Abstract
Background
High-risk medulloblastoma is defined by the presence of metastatic disease and/or incomplete resection and/or unfavorable histopathology and/or tumors with MYC amplification. We aimed to assess the 3-year progression-free survival (PFS) and define the molecular characteristics associated with PFS in patients aged 5–19 years with newly diagnosed high-risk medulloblastoma treated according to the phase II trial PNET HR+5.
Methods
All children received postoperative induction chemotherapy (etoposide and carboplatin), followed by 2 high-dose thiotepa courses (600 mg/m2) with hematological stem cell support. At the latest 45 days after the last stem cell rescue, patients received risk-adapted craniospinal radiation therapy. Maintenance treatment with temozolomide was planned to start between 1–3 months after the end of radiotherapy. The primary endpoint was PFS. Outcome and safety analyses were per protocol (all patients who received at least one dose of induction chemotherapy).
Results
Fifty-one patients (median age, 8 y; range, 5–19) were enrolled. The median follow-up was 7.1 years (range: 3.4–9.0). The 3 and 5-year PFS with their 95% confidence intervals (95% CI) were 78% (65–88) and 76% (63–86), and the 3 and 5-year OS were 84% (72–92) and 76% (63–86), respectively. Medulloblastoma subtype was a statistically significant prognostic factor (P-value = 0.039) with large-cell/anaplastic being of worse prognosis, as well as a molecular subgroup (P-value = 0.012) with sonic hedgehog (SHH) and group 3 being of worse prognosis than wingless (WNT) and group 4. Therapy was well tolerated.
Conclusions
This treatment based on high-dose chemotherapy and conventional radiotherapy resulted in a high survival rate in children with newly diagnosed high-risk medulloblastoma.
Keywords: children, high-risk medulloblastoma, phase II trial
Key Points.
PNET HR+5 trial improves significantly the outcome of patients with high- risk medulloblastoma.
Molecular subgroup has an impact on the outcome of patients with high-risk medulloblastoma.
Importance of the Study.
Long-term survival rates have increased from 20–40% to 60–70% in the last decade by using different intensive treatment strategies with high-dose chemotherapy or alternative radiation therapy fractionation or radiosensitizer chemotherapy. This phase II study is one of the largest studies to assess childhood high-risk medulloblastoma in a subgroup-specific manner. In this prospective trial, the outcome of patients treated with high-dose chemotherapy with stem cell rescue followed by conventional craniospinal radiation therapy and maintenance treatment was improved. Furthermore, we identified a proportion of children with excellent prognosis. All patients with group 4 medulloblastoma even if they had a metastatic disease survived without relapse. These findings suggest the possibility of a treatment effect in high-risk medulloblastoma because such intensive strategies as PNET HR+5 trial might overcome the negative effect of high-risk features, which could, in turn, be more relevant for some molecular subgroups than for others.
Medulloblastoma is the most common malignant brain tumor in children. Current international consensus recognizes 4 molecular subgroups of medulloblastoma: the wingless (WNT) and sonic hedgehog (SHH) signaling pathways as well as group 3 and group 4.1 Moreover, molecular heterogeneity exists within these consensus subgroups.2–4
For past and currently, ongoing trials, medulloblastoma in patients aged from 3–5 years to 21 years at diagnosis with gross total or near-total resection, with non-anaplastic and non-large cell histological subtype and without MYC or MYCN amplification has been considered as “standard-risk” disease, while the other patients are counted as “high-risk.”
As the result of a pilot study,5 we designed this phase II PNET HR+5 trial with the aim of assessing whether progression-free survival of children older than 5 years with high-risk medulloblastoma could be improved with tandem high-dose chemotherapy followed by conventional radiation therapy. Outcomes were analyzed according to clinical risk factors and molecular subgroups.
Methods
Study Design and Participants
This multicenter, phase II, open-label, single-arm study enrolled eligible patients in 18 French study centers (ClinicalTrials.gov identifier: NCT00936156).
Patients aged 5–20 years at diagnosis with centrally confirmed newly diagnosed high-risk medulloblastoma were eligible for this study. Medulloblastoma was considered high-risk if (1) there was a metastatic extension (M1–M3 as defined by modified Chang staging classification),6 or (2) the residual disease assessed within 48 h after surgery was ≥1.5 cm2, or (3) the histopathology review showed large-cell/anaplastic features (LCA MB) according to WHO classification7 or (4) MYCN or MYC genes were amplified. Additional criteria included a Lansky performance ≥60, normal renal function (creatinine <1.5 × normal for age), bone marrow function (absolute neutrophil count >1000/µl, platelet count >100 000/µl), and liver function (bilirubin <1.5 × normal, ALT <2.5 × normal). Key exclusion criteria included previous treatment with chemotherapy and/or radiotherapy, evidence of severe or uncontrolled systemic disease, extraneural metastasis.
