Abstract
Background: Cardiomyopathies (CMPs) due to myocytes involvement are among the leading causes of sudden adolescent death and heart failure. During the COVID-19 pandemic, there are limited data available on cardiac complications in patients with COVID-19, leading to severe outcomes.
Methods: We conducted a systematic search in Pubmed/Medline, Web of Science, and Embase databases up to August 2020, for all relevant studies about COVID-19 and CMPs.
Results: A total of 29 articles with a total number of 1460 patients were included. Hypertension, diabetes, obesity, hyperlipidemia, and ischemic heart disease were the most reported comorbidities among patients with COVID-19 and cardiomyopathy. In the laboratory findings, 21.47% of patients had increased levels of troponin. Raised D-dimer levels were also reported in all of the patients. Echocardiographic results revealed mild, moderate, and severe Left Ventricular (LV) dysfunction present in 17.13, 11.87, and 10% of patients, respectively.
Conclusions: Cardiac injury and CMPs were common conditions in patients with COVID-19. Therefore, it is suggested that cardiac damage be considered in managing patients with COVID-19.
Keywords: COVID-19, cardiomyopathy, cardiac injury and regeneration, systematic review, SARS-CoV-2
Introduction
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first reported on 31 December 2019 from Wuhan, China, resulted in an unprecedented outbreak of Coronavirus disease 2019 (COVID-19). The most common manifestation of COVID-19 is pulmonary complications. However, this novel disease's presentations have a broad spectrum of signs and symptoms from asymptomatic infection or mild flu-like symptoms to multiorgan failure resulting in death (1, 2). Cardiovascular disease (CVD) has been reported in patients infected with COVID-19 (3). Based on the literature, 20–30% of hospitalized patients showed cardiovascular manifestations associated with worse outcomes (4, 5). Cardiovascular complications of COVID-19
are thought to be a combination of direct viral injury and the host's immune response resulting in vascular inflammation, plaque instability, and myocardial inflammation (6–9). Cardiomyopathies (CMPs) which resulted from heart muscle involvement, are among the main causes of adolescent sudden death and heart failure (10). SARS-CoV-2 infection in patients suffering from CMPs represents an actual risk of exacerbating patient clinical status (11).
Although many authors have reported various aspects of respiratory-related symptoms of COVID-19, the increasing prevalence of cardiac complications in COVID-19 patients should be taken into considerations (12–17). Thus, this study was aimed to systematically review the current published literature to evaluate clinical and paraclinical characteristics of CMPs in patients infected with SARS-CoV-2.
Methods
Search Strategy
In the following bibliographic databases, we carried out a comprehensive systematic search of literature: PubMed/Medline, Embase, and Web of Science. We searched for any relevant articles published in English up to August 2020. The search included keywords including COVID-19, severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, in combinations with cardiomyopathy, or CMP, cardiomyopathies, myocardiopathy, cardiac injury, or myocarditis.
Additionally, all references of selected papers were searched manually for additional related articles. The present systematic review conforms to the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) statement (18).
Study Selection
Studies reported any data about CMPs in patients with confirmed COVID-19 were included. Abstracts, commentary, letter to editor, guidelines, and review articles were excluded.
All retrieved publications were screened for eligibility in two phases. First, two reviewers independently screened the titles and abstracts of potentially relevant articles identified in the primary search. Subsequently, a review of the full texts of all remaining articles was done by the same authors. Any discrepancy in the article selection or technical uncertainties were discussed and resolved between review authors.
Data Extraction
The following variables were extracted from all included studies: first author, year of publication, type of study, country where the research was conducted, study population, COVID-19 diagnosis technique, laboratory findings, treatment protocols, and type of CMPs. Two authors independently extracted the data from the selected studies. The data was jointly reconciled, and disagreements were discussed and resolved between review authors.
Results
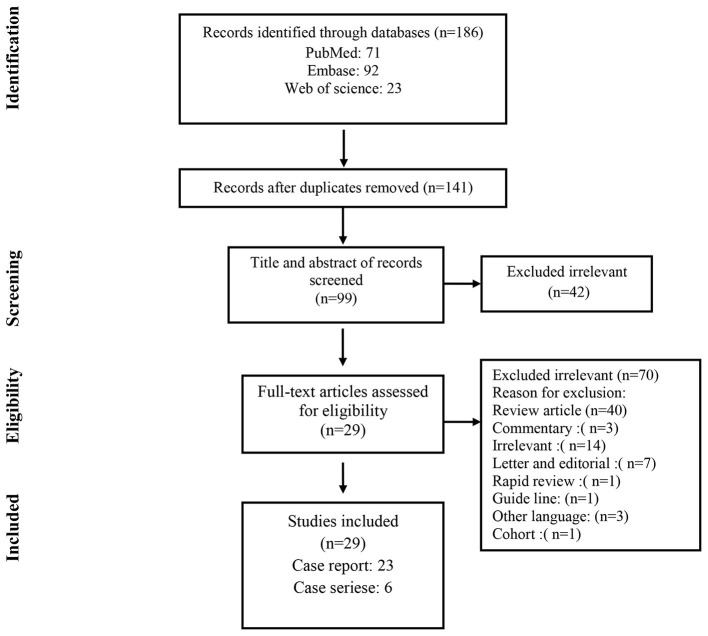
As shown in Figure 1, a total of 186 studies were identified from databases. After removing 45 duplicates, 141 non-duplicate studies remained for further assessments. After applying the inclusion/exclusion criteria, 29 articles (22 case reports and 7 case series) were included with a total number of 1460 unique cases of COVID-19 with a mean age of 58 years. The characteristics of the included studies are described in Table 1.
