Abstract
The clinical presentation of binge eating disorder (BED) and data emerging from task-based functional neuroimaging research suggests that this disorder may be associated with alterations in reward processing. However, there is a dearth of research investigating the functional organization of brain networks that mediate reward in BED. To address this gap, 27 adults with BED and 21 weight-matched healthy controls (WMC) completed a multimodel assessment consisting of a resting functional magnetic resonance imaging scan, behavioral tasks measuring reward-based decision-making (i.e., delay discounting and reversal learning), and self-report assessing clinical symptoms. A seed-based approach was employed to examine the resting state functional connectivity (rsFC) of the striatum (nucleus accumbens [NAcc] and ventral and dorsal caudate), a collection of regions implicated in reward processing. Compared with WMC, the BED group exhibited lower rsFC of striatal seeds, with frontal regions mediating executive functioning (e.g., superior frontal gyrus [SFG]) and posterior, parietal, and temporal regions implicated in emotional processing. Lower NAcc–SFG rsFC was associated with more difficulties with reversal learning and binge eating frequency in the BED group. Results suggest that hypoconnectivity of striatal networks that integrate self-regulation and reward processing may promote the clinical phenomenology of BED. Interventions for BED may benefit from targeting these circuit-based disturbances.
Keywords: binge eating, caudate, nucleus accumbens, resting state functional connectivity, reward
Introduction
Binge eating disorder (BED) is a psychiatric illness characterized by recurrent episodes of binge eating (i.e., consumption of an unusually large amount of food in a discrete time period accompanied by a sense of loss of control over eating) in the absence of the repeated compensatory behaviors that are associated with bulimia nervosa (APA 2013). Lifetime prevalence rates of BED are estimated between 1% and 4.7% (Smink et al. 2013), making it the most prevalent of the primary eating disorders. BED is associated with a wide range of negative psychological consequences, including mood, anxiety, and substance use disorders, as well as with an increased risk of suicide attempt (Udo et al. 2019; Udo and Grilo 2019). Furthermore, approximately 75% of individuals with the disorder are classified as either overweight (body mass index [BMI] of 25–29.9 kg/m2) or obese (BMI > 30 kg/m2) and, therefore, are subject to the physical sequelae associated with excess body weight, such as cardiovascular and metabolic disorders (Kessler et al. 2013; Udo and Grilo 2019). Although a number of treatments have been developed to target BED, only half or fewer of affected individuals attain binge eating abstinence following standard treatments (Brownley et al. 2016), and the mechanisms of action for these treatments are poorly specified (Kober and Boswell, 2018).
Relative to other eating disorders, very little research has been conducted to elucidate the neurobiology of BED (Steward et al. 2018). Therefore, the underlying mechanisms of this disorder remain poorly understood. Given the heightened drive toward palatable food that is characteristic of BED, it has been suggested that abnormalities in reward responding and corresponding patterns of striatal functioning may be implicated in this disorder, paralleling findings from the literature on addictive disorders (Volkow et al. 2013; Volkow and Baler 2015). In particular, consistent with other eating disorder and addiction models (Smith and Robbins 2013; Walsh 2013; Pearson et al. 2015), recently proposed neurobiological models of BED suggest that frontostriatal dysfunctions promote the transition from typical eating behavior to an impulsive-compulsive pattern of recurrent binge eating (Kessler et al. 2016). These theories posit that individuals with BED, when exposed to palatable foods, initially experience hyperresponsivity in the ventrally located regions of the striatum (e.g., nucleus accumbens [NAcc] and ventral portions of the caudate), which demonstrate greater associations with the emotional and motivational components of reward responding (Di Martino et al. 2008; Huang et al. 2017). Over time, there is hypothesized to be a shift toward greater reactivity of the dorsal regions of the striatum (e.g., dorsal caudate), which are linked to habitual motor and cognitive functions informed by prior reward learning. Additionally, individuals with BED have demonstrated impairments in executive functions (e.g., response inhibition) that are facilitated by the activity of prefrontal regions (Balodis et al. 2013), limiting the ability to constrain the drive toward rewards. Thus, the phenomenology of BED is suggested to result from inadequate reward circuit coordination between striatal and prefrontal inputs, yielding an imbalance between hedonic pursuit and inhibition.
Although this area of research is nascent, studies utilizing functional neuroimaging have demonstrated heightened striatal activity and diminished prefrontal activity for individuals with BED compared with overweight controls during exposure to high-caloric images (Weygandt et al. 2012; Lee et al. 2017). Preliminary evidence also suggests that reduced frontostriatal activity during processing of nonfood rewards, perhaps reflecting decreased sensitivity to disorder-irrelevant stimuli, is associated with the persistence of binge eating following treatment for BED (Balodis et al. 2014). Further, a broader body of neuroimaging research based on the food addiction model has highlighted the salience of frontostriatal dysfunction in relation to binge eating behavior (Smith and Robbins 2013). Most of the limited number of studies focused on the functional patterns of frontostriatal circuitry in BED have utilized task-evoked designs with food cues as stimuli, which provide valuable information about reward responsivity in disorder-salient contexts. However, such studies do not address whether there are more generalized disruptions in the functional architecture of frontostriatal circuitry or in associated mechanisms such as reward-related impulsivity or compulsivity (Berner et al. 2017).
Thus, there is a need to extend the existing literature beyond stimulus-evoked neural activity in BED to further investigate the underlying functional characteristics of brain networks that may subserve the behavioral dysregulation characterizing this disorder. Resting state functional connectivity (rsFC), which examines the correlational patterns of naturally occurring fluctuations in the blood oxygen level-dependent (BOLD) signal between brain regions, provides an opportunity to investigate the abnormalities in the synchrony of brain circuits independent of cue presentation that may reflect transdiagnostic neurobiological mechanisms shared with other psychiatric disorders. Indeed, prior research has provided evidence for reduced rsFC between the striatum and prefrontal regions in relation to a variety of addictive behaviors (Kühn and Gallinat 2014; Motzkin et al. 2014; Zhou et al. 2018). However, to date, only one study has examined the rsFC of frontostriatal networks in a sample that specifically included participants with BED (Baek et al. 2017). Using a data-driven approach informed by graph theory, these researchers found that individuals with obesity (with or without BED) exhibited lower frontostriatal rsFC relative to a comparison group (BMIs between 18.1 and 25.9 kg/m2). Lower frontostriatal rsFC was related to higher BMI across groups. However, because this study did not specifically examine how patterns of frontostriatal rsFC differed between participants with and without BED, it can provide only limited insight into the neural patterns specifically associated with recurrent binge eating. Additionally, this investigation employed a whole brain approach, precluding a theoretically guided probe of reward circuit functioning, and it did not examine associations between rsFC and the performance on reward-based behavioral paradigms, which could link connectivity patterns to clinically relevant neurocognitive processes. Therefore, more data are needed to determine how frontostriatal rsFC patterns relate to behavioral and clinical indicators of reward dysfunction and symptom expression in BED. Additionally, it would be valuable to determine if the patterns of neural synchrony differ across the ventral areas of the striatum traditionally linked to reward and/or dorsal areas linked with habitual motor functions, given the theorized dissociable role of different striatal regions in the development and maintenance of BED (Kessler et al. 2016).
