Abstract
Aims
The aim of this study was to derive and validate the SCORE2-Older Persons (SCORE2-OP) risk model to estimate 5- and 10-year risk of cardiovascular disease (CVD) in individuals aged over 70 years in four geographical risk regions.
Methods and results
Sex-specific competing risk-adjusted models for estimating CVD risk (CVD mortality, myocardial infarction, or stroke) were derived in individuals aged over 65 without pre-existing atherosclerotic CVD from the Cohort of Norway (28 503 individuals, 10 089 CVD events). Models included age, smoking status, diabetes, systolic blood pressure, and total- and high-density lipoprotein cholesterol. Four geographical risk regions were defined based on country-specific CVD mortality rates. Models were recalibrated to each region using region-specific estimated CVD incidence rates and risk factor distributions. For external validation, we analysed data from 6 additional study populations {338 615 individuals, 33 219 CVD validation cohorts, C-indices ranged between 0.63 [95% confidence interval (CI) 0.61–0.65] and 0.67 (0.64–0.69)}. Regional calibration of expected-vs.-observed risks was satisfactory. For given risk factor profiles, there was substantial variation across the four risk regions in the estimated 10-year CVD event risk.
Conclusions
The competing risk-adjusted SCORE2-OP model was derived, recalibrated, and externally validated to estimate 5- and 10-year CVD risk in older adults (aged 70 years or older) in four geographical risk regions. These models can be used for communicating the risk of CVD and potential benefit from risk factor treatment and may facilitate shared decision-making between clinicians and patients in CVD risk management in older persons.
Keywords: Risk prediction, Risk assessment, Cardiovascular disease, Primary prevention, 10-Year CVD risk, Older persons
Graphical Abstract
See page 2455 for the editorial comment on this article (doi: 10.1093/eurheartj/ehab310)
Introduction
Risk of cardiovascular disease (CVD) increases with age.1 The risk of non-CVD mortality generally also rises with age so that remaining life expectancy inevitably decreases with age. Hence, the treatment of important CVD risk factors needs to be carefully considered to balance the benefits and risks in this population. Meaningful treatment benefit is different in this population where life expectancy is limited,2 , 3 while older persons are generally at high risk of developing adverse drug events and side effects.4 , 5 It is thus important to identify those individuals who might benefit from preventive treatment.
For this purpose, CVD risk prediction models can be used to identify those at higher risk of CVD and those potentially benefiting the most from risk factor treatment.6 These prediction models may also aid in patient-centred clinical decision-making, taking into account other patient characteristics such as frailty, biological age, and patient preferences.7
Most of the 10-year CVD risk prediction models generally have a poor performance in older individuals for several reasons.8–11 First, the relationship between traditional risk factors and CVD attenuates with age,12 and traditional risk prediction models do not take into account competing risk of non-CVD mortality, leading to the overestimation of CVD risk and consequently overestimation of potential benefit from risk factor treatment in older persons.3 , 13 , 14 This overestimation may lead to unnecessary treatment in older persons, polypharmacy, increased risk of drug interactions, adverse events, reduced quality of life, and unnecessary costs.15 To deal with short-comings of traditional risk models, an older person-specific risk score should be used. However, previously developed risk models for older persons only estimate risk of cardiovascular mortality while non-fatal events are also of importance [e.g. stroke and heart failure (HF)]. Finally, previous models have not been extensively externally validated and shown to be applicable in different geographical risk regions where risk levels vary.2 , 16 , 17
We aimed to develop and validate a competing risk-adjusted model for individuals aged over 70 years without pre-existing CVD to estimate 5- and 10-year risk of incident CVD—the new SCORE2-Older Persons (SCORE2-OP). This risk model is calibrated to four different geographical risk regions using an approach based on aggregate level data that can be easily applied to further update the accuracy of risk predictions with changing CVD epidemiology in the future.
Methods
Study design
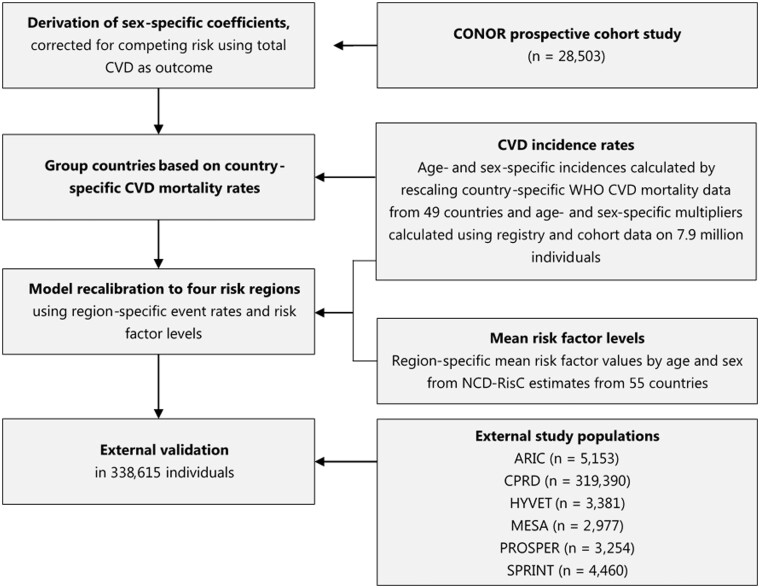
The SCORE2-OP project involved several interrelated components and data sources (Figure 1). The study design is closely related to the new SCORE2 model that estimates 10-year fatal and non-fatal CVD risk in individuals without previous CVD or diabetes aged 40–69 years.18 First, model coefficients were derived in the Cohort of Norway (CONOR) study.19 This study population was selected because it is a large, representative population-based cohort and has previously been used for model derivation.16 , 17 , 20 Second, the model was recalibrated to four geographical risk regions across Europe and beyond using estimated contemporary age- and sex-specific incidences and risk factor distributions. Third, external validation was performed in prospective cohorts from different risk regions. Finally, the model was applied to estimate individualized treatment benefit from blood pressure and cholesterol lowering to illustrate how SCORE2-OP can be used for treatment decision-making in clinical practice.
Figure 1.
Study design. ARIC, Atherosclerosis Risk in Communities; CONOR = Cohort of Norway; CPRD, Clinical Practice Research Datalink; CVD, cardiovascular disease; MESA, multi-ethnic study of atherosclerosis; NCD-RisC, Non-Communicable Disease Risk Factor Collaboration; PROSPER, PROspective Study of Pravastatin in Elderly at Risk; SPRINT, Systolic Blood Pressure Intervention Trial; WHO, World Health Organisation.
Sources of data
This study derived the risk model coefficients from the prospective CONOR study19 and used combined data from several cohort studies and clinical trials for external validation and testing: the Atherosclerosis Risk in Communities (ARIC) study,21 from which we used baseline data from visit 5 to include more individuals aged over 65 years; the Clinical Practice Research Datalink (CPRD);22 the Hypertension in the Very Elderly Trial (HYVET);23 the Multi-Ethnic Study of Atherosclerosis (MESA);24 the ‘PROspective Study of Pravastatin in Elderly at Risk’ (PROSPER) trial;25 and the Systolic Blood Pressure Intervention Trial (SPRINT).26 , 27 Details of the included studies can be found elsewhere and have been summarized in the Supplementary material online, Methods. The current study was conducted using data from the target population of individuals aged 65 years or over. Individuals with a history of CVD (i.e. coronary heart disease, stroke, or peripheral artery disease) were excluded from analysis. All included studies comply with the Declaration of Helsinki and were approved by local institutional review boards and all participants provided written informed consent.
