Abstract
Background
Microvascular dysfunction in the setting of ST-elevated myocardial infarction (STEMI) plays an important role in long-term poor clinical outcome. Coronary flow reserve (CFR) is a well-established physiological parameter to interrogate the coronary microcirculation. Together with hyperaemic average peak flow velocity, CFR constitutes the coronary flow capacity (CFC), a validated risk stratification tool in ischaemic heart disease with significant prognostic value. This mechanistic study aims to elucidate the time course of the microcirculation as reflected by alterations in microcirculatory physiological parameters in the acute phase and during follow-up in STEMI patients.
Methods
We assessed CFR and CFC in the culprit and non-culprit vessel in consecutive STEMI patients at baseline (n = 98) and after one-week (n = 64) and six-month follow-up (n = 65).
Results
A significant trend for culprit CFC in infarct size as determined by peak troponin T (p = 0.004), time to reperfusion (p = 0.038), the incidence of final Thrombolysis In Myocardial Infarction 3 flow (p = 0.019) and systolic retrograde flow (p = 0.043) was observed. Non-culprit CFC linear contrast analysis revealed a significant trend in C-reactive protein (p = 0.027), peak troponin T (p < 0.001) and heart rate (p = 0.049). CFC improved both in the culprit and the non-culprit vessel at one-week (both p < 0.001) and six-month follow-up (p = 0.0013 and p < 0.001) compared with baseline.
Conclusion
This study demonstrates the importance of microcirculatory disturbances in the setting of STEMI, which is relevant for the interpretation of intracoronary diagnostic techniques which are influenced by both culprit and non-culprit vascular territories. Assessment of non-culprit vessel CFC in the setting of STEMI might improve risk stratification of these patients following coronary reperfusion of the culprit vessel.
Keywords: Myocardial infarction, coronary flow reserve, coronary flow capacity
Introduction
Primary percutaneous coronary intervention (PCI) is considered the cornerstone for treating ST-segment elevation myocardial infarction (STEMI), and the implementation of dedicated revascularization networks has resulted in a remarkable decline in cardiac morbidity and mortality.1 Despite these advancements, a significant proportion of patients have a poor outcome, which is attributed to changes in the microvascular function and integrity due to the ischaemic event.2 It is increasingly recognized that the impact of the acute ischaemic event on the functional and structural integrity of the microcirculation may yield opportunities to further enhance clinical outcomes in STEMI patients.3
Coronary flow reserve (CFR) is a well-validated index that assesses the contribution of obstructive, diffuse and microcirculatory involvement to coronary flow impairment in ischaemic heart disease.4–6 In the past decades it has been extensively used to elucidate the role of microvascular dysfunction for the prognosis of myocardial infarction. However, assessing the coronary microcirculation solely by means of CFR is inherently cumbersome in STEMI patients, since residual effects of the ischaemic events and changes in (regional) cardiac workload may influence resting or hyperaemic flow and thereby obscure microvascular function assessment by CFR values.7
Recently the coronary flow capacity (CFC) concept has been validated as a cross modality platform for the diagnosis, prognosis and risk-stratification in ischaemic heart disease.7,8 It integrates both the coronary vasodilatory reserve as well as maximal achievable flow, thereby providing comprehensive insight into coronary haemodynamics.9 Accordingly, CFC was documented to be less prone to alterations in systemic haemodynamics.10 In the present study we aimed to document the impact of STEMI on CFC in 1) the ischaemic region of the myocardium and 2) in myocardial territories remote from the infarction at baseline, one-week and six-month follow-up.
Methods
Between April 1997 and August 2000, 98 consecutive patients with a first anterior wall STEMI treated by primary PCI were enrolled in the study, for whom the initial results have been reported previously.2,11 All patients were treated in the Amsterdam University Medical Centres – location AMC, a large tertiary referral centre in Amsterdam, The Netherlands.
Anterior STEMI was defined as chest pain lasting >30 min in the presence of persistent ST-segment elevation in ≥2 precordial leads. Primary PCI was performed within 6 h after the onset of symptoms according to standard clinical practice, with provisional bare metal stent implantation. Major exclusion criteria comprised prior anterior wall myocardial infarction, acute left-side heart failure (Killip class >II), prior coronary artery bypass grafting, known left ventricular ejection fraction of <40%, left ventricular hypertrophy, absence of thoracic windows for echocardiography, three-vessel coronary artery disease, Thrombolysis In Myocardial Infarction (TIMI) grade 2 or 3 flow at initial angiography before PCI, or unsuccessful PCI defined as TIMI grade 0 or 1 flow or >50% residual stenosis in the infarct-related artery after PCI. The study protocol was approved by the local ethics committee and all patients gave informed consent.