To address these gaps in the literature, we conducted the first seed-based investigation of rsFC focused on striatal regions of interest (ROIs) (i.e., the NAcc, ventral caudate, and dorsal caudate) to examine the organization of reward-related brain regions across the ventral through dorsal functional gradient from goal-oriented to habitual responding in adults with BED. Given the substantial overlap between BED and overweight/obesity, as well as the evidence regarding abnormal striatal activity in obesity (Volkow et al. 2013; Volkow and Baler 2015; Baek et al. 2017), the current study included a weight-matched control (WMC) group in order to provide findings specific to BED rather than excess weight more broadly. Based on the defining behavioral feature of BED (i.e., recurrent loss of control over consumption of a large amount of typically palatable food) and patterns of frontostriatal rsFC in additive disorders (Kühn and Gallinat 2014; Motzkin et al. 2014; Zhou et al. 2018), we hypothesized that individuals with BED would exhibit lower rsFC of all striatal ROIs with frontal regions involved in self-regulatory control.
In order to provide preliminary data regarding the clinical relevance of group differences in rsFC, we examined the correlations of rsFC with BED symptom severity (i.e., frequency of binge eating days). We also examined the relation between rsFC patterns and performance on neurocognitive paradigms that measure the tendency toward impulsive (i.e., delay discounting; Monterosso and Ainslie 1999) or compulsive (i.e., reversal learning; Izquierdo and Jentsch 2012) drive for reward. These measures were included due to the theoretical suggestion that BED is maintained by an impulsive and/or compulsive pattern of engagement toward food rewards, which may reflect more global reward response patterns (Kessler et al. 2016). These measures have been associated with externalizing behaviors in a variety of psychiatric disorders (van Timmeren et al. 2018; Lempert et al. 2019) and may capture a transdiagnostic bias toward the pathological pursuit of a range of rewards, including the reward of food consumption. Given the role of frontostriatal functioning in effective modulation of reward pursuit, it was hypothesized that reduced ventral and dorsal frontostriatal rsFC would be associated with a greater severity of binge eating and poorer performance on tasks assessing the tendency toward impulsivity and compulsivity in reward-seeking behaviors.
Materials and Methods
Participants
This case-controlled, cross-sectional study was approved by the Institutional Review Board at the University of Minnesota. Data used in the present study were obtained between December 2008 and July 2010 as a part of a broader study examining the differences in the dissociable influence of gamma-aminobutyric acid levels on the impulsivity between participants with BED or substance dependence and healthy controls. For this analysis, only BED and WMC participants were examined; results relevant to substance dependence have been previously reported (Camchong et al. 2011). Participants included 27 adults who met the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV; APA 2000) criteria for BED and 21 controls who were weight-, age-, gender-, and education-matched at the group level. Demographic and clinical characteristics of the sample are listed in Table 1. All inclusion and exclusion criteria are described in Section A and the matching procedures are described in Section B of the Supplementary Material. For female participants of childbearing potential, scanning and neurocognitive testing visits occurred in the luteal phase of the menstrual cycle. Written informed consent was obtained from all individuals prior to study enrollment, and the participants received financial compensation.
Table 1.
Demographic and clinical information for BED and WMC groupsa
| Variable | BED (n = 27) | WMC (n = 21) | Test statistic | p | Effect size |
|---|---|---|---|---|---|
| M (SD) or No. (%) | M (SD) or No. (%) | t/χ2 | d/V | ||
| Age (years) | 32.27 (8.54) | 30.90 (7.99) | 0.56 | 0.58 | 0.17 |
| Gender (% female) | 24 (88.9%) | 21 (100.0%) | 1.67 | 0.50 | 0.19 |
| Education (years) | 14.61 (2.09) | 14.75 (1.84) | −0.20 | 0.84 | 0.07 |
| BMI (kg/m2) | 33.19 (4.46) | 34.28 (4.62) | −0.80 | 0.43 | 0.25 |
| BDI | 35.04 (10.13) | 27.00 (4.64) | 3.34 | 0.002 | 1.01 |
| BAI | 32.67 (10.79) | 23.10 (2.36) | 3.98 | <0.001 | 1.19 |
| EDE binge eating frequency (days/month) | 12.08 (8.41) | — | — | — | — |
a d = Cohen’s d; V = Cramer’s V; gender not reported for one participant in the BED group.
Diagnostic Screening and Clinical Measures
All participants completed a full Structured Clinical Interview for DSM-IV Axis-I Disorders (SCID-IV; First et al. 1995) administered by an extensively trained member of the research staff to establish the eligibility criteria. Additionally, all participants with BED completed the Eating Disorder Examination-16 (EDE-16; Fairburn et al. 2008) and an investigator-led semi-structured interview assessing eating disorder symptoms to confirm BED diagnosis and obtain the number of days within the past month on which episodes of binge eating occurred, which served as our binge eating frequency variable in this study. The EDE-16 was not administered to WMC participants in order to reduce the measurement burden; however, the absence of binge eating in the WMC group was confirmed with the widely used questionnaire version of the EDE: the Eating Disorder Examination-Questionnaire 6.0 (EDE-Q; Fairburn and Beglin 2008). The EDE and EDE-Q have been shown to demonstrate generally high correspondence (Berg et al. 2012), and estimates of binge eating episode frequency have been shown to be slightly higher on the EDE-Q (Fairburn and Beglin 1994). Therefore, use of the EDE-Q to confirm the absence of recurrent binge eating is considered an adequate metric for determining a sample distinct from the BED. Participants also completed the Beck Depression Inventory (BDI; Beck et al. 1996) and the Beck Anxiety Inventory (BAI; Beck and Steer 1993) questionnaires; scores on these measures were used as covariates in analyses to determine if the group differences were impacted by depression or anxiety symptoms.
Behavioral Tasks
In a separate session prior to the functional magnetic resonance imaging (fMRI) scan, participants completed two behavioral tasks assessing the impulsive and compulsive reward pursuit in order to capture the transdiagnostic neurocognitive mechanisms related to dysfunctional reward seeking. Data were available for a subset of participants on these delay discounting (n = 33) and reversal learning (n = 34) tasks. See details about the group behavioral differences on these tasks in Section C of the Supplementary Material.
Delay Discounting Task
To assess the impulsive tendency toward immediate reward-related gratification, a delay discounting paradigm was used, which requires the participants to make a series of choices about whether to accept smaller immediate monetary rewards or larger monetary rewards administered on a delayed time scale. Participants were presented with two identical boxes on a computer screen and were instructed to make hypothetical choices between them by clicking a mouse: one with an immediate smaller monetary reward and another with a delayed larger monetary reward (e.g., “Would you rather have $5 now or $10 in 30 days?”). Although in some versions of delay discounting, financial payouts are given according to the participants’ choices, research suggests that choices on hypothetical and real scenarios are comparable (Johnson and Bickel 2002). The delay k parameter derived from this task represents the tendency toward immediate gratification. A higher delay k represents less willingness to delay gratification, which likely indexes more reward impulsivity. The delay k parameter is derived from the optimal discounting rate based upon minimizing the error around the five indifference points of the hyperbolic delay function.
