Abstract
Background
Therapeutic hypothermia (TH) is an established intervention to improve the outcome of neonates with moderate-to-severe hypoxic-ischemic encephalopathy resulting from perinatal asphyxia. Despite this beneficial effect, TH may further affect drug elimination pathways such as the glomerular filtration rate.
Objectives
The objective of this study was to quantify the effect of TH in addition to asphyxia on mannitol clearance as a surrogate for the glomerular filtration rate.
Methods
The effect of asphyxia and TH (mild vs moderate/severe) on mannitol clearance was assessed using a population approach, based on mannitol observations collected in the ALBINO (ALlopurinol in addition to TH for hypoxic-ischemic Brain Injury on Neurocognitive Outcome) trial, as some were exposed to a second dose of 10 mg/kg intravenous mannitol as placebo to ensure blinding. Pharmacokinetic analysis and model development were conducted using NONMEM version 7.4.
Results
Based on 77 observations from 17 neonates (TH = 13), a one-compartment model with first-order linear elimination best described the observed data. To account for prenatal glomerular filtration rate maturation, both birthweight and gestational age were implemented as clearance covariates using an earlier published three-quarters power function and a sigmoid hyperbolic function. Our final model predicted a mannitol clearance of 0.15 L/h for a typical asphyxia neonate (39.5 weeks, birthweight 3.25 kg, no TH), lower than the reported value of 0.33 L/h for a healthy neonate of similar age and weight. By introducing TH as a binary covariate on clearance, the additional impact of TH on mannitol clearance was quantified (60% decrease).
Conclusions
Mannitol clearance was decreased by approximately 60% in neonates undergoing TH, although this is likely confounded with asphyxia severity.
Trial Registration
ClinicalTrials.gov identifier NCT03162653.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40262-021-00991-6.
Key Points
| Therapeutic hypothermia (TH) improves the outcome of neonates with moderate-to-severe hypoxic-ischemic encephalopathy resulting from perinatal asphyxia, but this is associated with a reduced glomerular filtration rate. Mannitol clearance reflects the glomerular filtration rate. |
| Mannitol clearance of a typical asphyxiated neonate (39.5 weeks, birthweight 3.25 kg, no TH) was estimated at 0.15 L/h, lower than the reported mannitol clearance of a healthy neonate (0.33 L/h) of similar age and weight. |
| Mannitol clearance is further decreased by approximately 60% in neonates who undergo TH, but this is likely confounded with asphyxia severity, as TH is applied only to newborns with moderate or severe asphyxia. |
Introduction
Perinatal asphyxia is a clinical condition comprising perinatal hypoxia, hypercarbia, and combined metabolic and respiratory acidosis [1]. Perinatal asphyxia is a multi-organ disease with moderate-to-severe encephalopathy as a pivotal finding to initiate therapeutic hypothermia (TH) within 6 hours after birth in (near)term neonates [2]. This multi-organ disease includes renal impairment, with large inter-individual variability (IIV) in the glomerular filtration rate (GFR), in part explained by asphyxia severity [3]. In 36 asphyxiated neonates (mild, moderate, severe), acute kidney injury (AKI) was observed in 1/11 (9%) neonates with moderate sphyxia and in 12/25 (56%) neonates with severe asphyxia [4]. When we focus on cases that underwent TH (commonly restricted to moderate-to-severe HIE), AKI was observed in 35–42% [5–7]. Creatinine in the first days of life reflects to a certain extent maternal kidney function, assay-related differences still matter, while reference centile values following perinatal asphyxia do not yet exist. Consequently, their clinical use to assess individual renal impairment in early neonatal life remains poor [5].