Cardiac catheterization and periprocedural measurements
Five to 10 minutes after successful reperfusion, intracoronary blood flow velocity was measured in the infarct related artery using a 0.014-inch sensor equipped guide wire (Philips/Volcano, Rancho Cordova, California, USA). Additionally, measurements were performed in an angiographic normal non-culprit coronary artery, defined as a coronary artery with <30% diameter stenosis on visual estimation. Non-culprit vessel measurements were performed in the left circumflex coronary artery, unless a stenosis of >30% was present, in which case the right coronary artery was used. At one-week and six-month follow-up, 64 and 65 respectively patients underwent repeat angiography with assessment of intracoronary Doppler flow velocity, of which the initial results have been reported previously.2,11 The flow diagram in Figure 1 shows the number of patients included in the analysis at each time frame. Hyperaemia was induced by an intracoronary bolus of adenosine (40 µg). Before and after PCI, coronary angiography suitable for quantitative coronary angiographic analysis was performed for offline analysis of TIMI flow and myocardial blush grade. Left ventricular function was evaluated by means of echocardiographic 16-segment wall motion score index performed immediately before primary PCI.
Figure 1.
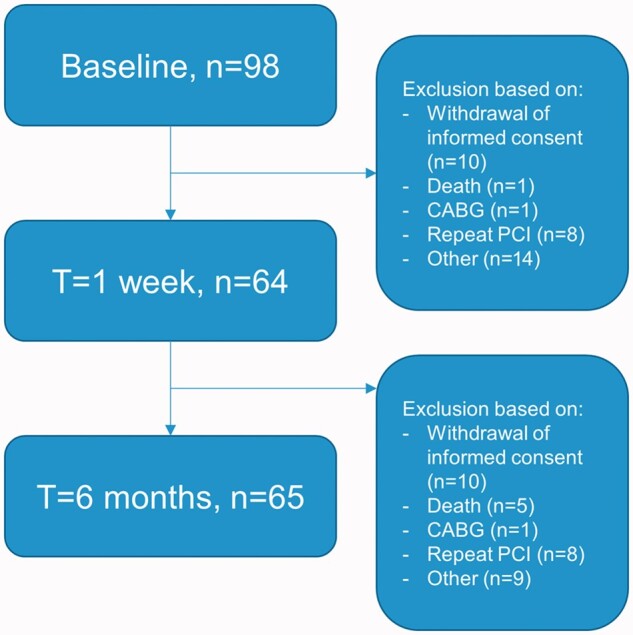
Flow diagram. CABG: coronary artery bypass graft; PCI: percutaneous coronary intervention
CFC
From the recorded data, CFR was calculated as the ratio of hyperaemic average peak flow velocity (hAPV) to baseline average peak flow velocity (bAPV). The CFC concept was applied according to that recently derived for invasive coronary flow measurements. Normal CFC was defined as a CFR ≥2.8, with its corresponding hAPV ≥ 49.0 cm/s.12 Mildly reduced CFC was defined as a CFR < 2.8 but >2.1, and corresponding hAPV < 49.0 and >33.0 cm/s, respectively. Moderately reduced CFC was defined as CFR ≤ 2.1 and >1.7, and the corresponding hAPV ≤33.0 and >26.0 cm/s, respectively.13 Finally, severely reduced CFC was defined as a CFR ≤ 1.7, and the corresponding hAPV ≤26.0 cm/s.5
Haemodynamic data analysis
Microvascular resistance was calculated at baseline and during hyperaemia, respectively the ratio between mean aortic pressure and mean distal flow velocity at baseline (BMR), and during hyperaemia (HMR), in the culprit and in the absence of significant epicardial disease in the non-culprit vessel. The delta microvascular resistance from resting to hyperaemic conditions (dMR) was determined by calculating the absolute difference between BMR and HMR.
Statistical analysis
Normality of the data was tested using the Shapiro–Wilk test, and homogeneity of variance was tested with Levene’s test. All continuous variables are presented as mean ± standard deviation or median (25th to 75th percentile) according to their normal or non-normal distribution. Categorical variables are presented as counts and percentages. Analyses of linear trends across CFC categories were performed with polynomial contrasts.
