1. Introduction
The terms ‘nutrigenomics’ and ‘nutrigenetics’ are often used to define the scientific study exploring the interplay between nutritional intake, genetic variation and subsequent gene expression (health outcomes).1 Recently, the broader term ‘lifestyle genomics’ has been used to define interactions between genetics and lifestyle factors (e.g., nutrition, physical activity, sleep, and smoking) and subsequent health outcomes.2 While early research was met with calls for tempering expectations about the ability for nutrigenomics to improve health outcomes, particularly in light of the complexities associated with changing lifestyle behaviors such as diet,3 more recent advances in the field have generated excitement about the potential for nutrigenomics to provide positive health benefits.
Genetic differences in caffeine metabolism provide a clear example of how the science of nutrigenomics can be translated into the provision of personalized nutrition recommendations. Genetic variation within the CYP1A2 gene at rs762551 can determine one’s risk of cardiovascular disease. Those who possess the AC or CC genotype are suggested to metabolize caffeine slowly, thus having a significantly increased risk of developing hypertension (HTN) and experiencing a myocardial infarction (MI), or heart attack, but only when caffeine intake exceeds 200 mg daily (the equivalent of about two cups of coffee). However, for those who metabolize caffeine quickly (AA genotype), consuming up to 400 mg of caffeine daily (four cups of coffee) does not appear to increase the risk of HTN or MI.4 Given that approximately 50% of the participants in these studies metabolized caffeine slowly, this genetic-based knowledge has considerable implications for individual nutrition guidelines as well as public health nutrition. While current Health Canada guidelines suggest that <400 mg caffeine per day for most adults is safe, nutrigenomics literature indicates that this level can in fact be harmful for approximately 50% of the population.5
Canadians express great interest in this area of nutrition,6 and with the ability to personalize nutrition recommendations based on genetic variation,7 this emerging area of healthcare has the potential to motivate behavior change and improve overall health and wellbeing. Currently, several companies offer nutrigenetic testing to Canadian consumers (Table 1), with prices ranging from approximately $90 up to approximately $450 CDN.8 Given that genetic testing for personalized nutrition is commercially available and increasingly popular among Canadian consumers, it is not surprising that there is a growing public and professional interest in research related to this type of genetic testing.9
Table 1.
Companies offering nutrigenetic testing services to Canadians
| Company name | Does the company offer direct-to-consumer (DTC) nutrigenetic testing? |
|---|---|
| 23andMe | Yes |
| Athletigen | Yes |
| CRI Genetics | Yes |
| DNAfit | Yes |
| dnaPower | Yes |
| EasyDNA | Yes |
| Gene Blueprint | Yes |
| MyDNA | Yes |
| Nutrigenomix, Inc. | No |
While consumers have expressed considerable interest in nutrigenomics, some healthcare professionals and academia have expressed greater skepticism.10 A recent study found that Canadian registered dietitians (RDs) were concerned about legal and ethical implications of this testing, but were still interested in learning more about the field of nutrigenomics.11 Similarly, food and nutrition students (many of whom may become RDs and other healthcare providers) have also expressed a keen interest to learn more, and overall demonstrated positive attitudes towards nutrigenomics testing. However, their understanding of potential ethical considerations of this type of testing was overall low.12
Canadian literature has lacked focus on legal and ethical considerations of nutrigenetic testing across the spectrum of nutrition care stemming from science and moving through to consumers. While the ‘early commercialization’ of nutrigenetic testing was critiqued over a decade ago, and the authors suggested that this was concerning, and warranted further policy consideration,13 concerns with lack of health policy and regulation in the field of nutrigenomics remain. More recently, insightful recommendations for healthcare professionals and future research endeavors have been articulated, but this perspective was more broad-spanning and not specific to the context of Canadian legislation.14 Despite minimal improvements to health policy and law, there have been significant scientific advancements in the field of nutrigenomics over the past decade. With scientific advancement, consumer interest and subsequent industry response, it is evident that a critical examination of legal and ethical considerations relating to nutrigenetic testing in Canada, and resulting recommendations for policy and legislation, is urgently needed.15
Therefore, the purpose of this paper is to bridge this research gap by providing a critical examination of legal and ethical considerations for nutrigenomics in Canada, while providing recommendations to improve Canadian regulation to positively affect each stage of the process—from science, through to consumers. The following sections provide an overview of nutrigenetic testing globally, followed by nutrigenomics in Canada and identify the current legislative regime governing this testing. Following this, we propose revisions to current health policy and law to help improve the field. Recommendations for health policy and law relate directly to five aspects of the nutrigenetic testing process: (i) the science behind nutrigenomics; (ii) the nutrigenetic testing industry; (iii) genetic testing laboratories; (iv) healthcare professionals (HCPs); and (v) protection of consumers. We suggest that enhancing the legal and ethical framework governing nutrigenomics will both directly and indirectly benefit the field and contribute to positive individual and public health outcomes.
2. Nutrigenomics Globally
Nutrigenomics is offered to consumers globally, but policy and regulation remain incomplete across the world.16 While there are several patents related to genetics and nutrigenomics,17 individual gene-nutrient-health outcome interactions generally cannot be patented; thus, companies are free to select such interactions to use in their tests as they wish. Companies provide a wide range of nutrigenetic information related to health topics such as weight management, taste preferences, cardiovascular health, food intolerances, and nutrient metabolism. Indeed, this information differs greatly between companies; for example, with respect to weight management, some companies provide information on fat storage, body size, and weight regain, while others provide information related to weight loss responses from consuming different quantities of macronutrients.18 Indeed, the scientific validity and clinical utility of tests available to consumers globally vary greatly. Researchers suggest that much regulation is required to help personalized nutrition reach its expected benefits.19
Currently, this regulation is highly variable around the world. In the US, the quality of laboratory testing is regulated through the ‘Clinical Laboratory Improvement Amendments (CLIA)’. CLIA aims to ensure accurate and reliable test results from human specimens and oversees more than 260,000 laboratories in the US.20 This regulatory action is certainly a step in the right direction for helping to ensure the accuracy of genetic testing. However, other regulatory actions are lacking. In the US and the United Kingdom (UK), consumers have expressed reservations about the secure handling of nutrigenomics-related data as well as the lack of regulation around scientific evidence supporting genetic tests, and with this, the importance of appropriate regulation has been noted.21 There has been some progress in this area, with the US Food and Drug Administration (FDA) now overseeing moderate-to-high risk medical DTC genetic tests. However, nutrigenetic tests typically do not fall in the category of moderate-to-high risk medical tests and thus the US FDA remain limited in their involvement regulating nutrigenetic DTC tests.22 On the other hand, the UK and Australia have developed specific governing bodies responsible for overseeing genetic testing processes. The UK’s Advisory Committee on Genetic Testing was established in 1996 and was responsible for overseeing issues related to public health and consumer protection.23 This advisory committee took an important step in developing a Code of Practice for genetic testing, which provided requirements regarding informed consent, genetic counseling, scientific validity, and the utility of genetic tests. Later, in 1999, this advisory committee was disbanded, and the Human Genetics Commission became responsible for the Code of Practice. Unfortunately, after one case of enforcing the code, the Human Genetics Commission concluded that they would no longer be responsible for enforcing this Code of Practice.24 As such, the established Code of Practice remains unenforced today.