Informed consent was received from parents and/or patients. Approval from Ethics committees was available.
Procedures
Pathology samples were centrally reviewed at diagnosis according to 2007 WHO classification7 and at the end of the study according to 2016 WHO classification.8 Immunohistochemical analyses of formalin-fixed, paraffin-wax-embedded tumor samples were done to determine the activation status of the WNT and SHH signaling pathways by use of routine methods with primary antibodies (beta-catenin, YAP1, Filamin A, GAB1). MYC and MYCN status were assessed by fluorescence in situ hybridization (FISH) or by array-CGH using Agilent arrays. Subgroup affiliation was established using nanoString targeted gene-expression profiling from paraffin-embedded or frozen samples, as previously described.9
When possible, surgical excision of the primary tumor was performed at diagnosis. To avoid delaying the start of chemotherapy, surgical excision of the primary tumor could be postponed after conventional chemotherapy or sequential high-dose chemotherapy in patients with persisting tumor. After the initial surgery, patients received 2 courses of carboplatin (160 mg/m2/day for 5 days) combined with etoposide (100 mg/m2/day for 5 days) with a 3-week interval. Before each chemotherapy course, neutrophils were required to be >800/µl and platelets >100 000/µl. Peripheral blood stem-cell (PBSC) collection was performed by leukapheresis either after mobilization with carboplatin-etoposide and G-CSF at 5 μg/kg or in steady-state after G-CSF at 10 μg/kg. At least 3 × 106 CD34 positive cells/kg had to be collected and cryopreserved for each stem cell rescue. After conventional chemotherapy, patients received 2 courses of high-dose thiotepa (200 mg/m2/ day, as a 1-h IV infusion for 3 consecutive days) with at least a 3-week interval. The time between courses was as short as possible when peripheral counts recovered with an absolute neutrophil >800/µl, platelets count >75 000/µl, and acquired platelet transfusion independence. After each course, PBSC were infused 24 or 48 h after completion of chemotherapy (day 0). Radiation therapy was started at the latest 45 days after the last stem cell rescue and 150 days after the initial surgery. Patients were treated in the prone position for all radiation fields. Radiation therapy was administered in daily fractions of 1.8 Gy, 5 days/week. For localized medulloblastoma with postoperative residual tumor and incomplete response before radiotherapy, radiation therapy consisted of 23.4 Gy on the craniospinal axis and 54 Gy on the primitive tumor bed. The other patients received 54 Gy delivered to the primitive tumor bed, 36 Gy on the craniospinal axis. For residual lesions in the posterior fossa or metastatic nodules before radiotherapy, another boost of 9 Gy could be delivered in 5 daily 1.8 Gy fractions. Radiation simulation films, and port films all were reviewed centrally as previously described.10 Maintenance treatment with temozolomide was planned at the dose of 150 mg/m2 during five consecutive days, every 28 days. A total of 6 cycles were scheduled. Maintenance treatment was begun as soon as the hematological criteria were respected (neutrophils >800/µl and platelets >100 000/µl and increasing), between 1 and 3 months after the end of radiotherapy.
During treatment, patients’ disease status and toxicity of treatment were monitored with appropriate laboratory assessments and imaging studies. Toxicities were graded using the National Cancer Institute’s Common Terminology Criteria (version 3.0) and Bearman grading for high-dose chemotherapy. Disease status was assessed by MRI of the head and spine. If tumor cells were identified in the cerebrospinal fluid (CSF) at diagnosis, CSF cytology analysis was also performed until normalization. Tumor response was assessed in each center by investigators, using MRI and CSF cytology taken at diagnosis, after surgery, before high-dose chemotherapy, after the second high-dose thiotepa, after radiotherapy, every 3 cycles of temozolomide, and at the end of treatment. An independent central review was performed at the end of the trial. Radiologic response was assessed according to the International Society of Pediatric Oncology criteria.11 Tumor response presented in the Results paragraph corresponds to the independent central review. Recurrence was classified as a disease that developed at a new site that had previously been tumor-free. After completion of treatment, follow-up examinations were done every 3 months for a period of 2 years, then every 6 months for 2 years, and then every year.
