Key Points
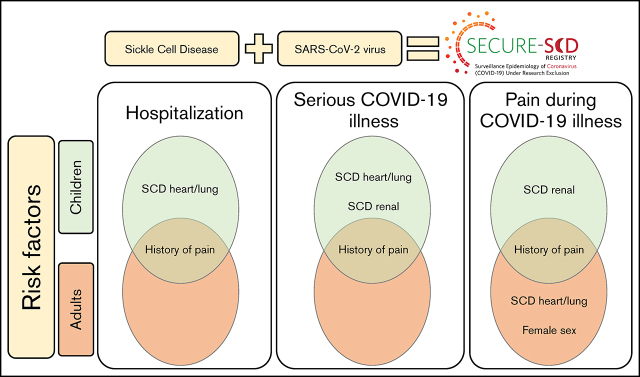
Children with SCD with history of pain and renal and heart/lung comorbidities are at higher risk of worse COVID-19 outcomes.
Adults with SCD with history of pain are at higher risk of worse COVID-19 outcomes.
Visual Abstract
Abstract
Patients with sickle cell disease (SCD) are at high risk of developing serious infections, therefore, understanding the impact that severe acute respiratory syndrome coronavirus 2 infection has on this population is important. We sought to identify factors associated with hospitalization and serious COVID-19 illness in children and adults with SCD.We established the international SECURE-SCD Registry to collect data on patients with SCD and COVID-19 illness. We used multivariable logistic models to estimate the independent effects of age, sex, genotype, hydroxyurea, and SCD-related and -nonrelated comorbidities on hospitalization, serious COVID-19 illness, and pain as a presenting symptom during COVID-19 illness. As of 23 March 2021, 750 COVID-19 illness cases in patients with SCD were reported to the registry. We identified history of pain (relative risk [RR], 2.15; P < .0001) and SCD heart/lung comorbidities (RR, 1.61; P = .0001) as risk factors for hospitalization in children. History of pain (RR, 1.78; P = .002) was also a risk factor for hospitalization in adults. Children with history of pain (RR, 3.09; P = .009), SCD heart/lung comorbidities (RR, 1.76; P = .03), and SCD renal comorbidities (RR, 3.67; P < .0001) and adults with history of pain (RR 1.94, P = .02) were at higher risk of developing serious COVID-19 illness. History of pain and SCD renal comorbidities also increased risk of pain during COVID-19 in children; history of pain, SCD heart/lung comorbidities, and female sex increased risk of pain during COVID-19 in adults. Hydroxyurea showed no effect on hospitalization and COVID-19 severity, but it lowered the risk of presenting with pain in adults during COVID-19.
Introduction
The COVID-19 pandemic has resulted in significant morbidity and mortality across the world. As of 13 April 2021, there were 135 million confirmed cases and 2.9 million deaths due to COVID-19 illness per the World Health Organization.1 Growing evidence has shown that in the general population,individuals hospitalized due to COVID-19 illness have a higher prevalence of comorbidities compared with individuals with COVID-19 illness who did not require hospitalization.2 Presence of hypertension, diabetes, cardiovascular disease, chronic obstructive pulmonary disease (COPD), cerebrovascular disease, chronic kidney disease, obesity, and malignancy are all associated with serious health outcomes during COVID-19 illness, including death, intensive care unit (ICU) admission, and need for mechanical ventilation.3
Sickle cell disease (SCD) is an inherited hemoglobinopathy that mostly occurs among persons of African ancestry.4 SCD affects nearly every organ system and is associated with early death, with average life expectancy estimates of ∽50 years.5 Patients with SCD are known to have increased risk of death due to pneumococcal sepsis from splenic impairment and have a higher risk of complications from influenza illness.6,7 Prior studies also suggest that patients with SCD have a higher prevalence of viral infections and are more likely to need acute health care due to these infections compared with the general population.8 Infection in patients with SCD can also lead to exacerbation of other SCD complications such as pain and acute chest syndrome.9
Due to the current COVID-19 pandemic and the potential for future infectious disease epidemics, it is important to understand the impact that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has on this medically vulnerable population. Recognizing the need to acquire rapid knowledge on this new infection to inform care, we established a registry for SCD patients with COVID-19 illness. This registry allowed us to show that although the majority of reported cases in the registry experienced only mild COVID-19 illness, patients with SCD were at increased risk of worse outcomes and had a higher case-fatality rate compared with the general population.10 This work led us to further seek to understand why some patients with SCD are at increased risk of worse outcomes from COVID-19 illness as compared with others who have a milder COVID-19 illness course. Therefore, the primary objective of this study was to identify the factors associated with hospitalization and serious COVID-19 illness in patients with SCD. We hypothesized that SCD-related comorbidities would be associated with hospitalization and serious COVID-19 illness. We also hypothesized that patients taking hydroxyurea would be less likely to be hospitalized and/or experience serious COVID-19 illness. Our secondary objective was to examine the relationship between COVID-19 illness and pain in patients with SCD. We hypothesized that a history of pain would increase the likelihood of presenting with pain as a symptom during COVID-19 illness. We also hypothesized that patients who use hydroxyurea are less likely to present with pain as a symptom during COVID-19 illness.