In Australia, the genetic testing industry (including nutrigenomics) remains closely regulated through the Therapeutic Goods Administration. However, this country’s regulatory oversight is challenged by offshore genetic testing companies who can access Australian consumers via the internet, but are not required to abide by Australian legislation.25 Thus, concerns remain related to privacy, the return of actionable genetic findings without appropriate genetic counseling and medical oversight, varying scientific validity, informed consent (or lack thereof), and others.26 Australia also struggles with a lack of genetic nondiscrimination regulation.27 While Australia is similar to Canada in this respect (as further discussed below), there are some states in the US and several countries worldwide that have developed laws surrounding genetic information nondiscrimination in addition to laws that ban DTC genetic testing.28 In Europe, seven countries have adapted legislation governing genetic testing, which relates to requirements for laboratories, the provision of diagnostic information, enforcing age restrictions on genetic testing, and others; while of some relevance to the field of nutrigenomics, none of this established legislation is specific to the unique field of nutrigenomics.29 In China, the Ministry of Health has made an attempt to oversee genetic counseling, with the development of guidelines for clinical genetic counseling. Furthermore, China recently announced that they are in the process of developing a guide for consumers to make informed decisions about nutrigenetic tests and for companies to provide information and messaging that are not misleading to the consumer.30 This is certainly a notable step towards improving regulatory oversight in the field.
Overall, it is evident that across the world, legislation related to genetic testing and nutrigenomics is highly variable. Thus, country-specific analyses of legislative considerations and recommendations are needed to help move forward with proper oversight and regulation.
3. Nutrigenomics in Canada
In Canada, minimal legislation exists specific to genetic testing in general, let alone nutrigenetic testing. In 2017, the Parliament of Canada provided Royal Assent to Bill S-201, the Genetic Non-Discrimination Act (GNDA), which prohibited and prevented genetic discrimination.31 This new law aimed to protect consumers from discrimination by employers based on the results of a genetic test, to eliminate the requirement for an individual to undergo genetic testing, and to prohibit insurance companies from demanding genetic test results.32 While the GNDA has been lauded as a critical law necessary to provide comprehensive protection to Canadians,33 the province of Quebec challenged the law, arguing that the federal government is encroaching on provincial jurisdiction. In December of 2018, the Quebec Court of Appeal held that Sections 4–9 of the GNDA were ‘ultra vires’—which is to say, these sections are unconstitutional.34 As a consequence, the GNDA is not a valid law, and the legitimacy of the GNDA will be determined by the Supreme Court of Canada.35
Provincial governments have also considered prohibiting genetic discrimination. For example, Bill 164 added genetic characteristics to the Ontario Human Rights Code, but the Bill only reached the second reading before the Liberal government at the time dissolved.36 Since then, two new bills have been introduced—Bill 3537 and Bill 4038—both of which seek to prohibit discrimination on the basis of genetic characteristics. It should be noted that existing human rights legislation may already protect individuals from being discriminated against on the basis of genetic information—and courts have considered genetics in their decisions.39 In fact, some researchers posit that it may be unnecessary to enact new legislation to directly prohibit genetic discrimination.40 Beyond genetic discrimination, more stringent oversight of nutrigenetic testing is necessary to protect the public as well as to ensure that the potential public health benefits of nutrigenetic testing will come to fruition.
Canadian-based policy and legislation could impact various stages of the nutrigenetic testing process. Figure 1 depicts a schematic representation of the current nutrigenetic testing process in Canada. First, research provides the scientific basis for nutrigenomics. This knowledge is then translated by industry, which may or may not comprise a team of HCPs and/or academic researchers. This allows for the provision of nutrigenetic testing to consumers across the country. Some nutrigenetic tests are sold to HCPs, who then sell these tests to clients and interpret the results for the client. The majority of companies offer DTC nutrigenetic tests whereby a HCP does not act as an intermediary between the company and the consumer. Thus, consumers’ first point of contact is either a nutrigenetic testing company or a HCP who offers nutrigenetic testing in their practice. Typically, it is the company that is responsible for coordinating the DNA laboratory analysis, which is often completed using buccal swabs or saliva samples. Once the laboratory analysis is complete, the company either sends the genetic results directly to the consumer for self-interpretation or the results are sent to the HCP who is responsible for connecting with the consumer to interpret their results. A consumer could also contact a HCP to review the results of a DTC genetic test if they are seeking out further information or interpretation of the test. The following provides an overview of legal and ethical implications across the spectrum of nutrigenetic testing, from science and research through to consumers (Figure 1), while providing legislative recommendations for Canada.
Figure 1.
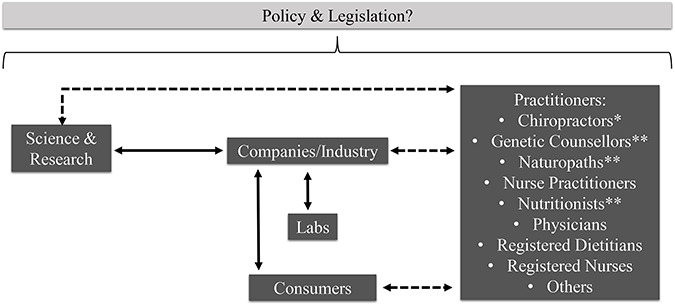
Schematic representation of key components of nutrigenomics practice in Canada, which could be influenced by policy/legislation. Solid arrows indicate typically occurring relationships. Dashed arrows indicate potentially occurring relationships. *Practitioners not regulated under provincial legislation to abide by ethical principles of clinical practice in all provinces/territories. **Practitioners not regulated under provincial legislation to abide by ethical principles of clinical practice in all provinces/territories and whose title is not legally protected in all provinces/territories
4. The Science
4.1. Overview of current nutrigenetic legal and ethical considerations
Although there are a number of genetic tests available to the public, through DTC services or provided by HCPs, perhaps one of the largest concerns is the lack of regulatory standards for genetic testing companies to follow in order to demonstrate that their tests are backed by robust scientific knowledge before going to market. Therefore, nutrigenetic testing companies can sell a test with little or no scientific evidence to support the information and recommendations provided.41 For example, one nutrigenetic testing company suggests that individuals with the CC genotype of CYP1A2 (rs762551) exhibit increased feelings of ‘jitteriness’ and recommend L-theanine supplementation to improve CYP1A2 function.42 Despite this claim and recommendation, there is a lack of scientific literature to support this information. Rather, research suggests that ‘jitteriness’ and other physical sensations related to caffeine consumption have been linked to genetic variation in the ADORA2A gene.43 This is one of many examples of results that are not based on robust scientific findings. While interpretation and extrapolation of scientific evidence is one issue, another challenge is determining the level of evidence considered sufficient to provide information and recommendations to consumers.44 In addition, scientific evidence changes with time, further complicating the process of ensuring scientific validity of consumer nutrigenetic tests. With many nutrigenomics studies funded by industry,45 conflicts of interest (COIs) are another consideration. Because of the multitude of factors impacting the science of nutrigenomics, this is certainly a challenging area to improve within the field.
4.2. Recommendations to improve the science
The current lack of regulation around the scientific validity of nutrigenetic testing inevitably poses a risk of harm. Therefore, determining appropriate standards for scientific validity is of utmost importance. While randomized controlled trials (RCTs) have been deemed the gold standard for original research, RCTs are often not feasible or ethical within the broad field of nutrition.46 Because of this, nutrition guidelines are often developed based on studies which are epidemiological and observational in nature.46 Therefore, we recommend that the level of evidence deemed sufficient in the context of nutrigenomics be comparable to the sufficient level of evidence set for population-based nutrition guidelines, as employed by organizations such as the Institute of Medicine or Health Canada. Details of proposed guidelines to determine the scientific validity and evidence for genotype-based dietary advice have recently been published elsewhere.47 While substantial knowledge exists to support the provision of nutrigenetic testing in clinical practice, we should consider COIs present in this body of research, similar to how COIs are considered in a broader nutrition research context. COIs in nutrition research have been a recent topic of discussion in the literature, with recommendations developed to avoid or limit bias in this work. These recommendations include advocating for increased federal funding, being transparent about industry funding, developing a prespecified research plan, and others.48
With evolving knowledge about the science of nutrigenomics, we must also consider the need to update nutrigenetic tests with the most current and robust scientific knowledge. In some cases, this may alter a consumer’s previous genetic results. However, it should be noted that this consideration is not specific to the field of personalized nutrition; rather, it is a consideration relevant for the field of nutrition as a whole. For example, it was formerly suggested that limiting the intake of dietary cholesterol would help to optimize cardiovascular health.49 With enhanced research, we now understand that dietary cholesterol has minimal to no impact on serum cholesterol levels and overall cardiovascular health50 and therefore focusing on restricting dietary cholesterol is no longer recommended to manage dyslipidemia and optimize cardiovascular health.51 Therefore, the genetic testing industry should not be vilified if/when the information in their reports change based on new knowledge. Rather, the industry should be recognized for helping to ensure a science-based approach.
