Abstract
Background
Knowledge on management of pediatric spinal cord low-grade glioma (LGG) is scarce.
Methods
We analyzed clinical datasets of 128 pediatric patients with spinal LGG followed within the prospective multicenter trials HIT-LGG 1996 (n = 36), SIOP-LGG 2004 (n = 56), and the subsequent LGG-Interim registry (n = 36).
Results
Spinal LGG, predominantly pilocytic astrocytomas (76%), harbored KIAA1549-BRAF fusion in 14/35 patients (40%) and FGFR1-TACC1 fusion in 3/26 patients (12%), as well as BRAFV600E mutation in 2/66 patients (3%). 10-year overall survival (OS) and event-free survival (EFS) was 93% ± 2% and 38% ± 5%, respectively. Disseminated disease (n = 16) was associated with inferior OS and EFS, while age ≥11 years and total resection were favorable factors for EFS. We observed 117 patients following total (n = 24) or subtotal/partial resection (n = 74), biopsy (n = 16), or radiologic diagnosis only (n = 3). Eleven patients were treated first with chemotherapy (n = 9) or irradiation (n = 2). Up to 20.8 years after diagnosis/initial intervention, 73/128 patients experienced one (n = 43) or up to six (n = 30) radiological/clinical disease progressions. Tumor resections were repeated in 36 patients (range, 2-6) and 47 patients required nonsurgical treatment (chemotherapy, n = 20; radiotherapy, n = 10; multiple treatment lines, n = 17). Long-term disease control for a median of 6.5 (range, 0.02-20) years was achieved in 73/77 patients following one (n = 57) or repeated (n = 16) resections, and in 35/47 patients after nonsurgical treatment.
Conclusions
The majority of patients experienced disease progression, even after years. Multiple interventions were required for more than a third, yet multimodal treatment enabled long-term disease control. Molecular testing may reveal therapeutic targets.
Keywords: children, low-grade gliomas, spinal cord glioma, surgery, therapy
Key Points.
1. Dissemination, young age, and subtotal resection were associated with inferior EFS in pediatric spinal LGG
2. About a third of patients require repeated resections and/or adjuvant treatment
3. Molecular alterations (40% KIAA1549-BRAF fusion) could not predict clinical outcome
Importance of the Study.
Due to the rarity of the disease, long-term results concerning comprehensive treatment strategies and outcome in pediatric spinal cord low-grade glioma (LGG) are scarce. We present 128 pediatric patients with spinal cord LGG followed by the German LGG study group within prospective multicenter studies for a median of 8.8 years. The study provides a detailed analysis of natural history, management strategies, treatment response, prognostic factors, and clinical outcome. Molecular-genetic analysis revealed KIAA1549-BRAF fusion in 40%, FGFR1-TACC1 fusion in 12%, and BRAFV600E mutation in 3% of spinal LGG tested. This molecular information did not correlate with outcome. Although the majority of pediatric patients with spinal LGG experienced disease progression, even after years, and multiple interventions were required for more than a third, long-term disease control and clinical improvement was achieved by a comprehensive treatment strategy.
Low-grade gliomas (LGG) of the spinal cord account for 4%-5% of all pediatric LGG.1–10 Resection is considered the treatment of choice for spinal LGG followed by close observation for asymptomatic or clinically stable patients.1–4,10–16 Nonsurgical treatment options have been recommended for tumors not amenable to radical resection or in case of dissemination or progression.1–4,6,9,11,16–24 Despite excellent overall patient survival, many LGG progress, even after long periods of stability.1–4,6,11–14,17–20
In 1996, the HIT-LGG study group launched its first multicenter clinical trial, establishing diagnostic and therapeutic standards for the management of children and adolescents with LGG.1 A randomized chemotherapy arm was embedded in the subsequent multicenter trial SIOP-LGG 2004.6 After closure of the SIOP-LGG 2004 trial, patients have been prospectively followed on a registrational basis. All three studies included pediatric spinal LGG.
Several risk factors for overall survival (OS) and event-free survival (EFS) including dissemination, age, histology, and extent of resection were found to be particularly relevant for prognosis in LGG.1–4,6,10–15,18,20,22,24,25 However, discerning outcomes and prognostic factors unique to pediatric spinal cord LGG is difficult owing to the rarity of the disease on the one hand and, on the other hand, due to mixed patient populations and tumor types or localizations included in previous studies.1–4,6–9,11–15,17–21,23,24,26
The objective of the present study was, therefore, (i) to describe the course of disease, (ii) to evaluate the treatment and prognostic factors including the results of molecular neuropathological investigations, and (iii) to report clinical and functional outcome within a large cohort of prospectively followed pediatric patients with spinal intramedullary LGG.
Patients and Methods
Inclusion Criteria
We included patients below the age of 16 years (below 18 years since 2007) with intramedullary spinal cord LGG WHO-grades I and II (n = 128) registered in the prospective multicenter trials HIT-LGG 1996 (n = 36), SIOP-LGG 2004 (n = 56), and the subsequent LGG-registry (n = 36) until December 31, 2016. The Institutional Review Board approved consecutive studies observed the Declaration of Helsinki (revised version Edinburgh, Scotland, 2000), and the WHO and European Community rules of “Good Clinical Practice” (effective January 17, 1997). The SIOP-LGG 2004 study was registered at the ClinicalTrials.gov PRS NCT00276640 (EudraCT number 2005-005377-29). Informed consent was obtained from all patients, parents, or legal guardians.1,6
Data Assessment
Data were collected prospectively for age, sex, the presence of neurofibromatosis/tuberous sclerosis, tumor location, neuropathologic and radiologic findings, presenting symptoms, extent of resection, and treatment strategy. Functional status during follow-up and long-term sequelae were analyzed retrospectively, depending on available medical records.
Central neuropathologic review was conducted at the German Society for Neuropathology and Neuroanatomy (DGNN) Reference Center for Brain Tumors, University of Bonn, Germany by a panel of at least three board-certified neuropathologists with extensive experience in the diagnosis of pediatric brain tumors. Central radiologic review was performed at the Reference Center for Neuroradiology of the German Society of Pediatric Oncology and Hematology (GPOH), Würzburg, Germany. Neuro-imaging at diagnosis, following surgical interventions, assessing response of nonsurgical treatment and during the further course of disease followed recommendations of the German pediatric brain tumor (HIT-) network and published consensus.27
Extent of resection was defined as biopsy (no significant volume change), partial resection (remaining tumor tissue more than a rim), subtotal resection (visible rim of less than 3 mm), or total resection (no visible tumor on postsurgical MRI).
Progressive disease (PD) was defined as radiologically confirmed growth (increase of tumor volume >25% following the volume formula “1/2 [A × B × C]”) or development of a new lesion, and/or significant clinical deterioration.27 Status of complete remission (CR, no evidence of tumor) or stable disease (SD, tumor volume between +25% and −25% compared to the reference MRI) at last follow-up was termed “disease control.” For assessment of baseline clinical status and functional outcome, a modified McCormick scale (MMCS), as previously proposed, was used (Supplementary Table S1).11,28 Clinical data were updated as of December 1, 2019.
Treatment Strategy
Following the treatment algorithm that applied to all three consecutive studies/registry (Supplementary Figure S1), best safe resection of the primary tumor was recommended at diagnosis. Severe initial symptoms or clinical/radiological progression during observation indicated the start of nonsurgical treatment (chemotherapy or radiotherapy) if resection remained infeasible.1,6,20
Vincristine (VCR)/carboplatin was administered as first-line chemotherapy in 29 patients (HIT-LGG 1996, n = 13; SIOP-LGG 2004, n = 7; LGG-registry, n = 9). Four patients were randomized to receive additional etoposide during induction in the SIOP-LGG 2004 trial.6 Radiotherapy was scheduled with a total dose of 50.4 (median, range 45-54) Gray using either photons or protons. Treatment for further clinical and radiologic progression was not standardized, but included all modalities following discussion in local and reference tumor boards.