Outcomes
The primary endpoint was progression-free survival (PFS) at 3 years. PFS was defined as the time from the start of conventional chemotherapy (etoposide-carboplatin) to the date of the first occurrence of recurrent or progressive disease or death from any cause or to the date of the last contact for patients without one of these events. Secondary endpoints included overall survival (OS), the complete response rate of chemotherapy, and feasibility of maintenance treatment. OS was defined as the time from the start of conventional chemotherapy to the date of death from any cause or last follow-up or to the date of the last contact for survivors. The complete response rate of chemotherapy was defined as the proportion of patients in complete response at the end of chemotherapy before radiotherapy. The feasibility of chemotherapy was assessed by the proportion of patients who received the study treatments as per protocol, including the 6 planned cycles of temozolomide. This endpoint was descriptive by nature. Given that there was no other study combining high-dose chemotherapy, radiotherapy, and temozolomide maintenance therapy, no threshold had been set a priori.
Statistical Analysis
The study was designed to include patients with high-risk medulloblastoma or CNS-PNET. Sample size was based on the assumption of a 20% increase (from 40% to 60%) of the 3-year PFS rate. Sixty-four patients had to be included in a single-stage Fleming design (one-sided type I error = 2.5%, type II error = 10%). Here, we report in detail the results of the fifty-one patients with high-risk medulloblastoma included in the study because this population represents the vast majority of the included patients and CNS-PNET cohort represents a small number of patients with heterogeneous histologies. We also present in the Supplementary Appendix the results for the patients included in the study with a PNET (n = 4) or a pineoblastoma (n = 9). Survival rates (PFS and OS) were estimated using the Kaplan-Meier method with Rothman’s 95% CI. Median follow-up was estimated using the reverse Kaplan-Meier method. The prognostic value of medulloblastoma subtype, stage, and molecular subgroup on PFS were tested using a two-sided log-rank test (univariable analysis). The first two analyses were preplanned in the protocol in 2008. The molecular subgroup analysis was foreseen after 2012 and before analyzing the data. To study whether the tumor response (complete or partial response versus stable disease) after conventional chemotherapy or after high dose chemotherapy predicted PFS, we performed a landmark analysis using as starting point the date of tumor assessment post-conventional chemotherapy or post-high dose chemotherapy respectively. Due to the explorative nature of the prognostic analysis and the small number of events, no adjustment for multiple testing was applied. Data were analyzed using SAS software v9.4 (SAS Institute, Cary, NC).
Results
Between January 19, 2009 and February 28, 2012, 51 children with newly diagnosed high-risk medulloblastoma were enrolled in this study. For the biological analyses, 46 (90%) of the 51 patients with medulloblastoma were included in the molecular analyses (no archival tissue available for nanoString targeted gene-expression profiling in 5 patients). Median age at diagnosis was 8 years (range, 5–19 years). Thirty-four patients were male and 17 were female. Table 1 shows the characteristics of the 51 patients according to metastatic status.
Table 1.
Clinical Characteristics of Enrolled Patients Integrated Histopathological and Genetic Classification of Medulloblastoma
| M0 (14) | M1 (3) | M2/M3 (34) | Total (51) | |
|---|---|---|---|---|
| Histology | ||||
| CMB | 5 | 1 | 28 | 34 |
| DNB | 1 | 1 | 2 | 4 |
| LCA | 7 | 1 | 3 | 11 |
| Not otherwise specified | 1 | 1 | 2 | |
| Molecular subgroup | ||||
| WNT | 1 | 2 | 3 | |
| SHH | 3 | 1 | 3 | 7 |
| Group 3 | 4 | 2 | 9 | 15 |
| Group 4 | 15 | 15 | ||
| Unknown | 6 | 5 | 11 | |
| Myc amplification | ||||
| Present | 5 | 2 | 7 | |
| Absent | 6 | 3 | 30 | 39 |
| Unknown | 3 | 2 | 5 | |
| P53 status | ||||
| Negative | 9 | 3 | 30 | 42 |
| Positive | 3 | 1 | 4 | |
| Unknown | 2 | 3 | 5 |
CMB, classic medulloblastoma; DNB, desmoplastic/nodular; LCA, large cell/anaplastic; WNT, Wingless; SHH, Sonic hedgehog.