Figure 1.
Flow chart of study selection for inclusion in the systematic review.
Table 1.
Characteristics of the included studies.
| References | Country | Type of study | No. of patients | Male/female | Mean age |
|---|---|---|---|---|---|
| Doyen et al. (19) | Italy | Case report | 1 | 1 M | 69 |
| Paul et al. (20) | France | Case report | 1 | 1 M | 35 |
| Huyut (21) | Turkey | Case report | 1 | 1 F | 59 |
| Pasqualetto et al. (22) | Italy | Case series | 3 | 2 M-1 F | 83.33 |
| Deng et al. (23) | China | Case series | 14 | 10 M-4 F | 74 |
| Taza et al. (24) | USA | Case report | 1 | 1 M | 52 |
| Roca et al. (25) | Italy | Case report | 1 | 1 F | 87 |
| Minhas et al. (26) | USA | Case report | 1 | 1 F | 52 |
| Juusela et al. (27) | USA | Case series | 2 | 2 F | 35.5 |
| Meyer et al. (28) | Switzerland | Case report | 1 | 1 F | 83 |
| Khalid et al. (29) | Italy | Case report | 1 | 1 F | 76 |
| Nguyen et al. (30) | Belgium | Case report | 1 | 1 F | 71 |
| Bonnet et al. (31) | France | Case report | 1 | 1 M | 27 |
| Zhang et al. (32) | Multicenter | Case series | 2 | 1 M-1 F | 59 |
| Dabbagh et al. (33) | USA | Case report | 1 | 1 F | 67 |
| Guo et al. (34) | China | Case series | 187 | 91 M-96 F | 58.5 |
| Tavazzi et al. (35) | Italy | Case report | 1 | 1 M | 69 |
| Hua et al. (36) | UK | Case report | 1 | 1 M | 47 |
| Villanueva et al. (37) | USA | Case report | 1 | 1 M | 68 |
| Kir et al. (38) | USA | Case report | 1 | 1 M | 49 |
| Dweck et al. (39) | Multicenter | Case series | 1209 | 844 M-365 F | 62 |
| Irabien-Ortiz et al. (40) | Spain | Case report | 1 | 1 M | 59 |
| Craver et al. (41) | USA | Case report | 1 | 1 M | 17 |
| Bobeck et al. (42) | USA | case report | 1 | 1 M | 80 |
| Arentz et al. (43) | USA | Case series | 21 | 10 M-11 F | 70 |
| Yildirim and Karaagac (44) | Turkey | Case report | 1 | 1 F | 7 |
| Chadha (45) | USA | Case report | 1 | 1 F | 85 |
| Kim et al. (46) | Korea | Case report | 1 | 1 F | 21 |
| Luetkens et al. (47) | Germany | Case report | 1 | 1 M | 79 |
Table 2 shows the outcomes and prognosis of CMPs in patients with COVID-19. 98 out of 1,212 evaluated patients developed cardiogenic shock (8.08%). Six studies reported mortality rates, showing 48 out of 192 (25%) of patients deceased.
Table 2.
The outcomes and prognosis of CMPs.
| Outcomes | No of study | n/N | Percentage (%) |
|---|---|---|---|
| Deceased | 6 | 48/192 | 25 |
| Cured | 13 | 14/16 | 87.5 |
| Prognosis | |||
| Cardiogenic shock | 4 | 98/1,212 | 8.08 |
| MODa | 3 | 81/191 | 42.40 |
| ARDSb | 8 | 67/215 | 31.16 |
n, number of patients with any variables; N, the total number of studied patients.
MOD,Multi organ disease;
ARDS, Adult respiratory distress syndrome.
As presented in Table 3, hypertension, diabetes, obesity, hyperlipidemia, ischemic disease, and obstructive sleep apnea were the most reported comorbidities among them.
Table 3.