Reversal Learning Task
The reversal learning task was used to assess the participants’ reward-based compulsivity versus flexibility in the ability to adapt their responses to shifting contingencies. In this task, which consists of three 5-min blocks with a maximum of 150 trials in each block, participants make choices between different stimuli to receive a reward. Participants were simultaneously presented with two visually dissimilar gray patterns. Participants had to respond to one of these objects by using either a left or a right button-press depending on whether their chosen object was on the left or right side of the screen. The screen gave feedback after each response to indicate if the reaction was correct or incorrect. After 10 correct responses, the strategy reversed and participants had to adapt their reactions and respond to the formerly wrong stimulus. To distract participants, false feedback was provided 20% of the time indicating a wrong choice despite a correct response. Although participants were informed that they would occasionally receive false feedback, they did not know how often this would occur. We examined trials to the first reversal, the number of trials required for the participant to first change behavior in response to a change in reward contingencies, which reflected the tendency to compulsively pursue previously rewarded cues.
Imaging Data Acquisition
Six-minute resting state fMRI data were collected using the vendor-supplied 12-channel receive-only head coil on a Siemens Tim Trio 3 T scanner. Participants were instructed to be as still as possible, keep their eyes closed, and stay awake. Participants were queried at the end of the scan to determine whether they had stayed awake. Sequence parameters included: gradient-echo echo-planar imaging (EPI) 180 volumes, time repetition (TR) = 2 s, time echo (TE) = 30 ms, flip angle = 90 °, 34 continuous anterior commissure–posterior cingulated cortex aligned axial slices with an interleaved acquisition, voxel size = 3.4 × 3.4 × 4.0 mm, and matrix = 64 × 64 × 34. For registration purpose, a high-resolution T1-weighted anatomical image was acquired using a magnetization prepared rapid gradient-echo sequence (TR = 2530 ms, TE = 3.65 ms, time to inversion [TI] = 1100 ms, flip angle = 7 °, and 1 mm isotropic voxel). A field map acquisition was collected and used to correct the fMRI data for geometric distortion caused by the magnetic field inhomogeneities. Time of day of fMRI acquisition was not standardized. Groups were matched on the average time of day at which imaging occurred; however, the BED group was scanned on average more recently to standard meal times (see Section D of the Supplementary Material).
fMRI Imaging Analysis
Data Preprocessing
The following fMRI data preprocessing steps were applied for each participant using FEAT (FMRIB’s Software Library [FSL]): deletion of the first three volumes (to account for magnetization stabilization), motion correction, B0 field map unwarping, slice-timing correction, nonbrain removal, spatial smoothing (with a 6-mm full-width half-maximum kernel), grand mean scaling, high-pass temporal filtering (100 Hz), and registration of all images to Montreal Neurological Institute (MNI) 2 × 2 × 2 mm standard space. The preprocessed fMRI data were used to calculate the individual level functional connectivity maps for each ROI. In order to remove all major sources of artifactual correlation in the rsFC data while preserving the integrity of the continuous time series, an Independent Component Analyses (ICA)-based denoising procedure was performed. The individual preprocessed 4D fMRI data sets were decomposed into independent spatiotemporal components using FSL MELODIC. Individual components were manually classified by an experienced rater (JC) as either noise or signal using spatial and temporal characteristics detailed in the MELODIC manual and previous methodological reports (Kelly et al. 2010). Components accounting for movement, respiration, heart rate, and head motion, and components localized to the ventricles and white matter signal were regressed out of the preprocessed data for each subject data during ICA denoising. BED and WMC groups did not differ in the percentage of components, explained variance, or total variance removed during ICA, t(46) = −0.14 to −0.37, ps = 0.478–0.890, indicating that noise was comparable between groups. Further, BED and WMC groups did not differ in the mean absolute value along the six motion parameters that characterize translations and rotations along x, y, and z dimensions, t(46) = −0.02 to −1.77, ps = 0.083–0.981, and no participant surpassed our threshold of >1.88 mm motion for removal (Camchong et al. 2017). Additional information characterizing the motion parameters can be found in Section E of the Supplementary Material.
ROI Generation
Figure 1 presents a visual representation of the NAcc, ventral caudate, and dorsal caudate seed maps with MNI coordinates. For all regions, we generated a spherical seed with 3.5 mm radius in the right and left hemispheres, which were then combined in the analysis. Center of mass MNI coordinates were as follows: x = ±12, y = 10, z = −9 (NAcc); x = ±10, y = 15, z = 0 (ventral caudate); and x = ±13, y = 15, z = 9 (dorsal caudate), established according to precedent from prior research that has parcellated the striatum (Di Martino et al. 2008; Camchong et al. 2013). Time series were extracted from each of these seeds for each participant.
Figure 1 .
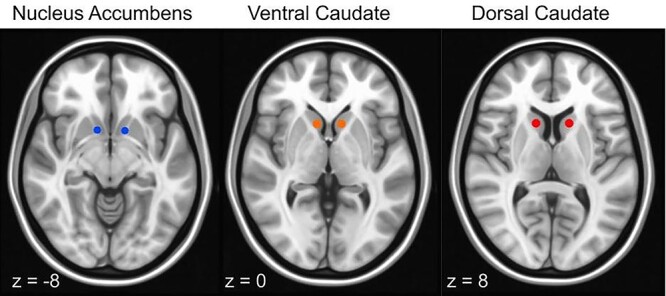
NAcc, ventral caudate, and dorsal caudate seed map: Image depicts nonoverlapping NAcc (blue) (right: x = 12, y = 10, z = −8; left: x = −10, y = 10, z = −8; only right is pictured); ventral caudate (orange) (bilateral, x = ±10, y = 15, z = 0); and dorsal caudate (red) (bilateral, x = ±13, y = 15, z = 9) seeds used to examine the strength of rsFC overlaid on MNI brain.
Resting-State Individual-Level Analysis
For each participant and each ROI (bilateral NAcc, ventral caudate, and dorsal caudate), we performed a multiple regression analysis on the denoised data. The correlation between the mean time course of each ROI and the time course of every voxel in the brain was calculated and Fisher z-transformed to standardized z values. Bilateral seeds were combined for each ROI (NAcc, ventral caudate, and dorsal caudate) to produce correlation maps reflecting connectivity associated with both left and right seeds. The resultant z maps showed the degree of positive or negative correlations between the corresponding NAcc and caudate seeds averaged time-series for each seed for each participant.