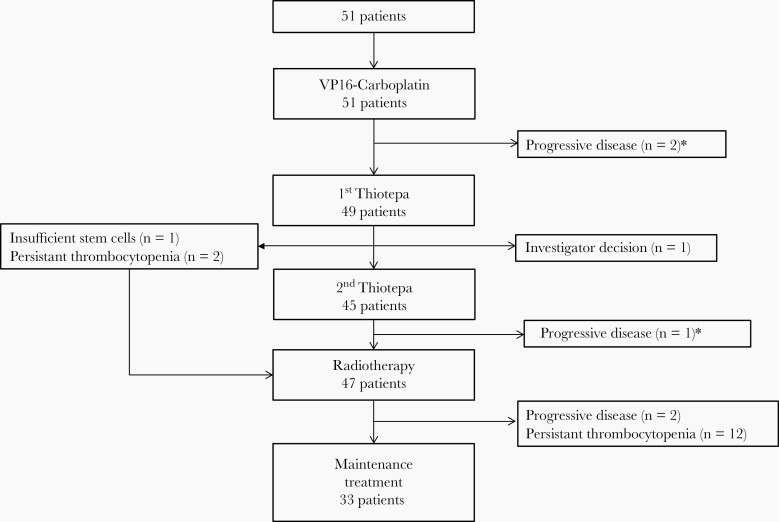
All patients but 2 received induction therapy with two courses of VP16-Carboplatin. The other 2 children received 3 courses of induction chemotherapy instead due to logistics’ difficulty for one and due to hydrocephalus requiring a ventriculoperitoneal shunt for the other one (Figure 1). After induction chemotherapy, 19 patients were in complete remission, 13 in partial remission, 16 patients had a stable disease, and 3 patients a progressive disease. The response rate (complete and partial response) of VP16-Carboplatin was 62.8%. After VP16-Carboplatin, 94 courses of high-dose thiotepa with stem cell support were delivered to 49 patients. Forty-five patients completed all 2 courses of high-dose chemotherapy. The schedule was modified for 4 patients who received only one course of high-dose chemotherapy because of insufficient stem cells (n = 1), persistent thrombocytopenia (n = 2), or a decision of an investigator to withdraw the patient from the study due to insufficient response after one course of high-dose chemotherapy (n = 1). Before radiotherapy, 25 patients had a complete remission, 13 had a partial remission, 8 had a stable disease and two were not assessed by central review. Forty-seven patients received radiation therapy. The median time between diagnosis and onset of radiation therapy was 146 days (range, 117–210) and in 16 patients (34%), this delay was greater than 150 days. At the end of radiotherapy, 35 patients achieved a complete remission, 7 children were in partial response and 3 had a stable disease. Two patients had a progressive disease after radiotherapy. Out of the 45 patients eligible for maintenance therapy, 12 patients received no temozolomide because of persistent thrombocytopenia. Thus, 193 cycles of temozolomide were delivered in 33 patients, of whom 32 patients completed all 6 cycles. One child received only one course of maintenance treatment because of progressive disease. In 16 patients, the dose of temozolomide was reduced at the beginning or during the maintenance treatment because of hematological criteria. At the end of treatment, complete remission was achieved in 37 patients. No relapse occurred in the 12 patients who did not receive maintenance treatment.
Fig. 1.
Trial profile. *The trial profile presents the treatments that were actually received by the patients, based on the results of the tumor assessments provided by local radiologists. A progression was identified by the central review on the post VP16-Carboplatin assessment of a third patient. The local radiologist identified this progression later, after the patient has received the second course of thiotepa.
At the time of the analysis, 34 patients were in first complete remission (median follow-up 7.1 years from the start of conventional chemotherapy (range, 3.4–9.0). Recurrences occurred in 15 patients at a median time of 1.7 years (range, 0.1–7.2) after the start of conventional chemotherapy. Patterns of recurrence were local in 6 children, metastatic in 4, and synchronous local and metastatic in 5. Among them, 14 patients died. Two other children died from a second malignancy without having relapsed. One child with SHH medulloblastoma in the context of Li-Fraumeni syndrome developed a metastatic osteosarcoma 4.4 years after the start of chemotherapy. The other patient with WNT medulloblastoma presented a glioblastoma 5.6 years after the start of chemotherapy.
The 3 and 5-years PFS with their 95% confidence intervals (95% CI) were 78% (65–88) and 76% (63–86), and the 3 and 5-years OS were 84% (72–92) and 76% (63–86), respectively (Figure 2).
Fig. 2.
Overall and progression-free survival.
Medulloblastoma histological subtype was a statistically significant prognostic factor of PFS (P-value = 0.039) with LCA MB being of worse prognosis (Figure 3A), as well as a molecular subgroup (P-value = 0.012) with SHH and group 3 being of worse prognosis than WNT and group 4 (Figure 3B). Stage was not a statistically significant prognostic factor (P-value = 0.42). Response after conventional chemotherapy (Figure 3C) or after high-dose chemotherapy (Figure 3D) did not predicted PFS (P-values = 0.38 and 0.80 respectively). The delay between diagnosis and onset of radiotherapy was not associated with PFS (P-value = 0.72).