Methods
Data collection
We established the Surveillance Epidemiology of Coronavirus Under Research Exclusion for Sickle Cell Disease (SECURE-SCD) Registry (covidsicklecell.org) to collect data on COVID-19 illness in patients with SCD in near real time.
Health care providers were asked to report only confirmed COVID-19 cases in patients with SCD after sufficient time had passed to observe the clinical course of the infection. Provider-completed questionnaires collected the following information: (1) reporter information (name, address, institution, and physician providing care), (2) patient demographics (age, country, sex, race, ethnicity, weight, genotype), (4) medical history (home SCD medications, SCD-related comorbidities, other comorbidities), and (5) COVID-19–related information (severity, presenting symptoms and complications, treatment, health care utilization, death, resolution). The full questionnaire can be found in the supplemental Material questionnaire file. Data are collected in a REDCap form that can be accessed through our registry Web site (covidsicklecell.org). REDCap is a secure, Web-based electronic data capture tool hosted at the Medical College of Wisconsin.11,12 We screened for potential duplicate records based on matching institution, age, sex, and other clinical variables. Duplicates were removed if identified. Reports from invalid e-mail addresses were also removed if we were not able to confirm legitimacy of reports upon further evaluation. We publish aggregated data weekly on our registry Web site to share our findings and promote further inclusion. Power BI is used to create visuals shared on the Web site.
The registry contains only deidentified data, in accordance with Health Insurance Portability and Accountability Act (HIPAA) Safe Harbor De-Identification standards. The Medical College of Wisconsin Human Research Protection Program has determined that storage and analysis of deidentified data does not constitute human subjects research as defined under federal regulations13 and does not require Institutional Review Board (IRB) approval. Providers interested in contributing cases to the registry were advised to consult with their local IRB and abide by their recommendations on whether institution-specific IRB approval was needed.
Study population
Children and adults with SCD and COVID-19 who were reported in SECURE-SCD from 20 March 2020 through 23 March 2021 were included in the analysis. Cases include international data from institutions in Belgium, Brazil, Canada, France, French Guiana, Greece, Guadeloupe, Lebanon, Nigeria, Oman, Saint Martin (French part), Sweden, Switzerland, and the United States. We excluded cases that were missing required data for confirmation of legitimacy of reports (name, e-mail address, and institution of the reporter and provider).
Study outcomes
Our primary outcomes were hospitalization and serious COVID-19 illness. The severity of COVID-19 illness was determined based on clinical manifestations during the infection using the established criteria outlined below14:
-
1.
Asymptomatic: no clinical signs or symptoms during the positive COVID-19 period;
-
2.
Mild: symptoms of acute upper respiratory tract infection, including fever, fatigue, myalgia, cough, sore throat, runny nose, and sneezing or gastrointestinal symptoms or digestive symptoms such as nausea, vomiting, abdominal pain, and diarrhea;
-
3.
Moderate: pneumonia with or without clinical symptoms, no hypoxia;
-
4.
Severe: early respiratory symptoms or gastrointestinal symptoms followed by dyspnea and hypoxia (o2 saturations <92%);
-
5.
Critical: acute respiratory distress syndrome, respiratory failure, encephalopathy, shock, coagulopathy, multiorgan impairment (lung, heart, kidney, brain) that may be life threatening.
For the analyses, we categorized the COVID-19 illness as being serious if a patient’s COVID-19 severity was categorized as moderate, severe, or critical.
Pain as a presenting symptom of COVID-19 illness was investigated as a secondary outcome of interest. We defined pain as a presenting symptom if the reported case included pain as 1 or more of the symptoms at the time of COVID-19 illness.