Group-Level Analysis
Group differences for each ROI were separately analyzed using 3dtest++ with AFNI, using the -clustsim option to calculate smoothness, given the non-Gaussian data distribution. Based on Cox et al. (2017) and addressing concerns of “inflated false-positive rates” raised by Eklund et al. (2016), Monte Carlo simulations (1000 iterations) accounted for the full-width half-maximum Gaussian filter (6 mm full-width at half-maximum [FWHM]; 3dFWHMx) and with a conservative connectivity radius of 5.6 mm, specifying that active voxels whose center of mass are less than 5.6 mm apart were considered as belonging to the same cluster. To avoid false positives, we selected the most stringent output (neural networks [NN] = 1 and bisided results) for significant clustering and thresholding. On the basis of these simulations, the familywise α of 0.01 was preserved with an a priori voxelwise probability of 0.005 and 3D clusters resulting in minimum volume criteria of 242 voxels for the NAcc, 83 voxels for the ventral caudate, and 83 voxels for the dorsal caudate. Using these minimum cluster size thresholds, clusters that survived correction for multiple comparisons were identified and used as masks from which individual mean z-scores were extracted for graphs visualization and for exploration of functional connectivity correlates. To examine whether results were impacted by symptoms of anxiety or depression, analyses of covariance were conducted to determine the group differences in rsFC after controlling for BDI and BAI scores, maintaining the familywise error rate, p < 0.01.
Associations of rsFC with Behavioral Tasks and Clinical Measures
To examine the relationship between rsFC strength from clusters that showed significant differences between the groups and measures of impulsive and compulsive reward pursuit, we extracted the average z-scores from clusters in Table 2. Separate Pearsons correlations (r) were conducted in the BED and WMC groups to examine the relations between rsFC z-scores within each of these clusters and: (1) behavioral measures representing the delay k parameter (delay discounting task) and (2) the average number of trials it took for an individual to perform first reversal (reversal learning task). Additionally, to examine the relationship between rsFC strength in ROIs and binge eating frequency, we conducted separate Pearson correlations (r) between the rsFC z-scores within each of the identified clusters and number of the past month binge eating days from the EDE-16 for the BED group only. Visual inspection and normality testing revealed that the delay k parameter was significantly skewed (Skewness statistic > 1.5; Shapiro–Wilk: p < 0.001); therefore, these data were log-transformed prior to correlational analyses. For all behavioral and self-report measures, significant outliers > 2.5 standard deviations (SD) above the mean were excluded from the correlation analyses, resulting in an exclusion of two BED participants from the correlations involving delay k and one BED participant from the correlations involving the reversal learning score. To avoid alpha inflation resulting from multiple separate statistical tests, all correlation analyses were two-tailed and corrected for familywise error using the Benjamini-Hochberg (1995) procedure.
Table 2.
Significant nodes of rsFC differences for NAcc, ventral caudate, and dorsal caudate seeds between individuals with BED (n = 27) and WMCs (n = 21)a
| Anatomical location (Brodmann area) | MNI Coordinates (mm) | Cluster (voxels) | t | p | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| NAcc seed | |||||||
| Frontal | L SFG (BA 9) | −6 | 52 | 43 | 963 | 6.09 | <0.001 |
| Posterior | L posterior cingulate (BA 23) | −2 | −54 | 18 | 269 | 3.88 | <0.001 |
| Ventral caudate seed | |||||||
| Frontal | L SFG (BA 9, 8) | −10 | 52 | 35 | 155 | 5.52 | <0.001 |
| 22 | 32 | 53 | 140 | ||||
| Dorsal caudate seed | |||||||
| Frontal | L SFG (BA 8, 6) | −10 | 50 | 44 | 302 | 5.48 | <0.001 |
| −26 | −6 | 68 | 288 | ||||
| L IFG (BA 47) | −18 | 30 | 52 | 234 | |||
| −50 | 36 | −6 | 103 | ||||
| Parietal | L inferior parietal lobule (BA 40) | −54 | −40 | 22 | 121 | 3.75 | <0.001 |
| Temporal | L middle temporal gyrus (BA 22) | −60 | −38 | 0 | 106 | 4.28 | <0.001 |
| R superior temporal gyrus (BA 41) | 48 | −40 | 20 | 100 | |||
Note: BA, Brodmann area; L, left; R, right.
a z-scores that survived thresholding and clustering (p < 0.01, corrected for multiple comparisons). In all results, WMCs > BED.
Results
Group Differences in Reward Circuitry rsFC
Table 2 provides descriptions and test statistics highlighting the rsFC nodes identified to be significantly different between the participants with BED and WMC for the NAcc, ventral caudate, and dorsal caudate seeds. Results did not vary according to gender when this variable was controlled (See Section F of the Supplementary Material).
NAcc
Significant differences were detected in the NAcc rsFC maps between the BED and WMC groups (see Fig. 2). Compared with WMCs, individuals with BED demonstrated lower rsFC between the NAcc and left superior frontal gyrus (SFG) and left posterior cingulate. Group differences in the NAcc–SFG (p < 0.001) and NAcc–posterior cingulate (p = 0.004) rsFC remained significant after controlling for BDI and BAI scores.
Figure 2 .
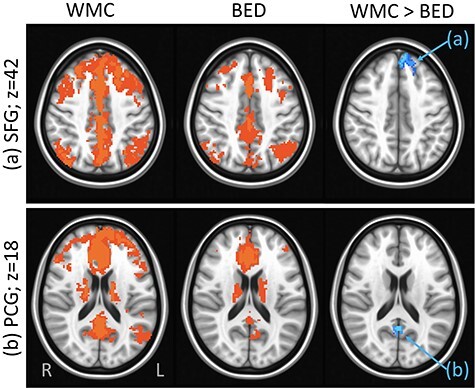
Group differences in rsFC from the NAcc seed: Results showing higher rsFC between NAcc and: (a) left SFG (Brodmann area 8, axial slice z = 42) and (b) posterior cingulate gyrus (PCG) (Brodmann area 23, axial slice z = 18) in WMC versus BED groups. Whole-brain rsFC maps showing regions with significant connectivity to NAcc, which survived thresholding and clustering to correct for multiple comparisons in WMC (first column) and BED (second column) groups. Third column shows whole-brain independent samples’ t-test results in which WMC had significantly higher rsFC than BED (p < 0.01, corrected for multiple comparisons). Functional maps are laid on MNI brains in radiological orientation, right (R) to left (L).
Ventral Caudate
Significant differences were detected in the ventral caudate rsFC maps between the BED and WMC groups (see Fig. 3). Compared with WMCs, individuals with BED showed lower rsFC between the ventral caudate and two clusters in the left SFG. Group differences in the ventral caudate–SFG (p < 0.001) rsFC remained significant after controlling for BDI and BAI scores.
Figure 3 .
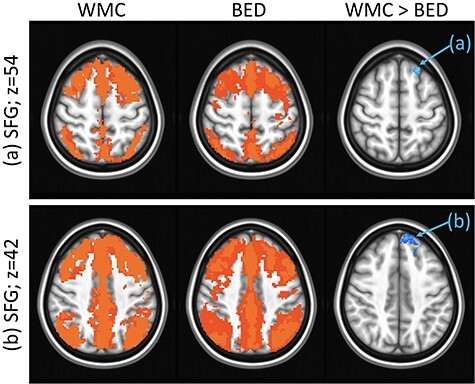
Group differences in rsFC from the ventral caudate seed: Results showing higher rsFC between ventral caudate and left SFG in (a) Brodmann area 8, axial slice z = 54 and (b) Brodmann area 9, axial slice z = 42 in WMCs versus BED groups. Whole-brain rsFC maps showing regions with significant connectivity to ventral caudate that survived thresholding and clustering to correct for multiple comparisons in WMC (first column) and BED (second column) groups. Third column shows whole-brain independent samples’ t-test results in which WMC had significantly higher rsFC than BED (p < 0.01, corrected for multiple comparisons). Functional maps are laid on MNI brains in radiological orientation, right (R) to left (L).
