Abstract
Dynamic modification of nuclear and cytoplasmic proteins with O-linked β-N-acetylglucosamine (O-GlcNAc) plays an important role in orchestrating the transcriptional activity of eukaryotic cells. Here, we report that the O-GlcNAc modification contributes to maintaining ocular surface epithelial homeostasis by promoting mucin biosynthesis and barrier function. We found that induction of human corneal epithelial cell differentiation stimulated the global transfer of O-GlcNAc to both nuclear and cytosolic proteins. Inflammatory conditions, on the other hand, were associated with a reduction in the expression of O-GlcNAc transferase at the ocular surface epithelia. Loss- and gain-of-function studies using small interfering RNA targeting O-GlcNAc transferase, or Thiamet G, a selective inhibitor of O-GlcNAc hydrolase, respectively, revealed that the presence of O-GlcNAc was necessary to promote glycocalyx barrier function. Moreover, we found that Thiamet G triggered a correlative increase in both surface expression of MUC16 and apical epithelial cell area while reducing paracellular permeability. Collectively, these results identify intracellular protein O-glycosylation as a novel pathway responsible for promoting the terminal differentiation of human corneal epithelial cells.
Keywords: cell differentiation, cornea, epithelium, O-GlcNAc modification, transmembrane mucin
Introduction
O-linked β-N-acetylglucosamine (O-GlcNAc) is a common carbohydrate modification that occurs on hundreds of proteins within the nuclear and cytoplasmic compartments of the cell (Zachara et al. 2015). Originally discovered in the early 1980s (Torres and Hart 1984), this residue cycles rapidly on and off most proteins in a process described as being analogous to phosphorylation. It is the combined action of two enzymes encoded by a single pair of genes that serves to regulate cellular O-GlcNAc levels (Vocadlo 2012). The O-GlcNAc transferase (OGT) is the glycosyltransferase that catalyzes the transfer of GlcNAc from UDP-GlcNAc to acceptor proteins, whereas a glycoside hydrolase known as O-GlcNAcase (OGA) catalyzes the cleavage of the O-GlcNAc moiety from proteins. Both OGT and OGA are ubiquitously expressed in mammalian tissues. Genetic abrogation of the Ogt gene in mouse results in embryonic lethality, highlighting the importance of this modification in cell survival (Shafi et al. 2000). Similarly, genetic disruption of Oga results in constitutive accumulation of O-GlcNAc in embryos, genomic instability, senescence and neonatal mortality (Yang et al. 2012).
The O-GlcNAc residue has been detected in nearly all functional classes of nucleocytoplasmic proteins (Love and Hanover 2005). Many of these proteins are phosphoproteins with important roles in gene regulation. Indeed, about one quarter of the O-GlcNAc-modified proteins function as transcription or translation regulatory factors. Additional substrate functions include control of signal transduction, cell cycle progression, protein processing and the stress response. The O-GlcNAc modification also occurs on several cytoskeletal proteins, such as those regulating structural integrity and cell shape (Cieniewski-Bernard et al. 2014; Tian and Qin 2019). Importantly, one of the major functions of O-GlcNAc is to serve as a nutrient sensor, since changes in UDP-GlcNAc concentrations directly affect the extent of protein O-GlcNAcylation (Hart 2019). Because of the dependence on glucose flux and substrate availability, there is a long-standing interest in understanding how O-GlcNAc contributes to metabolically related disorders including diabetes, cardiovascular disease and cancer (Vaidyanathan et al. 2014).
The cornea is a thin transparent tissue that covers the anterior segment of the eye. Its outermost layer consists of a nonkeratinized stratified epithelium with flattened squamous cells on its most apical portion. Focal connections between adjacent apical cells contain tight junctions that seal the intercellular space and serve as a barrier to the diffusion of molecules through the paracellular pathway (Mantelli et al. 2013). Additionally, apical cell membranes on superficial cells exhibit folds or ridges termed microplicae. A group of glycoproteins known as transmembrane mucins emanate from the tips of these microplicae to produce a protective glycocalyx that limits transport through the transcellular pathway (Fini et al. 2020). During normal homeostasis, basal and suprabasal cells reaching the apical portion of the cornea commit to terminal differentiation to fulfill the protective function of the corneal epithelium. Disruption of this process as a consequence of environmental insult or chronic inflammation has been associated with ocular surface disease (Mantelli et al. 2013; Nowell and Radtke 2017). Here, we identify a novel mechanism of terminal differentiation in corneal epithelial cells that involves modification of nuclear and cytoplasmic compartments with O-GlcNAc. Such modulation of intracellular proteins may be essential to sustain corneal epithelial integrity by influencing fundamental biological activities such as signaling and gene transcription.
Results
Induction of corneal epithelial differentiation stimulates the O-GlcNAc modification
Protocols have been established using defined cell culture environments to direct the differentiation of human corneal epithelial cells into mature, terminally differentiated stratified cells (Gipson et al. 2003; Postnikoff et al. 2014). To investigate how corneal differentiation influences the O-GlcNAc modification, we used an in vitro model in which cells were grown for 7 days in serum-containing media to promote stratification and the establishment of barrier function (Argueso et al. 2009; Argueso and Gipson 2012). These conditions resulted in the formation of flattened cells expressing the transmembrane mucin MUC16, a marker of terminal differentiation, on their apical surface (Figure 1A). The presence of MUC16 as a high molecular weight glycoprotein in cell lysates of differentiated cultures was confirmed by immunoblot (Figure 1B).