Fig. 3.
Progression-free survival (A) by histological subtype; (B) by molecular subgroup; (C) by tumor response after VP16-Carboplatin and (D) by tumor response after high-dose chemotherapy. DNB, desmoplastic/nodular medulloblastoma; MB NOS, medulloblastoma not otherwise specified; CMB, classic medulloblastoma; LCA, large cell/anaplastic medulloblastoma; WNT, Wingless; SHH, Sonic hedgehog.
Therapy was well-tolerated; no patients discontinued because of drug-related toxicity, and no protocol-related death was noted. The most common recorded adverse events are reported in Table 2. Unexpected grade III–IV toxic effect was thiotepa-related neurotoxicity. Two patients presented seizures during the administration of the first thiotepa course. Thiotepa was reintroduced during the second without a neurological event. One child presented tremor during the first thiotepa course.
Table 2.
Most Frequent Treatment-related Adverse Events
| Number of Cycles (%) | ||||
|---|---|---|---|---|
| VP16-CBP (104 cycles) | HDCT (94 cycles) | RT (47 RT) | TMZ (193 cycles) | |
| Hematological | ||||
| Leucopenia (<1000/µl) | 6 (6) | 94 (100) | 11 (23) | 0 |
| Neutropenia (<500/µl) | 56 (54) | 94 (100) | 11 (23) | 0 |
| Thrombocytopenia (<50 000/µl) | 85 (82) | 93 (99) | 35 (74) | 3 (2) |
| Non-hematological | ||||
| Febrile neutropenia | 12(12) | 48 (51) | 4 (9) | 0 |
| Mucositis | 1 (1)a | 40b (43) | 4 (9)a | 0a |
| Diarrhea | 0a | 49b (53) | 1 (2)a | 0a |
| Hepatic toxicity | 5 (5)a | 13b (14) | 1 (2)a | 0a |
| Neurological toxicity | 0a | 6b (7) | 0a | 0a |
aAdverse event ≥ Grade 2 NCI-CTCAE.
bAdverse event ≥ Grade 1 Bearman classification; One cycle with missing data.
Discussion
In contrast to standard-risk medulloblastoma, no gold standard treatment has been defined for high-risk medulloblastoma. With this prospective trial, we newly confirm previous results obtained from the pilot study.5 We have shown that high-dose chemotherapy with stem cell support followed by conventional craniospinal radiation therapy and maintenance treatment in children older than 5 years of age with high-risk medulloblastoma results in a 5-year PFS of 76% (95% CI, 63–86). The survival rates obtained in this trial are in the upper range of encouraging results recently obtained in different studies. In a limited institution trial (SJMB-96 trial), 48 patients with high-risk medulloblastoma treated with risk-adapted radiotherapy (36–39.6 Gy craniospinal) followed by 4 cycles of high-dose chemotherapy with stem cell rescue showed a 5-year event-free survival rate of 70% (95% CI, 54–84).12 A 5-year event-free survival of 70% (SE ± 8%) was reached by a monocentric protocol for 33 patients with metastatic medulloblastoma using neoadjuvant chemotherapy with methotrexate, etoposide, cyclophosphamide, and carboplatin followed by hyperfractionated accelerated radiotherapy (HART) with 39 Gy craniospinal in two daily fractions of 1.3 Gy and a posterior fossa boost to 60 Gy in two daily fractions of 1.5 Gy. Patients with less than complete remission before HART then received 2 courses of high-dose thiotepa, while those in complete remission were given maintenance chemotherapy with vincristine and lomustine.13 In the phase II COG 99701 trial (RP2D), 17 patients with metastatic disease treated with craniospinal irradiation (36 Gy) with boosts to the primary site and metastases, daily carboplatin and weekly vincristine during radiation and subsequent adjuvant chemotherapy with vincristine and cyclophosphamide showed 5-year PFS rate of 71% (SE ± 11%).14 In the prospective multicenter trial HIT 2000, 123 patients with metastatic medulloblastoma treated with neoadjuvant chemotherapy consisting of systemic and intraventricular chemotherapy followed by hyperfractionated nonaccelerated radiotherapy and maintenance chemotherapy showed a 5-year event-free survival rate of 62% (95% CI, 52.0–72.0).15
The strength of our study is that we report the impact of the molecular subgroup on outcome in the context of homogeneous clinical therapy. In contrast, as in the other studies on high-risk medulloblastoma,12–15 the value of our study is limited as a result of the sample size.
