Abstract
Background
Fecal incontinence (FI) is frequently reported in inflammatory bowel disease (IBD).
Methods
We retrospectively reviewed data from the Study of a Prospective Adult Research Cohort with IBD registry.
Results
Three hundred forty-seven patients had Crohn disease and 145 had ulcerative colitis. 14.2% of patients reported FI. FI was associated with active disease. FI was not associated with disease location, phenotype, or perianal involvement. Greater than 50 years of age or 15 years of disease increased the odds of FI and remission decreased the odds of FI.
Conclusions
Further research into the mechanism of FI in IBD is needed.
Keywords: quality of life, diarrhea, Crohn disease, ulcerative colitis, incontinence
INTRODUCTION
Inflammatory bowel disease (IBD) is associated with fecal urgency and diarrhea. Thinking about fecal urgency as a continuum from none to severe, fecal incontinence (FI) is the most extreme category. FI is defined as the involuntary loss of liquid or solid stool.1 It accounts for passive incontinence, which is involuntary discharge without awareness; urge incontinence, which is discharge despite active attempts to retain contents; and fecal leakage of stool with otherwise normal continence and evacuation.2 It is a sensitive issue that affects many patients with IBD and results in poor quality of life.3 Those affected have significant anxiety ensuring they will always have accessibility to restrooms. Severe symptoms can result in days off from work or cancelation of social events.
The current literature reports that patients with IBD are at an increased risk of FI compared to the general population, especially among patients with a history of perianal fistula or an ileal pouch anal anastomosis (IPAA).3 In the general population, the rate of FI ranges between 2.2% and 15%.3 Patients with a history of IPAA have an incidence of FI of approximately 30%.4 Patients without an IPAA have been surveyed about their symptoms to determine the prevalence of FI. The prevalence rates vary greatly, ranging between 20% and 73%.1,3 Prior studies have noted patients experiencing bothersome symptoms were more likely to respond to voluntary surveys skewing the true prevalence and creating large variations in results based on study design. Additionally, patients may under report symptoms due to the associated stigma, further making an accurate assessment of disease prevalence a challenge.1
There is currently a lack of information regarding FI in patients with IBD. The purpose of this study was to determine the prevalence and risk factors for FI in IBD by using both patient-reported outcome data and objective measures collected prospectively from patients enrolled in a research registry at one tertiary referral center.
MATERIALS AND METHODS
Initiated in 2016, the Study of a Prospective Adult Research Cohort with IBD (SPARC) registry has been collecting patient-reported outcomes data in real time as well as laboratory, endoscopic, and pathologic samples from 17 tertiary referral centers.
We retrospectively reviewed patient-reported outcomes data from the one SPARC site. Inclusion criteria were adult patients with IBD who answered these 2 questions: “During the last month, have you had leakage of stool while sleeping and/or while awake?” Data for this study were obtained from the IBD Plexus platform of the Crohn’s & Colitis Foundation on October 7, 2019. Patients were excluded if they currently had an ostomy present, had a previous IPAA, or had significant missing data that made it impossible to characterize the patient’s disease. Crohn disease (CD) location and phenotype were defined by the revised Montreal classification.5 Ulcerative colitis (UC) disease location included rectal, left-sided, extensive, and pancolonic. Disease activity was assessed using the short Crohn’s disease activity index (sCDAI) and 9-point ulcerative colitis disease activity index (UCDAI) scores.6,7
The analysis consisted of descriptive statistics such as means, medians, and frequency distributions, which were used to characterize the cohort. For comparative analysis, we performed chi-square and Fisher exact analyses, where P < 0.05 was significant. Each potential confounding variable was individually examined during bivariate analysis and included if adjustment to crude odds ratio increased by 10% or more by logistic regression. Statistics were performed using SAS statistical software, v. 9.4, Cary, NC.
ETHICAL CONSIDERATIONS
The SPARC IBD registry is approved by the Institutional Review Boards of The University of Pennsylvania, and all participants provided written informed consent to participate.