Fig. 1.

Induction of corneal epithelial differentiation promotes O-GlcNAcylation. (A) Phase contrast and immunofluorescence micrographs showing MUC16 distribution (green) in human corneal epithelial cells before and after induction of differentiation for 7 days. Nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI) (blue). Representative images are shown (bars, 100 μm). (B) Cell extracts were subjected to SDS-PAGE and immunoblot analysis using antibodies to MUC16 and GAPDH. (C) Protein isolated from cytoplasmic and nuclear fractions before and after induction of differentiation was subjected to immunoblot analysis using CTD110.6, GAPDH (cytoplasmic control) and HDAC1 (nuclear control) antibodies.
In subsequent experiments, we compared the pattern of endogenous O-GlcNAcylation in undifferentiated and differentiated cells using the CTD110.6 antibody. This antibody has been shown to recognize a wide range of O-GlcNAc-modified proteins and to be relatively less dependent on the protein structure than other antibodies (Ma and Hart 2014). Biochemical fractionation of corneal epithelial cells revealed an increase in the intensity and total number of O-GlcNAc bands in both the cytosol and nucleus following induction of differentiation with serum (Figure 1C). Of note is the marked upregulation of multiple bands in the nuclear fraction, which might suggest changes in the control of genome functions. Overall, these data evidence an association between the O-GlcNAc modification and the ability of corneal epithelial cells to acquire a differentiated phenotype.
Inflammatory stress impairs OGT expression at the ocular surface
OGT is the enzyme responsible for attaching O-GlcNAc to specific serine or threonine residues in nucleocytoplasmic proteins. Analysis of a public microarray depository revealed that the number of OGT transcripts was significantly downregulated in the conjunctival epithelium of patients with dry eye, a multifactorial disease of the ocular surface characterized by inflammation and epithelial damage, as shown by the increased permeability to the rose bengal diagnostic dye (Figure 2A). One of the proinflammatory cytokines implicated in pathogenesis of dry eye is tumor necrosis factor alpha (TNFα) (Yoon et al. 2007). We found that addition of TNFα to differentiated cultures of corneal epithelial cells decreased the expression of OGT (Figure 2B), suggesting that inflammatory stress affects the normal biosynthesis of O-GlcNAc-modified proteins in ocular surface disease.
Fig. 2.
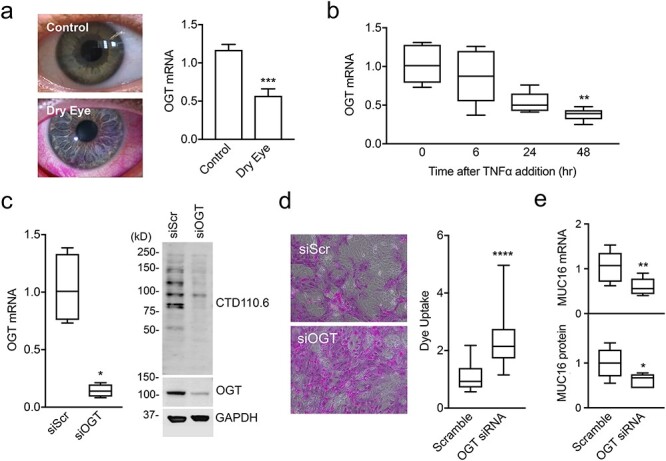
Inflammatory stress reduces OGT expression at the ocular surface. (A) Rose bengal staining of epithelial cells is observed at the ocular surface of a patient with dry eye. OGT expression in human conjunctival epithelium as determined using data from a public microarray depository. (B) RNA was isolated from differentiated cultures of human corneal epithelial cells treated with TNFα, and the OGT mRNA abundance was determined by quantitative polymerase chain reaction (qPCR). (C) Cells were transfected before differentiation with OGT-specific siRNA (siOGT) or scramble siRNA (siScr). After the treatments, OGT mRNA abundance was determined by qPCR and aliquots of cell lysates were resolved by SDS-PAGE and analyzed by immunoblot using CTD110.6, OGT and GAPDH antibodies. (D) Glycocalyx barrier function after transfection with siOGT or siScr was determined by the rose bengal penetration assay. (E) Expression and biosynthesis of MUC16 was determined by qPCR and immunoblot, respectively. All experiments were performed at least in triplicate. The data in (A) represent the mean ± SD. The box and whisker plots in B, C, D and E show the 25th and 75th percentiles (box), the median (horizontal line in box), and the minimum and maximum data values (whiskers). Significance was determined using unpaired t test (A and E), Kruskal–Wallis with Dunn’s post hoc test (B) or Mann Whitney test (c and d). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
To further examine the consequences of altered OGT expression to the maintenance of corneal barrier function, we abrogated OGT in epithelial cell cultures using small interfering RNA (siRNA). We found that siRNA successfully reduced the levels of OGT messenger RNA (mRNA) and OGT protein in these cells, concomitantly with a decrease in protein O-GlcNAcylation (Figure 2C). Importantly, we observed a significant uptake of the rose bengal diagnostic dye, an indicator of altered transcellular permeability, in OGT knockdown cells compared to scramble control (Figure 2D). Concomitantly, abrogation of OGT resulted in reduced expression and biosynthesis of MUC16 (Figure 2E). These results support the concept that the O-GlcNAc modification is necessary to provide glycocalyx barrier function in cornea.