We identified a proportion of children with excellent prognosis. All patients with group 4 medulloblastoma survived without relapse with a median follow-up of 7.2 years. Two subtypes (Group 4High-risk and Group 4Low-risk) were reported in one study,4 whereas another identified three subtypes (Group 4α, Group4ß and Group 4γ).3 These subtypes have variable cytogenetics and patient demographics, and the high-risk and low-risk subgroups were reported to display substantial differences in PFS.4 A designation of group 4High-risk medulloblastoma, 7q status, metastatic disease, and male sex were associated with poor PFS, whereas MYCN amplification, residual disease, and LCA pathology were not.4 Although these two studies are large to investigate additional substructure within the 4 consensus subgroups, the data are retrospective and from patients with heterogeneously treated medulloblastoma. In our study, all patients with group 4 medulloblastoma had a metastatic disease at diagnosis. Thus, our data suggest the possibility of a treatment effect in high-risk medulloblastoma because such intensive strategies as PNET HR+5 trial might overcome the negative effect of high-risk features, which could, in turn, be more relevant for some molecular subgroups than for others.
With this prospective trial, we newly confirm previous results obtained from retrospective series with heterogeneously treated patients. Pediatric patients with WNT-medulloblastoma have been shown to have an excellent prognosis.16,17 As in the HIT 2000 trial,15 our findings highlight the good prognosis of WNT subtype in a cohort of patients with high-risk medulloblastoma. The fact that the 3 patients with WNT-activated high-risk medulloblastoma are alive free of disease provides a rationale to investigate whether patients with WNT-activated high-risk medulloblastoma could receive less aggressive treatment and may be successfully treated by standard-risk strategy.
As previously reported,12–15 older children with LCA medulloblastoma have poorer outcomes than patients with classic medulloblastoma. Due to the small number of patients with MYC-amplified medulloblastoma, the impact of this molecular alteration on outcome was not assessed in this present trial.
The response rate of induction chemotherapy was comparable to response rates observed in HIT 2000 trial.15 However, the HIT 2000 study used intensified induction chemotherapy. In contrast to previous studies,15,18 earlier response to chemotherapy has not been identified as a prognostic factor for PFS in our study.
Timing of radiotherapy differed considerably between the several studies assessing preradiation chemotherapy. In our study, radiotherapy begun with a median delay of 146 days after diagnosis but for 16% of patients, it was started after day 210 (start according to protocol at day 150). For patients starting before or after day 150, no statistically difference in PFS was observed. In this phase II study, the progressive disease occurred before radiation therapy in 3 cases (3.9%). In HART study,13 the median interval between surgery and radiotherapy was 85 days and 5 (15.1%) of 33 patients had the progressive disease before radiation therapy. The duration of preradiation chemotherapy was 8 weeks in HIT 2000 trial and progressive disease occurred before radiotherapy in 14 (11%) of 123 patients.15 In SJMB-96 trial,12 31 patients with high-risk medulloblastoma received 6 weeks of topotecan treatment before radiotherapy and 3 of them had progressive disease.
In our study, high-dose chemotherapy and the whole therapeutic strategy were well tolerated; none of the patients in our series died from regimen-related toxic effects. Hematopoietic toxicity was the predominant side effect of the whole treatment strategy and 12 of 45 patients eligible for maintenance treatment could not receive temozolomide due to persistent thrombocytopenia. However, none recurrence occurred in these 12 patients which raises the question of the benefit of temozolomide in this strategy. Thiotepa-related neurotoxicity occurred in 6 courses and all these events had disappeared without late effect. In this study, the incidence of thiotepa-related neurotoxicity was lower than the published study.19 Thus and colleagues reported brain MRI abnormalities in 11 of 14 children receiving dose-intensive sequential, HART, and thiotepa.20 Ruben and colleagues found that chemotherapy after radiotherapy led to an approximately 5-fold increase in the risk of cerebral necrosis.21 In the HART study,13 all patients received radiotherapy after high-dose chemotherapy. Long-term treatment-related morbidity needs to be further assessed in PNET HR+5 trial. The rate of ototoxicity could be anticipated as being low given the use of carboplatin instead of cisplatinum. Pediatric patients with brain tumors are at high-risk for treatment-related hormone deficiencies. Delayed puberty in survivors of brain tumors may be due to gonadotropin deficiency (central or secondary) and/or direct damage to the gonad (primary). The risk of primary gonadal dysfunction is more frequent among female brain tumor survivors. Sterilization and loss of hormone production following treatment with alkylating agents and radiation therapy are usually concurrent in females because hormone production is closely linked to the presence of ova and maturation of the primary follicle.22 In SJMB-96 trial, the cumulative incidence of primary ovarian insufficiency 6 years after completion of radiation therapy was 82.8%.23 There were no significant differences between patients received that received ≥5 Gy and those that received <5 Gy in SJMB-96 trial23 and others reports,24,25 suggesting that ovarian insufficiency was due to alkylating agent treatment. In this French phase II trial, fertility preservation was recommended before high-dose thiotepa courses.