Dorsal Caudate
Significant differences were detected in the dorsal caudate rsFC maps between the BED and WMC groups (see Fig. 4). Compared with WMCs, individuals with BED showed lower rsFC between the dorsal caudate and seven clusters distributed across: (1) frontal regions (left SFG and left inferior frontal gyrus [IFG]); (2) left inferior parietal lobule; and (3) temporal regions (left middle temporal gyrus and right superior temporal gyrus). Group differences in all dorsal caudate and frontal (p < 0.001) and temporal (p = 0.002) rsFC remained significant, but dorsal caudate–left inferior parietal lobule rsFC was no longer significant (p = 0.020) after controlling for BDI and BAI scores.
Figure 4 .
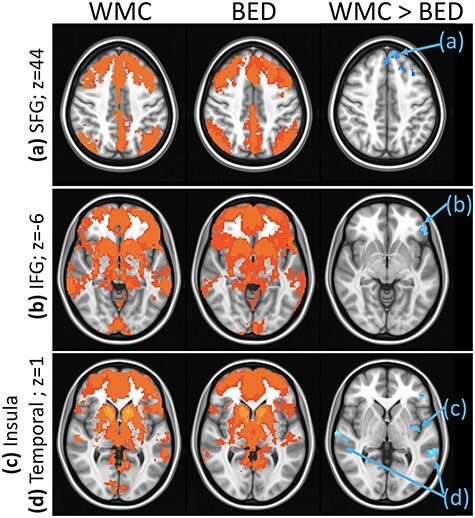
Group differences in rsFC from the dorsal caudate seed: Results showing higher rsFC between dorsal caudate and (a) left SFG (Brodmann area 8, axial slice z = 44), (b) left IFG (Brodmann area 47, axial slice z = −6), (c) left parietal lobule (Brodmann area 40, axial slice z = 1), (d) bilateral temporal gyrus (Brodmann areas 22 and 41, axial slice z = 1) in WMCs versus BED groups. Whole-brain rsFC maps showing regions with significant connectivity to dorsal caudate that survived thresholding and clustering to correct for multiple comparisons in WMC (first column) and BED (second column) groups. Third column shows whole-brain independent samples’ t-test results in which WMC had significantly higher rsFC than BED (p < 0.01, corrected for multiple comparisons). Functional maps are laid on MNI brains in radiological orientation, right (R) to left (L).
Associations Between rsFC and Reward-Based Behavioral Tasks
See Sections G–I of the Supplementary Material for Pearson correlations and p values for the associations between reward-based behavioral tasks and rsFC patterns across and within groups. No significant associations were detected between the delay discounting k parameter and any patterns of rsFC across or between groups. Across groups, there was a significant negative correlation between reversal learning scores and NAcc–SFG rsFC (r = −0.44, p = 0.005), indicating that individuals with lower rsFC connectivity between these regions displayed a more compulsive pattern of reward responding (higher number of trials before first reversal; see Fig. 5). Although it appeared that this association was stronger in participants with BED (r = −0.50, p = 0.045) versus WMCs (r = −0.25, p = 0.36), none of the correlations between rsFC and reversal learning in the BED or WMC groups separately were significant following familywise error correction.
Figure 5 .
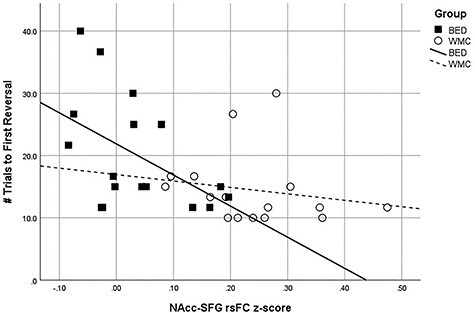
Associations between NAcc–SFG rsFC strength and reversal learning number of trials to first reversal: Results showing significant negative correlation between NAcc-SFG RSFC z-scores and reversal learning scores. BED group are depicted with squares and WMC group with circles.
Associations Between rsFC and Binge Eating
See Section H of the Supplementary Material for Pearson correlations and p values for the associations between rsFC patterns and binge eating frequency in the BED group. The number of past month binge eating days was significantly correlated with the rsFC strength between the NAcc and SFG (r = −0.44, p = 0.028) and posterior cingulate (r = −0.45, p = 0.025) such that the lower rsFC between these regions for the individuals with BED was associated with more days of binge eating. There were no significant associations between rsFC differences arising from the ventral (r = −0.32, p = 0.120) or dorsal (rs = −0.12 to −0.33, ps = 0.102–0.579) caudate and binge eating days.
Discussion
This study was the first to examine the underlying neurobiological disturbances in the functional organization of the striatum in BED using theoretically guided seed-based rsFC. Given theory and emerging evidence suggesting that reward-related deficits may characterize BED (Weygandt et al. 2012; Kessler et al. 2016; Lee et al. 2017), we investigated striatal rsFC along the ventral to dorsal continuum. This approach allowed us to detect disturbances in the circuitry mediating the distinct aspects of reward processing from initial responsivity through more overtrained, compulsive patterns. Consistent with our hypotheses, our findings identified significantly lower rsFC between the ventral through dorsal striatum and frontal regions in BED compared with WMC groups. Ventral frontostriatal hypoconnectivity was significantly associated with a poorer performance on a decision-making task measuring compulsive reward seeking (reversal learning) as well as with frequency of binge eating, the core behavioral symptom of BED. Individuals with BED further demonstrated hypoconnectivity between the striatum and posterior and temporal regions involved in a range of decision-making functions (Lee and Xue 2018). However, these findings were not specifically hypothesized or linked to the behavioral and clinical correlates in this study; therefore, the import of these patterns warrants further investigation. Taken together, our data suggest that symptoms of BED may arise from and be maintained by a disturbance in the functional architecture of the brain’s reward system and its connections to other regions guiding cognitive processing, paralleling other clinical disturbances characterized by behavioral dysregulation (Kühn and Gallinat 2014; Motzkin et al. 2014; Zhou et al. 2018).
Individuals with BED, compared with a WMC group, demonstrated lower rsFC between all striatal seeds and the left SFG, a frontal region involved with executive control (Lee and Xue 2018). These findings parallel data from a previous study conducted in a sample of participants with overweight/obesity, which included individuals with and without BED (Baek et al. 2017). Effective responding to rewarding stimuli requires an ability to sensitively coordinate automatic approach tendencies with cognitive and behavioral inhibition. Our data suggest that the brain regions subserving these functions are less synchronized at rest in BED compared with individuals of a similar body weight without an eating disorder. Prior research has reported similar patterns of frontostriatal hypoconnectivity in relation to higher weight and disinhibition over eating in adult and pediatric samples (Baek et al. 2017; Zhao et al. 2017; Shapiro et al. 2019). Thus, impairment in reward circuit functioning may be broadly involved in difficulties with self-regulating the food intake. Notably, because the frontostriatal circuit demonstrated a lower connectivity among individuals with BED compared with other individuals with a higher weight, this pattern of neural functioning may represent a mechanism of the heightened experience of dyscontrol over eating, which is characteristic of this disorder. Because this study was cross-sectional, however, it cannot be determined whether the frontostriatal hypoconnectivity reflects a risk or maintenance mechanism for BED, a consequence of the disorder, or perhaps both.