Improvement of CFC in the culprit and non-culprit vessel between baseline, one week and six months was assessed by a Kruskal–Wallis test with pairwise post hoc correction for multiple comparisons. A p-value < 0.05 was considered statistically significant.
The STATA version 13.1 (StataCorp, College Station, Texas, USA) software package was used to perform statistical analyses.
Results
In total, 98 patients were included in the study at baseline, for which the baseline characteristics are listed in Table 1. The mean age of this cohort was 56 ± 12 years, and 81% were male. Repeat coronary angiography and intracoronary measurements at one-week and six-month follow-up have been performed in a total of 64 and 65 patients respectively.
Table 1.
Baseline characteristics.
| Demographics | |
|---|---|
| n | 98 |
| Age, years | 56±12 |
| Male | 80 (81) |
| Risk factors | |
| Smoking | 52 (53) |
| Hypertension | 24 (24) |
| Family history | 40 (40) |
| Hyperlipidaemia | 26 (26) |
| Diabetes mellitus | 6 (6) |
| Prior medication use | |
| β-blocker | 13 (13) |
| Calcium antagonist | 8 (8) |
| Angiotensin-converting enzyme inhibitors | 5 (5) |
| Nitrates | 4 (4) |
| Lipid-lowering drugs | 8 (8) |
| Aspirin | 11 (11) |
Data are presented as mean ± SD or frequency (%).
Relationship of CFC with procedural characteristics
Across CFC groups determined in the culprit vessel directly after primary PCI, linear contrast analysis revealed a significant trend in infarct size as determined by peak troponin T (p = 0.004), time to reperfusion (p = 0.038), the incidence of respectively final TIMI 3 flow (p = 0.019) and systolic retrograde flow (p = 0.043) (Supplemental file 1 online). For CFC determined for the non-culprit vessel linear contrast analysis revealed a significant trend in C-reactive protein (p = 0.027), peak troponin T (p < 0.001) and heart rate (p = 0.049) across the different groups of CFC (Supplementary file 2).
Time course of culprit vessel CFC
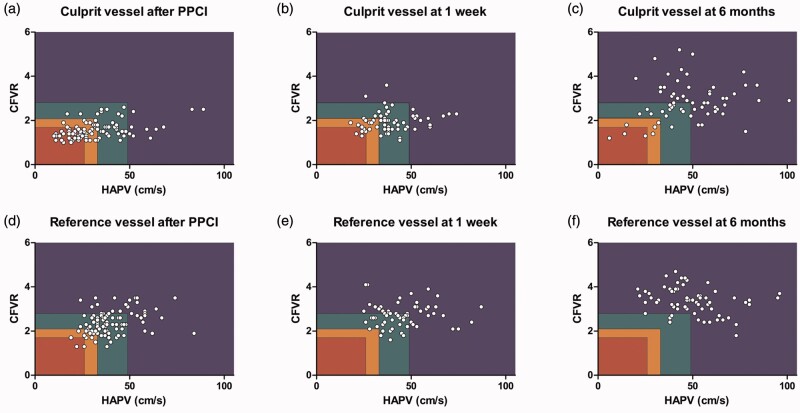
Figure 2(a) to (c) shows the scatterplots of the time course of CFC in the culprit vessel. At this stage of the procedure, 10% of the patients showed a normal CFC, 29% a mildly reduced CFC, 19% a moderately reduced CFC and 42% a severely reduced CFC (Supplementary file 3). A significant linear trend across CFC groups was observed for CFR, bAPV, hAPV, BMR, HMR and dMR (p < 0.001 for all measurements except for dMR, p = 0.002).
Figure 2.
Scatterplot of the time course of coronary flow capacity (CFC) in the culprit vessel ((a), (b) and (c)) and non-culprit vessel ((d), (e) and (f)) after primary percutaneous coronary intervention, at one-week follow-up and six-month follow-up. The rectangles represent CFC categories; blue: normal CFC; green: mildly reduced CFC; orange: moderately reduced CFC; red: severely reduced CFC. CFVR: coronary flow velocity reserve; hAPV: hyperaemic average peak flow velocity; PPCI: primary percutaneous coronary intervention.