Endpoint definitions
The primary endpoint was a composite of the first fatal or non-fatal CVD events in each study participant, defined as non-fatal myocardial infarction, non-fatal stroke, and cardiovascular mortality. Secondary endpoint included also hospitalization from HF, as this is an important source of morbidity and loss in quality of life in older persons.
The CVD mortality component of the primary and secondary outcomes resembles the endpoint definition of the original SCORE project, including death from coronary heart disease, HF, stroke, and sudden death. An overview of the ICD-10 codes included in both the fatal and non-fatal components of the composite endpoint can be found in Supplementary material online, Table S1. Deaths from non-CVD were treated as competing events. Follow-up time was defined as years until the first event, death, or end of the registration period.
Risk regions
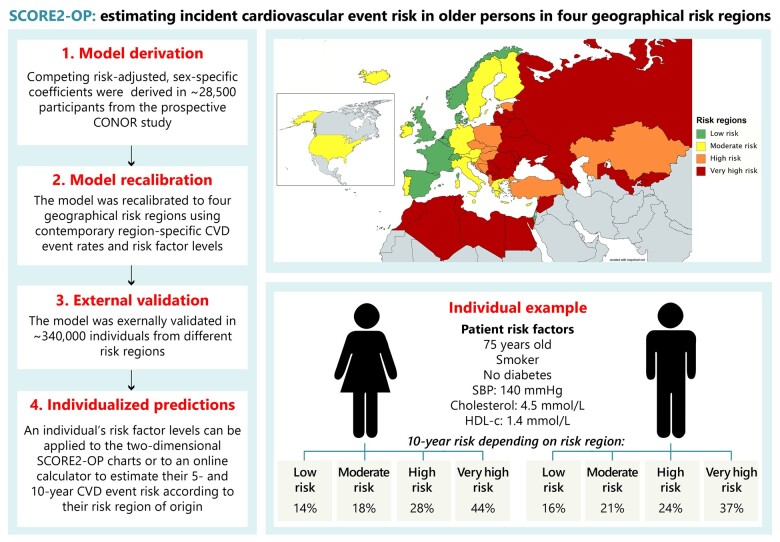
The four risk regions (low, moderate, high, and very high risk) were chosen based on the definition used in the newly developed SCORE2 risk model, according to the most recent overall age- and sex-standardized CVD mortality rates in all included countries (ICD 10 chapter IX, I00-I99). The following age-standardized rates were used for categorization: <100 CVD deaths per 100 000 (low risk), 100–149 CVD deaths per 100 000 (moderate risk), 150–299 CVD deaths per 100 000 (high risk), and ≥300 CVD deaths per 100 000 (very high risk). The four geographical risk regions are found in Supplementary material online, Figure S1 and Supplementary material online, Table S2.
Statistical analysis
Details of statistical analysis are provided in Supplementary material online, Methods. For model derivation, sex-specific coefficients were estimated in the CONOR study using competing risk-adjusted Fine and Gray proportional subdistribution hazards models. The models included the following pre-specified baseline predictors: age, current smoking, diabetes mellitus, systolic blood pressure (SBP), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-c). The risk factors were selected based on their predictive ability as well as availability in the derivation dataset and population statistics needed for model recalibration. Variable selection was not applied to prevent overfitting of the model to the derivation data (over-optimism). Age interaction terms were added as the effect of these risk factors may change with age.28 To maximise statistical power when estimating age-interactions, risk models were derived in participants aged 65 and older at baseline without previous CVD. However, SCORE2-OP risk models are intended for use in people aged over 70 years. In a parallel iniative a score for individuals aged below 70, SCORE2-OP, has been developed using similar methods.18 Continuous predictors were truncated at the 1st and 99th percentiles to minimize the influence of outliers in the model.29 Whether the association of continuous predictors with the outcome variable was adequately explained with a log-linear relationship was assessed using the Akaike information criterion. Internal model performance was assessed with Harrell’s C-index for discrimination, and visually with calibration plots of estimated vs. observed risk in a random sample with replacement of the CONOR study population to account for overfitting. The model was then recalibrated internally for the risk of the secondary CVD endpoint including HF using age- and sex-specific multiplication factors, using the same model coefficients.
Risk models were recalibrated to risk regions using age- and sex-specific mean risk factor levels and CVD incidence rates.30 Age-specific and sex-specific risk factor values were obtained from the Non-Communicable Disease Risk Factor Collaboration.31 , 32 We obtained country-, age-, and sex-specific CVD mortality rates reported by the World Health Organisation (WHO),33 and estimated fatal and non-fatal CVD incidences by using age- and sex-specific multipliers derived in the SCORE2 project in multiple cohorts from the different risk regions with a total of 4 056 218 men and 3 869 443 women, with 732 471 CVD events.18 The multipliers for fatal CVD to total CVD events per region are listed in Supplementary material online, Table S3.
External validation was performed in six studies, including the ARIC, MESA, and CPRD cohorts, and the combined study populations of the HYVET, PROSPER, and SPRINT trials (adding the trial treatment effect to account for differences in observed risk between the active treatment and control arm of the trials) as the separate trial populations have limited number of events in a short follow-up time. External model performance was assessed in terms of discrimination using Harrell’s C-index, and in terms of model calibration using plots of observed vs. estimated risks recalibrated using cohort-specific observed-vs.-expected ratios reflecting differences in baseline risk. SCORE2-OP was compared in terms of discrimination with the ASCVD (Atherosclerotic Cardiovascular Disease) risk calculator from AHA/ACC, an internationally widely used risk model for the general population also including older persons.34
All analyses were conducted with R-statistic programming (version 3.5.2, R Foundation for Statistical Computing, Vienna, Austria). Our approach to model development and validation complies with PROBAST guidelines35 and TRIPOD.36 The approaches used to handle missing data are described in the Supplementary material online, Methods.
Absolute CV event risk reduction from risk factor treatment in older people
SCORE2-OP can be used to estimate individualized treatment effect estimations from cardiovascular risk factor treatment,6 as described in detail in the Supplementary material online, Methods. To estimate the effect of blood pressure lowering on CVD, average relative treatment effects from large meta-analyses were added to SCORE2-OP. We estimated the absolute treatment effect from blood pressure lowering to the target of <140 mmHg in older persons with hypertension from the HYVET and SPRINT trials,26 , 37 using a hazard ratio (HR) of 0.80 per 10 mmHg SBP reduction from a large meta-analysis.38 For the effect of lipid lowering, an HR 0.78 per 1 mmol/L LDL-cholesterol lowering was used,39 and the absolute risk reduction (ARR) of lowering LDL-cholesterol to <2.6 mmol/L was estimated in participants with hypercholesterolaemia from the PROSPER trial.25 The ARR is defined as the baseline (‘untreated’) CVD risk minus the CVD risk with added risk factor management.
Results
A total of 211 184 women and 155 934 men aged 65 years or over from seven studies were included in the analysis for model derivation and validation. Study and baseline characteristics of all study populations are presented in Table 1.