Clinical and laboratories findings in patients with COVID-19.
| Variable | No of study | n/N | % | |
|---|---|---|---|---|
| Clinical manifestations | Chest pain | 9 | 128/1,232 | 10.38 |
| Dyspnea | 9 | 9/10 | 90 | |
| Shortness of breath | 11 | 39/45 | 88.66 | |
| Cough | 14 | 38/49 | 77.55 | |
| Fever | 14 | 40/51 | 78.43 | |
| Fatigue | 5 | 5/5 | 5 | |
| Tachypnea | 7 | 8/21 | 30.09 | |
| Crackles | 7 | 7/8 | 87.5 | |
| Diarrhea | 3 | 3/3 | 100 | |
| Nausea & vomiting | 4 | 5/6 | 83.33 | |
| Signs | Elevated pulse rate | 12 | 195/1,413 | 13.8 |
| Elevated temperature | 12 | 32/33 | 96.96 | |
| Comorbidities | Hypertension | 15 | 526/1,424 | 36.93 |
| Diabetes | 10 | 279/1,440 | 19.37 | |
| Obesity | 3 | 11/17 | 64.7 | |
| Hyperlipidemia | 5 | 5/5 | 100 | |
| Ischemic disease | 4 | 200–1,431 | 13.97 | |
| Obstructive sleep apnea | 2 | 10/35 | 25.57 | |
| COPDa | 3 | 12/222 | 5.4 | |
| CKDb | 3 | 17/209 | 8.13 | |
| CAc | 3 | 15/189 | 7.93 | |
| Laboratory findings | elevated NTproBNP | 11 | 26/214 | 12.2 |
| High IL-6 | 5 | 5/5 | 100 | |
| High D-dimer | 5 | 6/6 | 100 | |
| High ferritin | 6 | 6/6 | 100 | |
| High CRPd | 14 | 14/201 | 6.96 | |
| High Troponin | 18 | 307/1412 | 21.74 |
n, number of patients with any variables; N, the total number of studied patients.
COPD, Chronic obstructive pulmonary disease;
CKD, Chronic kidney disease;
CA, Copd/Asthma;
CRP, C-reactive protein.
As shown in Table 3, cough and fever were reported as the most prevalent symptoms in 14 out of 29 studies. Dyspnea was reported in 9 studies. According to these studies, 90% of the evaluated patients had this complication. Evaluation of laboratory findings showed elevated troponin levels in 18 studies with 308 out of 1,412 patients (21.47%). Increased D-dimer levels were reported in 5 case reports, of which six patients showed this elevated marker.
CMPs evidence in patients with COVID-19 indicates in Table 4. Common ECG findings were: tachycardia, premature beats, ST-segment elevation, blocks, and inverted T wave. Inverted T waves were seen in EKG findings of 9 studies (91.66% of evaluated patients). Left ventricular (LV) involvement is a hallmark of primary CMPs. Echocardiographic findings revealed mild (17.1%), moderate (11.85%), and severe (9.98%) LV dysfunction, which was discussed in 6, 4, and 11 studies, respectively. Aneurysm formation, a sign of stress-induced cardiomyopathy followed COVID-19, was found in all 11 evaluated patients (100%). Regional wall motion abnormalities (RWMA) as another sign were found in 46/1,217 (1.15%) patients. Right ventricular (RV) involvement and high pulmonary artery pressure (PAP) are signs of the destruction of the right heart. RV enlargement was presented in 14.88% of tested patients (181/1,216). RV dysfunction was also found in 26.01% (315/1,211) of patients' echocardiograms. Findings of Chest X-Ray (CXR) and Chest CT scan showed ground-glass opacification (GGO) patterns (26 of 34 patients) and consolidation (7 of 7 patients) as the most common findings (Table 4). Among the type of CMPs, COVID CMPs, and hypertrophic cardiomyopathy were among the most reported type in 39.13 and 18.75% cases, respectively.
Table 4.
Cardiomyopathy evidence in patients with COVID-19.
