Abstract
Rehabilitation device efficacy alone does not lead to clinical practice adoption. Previous literature identifies drivers for device adoption by therapists but does not identify the best settings to introduce devices, the roles of different stakeholders including rehabilitation directors, or specific criteria to be met during device development. The objective of this work was to provide insights into these areas to increase clinical adoption of post-stroke restorative rehabilitation devices. We interviewed 107 persons including physical/occupational therapists, rehabilitation directors, and stroke survivors and performed content analysis. Unique to this work, care settings in which therapy goals are best aligned for restorative devices were found to be outpatient rehabilitation, followed by inpatient rehabilitation. Therapists are the major influencers for adoption because they typically introduce new rehabilitation devices to patients for both clinic and home use. We also learned therapists’ utilization rate of a rehabilitation device influences a rehabilitation director’s decision to acquire the device for facility use. Main drivers for each stakeholder are identified, along with specific criteria to add details to findings from previous literature. In addition, drivers for home adoption of rehabilitation devices by patients are identified. Rehabilitation device development should consider the best settings to first introduce the device, roles of each stakeholder, and drivers that influence each stakeholder, to accelerate successful adoption of the developed device.
Index Terms—: stroke, stroke rehabilitation, rehabilitation device, occupational therapy, physical therapy, implementation, technology
I. Introduction
THERE are an estimated 7.8 million adult stroke survivors in the US with additional 795,000 strokes occurring each year [1], [2]. Two thirds of stroke survivors are left with some chronic impairment post stroke [3]. Post-stroke physical impairments diminish stroke survivors’ abilities for daily activities including self-care, hygiene, employment, and leisure, which decreases their independence and quality of life [4], [5].
Therefore, many sensorimotor rehabilitation technologies are being developed to enhance post-stroke patient outcomes. These technologies typically focus on increasing experience-dependent neuroplasticity and restoring function [6], [7]. Some examples include: virtual reality based rehabilitation games to increase repetitions of therapeutic movements to restore function [8], [9], wearable sensory stimulation to increase cortical activation during therapy [10], wearable devices that facilitate joint movements [11], [12], biofeedback [13], smart objects [14, 15], and vibratory cuing devices to remind stroke survivors to move their affected upper extremities more frequently [16], [17]. Many of these restorative rehabilitation devices have shown efficacy in enhancing motor outcomes of stroke survivors [18], [19], [20]. However, many devices do not translate to clinical practice, even with proven efficacy from large, well-designed clinical trials [21], [22]. This significant gap in translation from clinical trials to practice [23] indicates that there is a need to better understand what drives adoption of new rehabilitation devices [22].
Literature provides some insights to clinical adoption of new rehabilitation devices. First, a new rehabilitation device should be suitable and aligned with the treatment focus and approaches of the particular rehabilitation setting to which it is introduced [24]. However, existing literature does not specify which settings are most appropriate to introduce new restorative rehabilitation devices based on therapy approaches. Feasibility studies for new rehabilitation devices conducted in research labs [25], [26] do not replicate clinical settings and do not inform adoption feasibility in clinics. Additionally, many devices are designed for home use [27], [28] without the knowledge of whether home is the optimal place for introduction of the device.
Second, successful adoption requires consideration of stakeholders [29]. At the conceptual level, there are technology acceptance models [30], [31]. However, these models are for general technologies such as new computers or smartphones, and not specifically for rehabilitation devices. Thus, while these models provide a framework of how a single consumer adopts technology, they do not account for the dynamics in healthcare where multiple stakeholders are involved such as patients, healthcare providers, and rehabilitation facility administrators. At the practical level, drivers for rehabilitation device adoption have been obtained from surveys [24], [29], [32], [33], [34]. However, these studies do not describe the interdependencies and influences among stakeholders. In addition, viewpoints of rehabilitation directors (i.e., supervisors of therapists) have not been studied, despite the fact that therapists need support from rehabilitation directors to implement a new rehabilitation device [35]. Understanding roles and dynamics of multiple stakeholders in healthcare may elucidate how new devices should be developed to effectively address the needs of all parties involved for better adoption.
Third, while previous research provides general constructs of drivers for technology adoption for therapists and patients, the specific criteria to guide developers is unknown. For example, it is known that the less effort it takes to implement a device, the more likely the device would be adopted by patients [36] and therapists [28]. However, there is no information on how much effort is reasonable. Similarly, while low cost is better for adoption [22], [37], [38], the reasonable cost is unknown. Knowledge of the context for acceptable criteria is expected to guide new rehabilitation device development.
The objectives of this project were to use the I-Corps™ product development and market assessment approach to identify 1) the appropriate setting to introduce new restorative rehabilitation devices; 2) roles of multiple stakeholders including patients, therapists, and rehabilitation directors; and 3) drivers influencing each stakeholder with specific criteria. This approach generates new knowledge that integrates information on the main drives that need to be considered and integrated to accelerate translation of new rehabilitation devices into new products that are optimized for multiple stakeholders and minimize barriers to clinical adoption.
II. Methods
A. Interview Procedure
The keystone of the I-Corps™ approach is to accumulate a deep understanding of stakeholders’ perspectives on a new product. This is accomplished by interviewing at least 100 stakeholders in real-world settings. To obtain such rich contextual information to address the objectives, we performed interviews with stroke care stakeholders. The interviews were performed as part of the I-Corps™ at the National Institutes of Health (NIH) [39]. I-Corps™ is a federal initiative to increase the economic impact of federally funded research by engaging researchers and entrepreneurs in conversations with potential customers, partners, and competitors to accelerate translation of research technologies into the market. This work did not require an approval from the Institutional Review Board, as recommended by the NIH, since it is not a systematic investigation of generalizable knowledge [39].
The interview process was guided by the I-Corps™ at NIH program. Specifically, 3 interviewers were trained by the I-Corps™ at NIH program coaches who are experts in entrepreneurship with medical technology. Initially, 2–3 interviewers interviewed one person at a time, to ensure consistency for approaches and processes [40]. During this time, one interviewer led the conversation while the others took notes. Interviewers took turns in roles in subsequent interviews. Interviewers met with the I-Corps™ at NIH program coaches daily for 3 consecutive days to discuss each day’s interview processes (e.g., number of people interviewed, interview flow), results (e.g., new information relevant for the objectives), and the next day’s goals (e.g., what to find out next, who to interview, how to obtain contact for potential interviewees). Once consistency among interviewers was achieved, three interviewers conducted interviews separately. Each interviewer led the interview and took notes at the same time. Multiple people were interviewed each week. Every week, interviewers and the I-Corps™ at NIH program coaches met to discuss findings from the recent interviews and determine the next information that should be sought and who should be interviewed to obtain this data. We sought stakeholders who could further explain our newly learned information and also provide the next insights as we learned more about the ecosystem of stroke care, rehabilitation therapy, and rehabilitation devices [41], [42]. This process continued for 8 consecutive weeks.
We started with a general list of stakeholders identified from the literature and looked for representatives of these groups. Interviewees included healthcare professionals, patients/caregivers, and healthcare company representatives. Healthcare professionals and company representatives were contacted via existing relationships and word of mouth referrals. Stroke survivors and caregivers were contacted via solicitation for an interview in-person and via online stroke support groups, friends and families, and word of mouth referrals. Although we were encouraged to interview insurance representatives regarding insurance coverage for devices, we were unable to find insurance representatives who agreed to interview. The I-Corps™ at NIH program coaches assisted with emerging needs in the interview process and guided the progression of interviewee selection based on these needs.