Table 1.
Study and baseline patient characteristics of the included study populations
| Derivation population | External validation and testing populations |
||||||
|---|---|---|---|---|---|---|---|
| CONOR | ARIC | CPRD | HYVET | MESA | PROSPER | SPRINT | |
| N = 28 503 | N = 5153 | N = 319 390 | N = 3381 | N = 2977 | N = 3254 | N = 4460 | |
| Recruitment period | 1994–2003 | 2011–2013 | 2006a | 2001–2007 | 2000–2002 | 1997–1999 | 2010–2013 |
| Country | Norway | USA | UK | Eastern Europe (n = 1895), Western Europe (n = 84), others (n = 1402) | USA | UK (n = 1288), Ireland (n = 1339), Netherlands (n = 627) | USA |
| Baseline characteristics | |||||||
| Male sex (%) | 50 | 39 | 42 | 38 | 48 | 42 | 59 |
| Age (years) | 73 ± 5 | 75 ± 5 | 74 ± 6 | 83 ± 3 | 72 ± 5 | 75 ± 3 | 74 ± 6 |
| Current smoking (%) | 20 | 7 | 25 | 7 | 8 | 33 | 5 |
| SBP (mmHg) | 152 ± 23 | 130 ± 18 | 141 ± 16 | 173 ± 9 | 134 ± 22 | 157 ± 21 | 141 ± 15 |
| Total cholesterol (mmol/L) | 6.4 ± 1.2 | 4.8 ± 1.1 | 5.5 ± 1.2 | 5.3 ± 1.1 | 5.0 ± 0.9 | 5.7 ± 0.9 | 4.9 ± 1.0 |
| HDL cholesterol (mmol/L) | 1.5 ± 0.4 | 1.4 ± 0.4 | 1.6 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.4 ± 0.4 |
| Type 2 diabetes mellitus (%) | 6 | 31 | 10 | 9 | 15 | 12 | 0 |
| Lipid-lowering drugs use (%) | 9 | 49 | 21 | 0.3 | 22 | 49 | 44 |
| Median follow-up (IQI) | 13 (8–15) | 6 (5–6) | 7 (4–10) | 2 (1–3) | 13 (9–14) | 3 (3–4) | 3 (3–4) |
| Primary endpoint | 10 089 (35%) | 427 (8%) | 31 484 (10%) | 225 (7%) | 501 (17%) | 396 (12%) | 186 (4%) |
| Total mortality | 16 642 (58%) | 683 (13%) | 60 077 (19%) | 356 (11%) | 981 (33%) | 274 (8%) | 194 (4%) |
All data are expressed in n (%) or mean ± standard deviation unless stated otherwise.
Baseline for measurement of exposure was set at 1 January 2006.
HDL, high-density lipoprotein; IQI, interquartile interval; SBP, systolic blood pressure; CV, cardiovascular, IQR, interquartile range.
Model derivation and recalibration
A total of 10 089 non-fatal and fatal CVD events occurred in 305 640 person years of follow-up in the 28 503 participants included from the CONOR study, the derivation data. SCORE2-OP model coefficients and subdistribution hazard ratios for CVD events are shown in Table 2. Supplementary material online, Figure S2 shows the change in the effect of model predictors with increasing age.
Table 2.
Sex-specific coefficients and subdistribution hazard ratios for cardiovascular disease events of SCORE2-OP
| Men |
Women |
|||
|---|---|---|---|---|
| Coefficients (95% CI) | Subdistribution hazard ratios | Coefficients (95% CI) | Subdistribution hazard ratios | |
| Age (per year) | 0.063 (0.055 to 0.071) | 1.07 | 0.079 (0.070 to 0.087) | 1.08 |
| History of diabetes | 0.425 (0.305 to 0.544) | 1.50 | 0.601 (0.465 to 0.737) | 1.80 |
| History of diabetes × age (per year) | −0.017 (−0.040 to 0.005) | −0.011 (−0.032 to 0.011) | ||
| Current smoking | 0.352 (0.279 to 0.426) | 1.39 | 0.492 (0.398 to 0.587) | 1.59 |
| Current smoking × age (per year) | −0.025 (−0.040 to −0.009) | −0.026 (−0.043 to −0.008) | ||
| SBP (per 10 mmHg) |
0.094 (0.079 to 0.109) |
1.09 | 0.102 (0.085 to 0.119) | 1.10 |
| SBP (per 10 mmHg) × age (per year) | −0.005 (−0.008 to −0.002) | −0.004 (−0.007 to −0.002) | ||
| Total cholesterol (per 1 mmol/L) | 0.085 (0.054 to 0.116) | 1.10 | 0.060 (0.027 to 0.094) | 1.06 |
| Total cholesterol (per 1 mmol/L) × age (per year) | 0.007 (0.002 to 0.013) | −0.001 (−0.056 to 0.004) | ||
| HDL cholesterol (per 1 mmol/L) | −0.356 (−0.445 to −0.268) | 0.71 | −0.304 (−0.403 to −0.205) | 0.75 |
| HDL cholesterol (per 1 mmol/L) × age (per year) | 0.009 (−0.009 to 0.027) | 0.015 (0.0002 to 0.031) | ||
Sex-specific coefficients and subdistribution hazard ratios (SHRs) from Fine and Gray models predicted the risk of fatal and non-fatal CVD events as derived in the CONOR study. The SHRs are shown for age centred at 73 years, systolic blood pressure at 150 mmHg, total cholesterol at 6 mmol/L, and HDL cholesterol at 1.4 mmol/L. These SHRs are relevant for risk estimation only and have no aetiological interpretation.
CI, confidence interval; CVD, cardiovascular disease; HDL, high-density lipoprotein; SBP, systolic blood pressure.
In the internal validation set of the CONOR study, the 10-year estimated risk showed good agreement with the 10-year observed risk over all deciles for all outcomes of interest (Supplementary material online, Figure S3). C-index was 0.66 [95% confidence interval (CI) 0.65–0.66] for CVD events and 0.65 (95% CI 0.65–0.66) for CVD events including HF. The age- and sex-specific multiplication factors for estimating the risk of CVD events including HF can be found in Supplementary material online, Table S4.
Age- and sex-specific 10-year mortality CVD rates and derived incidence rates are shown for each region in Supplementary material online, Figure S4. The age- and sex-specific mean risk factor levels and estimated CVD event rates used for recalibration are presented by region in Supplementary material online, Table S5. After regional recalibration, SCORE2-OP estimated risks based on mean risk factor levels agreed well with the regional estimated CVD event incidence in the four risk regions across age-groups (Supplementary material online, Figure S5).
