Abstract
Background
Socioeconomic status (SES) is a determinant of cognitive and academic functioning among healthy and ill children; however, few pediatric oncology studies examine SES and long-term cognitive functioning. The current study systematically investigated SES as a predictor of cognitive outcomes among children treated for localized brain tumors (BT) with photon radiation therapy (RT).
Methods
248 children treated on a prospective, longitudinal, phase II trial of conformal RT (54-59.4 Gy) for ependymoma, low-grade glioma, or craniopharyngioma were monitored serially with cognitive assessments (intelligence quotient [IQ], reading, math, attention, adaptive function) for 10 years (2209 observations, median age at RT = 6.6 years, 48% male, 80% Caucasian). SES was derived from the Barratt Simplified Measure of Social Status, which incorporates parental occupation, education, and marital status.
Results
Overall, SES scores fell in the low range (Barratt median = 37). At pre-RT baseline, linear mixed models revealed significant associations between SES and IQ, reading, math, attention, and adaptive function, with higher SES associated with better performance (P < .005). SES predicted change over time in IQ, reading, and math; higher SES was associated with less decline (P < .001). Accounting for sex and age at RT, SES remained predictive of IQ, reading, and math. Analysis of variance revealed a greater relative contribution of SES than sex or age at RT to reading and math.
Conclusions
SES represents a novel predictor of cognitive performance before and after RT for pediatric BT. These findings have broad implications as high SES represents a protective factor. Developing interventions to mitigate the effects of low SES is warranted.
Keywords: brain tumor, cancer, cognitive outcomes, pediatric, socioeconomic status
Key Points.
SES predicts long-term cognitive outcomes of pediatric brain tumor survivors.
Higher SES is associated with better cognitive performance pre- and postirradiation.
SES is a protective factor and intervention target for cognitive late effects.
Importance of the Study.
This study is the first to systematically assess the impact of SES on cognitive outcomes among pediatric BT survivors. Previously, few pediatric oncology studies examined associations between SES and cognitive outcomes. Despite the greatest cognitive risk for BT survivors, previous studies focused on acute lymphoblastic leukemia and stem-cell transplant. In the present study, SES had a greater relative contribution to academic outcomes than other well-established risk factors, including age at RT. Interventions focused on mitigating cognitive late effects of cancer treatment often lack sociocultural and economic considerations. Broad policy changes to address social inequities and added education and support for low SES groups throughout treatment and into survivorship, are warranted and may significantly impact long-term quality of life. Findings highlight the need to identify and intervene in modifiable areas associated with low SES (eg, poorer quality early education, increased stress, nutritional deficits, parenting styles, health care access, treatment adherence, and advocacy for school supports).
Socioeconomic status (SES) has been identified in the literature as an important determinant of cognitive and academic functioning among healthy children and children with several health conditions.1,2 For example, longitudinal study of children with chronic iron deficiencies indicates all children exhibit cognitive declines; however, children from families of low SES have worse declines compared to children from families of middle SES, with the gap increasing 2- to 3-fold from infancy to age 19.3 Similarly, among children with sickle cell disease, SES is associated with attention, memory, and visuospatial reasoning, accounting for 18%-24% of variance in cognitive functioning.4 SES has also been shown to impact cognition in children with acquired neurologic insult. Among children with traumatic brain injuries (TBI), low SES has been associated with poorer expressive language,5 greater behavioral problems,6 and higher incidence of social skills deficits.7,8 Additionally, in a study of children with moderate and severe TBI, academic ratings declined more over time for low than high SES groups.7
Though few pediatric oncology studies have investigated relationships between SES and cognitive outcomes, those that have been conducted indicate a potential detrimental impact of low SES.9 For example, among stem-cell transplant (SCT) patients, SES was a leading predictor of cognitive performance wherein a 20-point IQ (intelligence quotient) difference was found between high and low SES groups.10 Likewise, among leukemia patients, using insurance status as a proxy for SES, investigators found those with US public insurance were at increased risk for lower IQ and working memory.11 SES has also been associated with worse performance on measures of verbal and nonverbal reasoning among survivors of leukemia, leading investigators to propose SES as an important moderator of neurocognitive functioning in this population. Further, these investigators found processing speed, a vulnerable cognitive skill for these patients, only declined in the low SES group.12 While provocative, these studies are limited by heterogeneous patient samples with respect to disease and treatment exposure,9,10 failure to include patients with brain tumor (BT) diagnoses,10–12 cross-sectional designs,9,11,12 substantial attrition if followed longitudinally,10 narrow cognitive assessments,9–12 and use of an indirect proxy for SES,11 which have precluded researchers from systematically evaluating the unique contribution of SES to long-term cognitive outcomes among childhood BT survivors.
It is well established that children treated for BT with radiation therapy (RT) are at cognitive risk, most commonly inattention, working memory problems, and decreased processing speed.13 The best-established cognitive risk factors include young age at treatment, longer time since treatment, and female sex.14,15 Additionally, children with medical complications including seizures,14 hydrocephalus16,17 or meningitis are at greater risk.18 SES as a potential protective factor has not been systematically studied among childhood BT survivors. Accordingly, the main objective of the present study was to investigate associations between SES and cognitive and academic outcomes in a large, prospectively followed, sample of patients with BT treated homogeneously with focal RT. A secondary objective was to assess the association of SES with demographic and clinical characteristics and to examine their relative contribution to cognitive outcomes. We hypothesized long-term cognitive outcomes of patients treated for BT would differ as a function of SES. Specifically, patients from low SES would demonstrate poorer cognitive and academic performance than their counterparts, both at pre-RT baseline and over time.
