Abstract
Background
Micronutrient deficiencies are common in patients with inflammatory bowel disease (IBD). To date, the literature has focused on vitamin D, vitamin B12, and iron deficiencies.
Methods
We report a case series of 20 patients with IBD and vitamin C deficiency treated at a single tertiary care center.
Results
Sixteen (80%) patients had symptoms of clinical scurvy, including arthralgia, dry brittle hair, pigmented rash, gingivitis, easy bruising, and/or brittle nails. Eighteen patients underwent a nutritional assessment, 10 (56%) patients reported complete avoidance of fruits and vegetables, and 3 (17%) reported reduced intake of fruits and vegetables.
Conclusions
Vitamin C deficiency should be considered in IBD patients, particularly those with reduced fruit/vegetable intake, as it can lead to significant signs and symptoms.
Keywords: vitamin C deficiency, inflammatory bowel disease, scurvy
INTRODUCTION
Patients commonly ask their gastroenterologists about the role of diet in inflammatory bowel disease (IBD) to alleviate symptoms. Ideally, patients are referred to nutrition experts, though a lack of access can sometimes lead to misinformation. It is well studied that many IBD patients have deficiencies in micronutrients and minerals including vitamins A, B12, D, K, iron, and zinc.1,2 The cause of these deficiencies may be due to malabsorption related to the underlying disease or specific dietary changes.
Vitamin C, or ascorbic acid, is a water-soluble micronutrient absorbed in the distal small bowel involved in the production of collagen, carnitine, and neurotransmitters.3 It is also essential in all phases of wound healing.4 Vitamin C is most commonly found in fruits and vegetables.5 Most patients diagnosed with vitamin C deficiency can be treated with an oral supplement (500 mg twice daily for 4 weeks) which can lead to improvement in constitutional symptoms within 24 hours, skin changes and gingival bleeding within a few weeks, and prevent long-term side effects including heart failure and severe hemorrhagic events.3
Clinical vitamin C deficiency, or scurvy, is no longer a disease of the 18th century with rising incidence in developed countries over the last several decades, affecting up to 7.1% of the general population in the United States based on serum vitamin C levels.6 It is unclear whether this is due to dietary changes, decreased health literacy surrounding nutrition or increased chronic illness leading to malabsorption and diet restriction. Scurvy is a clinical diagnosis confirmed with plasma vitamin C levels ≤0.2 mg/dL and clinical features including skin ecchymosis, petechiae, perifollicular hemorrhage, impaired wound healing, gingivitis, fatigue, dry brittle hair with hair loss, and/or arthralgia.7 Risk factors for scurvy include frequent dieting and chronic diseases such as Crohn disease (CD), celiac disease, anorexia nervosa, alcohol use disorder, psychiatric illness, and kidney failure.8 Due to the location of absorption, patients who have undergone surgical alteration of the small or large bowel may be at particular risk for developing scurvy, though nutritional guidelines do not recommend checking vitamin C in postoperative patients.9–11
Many foods that IBD patients may avoid are rich sources of vitamin C. However, little is known about vitamin C deficiency in IBD, with limited reports over the past few decades. The goal of this study was to report a case series of patients with IBD found to have vitamin C deficiency.
MATERIALS AND METHODS
We identified 20 patients with a diagnosis of IBD and vitamin C deficiency, defined as a concentration ≤0.2 mg/dL (reference range 0.2–2.0 mg/dL), who were treated at a single tertiary IBD center from February 2018 to October 2019. Patients with vitamin C deficiency were identified on routine clinical visits. Testing was prompted by symptoms, physical examination, and/or reports of restricted eating or weight loss. Demographics, disease characteristics, and laboratory data were collected from the electronic medical record. The nutritional assessments by a Registered Dietician nutritionist in the IBD center were reviewed. These included body mass index (BMI), diet recall, malnutrition assessment, multivitamin/supplement use, and recommendations.
ETHICAL CONSIDERATIONS
The study was approved by Mount Sinai Hospital Institutional Review Board. This case series does not meet NIH classification for a clinical trial and does not require registration.