RESULTS
Between June of 2016 and October of 2019, 524 cases were enrolled in the SPARC IBD registry at one SPARC site. Five hundred patients were included in the analysis; 7 patients were excluded due to missing data, 7 had undergone an IPAA, and 10 had a current ostomy. The clinical features of the cohort can be found in Table 1. Three hundred forty-seven (69.4%) patients had CD, 145 (29%) had UC, and 8 (1.6%) had indeterminate colitis (IC). Two hundred eighty-one were (56%) female, 394 (78.8%) were Caucasians, 78 (15.6%) were black, mean age was 40 [18–85] years-old, and the mean disease duration was 14 [0–55] years. Four hundred thirty-five patients (87.7%) were nonsmokers. In total, 331 (66.2%) patients had exposure to biologic therapy: 233 (47%) to infliximab, 160 (33%) to adalimumab, and 73 (15%) to vedolizumab. Other therapies included exposure to steroids in 342 (69.5%), 5-aminosalicylates and sulfasalazine in 289 (58.7%), methotrexate in 63 (12.8%), and thiopurines in 218 (44.3%) of patients.
Table 1.
Cohort Characteristics by IBD Diagnosis
| Variable | Overall (N = 500) | CD (N = 347) | UC (N = 145) | Indeterminant Colitis (N = 8) | P |
|---|---|---|---|---|---|
| Age, Avg (SD) | 40.2 (13.1) | 38.9 (12.1) | 43.2 (15.0) | 44.8 (14.4) | 0.0006 |
| Sex, N (%) | 0.27 | ||||
| Male | 219 (43.8) | 145 (41.8) | 69 (47.6) | 5 (62.5) | |
| Female | 281 (56.2) | 202 (58.2) | 76 (52.4) | 3 (37.5) | |
| Race | 0.26 | ||||
| White | 394 (78.8) | 266 (76.7) | 122 (84.1) | 6 (75.0) | |
| African American | 78 (15.6) | 64 (18.4) | 12 (8.3) | 2 (25.0) | |
| Asian | 6 (1.2) | 4 (1.2) | 2 (1.4) | 0 | |
| Other | 18 (3.6) | 11 (3.2) | 7 (4.8) | 0 | |
| Unknown | 4 (0.8) | 2 (0.6) | 2 (1.4) | 0 | |
| Smoking, N (%) | 0.19 | ||||
| Yes | 61 (12.3) | 48 (13.9) | 13 (9.1) | 0 | |
| No | 435 (87.7) | 297 (86.1) | 130 (90.9) | 8 (100) | |
| Duration of IBD, avg (SD) | 14.0 (9.8) | 14.9 (9.9) | 11.8 (8.9) | 13.5 (12.2) | 0.003 |
| Disease location | |||||
| Isolated ileal | 103 (29.7) | ||||
| Ileocolonic | 178 (51.3) | ||||
| Isolated colonic | 54 (15.6) | ||||
| Perianal disease | 69 (19.9) | ||||
| Upper tract | 2 (0.6) | ||||
| Rectum | 7 (4.8) | ||||
| Left-sided | 40 (27.4) | ||||
| Extensive | 16 (11.1) | ||||
| Pancolitis | 74 (51.4) | ||||
| Unknown | 8 (5.5) | ||||
| Disease phenotype | |||||
| Inflammatory | 131 (37.8) | ||||
| Penetrating | 102 (29.4) | ||||
| Stricturing | 110 (31.7) | ||||
| Unknown | 4 (1.2) | ||||
| Incontinence | |||||
| Daytime | 60 (12.0) | 43 (12.4) | 16 (11.0) | 1 (12.5) | 0.9 |
| Evening | 33 (6.6) | 25 (7.2) | 8 (5.6) | 0 | 0.59 |
| Any | 71 (14.2) | 50 (14.4) | 20 (13.8) | 1 (12.5) | 0.97 |
| Steroid exposure | |||||
| Any/ever | 342 (69.5) | 157 (45.2) | 95 (65.5) | ||
| Salicylates and sulfasalazine | |||||
| Any/ever | 289 (58.7) | 183 (52.7) | 106 (73.1) | ||
| Thiopurines | |||||
| Any/ever | 218 (44.3) | 159 (45.8) | 59 (40.7) | ||
| Methotrexate | |||||
| Any/ever | 63 (12.8) | 49 (14.1) | 14 (9.7) | 0.0004 | |
| Biologic exposure | |||||
| Any/ever | 331 (66.2) | 249 (71.8) | 78 (53.8) | 4 (50.0) |
UC was limited to the rectum in 7 (4.8%), left-sided in 40 (27.4%), extensive in 16 (11.1%), and pancolonic in 74 (51.4%) patients. The CD phenotype was inflammatory in 131 (37.8%), penetrating in 102 (29.4%), and stricturing in 110 (31.7%), and unknown in 4 (1.2%). CD disease location was ileal in 103 (29.7%), colonic in 54 (15.6%), ileocolonic in 178 (51.3%), and isolated upper tract in 2 (0.6%). Sixty-nine (19.9%) patients had perianal involvement.