Covariates of interest
Covariates of interest included age, sex, SCD genotype, hydroxyurea use, SCD-related comorbidities, and non-SCD comorbidities. Comorbidities were grouped according to organ systems, similarity in clinical presentation, and/or their causal relationship. SCD-related comorbidities for children and adults were combined into the following categories: (1) prior acute care visit for pain (hospitalization and/or emergency department [ED] treat-and-release visit requiring IV opioids for pain in the last 3 years), (2) brain (overt stroke, silent stroke, known central nervous system vasculopathy and/or abnormal transcranial Doppler result), (3) renal (albuminuria, decreased renal function and/or SCD nephropathy), and (4) heart/lung (acute chest syndrome in the last 3 years and/or pulmonary hypertension). Prior acute care visits for pain were considered frequent if they occurred >2 times in the last 3 years.
Non-SCD–related comorbidities in children were combined into the following categories: (1) neurobehavioral (anxiety, depression, and/or behavioral problems, attention deficit/hyperactivity disorder, developmental delay or learning disability, headaches, seizure disorder, and/or sleep disturbances) and (2) lung (asthma, current cigarette smoker, current user of other tobacco products other than cigarettes).
Non-SCD–related comorbidities in adults were combined into the following categories: (1) heart (cardiovascular disease including coronary artery disease, heart failure, arrhythmia, etc, and/or hypertension) and (2) lung comorbidities (asthma, COPD, other chronic lung disease, current cigarette smoker, and/or current user of other tobacco products other than cigarettes).
Statistical analysis
Categorical variables were summarized as number (percentage) and continuous variables as mean (standard deviation). We used χ2 or Fisher exact tests to compare those who: (1) required hospitalization vs not, (2) had serious COVID-19 illness vs less serious, and (3) experienced pain as a presenting COVID-19 symptom vs not. Generalized linear models with log link were used (binomial distribution for a binary outcome) to obtain relative risk (RR) with 95% confidence intervals (CIs). Univariate and multivariable models calculated for the RR were calculated with covariates: age, sex, SCD genotype, hydroxyurea, and SCD-related and -nonrelated comorbidities on outcomes of hospitalization, serious COVID-19 illness, and pain as a presenting symptom during COVID-19 illness. We also performed a subanalysis of the adult group that included patients from 19 to 55 years of age. Hydroxyurea was forced into models because of our a priori hypothesis. Statistical significance was set at a P value of .05. All analyses were conducted with SPSS software (version 24.0; IBM Corporation, Armonk, NY).
Results
Study population
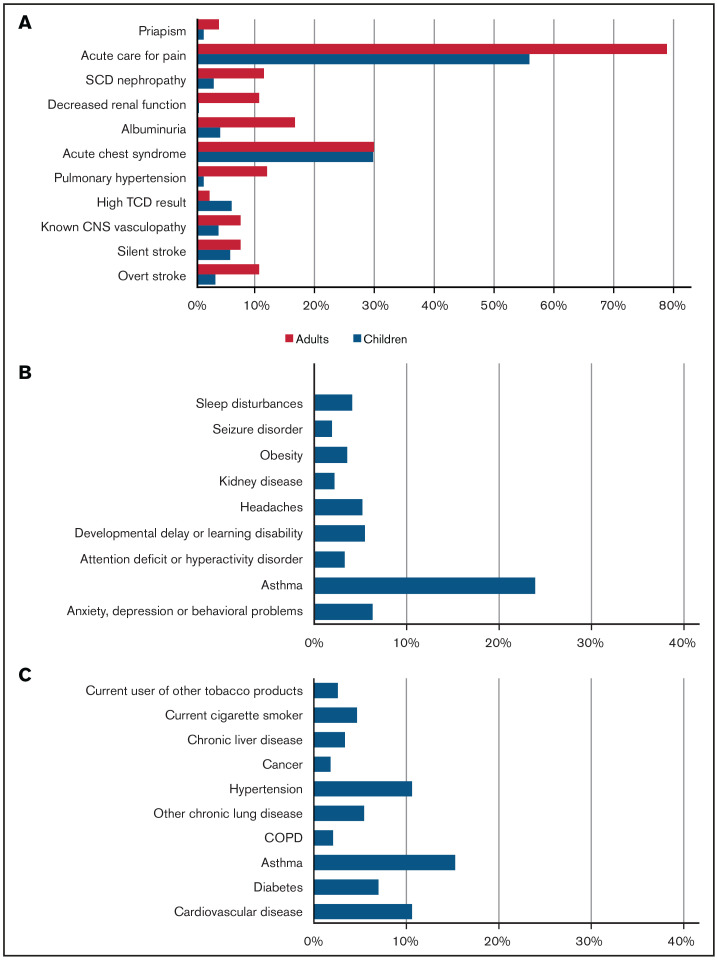