The O-GlcNAc modification functions during early differentiation
Transcriptional activity at early stages of corneal epithelial cell differentiation influences the biosynthesis of transmembrane mucins responsible for maintaining the barrier function of the glycocalyx (Xiong et al. 2011). Experiments in Figure 2D using siRNA indicate that early abrogation of OGT during differentiation compromises barrier formation. We used Thiamet G, a small and highly selective molecule inhibitor of OGA (Yuzwa et al. 2008), to determine whether early induction of O-GlcNAcylation would enhance glycocalyx barrier function in terminally differentiated corneal epithelial cells. We found that sustained treatment of immature cells with Thiamet G boosted the accumulation of O-GlcNAc-modified proteins in both the cytosol and nucleus of mature cells (Figure 3A). This increase in O-GlcNAc levels correlated with a significant decrease in rose bengal uptake, indicating that early O-GlcNAcylation contributes to enhance the glycocalyx barrier function of mature corneal epithelial cells. Strikingly, when Thiamet G was added to the cell culture following differentiation, there was no alteration in rose bengal uptake, despite the fact that Thiamet G successfully promoted the accumulation of O-GlcNAc-modified proteins. These results suggest that O-GlcNAcylation of nucleocytoplasmic proteins during early differentiation is essential for the induction of the transcellular barrier in mature cells, but has little effect once the cells have committed to differentiation.
Fig. 3.
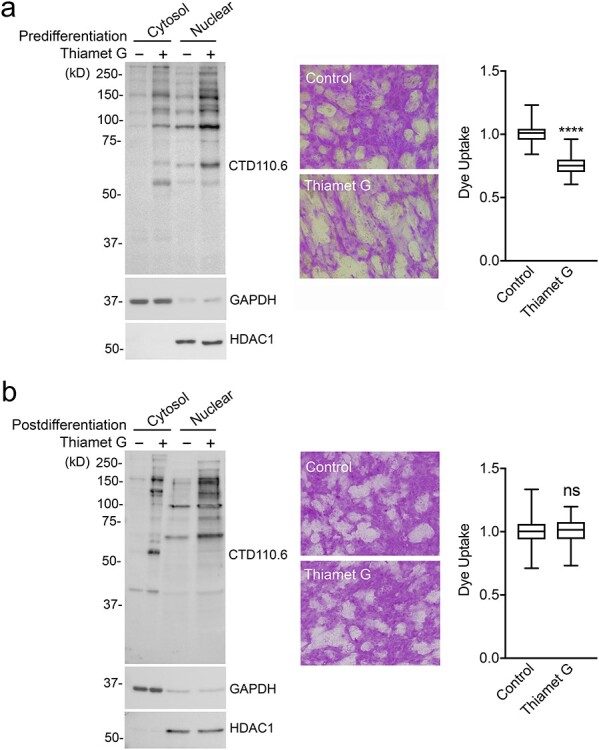
The O-GlcNAc modification functions during early differentiation. (A) Thiamet G was administered to human corneal epithelial cells before differentiation for 7 days. Protein isolated from cytoplasmic and nuclear fractions was subjected to immunoblot analysis using CTD110.6, GAPDH and HDAC1 antibodies. Glycocalyx barrier function was determined by the rose bengal penetration assay. (B) Same as (A) but Thiamet G was administered for 48 h after the induction of differentiation. All experiments were performed at least in triplicate. The box and whisker plots show the 25th and 75th percentiles (box), the median (horizontal line in box) and the minimum and maximum data values (whiskers). Significance was determined using unpaired t test. ****P < 0.0001; ns, nonsignificant.
Thiamet G stimulates mucin biosynthesis and paracellular barrier function
To gain insight into the mechanisms by which Thiamet G enhanced glycocalyx barrier function in corneal epithelial cells, we analyzed its effect on the biosynthesis of the MUC16 mucin. Consistent with the reduction in rose bengal uptake, we observed that supplementation of immature cells with Thiamet G over 7 days of culture enhanced the intensity of MUC16 staining in mature cells (Figure 4A). Moreover, we found a significant increase in apical cell surface area that correlated with the apical expression of the MUC16 mucin (Figure 4B). Importantly, the improvement in barrier function was not ascribed exclusively to the character of the apical cell surface but also affected the paracellular barrier. Transepithelial electrical resistance (TEER) is a measure of ionic conductance that reflects the integrity of tight junctions in cell culture models (Srinivasan et al. 2015). When plated on Transwell membrane inserts, the epithelial cell cultures showed remarkably higher TEER values in the presence of Thiamet G than controls (Figure 4C). We further evaluated the barrier function in this model by analyzing the penetration of fluorescein isothiocyanate (FITC)-dextran. Here, we found that the ability of FITC-dextran to pass across the stratified cell culture was significantly reduced following treatment with Thiamet G (Figure 4C). Together, these data demonstrate that induction of protein O-GlcNAcylation plays an important role in promoting transcellular and paracellular barrier function in differentiated human corneal epithelial cells.
