As of June, 2021, India is facing an overwhelming second wave of COVID-19. In the worst affected states, including Maharashtra and Uttar Pradesh, health-care resources are experiencing far greater strain than in the first wave of infection, which peaked in September, 2020. However, a crucial difference between then and now is the availability of at least two licensed vaccines domestically produced in India, ChAdOx1 nCoV-191 and BBV152.2 Until recently, and like many other countries, India's strategy for deployment of these vaccines had focused primarily on those most at risk of severe disease and mortality from COVID-19.3 Following coverage of key workers such as health-care personnel, vaccination was at first prioritised for people aged older than 60 years, with eligibility gradually widening in subsequent months. As of May, 2021, as one of the several measures to mitigate the ongoing second wave in the country, vaccine eligibility has been expanded to include all people aged 18 years or older.
These developments notwithstanding, we cannot ignore the practical challenges of mass vaccination in a country as large and complex as India. Achieving comprehensive vaccination coverage across the country will take several months, but the current epidemic crisis raises an urgent question: as part of the epidemic response, how can vaccination efforts be targeted to the areas of the country that are most in need? The current resurgence of infection is apparent in about half of the total 739 districts in India; other districts might also be at risk as local-level restrictions are lifted or in the event of a third wave in the future.
One strategy might be pre-emptively to identify and vaccinate those districts most at risk of resurgence—eg, through serological surveys to identify regions with lower levels of previous exposure. However, predicting risk in this way is far from straightforward; for example, major cities such as Mumbai and New Delhi were among the cities with the highest seroprevalence in India's first wave, and yet were the first to see resurgence in the ongoing second wave. It will be important to adjust for population density and other factors to make systematic comparisons of seroprevalence across different regions, but collection of the necessary evidence will take time. In the immediate term, more rapidly deployable strategies are urgently needed.
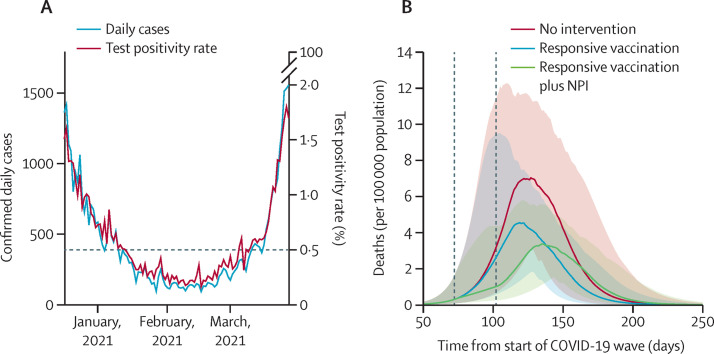
We argue that responsive vaccination, triggered by real-time data from sentinel sites (eg, test centres at district hospitals), can offer one approach for mitigating local resurgences of infection. Data from PCR and rapid antigen tests were used to calculate the test positivity rate for active SARS-CoV-2 infection in New Delhi, the country's capital. Despite the limitations of these tests, arising from their sensitivity and specificity, test positivity rate data in the period between the first and second waves suggest that a threshold value of 0·5% would have provided a timely alert for the emergence of the second wave (figure ).
Figure.
Feasibility and effects of rapid-response vaccination strategies
(A) Time course of test positivity rate in New Delhi (India), during the first quarter of 2021 (ie, between the first and second epidemic waves in the city). The horizontal, dashed line shows a test positivity rate of 0·5%; this threshold was crossed in early March, 2021, when the second wave was underway, illustrating the potential value of this threshold as a trigger for responsive vaccination. (B) Model projections for mortality during the second wave in a hypothetical district in India, where 25% of the population has previous immunity, with a basic reproduction number of 2, and under three different scenarios: no intervention; accelerated vaccination to cover 75% of the population aged 18 years or older within 1 month of exceeding a test positivity rate of 0·5%; and in addition to accelerated vaccination, implementing district-level non-pharmaceutical interventions (NPIs) to reduce transmission by 25%, for the duration of the vaccination campaign. Vertical dashed lines show the duration of interventions (vaccination and NPIs). The model assumes that the vaccine can reduce severe disease and mortality by 60%, but with no effect on acquisition of infection (see the appendix p 11 for alternative assumptions).
We investigated the potential epidemiological effect of rapid-response vaccination in a hypothetical district immediately upon the test positivity ratio crossing the example threshold of 0·5%, provided the testing rate in the population is sustained, using a simple, deterministic, compartmental model of SARS-CoV-2 transmission (figure; appendix pp 1–6). Consistent with the urgency of a rapid outbreak response, we concentrated on a vaccination strategy that prioritises the widest possible coverage of single-dose immunity (ie, ignoring follow-up activities after a month or more, to administer second doses). We assumed a scenario in which it takes 1 month to cover 75% of the population aged 18 years or older with a single dose. The model includes some realistic assumptions for the effect of immunisation, including that, once vaccinated, individuals take on average 3 weeks to develop immunity,4 and, conservatively, a single vaccine dose offers 60% protection against severe disease and death, but does not protect against infection (see the appendix p 11 for an alternative, infection-preventing scenario). The latter assumption of efficacy is based on published estimates of 76·0% (95% CI 59·3–85·9) protection from symptomatic infection, following a single dose of ChAdOx1 nCoV-19;4 to be conservative, we adopted an efficacy towards the lower end of the 95% CI. Overall, we found that such rapid, focused vaccination efforts alone could substantially reduce mortality, by up to 37% (95% uncertainty interval [UI] 21–50) in a district experiencing a resurgent infection with a basic reproduction number of 2 (R 0=2), and by 20% (10–34) for R 0=3 (figure).