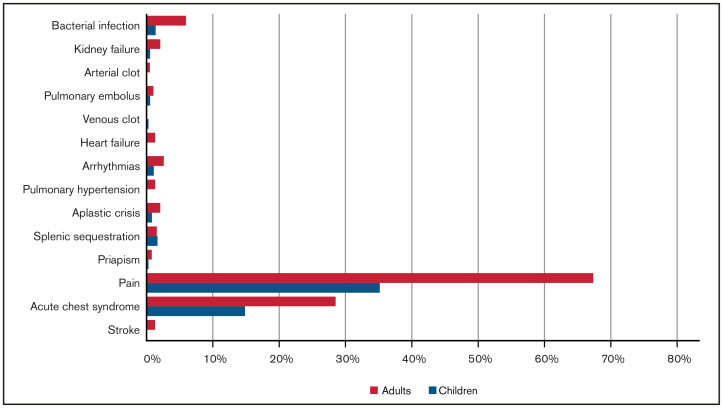
From 20 March 2020 to 23 March 2021, 750 cases were reported to SECURE-SCD (Table 1). In the majority of cases (∼90%), patients were Black; ∼7% were Hispanic or Latino. Of reported cases, 48.5% of patients were children (0-18 years) with a median age of 11 years (interquartile range [IQR], 6-15 years); 51.5% of patients were adults (>18 years) with a median age of 31 years (IQR, 24-40 years). Approximately one-half of children and adults were reported to be taking hydroxyurea. There was 1 death reported in children and 18 deaths in adults, making the overall death rate 2.5%. The most common SCD-related comorbidity in both children and adults was prior acute care visit for pain (55.5% of children, 78.5% of adults) (Figure 1). Acute chest syndrome in the previous 3 years was reported in approximately one-third of cases (29.4% of children, 29.5% of adults). The most common non-SCD comorbidity was asthma in both groups (23.9% of children, 15.3% of adults), followed by anxiety, depression, and/or behavioral problems in children (6.3%) and cardiovascular disease and hypertension in adults (10.6%). Pain was the most common presenting symptom during COVID-19 illness in both children and adults (35.2% of children, 67.4% of adults), followed by acute chest syndrome (14.8% of children, 28.5% of adults) (Figure 2). A small portion of individuals (17.4% of children and 35.2% of adults) without pain episodes in the last 3 years presented with pain for the first time during their COVID-19 illness.
Table 1.
Demographics and clinical characteristics of cases reported in SECURE-SCD from 20 March 2020 to 23 March 2021
| Variable | Children, 0-18 y | Adults, >18 y |
|---|---|---|
| Total, N (%) | 364 (48.5) | 386 (51.5) |
| Demographics | ||
| Median age (IQR), y | 11 (6-15) | 31 (24-40) |
| Sex, N (%) | ||
| Female | 176 (48.4) | 220 (57.0) |
| Male | 187 (51.4) | 159 (41.2) |
| Race, N (%) | ||
| Black | 330 (90.7) | 324 (83.9) |
| Ethnicity, N (%) | ||
| Hispanic/Latino | 20 (5.5) | 35 (9.1) |
| Genotype, N (%) | ||
| HbSS/HbSβ0 thalassemia | 263 (72.3) | 261 (67.6) |
| HbSC/HbSβ+ thalassemia | 98 (26.9) | 113 (29.3) |
| SCD-related therapy, N (%) | ||
| Hydroxyurea | 203 (55.8) | 191 (49.5) |
| Penicillin | 126 (34.6) | 21 (5.4) |
| Crizanlizumab | 10 (2.7) | 13 (3.4) |
| Voxelotor | 5 (1.4) | 16 (4.1) |
| Glutamine | 10 (2.7) | 14 (3.6) |
| Chronic RBC transfusion | 39 (10.7) | 53 (13.7) |
| COVID-19 severity,* N (%) | ||
| Asymptomatic | 93 (25.5) | 40 (10.4) |
| Mild | 205 (56.3) | 212 (54.9) |
| Moderate | 34 (9.3) | 67 (17.4) |
| Severe | 26 (7.1) | 55 (14.2) |
| Critical | 4 (1.1) | 11 (2.8) |
| COVID-19 outcomes, N (%) | ||
| ED visit | 215 (59.1) | 307 (79.5) |
| Hospitalization | 146 (40.1) | 231 (59.8) |
| ICU admission | 21 (5.8) | 34 (8.8) |
| Ventilator use | 4 (1.1) | 14 (3.6) |
| Death | 1 (0.3) | 18 (4.7) |
RBC, red blood cell.