| Variable | No of study | n/N | % | |
|---|---|---|---|---|
| EKG | Sinus tachycardia | 7 | 8/8 | 100 |
| Bradycardia | 2 | 2/2 | 100 | |
| Premature beats | 2 | 4/4 | 100 | |
| ST elevation | 5 | 5/5 | 100 | |
| ST depression | 2 | 2/2 | 100 | |
| Blocks | 2 | 4/4 | 100 | |
| Inverted T wave | 9 | 11/12 | 91.66 | |
| VTa | 2 | 49/1,403 | 3.49 | |
| Echocardiography | LVE (LVb enlargement) | 3 | 68/1,219 | 5.57 |
| Mild LV dysfunction | 6 | 208/1,216 | 17.10 | |
| Moderate LV dysfunction | 4 | 144/1,215 | 11.85 | |
| Severe LV dysfunction | 11 | 122/1,222 | 9.98 | |
| RVE (RVc enlargement) | 1 | 181/1,216 | 14.88 | |
| RV dysfunction | 3 | 315/1,211 | 26.01 | |
| High PAPd | 1 | 99/1,216 | 8.14 | |
| Aneurysm formation | 10 | 11/11 | 100 | |
| RWMAe | 10 | 46/1,217 | 1.15 | |
| Pericardial effusion | 3 | 3/3 | 100 | |
| LVHf | 4 | 4/4 | 100 | |
| Pericardial effusion | 3 | 3/3 | 100 | |
| Endocarditis | 1 | 14/1,216 | 1.15 | |
| Tamponade | 3 | 13/1,218 | 1.06 | |
| Echo MIg | 2 | 37/1,230 | 3 | |
| Echo Myocarditis | 1 | 35/1,216 | 2.87 | |
| D shap LV | 1 | 49/1,216 | 4.02 | |
| CXR | Diffuse involvement | 6 | 7/7 | 100 |
| Cardiomegaly | 2 | 2/3 | 66.66 | |
| CT scan | Ground-glass opacities | 11 | 26/34 | 76.47 |
| Consolidation | 5 | 7/7 | 100 | |
| Angiogram | Abnormal angiogram | 5 | 6/7 | 85.71 |
| Normal angiogram | 1 | 1/1 | 100 | |
| Type of cardiomyopathy | DCMh | 3 | 71/1,225 | 5.79 |
| HCMi | 3 | 3/16 | 18.75 | |
| Myocarditis | 8 | 55/1,229 | 4.47 | |
| Myocardial injury | 13 | 303/1,408 | 2.3 | |
| Takotsubo | 14 | 32/1,222 | 2.61 | |
| Ischemic after COVID | 1 | 36/1,216 | 2.96 | |
| COVID cardiomyopathy | 3 | 9/23 | 39.13 |
n, number of patients with any variables; N, the total number of studied patients.
VT, Ventricular tachycardia;
LV, Left ventricular;
RV, Right ventricular;
PAP, Pulmonary artery pressure;
RWMA, Regional wall motion abnormalities;
LVH, Left ventricular hypertrophy;
M, myocardial infarction;
DCM, Dilated cardiomyopathy;
HCM, Hyper trophic cardiomyopathy.
In terms of treatment, 10 out of 14 patients (71.42%) reported in 11 studies received β-Blocker as part of their treatment regimen. The use of Diuretic agents was reported in 7 studies which included 7 out of 9 (77.77%) patients (Table 5).
Table 5.
Treatment agents used in the included studies.
| Variable | No of study | n/N | % | ||
|---|---|---|---|---|---|
| Non-pharmacologic treatment | O2 nasal | 8 | 10/11 | 90.9 | |
| Intubation | 14 | 72/223 | 32.28 | ||
| Pericardiocentesis | 3 | 3/3 | 100 | ||
| Pharmacologic treatment | Antimicrobial agents | Antibacterial drugs | 6 | 188/193 | 97.4 |
| Azithromycin | 6 | 6/7 | 85.71 | ||
| Antiviral drugs | 4 | 171/192 | 89.06 | ||
| Immunomodulators | Hydroxychloroquine | 9 | 10/12 | 83.33 | |
| IVIGa | 3 | 23/189 | 12.16 | ||
| steroid | 8 | 113/194 | 58.24 | ||
| Tocilizumab | 4 | 4/5 | 80 | ||
| Anticoagulant | Fondaparinux | 3 | 4/5 | 80 | |
| Anti-platelet | 3 | 4/5 | 80 | ||
| Heparin/LMWHb | 6 | 6/7 | 85.71 | ||
| Others | ACE/ARBc | 4 | 4/4 | 100 | |
| β-Blocker | 10 | 10/14 | 71.42 | ||
| NEPd | 5 | 5/6 | 83.33 | ||
| Diuretic | 7 | 7/9 | 77.77 | ||
| Vasopressor | 5 | 5/5 | 100 |
IVIG, Intravenous immune globulin;
LMWH, Low molecular weight heparin;
ACE/ARB, angiotensin converting enzyme inhibitors/angiotensin-receptor blockers;
NEP, Norepinephrine.
Discussion
COVID-19 has resulted in other organ involvement, and CMPs are among the most significant complications of this rapidly emerging disease, causing more severe disease and increased mortality rates (48, 49). In this systematic review, we studied the cardiac injuries in patients with SARS-CoV-2 infection that resulted in CMPs. Echocardiographic results showed a range of mild to severe left ventricular dysfunction in 10% to 17.13% of the studied patients.
The patients' recovery and death rates were assessed in 20 studies that showed that 28.7% of patients with one type of CMPs died following SARS-CoV-2 infection. Patients with cardiovascular comorbidities had a higher risk of developing cardiac injury (50).
In a study on twenty-one critically ill patients admitted in intensive care units (ICU), one-third developed CMPs (51). Yang et al. showed 52 critically ill COVID-19 patients 12 (23%) presented with cardiac injury (52).
The results of a cohort study showed that 23% of patients experienced new heart failure or exacerbation of chronic heart failure, of which 28 survived, and 16 died (50).