To guide ethical and science-based practice, many areas of healthcare develop clinical practice guidelines (CPGs).52 However, despite wide-spread uptake in clinical practice, CPGs in nutrigenomics do not exist. The development of CPGs would be a positive step towards ensuring a high level of evidence is available to support nutrigenetic tests. Like all CPGs, these should be developed by expert researchers in the field and should provide a critical evidence summary of various gene-nutrient-health outcome interactions. Examples of such interactions of interest may relate to currently available nutrigenetic tests such as those related to weight management, taste preferences, cardiovascular health, food intolerances, and nutrient metabolism, among others. Furthermore, regulating the genetic testing industry to help ensure that only tests with established scientific/clinical validity and utility are offered promotes two key principles of ethics: beneficence and nonmaleficence.53 We recommend a regulatory process be set out by Health Canada, given Health Canada’s mission to ‘help the people of Canada maintain and improve their health’ as well as their core role to regulate the health industry in order to ‘facilitate the provision of products vital to the health and well-being of [Canadian] citizens.’ Health Canada should consider the abovementioned factors when determining scientific validity.54 It should also be noted that this concept of ensuring scientific/clinical validity and utility of health advice offered to the public certainly spans broader than just the nutrigenetic testing industry and is applicable to the nutrition industry as a whole.
From a public health perspective, we should further consider whether or not population-based recommendations should change, with increasing knowledge of the human genome and how genetic variation can affect nutritional guidelines. Using the previously mentioned caffeine and CYP1A2 example, Health Canada’s recommendation to consume less than 400 mg of caffeine per day for adult women (who are past child-bearing age) and men55 would pose a risk of harm to approximately 50% of the population. Erring on the side of caution and updating this population-based recommendation to no more than 200 mg of caffeine per day could help to enhance the health of the entire population, rather than just half of our population.56 This example demonstrates how nutrigenomics can help enhance our understanding of conflicting population-based nutrition research57 and justifies the importance of reviewing nutrigenomics literature in the development of updated population health guidelines; this field has been referred to as ‘public health genomics’.58
5. The Nutrigenetic Testing Industry
5.1. Overview of current legal and ethical considerations
Presently, genetic testing in Canada largely operates under the principle of ‘caveat emptor’—buyer beware. With the exception of broad provisions related to deceptive marketing outlined in the Competition Act59 and the regulation of medical devices,60 there is no specific nutrigenomics legislation in which these companies are obliged to abide by in Canada. The Medical Devices Regulations provide guidelines for the safety, effectiveness, and labeling, of only the medical device itself (i.e. the genetic testing kit). Genetic testing kits are considered Class III medical devices and therefore must be licensed before they can be used for the purposes of consumer genetic testing.38 These regulations do not indicate legal requirements for demonstrated safety, effectiveness, and/or scientific validity of the nutrigenetic results and nutrition recommendations provided to consumers (i.e. the genetic report). With respect to deceptive marketing, nutrigenetic products, like all products on the Canadian market, must not promote their tests, reports, and prices in a false or misleading way.59
Ethical considerations for the nutrigenetic testing industry largely relate to the provision of genetic testing results offered DTC.61 Currently, the majority of companies offering nutrigenetic testing do so via DTC genetic testing (Table 1). Notable concerns around DTC genetic testing relate to the informed consent process, validity of scientific evidence supporting the tests, consumer comprehension of the results, clinical validity and utility, and unsubstantiated advertising claims.62 Additionally, ethical considerations around privacy have been noted in the literature, as some companies share user data with biobanks and/or research studies.63 In response to the ethical considerations, several countries around the world have banned DTC genetic testing.64 The importance of these ethical considerations was exemplified when the US FDA shut down 23andMe in 2013 for unethical practices offered DTC. In particular, the FDA was concerned about the clinical validity and public health consequences of consumers receiving inaccurate results.65 Since 23andMe was permitted to continue seling DTC tests in Canada at a time when they were not permitted to do so in the US, this demonstrates that regulation of genetic testing in Canada is less stringent. After the FDA’s cease and desist letter to 23andMe, the company modified their genetic testing services and are now permitted to offer such testing again in the US. This example highlights that a lack of regulation can lead to potential harms for the general public.
5.2. Recommendations to improve the nutrigenetic testing industry
Many of the ethical issues noted above could be mitigated with the banning of DTC genetic testing, following suit with other countries. If nutrigenetic testing is only offered through qualified, regulated HCPs, this would allow for a more thorough consenting process, which should include a discussion of the nature and utility of the test, scope of the results, possible risks, and privacy/confidentiality information.66 Furthermore, regulated HCPs are required to abide by several laws to protect consumer privacy and confidentiality, as well as being required to uphold high standards of ethical practice.67 Examples of relevant legislation that governs healthcare professionals include the Personal Information Protection and Electronic Documents Act,68 the Personal Health Information Protection Act, 2004,69 the Dietetics Act,70 and the Regulated Health Professions Act.71 In addition to statutory requirements, regulated healthcare professionals are also bound by their respective colleges or licensing bodies, which often have codes of practice and ethics guidance that may govern how they use genetic testing in their practice. Noted ethical issues with unregulated HCPs in Canada will be discussed further on Healthcare Practitioners (HCPs).
Current regulations related to deceptive marketing and medical devices do not indicate any requirements for the safety, effectiveness and/or the scientific validity of the nutrigenetic information or nutritional guidelines provided to consumers. Offering genetic testing exclusively through regulated HCPs such as RDs could help to alleviate issues around this lack of regulation since RDs are required to practice using evidence-based, harm-reducing health information and advice.72 Conversely, with DTC genetic testing, we cannot expect a consumer to understand whether or not a test and subsequent nutrigenetic report includes evidence-based information.
6. Genetic Testing Laboratories
6.1. Overview of current legal and ethical considerations
The main issue to consider for genetic testing laboratories is the accuracy of the genetic analyses. Rather than regulating Canadian labs, provinces often govern the professionals who conduct laboratory tests. For example, the College of Medical Laboratory Technologists of Ontario regulates approximately 7000 medical laboratory technologists practicing in Ontario.73 Ontario has also enacted the Laboratory and Specimen Collection Centre Licensing Act.74 This act offers licensing for quality assurance for laboratories that undertake tests to obtain information for the purposes of diagnosis, prophylaxis, and treatment. This Act was a positive step for Ontario as it sets out standards of practice which licensed labs must follow. However, it is concerning to note that labs can still remain unlicensed, meaning that they are not required to follow robust (or any) standards of practice. These labs can be found in medical clinics, physicians’ offices, forensics, and occupational health centers.75 Laboratory regulation and certification varies from province-to-province with some provinces enacting formal legislation and others employing inspection programs where labs can opt to apply for quality certifications.76 Because of this, laboratory quality across Canada varies.