RESULTS
The median age was 27.5 years and 55% of patients were women (Table 1). Fifteen (75%) patients had CD and 5 (25%) had ulcerative colitis (UC) with a median disease duration disease of 3.9 years (range 0.4–56.3 years). Nine (45%) patients had undergone IBD-related surgery (Table 1). Thirteen (87%) patients with CD had active clinical disease (defined as a Harvey–Bradshaw Index >5) and 4 (80%) patients had active UC (defined as a partial Mayo Score ≥2). Fourteen (70%) patients were on biologic therapy and 8 (40%) were using corticosteroids. On nutritional assessment, 9 (45%) patients had a normal BMI and 5 (25%) were underweight (BMI <18.5).12,13
Table 1.
Characteristics of Patient Population
| Characteristics (Total n = 20) | |
|---|---|
| Female gender, n (%) | 11 (55) |
| Age, median years (range) | 27.5 (19–71) |
| Race | |
| White, n (%) | 13 (65) |
| Black, n (%) | 2 (10) |
| Asian, n (%) | 3 (15) |
| Hispanic, n (%) | 2 (10) |
| Diagnosis | |
| CD, n (%) | 15 (75) |
| UC, n (%) | 5 (25) |
| Disease Phenotype by Montreal Classification | |
| Age at diagnosis | |
| A1 <16 years old | 5 (25) |
| A2 17–40 years old | 12 (60) |
| A3 >40 years old | 3 (15) |
| Location | |
| L1 ileal | 6 (30) |
| L2 colonic | 5 (25) |
| L3 ileocolonic | 9 (45) |
| L4 isolated upper disease | 0 (0) |
| Behavior | |
| B1 nonstricturing, nonpenetrating | 10 (50) |
| B2 stricturing | 7 (35) |
| B3 penetrating | 3 (15) |
| Disease duration, median years (range) | 3.9 (0.4–56.3) |
| Prior IBD-related surgery | |
| None, n (%) | 11 (55) |
| Any prior surgery, n (%) | 9 (45) |
| Disease activity for CD (Harvey–Bradshaw Index) (n = 15) | |
| <5 remission (not active) | 2 (12) |
| >5 active | 13 (87) |
| Disease activity for UC (partial Mayo Score) (n = 5) | |
| <2 remission | 1 (20) |
| ≥2 active | 4 (80) |
| Current IBD medications | |
| None, n (%) | 4 (20) |
| Anti-TNF (adalimumab, infliximab), n (%) | 11 (55) |
| Oral corticosteroids (budesonide, prednisone), n (%) | 8 (40) |
| 5-ASA, n (%) | 5 (25) |
| Ustekinumab, n (%) | 2 (10) |
| AZA, 6-MP, n (%) | 1 (5) |
| Vedolizumab, n (%) | 1 (5) |
| Past IBD medications | |
| None, n (%) | 5 (25) |
| Corticosteroids (budesonide, prednisone), n (%) | 16 (80) |
| Anti-TNF (adalimumab, infliximab, certolizumab pegol), n (%) | 9 (45) |
| 5-ASA (mesalamine, sulfasalazine), n (%) | 7 (35) |
| AZA, 6-MP, n (%) | 6 (30) |
| Vedolizumab, n (%) | 2 (10) |
| Ustekinumab, n (%) | 1 (5) |
5-ASA, 5-aminosalicylic acid; 6-MP, 6-mercaptopurine; Anti-TNF, Anti-Tumor Necrosis Factor; AZA, azathioprine.