Patients and Methods
Participants
Patients between the ages of 1 and 25 years at irradiation were recruited to participate in an institutional phase II trial of conformal radiation therapy (CRT; RT1, NCT00187226) between August 1997 and June 2007. Eligible participants had diagnoses of intracranial ependymoma, low-grade glioma (LGG), or craniopharyngioma without evidence of dissemination, prior irradiation, or ongoing chemotherapy. Patients were required to be English-speaking to participate in neurocognitive assessments and were included in the analysis if they had at least one neurocognitive assessment, along with data needed to calculate SES (ie, parent occupation, education, and marital status). All patients had adequate hearing and vision to complete study tasks. Institutional Review Board approval was granted and all participants gave informed consent to participate.
Of 361 patients enrolled on the RT1 treatment protocol, 253 provided demographic information needed to calculate SES. Of these 253 patients, 248 participated in at least one neurocognitive assessment. Patients were no longer followed on study with neurocognitive assessments if recurrence or progression necessitated additional cancer-directed therapy. Comparisons between participants (n = 248) and nonparticipants (n = 113) failed to reveal a significant difference in sex or age at irradiation (P > .05), suggesting the analyzed subsample is representative of the larger study on variables most associated with cognitive risk. Participants were distributed across tumor groups as follows: ependymoma (n = 99), craniopharyngioma (n = 69), and LGG (n = 80).
Prior to RT, most patients (95.2%) underwent resection with only 19.0% undergoing chemotherapy. Patients received consistent, protocol-driven CRT, including intensity-modulated methods. Patients received CRT over 6-7 weeks with a prescribed total dose of 59.4 Gy (ependymoma) or 54 Gy (craniopharyngioma and LGG). Children who were <18 months old with ependymoma received 54.0 Gy after gross total resection (GTR). Target volume definitions and treatment parameters have been previously reported.13 Hydrocephalus was defined based on MRI at the time of diagnosis. Extent of surgical resection was defined as needle biopsy, less than GTR, or GTR based on residual disease on postoperative neuroimaging. Time interval from symptoms to diagnosis, age at diagnosis, age at treatment, and number of surgeries were extracted from the study database (see Table 1).
Table 1.
Participant Demographic and Clinical Characteristics
| Variable | Total, N = 248 | Ependymoma, N = 99 | Craniopharyngioma, N = 69 | Low-Grade Glioma, N = 80 | P Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| Sex | |||||||||
| Female | 129 | 52.02 | 47 | 47.47 | 37 | 53.62 | 45 | 56.25 | .481 |
| Male | 119 | 47.98 | 52 | 52.53 | 32 | 46.38 | 35 | 43.75 | |
| Race | |||||||||
| White | 199 | 80.24 | 81 | 81.82 | 54 | 78.26 | 64 | 80.00 | .608 |
| Black | 40 | 16.13 | 13 | 13.13 | 14 | 20.29 | 13 | 16.25 | |
| Other | 9 | 3.63 | 5 | 5.05 | 1 | 1.45 | 3 | 3.75 | |
| Hydrocephalus | |||||||||
| None | 112 | 45.16 | 31 | 31.31 | 30 | 43.48 | 51 | 63.75 | <.001* a |
| Diagnosis | 136 | 54.84 | 68 | 68.69 | 39 | 56.52 | 29 | 36.25 | |
| Chemotherapy prior to RT | |||||||||
| No | 201 | 81.05 | 84 | 84.85 | 67 | 97.10 | 50 | 62.50 | <.001* b |
| Yes | 47 | 18.95 | 15 | 15.15 | 2 | 2.90 | 30 | 37.50 | |
| NF-1 | |||||||||
| No | 70 | 87.50 | |||||||
| Yes | 10 | 12.50 | |||||||
| Tumor location | |||||||||
| Infratentorial | 93 | 37.50 | 73 | 73.74 | 1 | 1.45 | 19 | 23.75 | <.001* c |
| Supratentorial | 155 | 62.50 | 26 | 26.26 | 68 | 98.55 | 61 | 76.25 | |
| Pre-RT extent of resection | |||||||||
| Needle biopsy | 12 | 4.84 | 0 | 0.00 | 2 | 2.90 | 10 | 12.50 | <.001* c |
| Less than GTR | 142 | 57.26 | 10 | 10.10 | 67 | 97.10 | 65 | 81.25 | |
| GTR | 94 | 37.90 | 89 | 89.90 | 0 | 0.00 | 5 | 6.25 | |
| Variable | Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | P Value |
| Age at diagnosis | 6.67 (4.93) | 5.63 (0.01, 22.75) | 4.61 (4.67) | 2.64 (0.01, 22.75) | 8.52 (3.99) | 7.30 (2.50, 17.57) | 7.63 (5.10) | 6.54 (0.34, 19.92) | <.001* d |
| Age at treatment | 7.74 (4.95) | 6.62 (1.02, 22.92) | 4.97 (4.68) | 3.03 (1.02, 22.92) | 9.37 (3.98) | 8.52 (3.21, 17.63) | 9.76 (4.45) | 8.45 (2.19, 20.01) | <.001* d |
| Number of surgeries | 1.54 (1.03) | 1.00 (0.00, 7.00) | 1.44 (0.70) | 1.00 (1.00, 4.00) | 1.97 (1.41) | 1.00 (0.00, 7.00) | 1.28 (0.84) | 1.00 (0.00, 4.00) | .004* e |
| SES—BSMSS total score | 36.84 (12.75) | 37.00 (9.00, 66.00) | 38.07 (13.34) | 38.00 (9.00, 61.00) | 35.14 (12.26) | 35.50 (13.50, 64.50) | 36.78 (12.39) | 37.00 (12.00, 66.00) | .345 |
| Interval (years)—symptom to diagnosis | 0.40 (0.68) | 0.14 (0.00, 4.09) | 0.17 (0.24) | 0.08 (0.00, 1.85) | 0.55 (0.79) | 0.20 (0.00, 4.00) | 0.55 (0.84) | 0.26 (0.00, 4.09) | <.001* d |
Abbreviations: BSMSS, Barratt Simplified Measure of Social Status; GTR, gross total resection, based on gross residual disease on postoperative neuroimaging; LGG, low-grade glioma; NF-1, neurofibromatosis type 1; RT, radiation therapy; SES, socioeconomic status; other race, Black, Asian/Pacific Islander; hydrocephalus, defined based on MRI at the time of diagnosis; symptom to diagnosis, the time duration between patient experiencing symptoms to diagnosis.