6.2. Recommendations to improve genetic testing laboratories
Given the abovementioned variation in quality assurance policies across Canada and the existence of unlicensed labs not required to abide by any quality standards, there is a need for standardized policies to ensure high quality laboratory analyses. A lack of quality assurance policies increases the risk of a consumer receiving inaccurate genetic results. Therefore, we recommend the incorporation of a CLIA protocol for all Canadian labs conducting genetic analyses. In the US, CLIA certification was developed in order to improve the accuracy, reliability, and timeliness of patient results, in order to standardize laboratory practices across the country.77 A CLIA protocol is recommended in Canada for the same key reasons it was employed in the US.
7. Healthcare Practitioners (HCPs)
7.1. Overview of current legal and ethical considerations
While the majority of nutrigenetic testing in Canada is currently offered DTC, many HCPs are offering this type of testing in their practice. To our knowledge, only one company is offering nutrigenetic testing services exclusively through HCPs.78 These HCPs are both regulated and unregulated and include registered dietitians, chiropractors, nutritionists, wellness counselors, naturopaths, physicians, nurse practitioners, registered nurses, and genetic counselors, among others. Given the variety of HCPs offering nutrigenetic testing in their clinical practice, it is clear that there is a variety of training and educational backgrounds in genetics and nutrition among these providers. While this is an issue within the context of nutrigenomics, it is actually also a much larger issue in the field of nutrition as a whole. Many HCPs lack basic training in nutrition79 and currently, ‘medical nutrition therapy’ is not a controlled act for those qualified to perform it in their practice. Therefore, nutrition advice, including communicating nutrigenetic test results, can be completed by anyone regardless of whether or not they are regulated or qualified to offer such advice. Clearly, this is a major concern in the field of nutrition broadly. For example, research has demonstrated that non-RDs are providing false, misleading, and potentially harmful nutrition information and advice online.80 We recommend more stringent standards be put into place to protect the public against such false, misleading, harmful, and devious nutrition information and advice.
Some studies do suggest that HCPs do not feel confident or competent in their abilities to interpret the results of nutrigenetic tests.81 While this may seem concerning, it is actually not surprising given that the fields of nutrition and health are so large and broad that HCPs must specialize. For some perspective, a 2014 study found that 63% of private practice RDs reported low confidence in their ability to apply nutrigenetic testing to their practice.82 As a point of comparison, 54% of RDs reported low to no competence in developing food challenge protocols for food allergies—another specialized area of nutrition care.83 Perhaps RDs with additional training in nutritional genomics genetics are the best providers of nutrigenetic testing. Dietetic regulatory colleges do mandate that dietitians must feel competent in their abilities before engaging in any area of dietetic practice, including nutrigenomics. This helps to ensure that RDs are providing evidence-based, harm-reducing information and advice.
Given the reported interest in nutrigenomics among HCPs, consumers and students alike,84 major Canadian dietetics organizations including regulatory colleges have responded to the continuing education needs of HCPs by providing different sources of education in nutrigenomics.85 Additional training in genetics has also recently been established by Dietitians of Canada to help enhance competency for dietitians wishing to use nutrigenomics in their practice.86 Other universities and organizations have further developed nutrigenomics training to help meet the needs of HCPs.87 Moreover, the abovementioned studies (indicating low confidence and competence among HCPs) were conducted several years ago; thus, more current research is needed in this quickly-evolving field to determine how HCPs’ knowledge and competence may have changed with increased continuing education available.
7.2. Recommendations to improve nutrigenetic testing related to HCPs
To start, we recommend that ideally ‘medical nutrition therapy’ be included as a controlled act under dietetics legislation in each province and territory in Canada.88 In Canada, several Colleges of Dietitians recognize that nutrigenomics is an innovative approach to nutrition care that falls within RDs’ scope of practice.89 Of note, nutrigenetic test results tend to be nondiagnostic, meaning that HCPs other than medical doctors are able to provide an interpretation of the results. For example, CYP1A2 genetic variation can provide information about risk of developing HTN and MI based on caffeine consumption,90 but cannot be used to diagnose either of these conditions. Given RDs’ background knowledge and education in nutrition care, behavior change counseling, health policy, and genetics,91 coupled with the fact that RDs are regulated HCPs,92 RDs may be the most credible HCPs to communicate the results of nutrigenetic tests. In the context of nutrigenomics, where many ethical considerations come into play, at minimum, we recommend that only regulated HCPs should be permitted to offer this component of nutrition care in their practice. While all HCPs are required to uphold laws around patient confidentiality, ethics, security of documentation, and others,93 since unregulated HCPs have no oversight by a regulatory college, there are generally no negative repercussions if they do not follow such legislation. This highlights the importance of, at minimum, offering nutrigenetic testing exclusively through regulated HCPs—ideally RDs.
8. Consumers
8.1. Overview of current legal and ethical considerations
Canadians have demonstrated a high level of interest in nutrigenetic testing.94 While it is exciting to see consumer interest in an area of healthcare aimed at improving personal health and wellbeing, it is at the same time concerning given the lack of consumer protection via health policy and law. Again, this lack of regulation and oversight for the provision of ‘medical nutrition therapy’ in general is a concern for consumers interested in the broad field of nutrition, as further detailed above. The previously discussed GNDA aimed to protect consumers from discrimination by employers and health insurance and was perceived as a positive step for Canada towards ensuring the most ethical incorporation of nutrigenetic testing. With no explicit protection against genetic discrimination, consumers of genetic tests in Canada remain vulnerable. Responsibility may ultimately rest with provincial governments to legislate protection against genetic discrimination. Thus, one of the few avenues for recourse available to consumers is to make an argument under existing human rights legislation or to utilize difficult to enforce consumer protection laws. It is also possible for consumers to initiate a private lawsuit against a genetic testing company but in such cases, the consumer would not only need to show that the company was negligent in their action, but that they suffered a recoverable harm. Private law remedies are likely to be insufficient to provide meaningful protection to consumers. As a result, consumers are ultimately left exposed to the whims of the market.
8.2. Recommendations to improve nutrigenetic testing for consumers
First, if the Supreme Court of Canada does not uphold the GNDA as constitutional, it is recommended that the federal government work with provincial governments to enact something akin to the GNDA as a valid law in Canada, whether federally or within each province. This is of utmost importance given that there is no regulatory oversight of the science supporting consumer nutrigenetic tests (as discussed in `The Science').95 Because of this, it is possible for a consumer to be discriminated against based on the results of a genetic test, which may not actually have any scientific validity and could ultimately be inaccurate. Furthermore, the nature of nutrigenomics suggests that in order to understand one’s genetic risk of a specific disease or condition, their nutritional status must also be understood. Using the example detailed previously, CYP1A2 genetic variation demonstrates that half of the population is at risk for myocardial infarction, but only when caffeine intake exceeds 200 mg daily. Therefore, if an individual consumes less than 200 mg, they do not actually possess a high genetic risk. As current legislation stands, an individual could be discriminated against based on the results of a nutrigenetic test, even if they are following the genetically guided nutrition advice that mitigates their risk. Moreover, research suggests that actionable nutrigenetic testing results can motivate individuals to improve nutritional habits.96 However, if consumers are concerned about genetic discrimination, they may opt not to undergo nutrigenetic testing—an innovative component of nutrition care that could ultimately optimize health and well-being.