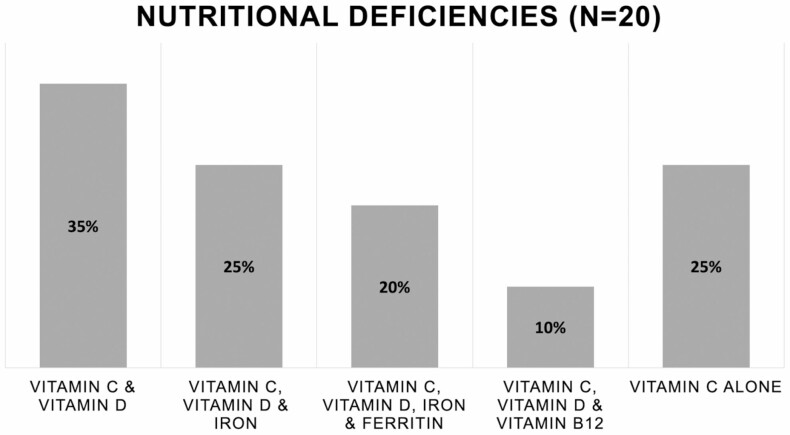
All patients were vitamin C deficient, with a mean concentration of 0.1 mg/dL (range 0.0–0.2 mg/dL). The median C-reactive protein was 5.7 mg/L (range 0.3–89.4 mg/L with the upper limit of normal being 5 mg/L) and 10 (50%) patients were anemic (hemoglobin <13 g/dL in men and <12 g/dL in women). The median ferritin was 23 ng/mL (range 4–433 ng/mL), and 7 (35%) patients had vitamin D deficiency (defined as vitamin D3 <20 ng/mL), and 5 (25%) had vitamin B12 deficiency (defined as B12 concentration <211 pg/mL). While 5 (25%) patients presented with vitamin C deficiency alone, the majority had other concomitant nutritional deficiencies (Table 2 and Fig. 1).
Table 2.
Nutritional Assessment
| Characteristics (Total n = 20) | |
|---|---|
| BMI (kg/m2) | |
| <18.5 underweight, n (%) | 5 (25) |
| 18.5–24.9 normal weight, n (%) | 9 (45) |
| 25–29.9 overweight, n (%) | 4 (20) |
| ≥30 obesity, n (%) | 2 (10) |
| Vitamin C plasma level (reference range 0.2–2.0 mg/dL) | |
| 0 mg/dL, n (%) | 8 (40) |
| 0.1 mg/dL, n (%) | 10 (50) |
| 0.2 mg/dL, n (%) | 2 (10) |
| Clinical signs and symptoms of scurvy | |
| Arthralgia, n (%) | 9 (45) |
| Dry brittle hair/hair loss, n (%) | 6 (30) |
| Pigmented rash, n (%) | 4 (20) |
| Dry skin, n (%) | 4 (20) |
| Gingivitis, n (%) | 3 (15) |
| Poor wound healing, n (%) | 2 (10) |
| Easy bruising, n (%) | 2 (10) |
| Fatigue, n (%) | 2 (10) |
| Brittle nails, n (%) | 1 (5) |
| No. clinical scurvy manifestations | |
| 0 signs/symptoms, n (%) | 4 (20) |
| 1 signs/symptoms, n (%) | 9 (45) |
| 2 signs/symptoms, n (%) | 1 (5) |
| 3 or more signs/symptoms, n (%) | 6 (30) |
| Fruit and vegetable avoidance (n = 18) | |
| Yes, n (%) | 10 (56) |
| No, n (%) | 5 (27) |
| Partial, n (%) | 3 (17) |
| Advised to avoid fruits and vegetables? (n = 9) | |
| Yes, n (%) | 2 (22) |
| No, n (%) | 7 (78) |
| Concomitant nutritional deficiencies | |
| Vitamin C and vitamin D, n (%) | 7 (35) |
| Vitamin C, vitamin D and iron, n (%) | 5 (25) |
| Vitamin C, vitamin D, iron and ferritin, n (%) | 4 (20) |
| Vitamin C, vitamin D and vitamin B12, n (%) | 2 (10) |
| Vitamin C alone, n (%) | 5 (25) |
Figure 1.
A case series (n = 20) of patients with IBD and vitamin C deficiency found to have concomitant nutritional deficiencies including vitamin D, vitamin B12, iron, and ferritin.
Signs and symptoms of scurvy were noted in 16 (80%) patients. These included 9 (45%) patients with arthralgia, 6 (30%) with dry brittle hair/hair loss, 4 (20%) with pigmented rash, 3 (15%) with gingivitis, 2 (10%) with poor wound healing, 2 (10%) with easy bruising, 2 (10%) with fatigue, and 1 (5%) with brittle nails.
During nutritionist evaluation, 10 (56%) patients reported complete avoidance of fruits and vegetables, and 3 (17%) reported reduced intake of fruits and vegetables. One (5%) patient reported taking a multivitamin. Of those who were avoiding these foods, 2 (22%) reported they were advised by a health care provider to avoid fruits and vegetables given their underlying IBD. Of note, some clinicians advise patients to temporarily limit fiber intake to alleviate symptoms during a flare, which may include fruits and vegetables, though patients may inadvertently remain on this diet indefinitely.
