Abstract
Abstract
Integrins are heterodimeric transmembrane cell adhesion molecules made up of alpha (α) and beta (β) subunits arranged in numerous dimeric pairings. These complexes have varying affinities to extracellular ligands. Integrins regulate cellular growth, proliferation, migration, signaling, and cytokine activation and release and thereby play important roles in cell proliferation and migration, apoptosis, tissue repair, as well as in all processes critical to inflammation, infection, and angiogenesis. This review presents current evidence from human and animal studies on integrin structure and molecular signaling, with particular emphasis on signal transduction in infants. We have included evidence from our own laboratory studies and from an extensive literature search in databases PubMed, EMBASE, Scopus, and the electronic archives of abstracts presented at the annual meetings of the Pediatric Academic Societies. To avoid bias in identification of existing studies, key words were short-listed prior to the actual search both from anecdotal experience and from PubMed’s Medical Subject Heading (MeSH) thesaurus.
Impact
Integrins are a family of ubiquitous αβ heterodimeric receptors that interact with numerous ligands in physiology and disease. Integrins play a key role in cell proliferation, tissue repair, inflammation, infection, and angiogenesis.
This review summarizes current evidence from human and animal studies on integrin structure and molecular signaling and promising role in diseases of inflammation, infection, and angiogenesis in infants.
This review shows that integrin receptors and ligands are novel therapeutic targets of clinical interest and hold promise as novel therapeutic targets in the management of several neonatal diseases.
Introduction
Integrins are a family of ubiquitous αβ heterodimeric receptors that exist in multiple conformations and interact with a diverse group of ligands. These molecules mediate interactions between cells and of these cells with the extracellular matrix (ECM) and thereby serve a critical role in signaling and homeostasis. By facilitating dynamic linkages between the intracellular actin cytoskeleton and the ECM, integrins also transduce both external and internal mechanochemical cues and bi-directional signaling across the plasma membrane.1,2 Integrins are involved in a diverse range of body processes, including cellular survival, inflammation, immunity, infection, thrombosis, angiogenesis, and malignancy. In this review, we highlight the structure and function of integrins; the mechanisms involved in integrin activation and signaling; their role in inflammation, infection, and angiogenesis; and discuss current advances in integrin-targeted therapies. Understanding the factors that regulate integrin structure, function, and signaling would enable us to identify new therapeutic targets.
Structure of integrins
In mammals, the family of integrins is comprised of 24 αβ pairs of heterodimeric transmembrane adhesion receptors and cell-surface proteins. These pairings are known to involve 18 α and 8 β subunits (Fig. 1),3 and their non-covalent associations involve an α and another β subunit (Fig. 2).4 The αβ pairings of integrin subunits dictate the specificity of the integrin to a particular ligand, modulate formation of intracellular adhesion complexes, and regulate downstream signaling.1 Six α (α1–6) and seven β (β1–7) subunits are known to form several unique αβ subunit associations (Fig. 1). Interestingly, the earliest discovered integrins, lymphocyte function-associated antigen 1 (integrin αLβ2) and macrophage antigen 1 (integrin αMβ2), derive their specificity from specific α subunits, but these share the same β subunit.5
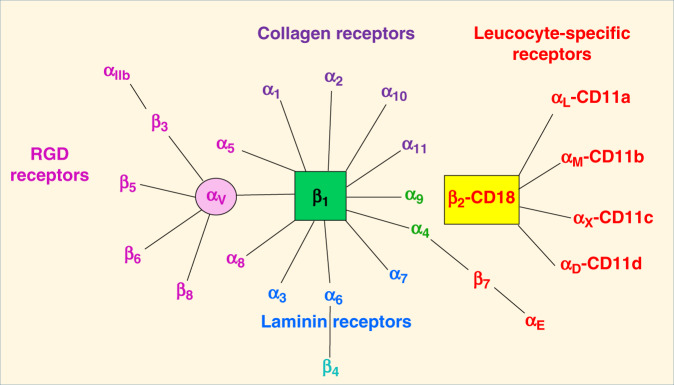
Fig. 1. Classification of integrin family.
Integrin heterodimers consists of numerous combinations of α and β subunits. With respect to ligand specificity, integrins are generally classified as collagen-binding integrins (α1β1, α2β1, α10β1, and α11β1), RGD-recognizing integrins (α5β1, αVβ1, αVβ3, αVβ5, αVβ6, αVβ8, and αIIbβ3), laminin-binding integrins (α3β1, α6β1, α7β1, and α6β4), and leukocyte integrins (αLβ2, αMβ2, αXβ2, and αDβ2). The β2 integrin subunit (CD18) can pair with one of the four α subunits (αL-CD11a, αM-CD11b, αX-CD11c, and αD-CD11d), forming leukocyte function-associated antigen-1, Mac1/CR3 (macrophage-1 antigen, complement receptor 3), 150.95/CR4 (complement receptor 4), and CD18/CD11d, respectively. CD11a/CD18 is expressed mainly on all leukocytes, while CD11b/CD18, CD11c/CD18, and CD11d/CD18 are expressed on myeloid cells.106,107 The αMβ2 integrin (also known as CR3, CD11b/CD18, or Mac-1) is found on phagocytic cells and implicated in the adhesion of leucocytes to endothelium and opsonization of microbes. Ligands for CR3 include the complement component iC3b, the intercellular adhesion molecule (1CAM-1), and coagulation factors like fibrinogen and factor X.
Fig. 2. Schematic of integrin structure and activation.
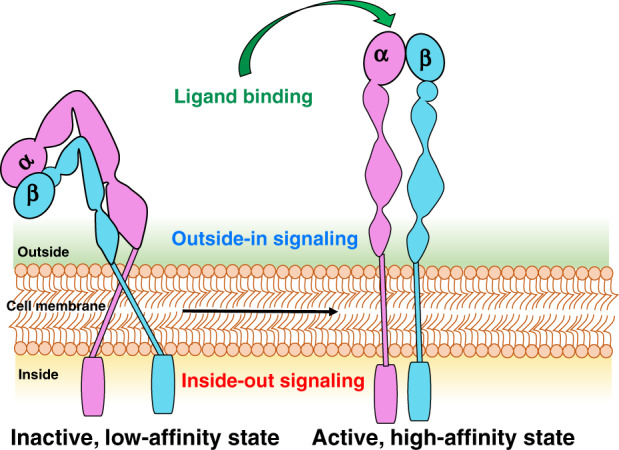
Structurally, the αβ integrin subunits are type 1 transmembrane proteins. Each subunit consists of one large multi-domain extracellular segment, one transmembrane helix, and a short cytoplasmic tail. The extracellular region interacts with ECM ligands and is composed of about 1104 (700–1100) residues in the α subunit and 778 residues in the β subunits32 and shorter cytoplasmic domains with 30–50 residues.108 The short cytoplasmic tails are composed of 20–70 amino acids and mediate interactions with intracellular cytoskeletal and signaling proteins.1 In response to intracellular or extracellular stimuli, integrin activation occurs by ligand binding or by the changes on the cytoplasmic domains, resulting in elongation and separation of the legs. Integrins appear in a closed or “bent” conformation on resting cells and display a low binding affinity for ligand rendering them inactive to ligand binding or signal transduction; while once activated, the integrin shape extends to an open conformation leading to a high affinity.109 In a closed conformation, integrins show low ligand-binding affinity, partly due to the bend in the center of the α and β subunits, which brings the ligand-binding site within 5 nm of the cell surface.110 However, when the conformation is open, the two subunits straighten with increased integrin affinity for the ligand.111 The initial binding of extracellular ligand effects separation of the cytoplasmic domains, allowing interaction with signal transduction and cytoskeletal molecules during outside-in signaling, while separation of the cytoplasmic domains by talin and other activators activates the head to enable ligand binding during inside-out signaling.4
Integrin α subunit family
The integrin α subunits carry a 200 amino acid “inserted” domain, the I-domain (αI). When present on an integrin, the αI domain is an exclusive ligand-binding site. αI integrins have 13 extracellular domains in 2 subunits, which interact with a variety of ligands. The I-domains are seen in 6 out of the 15 integrin α subunits.