*P < .05 based on Pearson’s chi-squared, exact, Kruskal-Wallis test, or one-way ANOVA, as appropriate.
Post-hoc analyses revealed the following differences: aLGG < craniopharyngioma < ependymoma, bCraniopharyngioma < ependymoma < LGG, cCraniopharyngioma < LGG < ependymoma [infratentorial/GTR], dEpendymoma < craniopharyngioma and LGG, eEpendymoma and LGG < craniopharyngioma.
Cognitive Measures
Measures for this study were selected from the larger RT1 cognitive battery based on the SES and acquired brain injury literature.1,2,7,9,18,19 Assessments were planned to be serially administered before RT, at 6 months, annually for 5 years, either at year 7 or 8, and year 10. All measures have age-specific norms from representative standardization samples and demonstrated reliability and validity.
An estimated IQ (EIQ) was derived from an age-appropriate Wechsler scale (WISC-III, WPPSI-R, WAIS-R)20–22 using scaled scores from the Information, Similarities, and Block Design subtests. This method for estimating IQ correlates highly with IQs derived from full administration.23 Academic skills were measured using Wechsler Individual Achievement Test (WIAT) Basic Reading and Mathematics Reasoning.24 The Vineland Adaptive Behavior Scales was administered as a semi-structured parent interview that measures adaptive or self-care skills in the child’s daily environment and produces an overall Adaptive Behavior Composite (ABC) score.25 These measures each provide an age-standardized score with a mean of 100 and standard deviation of 15 where higher scores represent better performance.
The Conners’ Continuous Performance Test (CPT) is a computerized task that measures sustained attention to letters presented individually on a computer screen.26 The CPT program produces age-standardized percentiles, with a mean of 50 and a standard deviation of 16. In the present study, omissions (inattention index) and reaction time (processing speed index) were analyzed. For omissions, higher scores indicate worse performance. Reaction time is bimodally interpreted with low scores indicative of slow processing speed.
SES Measurement
SES was measured using the Barratt Simplified Measure of Social Status (BSMSS)27 based on Hollingshead’s Four Factor Index.28 Scores were calculated using parental occupation, education level, and marital status. The BSMSS classification system was used to code occupations based on skill, power, and social position in society. For example, higher executives and owners of large businesses were placed in the highest occupational category while service workers such as dishwashers were placed in the lowest occupational category. Education was accounted for using level of school completed, with seventh grade and below receiving the lowest score and graduate degree or professional school beyond college receiving the highest score. For families headed by a single parent, or with multiple caregivers, scores were adjusted accordingly. Total composite scores range from 8 (low) to 66 (high).
Statistical Analyses
Descriptive analyses were conducted to examine demographic and clinical characteristics of participants from the RT1 sample, and investigate their association with SES. In univariate analyses, linear mixed models (LMM) with patient-specific intercepts (ie, intercept was included in the model as both fixed and random effect) were fitted to investigate the relationship between SES and baseline neurocognitive performance, as well as SES and neurocognitive scores over time since RT. Because of variable assessment times for each subject, time was treated as a continuous variable in LMMs. SES (BSMSS) was also treated as a continuous variable. Additionally, multivariate LMMs were fitted to examine the contribution of SES to cognitive performance after accounting for the effects of sex or age at RT for those outcomes that showed significance in univariate models. Finally, mean squares from analysis of variance (ANOVA) statistics were used to evaluate the relative contribution of SES, sex, and age at RT to cognitive outcomes. Results were considered significant at the P < .05 level.
Results
Table 1 provides demographic and clinical characteristics. For the three tumor groups combined, the majority of patients were White (80%), female (52%), and had supratentorial tumors (63%). The median age at diagnosis was 5.6 years (range = 0.0-22.8) and median age at time of irradiation was 6.6 years (range = 1.0-22.9), with only 7 participants (2.8% of the total sample) over age 18 at the time of treatment. Fifty-five percent of participants had hydrocephalus at diagnosis. Thirty-eight percent of participants had a GTR, 57% had less than a GTR, and 5% had a needle biopsy. The median number of surgeries per participant was one (range = 0-7). Of the 80 participants who had a LGG, 12.5% had a comorbid diagnosis of Neurofibromatosis Type 1.