On follow-up, 10 (50%) patients did not have repeat serum vitamin C, and 4 (20%) patients did not follow-up with a nutritionist. Of those that had repeat serum vitamin C testing, 6 (60%) had normalized following supplementation. Overall, 13 (65%) patients reported improved symptoms. It is important to note not all patients had appropriate follow-up within 4–6 weeks of starting vitamin C treatment. Some patients had multiple nutrition deficiencies, and were taking iron, vitamin D, vitamin B12, multivitamin, in addition to the vitamin C supplements. At this time, we cannot discern that the arthralgia, hair loss, and fatigue were solely attributable to vitamin C deficiency in patients with multiple deficiencies, however 25% had vitamin C deficiency alone with possible scurvy symptoms.
DISCUSSION
To the best of our knowledge, this is the largest case series to date of IBD patients with vitamin C deficiency. Importantly the signs and symptoms of scurvy occurred in 16 (80%) cases and the most common clinical features were arthralgia (45%), dry brittle hair/hair loss (30%), pigmented rash (20%), and gingivitis (15%). This case series helps emphasize the clinical implications of scurvy on patients with IBD and its potential to impact quality of life. The majority of patients (56% complete and 17% partial) in our case series reported avoidance of fruits and vegetables. We observed 2 patients whom reported being advised by a health care provider to avoid these foods, and we speculate there are some possible explanations including patients following advice from clinicians to temporarily limit fiber intake during a flare, patients self-restricting, patients receiving inappropriate dietary advice, patients unable to eat vitamin C rich foods due to disease activity, or a connection related to underlying IBD and/or inflammation. In general, up to 68% of IBD patients limit their diet in order to try to control their disease and manage symptoms.14
Prior literature on vitamin C in IBD is limited. Several studies from the 1970–80s highlight an early association between vitamin C deficiency in regional enteritis with fistulas, CD and 1 study even followed patients over a 6-month period to see if they could assess for risk factors and sufficient supplementation through diet.15–20 More recently, a few case reports in IBD patients indicate clinical signs of scurvy with more severe consequences including hemorrhagic and multiorgan dysfunction.21–26
The reasons for vitamin C deficiency in IBD patients are likely multifactorial, but potential risk factors include avoidance of fruits and vegetables, surgical resection, and disease activity. Only 1 (5%) patient reported taking a multivitamin at the time of initial vitamin C level. Due to the retrospective nature of the case series, it is difficult to know for certain who was taking a multivitamin since they are available over the counter and therefore subject to ascertainment bias. Many patients with IBD have tried a specific diet, most commonly the specific carbohydrate diet (SCD), a low FODMAP diet, CD-TREAT, or IBD-AID. In all of these diets, foods high in vitamin C are generally allowed, however in some, such as the IBD-AID diet, altered textures, such as puree, are suggested. Of all these diets, the SCD is likely the most restrictive although it specifically recommends vitamin C supplementation.27 One study evaluating nutritional adequacy of the SCD in children with IBD found 8 (100%) patients had adequate vitamin C levels with multivitamin.27 Patients are advised to adhere to these diets under the guidance of a nutritionist, but when they undertake these specific diets alone, they have the potential to restrict certain food groups and cause more harm than good.
While it is important to replete patients with vitamin C deficiency, it is key to maintain an adequate sustained level of vitamin C in the diet. We recommend that all of our IBD patients consume adequate fruits and vegetables, making note that heat can destroy the ascorbic acid content of many foods.5 Steaming and microwaving retain higher concentrations of ascorbic acid than boiling because of the reduced contact with water at lower temperatures. It is recommended to cook vitamin C rich fruits/vegetables for shorter time periods, at lower temperatures using minimal cooking water to retain higher concentrations.28 In patients with small bowel disease at risk for stricture, we recommend soft, fleshy fruits and vegetables without seeds or peels. If there is concern about potential obstructive symptoms, we recommend cooking vegetables until they are fork tender and/or making smoothies. If a patient has iron deficiency anemia, combining foods high in vitamin C with a source of iron will increase iron absorption.5 Importantly in 2020, The International Organization for the Study of Inflammatory Bowel Disease (IOIBD) published a resource on dietary guidance for patients with IBD and these recommendations included moderate to high consumption of fruits and vegetables in CD patients without stricturing disease, and no restriction of fruits and vegetables for patients with UC.29 It is important to ask all patients with IBD about dietary intake, particularly fruits and vegetables, not only those who report weight loss or have an abnormal BMI.