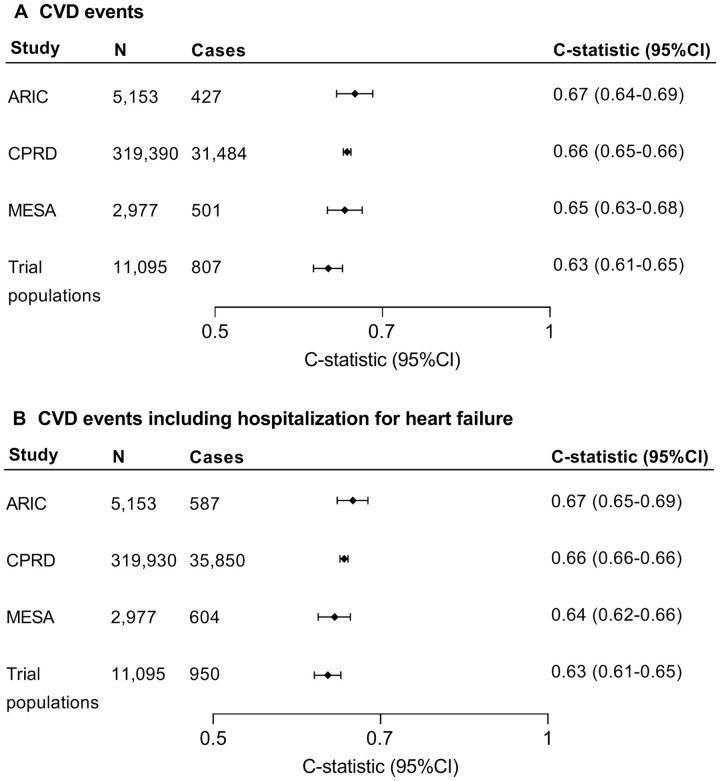
In the external validation study populations, a total of 33 219 primary outcome events were observed in 338 615 individuals in 2 259 933 person-years of follow-up. The external validation showed C-index for discrimination (Figure 2) ranging between 0.63 (95% CI 0.61–0.65) and 0.67 (95% CI 0.64–0.69). Calibration plots per study population after accounting for differences in baseline risk are shown in Supplementary material online, Figure S6. For the secondary CVD endpoint including HF, the external C-index ranged between 0.63 (95% CI 0.61–0.65) and 0.67 (95% CI 0.65–0.69). When we applied the recalibrated SCORE2-OP models from each risk region to individual risk factor data from participants from ARIC and MESA, the risk distribution varied greatly between risk regions (Supplementary material online, Figure S7). Comparison of SCORE2-OP and the ASCVD risk engine can be found in Supplementary material online, Table S6. C-index for SCORE2-OP was comparable to or higher than for ASCVD in the other study populations. In the external validation cohorts, the time-dependent ROC was comparable to or higher than Harrell’s C-index (Supplementary material online, Table S7).
Figure 2.
External validation of SCORE2-OP for (A) the estimation of risk for myocardial infarction, stroke, or cardiovascular disease mortality (primary endpoint) and (B) the estimation of risk for myocardial infarction, stroke, hospitalization for heart failure, or cardiovascular disease mortality (cardiovascular disease events including heart failure). Trial populations: HYVET, PROSPER, and SPRINT.
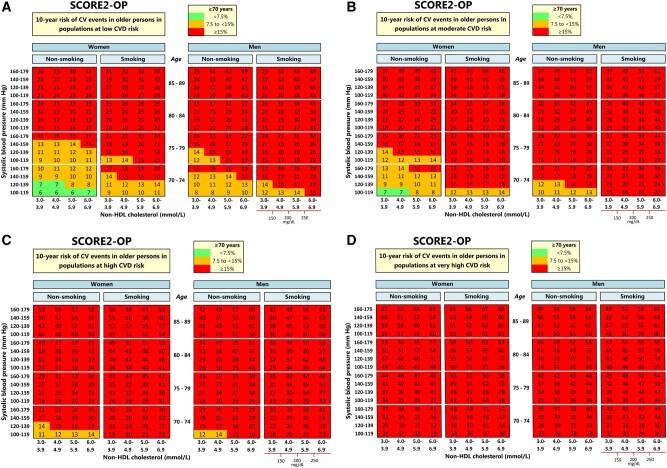
Two-dimensional risk charts of SCORE2-OP for all four risk regions are shown in the Figure 3, for practical purposes displayed according to non-HDL-c rather than TC and HDL-c. We have also added risk charts for the estimated 5-year risk charts are now in Supplementary material online, Figure S8, as this may fulfil a clinical need especially in the very old. The estimated absolute risk for a given age and combination of risk factors differed substantially across regions. For example, the estimated 10-year CVD risk for a 75-year-old male smoker with a systolic blood pressure of 150 mmHg, and a non-HDL-c of 4.5, ranged from 16% in a low risk country to 37% in a very high-risk country (Supplementary material online, Figure S9). Similarly, the 10-year risk for a 75-year-old woman with the same risk factor profile ranged from 14% in a low risk country to 44% in a very high-risk country. A sensitivity analysis taking into account uncertainty around individual predictions is described in the Supplementary material online, Methods and shown in Supplementary material online, Figure S10.
Figure 3.
Regional risk charts of predicted 10-year cardiovascular disease risks.
Absolute 10-year CVD event risk reduction from risk factor treatment in older people
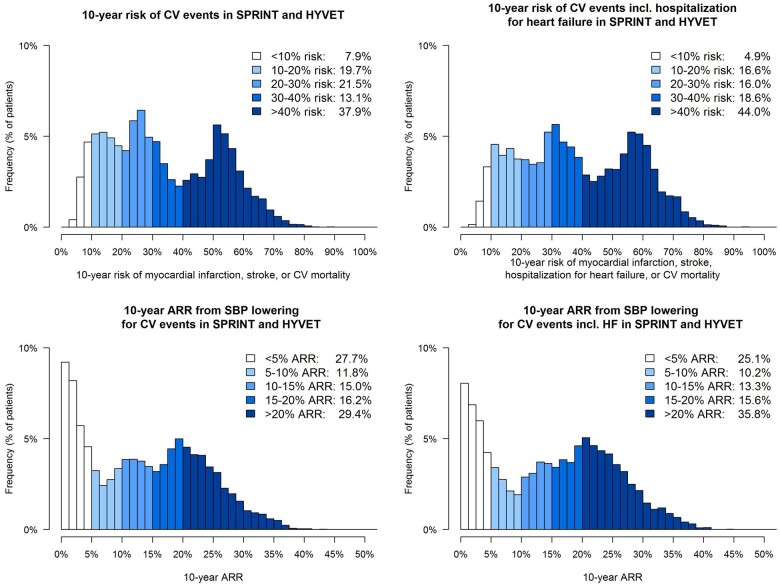
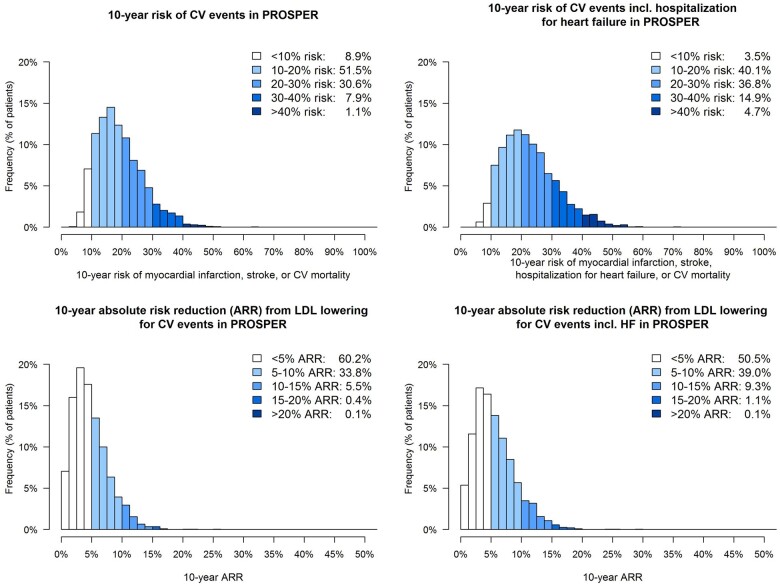
The distribution of individual estimated 10-year CVD risk and associated ARR for blood pressure lowering therapy when targeting an SBP of <140 mmHg in 5579 older persons with hypertension (SBP at baseline >140) in the SPRINT and HYVET blood pressure-lowering trials is shown in Figure 4. The overall median estimated 10-year risk for CVD events was 30% (IQR 19–50%); for CVD events including HF, this was 36% (22–55%). The overall median estimated individual 10-year ARR from blood pressure lowering for the primary endpoint CVD events was 13% (IQR 4–21%); for CVD events including HF, this was 16% (IQR 5–23%). The distribution of the individual estimated 10-year CV event risk and associated ARR for lipid-lowering therapy targeting an LDL-cholesterol <2.6 mmol/L in the PROSPER trial is shown in Figure 5. In these 3051 older persons, the overall median estimated 10-year risk for CVD events was 18% (IQR 13–24%), for CVD events including HF this was 21% (16–28%); the overall median estimated individual 10-year ARR from lipid lowering for the primary CVD endpoint was 4% (IQR 3–6%); for the secondary CVD endpoint including HF this was 5% (IQR 3–7%).