Integrin β subunit family
In humans, integrin β subunits have a cytoplasmic tail that have <75 amino acids in length, except the β4 tail that is about 1000 amino acids long (includes four fibronectin type III repeats).3 The integrin β tails have one or two NPxY/F motifs (x refers to any amino acid) that recognize protein modules, phosphotyrosine-binding domains, that are involved in several signaling and cytoskeletal proteins at the cytoplasmic face of the plasma membrane through phosphorylation of the tyrosine (Y) in the NPxY/F motif.3 The integrin β subunit family includes β1–7, which bind the α subunits in different combinations. The most frequently seen β subunit integrin heterodimers is β1. Although β2 integrins show functional overlap, the corresponding α subunit defines its individual functional properties.6 The β2/CD18 chain has also received attention because of its involvement in several inflammatory receptors such as CD11a/CD18, αLβ2, lymphocyte function-associated antigen-1 (LFA-1); CD11b/CD18, αMβ2, Mac-1, complement receptor 3 (CR3); CD11c/CD18 (αXβ2, p150.95, CR4); and CD11d/CD18, αDβ2; Fig. 1). In these β2 integrins, the α subunits bind specific ligands such as the intercellular adhesion molecules (ICAMs). The non-I-domain α subunits in other integrins, such as the laminin-binding α3, α6, and α7, and others that recognize the arginine (R), glycine (G), aspartic acid (D) (RGD) motif (αV, α8, α5, and αIIb), are also closely related to each other.7 The α subunit of each integrin is the primary determinant of its extracellular ligand specificity. The β chain binds acidic residues in ICAMs and in cytoplasmic adapters such as paxillin, talins, and kindlins to facilitate cellular adhesions with the ECM. Integrins interact with the actin cytoskeleton through the talin- and kindlin-binding motifs present in the cytoplasmic domains of their β subunits.8
Characteristics of specific integrin heterodimers
Integrin αβ heterodimers are divided into four classes (leukocyte, collagen-binding, Arg-Gly-Asp (RGD)-binding, and laminin-binding integrins; Fig. 1) based on evolutionary associations, ligand specificity, and restricted expression on white blood cells (β2 and β7 integrins). Leucocyte integrins have a common β2 chain that is linked to CD-18 and bind receptors such as ICAM and plasma proteins such as complement components C3b and C4b.9 Collagen-binding integrins have a common β1 chain that binds various α chains in integrins α1β1, α2β1, α10β1, and α11β1. The α2β1 integrin binds its primary ligand, collagen,10 and chondroadherin, a matrix protein.11 The RGD-binding integrins have a common αV chain or β1 chain. The RGD peptide motif was first discovered in fibronectin12 but was later found in several other ECM proteins, such as fibronectin,9 osteopontin,13 vitronectin,14 von Willebrand factor (VWF),15 and laminin.16 Among the 24 human integrin subtypes known to date, eight integrin dimers recognize the tripeptide RGD motif within ECM proteins, namely: αVβ1, αVβ3, αVβ5, αVβ6, αVβ8, α5β1, α8β1, and αIIbβ3. Laminin-binding integrins (α3β1, α6β1, α7β1, and α6β4) mediate the adhesion of cells to basement membranes in various tissues.9 The α4β1, α9β1, and α4β7 integrin family binds fibronectin in a RGD-independent manner.9
Integrin–ligand binding and consequent activation
The structure and function of integrins are complex. Integrins bind numerous extracellular ligands, intracellular signaling molecules, and the cytoskeleton in a bivalent-cation-dependent manner with varying specificities. Integrins also have many states with multiple conformations and affinities.
Mechanism of integrin ligand binding and conformational states
Integrins bind cell-surface ligands to promote cellular interactions with the ECM and with other cells in the transduction of complex signals that modulate many cellular processes, such as adhesion, migration, and differentiation. These soluble, ECM, or cell surface-bound ligands may include growth factors, structural constituents of the ECM, proteases, cytokines, plasma proteins, microbial pathogens, or receptors specific to immune cells. The affinity and avidity of a ligand may change actively by inside-out signaling in specific pathways. Ligand affinity may vary with the strength of interaction and dissociation of a monovalent protein and its ligand, where ligand avidity refers to its ability to form multiple combinations of bonds.17
Integrins exist primarily in three conformational states: bent–closed (inactive; the predominant state), extended–closed (active; low affinity or intermediate state), and the extended–open (active; high affinity).18 The affinity of integrins to various inhibitory and stimulatory ligands is modulated by bivalent cations, which induce a range of conformational changes in integrins ranging from a folded, inactive, and low-affinity state to a high-affinity conformation (Fig. 2).19 These conformational changes in the extracellular domains of integrins modulate both ligand binding and downstream cellular signaling.