Our case series had a number of limitations. First, all cases were collected at a single tertiary care center, and these results may not be generalizable to a broader IBD population. Second, it is difficult to differentiate some symptoms of scurvy from those of underlying IBD or IBD medication side effects (eg, arthralgias could be in the setting of IBD-associated arthropathy, myopathy secondary to steroids, and hair loss could be due to poor protein intake, or medications such as mercaptopurine or 5-aminosalicylic acid). Of note, 13 (65%) patients who received supplementation reported improvement in symptoms. This case series is not able to estimate an incidence and prevalence of vitamin C deficiency in IBD patients and this is an area that warrants further study. Finally, it is important to note the plasma vitamin C concentration can be read as normal (>0.2 mg/dL) in the setting of recent vitamin C intake, even if tissue levels are still deficient.5 Therefore, patients with underlying vitamin C deficiency can be missed. A better measurement of body stores of vitamin C is ascorbic acid in leukocytes, but this is not widely available for clinical use.5 While vitamin C has antioxidant qualities, in some cases it can be a pro-oxidant which may affect the quality of the serum vitamin C levels in patients with active inflammation.30 In this case series, 17 patients reported active disease activity at time of vitamin C deficiency (Table 1).
CONCLUSIONS
In conclusion, we report a case series of IBD patients with vitamin C deficiency. Most of these cases occurred in the setting of decreased fruit and vegetable intake. Health care practitioners should be mindful of this micronutrient deficiency when evaluating patients with IBD. Further prospective studies are needed to define the prevalence of vitamin C deficiency in patients with IBD and better understand the clinical implications and risk factors. This case series highlights that IBD patients can be deficient in the “forgotten micronutrient” vitamin C. These observations highlight the need for appropriate dietary counseling in patients with IBD and referral to a nutritionist should be encouraged, particularly for those with restricted diet patterns or weight loss. Further research is needed to understand the true prevalence and impact of vitamin C deficiency in IBD.
Funding: R.C.U. is supported by a National Institutes of Health K23 Career Development Award K23KD111995-01A1.
Conflict of Interest: K.A.D., L.M., S.G., and J.N. have no conflicts of interest. R.C.U. has served as an advisory board member or consultant for Eli Lilly, Janssen, Pfizer, and Takeda; research support from AbbVie, Boehringer Ingelheim, and Pfizer. J.F.C. reports receiving research grants from AbbVie, Janssen Pharmaceuticals, and Takeda; receiving payment for lectures from AbbVie, Amgen, Allergan, Inc., Ferring Pharmaceuticals, Shire, and Takeda; receiving consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene Corporation, Celltrion, Eli Lilly, Enterome, Ferring Pharmaceuticals, Genentech, Janssen Pharmaceuticals, Landos, Ipsen, Medimmune, Merck, Novartis, Pfizer, Shire, Takeda, Tigenix, and Viela bio; and hold stock options in Intestinal Biotech Development and Genfit.
Author Contribution: K.A.D.: study design, acquisition of data, data analysis, writing first draft, and final approval. R.U., J.F.C., and L.M.: study design, acquisition of data, data analysis, and revising the article and final approval. J.N.: study design, acquisition of data, and revising the article and final approval. S.G.: study design and revising the article and final approval.