At one-week follow-up, measurements in the culprit artery were obtained in 64 patients. In 28% of patients a normal CFC was found, in 44% a mildly reduced CFC, in 19% a moderately reduced and in 9% a severely reduced CFC. A significant linear trend across CFC groups was observed for CFR, bAPV and hAPV (p = 0.004, p < 0.001 and p < 0.001, respectively), but not for BMR (p = 0.183), HMR (p = 0.163) and dMR (p = 0.279). At six-month follow-up measurements in the culprit artery were obtained in 65 patients. In 69% of patients a normal CFC was found, in 20% a mildly reduced CFC, in 6% a moderately reduced and in 5% a severely reduced CFC (Supplementary file 3). A significant linear trend across CFC groups was observed for CFR, bAPV (p < 0.001), hAPV (p < 0.001), HMR (p < 0.001) and dMR (p = 0.02), but not for BMR (p = 0.142).
Time course of non-culprit vessel CFC
Figure 2(d) to (f) shows the scatterplots of the time course of CFC in the non-culprit vessel. At the index procedure, CFC was also determined post PCI in a non-culprit vessel derived from measurements obtained in 97 patients with angiographically normal coronary arteries ( < 30% diameter stenosis): the left circumflex coronary artery was assessed in 87 patients (90%) and the right coronary artery in 10 patients (10%) (Supplementary file 4). CFC in the non-culprit vessel was normal in 27%, mildly reduced in 45%, moderately reduced in 25% and severely reduced in 3% of patients. A significant linear trend was observed for CFR and hAPV (p < 0.001 and p < 0.001), but not for bAPV (p = 0.160). In addition, linear trend analysis of microvascular resistance parameters revealed a significant trend in HMR as well as in dMR (p < 0.001 and p < 0.001), but not in BMR (p = 0.428).
At one-week follow-up, CFC was derived from measurements obtained in 64 patients: the left circumflex coronary artery was assessed in 60 patients (94%), and the right coronary artery in four patients (6%). One week after acute myocardial infarction (AMI), CFC in the non-culprit vessel was normal in 45%, mildly reduced in 52%, and moderately reduced in 3% of patients. A statistically significant difference between normal and mildly reduced CFC was observed for CFR (p < 0.001) and hAPV (p < 0.001), but not for bAPV (p = 0.077). At six-month follow-up, non-culprit vessel measurements were obtained in the same non-culprit vessel as during one-week follow-up: in 65 patients. Six months after AMI, CFC in the non-culprit vessel was normal in 92% and mildly reduced in 8% of patients. A statistically significant difference between normal and mildly reduced CFC was observed for CFR (p = 0.003), hAPV (p = 0.003) and HMR (p < 0.001).
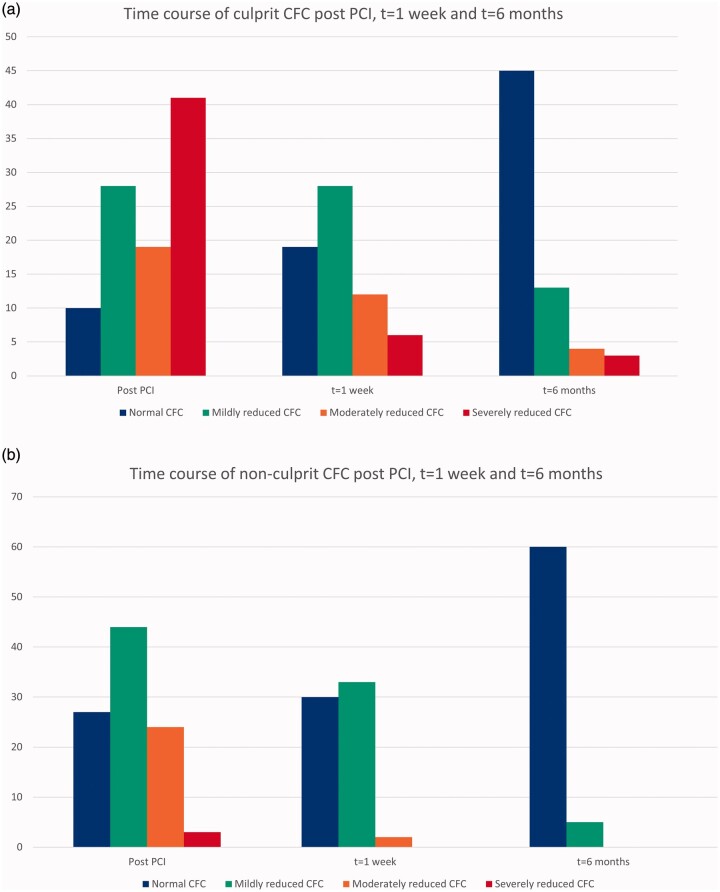
CFC improved significantly both in the culprit and the non-culprit vessel, when compared at baseline post PCI with one-week follow-up (p = 0.036 and p < 0.001), and one-week follow-up compared with six-month follow-up (p = 0.0013 and p < 0.001) (Figure 3; Supplementary file 5).