All interviews were with one interviewee at a time in order to obtain ‘expert opinions’ or ‘key informant perspectives’ that are not influenced by group discussion dynamics [43]. This method can provide richer information about individual experiences, thoughts, and feelings [44]. The interviews were completed in person, via teleconference or via phone call. In-person interviews took place in the interviewee’s preferred location such as their office, a coffee shop, or their home [45]. Interviews were requested for 15 minutes to discuss their view on “restorative rehabilitation devices”. Respondents were free to define this term according to their personal interest, but we used questions as needed to clarify the devices included. We allowed interviews to continue as longs as the respondent desired, and some interviews lasted for more than an hour.
Interviews were semi-structured in which the high-level key questions were predefined and asked to everyone, while interviewers or interviewees were allowed to ask follow-up questions in order to pursue an idea or response in more detail [40], [46]. This approach is frequently used in healthcare, as it provides interviewees with some guidance on the topics, and at the same time, provides flexibility for the discovery or elaboration of information that is important to interviewees but may not have previously been thought of as pertinent by the interviewers [46].
The high-level key questions were formulated to attain the objectives stated above. Specifically, the key questions for healthcare professionals were (i) the primary treatment focus and approaches of their respective settings and (ii) their roles in administering rehabilitation therapy or devices. If the participants were rehabilitation therapy providers, they were additionally asked for (iii) drivers and barriers to use rehabilitation devices. For patients/caregivers, key questions were about their post-stroke care and rehabilitation therapy experience, drivers and barriers to use rehabilitation devices, and how they received information for post-stroke rehabilitation treatment. In addition, to characterize the patient/caregiver interviewee pool with regards to rehabilitation needs, we asked for the top 3 problems they have struggled with post stroke, and the rehabilitation devices they have used. For healthcare company representatives, the key interview question was what methods they use to engage stakeholders in rehabilitation devices. Though not stakeholders, they were interviewed to capture their perception of the major product features for their devices, and the strategies they use to increase sales of devices.
B. Analysis
Knowledge from each week’s interviews were discussed through the weekly team meetings and documented in weekly presentations for the I-Corps™ at NIH program. Major learning points from all interviews were finalized for the final presentation of the I-Corps™ at NIH program, based on the review of the weekly presentation documents and guidance from the coaches.
A direct approach to content analysis [47] was used. Two authors created a preliminary code book for drivers of rehabilitation device adoption informed by previous literature (for healthcare providers [24], [32] and patients/caregivers [30]). The authors then independently coded all interview notes. New themes were allowed to emerge. The authors discussed codes when a new theme emerged. A high agreement [47] in coding between the two authors was found with a Cohen’s Kappa of 0.83.
In addition, codes were tallied to provide a ranking of themes [46]. Specifically, we reviewed the interview notes from healthcare providers who have a role in administering rehabilitation therapy and/or utilizing rehabilitation devices. We tallied the treatment approaches and drivers for rehabilitation device adoption mentioned. The number of interviewees who mentioned each treatment approach or each driver was divided by the total number of interviewees in that category to compute the percentage of interviewees who mentioned the treatment approach or driver. Similarly, we reviewed the interview notes from patients/caregivers and obtained the percent of patients/caregivers who mentioned each driver for rehabilitation device use and source of information.
III. Results
A. Interviewees
A total of 107 persons were interviewed, including 34 healthcare professionals, 54 stroke survivors and caregivers, and 19 healthcare company representatives across the U.S. (Table 1). We had a goal of 100 interviews. Through snowball sampling and data collection until theme saturation, our final number was 107. Caregivers responded for the stroke survivor they cared for.
Table 1.
Interviewees (total n=107)
| N | |
|---|---|
| Healthcare Professionals | Subtotal = 34 |
| Neurologists | 3 |
| Occupational therapists | 10 |
| Physical therapists | 4 |
| Physical therapy assistants | 2 |
| Speech therapists | 2 |
| Rehabilitation directors | 4 |
| Others (nurse, neurosurgeon, social worker, internal medicine physician, nursing home administrator, care coordinator, nursing assistant, neuropsychologist, telehealth clinical coordinator) | 9 |
| Stroke survivors | Subtotal = 54 |
| Female/Male | 30/24 |
| Age at stroke (Mean ± SD years) | 49±14.6 |
| Time since stroke | 0.5–23 years |
| Healthcare company | Subtotal = 19 |
| Leadership | 10 |
| Developers | 9 |
The healthcare providers with a role in rehabilitation mentioned experience with the types of rehabilitation devices including splints/braces/orthosis (63%), electrical stimulation (58%), app/games (33%), exercise equipment such as stationary hand/leg bikes (25%), activity monitor such as pedometer (25%), and workstations such as Dynavision™ (17%). Characteristics of stroke survivors interviewed are as follows. Their top 3 problems post stroke included: upper limb mobility/strength/dexterity (86% of the interviewees), lower limb strength/walking/balance (61%), pain (20%), numbness (18%), speech (16%), cognition (16%), emotion (16%), fatigue (12%), and social support (6%). They had some experience using therapy supplies and/or rehabilitation devices, such as therapy putty and bands (64%), splints/braces/orthosis (32%), electrical stimulation (32%), and stationary hand/leg bikes (14%). The healthcare company representatives included 10 persons in leadership roles (e.g., CEO, Board of Directors) and 9 developers (e.g., engineers).
B. Appropriate setting to introduce new rehabilitation devices based on therapy approaches
To determine the appropriate setting to introduce new restorative rehabilitation devices, treatment focus and approach of various rehabilitation care settings were clarified from interviews with healthcare professionals. Specifically, 5 settings in which stroke rehabilitation therapy is provided (3 inpatient, 1 outpatient, and 1 home health) were considered (Figure 1). We found that restorative rehabilitation supports the focus of outpatient rehabilitation better than those of other settings.