Ethical Approval: The Institutional Review Board at The Mount Sinai Hospital.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Ghishan FK, Kiela PR. Vitamins and minerals in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weisshof R, Chermesh I. Micronutrient deficiencies in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2015;18:576–581. [DOI] [PubMed] [Google Scholar]
- 3. Magiorkinis E, Beloukas A, Diamantis A. Scurvy: past, present and future. Eur J Intern Med. 2011;22:147–152. [DOI] [PubMed] [Google Scholar]
- 4. Angela M., Quain NMK. Nutrition in wound care management: a comprehensive overview. Wounds. 2015;27:327–335. [PubMed] [Google Scholar]
- 5. Padayatty SJ, Levine M. Vitamin C: the known and the unknown and Goldilocks. Oral Dis. 2016;22:463–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252–1263. [DOI] [PubMed] [Google Scholar]
- 7. Hodges RE, Hood J, Canham JE, et al. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24:432–443. [DOI] [PubMed] [Google Scholar]
- 8. Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician. 2008;54:1403–1406. [PMC free article] [PubMed] [Google Scholar]
- 9. Forbes A, Escher J, Hébuterne X, et al. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36:321–347. [DOI] [PubMed] [Google Scholar]
- 10. Hansen EP, Metzsche C, Henningsen E, et al. Severe scurvy after gastric bypass surgery and a poor postoperative diet. J Clin Med Res. 2012;4:135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J. 2011;437:357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US). Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6:51S. [PubMed] [Google Scholar]
- 13. WHO Consultation on Obesity. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i. [PubMed] [Google Scholar]
- 14. Limdi JK, Aggarwal D, McLaughlin JT. Dietary practices and beliefs in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:164–170. [DOI] [PubMed] [Google Scholar]
- 15. Gerson CD. Ascorbic acid deficiency in clinical disease including regional enteritis. Ann N Y Acad Sci. 1975;258:483–490. [DOI] [PubMed] [Google Scholar]
- 16. Gerson CD, Fabry EM. Ascorbic acid deficiency and fistula formation in regional enteritis. Gastroenterology. 1974;67:428–433. [PubMed] [Google Scholar]
- 17. Hughes RG, Williams N. Leucocyte ascorbic acid in Crohn’s disease. Digestion. 1978;17:272–274. [DOI] [PubMed] [Google Scholar]
- 18. Imes S, Dinwoodie A, Walker K, et al. Vitamin C status in 137 outpatients with Crohn’s disease. Effect of diet counseling. J Clin Gastroenterol. 1986;8:443–446. [DOI] [PubMed] [Google Scholar]
- 19. Linaker BD. Scurvy and vitamin C deficiency in Crohn’s disease. Postgrad Med J. 1979;55:26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pettit SH, Irving MH. Does local intestinal ascorbate deficiency predispose to fistula formation in Crohn’s disease. Dis Colon Rectum. 1987;30:552–557. [DOI] [PubMed] [Google Scholar]
- 21. Rotar Ž, Ferkolj I, Tomšič M, et al. Scurvy—surprisingly not yet extinct. Vasc Med. 2017;22:351–352. [DOI] [PubMed] [Google Scholar]
- 22. Broner J Jr, Reymann V, Guilpain P. Purpuric lesions in a 45-year old man. Eur J Intern Med. 2017;44:e7–e8. [DOI] [PubMed] [Google Scholar]
- 23. Berger MM, Pantet O, Schneider A, et al. Micronutrient deficiencies in medical and surgical inpatients. J Clin Med. 2019;8:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Branquinho DF, Pinto-Gouveia M, Mendes S, et al. From past sailors’ eras to the present day: scurvy as a surprising manifestation of an uncommon gastrointestinal disease. BMJ Case Rep. 2015:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levavasseur M, Becquart C, Pape E, et al. Severe scurvy: an underestimated disease. Eur J Clin Nutr. 2015;69:1076–1077. [DOI] [PubMed] [Google Scholar]
- 26. Olmedo JM, Yiannias JA, Windgassen EB, et al. Scurvy: a disease almost forgotten. Int J Dermatol. 2006;45:909–913. [DOI] [PubMed] [Google Scholar]
- 27. Braly K, Williamson N, Shaffer ML, et al. Nutritional adequacy of the specific carbohydrate diet in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2017;65:533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee S, Choi Y, Jeong HS, et al. Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Sci Biotechnol. 2018;27:333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levine A, Rhodes JM, Lindsay JO, et al. Dietary guidance from the International Organization for the Study of Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2020;18:1381–1392. [DOI] [PubMed] [Google Scholar]
- 30. Kazmierczak-Baranska J, Boguszewska K, Adamus-Grabicka A, et al. Two faces of vitamin C—antioxidative and pro-oxidative agent. Nutrients. 2020;12:1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.