Figure 4.
Distribution of estimated 10-year fatal and non-fatal cardiovascular disease events and estimated 10-year absolute risk reduction from blood-pressure lowering in older persons with hypertension (systolic blood pressure >140 mmHg) in the HYVET and SPRINT trials (n = 5579).
Figure 5.
Distribution of estimated 10-year non-fatal and fatal cardiovascular disease events and estimated 10-year absolute risk reduction from lipid lowering in older persons with cholesterol >2.6 mmol/L in the PROSPER trial (n = 3051).
Discussion
The current report describes the development, recalibration, and external validation of a new competing risk-adjusted model for older individuals aged over 70 years without pre-existing CVD—SCORE2-OP to estimate 5- and 10-year risk of incident CVD (Graphical Abstract). There is a wide range in estimated individual CVD event risk in older persons. Using SCORE2-OP, individualized effects of CVD risk factor treatment can be estimated, e.g. from blood pressure lowering or lipid lowering, which can be used for treatment decision-making in clinical practice. The full clinical tool for individualized estimations will be made available to use in online calculators.
Development process, risk regions and illustrative example for the SCORE2-OP algorithm.
In the SCORE2-OP project investigators from three previously published older person CV risk algorithms joined forces by combining datasets and using advanced methodology for data analyses. The original SCORE-OP model,16 derived in >40 000 European older individuals (including participants from the CONOR study) estimated risk of fatal CVD. However, it did not take into account non-fatal CVD events (such as non-fatal stroke) that are clinically relevant in older persons, and was not adjusted for competing non-CVD mortality risk. Another risk model derived in CONOR is the NORRISK2 model for CVD risk estimation in elderly men and women up to age 79 years.17 This risk score is competing risk adjusted, includes interaction terms with age, and was externally validated within Norway, but it was not recalibrated or externally validated outside Norway. In addition, it was not derived specifically in older persons, including persons aged <65 years.17 , 20 The older person-specific risk score derived in the PROSPER trial is competing risk adjusted, and estimates the risk of fatal and non-fatal CVD events.2 However, this risk model was derived in a relatively small study population from a randomized clinical trial and did not include age interactions.
The SCORE2-OP model has combined these previous efforts and as such has several important strengths and advantages. First, the coefficients been derived in a large population-based cohort study, specifically in older persons. The model has been externally validated in populations with different baseline risks including both cohorts and trials from several countries. It was shown that SCORE2-OP recalibrated to the different risk regions corresponds well to the regional estimated WHO incidence rates, suggesting that calibration between estimated and observed risk is good for all risk regions. Although the discrimination in the external study populations is only moderate, the excellent calibration shows that the risk model can be used for clinical decision-making and risk communication. For this purpose, calibration is arguably the more important metric than discrimination.40 Use of the risk model in regions outside of the included countries should be done with caution, as no validation has (yet) been performed outside of these regions.
Second, SCORE2-OP can be used to estimate the risk for the combined outcome of both fatal and non-fatal CVD events. Especially in older persons, non-fatal CVD events may be of clinical importance, as they may severely impact quality of life. The model also gives the option to include hospitalization for HF in the composite endpoint, which is an important source of morbidity in the older population.41 In clinical practice, this may therefore be a very relevant endpoint for older persons especially when considering the consequences of HF for quality of life.
Third, the model is competing risk adjusted and includes age-interactions for all risk factors to account for differences in the relationship between risk factors and outcomes across different ages. This allows for estimations of 5- and 10-year prognosis truly tailored to the individual person.
Fourth, the model has been recalibrated using contemporary CVD rates currently available for the different risk regions using WHO data. The method used for systematic recalibration has previously been shown to give reliable estimations with good agreement between estimated and observed risks.30 The recalibration methods avoid reliance on sparse or unreliable cohort or country-level data, providing stable recalibrations using age- and sex-specific CVD rates and risk factor levels of each risk region. Due to the flexible recalibration approach based on the most recent registry data, the model can easily be updated in the future to accommodate changes in CVD risk and risk factor levels in populations over time. If individual countries or even regions within a country have reliable data sources available, the model may even be recalibrated for even more precise risk estimations in that country or region. Because the same risk regions and data sources were used for systematic recalibration of SCORE2-OP as used in the SCORE2 project,18 these two models can be used next to each other with persons naturally progressing from the SCORE2 model to SCORE2-OP as they get older.
Finally, the model can be used to estimate the absolute CVD risk reduction from blood pressure and cholesterol lowering to blood pressure and LDL-cholesterol treatment goals, by applying the HRs from meta-analyses or clinical trials in older persons to the SCORE2-OP risk estimations. Higher levels of non-HDL-c confer a smaller increase in CV risk in older persons compared to young and middle-aged people. It should be noted that lowering cholesterol produces significant reductions in major vascular events irrespective of age, although there is still less direct evidence of benefit among people older than 75 years without a history of previous vascular disease.42 In general older persons are at high 10-year CVD risk as age is a major driver of risk. For older persons, there is currently no CVD risk threshold for initiating risk factor lowering treatment in international guidelines. Should those thresholds appear, these may differ according to age as both the potential harms and the gain in CVD-free life expectancy from preventive therapy heavily depend on age. National and international guidelines need to consider (different) treatment thresholds for young, middle-aged, and older persons. For example, the Norwegian guideline for the primary prevention of CVD has a graded recommendation for the consideration of intervention with pharmacological risk factor management (10-year CV risk over 5% in ages 45–54 years, over 10% in ages 55–64 years, and over 15% in ages 65–74 years).43 Using the SCORE2-OP model, no uncertainty regarding individual predictions was estimated. Ten-year risk of CVD events can already be hard to interpret in clinical practice and having to interpret confidence intervals as well might make risk communication even more difficult, rather than more informed. Clinicians who want to incorporate the uncertainty of treatment decisions could consider adding the confidence intervals from meta-analyses or trials in the calculation of the ARR.