Molecular Pathology
If sufficient archival tumor material (formalin-fixed paraffin-embedded specimens) was available, immunohistochemistry for H3K27M as well as BRAFV600E mutation and RNA-based fusion assay (NanoString nCounter system, nanoString Technologies, Seattle, WA, USA) for detection of 1 of the 88 most common brain tumor-related fusion transcripts (Supplementary Table S2) were performed at the DGNN Reference Center for Brain Tumors, University of Bonn, Germany, as described.29,30
Statistical Analysis
Statistical analyses were conducted using IBM SPSS statistics 26 (IBM, Armonk, NY, USA). Group comparisons were performed using Fisher’s exact test for categorical variables and using Mann-Whitney U test or Kruskal-Wallis test for continuous variables. OS was calculated by the Kaplan-Meier method from date of diagnosis until death (of any cause). EFS was calculated from date of diagnosis until event, defined as relapse or clinical/radiological progression, start of nonsurgical therapy, or death of any cause. To evaluate the variable resection, OS and EFS were instead calculated from the date of surgery. Progression-free survival (PFS) was calculated from start of nonsurgical therapy until event, as defined above. Uni- and multivariable Cox regression was used to analyze the prognostic value of variables on EFS. P values were considered as descriptive measures to detect and study meaningful effects. In particular, no significance level was fixed (see Supplementary Material, “Statistical analysis”).
Data Availability
The datasets of the current study are available from the corresponding author upon reasonable request and are in part provided in “Supplementary Dataset.”
Results
Patients and Disease Characteristics
Epidemiologic characteristics of study patients are presented in Table 1 and Supplementary Table S3. Median age at diagnosis was 8.1 (range, 0.7-16.2) years. Main tumor sites were the thoracic (n = 50), cervicothoracic (n = 42), and cervical (n = 25) spine. Tumors spanned a median of 5 (range, 1-23) vertebral levels, 75 patients having concomitant syringomyelia. Limb weakness (n = 87; legs, n = 77; arms, n = 38), back pain (n = 57), and torticollis (n = 43) were the most common presenting symptoms, followed by sensory deficits (n = 36), scoliosis (n = 31), and bladder dysfunction (n = 22). Pilocytic astrocytoma (PA) WHO-grade I was the predominant histologic subtype (n = 94), followed by diffuse glioma WHO-grade II (DG2, n = 12) and ganglioglioma (GG) WHO-grade I (n = 11). Central pathologic review was available for 107 cases and confirmed local histologic diagnosis in 85 cases (79%). Discrepancies were mainly caused by insufficient quantity of tumor material or uncommon diagnoses/histopathologic features.
Table 1.
Epidemiologic Data of the Spinal LGG Cohort (Chemotherapy or Radiotherapy as First Adjuvant Treatment)
| Total (n = 128) | Chemotherapy (n = 35) | Radiotherapy (n = 12) | |
|---|---|---|---|
| Sex | |||
| Female | 50 (39%) | 14 (40%) | 8 (67%) |
| Male | 78 (61%) | 21 (60%) | 4 (33%) |
| Neurofibromatosis NF-1 | 7/126 (6%) | 3/33 (9%) | 0/12 |
| Median age at diagnosis (y, range) | 8.1 (0.7-16.2) | 4.2 (0.7-15.6) | 9.8 (1.4-13.3) |
| Age group | |||
| 0-4 y | 42 (33%) | 19 (54%) | 1 (8%) |
| 5-10 y | 47 (37%) | 9 (26%) | 6 (50%) |
| ≥11 y | 39 (30%) | 7 (20%) | 5 (42%) |
| Localization | |||
| Cervical | 25 (20%) | 4 (11%) | 5 (42%) |
| Cervicothoracic | 42 (33%) | 14 (40%) | 1 (8%) |
| Thoracic | 50 (39%) | 15 (43%) | 4 (33%) |
| Thoraco-lumbar | 8 (6%) | 1 (3%) | 2 (17%) |
| Lumbar | 1 (1%) | - | - |
| Holocord, multifocal | 2 (2%) | 1 (3%) | - |
| Histology of primary tumor | |||
| PA | 94/124 (76%) | 27/34 (79%) | 7 (58%) |
| DG2 | 12/124 (10%) | 2/34 (6%) | 3 (25%) |
| GG | 11/124 (9%) | 2/34 (6%) | 2 (17%) |
| Othera | 7/124 (6%) | 3/34 (9%) | - |
| No histology | 4 | 1 | - |
| Point mutation statusb | |||
| BRAFV600E | 2/66 (3%) | 2/5 (40%) | 0/1 |
| Other | 2 | 2 | - |
| Fusion status (n = 35) | |||
| No fusion | 18/35 (51%) | 7/13 (54%) | 3/3 (100%) |
| KIAA1549-BRAF fusionc | 14/35 (40%) | 6/13 (46%) | 0/3 |
| FGFR1-TACC1 fusiond | 3/26 (12%) | 0/9 | 0/2 |
| Median follow-up time (y, range) | 8.8 (0.04-25.4) | 8.0 (0-21.1) | 9.9 (5.7-25.4) |
| Dissemination | 16/128 (12.5%) | 11/35 (31%) | 3/12 (25%) |
| Primary | 11 | 6 | 3 |
| Secondary | 5 | 5 | - |
| Extent of first resection | |||
| No resection | 4/128 | 1/35 | - |
| Biopsy | 22/128 | 13/35 | 4/12 |
| Partial resection | 64/128 | 16/35 | 6/12 |
| Subtotal resection | 14/128 | 3/35 | 1/12 |
| Total resection | 24/128 | 2/35 | 1/12 |
| Re-resection (≥1) | 36/112 | 14/25 | 5/10 |
| Median number of resections (range) | 1 (0-6) | 1 (0-4) | 1 (0-3) |
| Age at start of CT/RT (y, range) | 5.8 (0.9-17.1) | 13.5 (9.1-16.7) | |
| Time from diagnosis to CT/RT (y, range) | 0.6 (0.0-5.0) | 3.3 (0.1-12.5) | |
| First event after start of treatment | |||
| No event | 72 | 13 | 10 |
| Disease progression | 51 | 19 | 2 |
| Death | 3 | 2 | - |
| Other treatment (without progression) | 2 | 1 | - |
| Time to first event after start of treatment | 1.2 (0.0-12.3 | 3.4 (1.2-5.6) | |
| Multiple treatment lines (nonsurgical) | 17 | 15 | 2 |
| Multiple chemotherapy lines | 5 | - | |
| Multimodal treatment | 10 | 2 | |
| Status at last follow-up | |||
| Alive, complete remission | 34 | 7 | - |
| Alive, stable disease | 78 | 17 | 11 |
| Alive, disease progression | 3 | 2 | - |
| Alive, second malignant neoplasme | 2 | 1 | - |
| Alive, malignant transformationf | 3 | 1 | 1 |
| Dead, malignant transformationf | 3 | 3 | - |
| Dead, disease-relatedg | 5 | 4 |
Abbreviations: PA, pilocytic astrocytoma WHO-grade I; DG2, diffuse glioma WHO-grade II; GG, ganglioglioma WHO-grade I; CT/RT, nonsurgical treatment (chemotherapy/radiotherapy); HGG, high-grade glioma.
aDysembryoplastic neuroepithelial tumor (DNT) WHO-grade I, n = 1 (observation/resection); rosette-forming glioneural tumor (RGNT) WHO-grade I, n = 1 (observation/resection); LGG not otherwise specified, n = 5 (observation/resection, n = 2; chemotherapy as first-line nonsurgical treatment, n = 3 [suspected DLGNT, n = 1; radiological criteria not precluding DLGNT, n = 1]).