Using sCDAI scores, 217 (73%) CD patients were in remission (CDAI <150) and 80 (27%) CD patients had active disease. Using the 9-point UCDAI, 101 (81.4%) UC patients were in remission (UCDAI <2) and 23 (18.6%) had active disease. Seventy-one (14.2%) patients reported FI in the day or night: 20 (14%) with UC, 50 (14%) with CD, and 1 (13%) with IC. Daytime leakage was reported in 60 (12%) patients and nocturnal leakage was reported in 33 (6.6%) patients. There were only 3 patients who reported severe urgency with incontinence who did not also report either passive leakage in the day or night.
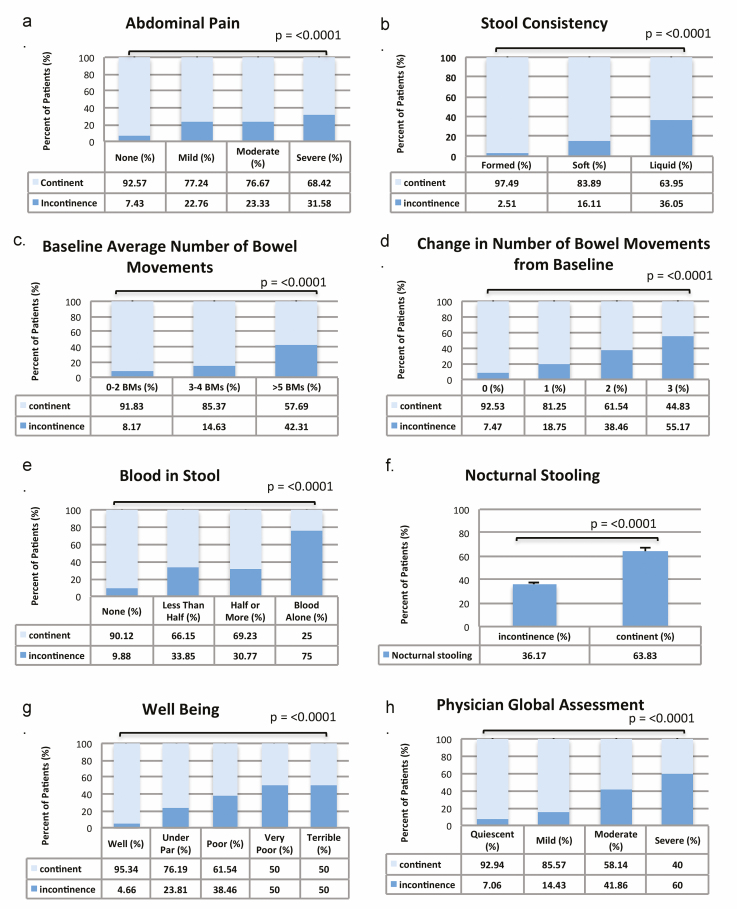
On univariate analysis, patients with FI had significantly more abdominal pain, liquid or softer stools, a higher number of baseline bowel movements, a greater change in the number of bowel movements from baseline, increased bloody stools, increased nocturnal stooling, lower general well-being, and a lower physician global assessment score (each P < 0.0001) (Fig. 1, composite A–H). FI was significantly associated with worsening disease activity by sCDAI scores (P = 0.0001) and UCDAI scores (P = 0.001). Of note, 19 (8%) CD and 2 (2%) UC patients in remission also reported FI. On univariate analysis, FI was also more common in patients who had undergone a colonic resection (P = 0.01), in adults age ≥50 years-old (P = 0.0005) and in patients with disease duration ≥15 (P = 0.037) but not ≥10 years duration (P = 0.47).
