Designed and managed with the support of patient and public representatives, the RATE-AF trial is the first head-to-head randomized trial of digoxin vs. beta-blockers in patients with atrial fibrillation and symptoms of heart failure
The RAte control Therapy Evaluation in permanent Atrial Fibrillation (RATE-AF) trial was designed as a pragmatic, healthcare-embedded clinical trial to address the concerns of patients and improve quality of life.1 With the main results recently published in JAMA,2 RATE-AF has demonstrated how patient and public involvement (PPI) in trial design and management can provide new opportunities and generate a robust evidence-base to guide routine clinical management.
Evidence for a patient and public involvement approach
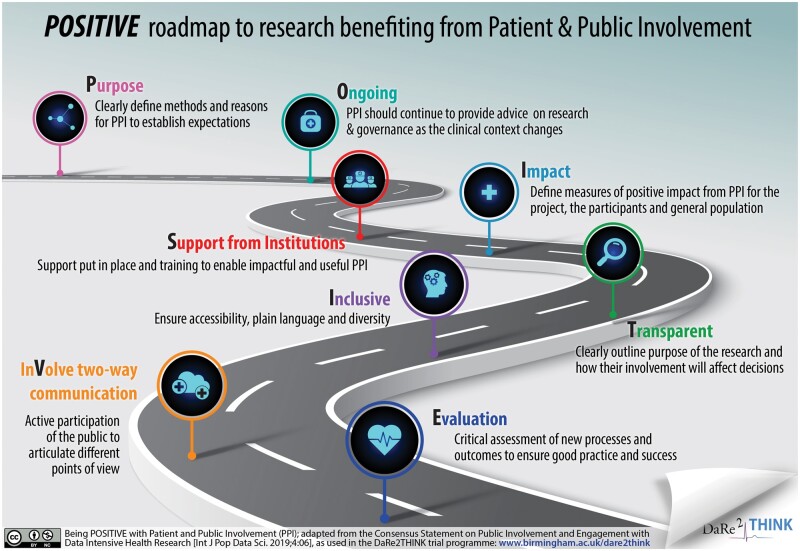
‘Research being carried out “with” or “by” members of the public (including patients and carers) rather than “to,” “about” or “for” them’ is the definition of patient and public involvement (www.invo.org.uk). Using PPI in trial development and management has the potential to augment patient recruitment in clinical trials, addressing the fact that one-third of clinical trials fail to reach their recruitment target. In a meta-analysis of 19 studies including a total of 178 921 participants, 11 out of 21 PPI interventions increased enrolment rates. In the seven randomized trials, the odds ratio (OR) of a patient enrolling was 1.16 compared to no PPI intervention (95% prediction interval 1.25–2.80; P = 0.04 with no heterogeneity).3 Across all study types, an even more substantial improvement in recruitment was demonstrated if the PPI member had the health condition of interest (OR 3.14, 1.89–5.22; vs. 1.07, 0.74–1.53 without the condition).3 Although the authors found no evidence of significant publication bias, data were insufficient to address whether participant retention was improved. Nevertheless, this adds to growing information that PPI, if properly supported (see Figure 1) can assist in the deployment of a clinical trial, ethical approval, avoidance of protocol amendments, enhanced participant adherence, and lead to reduced cost.4 Patient and public involvement is of particular importance in data-intensive healthcare research, where informed consent and privacy are fundamental to good governance and acceptance by potential participants.5 The ESC is actively involved in this critical area of big data research (www.escardio.org/bigdata). Finally, PPI provides a clear route to embedding the patient voice into clinical research, leading to benefit for all stakeholders.
Figure 1.
Being POSITIVE with Patient and Public Involvement.