*Asymptomatic: no clinical signs or symptoms during the positive COVID-19 period. Mild: symptoms of acute upper respiratory tract infection, including fever, fatigue, myalgia, cough, sore throat, runny nose, and sneezing or gastrointestinal symptoms or digestive symptoms such as nausea, vomiting, abdominal pain, and diarrhea. Moderate: pneumonia with or without clinical symptoms, no hypoxia. Severe: early respiratory symptoms or gastrointestinal symptoms followed by dyspnea and hypoxia (o2 saturations <92%). Critical: acute respiratory distress syndrome, respiratory failure, encephalopathy, shock, coagulopathy, multiorgan impairment (lung, heart, kidney, brain) that may be life-threatening.
Figure 1.
Prevalence of comorbidities in cases reported in SECURE-SCD from 20 March 2020 to 23 March 2021. (A) SCD-related comorbidities in children and adults. (B) Non-SCD comorbidities in children. (C) Non-SCD comorbidities in adults.
Figure 2.
Presenting symptoms and complications during COVID-19 illness.
Hospitalization during COVID-19 illness was more likely to occur in children and adults with SCD-related comorbidities
After adjusting for age, sex, genotype, hydroxyurea use, and comorbidities, multivariable analysis showed that hospitalization during COVID-19 illness was more likely to occur in children if they had frequent prior acute care visits for pain (2.15 times more likely) or SCD-related heart and lung comorbidities (1.61 times more likely). In adults, hospitalization during COVID-19 illness was 1.78 times more likely to occur if they had frequent prior acute care visits for pain (Table 2). Findings were the same in the adult subgroup of patients who were 19 to 55 years of age. Use of hydroxyurea did not show a significant effect on hospitalization during COVID-19 in children or adults. None of the other listed covariates were significant.
Table 2.
Multivariable model showing risk factors associated with hospitalization in children and adults
| Variable | Hospitalization | ||
|---|---|---|---|
| RR | 95% CI | P | |
| Children | |||
| Hydroxyurea | 0.92 | 0.73-1.15 | .45 |
| Prior acute care visits for pain,* >2 vs 0 | 2.15 | 1.69-3.44 | <.0001 |
| SCD heart/lung† | 1.61 | 1.26-2.04 | .0001 |
| Adults | |||
| Hydroxyurea | 0.92 | 0.77-1.10 | .35 |
| Prior acute care visits for pain,* >2 vs 0 | 1.78 | 1.24-2.57 | .002 |
P < .05 was considered significant.
*Hospitalization and/or ED treat-and-release visit requiring IV opioids for pain in the last 3 years.
†Acute chest syndrome in the last 3 years and/or pulmonary hypertension.
None of the other covariates were significant (SCD brain, SCD renal, non-SCD neurobehavioral, non-SCD lung, non-SCD heart comorbidities).
Serious COVID-19 illness was more likely to occur in children and adults with SCD-related comorbidities
Serious COVID-19 illness was more likely to occur in children with frequent prior acute care visits for pain (3.09 times more likely), SCD-related heart and lung comorbidities (1.76 times more likely), or SCD-related renal comorbidities (3.67 times more likely). In adults, serious COVID-19 illness was 1.94 times more likely to occur in patients with frequent prior acute care visits for pain (Table 3). Findings were the same in the subgroup of adults who were 19 to 55 years of age. Use of hydroxyurea did not show a significant effect on severity of COVID-19 in children or adults. None of the other listed covariates were significant.
Table 3.
Multivariable model showing risk factors associated with serious COVID-19 illness in children and adults
| Variable | Serious COVID-19 illness | ||
|---|---|---|---|
| RR | 95% CI | P | |
| Children | |||
| Hydroxyurea | 0.91 | 0.56-1.47 | .69 |
| Prior acute care visits for pain,* >2 vs 0 | 3.09 | 1.59-6.03 | .0009 |
| SCD renal† | 3.67 | 1.93-6.96 | <.0001 |
| SCD heart/lung‡ | 1.76 | 1.06-2.92 | .03 |
| Adults | |||
| Age | 1.04 | 1.01-1.06 | .002 |
| Hydroxyurea | 0.85 | 0.49-1.51 | .59 |
| Prior acute care visits for pain,* >2 vs 0 | 1.94 | 1.11-3.40 | .02 |
P < .05 was considered significant.
*Hospitalization and/or ED treat-and-release visit requiring IV opioids for pain in the last 3 years.