Figure 1.
FI by patient-reported outcome: abdominal pain (A), stool consistency (B), baseline number of bowel movements (C), change in number of bowel movements from baseline (D), blood in stool (E), nocturnal stooling (F), well-being (G), and physician global assessment (H).
FI was not associated with CD disease location (P = 0.23–0.48), phenotype (P = 0.10), nor perianal involvement (P = 0.72). FI was also not associated with UC disease location (P = 0.34). Previous exposure to biologic therapy (P = 0.17), a history of smoking (P = 0.07), and female gender (P = 0.62) were not associated with FI; however, there was a trend toward greater prevalence of FI in smokers.
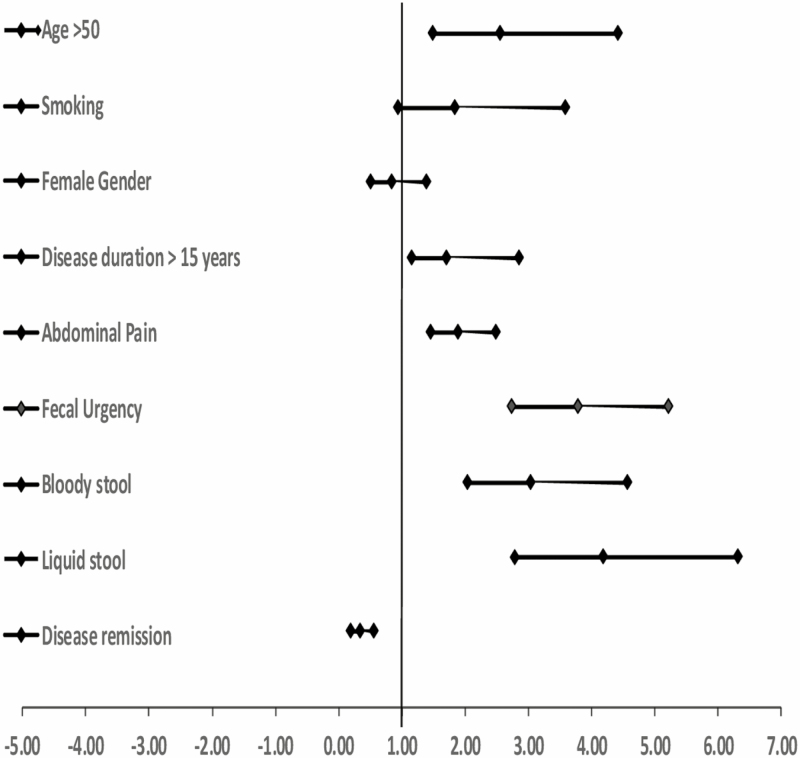
When evaluating these factors in a bivariate analysis (Fig. 2), we found that age greater than 50 years, disease duration 15 years and greater, and worse clinical symptoms significantly increased the odds of FI. Being in clinical remission (by sCDAI or UCDAI scores) significantly decreased the odds of FI. The significant variables were included in a logistic regression model (LR 88.95, P = 0.0001); patients with CD were 1.4 times more likely to have FI than patients with UC when accounting for all significant factors. Patients 50 years and older were 1.7 times more likely to have FI than those younger than 50 when accounting for all significant factors. Patients with fecal urgency were 2.7 times more likely to have FI than those without urgency.
Figure 2.
Forest plot depicting risk factors associated with FI in IBD.
On multivariable analysis in patients with CD only, FI was significantly more likely in patients greater than age 50, those with fecal urgency, liquid stools, higher sCDAI score, and worse physician global assessment (P < 0.05). FI was significantly less likely if the patient was in remission by sCDAI score (P < 0.05). Disease duration was not significant for CD as seen in Table 2. For patients with UC only, patients were significantly more likely to have FI if they had fecal urgency, liquid stools, a higher UCDAI score, and a worse physician global assessment (P < 0.05). They were significantly less likely to have FI if they were in remission by UCDAI score (P < 0.05) as seen in Table 3. Age and disease duration were not significant on multivariable analysis for UC.