Asphyxia with TH results in a clinically significant, transient decrease in mean GFR (− 40 to − 50%), with gestational age (GA) and hypoxic-ischemic encephalopathy (HIE) stage as additional covariates [3]. Mannitol, a hexitol, is eliminated exclusively by the kidneys where it undergoes free filtration through the glomeruli. While there is limited tubular reabsorption (10%) in adults, the tubular transporter processes in neonates are still immature. Moreover, mannitol is not metabolized, thereby fulfilling the criteria for being an exogenous substance for GFR measurement [8]. Consequently, mannitol clearance (CL) has been used to describe GFR, including maturation and growth from term neonatal age onwards [9–11]. The ALBINO (ALlopurinol in addition to TH for hypoxic-ischemic Brain Injury on Neurocognitive Outcome) trial is a randomized blinded placebo-controlled trial with mannitol administration in the placebo group, as this enabled visual blinding [12]. As pharmacokinetics was a predefined secondary endpoint of this trial, this provided us with the possibility to describe mannitol pharmacokinetics and its covariates as a GFR indicator and its variability in this specific population of neonates. Information on asphyxia severity and related covariates on GFR can subsequently be used to better predict renal drug CL, to improve pharmacotherapy or to explore physiology-based pharmacokinetic (PK) performance [1].
Methods
Setting and Study Design
(Near)term neonates who fulfilled perinatal asphyxia criteria and early signs of evolving encephalopathy (cfr infra) and were included in the ALBINO trial were administered an initial dose of study medication (intravenous, either 20 mg/kg of allopurinol or mannitol, placebo needed to enable blinding) shortly (i.e. within 45 min) after birth. This is because both the verum and the mannitol are provided as a freeze-dried sodium salt with the same visual aspect. A second dose of either allopurinol or mannitol (both 10 mg/kg, intravenous over 10 min) was administered 12 h after the first dose in neonates who subsequently underwent TH. The primary endpoint of this study is death or severe neurodevelopmental impairment vs survival without severe neurodevelopmental impairment at the age of 2 years [12].
Patients were eligible for the ALBINO trial if they fulfilled at least one of the perinatal asphyxia criteria: (1) pH < 7 or base deficit ≥ 16 mmol/L, (2) need for ongoing cardiac massage for ≥ 5 min postpartum; (3) need for adrenalin administration during resuscitation; and (4) Apgar score ≤ 5 after 10 min postpartum; in combination with two or more early signs of evolving encephalopathy: (1) altered state of consciousness; (2) hypotonia or hypertonia; (3) absent/insufficient spontaneous respiration requiring respiratory support for at least 10 min postpartum; and (4) abnormal primitive reflexes/abnormal movements (i.e., seizures). Further details of the study protocol have been published [12]. The most important exclusion criteria were a gestational age < 36 weeks, an estimated birth weight < 2500 g, or severe congenital abnormalities. Neonates who met the ALBINO inclusion criteria, but not the criteria to subsequently undergo TH within the first 6 h after birth because of a quick and spontaneous recovery (mild HIE), only received the first dose of either allopurinol or mannitol. Therapeutic hypothermia was initiated within 6 h after birth and aimed for a core temperature of 33.5 °C for 72 h. Subsequently, there was a slow rewarming to normothermia and after rewarming, body temperature was stabilized at 36.5 °C for 24 h in moderate-to-severe HIE cases in accordance with the published guidelines [2].
Pharmacokinetics, Blood Sampling, and Analysis
Pharmacokinetics was a predefined secondary outcome parameter of the ALBINO trial [12]. This sub-study was conducted in four specific centers (Amsterdam, Nürnberg, Tübingen, Utrecht) involved in the ALBINO trial. Blood sampling was combined with clinically indicated blood samples. To limit the number of blood samples per individual patient, two sample strategies were developed: A vs B at random for either neonates treated with TH or those who recovered quickly and did not undergo TH (Table 1) [13]. Exact timing of samples were documented and used in the analysis. Only samples of neonates exposed to mannitol (placebo, n = 17) were analyzed and reported in the current sub-study.
Table 1.