b BRAFV600E mutation (n = 2) in a 12-year-old/14-year-old boy with thoracic/thoraco-lumbar GG (both SD after chemotherapy); novel mutation (GGG>GAG) in codon 144 of IDH-2 in a 5-year-old boy with thoracic PA (SD after chemotherapy following PD/relapse after partial/total resection); CDKN2A/B deletion in a 10-year-old girl with NF-1 and cervical PA (SD after PD and chemotherapy).
c KIAA1549-BRAF fusion (ex15:9, n = 8; ex15:11, n = 2; ex16:9, n = 1) in 12 patients with PA (SD/CR after initial resection, n = 4; SD/CR following 1-6 progressions, n = 7 and death after PD and chemotherapy, n = 1), 1 patient with GG (SD after PD and second partial resection), 1 patient with cervical DG2 (CR after initial resection).
d FGFR1-TACC1 (ex17:7) fusion in 2 patients with PA (both SD after initial resection) and 1 patient with RGNT (SD following partial resection after PD).
eTwo patients (PA and DG2) developed second, independent malignancies of the brain (chondrosarcoma, n = 1; glioblastoma, n = 1); both were alive in SD/CR after HGG treatment.
fMalignant transformation (MT) of LGG to HGG (n = 6): 10 months after partial resection/observation (n = 1, PA/anaplastic oligo-astrocytoma WHO-grade III); during first-line consolidation chemotherapy (n = 1, disseminated PA/anaplastic PA WHO-grade III [dead]); during repeated chemotherapy (n = 1, disseminated LGG NOS [suspected DLGNT]/glioblastoma [dead]); 5.6 years following first-line craniospinal RT (n = 1, disseminated GG/MT of brain metastasis, glioblastoma); during second-line chemotherapy 2-3.6 years following second-line RT (n = 2; disseminated PA/anaplastic PA WHO-grade III, n = 1; DG2/glioblastoma [dead]) (no neurofibromatosis; H3K27M/BRAFV600E: 0/5, fusion: 0/2).
gTwo disseminated PA, one PA (later oligodendroglioma WHO-grade II) with multiple treatment lines and rapid tumor progression with brain stem infiltration 4.6 years after surgery and RT, and 1 patient with PA and SD with death of brain stem insult 1 week after completion of chemotherapy. One patient with CR of cervicothoracic DNT and central breathing regulation disorder died of aspiration 1.7 years after initial total resection.
After reviewing MRI findings and histopathology of patients with disseminated LGG for possible diagnosis of diffuse leptomeningeal glioneuronal tumor (DLGNT), we could not preclude DLGNT in 1 patient (LGG not otherwise specified [NOS]), and retrospectively suspected DLGNT in 1 patient meeting both radiologic and histopathologic criteria for DLGNT (LGG NOS; 1p loss after transformation to glioblastoma).
Molecular Pathology
Immunohistochemistry was negative for mutant H3K27M protein in all 72 tumors tested, and positive for BRAFV600E mutation in specimens of 2/66 patients (3%; Table 1). Fusion analysis detected KIAA1549-BRAF fusion transcripts in 14/35 LGG (40%) and FGFR1-TACC1 fusion transcripts in 3/26 LGG (11.5%; Table 1). Gene panel sequencing performed at diagnosis revealed point mutations in tumor specimens of 2 patients, one each harboring a CDKN2A/B deletion, and a novel IDH-2 variant (GGG>GAG in codon 144). Molecular-pathologic findings were not associated with the course of disease (Table 2).
Table 2.
Univariable Cox Regression of Event-Free Survival
| Parameter | Group Comparison | N | Events | Hazard Ratio | Lower 95% CL | Upper 95% CL | P |
|---|---|---|---|---|---|---|---|
| Dissemination (n = 128) | No dissemination | 112 | 61 | <.001 | |||
| Disseminated disease | 16 | 15 | 3.48 | 1.95 | 6.24 | ||
| Age at diagnosis (n = 128) | ≥11 y | 39 | 16 | .008 | |||
| 5-10 y | 47 | 29 | 1.75 | 0.95 | 3.22 | ||
| 0-4 y | 42 | 31 | 2.53 | 1.38 | 4.64 | ||
| Extent of resection (n = 121)a | Total resection | 24 | 8 | .001 | |||
| Subtotal resection | 14 | 8 | 2.09 | 0.79 | 5.58 | ||
| Partial resection | 63 | 38 | 2.73 | 1.27 | 5.87 | ||
| Biopsy | 20 | 17 | 5.50 | 2.36 | 12.83 | ||
| Histology (n = 124) | PA | 94 | 57 | .300 | |||
| DG2 | 12 | 5 | 0.59 | 0.24 | 1.48 | ||
| Other | 18 | 13 | 1.29 | 0.71 | 2.36 | ||
| Sex (n = 128) | Female | 50 | 31 | .342 | |||
| Male | 78 | 45 | 0.80 | 0.51 | 1.27 | ||
| Neurofibromatosis (n = 126) | Negative | 119 | 70 | .798 | |||
| NF-1 | 7 | 4 | 1.14 | 0.42 | 3.14 | ||
| Tumor volume (n = 80) | <7 mL | 42 | 20 | .126 | |||
| ≥7 mL | 38 | 24 | 1.59 | 0.88 | 2.90 | ||
| Localization (n = 125) | Cervical | 25 | 13 | .194 | |||
| Cervicothoracic | 42 | 30 | 1.66 | 0.86 | 3.18 | ||
| Thoracic | 50 | 29 | 1.16 | 0.60 | 2.24 | ||
| Thoraco-lumbar | 8 | 3 | 0.64 | 0.18 | 2.25 | ||
| MMCS at diagnosisb (n = 119) | I-II | 45 | 25 | .249 | |||
| III | 34 | 19 | 1.06 | 0.59 | 1.93 | ||
| IV-V | 40 | 27 | 1.55 | 0.90 | 2.68 | ||
| MIB-1 index (n = 109) | <1% | 18 | 10 | .411 | |||
| 1-5% | 71 | 40 | 1.06 | 0.53 | 2.13 | ||
| ≥5% | 20 | 14 | 1.60 | 0.71 | 3.61 | ||
| p53 accumulation (n = 91) | Negative | 74 | 46 | .163 | |||
| Positive | 17 | 8 | 0.60 | 0.28 | 1.28 | ||
| BRAFV600E status (n = 66) | No mutation | 64 | 43 | .633 | |||
| BRAFV600E mutation | 2 | 1 | 0.64 | 0.09 | 4.66 | ||
| Fusion status (n = 35) | No fusion | 18 | 12 | .566 | |||
| KIAA1549-BRAF fusion | 14 | 10 | 1.32 | 0.55 | 3.14 | ||
| FGFR1-TACC1 fusion | 3 | 2 | 0.61 | 0.14 | 2.78 |
Bold values indicate statistically significant findings.
aExclusion of 4 patients without resection and 3 patients with event before first resection.
bExclusion of 9 patients presenting with symptoms of increased intracranial pressure without motor/sensory deficits.
Course of Disease
Median follow-up was 8.8 (range, 0.0-25.4) years. At last follow-up, 120/128 patients were alive; 78 patients were alive with SD, another 34 with CR; seven of these patients developed spontaneous CR 0.7-7.7 years following last intervention/progression (PA, n = 6, GG, n = 1; all neurofibromatosis type 1 negative). Three patients had recent PD, 5 were alive with second/secondary high-grade neoplasms.
Overall, 73 patients experienced one (n = 43) or up to six (n = 30) disease progressions following first tumor resection (n = 52), biopsy (n = 10), radiological diagnosis (n = 4), or initial chemotherapy (n = 7). Median time to first progression was 8 months (range, 3 days-11 years). Disease progression was observed as late as 20.8 years after diagnosis, following surgery and two lines of chemotherapy in 1 patient with disseminated PA.