Figure 3.
Time course of coronary flow capacity (CFC) in the culprit (a) and the non-culprit vessel (b) post primary percutaneous coronary intervention (PCI), at one-week and at six-month follow-up. In the culprit vessel, CFC improved significantly post PCI compared with one-week and six-month follow-up (p < 0.001) and one-week compared with six-month follow-up (p = 0.0013). In the non-culprit vessel, CFC improved post PCI compared with one-week and six-month follow-up, and one-week compared with six-month follow-up (all p < 0.001).
Discussion
The present study is one of the first to document the impact of STEMI on myocardial perfusion using the validated CFC framework to comprehensively assess the consequences of focal obstructive, diffuse and microcirculatory causes of myocardial blood flow impairment. We have previously reported that microvascular function assessed by Doppler flow velocity is altered in the setting of STEMI, even in non-ischaemic regions at distance from the infarcted myocardial tissue and the independent association with long-term fatal cardiac events.
We observed a trend in infarct size for both the culprit vessel post PCI as well as the non-culprit vessel across CFC groups. In addition, an increase in time to reperfusion was associated with worsening of CFC determined after primary PCI in the both the culprit and the non-culprit vessel. CFC at the different time points resulted from an alternating contribution of the individual components that determine CFC group allocation; CFR, hAPV and bAPV. Of note, bAPV showed a significant trend across culprit vessel CFC groups after primary PCI and at one-week and six-month follow-up, but did not differ between groups in the non-culprit vessel.
CFC in the acute setting
First derived from positron emission tomography, the CFC concept integrates CFR with maximal hyperaemic flow velocity.7,9,14 It thereby captures all components of coronary flow physiology and provides a comprehensive tool to depict myocardial blood flow impairment due to a combination of obstructive, diffuse and microcirculatory involvement of the coronary vasculature. Hence, in the absence of epicardial disease the CFC concept provides insights into the microvascular function. In addition, it has been shown to provide an improvement in risk discrimination for adverse clinical outcomes compared with CFR alone.9
This concept is of particular interest when assessing microvascular function in the acute setting of STEMI, where mechanical and neurohumoral factors can have an effect on both resting and hyperaemic coronary flow,11 resulting in prolonged activation of the sympathetic nervous system,15,16 subsequently inducing a vasoconstrictive response of the coronary resistance vessels by upregulated catecholamines.3 The current study utilized the CFC concept to document the time course of microvascular function in the setting of STEMI in both the culprit and the non-culprit arteries.
It also revealed that despite restored epicardial patency of the culprit, a substantial number of patients remained having a severely reduced CFC, which improved over time. As previously documented for CFR, we also observed an impaired CFC in the non-culprit artery remote from the ischaemic region. However, compared with the culprit vessel, CFC in the non-culprit vessel was less impaired in the acute setting and improved more rapidly over time.
Previous studies on microvascular function in STEMI
Myocardial tissue perfusion remains compromised in 30–40% of STEMI patients despite rapid and successful mechanical revascularization.17,18 Whereas culprit vessel flow abnormalities have been ascribed to numerous pathophysiological mechanisms, including reperfusion injury, distal embolization of plaque and thrombus material, endothelial dysfunction, leucocyte plugging and external compression of the microvasculature, the pan-myocardial nature of microvascular dysfunction is less well-understood, but has partly been ascribed to metabolic consequences of STEMI.3,19 Microvascular dysfunction in the infarct related artery as well as remote regions from the infarct related myocardium observed after primary PCI are associated with a significantly increased long-term clinical outcome and mortality.11,20–23 In addition, CFR obtained directly after primary PCI is an independent predictor of long term global as well as regional recovery of left ventricular function.24,25 However, microvascular dysfunction in the setting of STEMI is often disclosed as a decrease in hyperaemic flow and increase in resting flow. The ratio of these, that is, the coronary flow reserve, does not provide insights into the relative contribution of both components.