Fig. 4.

Thiamet G stimulates mucin biosynthesis and paracellular barrier function. (A) Thiamet G was administered to human corneal epithelial cells before differentiation for 7 days. MUC16 (green) was localized by immunofluorescence microscopy and the fluorescence intensity per cell quantified using ImageJ. Representative en face images of nonpermeabilized epithelial cells are shown to the right. Nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI) (blue). Representative images are shown (bars, 200 μm). (B) Morphometric analyses of cell area were performed using ImageJ. A positive linear correlation was observed between MUC16 intensity and cell area following incubation with Thiamet G. (C) Thiamet G was added basolaterally to cells grown in Transwell cell culture inserts. Following a 7-day treatment, TEER values were significantly increased compared to control. Macromolecular permeability was estimated by measuring FD-4 in the basolateral compartment at the end of the experiments. For each condition in (A) and (B), eight images from two independent tissue chamber slides were analyzed. Experiments in (C) were performed at least in triplicate. The box and whisker plots show the 25th and 75th percentiles (box), the median (horizontal line in box) and the minimum and maximum data values (whiskers). Significance was determined using Mann Whitney test (A and B) or unpaired t test (C). **P < 0.01; ***P < 0.001; ****P < 0.0001.
Discussion
The simple modification of intracellular proteins with O-GlcNAc is rapidly emerging as a major mechanism for the control of cellular function. A number of environmental cues have been shown to impact the dynamic cycling of O-GlcNAc and subsequently induce biological activities such as immune cell maintenance and stem cell self-renewal (Bond and Hanover 2015). Here, we add to the list of functions triggered by protein O-GlcNAcylation by identifying corneal epithelial differentiation as a downstream effect of the O-GlcNAc modification. Our results indicate that sustained induction of O-GlcNAc during differentiation is necessary to promote the expression of the MUC16 mucin and the establishment of barrier function in mature human corneal epithelial cells.
The O-GlcNAc modification appears to have distinct effects on cell differentiation depending on tissue context and physiological state. Several studies so far have documented an important role of O-GlcNAcylation in maintaining cells in an undifferentiated state. Abrogation of OGT function by use of metabolic inhibitors or genetic knockdown accelerates the differentiation of embryonic stem cells and acute myeloid leukemia cells (Andres et al. 2017; Moon et al. 2017; Asthana et al. 2018). Similarly, O-GlcNAc levels have been shown to decrease during erythroid differentiation (Zhang et al. 2019). In this study, sustained treatment of G1E-ER4 cells prior to lineage commitment with Thiamet G increased the expression of progenitor cell surface markers. On the other hand, O-GlcNAcylation has been positively associated with cellular differentiation. Inhibition of OGA in MC3T3-E1 cells promoted the expression of osteogenic genes and extracellular matrix calcification, whereas inhibition of OGT suppressed them, suggesting a role for O-GlcNAc in triggering initial osteogenic gene regulatory programs and directing bone development (Koyama and Kamemura 2015). As mentioned previously, the discrepancy between these findings was not completely unexpected since it is recognized that O-GlcNAcylation can exert stimulatory or inhibitory effects on target gene transcription depending on cellular context (Baudoin and Issad 2014; Gu et al. 2018). Furthermore, the outcomes in these studies also rely on the different experimental approaches used. For instance, metabolic and pharmacological treatments, as well as genetic manipulation aimed to alter O-GlcNAc levels in cells, are not necessarily comparable as they might differentially affect O-GlcNAc cycling (Vaidyanathan and Wells 2014). We anticipate that future research exploring the contributions of individual O-GlcNAc-modified proteins to terminal differentiation will translate into a better understanding of the cell type-specific responses to this modification.
In our hands, induction of differentiation with serum stimulated global protein O-GlcNAcylation in corneal epithelial cells and the functional establishment of barrier function. The observed effect of serum is consistent with previously published data using mouse embryonic fibroblasts, where serum increased O-GlcNAcylation in a process relevant to the control of cell cycle entry (Yang et al. 2012). It has been recently shown that deficiency in intestinal epithelial O-GlcNAcylation predisposes to gut inflammation (Zhao et al. 2018). Herein, the authors found that the levels of OGT and protein O-GlcNAcylation were downregulated in patients with inflammatory bowel disease. Abrogation of Ogt in mice resulted in a permeable epithelial barrier, epithelial cell dysfunction and ultimately intestinal inflammation, whereas increasing O-GlcNAcylation with Thiamet G promoted barrier function and protected mice from chemical-induced inflammation. Similarly to these findings, we also found decreased OGT expression in patients with ocular inflammatory disease, and identified an association between the proinflammatory cytokine TNFα and impaired OGT expression in differentiated corneal epithelial cells. Although there is little evidence indicating that TNFα promotes rose bengal uptake in differentiated cells, our observations suggest that persistent inflammatory stress at the ocular surface affects immature cells located in subapical layers of the epithelium and their conversion into terminally differentiated cells with proper barrier function. The finding that the O-GlcNAc modification was important to promote glycocalyx barrier function during differentiation supports this hypothesis. Moreover, these data were further reinforced by the ability of Thiamet G to promote the formation of flattened superficial cells expressing MUC16, a heavily O-glycosylated transmembrane protein localized on the tips of the surface microplicae that restricts transcellular transport (Argueso et al. 2006; Blalock et al. 2007).