Estimation of absolute benefit may therefore guide treatment decisions in a shared decision-making process taking frailty, biological age, and patient preferences into account. Although on average the CVD risk is high in older persons, the current study shows that there is a wide distribution in 10-year CVD event risk in older persons and that risk factor treatment does not necessarily yield a clinically significant benefit in all older persons. Therefore, in the future, it might be interest to focus more on lifetime benefit from risk factor treatment based on lifetime CVD risk calculators.44–46
Several potential limitations of the current study should also be considered. First, the model was developed in a cohort study from the low-risk region alone. As such, the assumption is made that the model coefficients are transferrable to other risk regions. Previous studies have indeed shown homogeneity of model coefficients across different geographical regions and also across time for a CVD risk model, indicating transferability of model coefficients across different populations.18 , 28 Results from the current study have shown that discrimination was adequate in all countries where external validation was performed, indicating transferability of model coefficients was valid, although this validation could not be performed in all risk regions due to the lack of adequate data. Ideally, the SCORE2-OP algorithm should be validated in those regions as soon as reliable data are available in these regions.
Second, for the systematic recalibration approach estimated total CVD event incidence rates rather than observed CVD event incidence rates were used within the four risk regions by using a multiplier-based approach. This approach is based on the assumption that the multipliers are valid across all countries within the same risk region. Previous studies have shown that the multipliers showed good consistency across both different cohorts from the same region and across time.18 As such, we believe that this assumption is sufficiently met to give reliable estimations of total CVD event risk after systematic recalibration.
Third, part of the European validation data consisted of trial populations rather than unselected cohort data. Whereas the discrimination in our cohort populations was acceptable, especially compared to discrimination of a general risk model (namely ASCVD) in the same populations, slightly lower C-indices were reported in the external validation in the trial populations. Trial populations often make up a much more selected proportion of the population at large in comparison to cohort data (e.g. HYVET only contains patients aged 80 years or older, with SBP ranging from 156 to 200 mmHg) and the maximum C-index is strongly associated to the distribution of risk within a study population.40 Therefore, it is likely that the discrimination in these trials is an underestimation of the discrimination in real-life populations. As regional calibration (i.e. goodness of fit of the model) is satisfactory for all risk regions, the model can be used reliably for risk communication and treatment decisions in older persons.
Fourth, during model derivation in CONOR, no adjustment was made for treatment of risk factors at baseline. The assumption is made that, for example for cholesterol or blood pressure levels, the current risk factor level is predictive of the 10-year risk, regardless of whether this is treated or untreated. SCORE2-OP can thus be used for estimating 10-year risk in both untreated and treated individuals. However, caution should be given when risk factor treatment has been recently initiated. However, SCORE2-OP can be used for making treatment decisions in persons on a stable treatment regimen. Together with the fact that only one baseline risk factor measurement was used, which means that there may be underestimation of risk associations due to ‘regression dilution’,47 , 48 this may contribute to the relatively low discrimination. In addition, no adjustment was made for the potential initiation of risk factor treatment during study follow-up, which may also influence discrimination. However, it has been shown that accounting for statin drop-in during follow-up in model development had only a limited impact on model performance.49
Fifth, predictors related to co-morbidity or frailty (e.g. kidney function, height and body weight, co-morbidity at baseline) may be important determinants for CVD risk in older persons but were not included in SCORE2-OP due to the availability in the data sources. Including the number of drugs used as a measure of co-morbidity added to the predictive accuracy in the PROSPER older person score,25 but this variable was not available in all relevant data sources.
Finally, an inherent limitation of absolute risk estimations is that older individuals are invariably at higher risk for CVD than younger individuals with the same risk factors. As higher CVD risk translates to higher absolute risk reductions, this may give the impression that risk factors such as blood pressure and LDL-cholesterol should always be treated in the very old. It should be noted that 5- or 10-year CVD risk estimation should be combined with some assessment of treatment benefit, as life expectancy could be limited, together with patient preferences to make individual treatment decisions. For this purpose, lifetime treatment benefit approaches could be used, such as the LIFE-CVD model for primary prevention.44
In conclusion, the competing risk-adjusted SCORE2-OP model to estimate 5- and 10-year CVD event risk in persons aged over 70 years was derived, recalibrated, and externally validated in four risk regions. These models can be used for communicating the risk of CVD events and potential benefits from risk factor treatment and may facilitate shared decision-making in CVD risk management in older persons.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
This study was prepared using SPRINT-POP research materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the SPRINT-POP or the NHLBI. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The authors thank the staff and participants of the ARIC study, CPRD, HYVET trial, PROSPER trial, and SPRINT trial for their important contributions. Data from the Clinical Practice Research Datalink (CPRD) were obtained under licence from the UK Medicines and Healthcare products Regulatory Agency (protocol 162RMn2). CPRD uses data provided by patients and collected by the NHS as part of their care and support.
Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I. The MESA study research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The HYVET trial was funded by academic grants from the British Heart Foundation and Servier International to Imperial College London. The SPRINT trial was supported by contracts (HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C) and an interagency agreement (A-HL-13-002-001) from the NIH, including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Ageing, and the National Institute of Neurological Disorders and Stroke. The PROSPER study was supported by an investigator initiated grant obtained from Bristol-Myers Squibb. Prof. Dr. J.W. Jukema is an Established Clinical Investigator of the Netherlands Heart Foundation (grant 2001 D 032). Funding bodies had no role in the inception, design, completion, or publication of this work. Z.X. reports support from China Scholarship Council.
Contributors
All authors contributed to data collection, and the design, analysis, interpretation, and re-drafting of this paper. T.I., M.C., R.S., and S.H. conducted the combined statistical analysis. T.I., M.C., R.S., S.H., L.P., S.K., I.G., F.V., D.D.B., J.D., and E.D.A. drafted the study protocol and analysis plan. T.I., M.C., R.S., S.H., L.P., S.K., I.G., F.V., D.D.B., J.D., and E.D.A. drafted the manuscript. All other authors collected and re-analysed data and checked pooled data for the accuracy of information about their study.
Conflicts of interest: R.P. reports significant grant income from the Australian NHMRC. P.V. reports personal fees from Servier, Hygeia Hospital Groups Ltd and European Society of Cardiology. L.P. is funded by a British Heart Foundation Programme Grant (RG/18/13/33946). The other author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data availability
Data used for the current study are available upon reasonable request and approval of the individual cohorts or collaborative groups, please contact the individual cohorts used for the current study for details.