Based on the included studies that examined patients' mortality rate with CMPs and COVID-19, 25% of these patients were deceased. As a result, it can be inferred that cardiac injury is a significant predisposing factor for increasing the mortality rate of COVID-19.
Huang et al. demonstrated a “Cytokine storm” model that results in a pro-inflammatory markers surge that may lead to myocardial injury (53). Similar effects have been observed with MERS-CoV and SARS-CoV infections previously (54). Furthermore, the virus may be involved in a primary myocardial injury by entering the myocytes through the ACE-2 receptor (55).
Overall, SARS-CoV-2 can cause cardiac complications through the following pathways: (1) Indirect cardiac injury due to increased release of cytokines and inflammatory pathways. (2) Direct invasion of the SARS-COV-2 in cardiac myocytes. (3) Respiratory damage can cause hypoxia, myocardial supply-demand mismatch, followed by oxidative stress and damage to cardiomyocytes (56, 57).
There are different manifestations of cardiac involvement in COVID-19, including acute myocardial infarction, acute heart failure, cardiogenic shock, myocarditis, and fatal arrhythmias (58). Myocardial injury is a common condition in COVID-19 hospitalized, which is characterized by increased troponin levels (59). Another definition of cardiac injury is reported as abnormality in cardiac biomarkers, electrocardiography, or echocardiography relative to the patient's previous condition. In a cohort study of 416 patients, 19.7% of hospitalized patients had a cardiac injury (34).
Cardiomyopathy was defined as evidence of new left ventricular systolic dysfunction on trans-thoracic echocardiography with one of the following criteria: 1. Clinical signs of cardiogenic shock, 2. Increase in creatine kinase or troponin level, and 3. Reduction in oxygen saturation of the central vein below 70% (43).
Our results showed that ARDS was present in 31.45% of patients following COVID-19 and cardiomyopathy. The cardiogenic shock occurred in 8% of patients. Reported data from Germany and the United States (56, 60) showed that cardiogenic shock is a significant complication of COVID-19. According to the evaluated studies in our systematic review, ~8% of patients developed heart failure/cardiogenic shock as a manifestation of COVID-19.
We showed that common symptoms of COVID-19 in patients with cardiac injury include fever, cough, headache, and fatigue. These findings are broadly consistent with other studies examining clinical signs in patients with COVID-19 (13, 14).
Our review of published studies showed the most common abnormal laboratory findings in patients with cardiomyopathy were increased IL-6 level, elevated ferritin, and High D-dimer. Some studies were reported that Serum concentrations of IL-6 were higher in severe cases of COVID-19 compared with moderate cases. Moreover, in deceased patients, levels of this cytokine were substantially higher than in recovered ones. So, continuous measurement of IL-6 level for early prediction of severity of infection has been suggested (61, 62).
The elevated level of fibrin degradation products, especially D-dimers (>2590/ng·mL−1), was shown to be an indicator of pulmonary embolism in hospitalized COVID-19 patients. It also contributed to poor prognosis and high mortality in patients with a more severe form of COVID-19 (63).
Our systematic review showed that 21.7% of patients presented with high troponin levels that were investigated in 14 studies. Gue et al. indicated the importance of monitoring troponin levels to predict the likelihood of cardiovascular events. Patients with high troponin levels had higher levels of other cardiac biomarkers and more fatal arrhythmias (34).
The results of our analysis revealed that hypertension, obesity, and hyperlipidemia were the most common comorbidities among patients with COVID-19. The association between hypertension and inflammation is well-known; inflammatory responses increase the disease's severity and complications in patients (64, 65). In a systematic review study, hypertension was the most common underlying condition in CMPs following COVID-19, reported in 33% of patients (66). Moreover, the presence of hyperinflammatory conditions in the airways interferes with the virus's clearance (67). It is inferred that the potential synergistic effect of inflammation due to hypertension and COVID-19 can aggravate this effect on the heart and result in CMPs.
Studies have shown that obesity is a risk factor for developing ARDS in COVID-19 (68). Moreover, hyperlipidemia has been more prevalent among hospitalized and more severe cases of COVID-19 compared to non-hospitalized ones (69, 70).
One of the common diagnostic modalities for COVID-19 is CT-scan. Bilateral and peripheral predominant ground-glass opacity, multifocal patchy consolidation, and interstitial changes with the peripheral distribution are among these features (71).
According to the included articles in our study, 76.47% of evaluated patients demonstrated Ground-glass opacities in their chest CT scan examination.
Different pharmacological and non-pharmacological treatments have been studied and applied for COVID-19. The included studies showed that nasal oxygen and intubation were among the most common non-pharmacological treatments for patients. Hydroxychloroquine, azithromycin, antiviral drugs, and β-Blockers were the most common pharmacological treatments. Due to the wide range of disease symptoms and complications, further studies related to each organ involvement are required to manage the disease better and prevent the complications.