Sample timing and intervals
| Group | Samples |
|---|---|
| No hypothermia | |
| A | 15–60 min, 1.5–4 h, 8–12 h, 18–24 h, 60–72 h |
| B | 15–60 min, 1.5–4 h, 8–12 h, 36–48 h, 96–168 h |
| Hypothermia | |
| A | 15–60 min, 1.5–4 h, trough level t = 12 h, 13–14 h, 18–24 h, 60–72 h |
| B | 15–60 min, 1.5–4 h, trough level t = 12 h, 13–14 h, 36–48 h, 96–168 h |
h hours, min minutes
For the PK analyses, 0.5 mL of blood was collected in MiniCollect® lithium-heparin tubes (Greiner Bio One, Alphen aan den Rijn, the Netherlands). Samples were placed in melting ice immediately after collection and centrifuged with a speed of 1500–2000g for 10 min at 4–8 °C within 30 min after collection. Plasma was subsequently separated with a pipette and stored in a polypropylene crew cap at – 80 °C until analysis.
The Ardena Bioanalytical Laboratory determined mannitol concentrations in the plasma samples in accordance with Good Clinical Practice guidelines. The assay was validated according to the European Medicines Agency guidelines (International Conference on Harmonisation Good Clinical Practice regulations 2012). Following extraction of mannitol by protein precipitation and derivatization (2% acetic anhydride), liquid chromatography-mass spectrometry was used to determine total mannitol concentrations. The analytical range of the assay was 0.2–200 µg/mL, with the lower limit of quantificationbeing 0.2 µg/mL. Accuracy and precision of all quality control samples met the predefined acceptance criteria. The clinical characteristics were extracted from the study data registration files for all cases included in the mannitol PK study. Care was taken that investigators involved in patient recruitment remained blinded for treatment group assignment. The obtained concentration–time profiles of mannitol are illustrated in Fig. 1 of the Electronic Supplementary Material.
Ethics
Oral consent was obtained from at least one parent before administering the study medication. After the first dose, but before the (potential) second dose, full written parental consent was obtained [12]. In case parents did not sign the informed consent, blood samples already collected shortly after birth were destroyed. The medical ethics committee of the University Medical Centre Utrecht and the Central Committee on Human Research approved the study including the specific PK sub-study for the Netherlands (NL57237.041.16). The German Federal Authority (EudraCT 2016-000222-19) as well as the leading ethics committee of Tübingen approved the study for Germany.
PK Model Development
Pharmacokinetic analysis and model development were conducted using NONMEM version 7.4. Results were analyzed using R version 3.5.2 running under RStudio version 1.2.5042. Model building was performed in three different steps: (1) various structural models (i.e., one- and two-compartment models) were tested, after which the most appropriate model was selected; (2) a statistical sub-model was chosen; and (3) covariates were analyzed. The first-order conditional estimation with interaction was used throughout the model building process.
The minimum objective function value (OFV) was used to compare models. A decrease of 3.84 or greater in OFV was considered statistically significant at p < 0.05. Goodness-of-fit plots (both observed vs individual- and population-predicted concentrations, individual plots, and time as well as population predictions vs conditional weighted residuals) were evaluated as well.
Covariate Analysis
Covariates were plotted independently against individual estimates of IIV (i.e., η values) to screen for potential factors that affect mannitol pharmacokinetics (data not shown). Covariate-parameter relationships that showed a visual trend in the graphical assessment (i.e., birthweight [BW], lactate dehydrogenase (LDH), alanine aminotransferase (ALT), GA, and TH) were introduced into the base model. Therapeutic hypothermia was implemented as a binary covariate in the dataset and its parameter-covariate relationship was coded as follows (Eq. 1):
| 1 |
where Pp1 and Pp2 represent the population parameter estimates when cov is either equal to 0 or 1, Pi equals the individual parameter estimate of the ith subject, and cov is the covariate. BW, ALT, and LDH were implemented in the model using a power function (Eq. 2):
| 2 |
where Pp and Pi represent the population estimates and individual parameter estimates of the ith subject, cov is the covariate, and b is the power exponent. The relationship between GA and mannitol CL was investigated using a sigmoid hyperbolic function (Eq. 3) [14]:
| 3 |
where Pp and Pi represent the population estimates and individual parameter estimates of the ith subject, cov is the covariate, Hill (gamma) is the Hill coefficient, and TM50 the maturation half time.
The significance of the covariates was statistically tested using a stepwise covariate method involving testing of covariate relationships in a forward inclusion (reduction of OFV of 6.63; p < 0.01) and backward exclusion (reduction of OFV of 10.8; p < 0.001) [15]. Furthermore, some covariates (i.e., GA on CL, BW on CL, and TH on CL) were included into the final model based on scientific plausibility and prior knowledge [16–18].