With tumor groups divided, several expected demographic and clinical characteristic differences were revealed. Supratentorial tumors were most common in the craniopharyngioma group (99%), followed by the LGG group (76%), and then the ependymoma group (26%). Hydrocephalus at diagnosis was more common in the ependymoma (69%) and craniopharyngioma (57%) groups. Chemotherapy prior to RT was most common in the LGG group (38%), followed by the ependymoma group (15%) and was very rare in the craniopharyngioma group (3%). GTRs were most common in the ependymoma group (90%), were very rare in the LGG group (6%), and never occurred in the craniopharyngioma group. Patients in the ependymoma group were diagnosed more quickly after symptom onset than those in craniopharyngioma or LGG groups. Ependymoma patients were also younger at irradiation than those in craniopharyngioma and LGG groups.
The average SES score for the total sample (BSMSS Mean = 36.8; SD = 12.8; range = 9-66) fell in the low range and SES did not significantly differ across BT types (P = .345). White participants had a significantly higher SES compared to Black participants (P < .001). There was no association between SES and interval from symptom presentation to diagnosis, age at diagnosis, or age at time of treatment. The association between SES and hydrocephalus at diagnosis was approaching significance (P = .071), with higher SES associated with less likelihood of having hydrocephalus.
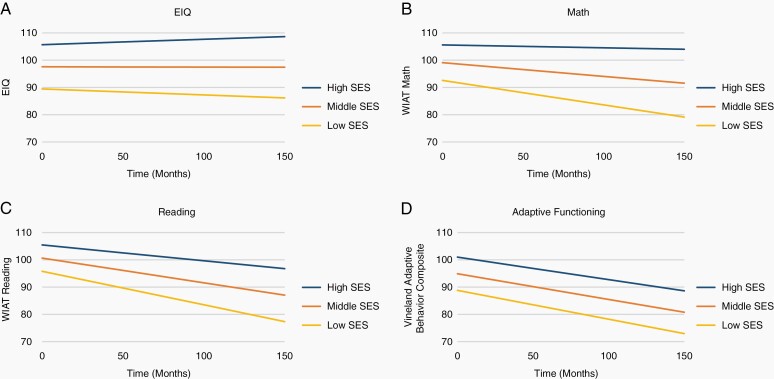
LMMs examining the association between SES and neurocognitive outcomes at pre-RT baseline (intercept) and change over time (slope) are presented in Table 2. On average each participant contributed 8.4 (1986/237) EIQ, 6.9 (1575/228) WIAT Reading, 6.9 (1571/228) WIAT Math, 5.7 (1285/224) CPT Omissions and CPT Reaction Time, and 6.1 (1468/241) Vineland ABC scores. Approximately 30% of subjects had 9 assessments or more, and only 3% had only one assessment. Based on LMMs for median SES, pre-RT EIQ and Vineland ABC scores were significantly less than normative expectations while WIAT Reading and Math were within normative expectations. LMMs also revealed CPT omissions were elevated and CPT Reaction Time was slow pre-RT relative to normative expectations. At pre-RT, significant positive associations between SES and EIQ, WIAT Reading, WIAT Math, CPT Reaction Time, and Vineland ABC were found (P < .005), with higher SES associated with better performance. SES also predicted change in EIQ, WIAT Reading, and WIAT Math over time (P < .001), with higher SES associated with less decline. Figure 1 depicts performance in EIQ, WIAT Reading, WIAT Math, and Vineland ABC for three SES levels based on BSMSS scores (low: 8-30, middle: 41-50, and high: 57-66). When BT groups were assessed separately, SES was associated with pre-RT EIQ, WIAT Reading, WIAT Math, and Vineland ABC among the ependymoma and LGG groups and pre-RT EIQ in the craniopharyngioma group (P < .009). Higher SES predicted less decline over time in EIQ and WIAT Reading among patients with ependymoma and craniopharyngioma, as well as WIAT Math among craniopharyngioma patients (P < .05; Supplementary Table 1).
Table 2.
The Impact of SES on Cognitive Performance over Time
| Normative Comparisona | Baseline (Intercept)b | Change (Slope)c | |||||
|---|---|---|---|---|---|---|---|
| Cognitive Variable | No. of Patients/Observations | Estimate | P Value | Estimate | P Value | Estimate | P Value |
| EIQ | 237/1986 | 97.5556 | .0327* | 0.54 | <.0001* | 0.001388 | .0003* |
| WIAT Reading | 228/1575 | 100.6027 | .5156 | 0.32 | <.0001* | 0.002159 | <.0001* |
| WIAT Math | 228/1571 | 99.0681 | .3816 | 0.43 | <.0001* | 0.002639 | <.0001* |
| CPT Omissions | 224/1285 | 81.3277 | <.0001* | −0.09 | .3703 | −0.00156 | .17 |
| CPT Reaction Time | 224/1285 | 32.3853 | <.0001* | 0.42 | .0048* | 0.000627 | .6483 |
| Vineland Adaptive Behavior Composite | 241/1468 | 94.8835 | <.0001* | 0.41 | <.0001* | 0.000780 | .2829 |
Abbreviations: CPT, Conner’s Continuous Performance Test; EIQ, estimated intelligence quotient; SES, socioeconomic status; WIAT, Wechsler Individual Achievement Test.
aScores represent estimated baseline performance for participants with a median SES score. EIQ, WIAT Reading, WIAT Math, and Vineland Adaptive Behavior Composite are compared to a normative mean of 100 and CPT Omissions and Reaction Time are compared to a normative mean of 50.
bBaseline represents the association between SES and baseline cognitive scores. A positive estimate indicates higher SES, higher baseline cognitive score, if significant.
cChange represents the association between SES and the change rate of cognitive scores. A positive estimate indicates higher SES, less decline, or more increase in outcome score over time, if significant.