In the end, it is necessary to point out the limitations of the present study. Since only case reports and case series studies have been selected for this review, this increases the potential risk of bias. Another issue is the small number of patients enrolled in the study. Due to the scarcity of randomized controlled trial (RCT)/quasi-randomized studies, we could not include them in the present study. We have not adopted the publications as abstracts or letters as data presented in this format is not high quality. Further investigations are required to include a broader range of studies, including clinical trials in patients with COVID-19 and CMPs.
In conclusion, cardiac injury and CMPs, including exacerbation of an underlying CMPs or the emergence of new CMPs, are common in COVID-19 patients. Moreover, they are associated with higher mortality and morbidity in these patients. Common fatal conditions in patients with COVID-19 CMPs include multiorgan damage, ARDS, and cardiogenic shock. Therefore, diagnostic measures of COVID-19 should consist of underlying cardiovascular comorbidities. History, signs, and symptoms of cardiac injury should be considered in evaluating these patients early in the course of this novel disease, and prompt therapeutic measures for the prevention of exacerbating cardiac condition should be sought.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
FO, MN, and BH: designed the study. FO, SK, AT, SR, AA, SH, MG, and FK: performed the search, study selection, and data synthesis. BH, FO, and MN: wrote the first draft of the manuscript. MN, BH, and MM: revised the article. All authors contributed to the paper and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Dadashi M, Hajikhani B, Yaslianifard S, Goudarzi M, Owlia P, Nasiri MJ, et al. COVID-19 and Skin manifestations: An overview of case reports/case series and meta-analysis of the prevalence studies. Front Med. (2020) 7:573188. 10.3389/fmed.2020.573188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajikhani B, Calcagno T, Nasiri MJ, Jamshidi P, Dadashi M, Goudarzi M, et al. Olfactory and gustatory dysfunction in COVID-19 patients: A meta-analysis study. Physiol Rep. (2020) 8:e14578. 10.14814/phy2.14578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020) 5:819–24. 10.1001/jamacardio.2020.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) 5:802–10. 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cizgici AY, Agus HZ, Yildiz M. COVID-19 myopericarditis: it should be kept in mind in today's conditions. Am J Emerg Med. (2020) 38:1547.e5-1547.e6. 10.1016/j.ajem.2020.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. (2020) 46:846–8. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Bermejo JA, Kang S, Rockwood SJ, Simoneau CR, Joy DA, Ramadoss GN, et al. SARS-CoV-2 infection of human iPSC-derived cardiac cells predicts novel cytopathic features in hearts of COVID-19 patients. BioRxiv [Preprint]. (2020). 10.1101/2020.08.25.265561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, et al. Recognizing COVID-19–related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. (2020) 17:1463–71. 10.1016/j.hrthm.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shafi AM, Shaikh SA, Shirke MM, Iddawela S, Harky A. Cardiac manifestations in COVID-19 patients—A systematic review. J Cardiac Surg. (2020) 35:1988–2008. 10.1111/jocs.14808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Limongelli G, Crotti L. COVID-19 pandemia and inherited cardiomyopathies and channelopathies: a short term and long term perspective. Orphanet J Rare Dis. (2020) 15:1–7. 10.1186/s13023-020-01444-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. (2020) 5:831–40. 10.1001/jamacardio.2020.1286 [DOI] [PubMed] [Google Scholar]
- 12.Nasiri MJ, Haddadi S, Tahvildari A, Farsi Y, Arbabi M, Hasanzadeh S, et al. COVID-19 clinical characteristics, and sex-specific risk of mortality: systematic review and meta-analysis. Front Med. (2020) 7:459. 10.3389/fmed.2020.00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tahvildari A, Arbabi M, Farsi Y, Jamshidi P, Hasanzadeh S, Calcagno TM, et al. Clinical features, diagnosis, and treatment of COVID-19 in hospitalized patients: a systematic review of case reports and case series. Front Med. (2020) 7:231. 10.3389/fmed.2020.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vetter P, Vu DL, L'Huillier AG, Schibler M, Kaiser L, Jacquerioz F. Clinical features of covid-19. Br Med J Publishing Group. (2020) 369:m1470. 10.1136/bmj.m1470 [DOI] [PubMed] [Google Scholar]
- 15.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. (2020) 323:2329–30. 10.1001/jama.2020.6825 [DOI] [PubMed] [Google Scholar]