Appendix
SCORE2-OP working group and ESC Cardiovascular risk collaboration
Writing committee: Tamar I. de Vries* (Department of Vascular Medicine, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands), Marie Therese Cooney* (St Vincent's University Hospital and School of Medicine, University College Dublin, Dublin, Ireland), Randi M. Selmer* (Division of Mental and Physical Health, Norwegian Institute of Public Health, Oslo, Norway), Steven H.J. Hageman* (Department of Vascular Medicine, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands), Lisa A. Pennells (Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK), Angela Wood (Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK), Stephen Kaptoge (Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK), Zhe Xu (Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK), Jan Westerink (Department of Vascular Medicine, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands), Kjersti S. Rabanal (Department of Public Health, Faculty of Health Sciences, University of Stavanger, Stavanger, Norway and Research Department, Stavanger University Hospital, Stavanger, Norway), Grethe S. Tell (Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway and Division of Mental and Physical Health, Norwegian Institute of Public Health, Oslo, Norway), Haakon E. Meyer (Division of Mental and Physical Health, Norwegian Institute of Public Health, Oslo, Norway and Department of Community Medicine and Global Health, Institute of Health and Society, University of Oslo, Oslo, Norway), Jannicke Igland (Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway), Inger Ariansen (Division of Mental and Physical Health, Norwegian Institute of Public Health, Oslo, Norway), Kunihiro Matsushita (Johns Hopkins University, Baltimore, USA), Michael J. Blaha (Johns Hopkins University, Baltimore, USA), Vijay Nambi (Michael E DeBakey Veterans Affairs Hospital and Baylor College of Medicine, Houston, USA), Ruth Peters (School of Public Health, Imperial College London, London, UK and Psychology, University of New South Wales, Sydney, Australia & Neuroscience Research Australia, Sydney, Australia), Nigel Beckett (Imperial Clinical Trials Unit, Imperial College London, London, UK), Riitta Antikainen (Center for Life Course Health Research/Geriatrics and Medical Research Center Oulu, University of Oulu, Oulu, Finland), Christopher J. Bulpitt (School of Public Health, Imperial College London, London, UK), Majon Muller (Department of Internal Medicine, section Geriatric Medicine, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, Amsterdam Cardiovascular Sciences, the Netherlands), Marielle H. Emmelot-Vonk (Department of Geriatrics, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands), Stella Trompet (Department of Internal Medicine, Section of Gerontology and Geriatrics, Leiden University Medical Center, Leiden, the Netherlands and Department of Cardiology, Leiden University Medical Center, Leiden, the Netherlands), Wouter Jukema (Department of Cardiology, Leiden University Medical Center, Leiden, the Netherlands), Brian A. Ference (Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK), Martin Halle (University Hospital ‘Klinikum rechts der Isar’, Technical University of Munich, Munich, Germany), Adam D. Timmis (Queen Mary University of London, London, UK), Panos E. Vardas (Hygeia Hospitals Group, Athens & Medical School, University of Crete, Greece), Jannick A.N. Dorresteijn (Department of Vascular Medicine, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands), Dirk De Bacquer † (Department of Public Health and Primary Care, Ghent University, Ghent, Belgium), Emanuele Di Angelantonio † (Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK), Frank L.J. Visseren † (Department of Vascular Medicine, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands), and Ian M. Graham † (School of Medicine, Trinity College Dublin, University of Dublin, Dublin, Ireland).
*Contributed equally.
†Contributed equally.
Contributor Information
SCORE2-OP working group and ESC Cardiovascular risk collaboration:
Tamar I de Vries, Marie Therese Cooney, Randi M Selmer, Steven H J Hageman, Lisa A Pennells, Angela Wood, Stephen Kaptoge, Zhe Xu, Jan Westerink, Kjersti S Rabanal, Grethe S Tell, Haakon E Meyer, Jannicke Igland, Inger Ariansen, Kunihiro Matsushita, Michael J Blaha, Vijay Nambi, Ruth Peters, Nigel Beckett, Riitta Antikainen, Christopher J Bulpitt, Majon Muller, Marielle H Emmelot-Vonk, Stella Trompet, Wouter Jukema, Brian A Ference, Martin Halle, Adam D Timmis, Panos E Vardas, Jannick A N Dorresteijn, Dirk De Bacquer, Emanuele Di Angelantonio, Frank L J Visseren, and Ian M Graham
References
- 1. North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res 2012;110:1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stam-Slob MC, Visseren FLJ, Jukema J, Graaf Y. V D, Poulter NR, Gupta A, Sattar N, Macfarlane PW, Kearney PM, Craen A. D, Trompet S. Personalized absolute benefit of statin treatment for primary or secondary prevention of vascular disease in individual elderly patients. Clin Res Cardiol 2017;106:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolbers M, Koller MT, Witteman JCM, Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology 2009;20:555–561. [DOI] [PubMed] [Google Scholar]
- 4. Hohl CM, Dankoff J, Colacone A, Afilalo M. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med 2001;38:666–671. [DOI] [PubMed] [Google Scholar]
- 5. Bourgeois FT, Shannon MW, Valim C, Mandl KD. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf 2010;19:901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dorresteijn JAN, Visseren FLJ, Ridker PM, Wassink AMJ, Paynter NP, Steyerberg EW, Y van der G, Cook NR. Estimating treatment effects for individual patients based on the results of randomised clinical trials. BMJ 2011;343:d5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kent DM, Steyerberg E, Klaveren D. V. Personalized evidence based medicine: predictive approaches to heterogeneous treatment effects. BMJ 2018;363:k4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sabayan B, Gussekloo J, Ruijter W. D, Westendorp RGJ, Craen A. D. Framingham stroke risk score and cognitive impairment for predicting first-time stroke in the oldest old. Stroke 2013;44:1866–1871. [DOI] [PubMed] [Google Scholar]
- 9. Ruijter W. D, Westendorp RGJ, Assendelft WJJ, Elzen W. D, Craen A. D, Cessie S. L, Gussekloo J. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ 2009;338:a3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodondi N, Locatelli I, Aujesky D, Butler J, Vittinghoff E, Simonsick E, Satterfield S, Newman AB, Wilson PWF, Pletcher MJ, Bauer DC. Framingham risk score and alternatives for prediction of coronary heart disease in older adults. PLoS One 2012;7:e34287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nanna MG, Peterson ED, Wojdyla D, Navar AM. The accuracy of cardiovascular pooled cohort risk estimates in U.S. older adults. J Gen Intern Med 2020;35:1701–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kannel WB, D’Agostino RB. The importance of cardiovascular risk factors in the elderly. Am J Geriatr Cardiol 1995;4:10–23. [PubMed] [Google Scholar]
- 13. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc 2010;58:783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Field TS, Gurwitz JH, Harrold LR, Rothschild J, DeBellis KR, Seger AC, Auger JC, Garber LA, Cadoret C, Fish LS, Garber LD, Kelleher M, Bates DW. Risk factors for adverse drug events among older adults in the ambulatory setting. J Am Geriatr Soc 2004;52:1349–1354. [DOI] [PubMed] [Google Scholar]