Integrin activation
The activation of integrins increases the affinity of these molecules to extracellular ligands. Integrin tail domains play a critical role in these steps, and any genetic mutations in these parts of integrins can disrupt downstream intracellular signaling.20 Integrin-mediated signaling across cell membranes is typically bi-directional and termed “outside-in” and “inside-out” signaling.20,21 When integrins interact with ECM ligands, a conformational change allows adherence to downstream adaptor molecules in the cell-membrane plane.22 Once clustered, integrins are able to recruit and activate kinases such as Src family kinases, focal adhesion and scaffold molecules such as the adaptor protein p130CRK-associated substrate/breast cancer anti-estrogen resistance 1 (p130CAS/BCAR1).22 These integrin-associated complexes include discrete active and inactive integrin organizations, which can activate unique signaling pathways.23,24
The extracellular domains of integrins are known to undergo a diverse range of conformational changes that alter the ligand-binding domains. In the cytoplasmic tails of integrins, α-helices are seen as heterodimers,25 and the β-strands often bind intracellular proteins, such as talin or filamin.26,27 The cytoplasmic tail may undergo several specific conformational changes to bind a range of other signal transducers.28,29
Integrin bi-directional inside-out and outside-in signaling
Mechanical stress30 and extracellular chemicals31 can induce rapid conformational changes to cause inside-out activation of integrins.32 Integrins display bi-directional signaling across the plasma membrane. Ligand binding induces extracellular-to-cytoplasm signal transduction, and inside-out signaling or priming regulates integrin-ligand binding conformations (Fig. 2). During integrin activation and signaling, the cytoplasmic tail acts as both a receptor and transmitter of signals. Specifically, during inside-out signaling, the activating signals make an impression on the cytoplasmic tail to induce large conformational changes to the extracellular domain, thereby transforming the integrin from a resting to an active state.33 During outside-in signaling, the binding of a ligand to the extracellular domain of active integrin transmits a conformational change to the cytoplasmic tail, which leads to the activation of kinases and adaptor molecules in the cytosol.1 In contrast, talin and kindlin interaction with the β-cytoplasmic tail can trigger inside-out signaling, leading to integrin activation, clustering, and recruitment of intercellular adaptor proteins to strengthen cellular adhesion. Talin is a large dimeric actin-binding protein and a major regulator of integrin activation, and the regulation of talin–integrin interactions is important in the control of integrin activation and signaling pathways.33 Direct interactions between the talin head and the short cytoplasmic tails of β integrin subunits disrupt inhibitory interactions between α and β integrin subunits.33 This leads to conformational changes in the integrin extracellular domains and consequent increase in their ligand affinity. The role of kindlins are not clearly defined, but they are structurally related to the talin head. The synergistic binding of talin and kindlin to β integrin cytoplasmic tails induces integrin activation by disrupting the α–β interactions at the transmembrane and the cytoplasmic domains.33,34
Integrins in inflammation and infection
In the resting state, β2 integrins are expressed specifically on leucocyte receptors. During inflammation, the inflammatory cytokines activate these integrins and promote cellular adherence to the counter-receptors such as ICAMs and promote phagocytosis and cytotoxic killing. Integrin receptors on leukocytes, such as the macrophage-1 antigen (Mac-1, also known as CR3, αMβ2, CD11b/CD18) interact with platelet antigens such as the glycoprotein Ibα (GPIbα) during inflammation. Integrins bind to the pro-domain of transforming growth factor (TGF)-β1 to activate it and promote its secretion. The pro-TGF-βs are biosynthesized and stored in tissues in latent forms, and integrins αVβ6 and αVβ8 can uniquely bind and activate pro-TGF-β1 and pro-TGF-β3. The αVβ6 integrin is known to specifically bind the RGDLXXL/I motif in TGF-β1 and TGF-β3.35
β2 integrins promote recruitment of leukocytes to the sites of inflammation by promoting the adhesion of circulating leukocytes to vascular endothelium, transendothelial migration,36,37 the formation of immunological synapses in leucocytes,38 and inflammatory signaling in involved cells.39 Activated β2 integrins on dendritic cells (DCs) may act as negative regulators of DC migration in certain conditions and may also regulate T cell activation.40,41 β integrins on the leukocyte surface are also involved in the tethering, rolling, and adhesion of leukocytes to activated endothelial cells.42 β2 integrins can also initiate intracellular signaling pathways in macrophages and neutrophils and stimulate cytokine secretion from these cells either directly or in synergy with Toll-like receptors (TLRs).43 Integrins may also integrate the impact of the epidermal growth factor receptor, platelet-derived growth factor receptor, insulin receptor, met receptor superfamily (hepatocyte growth factor receptor), and the vascular endothelial growth factor receptor (VEGFR) in inflammatory cells.44
β2 integrins are important regulators of adhesion, leukocyte recruitment, and immunological signaling. These integrins mediate adhesive interactions between myeloid cells, endothelial cells, antigen-presenting cells, T cells and the ECM.45 L-selectin, the CCR7 chemokine receptor, interacts with specific carbohydrate epitopes on the endothelium and promotes leukocyte rolling and transmigration through the vascular endothelium.46 Leukocyte rolling induces a rapid, although transient, increase in the affinity of the β1 and β2 integrins to the endothelial ligands.47,48 Conformational changes in the structure of the inserted (I) domain of the αL subunit of LFA-149 enhance firm leukocyte adhesion under shear flow.31,49
Role of integrins in neonatal organs during normal development and inflammation
Integrins in the lung during normal development and in inflammation
Integrins and receptor tyrosine kinases act with cytokine and growth factors to modulate the extracellular signal-regulated kinase and phosphatidylinositol 3-kinase (PI3K)-AKT signaling pathways during regeneration, inflammation, developmental, and pathological processes in the developing lung.2,44,50,51 The ECM in the lung contains collagen, fibronectin, laminin, and entactin,52 and alterations in the formation and structure of the ECM during normal development, healing from injury, or in chronic lung disease could lead to profound alterations in the lung structure.53,54 For instance, fibronectin in the ECM promotes integrin-mediated cellular migration and differentiation of cells during lung development.55 β1 integrin activates several signaling pathways, particularly the PI3K/AKT pathway activated during wound healing in the presence of collage VI in the lung ECM.56 β1 integrins play a critical role in alveolar homeostasis, as seen in chronic lung disease depicted in β1 integrin-deficient mice.57 In addition, β1 integrin-deficient alveolar epithelial cells produce excessive monocyte chemoattractant protein 1 and reactive oxygen species, suggesting that β1 integrins may be involved in alveolar homeostasis.58 In murine models of bronchopulmonary dysplasia, perinatal exposure to lipopolysaccharide and increased expression of interleukin-33 may activate neutrophils and promote fibronectin degradation in alveolar epithelial cells.59 Other studies have noted increased expression of integrin α2β1 on mast cells and activation/release of inflammatory cytokines.60,61 Similar findings have been noted in murine models with Listeria monocytogenes infections.62 Mice deficient in integrin α263 and integrin αIIb64 show defective platelet interaction with collagen. α2β1 integrin-null mice have normal angiogenesis but may have altered angiogenic responses during injury repair.65 In contrast, integrin β1 knockout mice may have altered development and are not viable, indicating an essential role of β1 during development. Table 1 outlines murine models of integrins, their target tissues, and signaling.
Table 1.