- 16.Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respirat Crit Care Med. (2020) 201:1560–4. 10.1164/rccm.202004-1163LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes?. Intensive Care Med. (2020) 46:1099–102. 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Cranio-Maxillofacial Surg. (2011) 39:91–2. 10.1016/j.jcms.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 19.Doyen D, Moceri P, Ducreux D, Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. (2020) 395:1516. 10.1016/S0140-6736(20)30912-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul J-F, Charles P, Richaud C, Caussin C, Diakov C. Myocarditis revealing COVID-19 infection in a young patient. Eur Heart J Cardiovasc Imaging. (2020) 21:776. 10.1093/ehjci/jeaa107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huyut MA. Novel coronavirus pneumonia and cardiomyopathy: a case report. Arq Bras Cardiol. (2020) 114:843–5. 10.36660/abc.20200268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasqualetto MC, Secco E, Nizzetto M, Scevola M, Altafini L, Cester A, et al. Stress cardiomyopathy in COVID-19 Disease. Eur J Case Rep Internal Med. (2020) 7:001718. 10.12890/2020_001718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W, et al. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. (2020) 311:116–21. 10.1016/j.ijcard.2020.03.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taza F, Zulty M, Kanwal A, Grove D. Takotsubo cardiomyopathy triggered by SARS-CoV-2 infection in a critically ill patient. BMJ Case Rep CP. (2020) 13:e236561. 10.1136/bcr-2020-236561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roca E, Lombardi C, Campana M, Vivaldi O, Bigni B, Bertozzi B, et al. Takotsubo syndrome associated with COVID-19. Eur J Case Rep Internal Med. (2020) 7:1665. 10.12890/2020_001665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minhas AS, Scheel P, Garibaldi B, Liu G, Horton M, Jennings M, et al. Takotsubo syndrome in the setting of COVID-19 infection. JACC: Case Rep. (2020) 2:1321–5. 10.1016/j.jaccas.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juusela A, Nazir M, Gimovsky M. Two cases of COVID-19 related cardiomyopathy in pregnancy. Am J Obstetri Gynecol MFM. (2020) 2:100113. 10.1016/j.ajogmf.2020.100113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer P, Degrauwe S, Van Delden C, Ghadri J-R, Templin C. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Euro Heart J. (2020) 41:1860. 10.1093/eurheartj/ehaa306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalid Y, Dasu N, Dasu K. A case of novel coronavirus (COVID-19)-induced viral myocarditis mimicking a Takotsubo cardiomyopathy. HeartRhythm Case Rep. (2020) 6:473. 10.1016/j.hrcr.2020.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen D, Nguyen T, De Bels D, Rodriguez JC. A case of Takotsubo cardiomyopathy with COVID 19. Euro Heart J Cardiovasc Imaging. (2020) 21:1052. 10.1093/ehjci/jeaa152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonnet M, Craighero F, Harbaoui B. Acute myocarditis with ventricular non compaction in a COVID-19 patient. Jacc Heart Failure. (2020) 8:599–600. 10.1016/j.jchf.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Wang B, Zhou J, Kirkpatrick J, Xie M, Johri AM. Bedside focused cardiac ultrasound in COVID-19 from the Wuhan epicenter: the role of cardiac point-of-care ultrasound, limited transthoracic echocardiography, and critical care echocardiography. J Am Soc Echocardiography. (2020) 33:676–82. 10.1016/j.echo.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabbagh MF, Aurora L, D'Souza P, Weinmann AJ, Bhargava P, Basir MB. Cardiac tamponade secondary to COVID-19. JACC: Case Rep. (2020) 2:1326–30. 10.1016/j.jaccas.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020) 5:811–8. 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Failure. (2020) 22:911–5. 10.1002/ejhf.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hua A, O'Gallagher K, Sado D, Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. (2020) 41:2130. 10.1093/eurheartj/ehaa253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villanueva D-DH, Lusby HP, Islam SP, Gupte AA, Beatty NL. Heart failure exacerbation as only presenting sign of COVID-19. IDCases. (2020) 21:e00870. 10.1016/j.idcr.2020.e00870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kir D, Mohan C, Sancassani R. Heart brake: an unusual cardiac manifestation of COVID-19. JACC: Case Rep. (2020) 2:1252–5. 10.1016/j.jaccas.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dweck MR, Bularga A, Hahn RT, Bing R, Lee KK, Chapman AR, et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. (2020) 21:949–58. 10.1093/ehjci/jeaa178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irabien-Ortiz Á, Carreras-Mora J, Sionis A, Pàmies J, Montiel J, Tauron M. Fulminant myocarditis due to COVID-19. Rev Española Cardiol. (2020) 73:503–4. 10.1016/j.rec.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craver R, Huber S, Sandomirsky M, McKenna D, Schieffelin J, Finger L. Fatal eosinophilic myocarditis in a healthy 17-year-old male with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2c). Fetal Pediatr Pathol. (2020) 39:26–8. 10.1080/15513815.2020.1761491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bobeck KA, Holtzclaw AW, Brown TE, Clark PA. Effective use of angiotensin ii in coronavirus disease 19–associated mixed shock state: a case report. A&a Practice. (2020) 14:e01221. 10.1213/XAA.0000000000001221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. (2020) 323:1612–4. 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yildirim AI, Karaagac AT. COVID-19 in a Young Girl with Restrictive Cardiomyopathy and Chronic Lung Disease. Indian Pediatri. (2020) 57:577. 10.1007/s13312-020-1863-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chadha S. ‘COVID-19 Pandemic'Anxiety induced Tako-tsubo Cardiomyopathy. QJM. (2020) 113:488–90. 10.1093/qjmed/hcaa135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim I-C, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. (2020) 41:1859. 10.1093/eurheartj/ehaa288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luetkens JA, Isaak A, Zimmer S, Nattermann J, Sprinkart AM, Boesecke C, et al. Diffuse myocardial inflammation in COVID-19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging. Circulation: Cardiovasc Imaging. (2020) 13:e010897. 10.1161/CIRCIMAGING.120.010897 [DOI] [PubMed] [Google Scholar]