Figure 1:
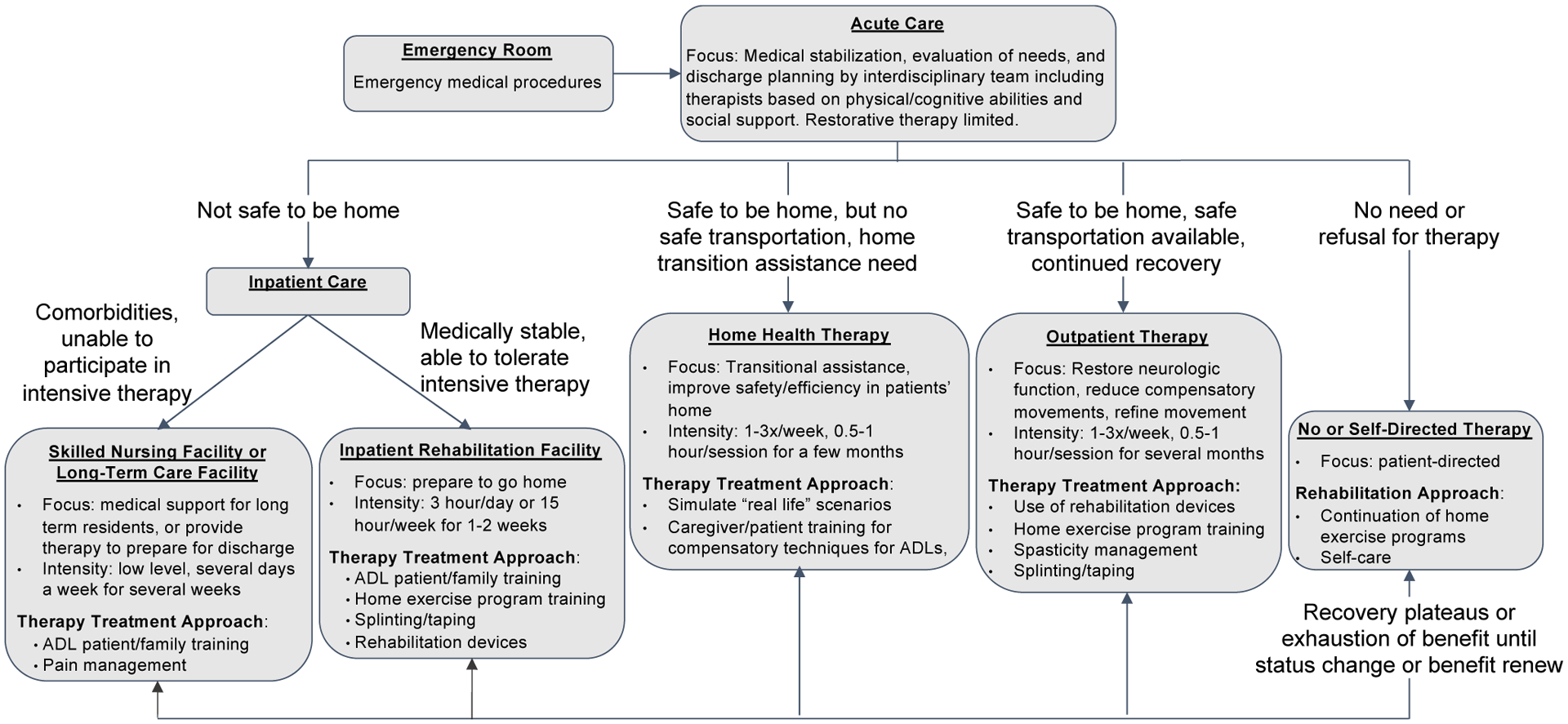
Stoke care flow describing transition from one care setting to another post stoke. Within each setting, the focus and therapy approaches relevant to use of rehabilitation devices are listed.
The focus of outpatient therapy is to preserve and restore neurologic function by driving neuroplasticity, such as reducing compensatory movements and refining movements since the basic living needs have been taken care of by previous settings. As such, outpatient therapists mentioned approaches including use of rehabilitation devices (100%), home exercise prescription and training (71%), spasticity management (57%), and splinting/taping (43%).
The secondary entry point may be inpatient rehabilitation facilities. Inpatient rehabilitation facilities house patients who are able to tolerate daily intensive therapy but are not yet safe to go home. Thus, the focus of treatment is to provide rigorous therapy to enable patients to return home [48]. Therapists in inpatient rehabilitation facilities mentioned that their primary treatment approaches include training in ADLs (Activities of Daily Living) (67%) including compensatory techniques and family and patient safety training such as car transfers, home exercise training (56%), splinting/taping (44%), and use of rehabilitation devices such as hand bikes and functional electrical stimulation (33%).
Other settings are less aligned for use of restorative rehabilitation devices. Specifically, skilled nursing and long-term care facilities have stroke patients who cannot tolerate intensive therapy due to severity of stroke or co-morbidities. In addition, therapy is typically provided by external contract companies that do not receive incentives for bringing additional rehabilitation devices. Home health is focused on patient safety at home and limited to devices that can be easily carried in and out of the therapists’ car or owned by patients.
C. Stakeholders relevant for rehabilitation devices
Healthcare professionals and patient interviewees mentioned that those who play a role in administration of sensorimotor rehabilitation therapy or devices are physicians, occupational and physical therapists and therapy assistants, and rehabilitation directors. Physicians in neurology and physical medicine and rehabilitation provide medical interventions such as spasticity management via oral medication or botulinum toxin injection, prescribe orthotics and functional electrical stimulation to be implemented by therapists, and refer patients to therapy. Some primary care physicians also refer patients to therapy per their patient’s request. Physicians and nurse practitioners also prescribe assistive devices that involve no more than low risk (e.g., FDA Class I devices such as walkers) [49].
Of all healthcare professionals involved in rehabilitation, physical/occupational therapists are the primary users of rehabilitation devices. If they believe the device will improve patients’ functional recovery, therapists advocate to rehabilitation directors for the need for rehabilitation devices to serve patients’ best interests. Rehabilitation directors are the decision makers to purchase rehabilitation devices to be owned by inpatient or outpatient rehabilitation facilities and used with their patients (Figure 2A).
Figure 2:
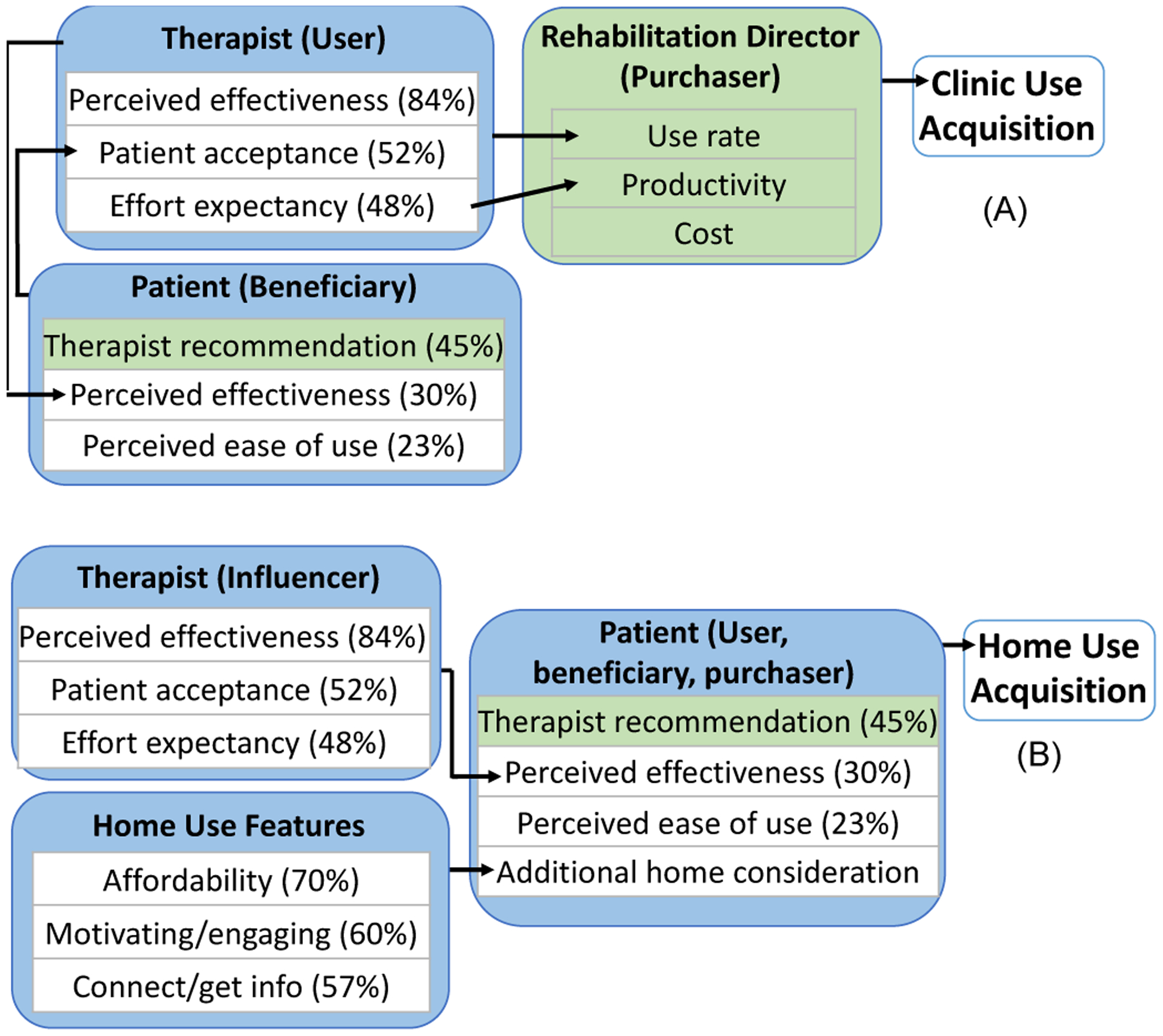
Drivers for multiple stakeholders for clinic (A) and home use (B). New results are indicated in green.