Integrin-targeted murine models and the effect of their signal modulation.
| Integrin | Tissue target | Effect of signal modulation | Mouse model |
|---|---|---|---|
| α3β1 | Endothelial cells | Inhibition of angiogenesis | Endothelial cells α3−/− knockout mice |
| α2β1 | Retinal Muller cells | Reduced neovascularization | α2β1 integrin deficient mice88 |
| α2β1 | Mast cells | Cytokine release following Listeria infection | α2β1 knockout mouse model of Listeria infection62 |
| αVβ6 | Epithelial cells of the lung | Activates transforming growth factor beta (TGF-β) to regulate pulmonary fibrosis and inflammation | Genetic knockdown116 |
| αV | Intestinal Th17 cells, colon | Decreased regulatory T (Treg) cells in the colon, leading to severe colitis, autoimmunity, and cancer | αv-deficient mice117 |
| β1 | Fibroblasts | Delayed cutaneous wound closure and reduced formation of granulation tissue and reduced ECM production | β1-deficient fibroblast-specific knockout mice118 |
| β3 | Fibroblasts, epithelial cells | Accelerated re-epithelialization, enhanced TGF-β signaling, dermal fibroblast infiltration | β3-deficient mice (genetic knockdown)119 |
Integrins in intestinal inflammation and in necrotizing enterocolitis (NEC)
The regulation of intestinal leukocyte responses is vital to maintaining immune homeostasis and prevention of intestinal inflammatory conditions. Integrin αvβ5 is expressed on neonatal intestinal macrophages; the expression is developmentally regulated and is not dependent on microbial colonization. These integrins bind different ECM components, such as laminins, collagens, and fibronectin, and are known to coordinate epithelial cell adhesion and movement.4,66 These integrins recognize the RGD tri-peptide sequence present in ECM proteins, such as fibronectin and vitronectin.67,68 The integrin αVβ5 can be found in both focal adhesions and in clathrin-coated membrane domains.69,70
Integrin αvβ8 plays an important role in epithelial homeostasis and is a major activator of TGF-β expression.71 α3 and β1 integrins, which are known to increase epithelial migration, are upregulated by bacterial products during NEC.72,73 TLR4 signaling on enterocytes promotes the efflux of β1 integrins from the cytoplasm toward the cell membrane and enhances cell–matrix contacts that limit cellular movement.74 In other studies, Besner and colleagues have examined the role of E-cadherin and integrins in NEC and showed that the growth factor, heparin-bound epidermal growth factor, can promote intestinal restitution in NEC through its effects on integrin–ECM interaction and intercellular adhesions.75 Intestinal epithelial cells also express α3β1, another set of integrins of translational importance. In NEC, increased epithelial expression of α3β1 may impair the migration of epithelial cells needed for mucosal wound healing.74 However, the same α3 integrins are also required for morphologic differentiation of the intestinal epithelium in the developing intestine.76 Despite the physiological needs of the β1 integrins, therapeutic targeting of these molecules may still be possible with information on the best timing and the possibility of regionally focused intervention.
Integrins in the developing eye and in retinopathy of prematurity
In the developing eye, disruption of the oxygen supply to the retina can disrupt neuronal dysfunction needed for transduction and transmission of photosensitive visual signals to the occipital lobe and other cognitive centers. Integrin α2β1 and VEGF interact closely in several intracellular angiogenic signaling (Fig. 3).77,78 Cyclic peptides selectively inhibit αVβ3 and αVβ5, and are potent inhibitors of endothelial cell invasion and differentiation induced by VEGF-A or fibroblast growth factor-2.78 Integrin αVβ3 works synergistically with VEGF to activate angiogenesis in endothelial cells via VEGFR‐2 phosphorylation.79 Endothelial cells are the primary cells expressing both VEGFR-2 and α2β1 integrin.80 Proteoglycans such as decorin and perlecan in the ECM of the eye can modulate α2β1 and play a vital role in angiogenesis.80 The C-terminal fragment of perlecan, known as endorepellin, has an opposite effect and blocks angiogenesis through antagonism of VEGFR-2 and α2β1 integrin on endothelial cells.80 Retinal pigment epithelial cells express beta‐8 integrin at the surface, and the knockdown of beta-8 integrin significantly decreased retinal pigment epithelial cell migration in wound-healing assays.81
Fig. 3. Schematic of integrin regulation of angiogenic signaling.
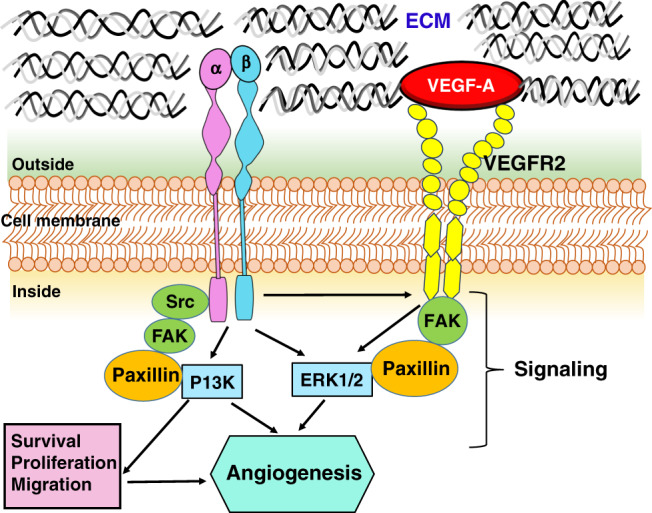
The schematic shows the interaction between the signaling pathways regulated by αβ integrins and the VEGF receptor. VEGF-A promotes angiogenesis through VEGF receptor-2 (VEGFR2), a tyrosine kinase receptor expressed by endothelial cells.112 When VEGF-A binds to VEGFR2, numerous intracellular signaling pathways are activated, such as phosphatidylinositol 3-kinase (PI3K), extracellular signal-regulated kinase (Erk), focal adhesion kinase (FAK), c-Src family, and paxillin, a signal transduction adaptor protein associated with focal adhesion.113,114 Specifically, FAK phosphorylates its substrate, paxillin, which activates ERK signaling.114 When integrins activate the tyrosine phosphorylation of FAK, it binds to signaling structural proteins, PI3K, and paxillin.115 Src family kinases (SFKs) play a critical role in cell adhesion, survival, and angiogenesis, interact with VEGF receptor, regulate gene expression of angiogenic growth factors, modulate cell proliferation via the mitogen-activated protein kinases (MAPK)-ERK pathway, and interact with integrins to regulate cell adhesion and migration. ECM extracellular matrix, VEGF vascular endothelial growth factor.