†Albuminuria, decreased renal function, and/or SCD nephropathy.
‡Acute chest syndrome in the last 3 years and/or pulmonary hypertension.
None of the other covariates were significant (SCD brain, non-SCD neurobehavioral, non-SCD lung, non-SCD heart comorbidities).
Children and adults with SCD-related comorbidities are more likely to present with pain during COVID-19 illness
In multivariable analysis, controlling for age, sex, SCD genotype, hydroxyurea use, and comorbidities, pain during COVID-19 illness was more likely to occur in children with frequent prior acute care visits for pain (6.69 times more likely) and in children with SCD-related renal comorbidities (1.5 times more likely). Furthermore, controlling for the same variables, pain during COVID-19 illness was more likely to occur in adults with frequent prior acute care visits for pain (2.19 times more likely) or SCD-related heart/lung comorbidities (1.12 times more likely). In adults, male patients had a lower risk (RR, 0.86; P = .04) of presenting with pain during COVID-19; however, in the subgroup of adults who were 19 to 55 years of age, this finding was not significant (Table 4). Use of hydroxyurea lowered the risk of presenting with pain during COVID-19 illness in adults (RR, 0.86; P = .02). None of the other listed covariates were significant.
Table 4.
Multivariable model showing risk factors associated with pain as presenting symptom during COVID-19 in children and adults
| Variable | Pain during COVID-19 | |||
|---|---|---|---|---|
| RR | 95% CI | P | ||
| Children | ||||
| Hydroxyurea | 0.96 | 0.73-1.26 | .76 | |
| Prior acute care visits for pain* | ||||
| 1-2 vs 0 | 4.46 | 2.48-8.02 | <.0001 | |
| >2 vs 0 | 6.69 | 3.88-11.54 | <.0001 | |
| SCD renal† | 1.50 | 1.10-2.05 | .0104 | |
| Adults | ||||
| Sex, male vs female | 0.86 | 0.75-0.99 | .04 | |
| Hydroxyurea | 0.86 | 0.76-0.98 | .02 | |
| Prior acute care visits for pain* | ||||
| 1-2 vs 0 | 1.92 | 1.28-2.88 | .002 | |
| >2 vs 0 | 2.19 | 1.51-3.16 | <.0001 | |
| SCD heart/lung‡ | 1.12 | 1.001-1.26 | .048 | |
P < .05 was considered significant
*Hospitalization and/or ED treat-and-release visit requiring IV opioids for pain in the last 3 years.
†Albuminuria, decreased renal function, and/or SCD nephropathy.
‡Acute chest syndrome in the last 3 years and/or pulmonary hypertension.
None of the other covariates were significant (SCD brain, non-SCD neurobehavioral, non-SCD lung, non-SCD heart comorbidities).
Discussion
Our data identified distinct risk factors for worse COVID-19 illness among children and adults with SCD. In children with SCD, we identified prior acute care visits for pain and SCD-related heart and lung comorbidities as risk factors for hospitalization during COVID-19 illness. Both of these, as well as SCD-related renal comorbidities were also identified as risk factors for serious COVID-19 illness in children. In adults with SCD, we identified prior acute care visits for pain as a risk factor for both hospitalization and serious COVID-19 illness.
Our study fills a gap regarding the limited knowledge of patient characteristics that can make a patient with SCD and COVID-19 more likely to be hospitalized and have serious COVID-19 illness. One prior study utilizing data from the research network TriNetX, which included 312 cases of COVID-19 in patients with SCD, suggests that patients with SCD are at increased risk of hospitalization compared with individuals without SCD, matched according to demographic and clinical characteristics.15 Prevalence of comorbidities, hospitalization, and mortality rates are similar to findings in our study. Another prior published study addresses clinical predictors of poor COVID-19 outcome in patients with SCD.16 This study included patients from the early period of the pandemic and only included 9 pediatric patients. Given the distinction between the clinical characteristics and comorbidities of adult and pediatric patients with SCD, we sought to identify age-related risk factors for the 2 different populations. Furthermore, this same study identified chronic kidney disease as a risk factor for hospitalization. Although our study did not find a relationship with hospitalization, SCD-related renal disease did increase the risk of serious COVID-19 illness in children only. Utilizing a large sample size, our study identified other risk factors using multivariable analyses that allowed us to adjust for more than just age.