The RATE-AF trial
The RATE-AF trial was a prospective, randomized, open-label, blinded endpoint trial addressing a major evidence gap in the management of AF, the issue of heart rate control.1 Previous studies were either observational (ignoring the fact that digoxin is often used as a second-line drug and therefore given to sicker patients6) or only demonstrating short-term outcomes such as an acute change in heart rate. In contrast, the RATE-AF trial randomized patients to either low-dose digoxin or beta-blockers, with outcomes at 6 and 12 months.1
One hundred and sixty patients aged 60 years or older with permanent AF and at least New York Heart Association Class II dyspnoea were recruited to the trial, with key findings summarized in Figure 2. There was no difference in the primary outcome of the physical component of quality of life, no difference in long-term heart rate control, and no deterioration in left ventricular ejection fraction comparing low-dose digoxin with beta-blockers.
Figure 2.
Key findings from the RATE-AF trial.
Patients randomized to the digoxin group had significantly better improvement in modified European Heart Rhythm Association (mEHRA) functional class, with 53% reporting a two-class improvement with digoxin at 6 months vs. 9% for beta-blockers (P < 0.001). A significant reduction in NT-pro-B-type natriuretic peptide at 12-months was seen in the digoxin group (P = 0.005 versus beta-blockers), with substantially less adverse events with digoxin compared to beta-blockers [29 events (25% of patients) vs. 142 events (64% of patients); P < 0.001]. Many of the patient-reported elements of general and treatment-specific quality of life also favoured the group randomized to digoxin.
Evolution of RATE-AF with patient and public involvement
The PPI team made considerable input to the design of the trial; as advocates for patients with AF, they impressed the importance of patient well-being as a priority, establishing the primary outcome as the patient-reported quality of life. The PPI team enhanced recruitment, for example, by producing a YouTube participant video, and boosted retention of participants [only three patients (1.9%) in the trial withdrew]. They also led the development of all patient-facing material, enabling clear and explanatory consent forms and information leaflets. This allowed participants to make truly informed consent and improved their adherence to study medications. Plain English summaries of the main results have led to dissemination to a wider audience, particularly for patients with AF in the community and their general practitioners. As members of the Trial Steering Committee, PPI input was essential for the progress of the trial, such as strategies to accelerate recruitment and the development of sub-studies. This included embedded studies on (i) wearable devices, where the PPI team helped to implement technology despite the older population being recruited; (ii) basic science experiments on cellular and mass-spectrometry endpoints; (iii) imaging studies to improve the reproducibility of echocardiography in AF7; and (iv) PPI-led qualitative studies to understand the importance of quality of life in the routine clinical assessment of patients with AF.8
Mary’s viewpoint as a patient on designing and managing RATE-AF
‘My involvement with the RATE-AF trial began after I was diagnosed and treated for paroxysmal atrial fibrillation. My fellow PPI team members and I have contributed to the design of the study, a successful application for funding, trial steering group meetings, and now the final rewarding results of many years of work. We also led focus groups exploring quality of life in patients with atrial fibrillation—which we went on to write up, publish, and present at the International Society of Quality of Life Research conference where we were awarded Patient Research Partner Scholarships.
It was these focus groups which gave me the most satisfaction—designing and running them, listening to patients talking about their quality of life, the neglect they felt at usual health consultations, and the impact that the trial was having on them. Many patients felt we had boosted their confidence and ability to self-manage their AF’.
Future perspectives
The involvement of patients, careers, and the public (and indeed anyone outside the usual clinical-academic framework) has the potential to improve the basis for clinical trials, support recruitment and retention of participants, and lead to more robust, and better-targeted evidence generation. Patient and public involvement is now commonplace in stakeholder meetings, the generation of clinical practice guidelines and efforts at dissemination. Being a mandatory part of developing and managing research studies internationally would seem the next sensible step to reducing the burden of cardiovascular disease.
On behalf of the RATE-AF Investigators (see Appendix).
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
RATE-AF Investigators are listed in Supplementary material online, Appendix: The RATE-AF team.
Funding
The RATE-AF trial and patient and public involvement were funded by the UK National Institute for Health Research (NIHR; CDF-2015-08-074). The opinions expressed are those of the authors and do not represent the NIHR or UK Department of Health and Social Care.