Clearly, maintenance of corneal homeostasis is going to require a balanced regulation of O-GlcNAcylation in the epithelium. Epithelial alterations such as superficial punctate keratitis and recurrent erosions are frequently observed in diabetic corneas (Ljubimov 2017). It has been hypothesized that hyperglycemic conditions contribute to the pathogenesis of diabetic keratopathy by promoting the accumulation of O-GlcNAc-modified proteins in cornea. Immunohistochemical studies in diabetic Goto-Kakizaki rats revealed increased levels of O-GlcNAc-modified proteins and Ogt in both the cytoplasm and nucleus of basal and suprabasal corneal epithelial cells (Akimoto et al. 2003). In these experiments, however, it should be noted that O-GlcNAc was robustly expressed in normal corneas, suggesting a physiological role for this modification in the maintenance of corneal homeostasis. Addition of the OGA inhibitor PUGNAc further increased O-GlcNAcylation in normal cornea, resulting in decreased number of hemidesmosomes and the detachment of the basement membrane from the epithelial basal cells, as seen in the diabetic cornea. A future task would be to fully integrate those changes that may occur as a consequence of altered O-GlcNAcylation in cornea and to define how the ocular surface withstands the therapeutic modulation of O-GlcNAc content.
Materials and methods
Cell culture
Telomerase-immortalized human corneal epithelial cells were grown in keratinocyte serum-free medium (Thermo Fisher Scientific; Rockford, IL) supplemented with bovine pituitary extract, 0.2 ng/mL epithelium growth factor (EGF) and 0.4 mM CaCl2 as described (Taniguchi et al. 2017). Once confluent, cells were switched to Dulbecco’s modified Eagles’s medium (DMEM)/F-12 supplemented with 10% newborn calf serum and 10 ng/mL EGF for 7 days to promote cell differentiation, stratification and establishment of barrier function (Argueso et al. 2009). This cell line was derived from normal tissue and produces the same mucin gene and keratin repertoire as native epithelium (Gipson et al. 2003). In these cells, MUC16 is localized to the tips of microplicae on the apical cell layer in a mosaic pattern representing different degrees of differentiation (Argueso et al. 2003; Blalock et al. 2007; Uchino 2018). Where indicated, cells were serum-starved for 1 h and incubated with TNFα (40 ng/mL; PeproTech, Inc.; Rocky Hill, NJ) in serum-free DMEM/F12. Medium along with fresh TNFα was replaced at 24 h. Thiamet G (20 μM; Sigma-Aldrich, St. Louis, MO) was administered before differentiation for 7 days (every other day for 4 days and daily afterwards) or after differentiation for 48 h (daily). Phase contrast imaging was performed using an inverted microscope (Eclipse TS100; Nikon, Inc., Melville, NY). Pictures were taken with a SPOT Insight Fire Wire Camera (Diagnostic Instruments, Inc.; Sterling Heights, MI).
Human microarray dataset
The number of OGT transcripts in the conjunctival epithelium of patients with dry eye was analyzed using data from a public microarray depository (available in the public domain at http://www.functionalglycomics.org). Details on sample collection, RNA labeling and chip hybridization have been previously described (Mantelli et al. 2009).
Electrophoresis and Immunoblotting
Protein from cell cultures was extracted using RIPA buffer (150 μM NaCl, 50 μM Tris, pH 8.0, 1% NP 40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) supplemented with Complete ethylenediaminetetraacetic acid (EDTA)-free Protease Inhibitor Cocktail (Sigma-Aldrich). After homogenization with a pellet pestle, the cell extracts were centrifuged at 17,000 × g for 45 min at 4°C, and the protein concentration of the supernatant determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto nitrocellulose membranes (Bio-Rad, Hercules, CA). Nonspecific binding to the membranes was blocked by incubation with 5% (wt/vol) bovine serum albumin (BSA) in Tris-buffered saline containing 0.1% Tween-20 (TTBS) for 1 h at room temperature. Membranes were then incubated with anti-O-GlcNAc CTD110.6 (1:1,000; Cell Signaling, Danvers, MA) or anti-OGT D1D8Q (1:1,000; Cell Signaling) in blocking buffer overnight at 4°C, followed by the corresponding horseradish peroxidase-conjugated secondary antibody. For analysis of MUC16, proteins were separated by 1% agarose gel electrophoresis and blotted onto nitrocellulose membranes using vacuum as described (Argueso et al. 2009). Nonspecific binding to the nitrocellulose was blocked by incubation with 5% nonfat dry milk in Tris buffered saline with Tween 20 (TTBS) for 1 h at room temperature. Membranes were then incubated with anti-MUC16 M11 (1:3,000; Neomarkers, Fremont, CA) in blocking buffer overnight at 4°C. Anti-glyceraldehyde-3-phosphate dehydrogenase (Anti-GAPDH, 1:5,000; FL-335; Santa Cruz Biotechnology; Dallas, TX) in 5% nonfat dry milk/TTBS or anti-HDAC1 (1:1,000; Cell Signaling, Danvers, MA) in 5% BSA/TTBS were used to control equal protein loading in cytosolic and nuclear fractions, respectively. Horseradish peroxidase activity was detected using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) and imaged using the Syngene G-Box (Syngene, Frederick, MD).