Table 2.
Odds Ratios for FI in Patients With CD
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age | |||
| <50 years | 1 | — | |
| >50 years | 2.74 | 1.41, 5.32 | 0.0029 |
| Gender | |||
| M | 1 | — | |
| F | 0.9 | 0.49, 1.65 | 0.73 |
| Smoking | |||
| No | 1 | — | |
| Yes | 2.03 | 0.95, 4.31 | 0.066 |
| Disease duration | |||
| <15 years | 1 | — | |
| >15 years | 1.55 | 0.95, 2.83 | 0.156 |
| Fecal urgency | |||
| None | 1 | — | |
| Increased severity | 3.82 | 2.58, 5.65 | <0.0001 |
| Physician global assessment | |||
| Quiescent | 1 | — | |
| Increasing severity | 2.44 | 1.69, 3.51 | <0.0001 |
| Stool description | |||
| Formed | 1 | — | |
| Liquid consistency | 3.475 | 2.16, 5.58 | <0.0001 |
| Biologic | |||
| None | 1 | — | |
| Any | 1.29 | 0.64, 2.59 | 0.472 |
| Disease location | |||
| Ileocolonic | 0.77 | 0.36, 1.68 | 0.524 |
| Colon only | 1.49 | 0.68, 3.27 | 0.313 |
| Any anal involvement | 1.14 | 0.55, 2.36 | 0.728 |
| Remission | |||
| N | 1 | — | |
| Y | 0.44 | 0.24, 0.83 | 0.012 |
| Disease severity (sCDAI) | |||
| None | 1 | — | |
| Increasing severity (sCDAI >150) | 2.71 | 1.91, 3.84 | <0.0001 |
CI, confidence interval.
Table 3.
Odds Ratios for FI in Patients With UC
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age | |||
| <50 years | 1 | — | |
| >50 years | 2.21 | 0.85, 5.73 | 0.105 |
| Gender | |||
| M | 1 | — | |
| F | 0.71 | 0.27, 1.83 | 0.476 |
| Smoking | |||
| No | 1 | — | |
| Yes | 1.21 | 0.25, 5.93 | 0.815 |
| Disease duration | |||
| <15 years | 1 | — | |
| >15 years | 2.21 | 0.82, 5.92 | 0.116 |
| Fecal urgency | |||
| None | 1 | — | |
| Increased severity | 3.68 | 2.09, 6.49 | <0.0001 |
| Physician global assessment | |||
| Quiescent | 1 | — | |
| Increasing severity | 4.29 | 2.43, 7.59 | <0.0001 |
| Stool description | |||
| Formed | 1 | — | |
| Liquid consistency | 6.71 | 2.97, 15.18 | <0.0001 |
| Biologic | |||
| None | 1 | — | |
| Any | 2.22 | 0.80, 6.26 | 0.124 |
| Disease location | |||
| Proctitis | 1 | — | |
| Left-sided | 0.316 | 0.03, 4.05 | 0.376 |
| Extensive | 1.39 | 0.12, 16.23 | 0.796 |
| Pancolitis | 1.16 | 0.13, 10.54 | 0.894 |
| Remission | |||
| N | 1 | — | |
| Y | 0.13 | 0.04, 0.42 | 0.0007 |
| Disease severity (9UCDAI) | |||
| None | 1 | — | |
| Increasing severity (UCDAI ≥2) | 5.03 | 2.71, 9.34 | <0.0001 |
CI, confidence interval.
DISCUSSION
The prevalence of FI in patients with IBD was 14%, equally affecting patients with UC, CD, and IC. FI was associated with increased age, disease duration, more severe symptoms, and disease activity scores. FI was not associated with female gender, disease location or phenotype, or perianal involvement as previously reported in the literature. Additionally, up to 8% of patients with CD experienced FI despite being in clinical remission by sCDAI score.