Aside from rehabilitation devices owned by rehabilitation facilities, patients are also able to purchase some rehabilitation devices for home use. Most patients used rehabilitation devices at home if instructed or recommended by their therapist (Figure 2B). Some patients mentioned that they proactively inquired their therapists about purchasing the rehabilitation devices that they had been using during therapy sessions so that they could continue using the devices at home even after discharge. Therapists facilitate the acquisition of devices by educating the patient on need, providing the vendor contact, and/or completing medical necessity paperwork. Caregivers/family members also assist with the acquisition process for patients with cognitive or communication deficits. Some patients mentioned finding out about new rehabilitation devices from stroke support group meetings, after they were already discharged from therapy. Upon learning about new devices, some patients contacted their previous therapists or physicians or performed a web search to find a device company representative to access the new device. However, the majority did not follow up since they were no longer in direct contact with therapists.
In summary, therapists are the primary influencers for rehabilitation device adoption for both clinic and home use. Rehabilitation directors are the decision makers for purchase of rehabilitation devices for rehabilitation facilities. Patients’ motivation plays a large role in home use of rehabilitation devices.
D. Drivers and barriers of rehabilitation device adoption
Drivers for adoption of rehabilitation devices are summarized for each stakeholder for clinic use (Figure 2A) and home use (Figure 2B), based on the interviews. They are detailed below.
1). Therapists’ drivers
For therapists, drivers for rehabilitation device use included perceived effectiveness (84% of respondents), patient acceptance of a rehabilitation device (52%), and effort expectancy for therapist (48%) (Figure 2A, Therapist). Perceived effectiveness entailed whether they think their patients will improve, whether the improvement will be faster and long-lasting, whether use of the rehabilitation device is expected to improve task performance in functional activities, perceived effectiveness from prior experience with the same or similar devices, and/or scientific evidence. Interestingly, scientific evidence was not the top driver for therapists’ perceived effectiveness. Even with strong scientific evidence, therapists may not use a treatment approach unless it aligns with their patients’ clinical disposition. For example, many therapists find it easy to conceptualize and apply functional electrical stimulation to stimulate patients’ nervous systems. However, some therapists are hesitant to use stimulation for sensory-impaired patients since these patients cannot verbalize if the stimulation is too high, which could damage tissues. Some therapists also do not use functional electrical stimulation with patients with flaccidity in their arm, as they perceive that patients without motor activity would not benefit from the stimulation. Additionally, although faster results are desired, outpatient therapists mentioned a willingness to try a device for 1–2 months before changing over to another treatment approach if they knew it would require long-term use to see benefits. For example, one therapist mentioned an infrared treatment for neuropathy for revascularization that required approximately 13 sessions for results but significantly decreased the patient’s pain, so it was therefore deemed worthwhile. In addition, therapists are more likely to use a treatment approach if they perceive that it would help improve functional ADL tasks that are meaningful to their patient.
Patient acceptance influences therapists’ adoption of rehabilitation devices, because therapists are trained to be client centered. Patient acceptance included not only device comfort and safety, but also increased motivation and excitement with new devices. If not accepted by enough patients, therapists may forget the device, resulting in discontinued use of the rehabilitation device.
Effort expectancy for therapists included ability to quickly access the device without spending time away from the patient, device portability, patient setup time, ease of use, training needs, and discovery of new devices. Therapists are under the pressure of high productivity demands. Specifically, they must maintain 75–85% billable patient hours out of total work hours. The remaining time is left for team meetings, patient care conferences, documentation not completed at point of care, walking from room to room, session clean up, and a myriad of other tasks. Importantly, time for device setup, training on new devices, and device troubleshooting is not billable time. Thus, therapists cannot spend extra time on device setup, unless they set up equipment concurrently while providing patient education verbally or supervising patients working on another therapeutic task. In addition, short training is preferred by most therapists such as during their typical 60-minute lunch break or after work. Therapists also prefer trainings that offer Continuing Education Units. Additionally, they have little time or means for troubleshooting rehabilitation devices. Therefore, any technical issues encountered with the device (e.g., screen freezing) typically lead to discontinued use. Therapists mentioned that they hear about new devices from peers, physicians, conferences, scientific journals, email lists, and vendors.
To reduce the effort needed for adoption, some device companies offer promotional trial periods so that therapists can trial the device in their clinics. This engagement of therapists is important as they play a role in administering or referring to rehabilitation device and have influence over patient decisions. Other engagement methods include in-service information sessions, providing specialized certifications and/or continuing education credits, and growing a brand with reliable customer service.
2). Rehabilitation directors’ drivers
For rehabilitation directors, drivers for adoption of rehabilitation devices were therapist use rate, impact on therapist productivity, and cost (Figure 2A, Rehabilitation Director). All 4 rehabilitation directors interviewed mentioned that their first consideration in purchase is their therapists’ needs. Specifically, they all mentioned that they buy devices that are projected to be used frequently based on therapist interest and an appropriate patient caseload (i.e., high number of patients with the diagnosis and functional level for which the device is effective in the facility). Therapists that have to borrow rehabilitation devices from each other and set sign-up schedules to share use demonstrate this demand.
For therapist productivity, rehabilitation directors consider time consumption for setup and training for devices. If the device is likely to negatively impact productivity, it may not lead to rehabilitation director approval.
As for cost, rehabilitation directors mentioned that low-cost items (such as therapy putty and bands, disposable electrodes for rehabilitation devices, and splinting supplies) can be readily purchased at their discretion if within their budget. However, high-cost items over $2,500–5,000 need to be requested during the following fiscal year budget planning and approved before the purchase can be made. The approval typically requires justification for how the new device will benefit patient outcomes, safety, or clinic marketability. Financially, the interviews indicated that the purchase of rehabilitation devices does not yield direct financial benefits to any party involved in rehabilitation, including patients, therapists, rehabilitation directors, or rehabilitation facilities. Indirect benefits may exist for rehabilitation facilities from potential patient referrals; however, such referrals typically only accrue from larger devices such bodyweight support systems or robotics.