- 48.Bonow RO, Fonarow GC, O'Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. (2020) 5:751–3. 10.1001/jamacardio.2020.1105 [DOI] [PubMed] [Google Scholar]
- 49.Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. (2020) 17:259–60. 10.1038/s41569-020-0360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu X-W, Wu X-X, Jiang X-G, Xu K-J, Ying L-J, Ma C-L, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. Bmj. (2020) 368:m606. 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respirat Med. (2020) 8:475–81. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahallawi WH Khabour OF Zhang Q Makhdoum HM Suliman BA . MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. (2018) 104:8–13. 10.1016/j.cyto.2018.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lang JP, Wang X, Moura FA, Siddiqi HK, Morrow DA, Bohula EA. A current review of COVID-19 for the cardiovascular specialist. Am Heart J. (2020) 226:29–44. 10.1016/j.ahj.2020.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bemtgen X, Krüger K, Supady A, Duerschmied D, Schibilsky D, Bamberg F, et al. First successful treatment of coronavirus disease 2019 induced refractory cardiogenic plus vasoplegic shock by combination of percutaneous ventricular assist device and extracorporeal membrane oxygenation: a case report. Asaio J. (2020) 66:607–9. 10.1097/MAT.0000000000001178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan W, Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int J Cardiol. (2020) 309:70–7. 10.1016/j.ijcard.2020.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fried JA, Ramasubbu K, Bhatt R, Topkara VK, Clerkin KJ, Horn E, et al. The variety of cardiovascular presentations of COVID-19. Circulation. (2020) 141:1930–6. 10.1161/CIRCULATIONAHA.120.047164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am College Cardiol. (2020) 75:2352–71. 10.1016/j.jacc.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harari R, Bangalore S, Chang E, Shah B. COVID-19 complicated by acute myocardial infarction with extensive thrombus burden and cardiogenic shock. Catheterization Cardiovasc Interven. (2020) 97:E661–6. 10.1002/ccd.28992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunologic features in severe and moderate forms of coronavirus disease. J Clin Invest. (2019) 130:2620–9. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao Y, Li T, Han M, Li X, Wu D, Xu Y, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. (2020) 92:791–6. 10.1002/jmv.25770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mouhat B, Besutti M, Bouiller K, Grillet F, Monnin C, Ecarnot F, et al. Elevated D-dimers and lack of anticoagulation predict PE in severe COVID-19 patients. Eur Respirat J. (2020) 56:2001811. 10.1183/13993003.01811-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jayedi A, Rahimi K, Bautista LE, Nazarzadeh M, Zargar MS, Shab-Bidar S. Inflammation markers and risk of developing hypertension: a meta-analysis of cohort studies. Heart. (2019) 105:686–92. 10.1136/heartjnl-2018-314216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circulat Res. (2015) 116:1022–33. 10.1161/CIRCRESAHA.116.303697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sawalha K, Abozenah M, Kadado AJ, Battisha A, Al-Akchar M, Salerno C, et al. Systematic review of COVID-19 related myocarditis: Insights on management and outcome. Cardiovasc Revasculariz Med. (2020) 23:107–13. 10.1016/j.carrev.2020.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trump S, Lukassen S, Anker MS, Chua RL, Liebig J, Thürmann L, et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat Biotechnol. (2020). 10.1038/s41587-020-00796-1. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 68.Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. (2010) 65:44–50. 10.1136/thx.2009.117572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iqbal Z, Ho JH, Adam S, France M, Syed A, Neely D, et al. Managing hyperlipidaemia in patients with COVID-19 and during its pandemic: An expert panel position statement from HEART UK. Atherosclerosis. (2020) 313:126–36. 10.1016/j.atherosclerosis.2020.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei X, Zeng W, Su J, Wan H, Yu X, Cao X, et al. Hypolipidemia is associated with the severity of COVID-19. J Clin Lipidol. (2020) 14:297–304. 10.1016/j.jacl.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nouri E, Delashoub O, Shahabi-Rabori MA, Afzalipour R, Jafari S. Interpretation of CT scan findings during the COVID-19 pandemic. Hormozgan Med J. (2020) 24:107909. 10.5812/hmj.10790933627933 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.