Furthermore, to uphold ethical standards of practice, consumers should undergo an informed consenting process in order to ensure they understand all potential implications of completing a genetic test. Indeed, this should ideally be completed in-person, with a qualified HCP,97 rather than over the internet where a consumer may choose to simply scroll through the information and click a button to accept the terms without thoroughly reviewing the implications noted. Informed consent should include explanations of the following important considerations: (i) the applicability of existing legislation; (ii) the potential to learn about family members, especially in cases where both parents and their child/children are undergoing a genetic test; (iii) that genetic information cannot be protected from disclosure by court order; (iv) whether or not third parties will have access to their genetic information; (v) that nutrigenetic tests are not intended to be used for diagnostic purposes; (vi) a discussion of possible incidental findings; and (vii) the nature of the test. Further considerations that could be discussed are outlined in detail elsewhere.98 It is recommended to include each of these seven considerations in an informed consent meeting to help ensure that consumers are making an informed decision to either proceed or not with the nutrigenomics process. A substantial body of literature has also critiqued current issues around lack of informed consent processes; this is a major concern in the genetic testing industry as a whole.99
9. Conclusion
Canadians express great interest in nutrition, genetics and correspondingly, in nutrigenetic testing. The growing field of nutrigenomics lacks direct oversight and regulation. Enhancing health policy and legislation could both directly and indirectly benefit both the field of nutrigenomics and, importantly, consumers. Our key recommendations, as summarized in Table 2, aim to protect consumers against devious, false, and/or misleading nutrigenetic testing processes, while ensuring consumer comprehension of ethical implications. As science and technology continue to advance, health policy and health law can play important roles in the ethical incorporation of novel healthcare strategies such as nutrigenetic testing. More stringent oversight of both the genetic testing industry and the field of nutrition (particularly pertaining to medical nutrition therapy) will help to ensure that the potential benefits of this science for individual and public health are realized.
Table 2.
Executive summary of recommendations to improve nutrigenetic testing in Canada
| Recommendation | Justification | |
|---|---|---|
| 1. Science | Improve regulation: Health Canada | • No regulation of the scientific validity or clinical utility of nutrigenetic testing currently exists; companies are free to base their tests on any level of scientific evidence |
| • Companies are providing results to consumers that are not evidence-based | ||
| Develop clinical practice guidelines (CPGs) in nutrigenomics by experts in the field | • CPGs are available in many areas of healthcare to guide ethical and science-based delivery of health practices • Uptake of nutrigenomics in clinical practice is high | |
| • Despite this, no CPGs exist in the field of nutrigenomics | ||
| • CPGs developed by experts in the field can help define evidence-based nutrigenomics tests while guiding ethical practice | ||
| Consider nutrigenomics research in the development of public health guidelines | • Nutrigenomics research can provide deeper insight into individual variation in nutrition guidelines, which can affect public health guidelines especially in cases of common SNPs (e.g. CYP1A2, caffeine, and cardiovascular disease) | |
| 2. Nutrigenetic Testing Industry | Ban DTC genetic testing in Canada | • Regulated HCPs are required by law and are overseen by regulatory colleges to abide by high standards of ethical and evidence-based practice |
| • Therefore, regulated HCPs are more likely to choose tests with strong scientific validity and clinical utility | ||
| • An informed consent process with a qualified HCP will improve consumer comprehension of the implications of genetic tests compared to online consent processes | ||
| 3. Genetic Testing Laboratories | Enact a nation-wide quality assurance program, similar to CLIA in the United States | • Quality standards vary from province to province, with some provinces enacting formal quality assurance legislation for labs, while others employ voluntary quality assurance certification |
| 4. Healthcare Practitioners | Ideally, protect ‘medical nutrition therapy’ as a controlled act for registered dietitians (RDs) | • RDs are the only regulated health professionals in the area of nutrition and have a strong scientific background in nutritional science• RDs are mandated to follow evidence-based, ethical practices, while reducing any risk of harm to the public |
| At minimum, mandate that only regulated health professionals are permitted to incorporate nutrigenetic testing in their practice | • Regulated health professionals are mandated by law to uphold high standards of patient privacy and confidentiality• Regulatory colleges oversee practices of regulated health professionals and employ quality assurance programs to ensure high standards of ethical practice are being followed | |
| 5. Consumers | Enact the GNDA as a valid law in Canada | • Consumers are currently at risk of discrimination by insurance companies and employers yet nutrigenetic tests often have poor scientific validity, and health risks are dependent on nutritional status |
| Mandate an informed consent process, ideally in-person through a regulated HCP | • Consumers have a right to understand major implications of nutrigenetic testing; undergoing an informed consent process with a regulated HCP helps to optimize consumer comprehension of legal and ethical considerations | |
| • Protecting ‘medical nutrition therapy’ (which includes nutrigenetic testing) as a controlled act for RDs would help ensure appropriate informed consent since all nutrigenetic testing would occur through RDs |
Funding
The first author was supported by a Doctoral Research Award (#395167) from the Canadian Institutes of Health Research.
Footnotes
As defined by Fenech M. et al., Nutrigenetics and Nutrigenomics: Viewpoints on the Current Status and Applications in Nutrition Research and Practice, 4 J. NUTRIGENET NUTRIGENOMICS 69–89 (2011).
Horne J. et al., A Systematic Review of Genetic Testing and Lifestyle Behaviour Change: Are We Using High-Quality Genetic Interventions and Considering Behaviour Change Theory?, 11 LIFESTYLE GENOMICS 49–63 (2018).
See, for example, Caulfield T. et al., Nutrigenomics and the Promise of Prevention: Representations and Realties, Special Ed. Health L. J. 41 (2008).
See landmark studies by: Cornelis M.C. et al., Coffee, CYP1A2 Genotype, and Risk of Myocardial Infarction, 295 JAMA 1135 (2006), and Palatini P. et al., CYP1A2 Genotype Modifies the Association Between Coffee Intake and the Risk of Hypertension, 27 J. HYPERTENS. 1594–1601 (2009).
Health Canada, Caffeine in Food—Government of Canada, https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-additives/caffeine-foods/foods.html (accessed Feb. 9, 2019); Cornelis M.C. et al., Coffee, CYP1A2 Genotype, and Risk of Myocardial Infarction, 295 JAMA 1135–1141 (2006); Palatini P. et al., CYP1A2 Genotype Modifies the Association Between Coffee Intake and the Risk of Hypertension, 27 J. Hypertens. 1594–1601 (2009).
Nielsen D.R., Shih S., El-Sohemy A., Perceptions of Genetic Testing for Personalized Nutrition: A Randomized Trial of DNA-Based Dietary Advice, 7 J. NUTRIGEN NUTRIGENOMICS, 94–104 (2014); Vallée Marcotte B. et al., Nutrigenetic Testing for Personalized Nutrition: An Evaluation of Public Perceptions, Attitudes, and Concerns in a Population of French Canadians. LIFESTYLE GENOM. (2019), DOI: 10.1159/000499626.
See, for example, Cornelis M.C. et al., Coffee, CYP1A2 Genotype, and Risk of Myocardial Infarction, 295 JAMA 1135–1141 (2006) and Palatini P. et al., CYP1A2 Genotype Modifies the Association Between Coffee Intake and the Risk of Hypertension, 27 J. HYPERTENS. 1594–1601 (2009).
Examples of Current Companies Offering Nutrigenetic Testing Services to Canadians Include: Nutrigenomix, https://www.nutrigenomix.com/ (accessed Feb. 6, 2019); 23andMe, Meet Your Genes, https://www.23andme.ca (accessed Feb. 6, 2019); Athletigen, https://athletigen.com (accessed Feb. 6, 2019); myDNA, https://www.mydna.life/en-ca/ (accessed Feb. 6, 2019); DNAfit, https://www.dnafit.com/ca/ (accessed June 18, 2019); CRI Genetics, https://www.crigenetics.com/ (accessed June 28, 2019); dnaPower, https://www.dnapower.com/ (accessed June 28, 2019); EasyDNA, https://www.easydna.ca/diet-and-nutrition-dna-test/ (accessed June 28, 2019); Gene Blueprint, https://geneblueprint.com/ (accessed June 28, 2019).