3). Stroke survivors and caregivers’ drivers
The primary driver for rehabilitation device utilization for patients was therapists’ recommendation (45%) (Figure 2A, Patient). The majority of patients used devices because they were recommended by their therapist. Throughout their rehabilitation, patients rely on therapists to recommend treatments and rehabilitation devices for the best possible outcomes.
Secondary drivers for patients were perceived effectiveness (30%) and ease of use (23%). Perceived effectiveness included instant gratification (e.g., the electrical stimulation made their hand open while they could not open the hand by themselves), device delivering the effect that it promises (e.g., a device for foot drop helps patients to not trip), instant feedback of their performance/improvement (e.g., game scores), and readiness for the next level of treatment. Ease of use included comfort (e.g., strap that does not cause pain), device function (e.g., a device broke; a step counter under-recording the step counts is frustrating) and convenience (e.g., having to place electrode pads for an electrical stimulation device at each use 3 times a day at home is inconvenient).
Many patients favored some therapeutic activities to complete at home as opposed to just during clinic visits (70%) to gain or maintain function. Additional drivers for home use of rehabilitation devices included affordability (70%), motivating/engaging (60%), and connecting with others and/or getting information (57%) (Figure 2B, Home Use Features). These drivers are specific to home use. In clinics, since devices are part of therapy service and therapists use devices on patients, patients are not concerned with affordability or other features.
For affordability, more than half of the respondents were upfront that they could afford nothing without insurance coverage as many of them are on Medicaid and/or Social Security Disability. Unfortunately, most rehabilitation devices are not reimbursed by insurance. Thus, some people liked a loaner device while they were in therapy but could not afford to own for home use. For a small portion of patients with financial resources to acquire new rehabilitation devices, the spending ranged from tens, hundreds, to thousands of dollars out of pocket for home devices.
For motivating/engaging, patients mentioned they would be motivated to improve functional use of the affected limb in ADLs (81%), upper/lower limb mobility (74%), dexterity (64%), strength (62%), and sensation (38%). Some mentioned that the typically prescribed exercise routine is boring, and they do not enjoy participating in exercise. Some people also did not like a device that gives a perception that they are impaired (e.g., walker). More acceptable examples include rehab devices that mimic common apparel, such as wristwatches, necklaces, or a device that can be hidden under regular clothing. Other patients mentioned that they got discharged from therapy because the therapist had run out of treatment options and/or the patient had plateaued but are still motivated if a new device provides a new opportunity. Additionally, 11% of respondents mentioned that they want stroke rehabilitation focused on younger people’s needs with age-relevant goals of returning to work and caring for family members.
Lastly, patients mentioned that they value connecting with peers and getting information. Their current methods for connecting with peers and getting information about stroke rehabilitation options included in-person or online stroke support groups (70%), therapists (56%), online searches (49%), primary care physicians (47%), neurologists (23%), and other professionals including acupuncturists, nurse practitioners, physician assistants, personal trainers/other fitness instructors (16%), and stroke magazine/emails (4%). The stroke support groups were the primary source of information, likely because the majority of patients interviewed were recruited from stroke support groups. Also, the majority of patients had already been discharged from therapy at the time of interview. Thus, therapists include not only those whom they are currently receiving services from, but also those whom they received service from in the past or know from stroke support group meetings or personally. Among those who see primary care physicians, 25% mentioned inadequate fit for their post-stroke care needs (e.g., their primary care physician could not answer all their questions about their rehabilitation needs).
The healthcare company representatives mentioned that they intend to support this interest of patients in connecting with others and getting information to engage patients for stronger client relationships and continued use of rehabilitation devices. Specifically, methods for engaging patients include helping patients understand their results, helping patients share their progress in communities, providing motivation to continue, and providing ongoing technical support as well as ongoing updates for new features. Digital platforms help deliver these engagement methods.
IV. Discussion
A. Best entry points for rehabilitation devices
We recommend new restorative rehabilitation devices to be first introduced in outpatient rehabilitation facilities because of the alignment with their treatment focus and approaches. Specifically, outpatient therapy is focused on maximizing neuroplasticity through functional restoration and refining skills (such as non-compensatory movements and fine motor control/dexterity) more than other settings. Thus, restorative rehabilitation devices are well suited to support the focus of outpatient therapy. Consistently, all outpatient therapist interviewees reported using rehabilitation devices (100% vs. 33% of inpatient therapists).
Moreover, the outpatient setting is practical to initiate self-directed home use of a rehabilitation device by patients. Since the initial stress of the new stroke has subsided; patients, caregivers, and therapists can now work on incorporating home therapy protocols into their new life routine. Therapists typically prescribe home therapy exercises for patients [50] to reach the repetitions necessary for neuroplasticity and functional recovery [51] and may recommend using a rehabilitation device at home if feasible. Once patients learn how to use the device at home via multiple consults with therapists, some patients continue using the device even after discharge. It is difficult to reach stroke survivors for new rehabilitation devices after they discharge from outpatient therapy.
Although the treatment focus is more on ADLs and transition to home, inpatient rehabilitation facilities may provide a secondary entry point for rehabilitation devices if the device can be incorporated in ADL/functional task practices. Acute care, home health, and skilled nursing facilities are less appropriate settings for restorative sensorimotor rehabilitation devices due to a lack of fit with the treatment focus.
B. Stakeholders
Another finding from this work is that developers should consider the diverse needs of all stakeholders (hierarchically based on their importance), because they all have roles in the adoption process. For clinic use of rehabilitation devices, therapists, patients, and rehabilitation directors are the primary stakeholders. Our results show therapists are the primary influencers for rehabilitation device adoption at rehabilitation facilities, by using devices with patients during therapy and requesting new devices from rehabilitation directors. Therapists’ need influences rehabilitation directors’ justification for purchasing devices for clinic use. Patients are the beneficiaries of devices. Typically, patients follow their therapists’ recommendation but voice their preferences, which influence therapists. There is no direct financial benefit of buying a rehabilitation device for any party including patients, therapists, rehabilitation directors, or facilities. Indirect financial benefits may include less spending from faster discharge for inpatient rehabilitation facilities and more referrals for outpatient rehabilitation facilities for potentially better outcomes. For home use of rehabilitation devices, patients decide to buy, typically out of pocket, due to recommendations from therapists.
C. Drivers for rehabilitation device adoption in clinics
1). Drivers for therapists for clinic use
Consistent with previous studies [24], [32], [34], [29], drivers for therapists’ device use in the clinic are perceived effectiveness, patient acceptance, and effort expectancy. However, our interviewees provided more specific criteria to assist device developers. For perceived effectiveness, first, therapists use a device only if they perceive the theoretical mechanism fits their patients’ needs. Second, therapists should perceive functional improvement in patients within 1–2 months of outpatient rehabilitation, even if therapists understand delayed gratification is expected. Third, devices that improve ADLs/functional activities are more desired. Finally, scientific evidence is helpful [52], but many therapists find it not directly translatable.
For effort expectancy, it is our opinion that device setup time should be less than 7 minutes. To bill for a full 4 units of therapy within an hour session, patients must be treated for minimum 53 of 60 minutes per Medicare’s 8-minute Rule [53]. It means that no more than 7 minutes could be spent for nonbillable time such as device setup. In addition, devices that are easy to use autonomously without cognitive demand (e.g. requiring only a few button clicks) are preferred because therapists typically educate patients and set up devices at the same time to ensure full billable units.