The identified pattern of lower rsFC between the brain regions involved in reward and self-control and decision-making found in the BED group in this study parallels findings from the investigations of substance abuse disorders (Camchong et al. 2013; Motzkin et al. 2014; Zhou et al. 2018) and behavioral addictions (Chen et al. 2016). Therefore, shared biological disturbances of the reward circuitry may underlie a range of psychiatric disorders that involved an excessive pursuit of different types of rewards (e.g., food and drugs). Considered in the context of this broader literature, the findings from our study also indicate that the reward disturbances that have been identified previously in BED using disorder-specific cues (Weygandt et al. 2012; Lee et al. 2017) may originate from dysfunctions in the basic functional architecture neural networks involved in reward responding. This could potentially account for the high levels of comorbidity between BED and other psychiatric disorders characterized by behavioral dysregulation (Ulfvebrand et al. 2015). However, because this study investigated responding during rest, data on functional connectivity during paradigms that are not disorder-specific are needed to determine the validity of this hypothesis. Further research, including longitudinal investigations of rsFC in BED during rest and task, are needed in order to more fully characterize the relation between frontostriatal synchrony and the psychopathology of BED as well as to better understand the similarities with and differences from other forms of addictive behavior.
The potential clinical implications of the rsFC group differences found here are strengthened through their significant associations with theoretically relevant neurocognitive tasks and clinical symptoms. Lower rsFC between the NAcc and SFG was associated with poorer performance on a reversal learning task across groups as well as with frequency of binge eating in the BED group. In contrast, despite prior research indicating that reward impulsivity and underlying circuit disturbances may distinguish individuals with BED from normal and higher weight controls (Davis et al. 2010; Bartholdy et al. 2017), the current study did not find any significant associations between the delay discounting k parameter and any of the identified rsFC patterns. Therefore, ventral frontostriatal dysconnectivity may drive a compulsive pattern of reward seeking characterized by an inability to disengage with previously rewarded experiences (rather than an immediate need for gratification) among individuals with higher weight. In BED, hypoconnectivity also was linked to frequency of the core clinical feature of binge eating, suggesting that this recurrent behavior may reflect an extension of the compromised ability to shift away from a reward response pattern that is no longer adaptive. Although the present findings are cross-sectional, prior research has found frontostriatal hypoconnectivity to longitudinally predict binge eating (Dunlop et al. 2015) as well as other addictive behaviors (Camchong et al. 2013; Berlingeri et al. 2017). Therefore, although additional prospective clinical research is needed, our results lend preliminary support to the notion that that targeted neuromodulation (Dunlop et al. 2015) and other interventions designed to strengthen frontostriatal connections (Eichen et al. 2017) may hold promise for decreasing ineffective and maladaptive persistence toward reward in this population.
In light of theoretical models conceptualizing BED as arising from both impulsive and compulsive processes (Kessler et al. 2016), we investigated the connectivity of reward-related striatal regions across the ventral regions which are more commonly associated with initial goal-oriented responding to rewards through to dorsal regions that are more commonly linked to habitual responses to reward. In our analyses, the degree of network disturbance increased dorsally along this axis, suggesting more widespread hypoconnectivity in the areas implicated in habitual reward learning (e.g., dorsal striatum). However, low synchrony between the NAcc (the most ventral region of the striatum commonly involved in goal-oriented behavior) and the prefrontal cortex was specifically associated with binge eating. These findings parallel a prior study from our group investigating rsFC in anorexia nervosa (Haynos et al. 2019). In that study, individuals with anorexia nervosa also demonstrated the most diffuse pattern of hypoconnectivity between the dorsal striatum and other brain regions; however, when examining clinical correlates, only NAcc–SFG connectivity was associated with eating disorder symptoms (measured by the EDE). Disruptions in both goal-directed and habit learning have been implicated across a range of eating disorders (Smith and Robbins 2013; Walsh 2013; Pearson et al. 2015). These findings suggest that disturbances in ventral frontostriatal circuitry involved in goal-directed learning may be a shared mechanism that is implicated in the core symptoms of eating disorders (i.e., binge eating and restraint).
The clinical impact of the low connectivity between the dorsal striatum and other brain regions in this study, as well as in our other study in anorexia nervosa (Haynos et al. 2019), remains unclear because these rsFC patterns were not significantly associated with our behavioral or clinical measures. However, our covariate analyses suggest that the dorsal striatal-parietal hypoconnectivity may be linked to elevated mood and anxiety symptoms in BED. In this study, we did not measure the duration of illness. It is possible that different illness stages may be associated with the different patterns of striatal functioning, as theoretical models suggest that the habit dysfunction of the dorsal striatum may be more characteristic of more chronic forms of disordered eating (Walsh 2013). Further investigation is needed to identify whether the low rsFC of the dorsal striatum and other brain regions is linked with illness duration, unmeasured symptoms, or eating disorder sequelae in BED and other eating disorder samples.
There are several strengths of this investigation, most notably the multimodal design integrating neuroimaging, neurocognitive tasks assessing different aspects of reward-based decision-making, and clinical measures. Further, the use of an age-, gender-, weight-, and education-matched control group allowed a stronger test of BED mechanisms independent of BMI. However, certain limitations also warrant consideration. There are several limitations related to the study sample. The study participants were limited in gender and ethnic diversity, and binge eating was assessed using different versions (interview or questionnaire) of the EDE, which may have yielded inconsistencies between groups. Although history of bulimia nervosa or other recurrent compensatory behavior was exclusionary for this study, unfortunately, information on other past eating disorder diagnoses (e.g., anorexia nervosa) was not available for this sample. Given that significant crossover between eating disorder diagnoses is common over time (Castellini et al. 2011), we cannot exclude the possibility that an undetected eating disorder history may have impacted patterns of the brain function. Additionally, confounding psychiatric comorbidities beyond the current major depressive disorder were excluded, which may have limited the generalizability of the sample. However, because unipolar depressive disorders are the most common comorbidity reported among individuals with BED (Ulfvebrand et al. 2015), we believe that the findings of this study extend to a substantial proportion of this population. Further, although a stable use of most classes of psychotropic medications was permitted among participants, we were unable to investigate the effects of medication use, dosage, or changes on the study findings. Given that prior research has identified psychotropic effects on rsFC (McCabe and Mishor, 2011), it is possible that medication differences between groups could have accounted for some of the study findings. More diverse samples with more thorough accounting of psychiatric history, comorbidities, and medication use are needed for future investigations.