Acute chest syndrome and pain are known markers for increased morbidity and mortality in patients with SCD.17 Although few published case series exist that describe outcomes of hospitalized patients with history of both acute chest syndrome and pain, to our knowledge, this is the first study that identifies pain as an independent risk factor for hospitalization and serious COVID-19 illness in both children and adults, and acute chest syndrome as a risk factor for both hospitalization and serious COVID-19 illness in children.16,18-25
We found that prior acute care visits for pain were significantly associated with increased risk of presenting with pain during COVID-19 illness in both children and adults. In addition, in some of our cases, patients without pain episodes in the last 3 years presented with pain for the first time during their COVID-19 illness, which suggests that COVID-19 could potentially trigger pain in patients with SCD. Although it is unknown whether SCD acute pain would occur in these patients in the absence of COVID-19, potential mechanisms support the concept that viral infection could precipitate acute SCD pain episodes.26,27 First, the ACE2 protein that serves as the receptor for SARS-CoV-2, the causative agent of COVID-19, is found in human dorsal root ganglion. It is possible that SARS-CoV-2 enters the nervous system through ACE2-expressing nociceptors in skin and mucosa, causing sensory neuronal infection and neuropathic pain.26 Furthermore, although SCD is recognized as a chronic inflammatory disease, infection triggers an acute-phase reaction, activates endothelium, and generates reactive oxygen species, further exacerbating inflammation. Additionally, any infection that targets the lungs, such as with COVID-19, can lead to decreased oxygenation and consequential peripheral blood hypoxia that promotes sickling, vaso-occlusive episodes, and pain.27 The odds of male patients presenting with pain are lower, likely due to the small number of cases. Our data suggest that patients with SCD who present with pain should be tested for COVID-19 because pain was the most common, and often only, symptom of COVID-19 in patients with SCD reported to our registry.
Our data suggest that hydroxyurea lowers the risk of presenting with pain during COVID-19 illness in adults; however, we did not find differences in COVID-19 illness severity and need for hospitalization in patients who were taking hydroxyurea as their disease-modifying therapy compared with those who were not. Previous studies on hydroxyurea and COVID-19 provide only descriptive results whereas our study also adjusts for other confounding factors such as age, sex, genotype, and medical history. A French study that included 83 hospitalized patients with SCD and COVID-19 illness describes no differences in proportions of ICU admissions between patients who received hydroxyurea compared with those who did not.19 A more recent study describes significant differences in hydroxyurea use between patients who died and those who survived (none of the patients who died were taking hydroxyurea) during the early phase of the pandemic; however, the authors raise caution in interpreting their finding due to possible confounding factors such as SCD genotype and contraindications for using the drug.16 Our findings implicate that, although hydroxyurea does not show a protective effect on COVID-19 outcomes besides presenting with pain, there is also no reason for discontinuation of therapy during COVID-19 illness.
Our study has some important limitations. Given the voluntary reporting system of the registry, it is possible that our study is impacted by reporting bias; there may be overrepresentation of severe cases and underrepresentation of cases from areas where testing is not available. In addition, we do not have information on date of testing and type of testing so we could not conduct time-trend analysis. We also did not look at death as an outcome due to the low number of death cases. Detailed laboratory data in our cases would allow for a more complete understanding of the pathophysiology underlying COVID-19 infections in individuals with SCD. However, to enable quick and real-time reporting, we did not include these data in our questionnaire. Also, we did not gather information on socioeconomic factors and other social determinants of health due to risk of case identifiability.
In conclusion, our findings show that history of pain, SCD-related heart and lung comorbidities, and SCD-related renal comorbidities are risk factors for worse outcomes of COVID-19 illness in children; history of pain is a risk factor in adults. We show that hydroxyurea does not influence hospitalization or severity of COVID-19 illness in patients with SCD, but it lowers the risk of presenting with pain in adults. COVID-19 illness might be triggering pain episodes in this population. Our data add valuable information to existing literature and emphasize the importance of further research in this vulnerable population to provide optimal clinical care for those with COVID-19 and to provide a better understanding for potential future infectious disease epidemics.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The SECURE-SCD Registry would not be possible without the contribution of reporters worldwide. The authors acknowledge the institutions and individuals for taking the time to contribute their case reports to the Registry. A full list of contributing reporters and institutions is available at covidsicklecell.org.
This work was supported by Doris Duke Charitable Foundation grant 2020079 and National Institutes of Health National Center for Advancing Translational Sciences award number UL1TR001436.