2). Drivers for patients for clinic use
This work found that the primary driver for patients to use rehabilitation devices in the clinic is therapist recommendation. While previous literature showed perceived effectiveness and ease of use as important drivers in technology acceptance [30], [34], [29], in the clinic, patients rely on therapists to decide and use devices. Thus, patients’ perceived effectiveness and ease of use play a lesser role in the clinic than in the home.
3). Drivers for rehabilitation directors for clinic acquisition
Unique to this work, drivers for rehabilitation directors were examined. Our results indicate their drivers are therapist use volume, productivity, and cost. Devices <$5,000 can be more easily authorized by the rehabilitation director than higher cost items [38] that require administrative approval.
D. Drivers for rehabilitation device adoption for home
Even though patients are the decision makers, payers, and beneficiaries for home use of rehabilitation devices, therapists still play a significant role in their acquisition decision. Many patients mentioned their reason for using new rehabilitation devices independently at home was by way of therapist recommendation. Stroke survivors also learn about new rehabilitation devices from support groups and online. However, without their trusted professional’s opinion, stroke survivors may not fully buy in to a device.
For perceived effectiveness, rehabilitation devices associated with immediate gratification and improved quality of life are easier to adopt. For devices that lack immediate gratification, patients may not know if they are using the device correctly in the absence of therapist guidance and may give up too early if they do not see immediate results. For those devices, patient education should clearly outline time needed to see results to minimize disappointment. For ease of use at home, patients desire “magic bullet” devices that involve no interruption to their daily routine, no setup, no maintenance, and no pain.
Additional drivers specific to home use include affordability, motivating/engaging devices, and ability to connect with others and get information. While these constructs have been identified before [54], [24], [33], our results provide detailed personal accounts. For example, more than half of stroke survivors in our interviews have financial difficulty, and only a small portion can afford rehabilitation devices. Some healthcare companies have dedicated case managers to assist patients with payment programs or scholarships/grants to increase rehabilitation device acquisition.
For motivation, most patients are motivated by a device that will help them improve functional use of their limbs. This is particularly important for younger stroke survivors with active lifestyles and familial roles. Additionally, rehabilitation devices can be motivating by providing new treatment options especially if a patient has plateaued in recovery. Some patients are motivated by new exercise routines with devices, while others consider exercise to be boring. Lastly, devices that mimic common apparel or can be hidden are preferred over devices that make patients appear impaired.
For connecting and getting information, some healthcare companies use online platforms to host patient interactions with their peers and engage patients with their companies’ rehabilitation devices. However, the success of these platforms is unclear for stroke survivors who are typically older adults [54].
E. Limitations
The focused probing and clarification techniques used did not include audio recording and transcription of the interviews which may increase the risk of subjective interpretation of the information. However, recording would have jeopardized anonymity of respondents because voice prints are identifiable. Exact length of time of interviews was not routinely documented. In addition, we were interested in U.S. market issues, so all interviewees were from the United States. Therefore, our findings are not generalizable to other countries and healthcare systems. The sample size of rehabilitation directors was low. People who agreed to interview may be more social and motivated and therefore might not represent all stakeholders. Interviewees had experienced different rehabilitation devices; therefore, drivers mentioned may be specific to certain devices and not generalizable. The scope of this work was limited to low-profile devices, as our interviewees’ experience was predominantly in low-profile devices. High-profile devices such as robotics systems will have different product characteristics of importance for adoption [55], [56]. We did not examine differences in themes by demographics, such as education level, socio-economic status, gender, level of technology anxiety, self-efficacy, stroke severity, and attitude. We did not complete a stakeholder analysis to rank importance of stakeholders’ drivers.
V. Conclusion
This project contributes several new ideas to improve rehabilitation device adoption. First, we recommend the best settings for introducing new low-profile restorative rehabilitation devices are outpatient followed by inpatient rehabilitation facilities due to their therapy focus on restoration of function. Second, we introduce rehabilitation directors as one of the stakeholders in rehabilitation device adoption. We also identify influences that stakeholder groups have on each other. Specifically, therapists are the primary influencers by advocating for device needs to rehabilitation directors for facility acquisitions, and also by recommending a rehabilitation device to patients for home use. In turn, patient acceptance influences therapists’ use of rehabilitation devices. Rehabilitation directors make purchase decisions based on therapists’ utilization rate, productivity, and budget.
Third, we separate stakeholders and drivers for clinic vs. home use of rehabilitation devices. Fourth, this work provides detailed accounts and contexts for developers to understand stakeholder needs. For example, we recommend device setup time to be less than 7 minutes to meet therapists’ productivity demands while abiding by the 8-minute Medicare Rule [53]. This provides an important setup benchmark for developers to consider. This project also specifies devices <$5,000 can be purchased at the discretion of rehabilitation directors without requiring higher administration approval. Purchase of a rehabilitation device results in only cost and no direct profit for all parties involved in rehabilitation. These new findings are expected to contribute to accelerating translation of rehabilitation devices in clinics and home to ultimately improve patients’ recovery.
Acknowledgments
This work was supported by the NIH/NCATS TL1 TR001451 & UL1 TR001450, NIH/NICHD 3R41HD090792-01A1S1, R41HD090792-01A1, R01HD094731-01A1 and NIH/NIGMS P20GM109040. The authors would like to acknowledge Jillian Harvey, Austen Hayes, Emily Case, and Kacie Neutz for their assistance with this project. The authors would also like to thank the interviewees for their insights and time.