- 16. Cooney MT, Selmer R, Lindman A, Tverdal A, Menotti A, Thomsen T, DeBacker G, Bacquer DD, Tell GS, Njolstad I, Graham IM. Cardiovascular risk estimation in older persons: SCORE O.P. Eur J Prev Cardiol 2016;23:1093–1103. [DOI] [PubMed] [Google Scholar]
- 17. Selmer R, Igland J, Ariansen I, Tverdal A, Njølstad I, Furu K, Tell GS, Klemsdal TO. NORRISK 2: a Norwegian risk model for acute cerebral stroke and myocardial infarction. Eur J Prev Cardiol 2017;24:773–782. [DOI] [PubMed] [Google Scholar]
- 18.SCORE2 Working Group. European Society of Cardiology SCORE2 risk prediction algorithms: revised models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naess O, Søgaard AJ, Arnesen E, Beckstrøm AC, Bjertness E, Engeland A, Hjort PF, Holmen J, Magnus P, Njølstad I, Tell GS, Vatten L, Vollset SE, Aamodt G. Cohort profile: cohort of Norway (CONOR). Int J Epidemiol 2008;37:481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rabanal KS, Igland J, Tell GS, Jenum AK, Klemsdal TO, Ariansen I, Meyer HE, Selmer RM. Validation of the cardiovascular risk model NORRISK 2 in South Asians and people with diabetes. Scand Cardiovasc J 2020;55:56–62. [DOI] [PubMed] [Google Scholar]
- 21.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 22. Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van ST, Smeeth L. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bulpitt C, Fletcher A, Beckett N, Coope J, Gil-Extremera B, Forette F, Nachev C, Potter J, Sever P, Staessen J, Swift C, Tuomilehto J. Hypertension in the Very Elderly Trial (HYVET): protocol for the main trial. Drugs Aging 2001;18:151–164. [DOI] [PubMed] [Google Scholar]
- 24. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DRJ, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 25. Shepherd J, Blauw GJ, Murphy MB, Bollen ELEM, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RGJ. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–1630. [DOI] [PubMed] [Google Scholar]
- 26. Group SR, Wright J, Williamson J, Whelton P, Snyger J, Sink K, Rocco M, Reboussin D, Rahman M, Oparil S, Lewis C, Kimmel P, Johnson K, Goff DJ, Fine L, Cutler J, Cushman W, Cheung A, Ambrosius W. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JTJ, Pajewski NM; for the SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >/=75 years: a randomized clinical trial. JAMA 2016;315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaptoge S, Pennells L, De Bacquer D, Cooney MT, Kavousi M, Stevens G, Riley LM, Savin S, Khan T, Altay S, Amouyel P, Assmann G, Bell S, Ben-Shlomo Y, Berkman L, Beulens JW, Björkelund C, Blaha M, Blazer DG, Bolton T, Bonita Beaglehole R, Brenner H, Brunner EJ, Casiglia E, Chamnan P, Choi Y-H, Chowdry R, Coady S, Crespo CJ, Cushman M, Dagenais GR, D'Agostino Sr RB, Daimon M, Davidson KW, Engström G, Ford I, Gallacher J, Gansevoort RT, Gaziano TA, Giampaoli S, Grandits G, Grimsgaard S, Grobbee DE, Gudnason V, Guo Q, Tolonen H, Humphries S, Iso H, Jukema JW, Kauhanen J, Kengne AP, Khalili D, Koenig W, Kromhout D, Krumholz H, Lam TH, Laughlin G, Marín Ibañez A, Meade TW, Moons KGM, Nietert PJ, Ninomiya T, Nordestgaard BG, O'Donnell C, Palmieri L, Patel A, Perel P, Price JF, Providencia R, Ridker PM, Rodriguez B, Rosengren A, Roussel R, Sakurai M, Salomaa V, Sato S, Schöttker B, Shara N, Shaw JE, Shin H-C, Simons LA, Sofianopoulou E, Sundström J, Völzke H, Wallace RB, Wareham NJ, Willeit P, Wood D, Wood A, Zhao D, Woodward M, Danaei G, Roth G, Mendis S, Onuma O, Varghese C, Ezzati M, Graham I, Jackson R, Danesh J, Di Angelantonio E. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Heal 2019;7:e1332–e1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation and Updating. New York, USA: Springer; 2009. [Google Scholar]
- 30. Pennells L, Kaptoge S, Wood A, Sweeting M, Zhao X, White I, Burgess S, Willeit P, Bolton T, Moons KGM, Schouw Y. V D, Selmer R, Khaw K-T, Gudnason V, Assmann G, Amouyel P, Salomaa V, Kivimaki M, Nordestgaard BG, Blaha MJ, Kuller LH, Brenner H, Gillum RF, Meisinger C, Ford I, Knuiman MW, Rosengren A, Lawlor DA, Volzke H, Cooper C, et al. Equalization of four cardiovascular risk algorithms after systematic recalibration: individual-participant meta-analysis of 86 prospective studies. Eur Heart J 2019;40:621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NCD Risk Factor Collaboration. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 2017;389:37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4·4 million participants. Lancet 2016;387:1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. WHO Mortality Database. https://www.who.int/data/data-collection-tools/who-mortality-database (7 December 2020).
- 34. Lloyd-Jones DM, Huffman MD, Karmali KN, Sanghavi DM, Wright JS, Pelser C, Gulati M, Masoudi FA, Goff DC. Estimating longitudinal risks and benefits from cardiovascular preventive therapies among medicare patients. J Am Coll Cardiol 2017;69:1617–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, Reitsma JB, Kleijnen J, Mallett S. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 2019;170:51. [DOI] [PubMed] [Google Scholar]
- 36. Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. BMC Med 2015;13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 38. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 39. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 2007;115:928–935. [DOI] [PubMed] [Google Scholar]
- 41. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Executive summary: heart disease and stroke statistics—2016. Update: a report from the American Heart Association . Circulation 2016;133:447–454. [DOI] [PubMed] [Google Scholar]
- 42.Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019;393:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Klemsdal TO, Gjelsvik B, Elling I, Johansen S, Kjeldsen SE, Kristensen Ø, Madsen S, Njølstad I, Selmer R, Tonstad S, Voie H. New guidelines for the prevention of cardiovascular disease. Tidsskr nor Laegeforen 2017;137: [DOI] [PubMed] [Google Scholar]
- 44. Jaspers NEM, Blaha MJ, Matsushita K, Schouw YT, van der Wareham NJ, Khaw K-T, Geisel MH, Lehmann N, Erbel R, Jöckel K-H. G, Y van der Verschuren WMM, Boer JMA, Nambi V, Visseren FLJ, Dorresteijn JAN. Prediction of individualized lifetime benefit from cholesterol lowering, blood pressure lowering, antithrombotic therapy, and smoking cessation in apparently healthy people. Eur Heart J 2019;31:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaasenbrood L, Bhatt DL, Dorresteijn JAN, Wilson PWF, D'Agostino RB, Massaro JM, van der Graaf Y, Cramer MJM, Kappelle LJ, de Borst GJ, Steg PG, Visseren FLJ. Estimated life expectancy without recurrent cardiovascular events in patients with vascular disease: the SMART-REACH model. J Am Heart Assoc 2018;7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dorresteijn JAN, Kaasenbrood L, Cook NR, Kruijsdijk RCM, van G, Y van der Visseren FLJ, Ridker PM. How to translate clinical trial results into gain in healthy life expectancy for individual patients. BMJ 2016;352:i1548. [DOI] [PubMed] [Google Scholar]
- 47. Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ 2010;340:c2289. [DOI] [PubMed] [Google Scholar]
- 48. Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 1999;150:341–353. [DOI] [PubMed] [Google Scholar]
- 49. Xu Z, Arnold M, Stevens D, Kaptoge S, Pennells L, Sweeting MJ, Barrett J, Angelantonio ED, Wood AM. Prediction of cardiovascular disease risk accounting for future initiation of statin treatment. Am J Epidemiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used for the current study are available upon reasonable request and approval of the individual cohorts or collaborative groups, please contact the individual cohorts used for the current study for details.