Sixteen patients had primary (n = 11) or developed secondary (n = 5) dissemination, unrelated to age. Dissemination was associated with more surgical interventions, necessity of nonsurgical treatment, malignant progression, and death. MMCS at diagnosis (P = .032) and after 5 years (P = .001) revealed more severe deficits, and clinical deterioration was observed more frequently in patients with dissemination compared to patients with localized disease (P = .003; Supplementary Table S4).
Malignant transformation (MT) of primary LGG emerged in 6 patients after up to 10.6 years from first diagnosis. Initial histologic diagnosis of these patients was PA (n = 3), DG2 (n = 1), LGG NOS (suspected DLGNT, n = 1), and GG (n = 1). Four of these patients had disseminated disease and three had received irradiation as part of their treatment 2-5.6 years before MT.
Two further patients developed second malignant neoplasm (SMN) of the brain (chondrosarcoma, glioblastoma) after 7.9-9.9 years from LGG diagnosis with (chemotherapy) or without previous adjuvant treatment. No H3K27M or BRAFV600E mutation was detected in patients with later MT or SMN (7/8 patients tested). After revelation of high-grade histology, all patients were treated accordingly and therefore censored for further analysis.
Eight patients died related to their disease, including three after MT (Table 1).
Survival and Risk Factor Analysis
OS and EFS of the entire cohort at 10 years were 93% ± 2% and 38% ± 5%, respectively (Fig. 1A and C). Patients with disseminated LGG had lower OS and EFS rates compared to patients with localized tumors (Fig. 1B and D). Uni- and multivariable Cox regression analysis revealed age ≥11 years (hazard ratio [HR] vs age group 0-4 years: 0.36; Tables 2 and 3, Fig. 1E) and total resection (HR vs biopsy: 0.21; Tables 2 and 3, Fig. 1F) to be associated with higher EFS. Disseminated disease was an independent prognostic factor for inferior EFS in uni-/multivariable analysis as well (HR 2.41; Tables 2 and 3).
Fig. 1.
Overall survival and event-free survival. (A) 10-year OS of the entire cohort (93% ± 2%); (B) 10-year OS in patients with localized disease (97% ± 2%), disseminated disease (68% ± 12%; P < .001); (C) 10-year EFS of the entire cohort (38% ± 5%); (D) 10-year EFS in patients with localized disease (43% ± 5%), disseminated disease (0%; P < .001); (E) 10-year EFS of patients aged 0-<5 years (27% ± 7%), 5-<11 years (34% ± 8%), ≥11 years (54% ± 9%; P = .008); (F) 10-year EFS according to extent of resection: biopsy (13% ± 8%), partial resection (33% ± 7%), subtotal resection (48% ± 14%), total resection (66% ± 10%; P = .001).
Table 3.
Multivariable Cox Regression of Event-Free Survival
| Parametera | Group Comparison | N | Events | Hazard Ratio | Lower 95% CL | Upper 95% CL | P |
|---|---|---|---|---|---|---|---|
| Dissemination | No dissemination | 112 | 61 | .011 | |||
| Disseminated disease | 16 | 15 | 2.41 | 1.28 | 4.57 | ||
| Age at diagnosis | ≥11 y | 39 | 16 | .002 | |||
| 5-10 y | 47 | 29 | 2.31 | 1.24 | 4.33 | ||
| 0-4 y | 42 | 31 | 2.74 | 1.48 | 5.06 | ||
| Extent of resection | Total resection | 24 | 8 | .008 | |||
| Subtotal resection | 14 | 8 | 1.77 | 0.66 | 4.77 | ||
| Partial resection | 63 | 38 | 2.78 | 1.29 | 6.00 | ||
| Biopsy | 20 | 17 | 4.67 | 1.89 | 11.53 |
Bold values indicate statistically significant findings.
aNo interactions were selected (P > .187 for all interaction terms).
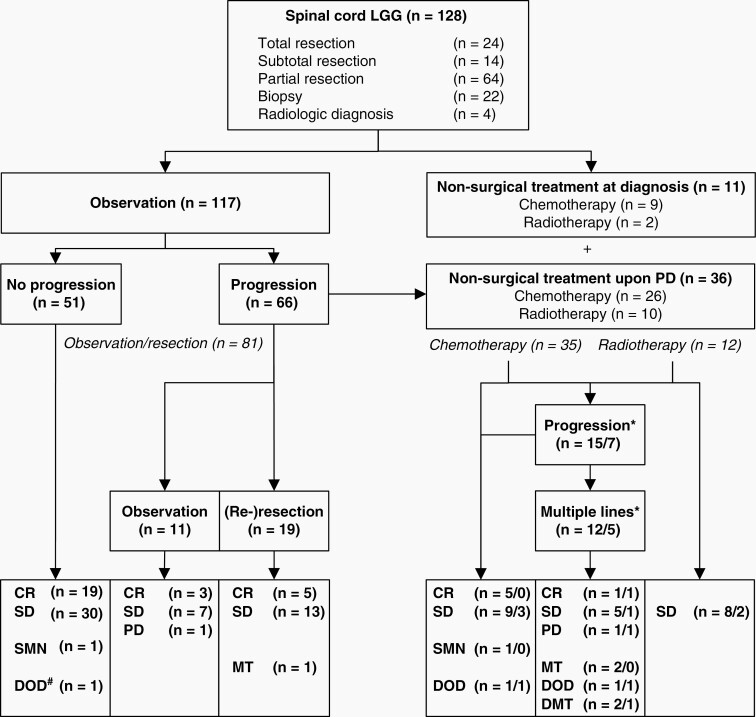
Initial Management
As depicted in Fig. 2, 117 patients were observed after first resection (total, n = 24; subtotal, n = 14, partial, n = 60), biopsy (n = 16), or radiologic diagnosis (n = 3). Forty-nine of them were alive and progression-free (CR, n = 19 [3 with spontaneous CR]; SD, n = 30) after a median follow-up of 7.2 (range, 2.1-19.9) years. One patient died in CR, another developed a SMN.
Fig. 2.
Treatment strategy and course of disease after initial resection (n = 124) or radiologic diagnosis (n = 4). Multiple treatment lines consisted of one or more courses of chemotherapy and/or radiotherapy ± surgical treatment. #Death of aspiration due to central respiratory dysregulation 1.7 years after initial total resection of cervicothoracic dysembryoplastic neuroepithelial tumor WHO-grade I. *Figures before the slash indicate numbers of patients initially observed, figures after the slash refer to numbers of patients who received initial nonsurgical treatment. Abbreviations: CR, complete remission; DMT, death of HGG after MT; DOD, death of disease; MT, malignant transformation with subsequent HGG treatment; PD, recent progressive disease; SD, stable disease; SMN, second malignant neoplasm.
Eleven patients received adjuvant treatment immediately following diagnosis (biopsy, n = 6; radiologic diagnosis, n = 1) or partial resection (n = 4). Two patients with localized LGG of the lower thoracic spine and imminent paraplegia were irradiated, achieving SD for 5.6-8.6 years. Chemotherapy was administered in 9 patients including 3 patients with dissemination. In 2 patients, SD was achieved for 3.3-13.2 years after start of chemotherapy.
Management of PD
After a median time of 0.6 (range, 0.1-11) years, 66/117 patients who were observed following initial diagnosis experienced relapse or disease progression (Fig. 2).
Seven of these 66 patients reached SD without further treatment; 3 patients with PA (one with KIAA1549-BRAF fusion) showed spontaneous CR after progression. One patient developed recent PD (no further information). Nineteen patients underwent one (n = 17), two (n = 1), or five (n = 1) additional resections for PD. Of these, all were alive with CR (n = 5) or SD (n = 13); in 1 patient, histopathology revealed MT at re-resection.
Thirty-six patients (chemotherapy, n = 26; radiotherapy, n = 10) proceeded to adjuvant treatment after disease progression (11 with disseminated disease); in 23 of these 36 patients, CR (n = 5; one with spontaneous CR 7.4 years after chemotherapy, two following further PD and resection) or SD (n = 17; one after PD and resection) was achieved after adjuvant treatment. One patient died related to disease, and 1 patient experienced a SMN.