References
- [1].N. C. f. H. Statistics, “Summary Health Statistics Tables for U.S. Adults: National Health Interview Survey,” ed: Centers for Disease Control and Prevention, 2018. [Google Scholar]
- [2].Benjamin EM, Paul; Alonso Alvaro; Bittencourt Marcio; Callaway Clifton; et al. , “Heart Disease and Stroke Statistics— 2019 Update,” American Heart Association, pp. 139:e56–e528, 2019, doi: : 10.1161/CIR.000000000000065. March 5, 2019. [DOI] [PubMed] [Google Scholar]
- [3].O’Mahony PG, Thomson RG, Dobson R, Rodgers H, and James OF, “The prevalence of stroke and associated disability,” (in eng), J. Public Health Med, vol. 21, no. 2, pp. 166–71, June 1999, doi: 10.1093/pubmed/21.2.166. [DOI] [PubMed] [Google Scholar]
- [4].Stewart JC and Cramer SC, “Patient-reported measures provide unique insights into motor function after stroke,” Stroke, vol. 44, no. 4, pp. 1111–6, April 2013, doi: 10.1161/STROKEAHA.111.674671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nakayama H, Jorgensen HS, Raaschou HO, and Olsen TS, “Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study,” (in eng), Arch Phys Med Rehabil, vol. 75, no. 4, pp. 394–8, April 1994. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/8172497. [DOI] [PubMed] [Google Scholar]
- [6].Brochard S, Robertson J, Médée B, and Rémy-Néris O, “What’s new in new technologies for upper extremity rehabilitation?,” (in eng), Curr. Opin. Neurol, vol. 23, no. 6, pp. 683–7, December 2010, doi: 10.1097/WCO.0b013e32833f61ce. [DOI] [PubMed] [Google Scholar]
- [7].Maier M, Ballester BR, and Verschure P, “Principles of Neurorehabilitation After Stroke Based on Motor Learning and Brain Plasticity Mechanisms,” (in eng), Front. Syst. Neurosci, vol. 13, p. 74, 2019, doi: 10.3389/fnsys.2019.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Friedman N et al. , “Retraining and assessing hand movement after stroke using the MusicGlove: comparison with conventional hand therapy and isometric grip training,” (in eng), J. Neuroeng. Rehabil, vol. 11, p. 76, April 30 2014, doi: 10.1186/1743-0003-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lin CY, Tsai CM, Shih PC, and Wu HC, “Development of a novel haptic glove for improving finger dexterity in poststroke rehabilitation,” (in eng), Technol. Health Care, vol. 24 Suppl 1, pp. S97–103, 2015, doi: 10.3233/thc-151056. [DOI] [PubMed] [Google Scholar]
- [10].Seo NJ et al. , “TheraBracelet Stimulation During Task-Practice Therapy to Improve Upper Extremity Function After Stroke: A Pilot Randomized Controlled Study,” (in eng), Phys. Ther, vol. 99, no. 3, pp. 319–328, March 1 2019, doi: 10.1093/ptj/pzy143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yue Z, Zhang X, and Wang J, “Hand Rehabilitation Robotics on Poststroke Motor Recovery,” (in eng), Behav. Neurol, vol. 2017, p. 3908135, 2017, doi: 10.1155/2017/3908135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang F, Jones CL, Shastri M, Qian K, Kamper DG, and Sarkar N, “Design and Evaluation of an Actuated Exoskeleton for Examining Motor Control in Stroke Thumb,” (in eng), Adv Robot, vol. 30, no. 3, pp. 165–177, 2016, doi: 10.1080/01691864.2015.1105867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Orand A, Aksoy EE, Miyasaka H, Levy C, Zhang X, and Menon C, “Bilateral tactile feedback-enabled training for stroke survivors using microsoft kinecttm,” Sensors (Switzerland), Article vol. 19, no. 16, 2019, Art no. 3474, doi: 10.3390/s19163474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bobin M, Bimbard F, Boukallel M, Anastassova M, and Ammi M, “SpECTRUM: Smart ECosystem for sTRoke patientós Upper limbs Monitoring,” Smart Health, vol. 13, p. 100066, 2019. [Google Scholar]
- [15].Bobin M, Anastassova M, Boukallel M, and Ammi M, “Design and Study of a Smart Cup for Monitoring the Arm and Hand Activity of Stroke Patients,” (in eng), IEEE journal of translational engineering in health and medicine, vol. 6, pp. 2100812–2100812, 2018, doi: 10.1109/JTEHM.2018.2853553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bai Z and Fong KNK, ““Remind-to-Move” Treatment Enhanced Activation of the Primary Motor Cortex in Patients with Stroke,” (in eng), Brain Topogr., February 13 2020, doi: 10.1007/s10548-020-00756-7. [DOI] [PubMed] [Google Scholar]
- [17].Fong KN et al. , “Effects of sensory cueing on voluntary arm use for patients with chronic stroke: a preliminary study,” (in eng), Arch. Phys. Med. Rehabil, vol. 92, no. 1, pp. 15–23, January 2011, doi: 10.1016/j.apmr.2010.09.014. [DOI] [PubMed] [Google Scholar]
- [18].Mehrholz J, Pohl M, Platz T, Kugler J, and Elsner B, “Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke,” (in eng), Cochrane Database Syst. Rev, vol. 9, no. 9, p. Cd006876, September 3 2018, doi: 10.1002/14651858.CD006876.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mehrholz J, Thomas S, and Elsner B, “Treadmill training and body weight support for walking after stroke,” (in eng), Cochrane Database Syst. Rev, vol. 8, no. 8, p. Cd002840, August 17 2017, doi: 10.1002/14651858.CD002840.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Veerbeek JM, Langbroek-Amersfoort AC, van Wegen EE, Meskers CG, and Kwakkel G, “Effects of Robot-Assisted Therapy for the Upper Limb After Stroke,” (in eng), Neurorehabil. Neural Repair, vol. 31, no. 2, pp. 107–121, February 2017, doi: 10.1177/1545968316666957. [DOI] [PubMed] [Google Scholar]
- [21].Krueger RB, Sweetman MM, Martin M, and Cappaert TA, “Occupational Therapists’ Implementation of Evidence-Based Practice: A Cross Sectional Survey,” (in eng), Occup Ther Health Care, pp. 1–24, May 5 2020, doi: 10.1080/07380577.2020.1756554. [DOI] [PubMed] [Google Scholar]
- [22].Turchetti G VN, Trieste L, Romiti S, Geisler E, Micera S, “Why effectiveness of robot-mediated neurorehabilitation does not necessarily influence its adoption,” Institute of Electrical and Electronics, vol. 7, pp. 143–153, 2014, doi: 10.1109/RBME.2014.2300234. [DOI] [PubMed] [Google Scholar]
- [23].Burridge JH and Hughes AM, “Potential for new technologies in clinical practice,” (in eng), Curr. Opin. Neurol, vol. 23, no. 6, pp. 671–7, December 2010, doi: 10.1097/WCO.0b013e3283402af5. [DOI] [PubMed] [Google Scholar]
- [24].Chen CC and Bode RK, “Factors influencing therapists’ decision-making in the acceptance of new technology devices in stroke rehabilitation,” (in eng), Am. J. Phys. Med. Rehabil, vol. 90, no. 5, pp. 415–25, May 2011, doi: 10.1097/PHM.0b013e318214f5d8. [DOI] [PubMed] [Google Scholar]
- [25].Lin LF, Lin YJ, Lin ZH, Chuang LY, Hsu WC, and Lin YH, “Feasibility and efficacy of wearable devices for upper limb rehabilitation in patients with chronic stroke: a randomized controlled pilot study,” (in eng), Eur. J. Phys. Rehabil. Med, vol. 54, no. 3, pp. 388–396, June 2018, doi: 10.23736/s1973-9087.17.04691-3. [DOI] [PubMed] [Google Scholar]