Isolation of nuclear and cytoplasmic fractions
Cytoplasmic and nuclear protein fractions were extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific). Cells were dissociated with TryplE (Thermo Fisher Scientific) and then centrifuged at 500 × g for 5 min. After washing with PBS, the cell pellet was suspended in ice-cold cytoplasmic extraction reagent I for 10 min, after which ice-cold cytoplasmic extraction reagent II was added for 1 min. The tube was mixed thoroughly using a Vortex mixer for 5 s before centrifugation (17,000 × g) at 4°C for 5 min. The supernatant containing the cytoplasmic extract was stored at −80°C. The nuclear pellets obtained were resuspended in ice-cold nuclear extraction reagent and kept on ice for 40 min with intermittent agitation. The samples were then subjected to centrifugation for 10 min at 4°C, and the supernatant was stored at −80°C.
OGT siRNA
Depletion of OGT was achieved using the Silencer Select Predesigned siRNA (Assay ID 112452; Life Technologies) targeting a sequence of human OGT mRNA. A nonspecific scramble siRNA (4390843; Life Technologies) served as negative control. Human corneal epithelial cells were transfected twice before differentiation—at confluence and 3 days postconfluence, by 6-h incubation with 650 nM siRNA in lipofectamine™ 2000 (Life Technologies, 2 μL) dissolved in 400 μL Opti-MEM (Life Technologies) reduced-serum medium GlutaMAX™ (Life Technologies). After the final transfection, cells were incubated in supplemented DMEM/F12 for three additional days.
Quantitative polymerase chain reaction
Total RNA was isolated from cell cultures using the RNeasy Micro Kit (Qiagen; Hilden, Germany) following the manufacturer’s instructions. Residual genomic DNA in the RNA preparation was eliminated by digestion with amplification-grade DNase I (Thermo Fisher Scientific). One μg total RNA was used for cDNA synthesis (iScript cDNA Synthesis; Bio-Rad; Hercules, CA). Gene expression was measured using the SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) in a Mastercycler ep realplex thermal cycler (Eppendorf, Hauppauge, NY). The following parameters were used: 30 sec at 95°C, followed by 40 cycles of 5 sec at 95°C and 30 sec at 60°C. Primers to determine OGT and MUC16 expression were obtained from Bio-Rad (catalog number qHsaCID0012537 and qHsaCID0018430, respectively). Expression values were normalized using the housekeeping gene GAPDH (catalog number qHsaCED0038674). The comparative CT method was used for relative quantitation of the number of transcripts. No template controls were run in each assay to confirm lack of DNA contamination in the reagents used for amplification.
Transcellular barrier function assay
Transcellular barrier function in cell culture was assayed with the rose bengal anionic dye (Acros Organics; Morris Plains, NJ) as previously described (Argueso and Gipson 2012). For dye penetrance assay, cells in tissue culture plates were rinsed with PBS and incubated for 5 min with a 0.1% solution of rose bengal. Afterwards, the dye was aspirated and the culture washed further with PBS. The extent of dye penetrance was assessed using an inverted microscope (Nikon Eclipse TS100). Images were taken at 10× magnification and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
Immunofluorescence microscopy
MUC16 was immunolocalized in cell cultures slides fixed in 4% paraformaldehyde using standard protocols. Slides were incubated with the M11 monoclonal antibody (1:300; NeoMarkers; Fremont, CA) overnight at 4°C followed by a goat anti-mouse secondary antibody conjugated to Alexa Fluor 488 (1:1000; Invitrogen; Carlsbad, CA). Slides were mounted in VectaShield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories; Burlingame, CA). Imaging was performed on a Zeiss Axio Observer Z1 inverted fluorescent microscope (Carl Zeiss Microimaging GmbH, Jena, Germany). Incubation with the primary antibody was routinely omitted in control experiments. ImageJ software was used to perform morphometric analyses of surface area in cells expressing the MUC16 mucin and MUC16 intensity by manually outlining and labeling individual cells. MUC16 intensity in each cell was calculated as the corrected total cell fluorescence (CTCF), where CTCF = integrated density − (area of selected cell × average mean of background).