Seven out of nine patients progressed after a median of 0.8 (range, 0.0-1.7) years following initial chemotherapy. One of these patients with disseminated DG2 and rapid progression died 3 days after initiation of chemotherapy. One achieved SD for 1.1 years after resection.
Seventeen patients ultimately required repeated courses of chemotherapy (n = 5) or multimodal treatment (ie, resection, chemotherapy, and irradiation, n = 12) due to multiple disease progressions. Following multiple treatment lines, eight were alive with CR (n = 2) or SD (n = 6), 1 patient with multifocal spinal LGG showed recent PD 4.8 years after second-line chemotherapy. Following MT, 2 patients were alive and 3 patients died. Two patients died related to their LGG (one disseminated).
Treatment and Disease Control
Of all 128 patients with spinal cord LGG, 81 patients remained observed after one resection (total, n = 20; subtotal, n = 8; partial, n = 32), with 2-6 therapeutic surgeries (n = 17), or without any tumor reductive surgery (radiological diagnosis, n = 3; biopsy, n = 1).
Nonsurgical treatment was required in 47 patients and given without resection (radiological diagnosis, n = 1; biopsy, n = 11), after one therapeutic surgery (total, n = 2; partial, n = 14) or with 2-4 concomitant resections (n = 19).
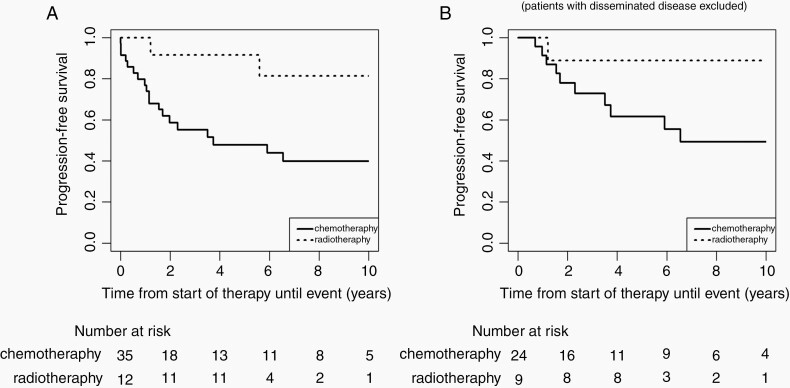
First-line adjuvant treatment consisted of chemotherapy in 35 patients and radiotherapy in 12 patients (Table 1). Five-year PFS following radiotherapy and chemotherapy was 92% ± 8% and 48% ± 9%, respectively (Fig. 3A). Excluding patients with disseminated disease, 5-year and 10-year PFS was 62% ± 11%, respectively, 49% ± 12% after first-line chemotherapy (Fig. 3B). Patients receiving first-line radiotherapy were older (median, 13.5 [range, 9.1-16.7] years) than those treated with front-line chemotherapy (median, 5.8 [range, 0.9-17.1] years; Table 1).
Fig. 3.
Progression-free survival of patients receiving chemotherapy or radiotherapy as first-line nonsurgical treatment. (A) 5-year PFS after radiotherapy (92% ± 8%) and chemotherapy (48% ± 9%) (not compared); (B) 5-year and 10-year PFS after radiotherapy (89% ± 10%) and chemotherapy (62% ± 11% and 49% ± 12%) excluding patients with disseminated disease (not compared).
Twenty patients received one course of chemotherapy (±resection), 10 received radiotherapy (±resection), and 17 underwent multiple lines of chemotherapy (n = 5) or multimodal treatment including surgery, radio- and chemotherapy (n = 12). Median age at start of second-line radiotherapy after chemotherapy was 8.8 (range, 4.4-12.4) years.
After last intervention, disease control (CR/SD) for a median of 6.5 (range, 0.02-20) years was achieved in 73/77 patients following one (n = 57) or repeated (n = 16) resections, and in 35/47 patients following adjuvant treatment (chemotherapy, n = 17/20; irradiation; n = 10/10; multiple treatment lines, n = 8/17), while 4 patients remained without therapy throughout follow-up.
Functional Outcome and Long-Term Sequelae
Data on functional outcome and long-term sequelae were available for 109 surviving patients at five, and for 67 at 10 years. According to MMCS, 75/109 patients (69%) had no or mild functional deficits, while 14 (13%) showed moderate and 20 (18%) severe neurological impairment after 5 years of follow-up (Table 4). The majority (n = 72; 66%) showed functional improvement, compared to their initial neurological status. Fifteen patients (14%) experienced clinical deterioration. MMCS at diagnosis was prognostic for functional outcome after 5 and 10 years (P = .002 and P = .001).
Table 4.
Symptoms at Diagnosis, Functional Outcome, and Long-Term Sequelae After 5 and 10 Years of Follow-Up
| Diagnosis (n = 128) | 5 y (n = 109) | 10 y (n = 67) | P value 5 y vs di | P value 10 y vs di | P value 10 y vs 5 y | |
|---|---|---|---|---|---|---|
| Duration of first symptoms (mo) | 4 (0.03-144) | - | - | - | - | - |
| Modified McCormick scale (n = 119)a | <.001 | <.001 | .531 | |||
| I-II | 45 (37%) | 75 (69%) | 47 (70%) | |||
| III | 34 (29%) | 14 (13%) | 5 (8%) | |||
| IV-V | 40 (34%) | 20 (18%) | 15 (22%) | |||
| Neurologic status compared to diagnosis | - | - | .813 | |||
| Improved | - | 72 (66%) | 45 (67%) | |||
| Stable | - | 22 (20%) | 14 (21%) | |||
| Deteriorated | - | 15 (14%) | 8 (12%) | |||
| Initial symptoms and long-term sequelae | ||||||
| Pain | 57 (45%) | 4 (4%) | 0 | <.001 | <.001 | <.001 |
| Torticollis | 43 (34%) | - | - | - | - | - |
| Neurologic | 101 (79%) | 78 (72%) | 47 (70%) | .200 | .049 | .375 |
| Normal neurologic status | 27 (21%) | 31 (28%) | 67 (30%) | .200 | .049 | .375 |
| Sensory deficits | 36 (28%) | 48 (44%) | 30 (45%) | .017 | .307 | .625 |
| Motor dysfunction | 87 (68%) | 62 (57%) | 37 (55%) | <.001 | <.001 | .012 |
| Mild | 17 (13%) | 29 (27%) | 18 (27%) | |||
| Moderate | 34 (27%) | 14 (13%) | 5 (8%) | |||
| Severe | 36 (28%) | 19 (17%) | 14 (21%) | |||
| Bladder/rectal dysfunction | 22 (17%) | 21 (19%) | 14 (21%) | .678 | 1.000 | 1.000 |
| ICP (intracranial pressure)/VP (ventriculoperitoneal) shunt | 21 (16%) | 8 (7%) | 6 (9%) | .057 | .453 | 1.000 |
| Horner syndrome | 1 (1%) | 5 (5%) | 6 (9%) | .219 | .063 | .500 |
| Hearing lossb | 0 | 0 | 1 (1.5%) | 1.000 | .344 | .381 |
| Symptomatic epilepsy | 2 (2%) | 2 (2%) | 2 (3%) | 1.000 | 1.000 | 1.000 |
| Orthopedic | 40 (31%) | 68 (62%) | 41 (61%) | <.001 | <.001 | .875 |
| Spine deformities | 31 (24%) | 60 (55%) | 38 (57%) | <.001 | <.001 | 1.000 |
| Others (feet, leg length difference) | 10 (8%) | 28 (26%) | 20 (30%) | <.001 | <.001 | .250 |
| Orthopedic surgery | 0 | 12 (11%) | 6 (9%) | <.001 | .001 | .800 |
| Others | 3 (2%) | 20 (18%) | 15 (22%) | <.001 | <.001 | .125 |
| Other surgeryc | 0 | 12 (11%) | 6 (9%) | <.001 | .001 | .800 |
| Psychologic/psychiatric | 2 (2%) | 8 (7%) | 8 (12%) | .070 | .016 | 1.000 |
| Thyroid glandd | 0 | 0 | 3 (5%) | 1.000 | .039 | .054 |
| Urinary tracte | 1 (1%) | 2 (2%) | 0 | 1.000 | 1.000 | .500 |
| Second malignancyf | 0 | 0 | 2 (3%) | 1.000 | .117 | .144 |
aExclusion of n = 9 patients presenting with symptoms of increased intracranial pressure without motor/sensory deficits.