Clinical implication
Risk stratification in the setting of AMI has long remained to be elucidated, and recent findings of large clinical trials have led to a revived interest in the approach to STEMI patients with multivessel disease. Revascularization of multivessel disease in STEMI patients roughly has three different approaches: angiography, optical coherence tomography (OCT) and invasive coronary physiology assessment. The COMPLETE (Complete vs Culprit-only Revascularization to Treat Multi-vessel Disease After Early PCI for STEMI) trial suggests complete revascularization in STEMI patients with multivessel disease based on angiography, independent of infarct size.26 A sub study of the COMPLETE trial and several other studies suggest OCT assessment of obstructive non-culprit lesions containing complex vulnerable plaque morphology and subsequent treatment of these lesions.27–29 Coronary physiology assessment by using Fractional Flow Reserve (FFR) in STEMI patients with multivessel disease has been evaluated in several trials, and showed a decrease in major adverse cardiac events for FFR-guided PCI of the non-culprit; however, this effect is mainly driven by the complete revascularization at baseline and subsequent prevention of inevitable revascularization at a later stadium.30,31 Additionally, non-culprit instantaneous wave-free ratio (iFR) has been assessed in the iSTEMI trial, during the acute ischaemic event and ≥16 days post-STEMI. iFR was significantly lower during the acute ischaemic event compared with follow-up, potentially due to a higher baseline flow in the setting of STEMI, resulting in a potential overtreatment of these lesions compared with FFR.32 The ongoing trials iModern (iFR Guided Multi-vessel Revascularization During Percutaneous Coronary Intervention for Acute Myocardial Infarction, NCT03298659) and FRAME-AMI (FFR Versus Angiography-Guided Strategy for Management of AMI With Multivessel Disease, NCT02715518) both evaluate non-culprit lesions with iFR and/or FFR in the setting of AMI. However, certainly FFR, and potentially to a lesser extent iFR, are affected by the coronary microcirculation and microvascular resistance in particular, so these indices have to be interpreted cautiously if these are assessed in the setting of STEMI.33,34 On the contrary, non-culprit vessel CFR has important prognostic value as reflected by a 4.09-fold increase in long-term cardiac mortality if non-culprit vessel CFR < 2.0 in STEMI patients with multivessel disease.11 Non-culprit vessel CFC assessment post primary PCI of the culprit has a significant benefit to determine long term prognosis and clinical outcome. Hence, patients with lower CFC in the non-culprit vessel after primary PCI of the culprit in the setting of STEMI require more intensive treatment and monitoring.
Limitations
There has been an extensive debate on the amount of adenosine needed to achieve a maximally vasodilated state. More recently, the dose–response relationship of intracoronary hyperaemia has been investigated, and no significant differences in FFR-values between low and high dose intracoronary adenosine were documented.35 In this study we used an intracoronary bolus of 40 µg adenosine, which induced a sufficient state of hyperaemie to allow accurate assessment of coronary flow characteristics.
The acquisition of coronary flow velocity was performed by a sensor-equipped guidewire that assessed only coronary flow. We assessed only non-culprit vessel haemodynamics in coronary arteries without significant epicardial narrowing and assumed distal pressure to equal aortic pressure. Therefore, a potential role of subclinical atherosclerosis of the conduit artery in the absence of focal narrowing in the impairment of non-culprit vessel flow and pressure cannot be excluded. However, resting coronary flow is unlikely to be disturbed by coronary stenoses up to 85% of the vessel diameter, without interference of compensatory vasodilation of the distal vascular bed.36
Conclusion
These observations underline the impact of the coronary microcirculation both in the culprit and non-culprit vessel in the setting of STEMI on intracoronary diagnostic techniques. The coronary microcirculation recovers over time at six-month follow-up, as shown by an improvement in CFC. Both culprit and non-culprit vessel CFC assessment in the setting of STEMI might provide valuable insight into the recovery of the coronary circulation, emphasizing the importance of intracoronary physiology assessment following primary PCI in AMI.
Supplemental material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care online.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the European Community’s Seventh Framework Program (FP7/2007–2013) under grant agreement no. 224495 (euHeart project), and by a grant from the Dutch Heart Foundation (2006B186).