The retinal tissue has one of the body’s highest metabolic demands, placing it at risk of injury from oxidative stress, metabolic derangements, and consequent pathologic neovascularization seen in retinopathy of prematurity (ROP) and other proliferative retinal vitreoretinopathies. ROP is a bi-phasic disease of retinal vascular development due to dysregulation of VEGF.82,83 In phase 1, VEGF is downregulated during exposure to hyperoxia, while in phase 2, VEGF is upregulated in relative/true hypoxia. VEGF is known to have several isoforms; VEGFA165 is the predominant isoform in the eye with multiple pro- and anti-angiogenic splice variants.84 In a newborn mouse model of oxygen-induced retinopathy (OIR), oxidative stress from fluctuating hyperoxia and hypoxia leads to altered vascular development with tortuous arteries, dilated veins, and capillary attrition, akin to human ROP.82,85 These changes persist in adult mice with long-term abnormalities in vascularization, structure, and function both in vivo and histologically.86,87
Integrin-targeted therapy holds promise in ROP. Targeting α2β1 integrin expression on endothelial cells mitigates OIR,88 and the administration of 3-[3-(6-guanidino-1-oxoisoindolin-2-yl) propanamido]-3-(pyridin-3-yl) propanoic acid dihydrochloride, a novel non-peptide αvβ3 antagonist, can inhibit retinal neovascularization.89 There are exciting possibilities that endothelial α2β1 may be therapeutic target in pathological angiogenesis.
Integrins in thrombosis and fibrosis
Platelet adhesion and signaling play key roles in hemostasis and thrombosis. Two platelet receptors, integrin αIIbβ3 and GPIbα, mediate the early and mid-stages of platelet adhesion in the vascular environment.90 GPIbα is a key part of the receptor for VWF, and its binding to VWF enables platelet rolling during the formation of thrombotic plugs at the sites of vascular injury.91–93 αIIbβ3 is expressed on both platelets and the endothelium, and upon activation, it promotes platelet adhesion and aggregation by cross-linking with soluble fibrinogen, fibronectin, and VWF.
In alloimmune thrombocytopenia, autoantibodies are frequently seen against integrin β3 and GPIbα.94,95 Intracranial hemorrhages may be seen more frequently in infants with anti-β3 integrin antibodies than in those with antibodies against GPIbα.96 Existing in vitro and in vivo data suggest that the β3 integrin may bind a wider range of ligands, including fibrinogen and VWF, and autoantibodies that block its function may induce a deeper functional deficit than the anti-GPIbα antibodies.97
Integrin-targeted therapies
Integrin dysregulation is implicated in the pathogenesis of numerous diseases with altered angiogenesis, inflammation, or in infectious diseases. In these conditions, therapeutic strategies may either directly target integrins or their ligands. Out of the 24 known human integrins, many have already been identified as therapeutic targets for monoclonal antibodies, peptides, and/or small molecules. In adult subjects, efforts are ongoing to target platelet integrin αIIbβ3 to prevent thrombotic complications after percutaneous vascular interventions, lymphocyte α4β1 and α4β7 integrins in the treatment of multiple sclerosis, and β7 integrins (α4β7 and αEβ7 integrins) in inflammatory bowel disease.98 Specifically, a humanized anti-α4 antibody (Natalizumab) works in reduction of inflammation in multiple sclerosis by blocking the α4β1–vascular cell adhesion molecule interaction or the α4β7–mucosal addressin cell adhesion molecule interaction on mucosal endothelium and blocking leukocyte trafficking across the blood–brain barrier.99 In another study, a micellar delivery vehicle decorated with an anti-angiogenic peptide has been shown to inhibit αVβ3-mediated neovascularization in endothelial cells.100 Several anticancer drugs have also been developed against integrin ligands or by using integrin-targeted encapsulated nanoparticles as vehicles to unload drugs into the vasculature of several tumors.101 In a mouse model of hepatic fibrosis, cyclic peptide-guided liposomes preferentially targeted the activated hepatic stellate cells (not quiescent ones) to treat the fibrotic phenotype.102 αVβ3 antagonists are being tried for the inhibition of retinal neovascularization and may have therapeutic value in ROP.89 In a mouse model of laser-induced choroidal neovascularization, intravenous injection of irradiated nanoparticles loaded with doxorubicin allowed nanoparticle accumulation in the neovascular lesions and reduced the size of neovascular lesions.103 Integrin antagonists may also be used in fibrotic diseases; IDL-2965 is being studied as a selective, highly potent, anti-fibrotic integrin antagonist in idiopathic pulmonary fibrosis.104 Small molecule pure antagonists, TDI-4161 and TDI-3761, have been designed to inhibit αVβ3-mediated cell adhesion to αVβ3 ligands.105 Further studies are needed to improve the specificity of anti-integrin drugs to improve both the safety profile and therapeutic success of these agents.
Conclusions
Enhanced understanding of integrin ligand interactions will enable development of therapies targeting specific receptors in order to modulate angiogenic, thrombotic, infections, and inflammatory disorders. Although numerous animal studies have shown promise in the clinical use of integrins as therapeutic targets, there is a need for clinical studies to confirm efficacy and safety in neonates and young infants. In this review, we have summarized and outlined the roles of integrins in inflammation, angiogenesis, and infectious conditions. Therapies could be targeted specifically to alpha subunits, but their overlapping roles are a critical factor to be considered. Further studies are needed both on molecular signaling and regulatory mechanisms of integrin function and the safety and efficacy in clinical settings.