bHigh-frequency hearing loss after chemotherapy (stable).
cTethered cord + cerebrospinal fluid (CSF) leak (n = 1); CSF leak (n = 5); syringomyelia (n = 3); nephrectomy (n = 1); hemithyroidectomy (n = 1); temporary tracheostoma (n = 1); percutaneous endoscopic gastrostomy (PEG) during chemotherapy (n = 1).
dHypothyroidism (n = 2; one after irradiation, one after radio-chemotherapy), microfollicular adenoma after chemotherapy requiring resection (n = 1).
eRecurrent pyelonephritis due to bladder dysfunction requiring unilateral nephrectomy (n = 1), lithotripsy of bladder stone (n = 1).
fChondrosarcoma of the skull-base (no previous adjuvant treatment; n = 1), glioblastoma of the brain after chemotherapy (n = 1).
Moderate to severe neurological long-term sequelae were present in 19/67 patients (28%) after 10 years of follow-up (Table 4). More than 60% of all patients (68/109 and 41/67) suffered from secondary spine deformities, mainly kyphoscoliosis. Spine deformities were present at diagnosis in 31/128 patients (24%), and developed in another 24/85 patients (28%) after first resection (mild-moderate, n = 19), plus in 11/34 patients (32%) after adjuvant/multimodal treatment. Orthopedic spine surgery had been reported for 12/109 patients at 5 and 6/67 at 10 years.
Discussion
We present 128 pediatric patients with spinal cord LGG followed by the German LGG study group within prospective multicenter studies covering a time frame of two decades. The large size of the cohort, treatment according to a comprehensive strategy, and long follow-up time allow meaningful analysis of these patients afflicted by rare and slowly growing neoplasms, though molecular analysis was not routinely performed for most tumors and the respective studies lacked prospective standardized assessment of clinical features during follow-up.
The excellent OS rate of our cohort corroborates previous findings, with 5- and 10-year OS rates ranging between 85% and 100% for children and adolescents with spinal cord LGG.1–4,8,12,13,17,19,31 In contrast, the 10-year EFS/PFS rates of 23%-50% in this subgroup (38.5% in our cohort) were lower compared to LGG of other locations.1,3,4,17,19 While disease progression occurred early in more than half of our patients, it was detected more than 20 years after diagnosis, as well, confirming reports of progression beyond 10 years of follow-up.11,17,19 These findings emphasize the necessity of a comprehensive treatment strategy and the importance of extended long-term follow-up in patients with spinal cord LGG.
Three factors predicted events and were confirmed by multivariate analysis. First, dissemination was shown to be associated with both inferior OS and EFS. The percentage of patients with disseminated disease (12.5%) was remarkably higher in our study than in the entire HIT-LGG 1996 cohort (4.3%-5.2%),1,22 indicating that spinal LGG may have a higher propensity to disseminate than LGG of other locations. Although metastatic LGG frequently recur or progress,1,6,13,22,32,33 two-thirds of patients with disseminated disease were alive after multimodal treatment in the present cohort. Following our observations that DLGNT could be suspected retrospectively in 2 of these 16 patients and four progressed to high-grade glioma (HGG), we recommend close follow-up and comprehensive diagnostic workup including molecular analysis for patients with disseminated LGG.
Young age (especially age <1 year) was shown to be associated with worse prognosis in various cohorts of patients with LGG of all locations.1–3,6,24,25 However, the prognostic impact of older age in children with LGG is discussed controversially.1–4,13,14 We identified older age (≥11 years in our cohort) as favorable prognostic parameter, probably reflecting a subgroup of LGG less prone to growth and progression. Conflicting results within published series concerning the prognostic relevance of age in pediatric LGG emphasize the need for careful risk stratification in future clinical trials.
In accordance with numerous previous studies, a higher extent of resection correlated with better EFS in our population,1–4,9–13,15,20 emphasizing the impact of primary maximal safe resection. However, infiltrative spinal cord tumors are often not amenable to complete resection. Complete resection rates of 53% and 66% were reported for pediatric LGG of the cerebral hemispheres and the cerebellum in the HIT-LGG 1996 cohort, while total resection was only achieved in 28% of patients with spinal cord LGG, and in 25% in a recent retrospective analysis of 40 pediatric patients with spinal cord LGG.1,31 In the present study, initial total resection was achieved in only 19% of all patients. However, modern technical adjuncts such as intraoperative imaging and intraoperative neurophysiological monitoring allow for multiple resections in spinal cord tumors even in the pediatric age group. Prolonged disease control was achieved following one or repeated resections without need of adjuvant treatment in two-thirds of patients in the present cohort. Careful planning of neurosurgical interventions performed in specialized centers is therefore mandatory in order to guarantee for maximal safe resection of spinal LGG. Moreover, resection and follow-up should not only consider neurological issues, but also focus on orthopedic problems, such as spinal deformities, which developed in almost 30% of patients following first resection, though remaining mainly mild/moderate.
For 47 patients requiring nonsurgical treatment, disease control was achieved in 75%, and long-term control could even be attained following multiple progressions with multiple and multimodal treatment lines in a subgroup of patients.
In a study by Scheinemann et al., 48% of patients required radio-and/or chemotherapy.4 In contrast, only 47 patients (37%) received nonsurgical treatment in our series. This difference may be explained by a higher rate of neurosurgical re-interventions and by consistent recommendations for the start of nonsurgical treatment, being restricted to non-resectable (progressive) disease and severe/progressing symptoms.
Progression rates following chemotherapy are reportedly higher than for radiotherapy, especially in the young.1,20,25 The present results confirm these findings, although direct comparison of PFS following chemo- or radiotherapy is prohibited due to differences in patient allocation (eg, concerning age or disseminated disease). Excluding patients with disseminated disease, almost two-thirds of patients remained progression-free after 5 years strengthening the role of chemotherapy as first-line adjuvant treatment, especially in extensive or disseminated disease. Although a high proportion of these patients may eventually experience further progression, chemotherapy avoids early irradiation in younger children sparing possible adverse effects on spinal growth (this aspect was not prospectively assessed in our cohort).1,4,6,19,34 Furthermore, we observed hypothyroidism in 2 patients after photon irradiation of cervical tumors, as well as malignant progression in 3 patients 2-5.6 years following first- or second-line radiotherapy. However, given the excellent PFS and long-term disease control rate observed in our cohort, radiotherapy should be considered both as first-line and salvage nonsurgical treatment of localized spinal LGG not amenable to complete resection in children and adolescents with imminent clinical deterioration, for example, paraplegia. The use of proton irradiation may further help to decrease the risk of long-term sequelae. At last, possible risks should be carefully weighed up against potential benefits of adjuvant treatment.
About 69%-95% of pediatric patients with spinal tumors improve or remain functionally stable during long-term follow-up.4,10,11,35 Likewise, functional improvement or at least stability were achieved in the majority (86%) of patients in our series. In contrast, severe functional deficits persisted in approximately every fifth patient. More than 60% of patients suffered from well-known orthopedic long-term complications, mainly scoliosis, often with need of further surgical intervention.4,17,27,35 Kyphoscoliosis was present in a quarter of patients at diagnosis, whereas another 30% developed spine deformities following treatment. Most of these patients, however, did not require any interventions. Although the assessment of clinical outcome in this study was not standardized and therefore dependent on the treating physicians’ documentation and the MMCS grading was done retrospectively, we confirm the relevance of the preoperative clinical status for functional long-term outcome,10,11,15,36 and advocate that the initiation of nonsurgical treatment be guided by the severity of symptoms and not by the extent of the tumor. As with intracranial LGG, a watch and wait strategy may be appropriate in patients with only mild clinical symptoms.