Model Evaluation
The final PK model was evaluated using two methods: (1) the sampling importance resampling (SIR) method and (2) the normalized prediction distribution error (NPDE) method. The SIR procedure was used to assess the uncertainty and to calculate the 95% confidence intervals (CIs) of the parameter estimates of the final model. The procedure was run with 2000 final samples and 1000 resamples. A covariance matrix from the successful final model run was used as a proposal distribution. Results of the SIR procedure were evaluated with the graphical diagnostic methods provided by the sir package in PsN version 5.0.0. To determine the accuracy of the model predictions, a NPDE analysis was performed using the NPDE package in R version 3.5.2 [19]. Computation of NPDEs has been described previously by Brendel et al. [20]. Briefly, prediction discrepancies were obtained as the percentile of each observation within its predicted distribution. After decorrelation of the observations and the predictions, prediction distribution errors were calculated. These were then normalized by inverting the cumulative density function, after which NPDEs were obtained. A model describes the data well if the calculated NPDEs follow a normal distribution with a mean of 0 and a variance of 1.
Results
Patients and Clinical Characteristics
The PK analysis was based on a total of 77 observations from 17 neonates of whom 13 underwent TH. Table 2 summarizes all patient characteristics.
Table 2.
Clinical characteristics of patients included in the study
| Characteristic | Median [IQR] |
|---|---|
| Patients (n) | 17 |
| Gestational age (weeks) | 39.5 [1] |
| Birthweight (kg) | 3.4 [0.5] |
| Height (cm) | 54.1 [3] |
| Age at start of cooling (h) | 2.1 [4.8] |
| Lactate dehydrogenase (U/L) | 1520 [746] |
| Alanine aminotransferase (U/L) | 63.8 [44.8] |
| Thompson score | 9.5 [9] |
| Therapeutic hypothermia (n [%]) | 13 [76.5] |
| Inotropes (n [%]) | 8 [47.1] |
IQR interquartile range, absolute difference between Q3 and Q1
PK Model Building
A one-compartment model, which was parameterized in terms of CL and V, was preferred over a two-compartment model as it described the data more accurately. Indeed, the OFV of the one-compartment model (OFV = 450) was significantly lower (p < 0.05) as compared with the OFV of the two-compartment model (OFV = 454). Moreover, the decrease in OFV was accompanied with improved goodness-of-fit plots (data not shown). In addition, various residual error models (i.e., additive, proportional, and combined) were evaluated. The combined additive and proportional error model best described the residual variability as reflected by the lower OFV and improved goodness-of-fit plots (data not shown). Table 3 depicts the population PK parameters that were derived from the base model.
Table 3.
Estimated parameters of the base model and final pharmacokinetic model
| Parameter | Units | Base model | Final pharmacokinetic model | SIR final pharmacokinetic model | |
|---|---|---|---|---|---|
| Mean (% RSE) | Mean (% RSE) | Median | 95% confidence interval | ||
| Structural model parameters | |||||
| CL | L/h | 0.0760 (33) | 0.441 (10) | 0.440 | 0.320–0.544 |
| V | L | 1.440 (12) | 1.400 (13) | 1.415 | 1.132–1.705 |
| Covariates | |||||
| Asphyxia treated with hypothermia (θTH) | 0.399 (39)a | 0.395 | 0.194–0.616 | ||