Author contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published: both authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morse EM, Brahme NN, Calderwood DA. Integrin cytoplasmic tail interactions. Biochemistry. 2014;53:810–820. doi: 10.1021/bi401596q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper J, Giancotti FG. Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell. 2019;35:347–367. doi: 10.1016/j.ccell.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 5.Kurzinger K, Ho MK, Springer TA. Structural homology of a macrophage differentiation antigen and an antigen involved in T-cell-mediated killing. Nature. 1982;296:668–670. doi: 10.1038/296668a0. [DOI] [PubMed] [Google Scholar]
- 6.Bednarczyk M, Stege H, Grabbe S, Bros M. β2 Integrins—multi-functional leukocyte receptors in health and disease. Int. J. Mol. Sci. 2020;21:1402. doi: 10.3390/ijms21041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JO, Bankston LA, Arnaout MA, Liddington RC. Two conformations of the integrin A-domain (I-domain): a pathway for activation? Structure. 1995;3:1333–1340. doi: 10.1016/s0969-2126(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 8.McCarty JH, Cook AA, Hynes RO. An interaction between αvβ8 integrin and Band 4.1B via a highly conserved region of the band 4.1 C-terminal domain. Proc. Natl Acad. Sci. USA. 2005;102:13479–13483. doi: 10.1073/pnas.0506068102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koivisto L, Heino J, Häkkinen L, Larjava H. Integrins in wound healing. Adv. Wound Care. 2014;3:762–783. doi: 10.1089/wound.2013.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddington RC. Structural basis of collagen recognition by integrin alpha2beta1. Cell. 2000;101:47–56. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- 11.Haglund L, et al. Identification and characterization of the integrin alpha2beta1 binding motif in chondroadherin mediating cell attachment. J. Biol. Chem. 2011;286:3925–3934. doi: 10.1074/jbc.M110.161141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 13.Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc. Natl Acad. Sci. USA. 1986;83:8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki S, Oldberg A, Hayman EG, Pierschbacher MD, Ruoslahti E. Complete amino acid sequence of human vitronectin deduced from cDNA. Similarity of cell attachment sites in vitronectin and fibronectin. EMBO J. 1985;4:2519–2524. doi: 10.1002/j.1460-2075.1985.tb03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plow EF, Pierschbacher MD, Ruoslahti E, Marguerie GA, Ginsberg MH. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc. Natl Acad. Sci. USA. 1985;82:8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant DS, et al. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989;58:933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- 17.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishida N, et al. Activation of leukocyte β2 integrins by conversion from bent to extended conformations. Immunity. 2006;25:583–594. doi: 10.1016/j.immuni.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Mould AP, Garratt AN, Puzon-McLaughlin W, Takada Y, Humphries MJ. Regulation of integrin function: evidence that bivalent-cation-induced conformational changes lead to the unmasking of ligand-binding sites within integrin alpha5 beta1. Biochem. J. 1998;331(Pt 3):821–828. doi: 10.1042/bj3310821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calderwood DA. Integrin activation. J. Cell Sci. 2004;117:657–666. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- 21.Van Agthoven JF, et al. Structural basis for pure antagonism of integrin alphaVbeta3 by a high-affinity form of fibronectin. Nat. Struct. Mol. Biol. 2014;21:383–388. doi: 10.1038/nsmb.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer. 2010;10:9. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Springer TA. Integrin extension enables ultrasensitive regulation by cytoskeletal force. Proc. Natl Acad. Sci. USA. 2017;114:4685–4690. doi: 10.1073/pnas.1704171114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Mets R, et al. Cellular tension encodes local Src-dependent differential beta1 and beta3 integrin mobility. Mol. Biol. Cell. 2019;30:181–190. doi: 10.1091/mbc.E18-04-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhunia A, Tang XY, Mohanram H, Tan SM, Bhattacharjya S. NMR solution conformations and interactions of integrin alphaLbeta2 cytoplasmic tails. J. Biol. Chem. 2009;284:3873–3884. doi: 10.1074/jbc.M807236200. [DOI] [PubMed] [Google Scholar]
- 26.Kiema T, et al. The molecular basis of filamin binding to integrins and competition with talin. Mol. Cell. 2006;21:337–347. doi: 10.1016/j.molcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Ellis SJ, et al. The talin head domain reinforces integrin-mediated adhesion by promoting adhesion complex stability and clustering. PLoS Genet. 2014;10:e1004756. doi: 10.1371/journal.pgen.1004756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beglova N, Blacklow SC, Takagi J, Springer TA. Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat. Struct. Biol. 2002;9:282–287. doi: 10.1038/nsb779. [DOI] [PubMed] [Google Scholar]
- 29.Armulik A, Nilsson I, von Heijne G, Johansson S. Determination of the border between the transmembrane and cytoplasmic domains of human integrin subunits. J. Biol. Chem. 1999;274:37030–37034. doi: 10.1074/jbc.274.52.37030. [DOI] [PubMed] [Google Scholar]
- 30.Zwartz GJ, et al. Real-time analysis of very late antigen-4 affinity modulation by shear. J. Biol. Chem. 2004;279:38277–38286. doi: 10.1074/jbc.M402944200. [DOI] [PubMed] [Google Scholar]
- 31.Constantin G, et al. Chemokines trigger immediate β2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13:759–769. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- 32.Arnaout MA, Mahalingam B, Xiong J-P. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 33.Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 2013;14:503–517. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim C, Ye F, Ginsberg MH. Regulation of integrin activation. Annu. Rev. Cell Dev. Biol. 2011;27:321–345. doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- 35.Dong X, Hudson NE, Lu C, Springer TA. Structural determinants of integrin beta-subunit specificity for latent TGF-beta. Nat. Struct. Mol. Biol. 2014;21:1091–1096. doi: 10.1038/nsmb.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Andrian UH, et al. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc. Natl Acad. Sci. USA. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lammermann T, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 38.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 39.Szukiewicz D, et al. Chorioamnionitis (ChA) modifies CX3CL1 (fractalkine) production by human amniotic epithelial cells (HAEC) under normoxic and hypoxic conditions. J. Inflamm. 2014;11:12. doi: 10.1186/1476-9255-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varga G, et al. Active MAC-1 (CD11b/CD18) on DCs inhibits full T-cell activation. Blood. 2007;109:661–669. doi: 10.1182/blood-2005-12-023044. [DOI] [PubMed] [Google Scholar]
- 41.Schittenhelm L, Hilkens CM, Morrison VL. β2 integrins as regulators of dendritic cell, monocyte, and macrophage function. Front. Immunol. 2017;8:1866. doi: 10.3389/fimmu.2017.01866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herter J, Zarbock A. Integrin regulation during leukocyte recruitment. J. Immunol. 2013;190:4451–4457. doi: 10.4049/jimmunol.1203179. [DOI] [PubMed] [Google Scholar]