There were additional limitations related to the methods of these investigations. This research was cross-sectional, limiting the ability to establish causal links between neurobiological indices and clinical measures. Although 6 min was a typical length of time for an rsFC scan when this study was conducted, recent analyses suggest a longer data collection is associated with greater inter- and intrasession reliability (Birn et al. 2013), and attention checks were not performed to ensure consistent alertness. Additionally, food consumption prior to imaging was not controlled, and no data are available on consumption or hunger and satiety levels prior to the scan. Based on the timing of the fMRI scan, there was some indication that individuals in the BED group may have been scanned in closer time proximity to a recent meal; however, we could not definitively confirm this. Previous research in remitted anorexia nervosa and bulimia nervosa samples has suggested that brain activity in reward regions may differ between fed and fasting states (Ely et al. 2017; Kaye et al. 2020); therefore, between-subject differences in hunger states may have impacted the reward network activity. Finally, different methods exist to examine the rsFC patterns, including those that can interrogate fully established circuits (e.g., salience, default mode, or frontoparietal networks). Future research on rsFC in BED would benefit from longitudinal investigations with greater experimental control over hunger/satiety and a more diverse set of analysis approaches.
In conclusion, BED is associated with a broad range of psychiatric comorbidities and a heightened risk of overweight and obesity (Kessler et al. 2013; Udo et al. 2019; Udo and Grilo 2019), necessitating an urgent need for effective clinical intervention. The results of this investigation implicate an altered functional organization of the striatum, especially the more ventral regions, as a key mechanism involved in the excess pursuit of food rewards in BED. Thus, interventions aimed at enhancing frontostriatal connections and the underlying ability to balance the drive toward reward consumption with executive functions are likely to hold promise for this serious and prevalent eating disorder.
Supplementary Material
Contributor Information
Ann F Haynos, Department of Psychiatry and Behavioral Sciences, University of Minnesota, Minneapolis, 55454 MN, USA.
Jazmin Camchong, Department of Psychiatry and Behavioral Sciences, University of Minnesota, Minneapolis, 55454 MN, USA.
Carolyn M Pearson, Department of Psychiatry and Behavioral Sciences, University of Minnesota, Minneapolis, 55454 MN, USA.
Jason M Lavender, Military Cardiovascular Outcomes Research (MiCOR) Program, Department of Medicine, Uniformed Services University of the Health Sciences, Bethesda, 20814 MD, USA; Metis Foundation, San Antonio, 78205 TX, USA.
Bryon A Mueller, Department of Psychiatry and Behavioral Sciences, University of Minnesota, Minneapolis, 55454 MN, USA.
Carol B Peterson, Department of Psychiatry and Behavioral Sciences, University of Minnesota, Minneapolis, 55454 MN, USA.
Sheila Specker, Department of Psychiatry and Behavioral Sciences, University of Minnesota, Minneapolis, 55454 MN, USA.
Nancy Raymond, Department of Psychiatry, University of Wisconsin, Madison, 53719 WI, USA.
Kelvin O Lim, Department of Psychiatry and Behavioral Sciences, University of Minnesota, Minneapolis, 55454 MN, USA; Minneapolis VA Health Care System, Minneapolis, 55417 MN, USA.
Funding
National Institute of Health (grants R01DA038984, P20DA024196, KL2TR000113, K23MH112867, K23MH101342, and T32MH082761).
Notes
The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the National Institute of Health, Uniformed Services University, or the Department of Defense. Conflict of Interest: None declared.
References
- American Psychiatric Association . 2000. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: APA. [Google Scholar]
- American Psychiatric Association . 2013. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: APA. [Google Scholar]
- Baek K, Morris LS, Kundu P, Voon V. 2017. Disrupted resting-state brain network properties in obesity: decreased global and putaminal cortico-striatal network efficiency. Psychol Med. 47:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Grilo CM, Kober H, Worhunsky PD, White MA, Stevens MC, Pearlson GD, Potenza MN. 2014. A pilot study linking reduced fronto-striatal recruitment during reward processing to persistent bingeing following treatment for binge-eating disorder. Int J Eat Disord. 47:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Molina ND, Kober H, Worhunsky PD, White MA, Sinha R, Grilo CM, Potenza MN. 2013. Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity. 21:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholdy S, Rennalls S, Danby H, Jacques C, Campbell IC, Schmidt U, O'Daly OG. 2017. Temporal discounting and the tendency to delay gratification across the eating disorder spectrum. Eur Eat Disord Rev. 25:344–350. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. 1993. Beck Anxiety Inventory manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beck AT, Steer RA, Brown GK. 1996. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodology. 57:289–300. [Google Scholar]
- Berg KC, Stiles-Shields EC, Swanson SA, Peterson CB, Lebow J, Le Grange D. 2012. Diagnostic concordance of the interview and questionnaire versions of the eating disorder examination. Int J Eat Disord. 45:850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlingeri M, Losasso D, Girolo A, Cozzolino E, Masullo T, Scotto M, Sberna M, Bottini G, Paulesu E. 2017. Resting state brain connectivity patterns before eventual relapse into cocaine abuse. Behav Brain Res. 327:121–132. [DOI] [PubMed] [Google Scholar]
- Berner LA, Winter SR, Matheson BE, Benson L, Lowe MR. 2017. Behind binge eating: a review of food-specific adaptations of neurocognitive and neuroimaging tasks. Physiol Behav. 176:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, Nair VA, Meyerand ME, Vivek P. 2013. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 83:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownley KA, Berkman ND, Peat CM, Lohr KN, Cullen KE, Bann CM, Bulik CM. 2016. Binge-eating disorder in adults: a systematic review and meta-analysis. Ann Intern Med. 165:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Lim KO, Kumra S. 2017. Adverse effects of cannabis on adolescent brain development: a longitudinal study. Cereb Cortex. 27:1922–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, MacDonald AW 3rd, Nelson B, Bell C, Mueller BA, Specker S, Lim KO. 2011. Frontal hyperconnectivity related to discounting and reversal learning in cocaine subjects. Biol Psychiatry. 69:1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger A, Fein G. 2013. Resting-state synchrony during early alcohol abstinence can predict subsequent relapse. Cereb Cortex. 23:2086–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellini G, Lo Sauro C, Mannucci E, Ravaldi C, Rotella CM, Faravelli C. 2011. Diagnostic crossover and outcome predictors in eating disorders according to DSM-IV and DSM-V proposed criteria: a 6-year follow-up study. Psychosom Med. 73:270–279. [DOI] [PubMed] [Google Scholar]
- Chen CY, Yen JY, Wang PW, Liu GC, Yen CF, Ko CH. 2016. Altered functional connectivity of the insula and nucleus accumbens in internet gaming disorder: a resting state fMRI study. Eur Addict Res. 22:192–200. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. 2017. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 7:152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Patte K, Curtis C, Reid C. 2010. Immediate pleasures and future consequences. a neuropsychological study of binge eating and obesity. Appetite. 54:208–213. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. 2008. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 18:2735–2747. [DOI] [PubMed] [Google Scholar]