The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: L.M., A.M.B., A.S., and J.A.P. designed the project; S.F.M., B.W.T., K.J.W., and F.I.Y. helped with collecting data; M.D. and P.M.S. performed statistical analysis; L.M., A.M.B., and J.A.P. wrote the first version of manuscript; and L.M., A.M.B., M.D., S.F.M., P.M.S., A.S., B.W.T., K.J.W., F.I.Y., and J.A.P. edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.World Health Organization. Weekly epidemiological update on COVID-19 - 13 April 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---13-april-2021. Accessed 13 April 2021.
- 2.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC.. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782-793. [DOI] [PubMed] [Google Scholar]
- 3.Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY). 2020;12(13):12493-12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(suppl 4):S512-S521. [DOI] [PubMed] [Google Scholar]
- 5.Lubeck D, Agodoa I, Bhakta N, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw Open. 2019;2(11):e1915374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oligbu G, Fallaha M, Pay L, Ladhani S.. Risk of invasive pneumococcal disease in children with sickle cell disease in the era of conjugate vaccines: a systematic review of the literature. Br J Haematol. 2019;185(4):743-751. [DOI] [PubMed] [Google Scholar]
- 7.Strouse JJ, Reller ME, Bundy DG, et al. Severe pandemic H1N1 and seasonal influenza in children and young adults with sickle cell disease. Blood. 2010;116(18):3431-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reeves SL, Jary HK, Gondhi JP, Kleyn M, Dombkowski KJ.. Health outcomes and services in children with sickle cell trait, sickle cell anemia, and normal hemoglobin. Blood Adv. 2019;3(10):1574-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannas G, Merazga S, Virot E.. Sickle cell disease and infections in high- and low-income countries. Mediterr J Hematol Infect Dis. 2019;11(1):e2019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panepinto JA, Brandow A, Mucalo L, et al. Coronavirus disease among persons with sickle cell disease, United States, March 20-May 21, 2020. Emerg Infect Dis. 2020;26(10):2473-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services. Title 45: Public Welfare. Part 46—Protection of Human Subjects. §46.102. Definitions for Purposes of this Policy. Washington, DC: Office for Human Research Protections. 45 CFR 46.102. [Google Scholar]
- 14.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. [DOI] [PubMed] [Google Scholar]
- 15.Singh A, Brandow AM, Panepinto JA.. COVID-19 in individuals with sickle cell disease/trait compared with other Black individuals. Blood Adv. 2021;5(7):1915-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minniti CP, Zaidi AU, Nouraie M, et al. Clinical predictors of poor outcomes in patients with sickle cell disease and COVID-19 infection. Blood Adv. 2021;5(1):207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639-1644. [DOI] [PubMed] [Google Scholar]
- 18.Hussain FA, Njoku FU, Saraf SL, Molokie RE, Gordeuk VR, Han J.. COVID-19 infection in patients with sickle cell disease. Br J Haematol. 2020;189(5):851-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arlet J-B, de Luna G, Khimoud D, et al. Prognosis of patients with sickle cell disease and COVID-19: a French experience. Lancet Haematol. 2020;7(9):e632-e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakravorty S, Padmore-Payne G, Ike F, et al. COVID-19 in patients with sickle cell disease - a case series from a UK tertiary hospital. Haematologica. 2020;105(11):2691-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Telfer P, De la Fuente J, Sohal M, et al. Real-time national survey of COVID-19 in hemoglobinopathy and rare inherited anemia patients. Haematologica. 2020;105(11):2651-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AbdulRahman A, AlAli S, Yaghi O, et al. COVID-19 and sickle cell disease in Bahrain. Int J Infect Dis. 2020;101:14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appiah-Kubi A, Acharya S, Fein Levy C, et al. Varying presentations and favourable outcomes of COVID-19 infection in children and young adults with sickle cell disease: an additional case series with comparisons to published cases. Br J Haematol. 2020;190(4):e221-e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balanchivadze N, Kudirka AA, Askar S, et al. Impact of COVID-19 infection on 24 patients with sickle cell disease. One Center Urban Experience, Detroit, MI, USA. Hemoglobin. 2020;44(4):284-289. [DOI] [PubMed] [Google Scholar]
- 25.Ramachandran P, Perisetti A, Kathirvelu B, et al. Low morbidity and mortality with COVID-19 in sickle cell disease: a single center experience. eJHaem. 2020;1(2):608-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiers S, Ray PR, Wangzhou A, et al. ACE2 and SCARF expression in human dorsal root ganglion nociceptors: implications for SARS-CoV-2 virus neurological effects. Pain. 2020;161(11):2494-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed SG. The role of infection in the pathogenesis of vaso-occlusive crisis in patients with sickle cell disease. Mediterr J Hematol Infect Dis. 2011;3(1):e2011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.