| Birthweight on CL (θCL) | 0.75 Fixeda | N.D. | N.D. | ||
| Birthweight on V (θV) | 1 Fixedb | N.D. | N.D. | ||
| Hill coefficient | 3.40 Fixeda, c | N.D. | N.D. | ||
| Maturation half time (TM50) | Weeks | 47.7 Fixeda, c | N.D. | N.D. | |
| Inter-individual variability | |||||
| CL | CV% | 176 (32) | 137 (65) | 1.093 | 0.547–2.165 |
| V | CV% | 49.7 (19) | 41.9 (40) | 0.170 | 0.0846–0.316 |
| Residual variability | |||||
| Proportional | % | 29.2 (22) | 29.2 (23) | 29.4 | 24.1–35.5 |
| Additive | µg/mL | 0.358 (53) | 0.358 (36) | 0.359 | 0.184–0.484 |
BW birthweight, CL clearance, CV coefficient of variation, GA gestational age, N.D. not determined, Pop population, RSE relative standard error, SIR sampling importance resampling procedure (the median birth weight in this cohort was 3.4 kg), V volume of distribution
aCL = PopCL × (BW/median BW)θCL × ((GAHill)/(TM50Hill + GAHill)) × θTH
bV = PopV × (BW/median BW)θV
cValues were fixed according to [14]
Systematic Covariate Analysis
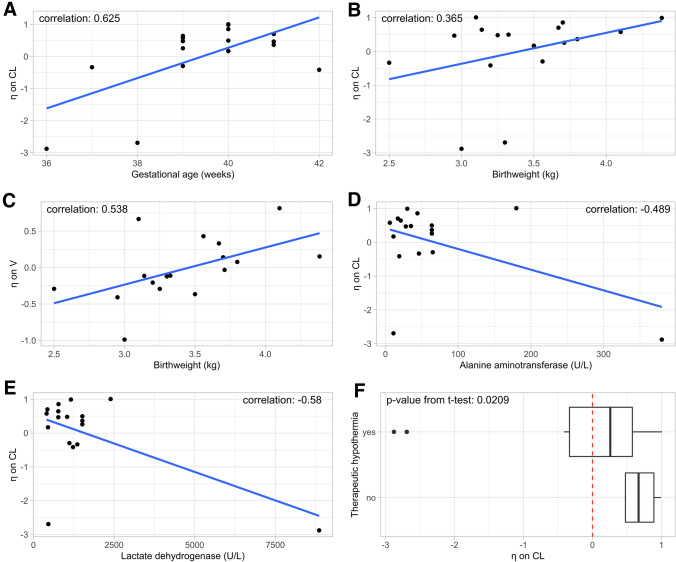
Following visual inspection of the covariate-η plots, the covariates GA, BW, ALT, LDH, and TH were selected for further evaluation (Fig. 1). However, because of the limited amount of data available, none of the tested covariates significantly affected either CL or V, as determined by the stepwise covariate method. We therefore included covariates based on prior knowledge and scientific plausibility. More specifically, the allometric relationship between BW and PK parameters such as CL and V is well documented. Consequently, we decided to implement BW as a covariate on both CL and V using an allometric equation with exponents fixed to 0.75 for CL and 1 for V. This resulted in a drop in the OFV of 5 points (p < 0.05) and reduced the unexplained variability on CL from 1.41 to 1.35 (7%) and on V from 0.221 to 0.165 (7.5%).
Fig. 1.
a–f Covariate-η plots of the covariates (gestational age, birthweight, alanine aminotransferase, lactate dehydrogenase, and therapeutic hypothermia) that showed a clear trend (|corr| > 0.3 or p < 0.05). CL clearance, V volume of distribution
Rhodin et al. showed that postmenstrual age (PMA) with a sigmoid hyperbolic function well described GFR maturation across the entire pediatric population. TM50, the time at which maturation reaches half the adult value, was estimated at 47.7 weeks, whereas the Hill coefficient, which describes the slope of the sigmoidal curve, was estimated at 3.4 [14]. In our study, PMA (i.e., the sum of GA and postnatal age [PNA]) was almost fully determined by GA as data were collected until 3 days after birth. Consequently, to describe GFR maturation, we introduced GA as a covariate in the model using the aforementioned sigmoid hyperbolic function, with TM50 and the Hill coefficient fixed to 47.7 and 3.4, respectively. Although the OFV did not drop to a statistically significant extent, the unexplained variability on CL did decrease from 1.41 to 1.27 (16%). Furthermore, various studies have previously shown that TH affects renal CL in neonates [16, 17]. Based on these observations, we decided to include TH as a covariate on CL. This led to a considerable decrease in the unexplained variability in CL (from 1.41 to 1.22 [22%]), whereas the OFV remained virtually unchanged.