- [26].Lakshminarayanan K, Wang F, Webster JG, and Seo NJ, “Feasibility and usability of a wearable orthotic for stroke survivors with hand impairment,” Disability and rehabilitation. Assistive technology, vol. 12, no. 2, pp. 175–183, February 2017, doi: 10.3109/17483107.2015.1111945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Parker J, Powell L, and Mawson S, “Effectiveness of Upper Limb Wearable Technology for Improving Activity and Participation in Adult Stroke Survivors: Systematic Review,” (in eng), J. Med. Internet Res, vol. 22, no. 1, p. e15981, January 8 2020, doi: 10.2196/15981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lum PS, Taub E, Schwandt D, Postman M, Hardin P, and Uswatte G, “Automated Constraint-Induced Therapy Extension (AutoCITE) for movement deficits after stroke,” (in eng), J. Rehabil. Res. Dev, vol. 41, no. 3a, pp. 249–58, May 2004, doi: 10.1682/jrrd.2003.06.0092. [DOI] [PubMed] [Google Scholar]
- [29].Shirota C, Balasubramanian S, and Melendez-Calderon A, “Technology-aided assessments of sensorimotor function: current use, barriers and future directions in the view of different stakeholders,” (in eng), J. Neuroeng. Rehabil, vol. 16, no. 1, pp. 53-53, 2019, doi: 10.1186/s12984-019-0519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Davis FD, Bagozzi RP, & Warshaw PR, “User Acceptance of Computer Technology: A Comparison of Two Theoretical Models,” Economics, 1989. [Google Scholar]
- [31].Venkatesh V, Morris MG, Davis GB, and Davis FD, “User Acceptance of Information Technology: Toward a Unified View,” MIS Quarterly, vol. 27, no. 3, pp. 425–478, 2003, doi: 10.2307/30036540. [DOI] [Google Scholar]
- [32].Liu L, Miguel Cruz A, Rios Rincon A, Buttar V, Ranson Q, and Goertzen D, “What factors determine therapists’ acceptance of new technologies for rehabilitation - a study using the Unified Theory of Acceptance and Use of Technology (UTAUT),” (in eng), Disabil. Rehabil, vol. 37, no. 5, pp. 447–55, 2015, doi: 10.3109/09638288.2014.923529. [DOI] [PubMed] [Google Scholar]
- [33].Hochstenbach-Waelen A and Seelen H, “Embracing change: Practical and theoretical considerations for successful implementation of technology assisting upper limb training in stroke,” J. Neuroeng. Rehabil, vol. 9, p. 52, 08/February 2012, doi: 10.1186/1743-0003-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kerr A, Smith M, Reid L, and Baillie L, “Adoption of Stroke Rehabilitation Technologies by the User Community: Qualitative Study,” JMIR rehabilitation and assistive technologies, vol. 5, no. 2, pp. e15–e15, 2018, doi: 10.2196/rehab.9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Swank C, Sikka S, Driver S, Bennett M, and Callender L, “Feasibility of integrating robotic exoskeleton gait training in inpatient rehabilitation,” (in eng), Disabil Rehabil Assist Technol, vol. 15, no. 4, pp. 409–417, May 2020, doi: 10.1080/17483107.2019.1587014. [DOI] [PubMed] [Google Scholar]
- [36].Wang Q et al. , “Stroke Patients’ Acceptance of a Smart Garment for Supporting Upper Extremity Rehabilitation,” (in eng), IEEE J Transl Eng Health Med, vol. 6, p. 2101009, 2018, doi: 10.1109/jtehm.2018.2853549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Backus D, Winchester P, and Tefertiller C, “Translating Research Into Clinical Practice: Integrating Robotics Into Neurorehabilitation for Stroke Survivors,” Top. Stroke Rehabil, vol. 17, no. 5, pp. 362–370, 2010/September/01 2010, doi: 10.1310/tsr1705-362. [DOI] [PubMed] [Google Scholar]
- [38].Jones M, Mueller J, and Morris J, “Advanced technologies in stroke rehabilitation and recovery,” (in eng), Top. Stroke Rehabil, vol. 17, no. 5, pp. 323–7, Sep-Oct 2010, doi: 10.1310/tsr1705-323. [DOI] [PubMed] [Google Scholar]
- [39].I.-C. a. NIH. “Funding Opportunity Announcement (FOA) PA-19–029.” https://sbir.cancer.gov/programseducation/icorps (accessed.
- [40].Harrell MC and Bradley MA, “Data collection methods. Semi-structured interviews and focus groups,” DTIC Document, 2009. [Google Scholar]
- [41].Creswell J, Qualitative inquiry and research design, choosing among five approaches. Thousand Oaks: Sage Publications, Inc, 2013. [Google Scholar]
- [42].Bloomberg LV, M, 1, Ed. Completing your qualitative dissertation, a roadmap from beginning to end. Thousand Oaks: Sage Publications, Inc., 2008. [Google Scholar]
- [43].Richards, J. LM, Readme First for a User’s Guide to Qualitative Methods, 3rd ed. Thousand Oaks: Sage, 2013. [Google Scholar]
- [44].Krause N, “A comprehensive strategy for developing closed-ended survey items for use in studies of older adults,” (in eng), J. Gerontol. B Psychol. Sci. Soc. Sci, vol. 57, no. 5, pp. S263–74, September 2002, doi: 10.1093/geronb/57.5.s263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Smith J, Flowers P, Larkin M, Interpretative phenomenological analysis: Theory, method, and research. London: Sage, 2009. [Google Scholar]
- [46].Gill P, Stewart K, Treasure E, and Chadwick B, “Methods of data collection in qualitative research: interviews and focus groups,” Br Dent J, vol. 204, no. 6, pp. 291–5, March 22 2008, doi: 10.1038/bdj.2008.192. [DOI] [PubMed] [Google Scholar]
- [47].Hsieh HF and Shannon SE, “Three approaches to qualitative content analysis,” (in eng), Qual. Health Res, vol. 15, no. 9, pp. 1277–88, November 2005, doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- [48].Inpatient Rehabilitation Facility Medical Necessity Criteria, C. f. M. a. M. Services; 110.2, 2017. [Google Scholar]
- [49].Yock, J. PZ; Makower T; Brinton U; Kumar J; Watkins J; Denend L; Krummel T; Kurihara C, Biodesign: The Process of Innovating Medical Technologies, 2nd ed. Cambridge: Cambridge University Press, 2015, pp. 321–322. [Google Scholar]
- [50].Proffitt R, “Relationships between Occupational Therapy Practitioner Characteristics and Home Exercise Program Prescription for Clients with Neurological Injuries,” (in eng), Occup Ther Health Care, vol. 33, no. 4, pp. 381–393, October 2019, doi: 10.1080/07380577.2019.1649786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kimberley TJ, Samargia S, Moore LG, Shakya JK, and Lang CE, “Comparison of amounts and types of practice during rehabilitation for traumatic brain injury and stroke,” (in eng), J. Rehabil. Res. Dev, vol. 47, no. 9, pp. 851–62, 2010, doi: 10.1682/jrrd.2010.02.0019. [DOI] [PubMed] [Google Scholar]
- [52].Stein J, “Adopting new technologies in stroke rehabilitation: the influence of the US health care system,” (in English), Eur. J. Phys. Rehabil. Med, vol. 45, no. 2, pp. 255–258, June 2009. 2009. [PubMed] [Google Scholar]
- [53].Jannenga H. “Physical Therapists’ Guide to the 8-Minute Rule.” https://www.webpt.com/8-minute-rule/ (accessed.
- [54].Lemke M, Rodríguez Ramírez E, Robinson B, and Signal N, “Motivators and barriers to using information and communication technology in everyday life following stroke: a qualitative and video observation study,” Disabil. Rehabil, vol. 42, no. 14, pp. 1954–1962, 2020, doi: 10.1080/09638288.2018.1543460. [DOI] [PubMed] [Google Scholar]
- [55].Lo K, Stephenson M, and Lockwood C, “Adoption of robotic stroke rehabilitation into clinical settings: a qualitative descriptive analysis,” International journal of evidence-based healthcare, vol. Publish Ahead of Print, 2020, doi: 10.1097/XEB.0000000000000231. [DOI] [PubMed] [Google Scholar]
- [56].Maciejasz P, Eschweiler J, Gerlach-Hahn K, Jansen-Troy A, and Leonhardt S, “A survey on robotic devices for upper limb rehabilitation,” (in eng), J. Neuroeng. Rehabil, vol. 11, p. 3, January 9 2014, doi: 10.1186/1743-0003-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