Such an impact could be substantially increased by concomitant implementation of non-pharmaceutical interventions such as mandated mask wearing, restrictions on gatherings, and stay-at-home measures through community engagement. In a conservative example in which these non-pharmaceutical interventions reduce transmission by 25%, and are implemented for 1 month in the district at the same time as the vaccination drive, model projections suggest that overall deaths could be reduced by up to 45% (95% UI 32–54) for an infection with R 0=2, and by 30% (21–41) with R 0=3 (figure). Additional results in the appendix (p 10) show that even stronger effects would arise from a vaccine that prevents infection, but that it remains crucial to initiate vaccination as rapidly as possible, upon crossing the test positivity rate threshold of 0·5%.
Currently, there is a mixed picture on single-dose efficacy of currently available vaccines against B.1.617.2, the dominant variant now circulating in India. Early findings from the UK suggest that for ChAdOx1 nCoV-19, single-dose efficacy against this variant is substantially reduced when considering symptomatic infection as an endpoint;5 however, more recent findings suggest that protection against hospitalisation is as high as 71%.6 Similar, single-dose protection against hospitalisation has been reported among health-care workers in India.7 Nonetheless, additional analysis illustrates that rapid-response vaccination could still have a substantial effect on deaths, even if single-dose vaccine efficacy were reduced to 30% (appendix p 11).
In sensitivity analyses, we varied the test positivity rate thresholds to trigger vaccination from 0·3% to 2·0%. For example, with R 0=2, a threshold test positivity rate of 0·3% would avert 48% (95% UI 38–56) of deaths (appendix pp 10–11). In another sensitivity analysis, in a scenario in which the post-dose delay to acquiring immunity is 2 weeks, and not 3 weeks, and R 0=2, a threshold test positivity rate of 0·5% would avert 49% (95% UI 35–56) of deaths (appendix p 11).
Although a test positivity rate of 0·5% would have been predictive of the second wave in New Delhi, this threshold is by no means universal. Appropriate thresholds for any given district in India are likely to depend on various factors, such as population density and local testing strategies. Nonetheless, examination of past data such as that presented in the figure can allow a systematic estimation of appropriate thresholds for different districts. Other, more sophisticated approaches might also be helpful in rapid response by district level teams. For example, monitoring the doubling time of the test positivity rate will offer important additional information on the rate of increase in infection as well as its absolute level. These alternative strategies notwithstanding, the key message remains that focused vaccination efforts, triggered as a rapid outbreak response, could have a substantial effect in mitigating the health burden of local surges of infection.
In practice, successful implementation of such strategies would depend on immunisation resources that are rapidly deployable anywhere in the country. In devolved administrations such as in India, coordination with state-level and district-level health authorities will be the key. India's current COVID-19 vaccination programme requires that all vaccine recipients be observed for 30 min at the vaccination session sites after receiving a dose to identify and manage any adverse events. Thus, door-to-door vaccination strategies would not be a feasible option under current guidelines. However, other strategies can also be helpful in achieving rapid coverage in a defined geographical location. Innovative measures currently being tried in India include establishing satellite vaccination centres closer to hamlets in rural settings and resident welfare associations in urban areas; converting community halls and using large parking spaces for drive-in vaccination; and using mobile vaccination facilities to cover populations that do not live within easy access of vaccination centres.8 Such community-based outreach activities, along with transport facilities for older and less physically able individuals (from their homes to satellite vaccination sites), will reduce the distance between main vaccination session sites and the potential recipients. Active engagement of community-based organisations in such planning and execution will also be crucial, not only for successful implementation of vaccination but also in addressing vaccine hesitancy.9 As India has already administered 199 million doses of vaccine, and records for adverse events following immunisation have reassuringly captured a very low rate of side-effects, the possibility of shortening the observation period following vaccination is being discussed; such measures will help to speed up vaccination, while maintaining safety.
Managing the vaccine supply chain also merits careful consideration to facilitate the rapid deployment of vaccination resources wherever needed. In this respect, India benefits from a strong pre-existing infrastructure under the Expanded Programme of Immunisation with more than 26 000 cold-chain points across the country, the vast majority (97%) of which are at subdistrict level. These facilities are appropriate for the storage of COVID-19 vaccines currently in use in India. Moreover, rapid-response vaccination might require a dedicated stockpile of vaccines, with careful consideration of whether this stockpile would be held at the district level or instead at state-level storage depots for rapid mobilisation.