Conflict of interest: K.B. received funding from the National Institute for Health Research during the study (CDF-2015-08-074). O.T. receives funding from the EU/EFPIA Innovative Medicines Initiative (BigData@Heart 116074), outside of the study. D.K. acknowledges grants from the National Institute for Health Research during the study (NIHR CDF-2015-08-074); and outside of the study from: the National Institute for Health Research (NIHR-130280 DaRe2THINK NCT04700826); the British Heart Foundation (PG/17/55/33087 and AA/18/2/34218); EU/EFPIA Innovative Medicines Initiative (BigData@Heart 116074); the European Society of Cardiology supported by educational grants from Boehringer Ingelheim/BMS-Pfizer Alliance/Bayer/Daiichi Sankyo/Boston Scientific, the NIHR/University of Oxford Biomedical Research Centre and the British Heart Foundation/University of Birmingham Accelerator Award (STEEER-AF NCT04396418); Amomed Pharma and IRCCS San Raffaele/Menarini (Beta-blockers in Heart Failure Collaborative Group NCT0083244); in addition to personal fees from Bayer, AtriCure, Amomed, Protherics Medicines Development and Myokardia. M.S. has no disclosures.

References
- 1. Kotecha D, Calvert M, Deeks JJ, Griffith M, Kirchhof P, Lip GY, Mehta S, Slinn G, Stanbury M, Steeds RP, Townend JN.. A review of rate control in atrial fibrillation, and the rationale and protocol for the RATE-AF trial. BMJ Open 2017;7:e015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kotecha D, Bunting KV, Gill SK, Mehta S, Stanbury M, Jones JC, Haynes S, Calvert MJ, Deeks JJ, Steeds RP, Strauss VY, Rahimi K, Camm AJ, Griffith M, Lip GYH, Townend JN, Kirchhof P; Rate Control Therapy Evaluation in Permanent Atrial Fibrillation (RATE-AF) Team. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomized clinical trial. JAMA 2020;324:2497–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crocker JC, Ricci-Cabello I, Parker A, Hirst JA, Chant A, Petit-Zeman S, Evans D, Rees S.. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis. BMJ 2018;363:k4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levitan B, Getz K, Eisenstein EL, Goldberg M, Harker M, Hesterlee S, Patrick-Lake B, Roberts JN, DiMasi J.. Assessing the financial value of patient engagement: a quantitative approach from CTTI's patient groups and clinical trials project. Ther Innov Regul Sci 2018;52:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aitken M, Tully MP, Porteous C, Denegri S, Cunningham-Burley S, Banner N, Black C, Burgess M, Cross L, Van Delden J, Ford E, Fox S, Fitzpatrick N, Gallacher K, Goddard C, Hassan L, Jamieson R, Jones KH, Kaarakainen M, Lugg-Widger F, McGrail K, McKenzie A, Moran R, Murtagh MJ, Oswald M, Paprica A, Perrin N, Richards EV, Rouse J, Webb J, Willison DJ.. Consensus statement on public involvement and engagement with data-intensive health research. Int J Pop Data Sci 2019;4:06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ziff OJ, Lane DA, Samra M, Griffith M, Kirchhof P, Lip GY, Steeds RP, Townend J, Kotecha D.. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ 2015;351:h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunting KV, Gill S, Sitch A, Mehta S, O’Connor K, Lip GYH, Kirchhof P, Strauss VY, Rahimi K, Camm AJ, Stanbury M, Griffith M, Townend JN, Gkoutos GV, Karwath A, Steeds RP, Kotecha D. Improving the diagnosis of heart failure in patients with atrial fibrillation. Heart 2021; doi: heartjnl-2020-318557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones J, Stanbury M, Haynes S, Bunting KV, Lobban T, Camm AJ, Calvert MJ, Kotecha D; on behalf of the RAte control Therapy Evaluation in permanent Atrial Fibrillation (RATE-AF) trial group. Importance and assessment of quality of life in symptomatic permanent atrial fibrillation: patient focus groups from the RATE-AF Trial. Cardiology 2020;145:666–675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.