TEER
TEER was determined on differentiated cells grown in Transwell cell culture inserts with an Evom2 Epithelial Voltohmmeter (World Precision Instruments, Sarasota, FL) as described (Guzman-Aranguez et al. 2012). Before each measurement, the Evom2 was “zeroed” according to the manufacturer’s instructions. One Transwell insert was left empty as a control to determine the intrinsic resistance of the filter, which was subtracted from all readings. Experiments were performed in triplicate and the transepithelial resistance (ohm-square centimeters) was calculated by multiplying the measured electrical resistance by the area of the filter (1.12 cm2).
Measurement of macromolecular permeability
Paracellular permeability was determined by measuring apical to basolateral flux of FITC-conjugated dextran (FD-4, Mr 4; Sigma-Aldrich). Differentiated epithelial cultures on 0.4 μm pore size permeable supports were incubated with 1 mL DMEM/F-12 medium in the basolateral compartment and with 500 μL of 2 mg/mL FD-4 on the apical portion for 5 h at 37°C. Media samples taken from the basolateral compartment in triplicate were placed on opaque 96-well Nunc plates (Thermo Fisher Scientific). Fluorescence intensity of each sample was measured on a fluorimeter at excitation and emission wavelengths of 485 nm and 528 nm, respectively, and FITC-dextran concentrations were determined from standard curves generated by serial dilution of FITC-dextran (1 to 1.95 × 10−3 mg/mL). The basolateral measurements were corrected for dilution factor by dividing the volume of the basolateral medium by the volume of the apical medium and multiplying the readings by this number (Hardyman et al. 2013).
Statistical analyses
Statistical analyses were performed using Prism 7 (Graphpad Software, San Diego, CA) for Mac OSX.
Acknowledgements
The authors thank Drs Ilene K. Gipson and Stefano Bonini for providing the human corneal epithelial cell line and clinical images of the cornea, respectively. This work was supported by the National Eye Institute, National Institutes of Health, Bethesda, Maryland (grants no.: R01EY026147 and P30EY003790).
Conflict of interest statement
None declared.
References
- Akimoto Y, Kawakami H, Yamamoto K, Munetomo E, Hida T, Hirano H. 2003. Elevated expression of O-GlcNAc-modified proteins and O-GlcNAc transferase in corneas of diabetic Goto-Kakizaki rats. Invest Ophthalmol Vis Sci. 44:3802–3809. [DOI] [PubMed] [Google Scholar]
- Andres LM, Blong IW, Evans AC, Rumachik NG, Yamaguchi T, Pham ND, Thompson P, Kohler JJ, Bertozzi CR. 2017. Chemical modulation of protein O-GlcNAcylation via OGT inhibition promotes human neural cell differentiation. ACS Chem Biol. 12:2030–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso P, Gipson IK. 2012. Assessing mucin expression and function in human ocular surface epithelia in vivo and in vitro. Methods Mol Biol. 842:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. 2009. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem. 284:23037–23045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. 2003. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 44:2487–2495. [DOI] [PubMed] [Google Scholar]
- Argueso P, Tisdale A, Spurr-Michaud S, Sumiyoshi M, Gipson IK. 2006. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Invest Ophthalmol Vis Sci. 47:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthana A, Ramakrishnan P, Vicioso Y, Zhang K, Parameswaran R. 2018. Hexosamine biosynthetic pathway inhibition leads to AML cell differentiation and cell death. Mol Cancer Ther. 17:2226–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudoin L, Issad T. 2014. O-GlcNAcylation and inflammation: A vast territory to explore. Front Endocrinol (Lausanne). 5:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock TD, Spurr-Michaud SJ, Tisdale AS, Heimer SR, Gilmore MS, Ramesh V, Gipson IK. 2007. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 48:4509–4518. [DOI] [PubMed] [Google Scholar]
- Bond MR, Hanover JA. 2015. A little sugar goes a long way: The cell biology of O-GlcNAc. J Cell Biol. 208:869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieniewski-Bernard C, Lambert M, Dupont E, Montel V, Stevens L, Bastide B. 2014. O-GlcNAcylation, contractile protein modifications and calcium affinity in skeletal muscle. Front Physiol. 5:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini ME, Jeong S, Gong H, Martinez-Carrasco R, Laver NMV, Hijikata M, Keicho N, Argueso P. 2020. Membrane-associated mucins of the ocular surface: New genes, new protein functions and new biological roles in human and mouse. Prog Retin Eye Res. 75:100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. 2003. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 44:2496–2506. [DOI] [PubMed] [Google Scholar]
- Gu H, Song M, Boonanantanasarn K, Baek K, Woo KM, Ryoo HM, Baek JH. 2018. Conditions inducing excessive O-GlcNAcylation inhibit BMP2-induced Osteogenic differentiation of C2C12 cells. Int J Mol Sci. 19:E202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Aranguez A, Woodward AM, Pintor J, Argueso P. 2012. Targeted disruption of core 1 beta1,3-galactosyltransferase (C1galt1) induces apical endocytic trafficking in human corneal keratinocytes. PLoS One. 7:e36628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardyman MA, Wilkinson E, Martin E, Jayasekera NP, Blume C, Swindle EJ, Gozzard N, Holgate ST, Howarth PH, Davies DE, et al. 2013. TNF-alpha-mediated bronchial barrier disruption and regulation by src-family kinase activation. J Allergy Clin Immunol. 132:665–675 e668. [DOI] [PubMed] [Google Scholar]