Morin K., Knowledge and Attitudes of Canadian Consumers and Health Care Professionals Regarding Nutritional Genomics, 13 OMICS 37–41 (2009); Vallée Marcotte B. et al., Nutrigenetic Testing for Personalized Nutrition: An Evaluation of Public Perceptions, Attitudes, and Concerns in a Population of French Canadians, LIFESTYLE GENOM. (2019), DOI: 10.1159/000499626.
Caulfield T., Nutrigenomics Patents and Commercialization: Old Wine in a New Bottle?, 13 OMICS 63–67 (2009); Stenne R., Hurlinmann T., Godard B., Are Research Papers Reporting Results from Nutrigenetics Clinical Research a Potential Source of Biohype?, 19 ACCOUNT RES. 285–307 (2012); Caulfield T. et al., Nutrigenomics and the Promise of Prevention: Representations and Realties, Special Ed. HEALTH L. J. 41 (2008).
Cormier et al., Nutrigenomics—Perspectives from Registered Dietitians: A Report from the Quebec-wide e-Consultation on Nutrigenomics among Registered Dietitians, 27 J. HUM. NUTR. DIET 391–400 (2014).
Horne J., Madill J., O’Connor C., Exploring Knowledge and Attitudes of Personal Nutrigenomics Testing among Dietetic Students and its Value as a Component of Dietetic Education and Practice, 4 CANAD. J. CLIN. NUTR. 50–62 (2016).
Caulfield T., Nutrigenomics Patents and Commercialization: Old Wine in a New Bottle?, 13 OMICS 63–67 (2009).
Hurlimann et al., Ethical Considerations in the Implementation of Nutrigenetics/Nutrigenomics, 14 PER. MED. 75–83 (2017).
Similar research has been undertaken in other jurisdictions, see for example: Philips A.M., Only a Click Away—DTC Genetics for Ancestry, Health, Love… and More: A View of the Business and Regulatory Landscape, 8 ATG 16–22 (2016).
Ahlgren J. et al., Consumers on the Internet: Ethical and Legal Aspects of Commercialization of Personalized Nutrition, 8 GENES NUTR. 349–355 (2013).
Preuss C., Das M.K., Pathak Y.V., Genomics and Natural Products: Role of Bioinformatics and Recent Patents, 8 RECENT PAT. BIOTECHNOL. 144–151 (2014).
Examples of Current Companies Offering Nutrigenetic Testing Services to Canadians Include: Nutrigenomix, https://www.nutrigenomix.com/ (accessed Feb. 6, 2019); 23andMe, Meet Your Genes, https://www.23andme.ca (accessed Feb. 6, 2019); Athletigen, https://athletigen.com (accessed Feb. 6, 2019); myDNA, https://www.mydna.life/en-ca/ (accessed Feb. 6, 2019); DNAfit, https://www.dnafit.com/ca/ (accessed June 18, 2019); CRI Genetics, https://www.crigenetics.com/ (accessed June 28, 2019); dnaPower, https://www.dnapower.com/ (accessed June 28, 2019); EasyDNA, https://www.easydna.ca/diet-and-nutrition-dna-test/ (accessed June 28, 2019); Gene Blueprint, https://geneblueprint.com/ (accessed June 28, 2019).
Ordovas J.M. et al., Personalised Nutrition and Health, BMJ 361 (2018).
CLIA, Clinical Laboratory Improvement Amendments, https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/index.html?redirect=/CLIA (accessed Feb. 9, 2019).
Ahlgren J. et al., Consumers on the Internet: Ethical and Legal Aspects of Commercialization of Personalized Nutrition, 8 GENES NUTR. 349–355 (2013); Bollinger J.M., Green R.C., Kaufman D., Attitudes about Regulation among Direct-to-Consumer Genetic Testing Customers, 17 GEN. TEST MOLEC. BIOMARKERS 424–428 (2013); Stewart-Knox B. et al., Promoting Healthy Dietary Behaviour Through Personalised Nutrition: Technology Push or Technology Pull?, 74 PROC. NUTR. SOCIETY 171–176 (2015).
United States Food and Drug Administration. Direct-to-Consumer Tests, https://www.fda.gov/medical-devices/vitro-diagnostics/direct-consumer-tests (accessed Dec. 2, 2019)
Hogarth et al., The Current Landscape for Direct-to-Consumer Genetic Testing: Legal, Ethical and Policy Issues, ANNU. REV. GENOMICS HEALTH GENET. (2008); Hogarth S. et al., The Regulation of Commercial Genetic Testing Services in the UK: A Briefing for the Human Genetics Commission. Cambridge (2005).
Hogarth et al., The Current Landscape for Direct-to-Consumer Genetic Testing: Legal, Ethical and Policy Issues, ANNU. REV. GENOMICS HEALTH GENET. (2008).
Tiller J. & Lacaze P., Regulation of Internet-Based Genetic Testing: Challenges for Australia and Other Jurisdictions, 6 FRONT PUBLIC HEALTH 1–6 (2018).
Tiller J. & Lacaze P., Regulation of Internet-Based Genetic Testing: Challenges for Australia and Other Jurisdictions, 6 FRONT PUBLIC HEALTH 1–6 (2018).
Otlowski M. et al., Genetic Testing and Insurance in Australia, 48 AJGP 96–99 (2019).
Hogarth et al., The Current Landscape for Direct-to-Consumer Genetic Testing: Legal, Ethical and Policy Issues, ANNU. REV. GENOMICS HEALTH GENET. (2008).
Id.
Sun L. et al., The Rise of the Genetic Counseling Profession in China, 181C AJMG 170–6 (2019); Sui S., The Practice of Genetic Counselling: A Comparative Approach to Understanding Genetic Counselling in China, 4 BIOSOCIETIES 391–405 (2009) and as reported in Channel News Asia. MOH to Develop Guidelines for Firms that Provide Non-Clinical Genetic Testing (2019), https://www.channelnewsasia.com/news/singapore/moh-guide-provide-non-clinical-genetic-testing-health-12035952 (accessed Dec. 2, 2019).
Bill S-201, An Act to Prohibit and Prevent Genetic Discrimination, 1st Sess, 42th leg, 2017, (Assented May 4, 2017).
For a discussion about the potential for genetic discrimination, see: Adjin-Tettey E., Potential for Genetic Discrimination in Access to Insurance: Is there a Dark Side to Increased Availability of Genetic Information?, 50 ALTA L. REV. 577–614 (2013).
Bombard Y., Heim-Myers B., The Genetic Non-Discrimination Act: Critical for Promoting Health and Science in Canada, 190 CMAJ E579 (2018).
Genetic Non-Discrimination Act, 2018 QCCA 2193 at para 1 [Reference Re Genetic Non-Discrimination Act].
Schmitz C., SCC to Look at Constitutionality of Genetic Discrimination Ban Nixed by ex-AG Canada but Supported by New AG, The Lawyer’s Daily, https://www.thelawyersdaily.ca/articles/9895/scc-to-look-at-constitutionality-of-genetic-discrimination-ban-nixed-by-ex-ag-canada-but-supported-by-new-ag (accessed Jan. 24, 2019).
Gillmore M., Genetic Discrimination in Provincial Law, Law Times, https://www.lawtimesnews.com/article/genetic-discrimination-unclear-in-provincial-law-16183/ (accessed Sept. 10, 2018).
Human Rights Code Amendment Act, 2018. As a private member’s bill, introduced by a Liberal MPP, it is unlikely to pass. Bill 35 is ambitious, as it seeks to bar discrimination on the basis of immigration status, genetic characteristics, police records and social conditions.