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1. Keeley EC, Boura JA, Grines CL.. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet 2003;361:13–20. [DOI] [PubMed] [Google Scholar]
- 2. Bax M, de Winter RJ, Koch KT, et al. Time course of microvascular resistance of the infarct and noninfarct coronary artery following an anterior wall acute myocardial infarction. Am J Cardiol 2006;97:1131–1136. [DOI] [PubMed] [Google Scholar]
- 3. Schafer U, Kurz T, Jain D, et al. Impaired coronary flow and left ventricular dysfunction after mechanical recanalization in acute myocardial infarction: role of neurohumoral activation? Basic Res Cardiol 2002;97:399–408. [DOI] [PubMed] [Google Scholar]
- 4. De Bruyne B, Baudhuin T, Melin JA, et al. Coronary flow reserve calculated from pressure measurements in humans. Validation with positron emission tomography. Circulation 1994;89:1013–1022. [DOI] [PubMed] [Google Scholar]
- 5. Johnson NP, Gould KL.. Physiological basis for angina and ST-segment change PET-verified thresholds of quantitative stress myocardial perfusion and coronary flow reserve. JACC Cardiovasc Imaging 2011;4:990–998. [DOI] [PubMed] [Google Scholar]
- 6. Joye JD, Schulman DS, Lasorda D, et al. Intracoronary Doppler guide wire versus stress single-photon emission computed tomographic thallium-201 imaging in assessment of intermediate coronary stenoses. J Am Coll Cardiol 1994;24:940–947. [DOI] [PubMed] [Google Scholar]
- 7. Johnson NP, Gould KL.. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging 2012;5:430–440. [DOI] [PubMed] [Google Scholar]
- 8. Gould KL, Johnson NP, Roby AE, et al. Regional, Artery-specific thresholds of quantitative myocardial perfusion by PET associated with reduced myocardial infarction and death after revascularization in stable coronary artery disease. J Nucl Med 2019;60:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van de Hoef TP, Echavarria-Pinto M, van Lavieren MA, et al. Diagnostic and prognostic implications of coronary flow capacity: A comprehensive cross-modality physiological concept in ischemic heart disease. JACC Cardiovasc Inter 2015;8:1670–1680. [DOI] [PubMed] [Google Scholar]
- 10. Stegehuis VE, Wijntjens GWM, Bax M, et al. Impact of clinical and hemodynamic factors on coronary flow reserve and invasive coronary flow capacity in non-obstructed coronary arteries – a patient level pooled analysis of the DEBATE and ILIAS studies. Eurointervention. Epub ahead of print 18 January 2020. DOI: 10.4244/eij-d-19-00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van de Hoef TP, Bax M, Meuwissen M, et al. Impact of coronary microvascular function on long-term cardiac mortality in patients with acute ST-segment-elevation myocardial infarction. Circ Cardiovasc Intervs 2013;6:207–215. [DOI] [PubMed] [Google Scholar]
- 12. Kern MJ, Bach RG, Mechem CJ, et al. Variations in normal coronary vasodilatory reserve stratified by artery, gender, heart transplantation and coronary artery disease. J Am Coll Cardiol 1996;28:1154–1160. [DOI] [PubMed] [Google Scholar]
- 13. Meuwissen M. Role of fractional and coronary flow reserve in clinical decision making in intermediate coronary lesions. Interv Cardiol 2009;1:237–255. [Google Scholar]
- 14. De Bruyne B. FAME II: FFR pinpoints stable CAD patients who fare worse with OMT. http://www.theheart.org/article/1398627.do (2012, accessed 18 May 2018).