FI was more common in adults older than 50 years-old which is consistent with findings reported both in the general population and the IBD population.3,8–10 With increased age, patients often have an increased number of medical and psychiatric comorbidities, history of polypharmacy, and decreased mobility, which can contribute to their inability to make it to the bathroom in time.8,10 Although we looked at exposure to various IBD medications, we did not look at psychiatric or medical comorbidities, mobility, or non-IBD medications which may contribute to the development of FI at older ages. FI was also associated with disease duration greater than 15 years. With each additional year of disease, patients are at increased risk for complications such as defecatory dysfunction and structural damage, the latter sometimes requiring surgery. Defecatory dysfunction may be a result of years of maladaptive defecatory behaviors. Defecatory dysfunction in IBD has been associated with decreased rectal sensation, poor rectal distensibility, reduced anal resting pressure, and loss of the anorectal inhibitory reflex.11–13 Patients may also develop increased anal sphincter fatigueability, which is found to occur independent of external anal sphincter defects.14 Persistent defecatory dysfunction in the absence of active inflammation may explain why up to 8% of patients with CD in symptomatic clinical remission had FI. Up to 77% of patients with FI and IBD respond favorably to gut-directed behavioral therapy including biofeedback therapy further supporting a role for defecatory dysfunction in FI in IBD.15 With increased disease duration, patients with UC and CD are also more likely to require surgery. Approximately 15.6% of patients with UC and 46.6% of patients with CD will require surgery 10 years after diagnosis.16 Various surgical procedures have been linked to both storage and evacuation dysfunction. When looking at surgical history as a possible reason for why patients with long-standing disease develop FI, we noted that patients with colonic resections had significantly more FI than those without colonic resections (P = 0.01) though our numbers were too small to make a reliable conclusion. It is possible that surgery predisposes patients to small intestinal bacterial overgrowth, malabsorption, or dysmotility placing them at higher risk for FI.
Disease activity has been associated with FI in the IBD literature as well as in our cohort.3,17 Patients with FI reported more abdominal pain, more liquid stools, a higher number of baseline stools, a greater increase in stools from baseline, more bloody stools, nocturnal awakening to defecate, and poor well-being. Additionally when looking at more symptomatic patients such as those with loose stools, 36% of patients reported FI. Similarly, 31% of patients with severe abdominal pain, and 75% of patients who passed blood alone in their bowel movements reported FI. Ongoing inflammation not only causes frequent liquid stools in many cases, but it is also thought to cause neuropathic changes in the enteric nervous system.18 This can lead to disruptions in both the motor and sensory function of the bowel.13 Patients with both UC and CD were significantly less likely to have FI if they were in symptomatic clinical remission, with an odds ratio of 0.33.
A few discrepancies exist in our data compared to that previously reported in literature. Prior studies demonstrated an association between FI and gender, even in non-IBD populations.10 We did not find that women with IBD were more likely to have FI than men. Previous reports have theorized that women with diarrhea were more likely to have FI than men due to a longer and more complete anal sphincter in men, protecting them against FI related to diarrhea.3 A prior history of vaginal delivery has been inconsistently shown to be associated with FI. Childbirth becomes a risk factor for FI when there is injury to the anal sphincter or performance of a midline episiotomy for vaginal delivery.19 We also did not find that disease location had an impact on the prevalence of FI. Previous studies found that FI is more common in patients with perianal disease, however our data did not support this finding.20,21 Perianal disease consists of fistulas, strictures, and abscess that can cause damage to the anal sphincter complex; additionally, surgical intervention to treat complications of perianal disease can result in injury of the anal sphincter.21 Unfortunately, we were unable to identify patients with perianal disease who had a perianal procedure or surgery from this dataset so we could not explore this hypothesis. Additionally, we were unable to differentiate patients with a history of perianal disease vs those with active perianal symptoms. We speculate that patients with active perianal disease, particularly those with rectal involvement, are more likely to experience FI. This needs to be confirmed in future studies. Another discrepancy is the overall prevalence of FI of 14% in our study, which was much lower compared to that previously reported in literature. This may in part be due to the definition of FI used in other studies, which included leakage of intestinal gas as part of the definition, thereby increasing prevalence rates.19,22 Alternatively, it can be due to a higher proportion of patients in our cohort having quiescent or mild disease activity. Although referral centers accrue IBD patients with more complicated disease courses and severe symptoms, we have observed a trend toward recruitment of “well” patients in the SPARC registry and others. It is possible that our findings are not generalizable to those of the general population of patients with IBD and represent a worst-case scenario. Arguing against this is the fact that the majority of patients enrolled at our site were in clinical remission at baseline which should mitigate some of the biases inherent in referral center studies.