Human Rights Code Amendment Act (Genetic Characteristics), 2018. Introduction by a Progressive Conservative MPP, Bill 40 is more likely to be passed than Bill 35. It is also more restrictive, limited to genetic characteristics.
Pioro M. et al., Understanding the Use of ‘Genetic Predisposition’ in Canadian Legal Decision, 7 MJLH 1–65 (2013).
Nicholis S. & Farfard P., Genetic Discrimination Legislation in Canada: Moving from Rhetoric to Real Debate, 188 CMAJ 778–89 (2016).
This law regulates medical devices, including genetic test kits, but does not provide regulatory oversight of the scientific evidence used as the basis for the genetic report: Government of Canada, Medical Devices Regulations. SOR/98-282 (2011).
This sample recommendation is provided by the nutrigenetic industry, https://athletigen.com/products/ (2019). See lack of scientific support via PubMed searches for: ‘CYP1A2 AND L-theanine’ revealing zero results, https://www.ncbi.nlm.nih.gov/pubmed/?term=CYP1A2+AND+L-theanine (accessed Feb. 6, 2019) and ‘CYP1A2 AND “jitter*”’ revealing zero results, https://www.ncbi.nlm.nih.gov/pubmed/?term=CYP1A2+AND+%22jitter*%22 (accessed Feb. 6, 2019).
Rather, ADORA2A genetic variation appears to likely be linked to physical sensations associated with caffeine consumption: Landolt H.P., No Thanks, Coffee Keeps Me Awake: Individual Caffeine Sensitivity Depends on ADORA2A Genotype, 35 SLEEP 899–900 (2012); Domschke K. et al., ADORA2A Gene Variation, Caffeine, and Emotional Processing: A Multi-Level Interaction on Startle Reflex, 37 Neuropsychopharmacology 759–69 (2012).
Blumberg J. et al., Evidence-Based Criteria in the Nutritional Context, 68 NUTR. REV. 478–84 (2010).
Horne J. et al., A Systematic Review of Genetic Testing and Lifestyle Behaviour Change: Are We Using High-Quality Genetic Interventions and Considering Behaviour Change Theory?, 11 LIFESTYLE GENOM. 49–63 (2018).
Blumberg J. et al., Evidence-Based Criteria in the Nutritional Context, 68 NUTR. REV. 478–84 (2010).
Grimaldi et al., Proposed guidelines to evaluate scientific validity and evidence for genotype-based dietary advice, GENES NUTR., 2017. 12(1):35.
See complete list of recommendations in: Mozaffarian D., Conflict of Interest and the Role of the Food Industry in Nutrition Research, 317 JAMA 1755–56 (2017).
Shekelle R.B. et al., Diet, Serum Cholesterol and Death from Coronary Heart Disease: The Western Electric Study, 304 NEJM 65–70 (1981).
McGEE D.L. et al., Ten-Year Incidence of Coronoary Heart Disease in the Honolulu Heart Program: Relationship to Nutrient Intake, 119 AM. J. EPIDEMIOLOGY 667–76 (1984).
Anderson T.J. et al., 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult, 32 Can. J. Cardiol. 1263–82 (2016).
See, for example: The College of Family Physicians of Canada. Clinical Practice Guidelines, https://www.cfpc.ca/clinicalpracticeguidelines/ (accessed June 19, 2019).
See, for example: The College of Family Physicians of Canada. Clinical Practice Guidelines, https://www.cfpc.ca/clinicalpracticeguidelines/ (accessed June 19, 2019).
Health Canada, About Mission, Values, Activities, https://www.canada.ca/en/health-canada/corporate/about-health-canada/activities-responsibilities/mission-values-activities.html (accessed June 19, 2019).
Health Canada, Caffeine in Food, Government of Canada, https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-additives/caffeine-foods/foods.html (accessed Feb. 9, 2019).
Id.; Cornelis M.C. et al., Coffee, CYP1A2 Genotype, and Risk of Myocardial Infarction, 295 JAMA 1135–41 (2006); Palatini P. et al., CYP1A2 Genotype Modifies the Association Between Coffee Intake and the Risk of Hypertension, 27 J. HYPERTENS. 1594–1601 (2009); Palatini P. et al., Association of Coffee Consumption and CYP1A2 Polymorphism with Risk of Impaired Fasting Glucose in Hypertensive Patients, 30 EUR. J. EPIDEMIOL. 209–17 (2015).
See, for example: James J., Critical Review of Dietary Caffeine and Blood Pressure: A Relationship that should be taken more Seriously, 66 Psychosom. Med. 63–71 (2004) and Chrysant S.G., The Impact of Coffee Consumption on Blood Pressure, Cardiovascular Disease and Diabetes Mellitus, 15 Expert Rev. Cardiovasc. Ther. 151–56 (2017).
Khoury M.J. et al., From Public Health Genomics to Precision Public Health: A 20-Year Journey, 20 GENET. MED. 574–82 (2017).
Competition Act, R.S.C., 1985, c. C-34, s. 1.
Government of Canada, Medical Devices Regulations. SOR/98-282 (2011).
Ries N.M. & Castle D., Nutrigenomics and Ethics Interface: Direct-to Consumer Services and Commercial Aspects, 12 OMICS 245–55 (2008).
Nelson T.N. et al., CCMG Statement on Direct-to-Consumer Genetic Testing, 81 CLIN. GENET. 1–3 (2011); Badalato L., Kalokairinou L., Borry P., Third Party Interpretation of Raw Genetic Data: An Ethical Exploration, 25 EUR. J. HUM. GENET. 1189–94 (2017).
Caulfield T. & Murdoch B., Genes, Cells, and Biobanks: Yes, There’s Still a Consent Problem, 15 PLOS BIOL. e2002654 (2017); Vayena E. & Gasser U., Between Openness and Privacy in Genomics, 13 PLOS MED. e1001937 (2016).
Hogarth S. et al., The Current Landscape for Direct-to-Consumer Genetic Testing: Legal, Ethical, and Policy Issues, 9 ANNU. REV. GENOMICS HUM. GENET. 161–82 (2008).
Inspections, Compliance, Enforcement, and Criminal Investigations, 23andMe, Inc., U.S. F.D.A, https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm376296.htm (accessed Nov. 22, 2013).
Hurlimann et al., Ethical Considerations in the Implementation of Nutrigenetics/Nutrigenomics, 14 PER. MED. 75–83 (2017).
Mcmillan LLC, Health law in Canada, Mcmillian.ca, https://mcmillan.ca/files/Health_Law_in_Canada.pdf (2019).
Office of the Privacy Commissioner of Canada, The Personal Information Protection and Electronic Documents Act, Government of Canada, https://www.priv.gc.ca/en/privacy-topics/privacy-laws-in-canada/the-personal-information-protection-and-electronic-documents-act-pipeda/ (accessed Dec. 12, 2018).
Personal Health Information Protection Act, S.O. 2004, C. 4 Sched. A.
Dietetics Act, S.O. 1991, C. 26.
Regulated Health Professions Act, S.O. 1991, c. 18.
See, for example: Health Professions Act, Alberta, http://www.qp.alberta.ca/documents/Acts/h07.pdf (2000) and Regulated Health Professions Act, S.O. 1991, c. 18.
College of Medical Laboratory Technologists of Ontario, About CMLTO, http://www.cmlto.com/index.php?option=com_content&view=article&id=1214&Itemid=68 (accessed Feb. 9, 2019).
Laboratory and Specimen Collection Centre Licensing Act, R.S.O. 1990, C. L. 1.
College of Medical Laboratory Technologists of Ontario, About Labs, http://www.cmlto.com/index.php?option=com_content&view=article&id=1247&Itemid=560 (accessed June 19, 2019).