Our analysis serves as an illustration of principle, leaving several areas for future, more fine-grained analysis. First, our projections were not based on formally calibrated models, as any such calibrations will necessarily be applicable only to the region from which data arise. Instead, for illustration, our approach was based on assumptions for plausible ranges of R 0 in the second wave, to reflect the different levels of transmission intensity that might be expected across such a large and diverse country as India. Second, our analysis focused on reduction of deaths, although another key outcome is demand for hospital-based care, including oxygen support. Further work could extend the analysis shown here to incorporate these considerations, including the need to avoid overwhelming existing health-care capacity. Finally, we modelled rapid-response vaccination as proceeding uniformly across the population within a district, thus ignoring the potential effect of prioritising vaccination efforts in specific subgroups of the population. For example, recent modelling work has highlighted how prioritising people aged older than 60 years would have greatest effect on deaths, while also potentially permitting ongoing opportunities for transmission.10 Further work should examine how these results would translate to rapid vaccination strategies, in the face of rapid epidemic growth. Spatial targeting is another possible implementation strategy. For example, in situations in which infection in a district appears to be geographically localised, outbreak response efforts might adopt a ring vaccination strategy by focusing first on the neighbourhood of this infection activity, before expanding to cover the rest of the district.
Overall, our analysis offers a demonstration of principle that even limited vaccination resources could be marshalled for maximum impact, if deployed flexibly in response to a rapidly evolving epidemic. Looking ahead, experience from influenza pandemics in 1918 and 2009, as well as the current COVID-19 pandemic in other countries, highlights the potential for not just two, but even subsequent waves, of infection.11, 12, 13 Flexible, agile vaccination strategies could thus play an important part in protecting lives and livelihoods as the COVID-19 pandemic continues to unfold.
NA is supported by the UK Medical Research Council and the Bill & Melinda Gates Foundation. SM, BB, and SP are supported by the Indian Council of Medical Research.
Supplementary Material
References
- 1.Voysey M, Clemens SAC, Madhi SA. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ella R, Reddy S, Jogdand H. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21:950–961. doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandal S, Arinaminpathy N, Bhargava B, Panda S. India's pragmatic vaccination strategy against COVID-19: a mathematical modelling based analysis. medRxiv. 2021 doi: 10.1101/2021.05.07.21256742. published online May 10. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Costa Clemens SA, Madhi SA. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernal JL, Andrews N, Gower C. Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. medRxiv. 2021 doi: 10.1101/2021.05.22.21257658. published online May 24. (preprint). [DOI] [PubMed] [Google Scholar]
- 6.Stowe J, Andrews N, Gower C. Effectiveness of COVID-19 vaccines against hospital admission with the Delta (B.1.617.2) variant. https://khub.net/web/phe-national/public-library/-/document_library/v2WsRK3ZlEig/view_file/479607329?_com_liferay_document_library_web_portlet_DLPortlet_INSTANCE_v2WsRK3ZlEig_redirect=https%3A%2F%2Fkhub.net%3A443%2Fweb%2Fphe-national%2Fpublic-library%2F-%2Fdocument_library%2Fv2WsRK3ZlEig%2Fview%2F479607266 preprint.
- 7.Victor J, Mathews P, Paul H, Murugesan M, Mammen JJ. Protective effect of COVID-19 vaccine among health care workers during the second wave of the pandemic in India. Mayo Clin Proc (in press). [DOI] [PMC free article] [PubMed]
- 8.Asian News International Planning to deploy mobile vans for Phase 3 COVID-19 vaccination: Mumbai Mayor Kishori Pednekar. April 25, 2021. https://mumbaimirror.indiatimes.com/coronavirus/news/planning-to-deploy-mobile-vans-for-phase-3-covid-19-vaccination-mumbai-mayor-kishori-pednekar/articleshow/82242440.cms
- 9.Panda S. Tackling vaccine hesitancy. April 21, 2021. https://www.thehindu.com/opinion/op-ed/tackling-vaccine-hesitancy/article34369937.ece
- 10.Foy BH, Wahl B, Mehta K, Shet A, Menon GI, Britto C. Comparing COVID-19 vaccine allocation strategies in India: a mathematical modelling study. Int J Infect Dis. 2021;103:431–438. doi: 10.1016/j.ijid.2020.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taubenberger JK, Morens DM. 1918 influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12:15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorigatti I, Cauchemez S, Ferguson NM. Increased transmissibility explains the third wave of infection by the 2009 H1N1 pandemic virus in England. Proc Natl Acad Sci USA. 2013;110:13422–13427. doi: 10.1073/pnas.1303117110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seong H, Hyun HJ, Yun JG. Comparison of the second and third waves of the COVID-19 pandemic in South Korea: Importance of early public health intervention. Int J Infect Dis. 2021;104:742–745. doi: 10.1016/j.ijid.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.