75.4853 . [DOI] [PubMed] [Google Scholar]
- 35.Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650–9. Epub 2020/03/28. doi: 10.1001/jamaoncol.2020.0147 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rauwerdink DJW, Molina G, Frederick DT, Sharova T, van der Hage J, Cohen S, et al. Mixed Response to Immunotherapy in Patients with Metastatic Melanoma. Ann Surg Oncol. 2020;27(9):3488–97. Epub 2020/05/31. doi: 10.1245/s10434-020-08657-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–8. Epub 2019/04/16. doi: 10.1016/S0140-6736(18)32487-5 . [DOI] [PubMed] [Google Scholar]
- 38.Stone E, Vinod SK. Oligometastatic Disease in NSCLC - Not Just Wishful Thinking? J Thorac Oncol. 2019;14(12):2042–5. Epub 2019/11/24. doi: 10.1016/j.jtho.2019.10.008 . [DOI] [PubMed] [Google Scholar]
- 39.Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol. 2018;4(11):1543–52. Epub 2018/09/08. doi: 10.1001/jamaoncol.2018.3676 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan RL, Camidge DR. Reviewing RECIST in the Era of Prolonged and Targeted Therapy. J Thorac Oncol. 2018;13(2):154–64. Epub 2017/11/09. doi: 10.1016/j.jtho.2017.10.015 . [DOI] [PubMed] [Google Scholar]
- 41.An MW, Han Y, Meyers JP, Bogaerts J, Sargent DJ, Mandrekar SJ. Clinical Utility of Metrics Based on Tumor Measurements in Phase II Trials to Predict Overall Survival Outcomes in Phase III Trials by Using Resampling Methods. J Clin Oncol. 2015;33(34):4048–57. Epub 2015/10/28. doi: 10.1200/JCO.2015.60.8778 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An MW, Dong X, Meyers J, Han Y, Grothey A, Bogaerts J, et al. Evaluating Continuous Tumor Measurement-Based Metrics as Phase II Endpoints for Predicting Overall Survival. J Natl Cancer Inst. 2015;107(11). doi: 10.1093/jnci/djv239 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandrekar SJ, An MW, Meyers J, Grothey A, Bogaerts J, Sargent DJ. Evaluation of alternate categorical tumor metrics and cut points for response categorization using the RECIST 1.1 data warehouse. J Clin Oncol. 2014;32(8):841–50. doi: 10.1200/JCO.2013.52.3019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen Y, Anderson A, Sinha R, Li Y. Joint modeling tumor burden and time to event data in oncology trials. Pharm Stat. 2014;13(5):286–93. doi: 10.1002/pst.1629 . [DOI] [PubMed] [Google Scholar]
- 45.Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e52. Epub 2017/03/09. doi: 10.1016/S1470-2045(17)30074-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bohnsack O, Hoos A, Ludajic K. Adaptation and modification of the immune related response criteria (IRRC): IrRECIST. Journal of Clinical Oncology. 2014;32(15_suppl):e22121–e. doi: 10.1200/jco.2014.32.15_suppl.e22121 [DOI] [Google Scholar]
- 47.van Kessel CS, Samim M, Koopman M, van den Bosch MA, Borel Rinkes IH, Punt CJ, et al. Radiological heterogeneity in response to chemotherapy is associated with poor survival in patients with colorectal liver metastases. European journal of cancer. 2013;49(11):2486–93. Epub 2013/05/23. doi: 10.1016/j.ejca.2013.03.027 . [DOI] [PubMed] [Google Scholar]
- 48.Counago F, Luna J, Guerrero LL, Vaquero B, Guillen-Sacoto MC, Gonzalez-Merino T, et al. Management of oligometastatic non-small cell lung cancer patients: Current controversies and future directions. World J Clin Oncol. 2019;10(10):318–39. Epub 2019/12/05. doi: 10.5306/wjco.v10.i10.318 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbasi AW, Westerlaan HE, Holtman GA, Aden KM, van Laar PJ, van der Hoorn A. Incidence of Tumour Progression and Pseudoprogression in High-Grade Gliomas: a Systematic Review and Meta-Analysis. Clin Neuroradiol. 2018;28(3):401–11. Epub 2017/05/04. doi: 10.1007/s00062-017-0584-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goto Y, Tanai C, Yoh K, Hosomi Y, Sakai H, Kato T, et al. Continuing EGFR-TKI beyond radiological progression in patients with advanced or recurrent, EGFR mutation-positive non-small-cell lung cancer: an observational study. ESMO Open. 2017;2(4):e000214. Epub 2017/10/12. doi: 10.1136/esmoopen-2017-000214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park K, Yu CJ, Kim SW, Lin MC, Sriuranpong V, Tsai CM, et al. First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer: The ASPIRATION Study. JAMA Oncol. 2016;2(3):305–12. Epub 2016/01/01. doi: 10.1001/jamaoncol.2015.4921 . [DOI] [PubMed] [Google Scholar]
- 52.Won SE, Park HJ, Byun S, Pyo J, Kim JH, Choi CM, et al. Impact of pseudoprogression and treatment beyond progression on outcome in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Oncoimmunology. 2020;9(1):1776058. Epub 2020/09/15. doi: 10.1080/2162402X.2020.1776058 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.