- 15. Guzzetti S, Spyrou N, Rosen SD, et al. Low frequency spectral component of heart rate variability and myocardial beta-adrenoceptor density after acute myocardial infarction. Basic Res Cardiol 2002;97:97–104. [DOI] [PubMed] [Google Scholar]
- 16. Heusch G. Adenosine and maximum coronary vasodilation in humans: Myth and misconceptions in the assessment of coronary reserve. Basic Res Cardiol 2010;105:1–5. [DOI] [PubMed] [Google Scholar]
- 17. Dibra A, Mehilli J, Dirschinger J, et al. Thrombolysis in myocardial infarction myocardial perfusion grade in angiography correlates with myocardial salvage in patients with acute myocardial infarction treated with stenting or thrombolysis. J Am Coll Cardiol 2003;41:925–929. [DOI] [PubMed] [Google Scholar]
- 18. Tarantini G, Cacciavillani L, Corbetti F, et al. Duration of ischemia is a major determinant of transmurality and severe microvascular obstruction after primary angioplasty: A study performed with contrast-enhanced magnetic resonance. J Am Coll Cardiol 2005;46:1229–1235. [DOI] [PubMed] [Google Scholar]
- 19. Rezkalla SH, Kloner RA.. Coronary no-reflow phenomenon. Curr Treat Options Cardiovasc Med 2005;7:75–80. [DOI] [PubMed] [Google Scholar]
- 20. Alexanderson E, Jacome R, Jimenez-Santos M, et al. Evaluation of the endothelial function in hypertensive patients with 13N-ammonia PET. J Nucl Cardiol 2012;19:979–986. [DOI] [PubMed] [Google Scholar]
- 21. Fearon WF, Low AF, Yong AS, et al. Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation 2013;127:2436–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furber AP, Prunier F, Nguyen HC, et al. Coronary blood flow assessment after successful angioplasty for acute myocardial infarction predicts the risk of long-term cardiac events. Circulation 2004;110:3527–3533. [DOI] [PubMed] [Google Scholar]
- 23. Takahashi T, Hiasa Y, Ohara Y, et al. Usefulness of coronary flow reserve immediately after primary coronary angioplasty for acute myocardial infarction in predicting long-term adverse cardiac events. Am J Cardiol 2007;100:806–811. [DOI] [PubMed] [Google Scholar]
- 24. Kitabata H, Kubo T, Ishibashi K, et al. Prognostic value of microvascular resistance index immediately after primary percutaneous coronary intervention on left ventricular remodeling in patients with reperfused anterior acute ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2013;6:1046–1054. [DOI] [PubMed] [Google Scholar]
- 25. Bax M, de Winter RJ, Schotborgh CE, et al. Short- and long-term recovery of left ventricular function predicted at the time of primary percutaneous coronary intervention in anterior myocardial infarction. J Am Coll Cardiol 2004;43:534–541. [DOI] [PubMed] [Google Scholar]
- 26. Mehta SR, Wood DA, Storey RF, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med 2019;381:1411–1421. [DOI] [PubMed] [Google Scholar]
- 27. Iannaccone M, Souteyrand G, Niccoli G, et al. Clinical impact of optical coherence tomography findings on culprit plaque in acute coronary syndrome: The OCT-FORMIDABLE study registry. Catheter Cardiovasc Interv 2018;92:E486–E492. [DOI] [PubMed] [Google Scholar]
- 28. Kajander OA, Pinilla-Echeverri N, Jolly SS, et al. Culprit plaque morphology in STEMI – an optical coherence tomography study: Insights from the TOTAL-OCT substudy. Eurointervention 2016;12:716–723. [DOI] [PubMed] [Google Scholar]
- 29. Pinilla-Echeverri N. Non-culprit lesion plaque morphology in patients with ST-segment elevation myocardial infarction: Results from the COMPLETE trial optical coherence tomography (OCT) substudy. Presented at The American Heart Association (AHA) 2019, Philadelphia, PA, USA, 17 November 2019.
- 30. Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med 2017;376:1234–1244. [DOI] [PubMed] [Google Scholar]
- 31. Engstrom T, Kelbaek H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): An open-label, randomised controlled trial. Lancet 2015;386:665–671. [DOI] [PubMed] [Google Scholar]
- 32. Thim T, Gotberg M, Frobert O, et al. Nonculprit stenosis evaluation using instantaneous wave-free ratio in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2017;10:2528–2535. [DOI] [PubMed] [Google Scholar]
- 33. Mejia-Renteria H, Lee JM, van der Hoeven NW, et al. Coronary microcirculation downstream non-infarct-related arteries in the subacute phase of myocardial infarction: Implications for physiology-guided revascularization. J Am Heart Assoc 2019;8:e011534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cuculi F, de Maria GL, Meier P, et al. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction. J Am Coll Cardiol 2014;64:1894–1904. [DOI] [PubMed] [Google Scholar]
- 35. Wijntjens GWM, van Uffelen EL, Echavarria-Pinto M, et al. Individual lesion-level meta-analysis comparing various doses of intracoronary bolus injection of adenosine with intravenous administration of adenosine for fractional flow reserve assessment. Circ Cardiovasc Interv 2020;13:e007893. [DOI] [PubMed] [Google Scholar]
- 36. Gould KL, Lipscomb K, Calvert C.. Compensatory changes of the distal coronary vascular bed during progressive coronary constriction. Circulation 1975;51:1085–1094. [DOI] [PubMed] [Google Scholar]