*P < .05.
Fig. 1.
Cognitive outcomes by SES level over time.
SES based on BSMSS scores where low = 8-30; middle = 41-50; high = 57-66. Abbreviations: BSMSS, Barratt Simplified Measure of Social Status; SES, socioeconomic status.
Based on the established importance of age at RT and sex as predictors of cognitive outcomes following RT,14,15,29–33 separate multivariate LMMs were conducted with EIQ, WIAT Reading, and WIAT Math as outcomes that included age at RT and SES, or sex and SES, as covariates. When multivariate LMMs included age at RT and SES, SES remained predictive of baseline EIQ, WIAT Reading, and WIAT Math with higher SES associated with better performance (P < .001). SES also remained predictive of change in EIQ, WIAT Reading, and WIAT Math with higher SES predictive of less declines over time (P <.001). When multivariate LMMs included sex and SES, SES remained predictive of baseline EIQ, WIAT Reading, and WIAT Math with higher SES associated with better performance for both males and females (P < .05). SES also remained predictive of change in WIAT Reading and WIAT Math with higher SES associated with less decline for both males and females (P < .05). LMMs revealed a different pattern in change in EIQ based on sex. For males, SES was not associated with change in IQ over time (P = .196); whereas, for females, higher SES was predictive of less decline in EIQ over time (P < .001; Table 3).
Table 3.
Parameter Estimates of Multivariate Linear Mixed Models with SES, Age at RT, Sex, and Neurocognitive Change over Time
| Outcome and Effect | No. of Participants | No. of Observations | Estimate | SE | P Value |
|---|---|---|---|---|---|
| EIQ | 237 | 1986 | |||
| Intercept | 97.42 | 1.1490 | .0259a | ||
| Time | −0.01533 | 0.005063 | .0025 | ||
| SES | 0.5058 | 0.09000 | <.0001 | ||
| Age | 0.3313 | 0.2265 | .1437 | ||
| Time × SES | 0.001930 | 0.000399 | <.0001 | ||
| Time × Age | 0.008265 | 0.000987 | <.0001 | ||
| SES × Age | 0.02806 | 0.01796 | .1184 | ||
| Time × SES × Age | −0.00023 | 0.000076 | .0023 | ||
| WIAT Reading | 228 | 1575 | |||
| Intercept | 101.5 | 0.9513 | .1174a | ||
| Time | −0.09294 | 0.005082 | <.0001 | ||
| SES | 0.2690 | 0.07508 | .0004 | ||
| Age | −0.6374 | 0.2084 | .0023 | ||
| Time × SES | 0.002358 | 0.000408 | <.0001 | ||
| Time × Age | 0.004165 | 0.001691 | .0139 | ||
| SES × Age | 0.03136 | 0.01579 | .0472 | ||
| Time × SES × Age | −0.00017 | 0.000128 | .1880 | ||
| WIAT Math | 228 | 1571 | |||
| Intercept | 98.74 | 1.1057 | .2539a | ||
| Time | −0.04931 | 0.006122 | <.0001 | ||
| SES | 0.3982 | 0.08725 | <.0001 | ||
| Age | 0.1788 | 0.2425 | .4611 | ||
| Time × SES | 0.002642 | 0.000492 | <.0001 | ||
| Time × Age | −0.00624 | 0.002034 | .0022 | ||
| SES × Age | 0.02414 | 0.01837 | .1890 | ||
| Time × SES × Age | −0.00017 | 0.000154 | .2808 | ||
| EIQ | 237 | 1986 | |||
| Intercept | 97.42 | 1.6347 | .1162a | ||
| Time | 0.02365 | 0.007051 | .0008 | ||
| SES | 0.4325 | 0.1312 | .0010 | ||
| Sex (female vs maleb) | 0.1192 | 2.2661 | .9581 | ||
| Time × SES | 0.000709 | 0.000549 | .1964 | ||
| Time × Sex | −0.04748 | 0.009711 | <.0001 | ||
| SES × Sex | 0.1954 | 0.1779 | .2723 | ||
| Time × SES × Sex | 0.001448 | 0.000758 | .0563 | ||
| WIAT Reading | 228 | 1575 | |||
| Intercept | 100.5183 | 1.3310 | .6974a | ||
| Time | −0.07732 | 0.007270 | <.0001 | ||
| SES | 0.2742 | 0.1070 | .0105 | ||
| Sex (female vs maleb) | 0.04532 | 1.8474 | .9804 | ||
| Time × SES | 0.001473 | 0.000574 | .0104 | ||
| Time × Sex | −0.02455 | 0.01008 | .0150 | ||
| SES × Sex | 0.09406 | 0.1450 | .5166 | ||
| Time × SES × Sex | 0.001306 | 0.000805 | .1049 | ||
| WIAT Math | 228 | 1571 | |||
| Intercept | 99.35 | 1.5136 | .6673a | ||
| Time | −0.01930 | 0.008693 | .0265 | ||
| SES | 0.3068 | 0.1216 | .0117 | ||
| Sex (female vs maleb) | −0.6767 | 2.1002 | .7474 | ||
| Time × SES | 0.002782 | 0.000687 | <.0001 | ||
| Time × Sex | −0.05980 | 0.01207 | <.0001 | ||
| SES × Sex | 0.2470 | 0.1648 | .1342 | ||
| Time × SES × Sex | −0.00039 | 0.000965 | .6841 |
Abbreviations: EIQ, estimated intelligence quotient; RT, radiation therapy; SES, socioeconomic status; WIAT, Wechsler Individual Achievement Test.