- 43.Wolf D, et al. A ligand-specific blockade of the integrin Mac-1 selectively targets pathologic inflammation while maintaining protective host-defense. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-018-02896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu, Y., Rose, D. M. & Ginsberg, M. H. Integrins in the immune system. Adv. Immunol. 72, 325–380 (1999). [DOI] [PubMed]
- 46.Lasky LA, et al. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell. 1992;69:927–938. doi: 10.1016/0092-8674(92)90612-g. [DOI] [PubMed] [Google Scholar]
- 47.Grabovsky V, et al. Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J. Exp. Med. 2000;192:495–506. doi: 10.1084/jem.192.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen C, et al. High affinity very late antigen-4 subsets expressed on T cells are mandatory for spontaneous adhesion strengthening but not for rolling on VCAM-1 in shear flow. J. Immunol. 1999;162:1084–1095. [PubMed] [Google Scholar]
- 49.Salas A, Shimaoka M, Chen S, Carman CV, Springer T. Transition from rolling to firm adhesion is regulated by the conformation of the I domain of the integrin lymphocyte function-associated antigen-1. J. Biol. Chem. 2002;277:50255–50262. doi: 10.1074/jbc.M209822200. [DOI] [PubMed] [Google Scholar]
- 50.Danen EH, Yamada KM. Fibronectin, integrins, and growth control. J. Cell. Physiol. 2001;189:1–13. doi: 10.1002/jcp.1137. [DOI] [PubMed] [Google Scholar]
- 51.Mižíková I, Morty RE. The extracellular matrix in bronchopulmonary dysplasia: target and source. Front. Med. 2015;2:91. doi: 10.3389/fmed.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kahsai TZ, et al. Seminiferous tubule basement membrane. Composition and organization of type IV collagen chains, and the linkage of alpha3(IV) and alpha5(IV) chains. J. Biol. Chem. 1997;272:17023–17032. doi: 10.1074/jbc.272.27.17023. [DOI] [PubMed] [Google Scholar]
- 53.Vlahovic G, Russell ML, Mercer RR, Crapo JD. Cellular and connective tissue changes in alveolar septal walls in emphysema. Am. J. Respir. Crit. Care Med. 1999;160:2086–2092. doi: 10.1164/ajrccm.160.6.9706031. [DOI] [PubMed] [Google Scholar]
- 54.Burgstaller G, et al. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur. Respir. J. 2017;50:1601805. doi: 10.1183/13993003.01805-2016. [DOI] [PubMed] [Google Scholar]
- 55.Roy DC, Hocking DC. Recombinant fibronectin matrix mimetics specify integrin adhesion and extracellular matrix assembly. Tissue Eng. Part A. 2013;19:558–570. doi: 10.1089/ten.tea.2012.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mereness JA, et al. Type VI collagen promotes lung epithelial cell spreading and wound-closure. PLoS ONE. 2018;13:e0209095. doi: 10.1371/journal.pone.0209095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plosa, E. J. et al. β1 integrin regulates adult lung alveolar epithelial cell inflammation. JCI Insight5, e129259 (2020). [DOI] [PMC free article] [PubMed]
- 58.Plosa EJ, et al. Epithelial β1 integrin is required for lung branching morphogenesis and alveolarization. Development. 2014;141:4751–4762. doi: 10.1242/dev.117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin R, et al. IL-33-induced neutrophil extracellular traps degrade fibronectin in a murine model of bronchopulmonary dysplasia. Cell Death Discov. 2020;6:33. doi: 10.1038/s41420-020-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ringel-Scaia, V. M., Powell, M. D., Read, K. A., Allen, I. C. & Oestreich, K. J. Systemic Listeria monocytogenes infection as a model to study T helper cell immune responses. Methods Mol. Biol. 1960, 149–160 (2019). [DOI] [PubMed]
- 61.McCall-Culbreath KD, Li Z, Zutter MM. Crosstalk between the α2β1 integrin and c-met/HGF-R regulates innate immunity. Blood. 2008;111:3562–3570. doi: 10.1182/blood-2007-08-107664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edelson BT, Li Z, Pappan LK, Zutter MM. Mast cell–mediated inflammatory responses require the α2β1 integrin. Blood. 2004;103:2214–2220. doi: 10.1182/blood-2003-08-2978. [DOI] [PubMed] [Google Scholar]
- 63.Holtkotter O, et al. Integrin alpha 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J. Biol. Chem. 2002;277:10789–10794. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- 64.Bouvard D, et al. Functional consequences of integrin gene mutations in mice. Circ. Res. 2001;89:211–223. doi: 10.1161/hh1501.094874. [DOI] [PubMed] [Google Scholar]
- 65.Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am. J. Pathol. 2002;161:337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bodary S, McLean JW. The integrin beta 1 subunit associates with the vitronectin receptor alpha v subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J. Biol. Chem. 1990;265:5938–5941. [PubMed] [Google Scholar]
- 68.Charo IF, Nannizzi L, Smith JW, Cheresh DA. The vitronectin receptor avb3 binds fibronectin and acts in concert with a5b1 in promoting cellular attachment and spreading on fibronectin. J. Cell Biol. 1990;111:2795–2800. doi: 10.1083/jcb.111.6.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grove J, et al. Flat clathrin lattices: stable features of the plasma membrane. Mol. Biol. Cell. 2014;25:3581–3594. doi: 10.1091/mbc.E14-06-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lampe M, Vassilopoulos S, Merrifield C. Clathrin coated pits, plaques and adhesion. J. Struct. Biol. 2016;196:48–56. doi: 10.1016/j.jsb.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 71.Sherman MP. New concepts of microbial translocation in the neonatal intestine: mechanisms and prevention. Clin. Perinatol. 2010;37:565–579. doi: 10.1016/j.clp.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neu J., Pammi M. Pathogenesis of NEC: impact of an altered intestinal microbiome. Semin. Perinatol. 41, 29–35 (2017). [DOI] [PubMed]
- 73.Fundora, J. B., Guha, P., Shores, D. R., Pammi, M. & Maheshwari, A. Intestinal dysbiosis and necrotizing enterocolitis: assessment for causality using Bradford Hill criteria. Pediatr. Res. 87, 235–248 (2019). [DOI] [PMC free article] [PubMed]
- 74.Qureshi FG, et al. Increased expression and function of integrins in enterocytes by endotoxin impairs epithelial restitution. Gastroenterology. 2005;128:1012–1022. doi: 10.1053/j.gastro.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 75.Su Y, Yang J, Besner GE. HB-EGF promotes intestinal restitution by affecting integrin–extracellular matrix interactions and intercellular adhesions. Growth Factors. 2013;31:39–55. doi: 10.3109/08977194.2012.755966. [DOI] [PubMed] [Google Scholar]
- 76.Zhang X, Cromwell JW, Kunjummen BD, Yee D, Garcia-Aguilar J. The alpha2 and alpha3 integrins are required for morphologic differentiation of an intestinal epithelial cell line. Surgery. 2003;133:429–437. doi: 10.1067/msy.2003.107. [DOI] [PubMed] [Google Scholar]
- 77.Campochiaro PA, Aiello LP, Rosenfeld PJ. Anti–vascular endothelial growth factor agents in the treatment of retinal disease: from bench to bedside. Ophthalmology. 2016;123:S78–S88. doi: 10.1016/j.ophtha.2016.04.056. [DOI] [PubMed] [Google Scholar]
- 78.Nisato RE, Tille J-C, Jonczyk A, Goodman SL, Pepper MS. αvβ3 and αvβ5 integrin antagonists inhibit angiogenesis in vitro. Angiogenesis. 2003;6:105–119. doi: 10.1023/B:AGEN.0000011801.98187.f2. [DOI] [PubMed] [Google Scholar]
- 79.Soldi R, et al. Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor‐2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Douglass S, Goyal A, Iozzo RV. The role of perlecan and endorepellin in the control of tumor angiogenesis and endothelial cell autophagy. Connect. Tissue Res. 2015;56:381–391. doi: 10.3109/03008207.2015.1045297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caceres PS, Hanke-Gogokhia C, Ridano ME, Zobel G, Rodriguez-Boulan E. Cell‐cell and cell‐extracellular matrix communication pathways identified in the polarized surface proteome of retinal pigment epithelial cells. FASEB J. 2020;34:1–1. [Google Scholar]