Our study includes several strengths and few weaknesses. Our study includes a large sample size consisting of 500 patients providing power to detect small to moderate associations between important variables and FI. Patients included in our study were “deeply phenotyped” using prior endoscopy, pathology, imaging, and operative data. We also included valid disease activity indices to assess disease activity. Additionally, participation in the SPARC registry required all patients fill out the same questionnaire at each visit, preventing voluntary response bias. Lastly, we conducted adjusted analyses to minimize confounding. A few limitations of our study include the lack of differentiation of stool consistency in those with FI; we do not have data differentiating liquid vs solid stool incontinence. Another limitation in our study is the lack of detailed surgical data of perianal intervention, limiting our ability to draw a meaningful conclusion regarding the role surgery plays in FI. Although IBD medications are documented in the SPARC database, we were not able to determine if there was a temporal relationship between certain treatments and the development or resolution of FI. We did not collect information regarding childbirth or other medical comorbidities which may have also contributed to the development of FI. Lastly, data from only one SPARC site were analyzed. To ensure that our site did not differ significantly from the total cohort, we compared the demographic and clinical features of our cohort to the total population. We did not find differences in gender, age, disease duration, and medication history. There was a higher percentage of patients with complicated CD and a higher percentage with perianal involvement. Patients with UC had more colonic involvement at our site compared to the population overall. Our cohort also had twice as many black patients compared to the cohort overall. Despite these differences, a higher percentage of our patients were in symptomatic remission at the baseline visit. Given no major differences in age and disease duration, which were the 2 major determinants of FI in our study, we suspect that our conclusions regarding FI would be similar to the SPARC population overall. As we had more patients in symptomatic remission at baseline, it is possible that FI may be more common in the overall population; however, our results still highlight a fairly high rate of FI in the IBD population.
We anticipate that our results will be of interest to providers that care for patients with IBD. FI is common, affecting one in 6 patients with IBD. Physicians should inquire about FI in all patients with IBD, particularly in older patients, those with long-standing disease, and those with significant symptoms. Although age and disease duration are nonmodifiable risk factors, physicians should develop treatment plans to achieve clinical remission as this is protective against FI. Lastly, if a patient continues to report FI despite resolution of inflammation, an evaluation for defecatory dysfunction is warranted.
CONCLUSIONS
Overall, FI is common in IBD, affecting 1 in 6 patients. We found that patients with UC and CD are affected equally. Gender, disease location and phenotype, and perianal disease do not appear to be risk factors for FI. We concluded that disease duration >15 years, increased disease activity by sCDAI and UCDAI, and older age are significant risk factors for FI. A better understanding of the factors that place patients at risk for FI is imperative to improve outcomes in patients with IBD.
ACKNOWLEDGMENTS
A special thanks to Cibi Alexander from the IBD Plexus program for gathering the data from the SPARC database.
Funding: This work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases [T32 DK067872 to J.W.].
Conflict of Interest: None of the authors have any conflicts of interest to disclose.
Author Contribution: All authors have made significant contributions to this manuscript and have approved the final version to be submitted. Natasha Kamal: study conception and design, interpretation of data, and drafting of article. Kiran Motwani: data acquisition and organization, and drafting of the article. Jennifer Wellington: data analysis and interpretation, and revision of the article for important intellectual content. Uni Wong: revision of the article for important intellectual content. Raymond K. Cross: acquisition of data, data interpretation, and revision of the article for important intellectual content.
DATA AVAILABILITY
Data are available by request to Sirimon O’Charoen from the IBD plexus program.