In the present trial, 2 patients (3.9%) developed secondary tumors, one in the context of Li-Fraumeni syndrome. This incidence of second malignancies corroborates reports of a higher risk for second malignancies with adjuvant chemotherapy for medulloblastoma.26–29 The long-term follow-up study of Children’s Oncology Group trial A9961 estimated a cumulative 10-year incidence rate of secondary malignancies of 4.2% (95% CI, 1.9% to 6.5%).26 Among 280 patients with medulloblastoma in HIT 91 trial, a second tumor occurred in 12 (4.3%) cases.27 The 10-year cumulative incidence rate of second malignancies among medulloblastoma survivors of multimodal therapy at St Jude Children’s Research Hospital was 5.5% (95% CI, 2.8% to 9.6%).28 The increased cumulative incidence of second malignancies after multimodal therapy may be partially attributable to the improvement in neuro-imaging techniques to detect indolent tumors. The etiology of subsequent neoplasms is multifactorial and includes primary cancer therapy as well as genetic susceptibility. In our study, one of the two patients with second malignancies had a known tumor predisposition syndrome.
In conclusion, the treatment regimen is safe, feasible (even though 25% of the patients were not able to receive maintenance treatment due to persistent thrombocytopenia) and results in encouraging survival rates. Long-term treatment-related morbidity and neurocognitive outcomes need to be further assessed. The future SIOPe trial for high-risk medulloblastoma for children above 3 years old will be conducted to compare HART approach versus PNET HR+5 strategy versus conventional chemotherapy with conventional radiotherapy.
Supplementary Material
Acknowledgments
We would like to thank Enfants et Santé, ARTC, les 111 des arts for their supports. We thank the patients and their families, all investigators and all pathologists (MP Chenard, CD Chiforeanu, MC copin, S Eimer, G Gauchotte, A Jouvet, A Laquerriere, MC Machet, CA Maurage, S Michalak, D Meyronet, C Miquel, M Peoc’h, M Polivka, V Rigau, G Saint-Pierre, H Sevestre, N Streichenberg, F Vandenhos, P Varlet, C Villa, A Vital, E Uro-Coste and the GENOP) involved in the study. We would like to thank Najat Loukh, Melanie Pucelle, and Sandrine Bedin. We would like to thank Sylviane Iacobelli for the data management of the study.
Funding
This study was supported by Amgen, MSD, Enfants et Santé, ARTC and “les 111 des arts.”
Conflict of interest statement. The authors declare that they have no conflict of interest
Authorship statement. Data collection: C.D., L.G.R., C.F.C., C.I., A.I.B., P.L., T.A., F.B., N.A., C.C., P.S., E.D.C., P.C., C.B., J..L, C.S., N.E.W. Data analysis and interpretation: C.D., S.F., A.G., J.M.P., D.F.B., M.B.D.. Report writing: C.D., S.F., A.G., J.M.P., D.F.B., M.B.D. Proofreading and approval: C.D., S.F., A.G., J.M.P., D.F.B., L.G.R., C.F.C., C.I., A.I.B., P.L., T.A., F.B., N.A., C.C., P.S., E.D.C., P.C., C.B., J.L., C.S., N.E.W., M.B.D.