Time represents time since radiation therapy and is treated as a continuous variable. SES was treated as a continuous variable and was centered at the median SES score of the participant cohort. For models with age, age (at RT) was centered at the median age at treatment of the participant cohort; intercept indicates the model estimate of baseline score for median age with median SES; time indicates the effect of time for median age with median SES. For models with sex, intercept indicates the model estimate of baseline score for males with median SES; time indicates the effect of time for males with median SES. Interactions were used to account for the contribution of different covariate levels in model-estimated baseline score and time effect. “Time × Sex” interaction indicates whether cognitive decline was significantly different between females vs males; a negative estimate indicates less decline among females than males.
aFor intercept effect in each model, P value was calculated by comparing intercept estimate with 100, the mean value of the normative comparison group.
bUsed as reference level.
ANOVA was used to investigate the amount of variance in cognitive outcomes accounted for by SES and age at RT or SES and sex. Comparison of mean squares from ANOVAs indicates a greater relative contribution of age at RT and sex than SES to change in EIQ over time. In contrast, findings suggest SES has a greater relative contribution than age at RT and sex to WIAT Reading and WIAT Math over time (Table 4).
Table 4.
Relative Contribution of Age at RT, Sex, and SES to Cognitive Outcomes over Time
| Outcome | Source of Variation (Mean Squares) | |||
|---|---|---|---|---|
| Age at RT | SES | Sex | SES | |
| IQ | 3906.1a | 823.6 | 1428.1a | 814.5 |
| Reading | 237.3 | 1157.7a | 226.6 | 1161.0a |
| Math | 559.2 | 1733.1a | 1425.2 | 1734.0a |
Abbreviations: IQ, intelligence quotient; RT, radiation therapy; SES, socioeconomic status.
aIndicates which variable accounted for a larger portion of variance.
The results in this table came from ANOVA tables after linear mixed models were fitted. Each ANOVA table contained a sequential decomposition of the contribution from all fixed-effect terms in the model, by evaluating variance or deviance of fixed-effect terms one by one.
Discussion
In this study of children uniformly treated for pediatric BT, higher SES was predictive of better IQ, academic achievement in reading and math, processing speed, and adaptive functioning prior to treatment exposure. Prior to RT, differences between high and low SES groups on IQ (17 points) and math (13 points) were near one standard deviation. High and low SES groups also demonstrated a 10-point reading difference prior to treatment. Additionally, change over time in IQ and academic achievement in math and reading was predicted by SES. High SES groups exhibited less decline than low SES groups. As time progressed, score discrepancies between high and low SES groups widened markedly (23 points in IQ; 20 points in reading; 25 points in math), with differences approximating 1.5 standard deviations.
Younger age at treatment, longer time since treatment, and female sex are well-established predictors of cognitive outcomes in the pediatric oncology literature.14,15 Even with age at RT and sex accounted for, higher SES was predictive of better IQ, math, and reading over time. Notably, SES was a stronger predictor of academic outcomes than sex or age at RT. SES appears to be a novel, useful predictor of cognitive outcomes both pretreatment and over time.
Results are consistent with findings from the few pediatric oncology studies previously investigating the relationship between SES and cognitive outcomes.9–12 To our knowledge, this study is the first to systematically assess SES specifically amongst pediatric BT survivors, despite greatest cognitive risk. While one previous study included children treated for leukemia or BT,9 other oncology studies focused on patients with acute lymphoblastic leukemia or SCT.10–12 Given children were seen regardless of ability to pay for treatment, our sample is likely more variable in SES than most samples affording greater exploration of statistical associations. No previous studies have enrolled patients treated homogenously with CRT or followed patients as long using a comprehensive cognitive battery.
Interventions focused on mitigating cognitive late effects often lack consideration of sociocultural and economic factors.9 Accordingly, SES as a marker for targeted intervention may be instrumental in guiding clinical conversations throughout the course of treatment and into the survivorship period. Investigators speculate that children and families from low SES may differ from those in high SES circumstances prior to illness, throughout treatment, and in survivorship.7,10,11 Specifically, children from low SES may experience poorer quality and quantity of early education, increased exposure to toxins, chronic stress, nutritional deficits, differences in parenting styles, and less academic stimulation in-home, which may impact cognitive reserve.1,2,4 Thus, children from high SES may be more resilient to treatment-related toxicities.
Notably, Black participants had significantly lower SES as compared to their White counterparts. However, since only 16% of this sample identified as Black, the impact of race and subsequent disparities on cognitive outcomes could not be assessed. Future research is needed to evaluate the impact of systemic racism on cognitive and academic outcomes following treatment for pediatric BT. Findings point to the need for broad policy change to improve social determinants of health, such as education, income/wealth, health systems and services, housing, physical and social environments, transportation, public safety, and employment.34 Specifically, policies and programs should focus on reducing concentrated poverty and exposure to environmental hazards, and increasing social cohesion, access to educational and health care resources, and attention to preventative care.
Due to barriers to health care access (eg, high cost of care, inadequate insurance coverage, lack of culturally competent care), families from low SES may be less likely to pursue medical treatment early, leading to delayed diagnosis and greater complications. While in the current study, SES was not associated with a longer symptom-to-diagnosis interval, the association between hydrocephalus at diagnosis and SES was approaching significance and may suggest longer time to diagnosis. Children from low SES may face reduced access to medical care and their families may have greater difficulty understanding and meeting their child’s medical needs, due in part to lower literacy rates. Additionally, parents from low SES may exhibit less medical trust,35 in turn, influencing their treatment adherence and willingness to consistently engage in follow-up medical appointments and cognitive interventions. Accordingly, the need for cognitive monitoring and attention to treatment adherence among low SES groups may be particularly crucial.