Progression to HGG was observed in 6 patients in our cohort (four with dissemination, three with previous irradiation), after up to 10 years, exceeding reported MT rates of about 2%-3% in pediatric LGG of all locations and histologies.20,37 While exact incidence and underlying mechanisms of MT of pediatric LGG are still largely unknown and matter of ongoing research, these findings emphasize the importance of re-resection/biopsy in case of tumor relapse or progression whenever feasible and stress the importance of molecular-genetic testing, in order to possibly identify “high-risk” patients.
Retrospective molecular-pathological analysis was restricted to the subset of patients in our study for whom tumor tissue in sufficient quantity for testing was available. Immunohistochemistry excluded histone3K27M mutation, thus precluding malignant midline gliomas, in all tumor specimens tested.29,38 Systematic fusion analysis revealed KIAA1549-BRAF fusion in 40% of patients tested, consistent with reports describing this fusion as most common genetic alteration in pediatric (spinal cord) LGG, especially PA.39–42 However, in contrast to the findings of large molecular and clinical analyses of pediatric LGG of all locations by Ryall et al.,40,42 we found only one tumor with fusion involving exon 16 in KIAA1549 and exon 9 in BRAF, while KIAA1549-BRAF ex15:9 was the most common fusion in our cohort with spinal LGG (n = 8). In consistence with previous findings,42 three of them had aggressive courses requiring multimodal treatment, one even succumbing to disease. The other 5 patients, however, achieved SD, 1 patient even experienced spontaneous CR. The observed rate of 12% of spinal LGG harboring a FGFR1-TACC1 fusion in our cohort clearly exceeds the reported range of 1.5%-7.6%.40,42 None of these 3 patients had disseminated disease and all achieved SD after surgery. Other mutations represented the previously described spectrum with mutation of BRAFV600E in 3% (GG, both SD after chemotherapy) and loss of CDKN2A/B, but also a novel IDH-2 variant in a 5-year-old boy with PA.39–42 Altogether, we did not observe any correlation between molecular-pathologic findings and the clinical course of patients in our study, probably due to the small sample size. Routine application of molecular profiling is mandatory to increase our knowledge about the relationship of distinct molecular findings and clinical parameters in pediatric spinal cord LGG and in order to reveal possible therapeutic targets.
Conclusion
This large multicenter series with extended long-term follow-up demonstrated that the majority of pediatric patients with spinal LGG experienced disease progression, even after years, and multiple interventions were required for more than a third. Yet, almost two-thirds of patients were managed by surgery and observation, and multimodal adjuvant treatment enabled long-term disease control even in patients with multiple progressions.
Maximum safe resection should be intended, whenever feasible, taking into account not only neurologic, but orthopedic sequelae, as well. For patients with severe symptoms or PD without option for complete resection, nonsurgical treatment should be considered. Chemotherapy (VCR/carboplatin) may be the best option for first-line adjuvant treatment in patients with extended tumors, disseminated disease, and in young children, even if further treatment may be necessary due to disease progression. Radiotherapy can achieve high and long-lasting local control rates and should be considered as adjuvant therapy especially for the older subgroup with localized tumors. Given the “chronic” nature of the disease, possible benefits of treatment must be carefully weighed up against potential (long-term) side effects. Molecular testing should confirm low-grade diagnosis and may identify therapeutic targets providing an additional option to achieve disease control.
Supplementary Material
Funding
The German low-grade glioma study center (A.K.G., D.K.) was supported by the German Cancer Aid (Deutsche Krebshilfe) for the HIT-LGG 1996 study (Grant no. 70-2218-Gn1) and by the German Children’s Cancer Foundation (Deutsche Kinderkrebsstiftung) for the SIOP-LGG 2004 trial and LGG-registry (Grant nos. 2004.5, 2007.10, and 2010.08, 2011.16, 2013.19, 2015.19, 2017.13). The Brain Tumor Reference Center of the DGNN, Institute of Neuropathology, University of Bonn (Grant nos. 2006.03, 2009.19, 2011.01 and 2014.17 to T.PT.), Institute of Biostatistics and Clinical Research, University of Münster (Grant no. 2017.13 to R.S.), and the Neuroradiologic Reference Center of the HIT-Consortium, University Würzburg (Grant nos. 2001.05, 2003.09, 2005.07, 2008.07, 2011.02, 2013.21, 2014.15, 2017.07, 2018.02 to M.W.M., B.B.) were supported by the German Children’s Cancer Foundation (Deutsche Kinderkrebsstiftung). T.PW. and M.B. receive support from the Styrian Childhood Cancer Foundation (Steirische Kinderkrebshilfe).
Conflict of interest statement. The authors declare no conflict of interest.
Previous presentations. Portions of this work were presented at the 18th International Symposium on Pediatric Neuro-Oncology, Denver, CO, USA, June 29-July 3, 2018.
Authorship statement. Conception and design: T.PW., M.B., T.PT., A.K.G., and D.K. Acquisition of data: T.PW., T.PT., and D.K. Neuropathological review and molecular analysis: T.PT. Neuroradiological review: B.B. and M.W.M. Radiooncological review: R.D.K. and B.T. Statistical analysis: R.S. and F.Q. Analysis and interpretation: T.PW., M.B., T.PT., A.K.G., and D.K. Drafting article and critically revising: all authors.