- 82.Mezu-Ndubuisi OJ. In vivo angiography quantifies oxygen-induced retinopathy vascular recovery. Optom. Vis. Sci. 2016;93:1268–1279. doi: 10.1097/OPX.0000000000000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mezu-Ndubuisi OJ, et al. Intravitreal delivery of VEGF-A165-loaded PLGA microparticles reduces retinal vaso-obliteration in an in vivo mouse model of retinopathy of prematurity. Curr. Eye Res. 2019;44:275–286. doi: 10.1080/02713683.2018.1542736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao M, et al. Expression of pro-and anti-angiogenic isoforms of VEGF in the mouse model of oxygen-induced retinopathy. Exp. Eye Res. 2011;93:921–926. doi: 10.1016/j.exer.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 85.Mezu-Ndubuisi OJ, et al. In vivo retinal vascular oxygen tension imaging and fluorescein angiography in the mouse model of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2013;54:6968–6972. doi: 10.1167/iovs.13-12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mezu-Ndubuisi OJ, Adams T, Taylor LK, Nwaba A, Eickhoff J. Simultaneous assessment of aberrant retinal vascularization, thickness, and function in an in vivo mouse oxygen-induced retinopathy model. Eye. 2019;33:363–373. doi: 10.1038/s41433-018-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mezu-Ndubuisi OJ, et al. Long-term evaluation of retinal morphology and function in a mouse model of oxygen-induced retinopathy. Mol. Vis. 2020;26:257–276. [PMC free article] [PubMed] [Google Scholar]
- 88.Madamanchi A, et al. Mitigation of oxygen-induced retinopathy in α2β1 integrin-deficient mice. Investig. Ophthalmol. Vis. Sci. 2014;55:4338–4347. doi: 10.1167/iovs.14-14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y-J, et al. Therapeutic efficacy of a novel non-peptide αvβ3 integrin antagonist for pathological retinal angiogenesis in mice. Exp. Eye Res. 2014;129:119–126. doi: 10.1016/j.exer.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 90.Monti M, et al. Integrin-dependent cell adhesion to neutrophil extracellular traps through engagement of fibronectin in neutrophil-like cells. PLoS ONE. 2017;12:e0171362. doi: 10.1371/journal.pone.0171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ruggeri ZM. Mechanisms initiating platelet thrombus formation. Thrombosis Haemost. 1997;78:611–616. [PubMed] [Google Scholar]
- 92.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate–receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–666. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 93.Tsuji S, et al. Real-time analysis of mural thrombus formation in various platelet aggregation disorders: distinct shear-dependent roles of platelet receptors and adhesive proteins under flow. Blood. J. Am. Soc. Hematol. 1999;94:968–975. [PubMed] [Google Scholar]
- 94.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 95.Di Q, et al. Impaired cross-activation of β3 integrin and VEGFR-2 on endothelial progenitor cells with aging decreases angiogenesis in response to hypoxia. Int. J. Cardiol. 2013;168:2167–2176. doi: 10.1016/j.ijcard.2013.01.240. [DOI] [PubMed] [Google Scholar]
- 96.Yougbaré I, et al. Maternal anti-platelet β3 integrins impair angiogenesis and cause intracranial hemorrhage. J. Clin. Investig. 2015;125:1545–1556. doi: 10.1172/JCI77820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang H, et al. Fibrinogen and von Willebrand factor‐independent platelet aggregation in vitro and in vivo. J. Thrombosis Haemost. 2006;4:2230–2237. doi: 10.1111/j.1538-7836.2006.02116.x. [DOI] [PubMed] [Google Scholar]
- 98.Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat. Rev. Drug Discov. 2016;15:173. doi: 10.1038/nrd.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O’Connor P. Natalizumab and the role of α4-integrin antagonism in the treatment of multiple sclerosis. Expert Opin. Biol. Ther. 2007;7:123–136. doi: 10.1517/14712598.7.1.123. [DOI] [PubMed] [Google Scholar]
- 100.Nagaraj R, et al. High density display of an anti-angiogenic peptide on micelle surfaces enhances their inhibition of αvβ3 integrin-mediated neovascularization in vitro. Nanomaterials. 2020;10:581. doi: 10.3390/nano10030581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Majumder P. Integrin-mediated delivery of drugs and nucleic acids for anti-angiogenic cancer therapy: current landscape and remaining challenges. Bioengineering. 2018;5:76. doi: 10.3390/bioengineering5040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y, et al. An integrin-based nanoparticle that targets activated hepatic stellate cells and alleviates liver fibrosis. J. Controlled Release. 2019;303:77–90. doi: 10.1016/j.jconrel.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y, et al. Intravenous treatment of choroidal neovascularization by photo-targeted nanoparticles. Nat. Commun. 2019;10:1–9. doi: 10.1038/s41467-019-08690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kossen, K. et al. IDL-2965: a selective, highly-potent, oral Integrin antagonist for IPF. Eur. Respir. J. 54, PA5374 (2019).
- 105.Li J, et al. Novel pure αVβ3 integrin antagonists that do not induce receptor extension, prime the receptor, or enhance angiogenesis at low concentrations. ACS Pharmacol. Transl. Sci. 2019;2:387–401. doi: 10.1021/acsptsci.9b00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tan S-M. The leucocyte β2 (CD18) integrins: the structure, functional regulation and signalling properties. Biosci. Rep. 2012;32:241–269. doi: 10.1042/BSR20110101. [DOI] [PubMed] [Google Scholar]
- 107.Fagerholm, S. C., Guenther, C., Asens, M. L., Savinko, T. & Uotila, L. M. Beta2-integrins and interacting proteins in leukocyte trafficking, immune suppression, and immunodeficiency disease. Front. Immunol. 10, 254 (2019). [DOI] [PMC free article] [PubMed]
- 108.Humphries MJ. Integrin structure. Biochem. Soc. Trans. 2000;28:311–339. [PubMed] [Google Scholar]
- 109.Luo B-H, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xiong JP, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 111.Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–511. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- 112.Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 113.Abhinand CS, Raju R, Soumya SJ, Arya PS, Sudhakaran PR. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J. Cell Commun. Signal. 2016;10:347–354. doi: 10.1007/s12079-016-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang WJ, et al. Paxillin regulates vascular endothelial growth factor A-induced in vitro angiogenesis of human umbilical vein endothelial cells. Mol. Med. Rep. 2015;11:1784–1792. doi: 10.3892/mmr.2014.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sin, C. C. Effect of PI3K δ Isoform on the Expression of Integrin Beta 3 and p130cas in Glioblastoma Multiforme. MSc thesis, Hong Kong Polytechnic Univ. (2016).
- 116.Munger J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: A mechanism for regulating pulmonary inflammation and fibrosis. Cel. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 117.Acharya M, et al. αv Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120:4445–4452. doi: 10.1172/JCI43796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu S, et al. Expression of integrin β1 by fibroblasts is required for tissue repair in vivo. J Cell Sci. 2010;123:3674–3682. doi: 10.1242/jcs.070672. [DOI] [PubMed] [Google Scholar]
- 119.Reynolds L, et al. Accelerated re-epithelialization in β 3-integrin-deficient-mice is associated with enhanced TGF-β1 signaling. Nat Med. 2005;11:167–174. doi: 10.1038/nm1165. [DOI] [PubMed] [Google Scholar]