Parents from low SES may demonstrate differences in school advocacy patterns in comparison to parents from families of high SES.36,37 Pinpointing efficacious strategies in advocacy efforts may lead to more successful cognitive outcomes. Families from low SES may also have more difficulty accessing quality resources (eg, parks, libraries) due to environmental factors (ie, incidental barriers, transportation), which may be inconducive to development of compensatory skills. Recommendations focused on providing an enriching environment for fostering cognitive development such as regular reading at home, educational outings such as visiting museums, and rehabilitation service adherence may offset some of the detrimental impact of low SES. Additionally, the impact of special education services, tutoring, and specific educational interventions on the relationship between SES and academic outcomes is worthy of future research.
Some methodological issues limit the generalizability of these findings. First, this study was conducted using a predominantly American patient sample; thus, findings may not generalize to other countries that have stronger social safety nets.38 Notably, there have been changes in front-line therapy for treatment of BT, such as greater availability of proton RT, since initiation of this 10-year longitudinal trial. While these findings should be replicated in a sample treated with modern therapy, many children are still being treated with CRT. Findings are also relevant for a large number of long-term pediatric BT survivors. An additional limitation is missing SES data due to failure of some families to complete the required demographic form. Finally, EIQ can underestimate declines in IQ relative to FSIQ when processing speed is not incorporated.39 However, in this study, a timed measure (ie, block design) was included in the IQ estimate. It is also unlikely that the use of EIQ is accounting for the relationship between SES and cognitive outcomes.
In sum, SES was demonstrated to be a novel predictor of cognitive outcomes for pediatric BT patients both at treatment initiation and over time. SES appears to serve as a protective factor mitigating harmful effects of treatment on cognitive functioning. Going forward, SES may represent a useful focal point for improving interventions as those in low SES groups may be better served through broad policy change, education, and support.
Funding
This work was supported in part by the National Cancer Institute (St. Jude Cancer Center Support [CORE] Grant [P30 CA21765]) and the American Lebanese Syrian Associated Charities (ALSAC). Portions of this manuscript were presented at the annual meeting of the International Neuropsychological Society, Denver, CO, USA, February 2020.
Supplementary Material
Acknowledgments
The authors thank the patients and their families who volunteered their time to participate in neurocognitive assessments during their enrollment on the RT1 protocol. We also thank our Psychological Examiners (Maggi Dunavant, MS, Charlotte Fineberg-Buchner, MA, David Hopper, EdS, Deborah B. Stewart, Med) and Supervising Clinical Psychologists, including Niki Jurbergs, PhD, for their valuable contributions to this work. We thank Sean Phipps, PhD for his editorial input. Portions of this manuscript were presented at the annual meeting of the International Neuropsychological Society, Denver, CO, USA, February 2020.
Conflict of interest statement. The authors declare that there is no conflict of interest.
Authorship statement. Acquired study data and provided valuable insight into interpretation of results: T.E.M.; Originally conceived the idea for the study: H.M.C.; Involved in all aspects of the present study including design of the work, data analysis, interpretation, and final manuscript preparation: H.M.C., V.A.T., and J.M.A.; Helped to derive the BSMSS variable: E.W.; Substantially contributed to analysis and interpretation of data for the work: J.X. and H.Z.; Prepared figures and tables: V.A.T., J.M.A., J.X., and H.Z.; Contributed to writing and editing the final manuscript and have read and approved the final version of this manuscript: V.A.T., J.M.A., E.W., J.X., H.Z., T.E.M., and H.M.C.
References
- 1. Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399. [DOI] [PubMed] [Google Scholar]
- 2. Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13(2):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med. 2006;160(11):1108–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tarazi RA, Grant ML, Ely E, Barakat LP. Neuropsychological functioning in preschool-age children with sickle cell disease: the role of illness-related and psychosocial factors. Child Neuropsychol. 2007;13(2):155–172. [DOI] [PubMed] [Google Scholar]
- 5. Taylor HG, Swartwout MD, Yeates KO, Walz NC, Stancin T, Wade SL. Traumatic brain injury in young children: postacute effects on cognitive and school readiness skills. J Int Neuropsychol Soc. 2008;14(5):734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yeates KO, Taylor HG, Walz NC, Stancin T, Wade SL. The family environment as a moderator of psychosocial outcomes following traumatic brain injury in young children. Neuropsychology. 2010;24(3):345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term outcomes after traumatic brain injury in children: behavior and achievement. Neuropsychology. 2002;16(1):15–27. [DOI] [PubMed] [Google Scholar]
- 8. Yeates KO, Swift E, Taylor HG, et al. Short- and long-term social outcomes following pediatric traumatic brain injury. J Int Neuropsychol Soc. 2004;10(3):412–426. [DOI] [PubMed] [Google Scholar]
- 9. Butler RW, Fairclough DL, Katz ER, et al. Intellectual functioning and multi-dimensional attentional processes in long-term survivors of a central nervous system related pediatric malignancy. Life Sci. 2013;93(17):611–616. [DOI] [PubMed] [Google Scholar]