- Dunlop K, Woodside B, Lam E, Olmsted M, Colton P, Giacobbe P, Downer J. 2015. Increases in frontostriatal connectivity are associated with response to dorsomedial repetitive transcranial magnetic stimulation in refractory binge/purge behaviors. Neuroimage Clin. 8:611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichen DM, Matheson BE, Appleton-Knapp SL, Boutelle KN. 2017. Neurocognitive treatments for eating disorders and obesity. Curr Psychiatry Rep. 19:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely AV, Wierenga CE, Bischoff-Grethe A, Bailer UF, Berner LA, Fudge JL, Paulus MP, Kaye WH. 2017. Response in taste circuitry is not modulated by hunger and satiety in women remitted from bulimia nervosa. J Abnorm Psychol. 126:519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, O’Connor ME. 2008. Eating Disorder Examination (16.0D). In: Fairburn CG, editor. Cognitive behavior therapy and eating disorders. New York: Guilford Press. [Google Scholar]
- Fairburn CG, Beglin SJ. 1994. Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord. 16:363–370. [PubMed] [Google Scholar]
- Fairburn CG, Beglin S. 2008. The Eating Disorder Examination Questionnaire (EDE-Q 6.0). In: Fairburn CG, editor. Cognitive behavior therapy and eating disorders. New York: Guilford Press, pp. 309–314. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. 1995. Structured clinical interview for Axis I DSM-IV disorders. Patient edition (SCID-I/P, version 2.0). New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Haynos AF, Hall LMJ, Lavender JM, Peterson CB, Crow SJ, Klimes-Dougan B, Cullen KR, Lim KO, Camchong J. 2019. Resting state functional connectivity of networks associated with reward and habit in anorexia nervosa. Hum Brain Mapp. 40:652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Nguyen PT, Schwab NA, Tanner JJ, Price CC, Ding M. 2017. Mapping dorsal and ventral caudate in older adults: method and validation. Front Aging Neurosci. 9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Jentsch DJ. 2012. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl). 219:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. 2002. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 77:129–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bischoff-Grethe A, Berner LA, Ely AV, Bailer UF, Paulus MP, Fudge JL. 2020. Neural insensitivity to the effects of hunger in women remitted from anorexia nervosa. Am J Psychiatry. doi: 10.1176/appi.ajp.2019.19030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RE, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ. 2010. Visual inspection of independent components: defining a procedure for artifact removal from fmri data. J Neurosci Methods. 189:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Chiu WT, Deitz AC, Hudson JI, Shahly V, Aguilar-Gaxiola S, Alonso J, Angermeyer MC, Benjet C et al. 2013. The prevalence and correlates of binge eating disorder in the World Health Organization world mental health surveys. Biol Psychiatry. 73:904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RM, Hutson PH, Herman BK, Potenza MN. 2016. The neurobiological basis of binge-eating disorder. Neurosci Biobehav Rev. 63:223–238. [DOI] [PubMed] [Google Scholar]
- Kober H, Boswell RG. 2018. Potential psychological & neural mechanisms in binge eating disorder: implications for treatment. Clin Psychol Rev. 60:32–44. [DOI] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. 2014. Brain structure and functional connectivity associated with pornography consumption: the brain on porn. JAMA Psychiat. 71:827–834. [DOI] [PubMed] [Google Scholar]
- Lee JE, Namkoong K, Jung YC. 2017. Impaired prefrontal cognitive control over interference by food images in binge-eating disorder and bulimia nervosa. Neurosci Lett. 651:95–101. [DOI] [PubMed] [Google Scholar]
- Lee TW, Xue SW. 2018. Does emotion regulation engage the same neural circuit as working memory? A meta-analytical comparison between cognitive reappraisal of negative emotion and 2-back working memory task. PLoS One. 13:e0203753. doi: 10.1371/journal.pone.0203753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempert KM, Steinglass JE, Pinto A, Kable JW, Simpson HB. 2019. Can delay discounting deliver on the promise of RDoC? Psychol Med. 49:190–199. [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z. 2011. Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. Neuroimage. 57:1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso J, Ainslie G. 1999. Beyond discounting: possible experimental model of impulse control. Psychopharmacology. 146:339–347. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Baskin-Sommers A, Newman JP, Kiehl KA, Koenigs M. 2014. Neural correlates of substance abuse: reduced functional connectivity between areas underlying reward and cognitive control. Hum Brain Mapp. 35:4282–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CM, Wonderlich SA, Smith GT. 2015. A risk and maintenance model for bulimia nervosa: from impulsive action to compulsive behavior. Psychol Rev. 122:516–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ALB, Johnson SL, Sutton B, Legget KT, Dabelea D, Tregellas JR. 2019. Eating in the absence of hunger in young children is related to brain reward network hyperactivity and reduced functional connectivity in executive control networks. Pediatr Obes. 14:e12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smink FRE, van Hoeken D, Hoek HW. 2013. Epidemiology, course, and outcome of eating disorders. Curr Opin Psychiatry. 26:543–548. [DOI] [PubMed] [Google Scholar]
- Smith DG, Robbins TW. 2013. The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol Psychiatry. 73:804–810. [DOI] [PubMed] [Google Scholar]
- Steward T, Menchon JM, Jiménez-Murcia S, Soriano-Mas C, Fernandez-Aranda F. 2018. Neural network alterations across eating disorders: a narrative review of fMRI studies. Curr Neuropharmacol. 16:1150–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo T, Bitley S, Grilo CM. 2019. Suicide attempts in US adults with lifetime DSM-5 eating disorders. BMC Med. 17:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo T, Grilo CM. 2019. Psychiatric and medical correlates of DSM-5 eating disorders in a nationally representative sample of adults in the United States. Int J Eat Disord. 52:42–50. [DOI] [PubMed] [Google Scholar]
- Ulfvebrand S, Birgegård A, Norring C, Högdahl L, von Hausswolff-Juhlin Y. 2015. Psychiatric comorbidity in women and men with eating disorders results from a large clinical database. Psychiatry Res. 230:294–299. [DOI] [PubMed] [Google Scholar]
- van Timmeren T, Daams JG, van Holst RJ, Goudriaan AE. 2018. Compulsivity-related neurocognitive performance deficits in gambling disorder: A systematic review and meta-analysis. Neurosci Biobehav Rev. 84:204–217. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD. 2015. NOW vs LATER brain circuits: implications for obesity and addiction. Trends Neurosci. 38:345–352. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. 2013. Obesity and addiction: neurobiological overlaps. Obes Rev. 14:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh BT. 2013. The enigmatic persistence of anorexia nervosa. Am J Psychiatry. 170:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weygandt M, Schaefer A, Schienle A, Haynes JD. 2012. Diagnosing different binge-eating disorders based on reward-related brain activation patterns. Hum Brain Mapp. 33:2135–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Li M, Zhang Y, Song H, von Deneen KM, Shi Y, Liu Y, He D. 2017. Intrinsic brain subsystem associated with dietary restraint, disinhibition and hunger: an fMRI study. Brain Imaging Behav. 11:264–277. [DOI] [PubMed] [Google Scholar]
- Zhou F, Zimmermann K, Xin F, Scheele D, Dau W, Banger M, Weber B, Hurlemann R, Kendrick KM, Becker B. 2018. Shifted balance of dorsal versus ventral striatal communication with frontal reward and regulatory regions in cannabis-dependent males. Hum Brain Mapp. 39:5062–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