Alberta Health Services, Accreditation: ProvLab, Laboratory Services, https://www.albertahealthservices.ca/lab/Page14602.aspx (accessed June 19, 2019).
Department of Health and Human Services USA, Clinical Laboratory Improvement Amendments (CLIA), https://www.cms.gov/regulations-and-guidance/legislation/clia/downloads/howobtaincliacertificate.pdf (accessed June 19, 2019).
Nutrigenomix, Inc. Genetic Testing for personalized Nutrition, https://www.nutrigenomix.com/ (accessed June 19, 2019); Nutrigenomix, Inc. Locate a clinic. https://www.nutrigenomix.com/locate-a-clinic (accessed June 19, 2019).
Mogre V., Why Nutrition Education is Inadequate in the Medical Curriculum: A Qualitative Study of Students’ Perspectives on Barriers and Strategies, 18 BMC MED. EDUC. 26 (2018); Kris-Etherton P.M. et al., The Need to Advance Nutrition Education in the Training of Health Care Professionals and Recommended Research to Evaluate Implementation and Effectiveness, 99 AM. J. CLIN. NUTR. 1153S–66S (2014).
See, for example: Toth J. et al., “Detoxify or Die”: Qualitative Assessments of Ontario Nutritionists’ and Dietitians’ Blog Posts Related to Detoxification Diets, CAN. J. DIET PRACT. RES. 80 (2019), DOI: 10.3148/cjdpr-2018-047 and Sabbagh C. et al., Assessing Credibility of Online Nutritional Information: Analysis of Key UK Social Media Influencers’ Weight-Management Blogs [Abstract], in OBESITY FACTS. 26th European Congress on Obesity (ECO 2019); 28 Apr–01 May, 2019; Glasgow, UK. Abstract OS8.06.
Cormier et al., Nutrigenomics—Perspectives from Registered Dietitians: A Report from the Quebec-Wide e-Consultation on Nutrigenomics among Registered Dietitians, 27 J. HUM. NUTR. DIET 391–400 (2014); Wright O.R.L., Systematic Review of Knowledge Confidence and Education in Nutritional Genomics for Students and Professionals in Nutrition and Dietetics, 27 J. HUM. NUTR. DIET 298–307 (2014).
Cormier et al., Nutrigenomics—Perspectives from Registered Dietitians: A Report from the Quebec-Wide e-Consultation on Nutrigenomics among Registered Dietitians, 27 J. HUM. NUTR. DIET 391–400 (2014).
Maslin K., Food Allergy Competencies of Dietitians in the United Kingdom, Australia and United States of America, 4 Clin. Transl. Allergy 37 (2014).
Cormier et al., Nutrigenomics—Perspectives from Registered Dietitians: A Report from the Quebec-Wide e-Consultation on Nutrigenomics among Registered Dietitians, 27 J. HUM. NUTR. DIET 391–400 (2014); Horne J., Madill J., O’Connor C., Exploring Knowledge and Attitudes of Personal Nutrigenomics Testing among Dietetic Students and its Value as a Component of Dietetic Education and Practice, 4 CANAD. J. CLIN. NUTR. 50–62 (2016); Vallée Marcotte B. et al., Nutrigenetic Testing for Personalized Nutrition: An Evaluation of Public Perceptions, Attitudes, and Concerns in a Population of French Canadians, LIFESTYLE GENOM. (2019), DOI: 10.1159/000499626.
Dietitians of Canada, Nutrigenomics: Genetic Testing for Personalized Nutrition, https://www.dietitians.ca/Learn/Distance-Learning/LODStoreProduct.aspx?guid=cd361e54-9fee-4271-b172-18a5a38fe2af (accessed Feb. 9, 2019); College of Dietitians of Alberta, Nutrigenomics in dietetic practice: College of Dietitians of Alberta Position Statement (March 2015), http://www.collegeofdietitians.ab.ca/wp-content/uploads/2017/01/CDA-Nutrigenomics-Guidelines-2015.pdf (accessed Feb. 9, 2019); Cohen D., College of Dietitians of Ontario. Keeping Pace with Innovations in Nutrition Care. Professional Practice: Résumé. Winter 2014; Dietitians of Canada, The NOW Trial: Nutrigenomics, Overweight/Obesity and Weight Management, https://www.dietitians.ca/Learn/Distance-Learning/LODStoreProduct.aspx?guid=b094d0ff-53b0-40d8-b686-a5716d428fae (accessed Feb. 9, 2019).
Dietitians of Canada, Nutrigenomics: Genetic Testing for Personalized Nutrition, https://www.dietitians.ca/Learn/Distance-Learning/LODStoreProduct.aspx?guid=cd361e54-9fee-4271-b172-18a5a38fe2af (accessed Feb. 9, 2019).
Collins A., Twohig C., Murgia C., Opportunities for Training for Nutritional Professionals in Nutritional Genomics: What is Out There?, 75 NUTR. DIET 206–18 (2018).
See, for example, Ontario legislation: Dietetics Act, S.O. 1991, C. 26.
Cohen D., College of Dietitians of Ontario. Keeping Pace with Innovations in Nutrition Care. Professional Practice: Résumé. Winter (2014); College of Dietitians of Alberta, Nutrigenomics in dietetic practice: College of Dietitians of Alberta Position Statement (March 2015), http://www.collegeofdietitians.ab.ca/wp-content/uploads/2017/01/CDA-Nutrigenomics-Guidelines-2015.pdf (accessed Feb. 9, 2019).
See landmark studies by: Cornelis M.C. et al., Coffee, CYP1A2 Genotype, and Risk of Myocardial Infarction, 295 JAMA 1135 (2006) and Palatini P. et al., CYP1A2 Genotype Modifies the Association Between Coffee Intake and the Risk of Hypertension, 27 J. HYPERTENS. 1594–1601 (2009).
Partnership for Dietetic Education and Practice, https://www.pdep.ca/ (accessed Feb. 9, 2019).
Regulated Health Professions Act, S.O. 1991, c. 18.
See, for example: Office of the Privacy Commissioner of Canada, The Personal Information Protection and Electronic Documents Act, Government of Canada, https://www.priv.gc.ca/en/privacy-topics/privacy-laws-in-canada/the-personal-information-protection-and-electronic-documents-act-pipeda/ (accessed Dec. 12, 2018) and Regulated Health Professions Act, S.O. 1991, c. 18.
Nielsen D.R., Shih S., El-Sohemy A., Perceptions of Genetic Testing for Personalized Nutrition: A Randomized Trial of DNA-based Dietary Advice, 7 J. NUTRIGEN NUTRIGENOMICS 94–104 (2014).
This law regulates medical devices, including genetic test kits, but does not provide regulatory oversight of the scientific evidence used as the basis for the genetic report: Government of Canada, Medical Devices Regulations, 2011. SOR/98-282.
Horne J. et al., A Systematic Review of Genetic Testing and Lifestyle Behaviour Change: Are We Using High-Quality Genetic Interventions and Considering Behaviour Change Theory?, 11 LIFESTYLE GENOM. 49–63 (2018).
Hurlimann et al., Ethical Considerations in the Implementation of Nutrigenetics/Nutrigenomics, 14 PER. MED. 75–83 (2017); Spector-Bagdady K., Reconceptualizing Consent for Direct-to-Consumer Health Services, 41 AM. J. LAW MED. 568–616 (2015); Hogarth S., Javitt G., Melzer D., The Current Landscape for Direct-to-Consumer Genetic Testing: Legal, Ethical and Policy Issues, 9 ANNU. REV. GENOMICS HUM. GENET. 161–82 (2008).
Hurlimann et al., Ethical Considerations in the Implementation of Nutrigenetics/Nutrigenomics, 14 PER. MED. 75–83 (2017).
Id.