References
- 1. Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14(10):1265–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(21):2641–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stokland T, Liu JF, Ironside JW, et al. A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702). Neuro Oncol. 2010;12(12):1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scheinemann K, Bartels U, Huang A, et al. Survival and functional outcome of childhood spinal cord low-grade gliomas. Clinical article. J Neurosurg Pediatr. 2009;4(3):254–261. [DOI] [PubMed] [Google Scholar]
- 5. Arnautovic A, Billups C, Broniscer A, Gajjar A, Boop F, Qaddoumi I. Delayed diagnosis of childhood low-grade glioma: causes, consequences, and potential solutions. Childs Nerv Syst. 2015;31(7):1067–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gnekow AK, Walker DA, Kandels D, et al. ; of the Low Grade Glioma Consortium and the participating centers . A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤16 years) low grade glioma - a final report. Eur J Cancer. 2017;81:206–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Engelhard HH, Villano JL, Porter KR, et al. Clinical presentation, histology, and treatment in 430 patients with primary tumors of the spinal cord, spinal meninges, or cauda equina. J Neurosurg Spine. 2010;13(1):67–77. [DOI] [PubMed] [Google Scholar]
- 8. Hayden Gephart MG, Lober RM, Arrigo RT, et al. Trends in the diagnosis and treatment of pediatric primary spinal cord tumors. J Neurosurg Pediatr. 2012;10(6):555–559. [DOI] [PubMed] [Google Scholar]
- 9. Kutluk T, Varan A, Kafalı C, et al. Pediatric intramedullary spinal cord tumors: a single center experience. Eur J Paediatr Neurol. 2015;19(1):41–47. [DOI] [PubMed] [Google Scholar]
- 10. Choi GH, Oh JK, Kim TY, et al. The clinical features and surgical outcomes of pediatric patients with primary spinal cord tumor. Childs Nerv Syst. 2012;28(6):897–904. [DOI] [PubMed] [Google Scholar]
- 11. Ahmed R, Menezes AH, Awe OO, Torner JC. Long-term disease and neurological outcomes in patients with pediatric intramedullary spinal cord tumors. J Neurosurg Pediatr. 2014;13(6):600–612. [DOI] [PubMed] [Google Scholar]
- 12. Fakhreddine MH, Mahajan A, Penas-Prado M, et al. Treatment, prognostic factors, and outcomes in spinal cord astrocytomas. Neuro Oncol. 2013;15(4):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luksik AS, Garzon-Muvdi T, Yang W, Huang J, Jallo GI. Pediatric spinal cord astrocytomas: a retrospective study of 348 patients from the SEER database. J Neurosurg Pediatr. 2017;19(6):711–719. [DOI] [PubMed] [Google Scholar]
- 14. Milano MT, Johnson MD, Sul J, et al. Primary spinal cord glioma: a Surveillance, Epidemiology, and End Results database study. J Neurooncol. 2010;98(1):83–92. [DOI] [PubMed] [Google Scholar]
- 15. Samuel N, Tetreault L, Santaguida C, et al. Clinical and pathological outcomes after resection of intramedullary spinal cord tumors: a single-institution case series. Neurosurg Focus. 2016;41(2):E8. [DOI] [PubMed] [Google Scholar]
- 16. Tallen G, Resch A, Calaminus G, et al. ; German Paediatric Brain Tumour Consortium (HIT-Network) . Strategies to improve the quality of survival for childhood brain tumour survivors. Eur J Paediatr Neurol. 2015;19(6):619–639. [DOI] [PubMed] [Google Scholar]
- 17. McAbee JH, Modica J, Thompson CJ, et al. Cervicomedullary tumors in children. J Neurosurg Pediatr. 2015;16(4):357–366. [DOI] [PubMed] [Google Scholar]
- 18. Bouffet E, Pierre-Kahn A, Marchal JC, et al. Prognostic factors in pediatric spinal cord astrocytoma. Cancer. 1998;83(11):2391–2399. [DOI] [PubMed] [Google Scholar]
- 19. Guss ZD, Moningi S, Jallo GI, Cohen KJ, Wharam MD, Terezakis SA. Management of pediatric spinal cord astrocytomas: outcomes with adjuvant radiation. Int J Radiat Oncol Biol Phys. 2013;85(5):1307–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kandels D, Pietsch T, Bison B, et al. Loss of efficacy of subsequent nonsurgical therapy after primary treatment failure in pediatric low-grade glioma patients - report from the German SIOP-LGG 2004 cohort. Int J Cancer. 2020;147(12):3471–3489. [DOI] [PubMed] [Google Scholar]
- 21. Kahn J, Loeffler JS, Niemierko A, Chiocca EA, Batchelor T, Chakravarti A. Long-term outcomes of patients with spinal cord gliomas treated by modern conformal radiation techniques. Int J Radiat Oncol Biol Phys. 2011;81(1):232–238. [DOI] [PubMed] [Google Scholar]
- 22. von Hornstein S, Kortmann RD, Pietsch T, et al. Impact of chemotherapy on disseminated low-grade glioma in children and adolescents: report from the HIT-LGG 1996 trial. Pediatr Blood Cancer. 2011;56(7):1046–1054. [DOI] [PubMed] [Google Scholar]
- 23. Müller K, Gnekow A, Falkenstein F, et al. Radiotherapy in pediatric pilocytic astrocytomas. A subgroup analysis within the prospective multicenter study HIT-LGG 1996 by the German Society of Pediatric Oncology and Hematology (GPOH). Strahlenther Onkol. 2013;189(8):647–655. [DOI] [PubMed] [Google Scholar]
- 24. Mirow C, Pietsch T, Berkefeld S, et al. Children <1 year show an inferior outcome when treated according to the traditional LGG treatment strategy: a report from the German multicenter trial HIT-LGG 1996 for children with low grade glioma (LGG). Pediatr Blood Cancer. 2014;61(3):457–463. [DOI] [PubMed] [Google Scholar]
- 25. Gnekow AK, Kortmann RD, Pietsch T, Emser A. Low grade chiasmatic-hypothalamic glioma-carboplatin and vincristin chemotherapy effectively defers radiotherapy within a comprehensive treatment strategy – report from the multicenter treatment study for children and adolescents with a low grade glioma – HIT-LGG 1996 – of the Society of Pediatric Oncology and Hematology (GPOH). Klin Padiatr. 2004;216(6):331–342. [DOI] [PubMed] [Google Scholar]
- 26. Ardeshiri A, Chen B, Hütter BO, et al. Intramedullary spinal cord astrocytomas: the influence of localization and tumor extension on resectability and functional outcome. Acta Neurochir (Wien). 2013;155(7):1203–1207. [DOI] [PubMed] [Google Scholar]
- 27. Gnekow AK, Kandels D, Tilburg CV, et al. SIOP-E-BTG and GPOH guidelines for diagnosis and treatment of children and adolescents with low grade glioma. Klin Padiatr. 2019;231(3):107–135. [DOI] [PubMed] [Google Scholar]
- 28. Benesch M, Frappaz D, Massimino M. Spinal cord ependymomas in children and adolescents. Childs Nerv Syst. 2012;28(12):2017–2028. [DOI] [PubMed] [Google Scholar]
- 29. Gessi M, Gielen GH, Dreschmann V, Waha A, Pietsch T. High frequency of H3F3A (K27M) mutations characterizes pediatric and adult high-grade gliomas of the spinal cord. Acta Neuropathol. 2015;130(3):435–437. [DOI] [PubMed] [Google Scholar]
- 30. Falkenstein F, Gessi M, Kandels D, et al. Prognostic impact of distinct genetic entities in pediatric diffuse glioma WHO-grade II - report from the German/Swiss SIOP-LGG 2004 cohort. Int J Cancer. 2020;147(8):2159–2175. [DOI] [PubMed] [Google Scholar]
- 31. Carey SS, Sadighi Z, Wu S, et al. Evaluating pediatric spinal low-grade gliomas: a 30-year retrospective analysis. J Neurooncol. 2019;145(3):519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsang DS, Murphy ES, Ezell SE, Lucas JT Jr, Tinkle C, Merchant TE. Craniospinal irradiation for treatment of metastatic pediatric low-grade glioma. J Neurooncol. 2017;134(2):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dodgshun AJ, SantaCruz N, Hwang J, et al. Disseminated glioneuronal tumors occurring in childhood: treatment outcomes and BRAF alterations including V600E mutation. J Neurooncol. 2016;128(2):293–302. [DOI] [PubMed] [Google Scholar]
- 34. Shalet SM, Gibson B, Swindell R, Pearson D. Effect of spinal irradiation on growth. Arch Dis Child. 1987;62(5):461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spacca B, Giordano F, Donati P, Genitori L. Spinal tumors in children: long-term retrospective evaluation of a series of 134 cases treated in a single unit of pediatric neurosurgery. Spine J. 2015;15(9):1949–1955. [DOI] [PubMed] [Google Scholar]
- 36. Houten JK, Weiner HL. Pediatric intramedullary spinal cord tumors: special considerations. J Neurooncol. 2000;47(3):225–230. [DOI] [PubMed] [Google Scholar]
- 37. Mistry M, Zhukova N, Merico D, et al. BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. J Clin Oncol. 2015;33(9):1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 39. Shankar GM, Lelic N, Gill CM, et al. BRAF alteration status and the histone H3F3A gene K27M mutation segregate spinal cord astrocytoma histology. Acta Neuropathol. 2016;131(1):147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ryall S, Arnoldo A, Krishnatry R, et al. Multiplex detection of pediatric low-grade glioma signature fusion transcripts and duplications using the nanostring nCounter system. J Neuropathol Exp Neurol. 2017;76(7):562–570. [DOI] [PubMed] [Google Scholar]
- 41. Jones DTW, Kieran MW, Bouffet E, et al. Pediatric low-grade gliomas: next biologically driven steps. Neuro Oncol. 2018;20(2):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ryall S, Zapotocky M, Fukuoka K, et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell. 2020;37(4):569–583.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets of the current study are available from the corresponding author upon reasonable request and are in part provided in “Supplementary Dataset.”