REFERENCES
- 1. Gu P, Kuenzig ME, Kaplan GG, et al. Fecal incontinence in inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2018;24:1280–1290. [DOI] [PubMed] [Google Scholar]
- 2. Saldana Ruiz N, Kaiser AM. Fecal incontinence—challenges and solutions. World J Gastroenterol. 2017;23:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Norton C, Dibley LB, Bassett P. Faecal incontinence in inflammatory bowel disease: associations and effect on quality of life. J Crohns Colitis. 2013;7:e302–e311. [DOI] [PubMed] [Google Scholar]
- 4. Chang S, Shen B, Remzi F. When not to pouch: important considerations for patient selection for ileal pouch-anal anastomosis. Gastroenterol Hepatol (N Y). 2017;13:466–475. [PMC free article] [PubMed] [Google Scholar]
- 5. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(suppl A):5A–36A. [DOI] [PubMed] [Google Scholar]
- 6. Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 7. Sutherland LR, Martin F, Greer S, et al. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92:1894–1898. [DOI] [PubMed] [Google Scholar]
- 8. Nelson RL. Epidemiology of fecal incontinence. Gastroenterology. 2004;126:S3–S7. [DOI] [PubMed] [Google Scholar]
- 9. Vollebregt PF, Visscher AP, van Bodegraven AA, et al. Validation of risk factors for fecal incontinence in patients with Crohn’s disease. Dis Colon Rectum. 2017;60:845–851. [DOI] [PubMed] [Google Scholar]
- 10. Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005–2010. Clin Gastroenterol Hepatol. 2014;12:636–643.e1. [DOI] [PubMed] [Google Scholar]
- 11. Barros LL, Farias AQ, Rezaie A. Gastrointestinal motility and absorptive disorders in patients with inflammatory bowel diseases: prevalence, diagnosis and treatment. World J Gastroenterol. 2019;25:4414–4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kangas E, Hiltunen KM, Matikainen M. Anorectal function in Crohn’s disease. Ann Chir Gynaecol. 1992;81:43–47. [PubMed] [Google Scholar]
- 13. Bassotti G, Antonelli E, Villanacci V, et al. Gastrointestinal motility disorders in inflammatory bowel diseases. World J Gastroenterol. 2014;20:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papathanasopoulos AA, Katsanos KH, Tatsioni A, et al. Increased fatigability of external anal sphincter in inflammatory bowel disease: significance in fecal urgency and incontinence. J Crohns Colitis. 2010;4:553–560. [DOI] [PubMed] [Google Scholar]
- 15. Khera AJ, Chase JW, Salzberg M, et al. Gut-directed pelvic floor behavioral treatment for fecal incontinence and constipation in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:620–626. [DOI] [PubMed] [Google Scholar]
- 16. Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. [DOI] [PubMed] [Google Scholar]
- 17. Papathanasopoulos A, Van Oudenhove L, Katsanos K, et al. Severity of fecal urgency and incontinence in inflammatory bowel disease: clinical, manometric and sonographic predictors. Inflamm Bowel Dis. 2013;19:2450–2456. [DOI] [PubMed] [Google Scholar]
- 18. Nigam GB, Limdi JK, Vasant DH. Current perspectives on the diagnosis and management of functional anorectal disorders in patients with inflammatory bowel disease. Therap Adv Gastroenterol. 2018;11:1756284818816956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chin K. Obstetrics and fecal incontinence. Clin Colon Rectal Surg. 2014;27:110–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vollebregt PF, van Bodegraven AA, Markus-de Kwaadsteniet TML, et al. Impacts of perianal disease and faecal incontinence on quality of life and employment in 1092 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47:1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alvarez JA, Bermejo F, Algaba A, et al. Surgical repair and biological therapy for fecal incontinence in Crohn’s disease involving both sphincter defects and complex fistulas. J Crohns Colitis. 2011;5:598–607. [DOI] [PubMed] [Google Scholar]
- 22. Petryszyn PW, Paradowski L. Stool patterns and symptoms of disordered anorectal function in patients with inflammatory bowel diseases. Adv Clin Exp Med. 2018;27:813–818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available by request to Sirimon O’Charoen from the IBD plexus program.