References
- 1. Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Northcott PA, Buchhalter I, Morrissy AS, et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547(7663):311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cavalli FMG, Remke M, Rampasek L, et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell. 2017;31(6):737–754.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwalbe EC, Lindsey JC, Nakjang S, et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: a cohort study. Lancet Oncol. 2017;18(7):958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dufour C, Kieffer V, Varlet P, et al. Tandem high-dose chemotherapy and autologous stem cell rescue in children with newly diagnosed high-risk medulloblastoma or supratentorial primitive neuro-ectodermic tumors. Pediatr Blood Cancer. 2014;61(8):1398–1402. [DOI] [PubMed] [Google Scholar]
- 6. Chang CH, Housepian EM, Herbert C Jr. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93(6):1351–1359. [DOI] [PubMed] [Google Scholar]
- 7. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 9. Northcott PA, Shih DJ, Remke M, et al. Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 2012;123(4):615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carrie C, Grill J, Figarella-Branger D, et al. Online quality control, hyperfractionated radiotherapy alone and reduced boost volume for standard risk medulloblastoma: long-term results of MSFOP 98. J Clin Oncol. 2009;27(11):1879–1883. [DOI] [PubMed] [Google Scholar]
- 11. Gnekow AK. Recommendations of the Brain Tumor Subcommittee for the reporting of trials. SIOP Brain Tumor Subcommittee. International Society of Pediatric Oncology. Med Pediatr Oncol. 1995;24(2):104–108. [DOI] [PubMed] [Google Scholar]
- 12. Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 13. Gandola L, Massimino M, Cefalo G, et al. Hyperfractionated accelerated radiotherapy in the Milan strategy for metastatic medulloblastoma. J Clin Oncol. 2009;27(4):566–571. [DOI] [PubMed] [Google Scholar]
- 14. Jakacki RI, Burger PC, Zhou T, et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children’s Oncology Group Phase I/II study. J Clin Oncol. 2012;30(21):2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. von Bueren AO, Kortmann RD, von Hoff K, et al. Treatment of children and adolescents with metastatic medulloblastoma and prognostic relevance of clinical and biologic parameters. J Clin Oncol. 2016;34(34):4151–4160. [DOI] [PubMed] [Google Scholar]
- 16. Clifford SC, Lannering B, Schwalbe EC, et al. ; SIOP-Europe PNET Group . Biomarker-driven stratification of disease-risk in non-metastatic medulloblastoma: results from the multi-center HIT-SIOP-PNET4 clinical trial. Oncotarget. 2015;6(36):38827–38839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dufour C, Beaugrand A, Pizer B, et al. Metastatic Medulloblastoma in childhood: chang’s classification revisited. Int J Surg Oncol. 2012;2012:245385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maritaz C, Lemare F, Laplanche A, Demirdjian S, Valteau-Couanet D, Dufour C. High-dose thiotepa-related neurotoxicity and the role of tramadol in children. BMC Cancer. 2018;18(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thust SC, Blanco E, Michalski AJ, et al. MRI abnormalities in children following sequential chemotherapy, hyperfractionated accelerated radiotherapy and high-dose thiotepa for high-risk primitive neuroectodermal tumours of the central nervous system. J Med Imaging Radiat Oncol. 2014;58(6):683–690. [DOI] [PubMed] [Google Scholar]
- 21. Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65(2):499–508. [DOI] [PubMed] [Google Scholar]
- 22. Gleeson HK, Shalet SM. The impact of cancer therapy on the endocrine system in survivors of childhood brain tumours. Endocr Relat Cancer. 2004;11(4):589–602. [DOI] [PubMed] [Google Scholar]
- 23. DeWire M, Green DM, Sklar CA, et al. Pubertal development and primary ovarian insufficiency in female survivors of embryonal brain tumors following risk-adapted craniospinal irradiation and adjuvant chemotherapy. Pediatr Blood Cancer. 2015;62(2): 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clayton PE, Shalet SM, Price DA, Jones PH. Ovarian function following chemotherapy for childhood brain tumours. Med Pediatr Oncol. 1989;17(2):92–96. [DOI] [PubMed] [Google Scholar]
- 25. Ahmed SR, Shalet SM, Campbell RH, Deakin DP. Primary gonadal damage following treatment of brain tumors in childhood. J Pediatr. 1983;103(4):562–565. [DOI] [PubMed] [Google Scholar]
- 26. Packer RJ, Zhou T, Holmes E, Vezina G, Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children’s Oncology Group trial A9961. Neuro Oncol. 2013;15(1):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoff KV, Hinkes B, Gerber NU, et al. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT’91. Eur J Cancer. 2009;45(7):1209–1217. [DOI] [PubMed] [Google Scholar]
- 28. Tsui K, Gajjar A, Li C, et al. Subsequent neoplasms in survivors of childhood central nervous system tumors: risk after modern multimodal therapy. Neuro Oncol. 2015;17(3):448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salloum R, Chen Y, Yasui Y, et al. Late morbidity and mortality among medulloblastoma survivors diagnosed across three decades: a report from the childhood cancer survivor study. J Clin Oncol. 2019;37(9):731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.