- 10. Phipps S, Rai SN, Leung WH, Lensing S, Dunavant M. Cognitive and academic consequences of stem-cell transplantation in children. J Clin Oncol. 2008;26(12):2027–2033. [DOI] [PubMed] [Google Scholar]
- 11. Hardy KK, Embry L, Kairalla JA, et al. Neurocognitive functioning of children treated for high-risk B-acute lymphoblastic leukemia randomly assigned to different methotrexate and corticosteroid treatment strategies: a report from the Children’s Oncology Group. J Clin Oncol. 2017;35(23):2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel SK, Fernandez N, Dekel N, et al. Socioeconomic status as a possible moderator of neurocognitive outcomes in children with cancer. Psychooncology. 2016;25(1):115–118. [DOI] [PubMed] [Google Scholar]
- 13. Merchant TE. Current management of childhood ependymoma. Oncology (Williston Park). 2002;16(5):629–642, 644; discussion 645-6, 648. [PubMed] [Google Scholar]
- 14. Willard VW, Conklin HM, Wu S, Merchant TE. Prospective longitudinal evaluation of emotional and behavioral functioning in pediatric patients with low-grade glioma treated with conformal radiation therapy. J Neurooncol. 2015;122(1):161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE. Longitudinal investigation of adaptive functioning following conformal irradiation for pediatric craniopharyngioma and low-grade glioma. Int J Radiat Oncol Biol Phys. 2013;85(5):1301–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conklin HM, Li C, Xiong X, Ogg RJ, Merchant TE. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol. 2008;26(24):3965–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Pinto M, Conklin HM, Li C, Xiong X, Merchant TE. Investigating verbal and visual auditory learning after conformal radiation therapy for childhood ependymoma. Int J Radiat Oncol Biol Phys. 2010;77(4):1002–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duffner PK. Risk factors for cognitive decline in children treated for brain tumors. Eur J Paediatr Neurol. 2010;14(2):106–115. [DOI] [PubMed] [Google Scholar]
- 19. Rivara JB, Jaffe KM, Polissar NL, et al. Family functioning and children’s academic performance and behavior problems in the year following traumatic brain injury. Arch Phys Med Rehabil. 1994;75(4):369–379. [DOI] [PubMed] [Google Scholar]
- 20. Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- 21. Wechsler D. Wechsler Preschool and Primary Scale of Intelligence, Revised. San Antonio, TX: The Psychological Corporation; 1989. [Google Scholar]
- 22. Wechsler D. Wechsler Adult Intelligence Scale: Revised. New York, NY: The Psychological Corporation; 1981. [Google Scholar]
- 23. Sattler JM. Assessment of Children. 3rd ed. San Diego, CA: Jerome M. Sattler, Publisher, Inc.; 1992. [Google Scholar]
- 24. Wechsler D. Wechsler Individual Achievement Test. San Antonio, TX: The Psychological Corporation; 1992. [Google Scholar]
- 25. Sparrow SS, Balla D, Cicchetti D.. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- 26. Conners CK. Conners’ Continuous Performance Test Computer Program: User’s Manual. Toronto: Multi-Health Systems; 1995. [Google Scholar]
- 27. Barratt W. The Barratt Simplified Measure of Social Status (BSMSS): measuring SES.http://socialclassoncampus.blogspot.com/2012/06/barratt-simplified-measure-of-social.html. Published 2006. Accessed August 12, 2019.
- 28. Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- 29. Landau E, Boop FA, Conklin HM, Wu S, Xiong X, Merchant TE. Supratentorial ependymoma: disease control, complications, and functional outcomes after irradiation. Int J Radiat Oncol Biol Phys. 2013;85(4):e193–e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Merchant TE, Sharma S, Xiong X, Wu S, Conklin H. Effect of cerebellum radiation dosimetry on cognitive outcomes in children with infratentorial ependymoma. Int J Radiat Oncol Biol Phys. 2014;90(3):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE. A 5-year investigation of children’s adaptive functioning following conformal radiation therapy for localized ependymoma. Int J Radiat Oncol Biol Phys. 2012;84(1):217–223.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Willard VW, Conklin HM, Boop FA, Wu S, Merchant TE. Emotional and behavioral functioning after conformal radiation therapy for pediatric ependymoma. Int J Radiat Oncol Biol Phys. 2014;88(4):814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Academies of Sciences, Engineering, and Medicine. Chapter 3: the root causes of health inequity. In: Baciu A, Negussie Y, Geller A, Weinstein JN, eds. Communities in Action: Pathways to Health Equity. Washington, DC: National Academies Press; 2017:99–184. [PubMed] [Google Scholar]
- 35. Armstrong K, Ravenell KL, McMurphy S, Putt M. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97(7):1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. LaRocque M, Kleiman I, Darling SM. Parental involvement: the missing link in school achievement. Prev Sch Fail. 2011;55(3):115–122. [Google Scholar]
- 37. Bennett PR, Lutz A, Jayaram L. Beyond the schoolyard: the contributions of parenting logics, financial resources, and social institutions to the social class gap in structured activity participation. Sociol Educ. 2012;85(2):131–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eilertsen T, Thorsen AL, Holm SE, Bøe T, Sørensen L, Lundervold AJ. Parental socioeconomic status and child intellectual functioning in a Norwegian sample. Scand J Psychol. 2016;57(5):399–405. [DOI] [PubMed] [Google Scholar]
- 39. Burgess L, Pulsifer MB, Grieco JA, et al. Estimated IQ systematically underestimates neurocognitive sequelae in irradiated pediatric brain tumor survivors. Int J Radiat Oncol Biol Phys. 2018;101(3):541–549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.