- Hart GW. 2019. Nutrient regulation of signaling and transcription. J Biol Chem. 294:2211–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Kamemura K. 2015. Global increase in O-linked N-acetylglucosamine modification promotes osteoblast differentiation. Exp Cell Res. 338:194–202. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV. 2017. Diabetic complications in the cornea. Vision Res. 139:138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love DC, Hanover JA. 2005. The hexosamine signaling pathway: Deciphering the "O-GlcNAc code". Sci STKE. 2005:re13. [DOI] [PubMed] [Google Scholar]
- Ma J, Hart GW. 2014. O-GlcNAc profiling: From proteins to proteomes. Clin Proteomics. 11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelli F, Mauris J, Argueso P. 2013. The ocular surface epithelial barrier and other mechanisms of mucosal protection: From allergy to infectious diseases. Curr Opin Allergy Clin Immunol. 13:563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelli F, Schaffer L, Dana R, Head SR, Argueso P. 2009. Glycogene expression in conjunctiva of patients with dry eye: Downregulation of notch signaling. Invest Ophthalmol Vis Sci. 50:2666–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S, Lee YK, Lee SW, Um SJ. 2017. Suppressive role of OGT-mediated O-GlcNAcylation of BAP1 in retinoic acid signaling. Biochem Biophys Res Commun. 492:89–95. [DOI] [PubMed] [Google Scholar]
- Nowell CS, Radtke F. 2017. Corneal epithelial stem cells and their niche at a glance. J Cell Sci. 130:1021–1025. [DOI] [PubMed] [Google Scholar]
- Postnikoff CK, Pintwala R, Williams S, Wright AM, Hileeto D, Gorbet MB. 2014. Development of a curved, stratified, in vitro model to assess ocular biocompatibility. PLoS One. 9:e96448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi R, Iyer SP, Ellies LG, O'Donnell N, Marek KW, Chui D, Hart GW, Marth JD. 2000. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci U S A. 97:5735–5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan B, Kolli AR, Esch MB, Abaci HE, Shuler ML, Hickman JJ. 2015. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 20:107–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Woodward AM, Magnelli P, McColgan NM, Lehoux S, Jacobo SMP, Mauris J, Argueso P. 2017. N-glycosylation affects the stability and barrier function of the MUC16 mucin. J Biol Chem. 292:11079–11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JL, Qin H. 2019. O-GlcNAcylation regulates primary ciliary length by promoting microtubule disassembly. iScience. 12:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres CR, Hart GW. 1984. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 259:3308–3317. [PubMed] [Google Scholar]
- Uchino Y. 2018. The ocular surface glycocalyx and its alteration in dry eye disease: A review. Invest Ophthalmol Vis Sci. 59:DES157–DES162. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan K, Durning S, Wells L. 2014. Functional O-GlcNAc modifications: Implications in molecular regulation and pathophysiology. Crit Rev Biochem Mol Biol. 49:140–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan K, Wells L. 2014. Multiple tissue-specific roles for the O-GlcNAc post-translational modification in the induction of and complications arising from type II diabetes. J Biol Chem. 289:34466–34471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocadlo DJ. 2012. O-GlcNAc processing enzymes: Catalytic mechanisms, substrate specificity, and enzyme regulation. Curr Opin Chem Biol. 16:488–497. [DOI] [PubMed] [Google Scholar]
- Xiong L, Woodward AM, Argueso P. 2011. Notch signaling modulates MUC16 biosynthesis in an in vitro model of human corneal and conjunctival epithelial cell differentiation. Invest Ophthalmol Vis Sci. 52:5641–5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YR, Song M, Lee H, Jeon Y, Choi EJ, Jang HJ, Moon HY, Byun HY, Kim EK, Kim DH, et al. 2012. O-GlcNAcase is essential for embryonic development and maintenance of genomic stability. Aging Cell. 11:439–448. [DOI] [PubMed] [Google Scholar]
- Yoon KC, Jeong IY, Park YG, Yang SY. 2007. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea. 26:431–437. [DOI] [PubMed] [Google Scholar]
- Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, McEachern EJ, Davies GJ, et al. 2008. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 4:483–490. [DOI] [PubMed] [Google Scholar]
- Zachara N, Akimoto Y, Hart GW. 2015. The O-GlcNAc Modificationrd. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, et al., editors. Essentials of Glycobiology. New York (USA): Cold Spring Harbor. p. 239–251. [Google Scholar]
- Zhang Z, Parker MP, Graw S, Novikova LV, Fedosyuk H, Fontes JD, Koestler DC, Peterson KR, Slawson C. 2019. O-GlcNAc homeostasis contributes to cell fate decisions during hematopoiesis. J Biol Chem. 294:1363–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Xiong X, Ren K, Xu B, Cheng M, Sahu C, Wu K, Nie Y, Huang Z, Blumberg RS, et al. 2018. Deficiency in intestinal epithelial O-GlcNAcylation predisposes to gut inflammation. EMBO Mol Med. 10:e8736. [DOI] [PMC free article] [PubMed] [Google Scholar]