Final PK Model and Internal Evaluation
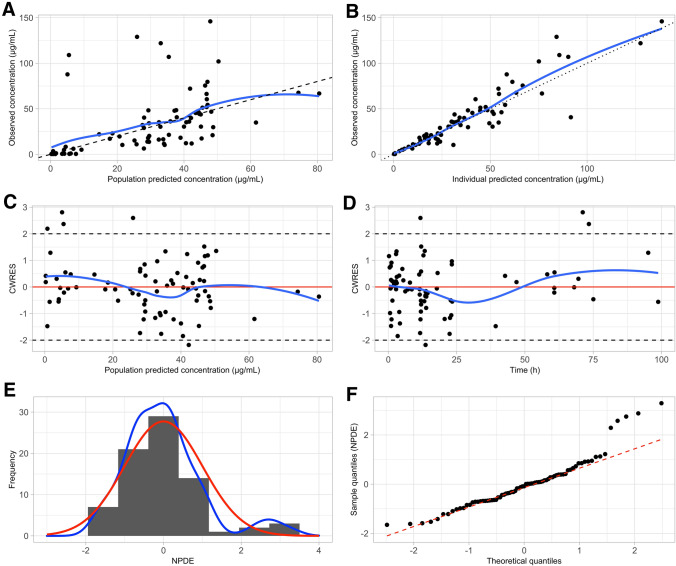
Table 3 summarizes the estimated population PK parameters of both the base model and the final model together with the values obtained from the SIR analysis. Therapeutic hypothermia was found to decrease mannitol CL, and thus GFR, by approximately 60% (39% relative standard error) compared with the estimated mannitol CL of neonates who did not undergo TH. The SIR analysis confirmed the precision of the parameter estimates that were obtained from the final model as the SIR medians were close to the estimates and were within the 95% confidence interval. Moreover, the observed vs population- and individual-predicted concentration plots (Fig. 2a and b) as well as the conditional weighted residuals vs population-predicted concentration and time plots (Fig. 2c and d) did not show any considerable trends or bias.
Fig. 2.
a Observed vs population-predicted concentrations and b observed vs individual-predicted concentrations. The solid blue line represents loess smoothing. c Conditional weighted residuals (CWRES) vs population-predicted concentrations and d time. The solid blue line represents loess smoothing. e Distribution of the normalized prediction distribution errors (NPDEs). The solid red line represents a normal distribution, whereas the solid blue line represents the actual distribution of the NPDEs. f QQ plot of NPDEs
In addition, both the mean (0.004) and variance (1.003) of the NPDEs did not differ significantly from 0 and 1 (p > 0.05), indicating that the model describes the data well. However, both the histogram and QQ plot of the NPDEs did show a slight deviation from normality (Fig. 2e and f).
Discussion
Although TH has demonstrated to have beneficial effects on neurological outcomes, reducing a neonate’s body temperature may further affect physiological processes such as those involved in drug elimination [21]. The current study therefore aimed to quantify the impact of TH in addition to asphyxia on mannitol CL, as a surrogate for GFR. Because TH was only started in infants with moderate-to-severe HIE according to current guidelines, and non-TH infants studied herein only had mild HIE, it was only possible to compare the combined effect of TH and more severe asphyxia with no TH and milder asphyxia.
A PK model was developed based on 77 observations that originated from 17 (near)term neonates. A one-compartment model with linear first-order elimination described the data best as reflected by the low OFV and improved goodness-of-fit plots (data not shown). Interestingly, none of the investigated covariates significantly affected CL or V, as determined by the stepwise covariate method. This may be a problem of a small sample size, but as we had no access to additional data, we decided to introduce covariates in the model based on prior knowledge and scientific plausibility rather than statistical significance. This is because previous studies have clearly indicated that both maturation and growth are implicated in the increase in neonatal glomerular function [10, 14, 18]. Maturation is typically described using an age-related factor, such as PMA, whereas the most common descriptor for growth is BW [22, 23]. There are, however, different views on whether age-related covariates should be used to describe prenatal GFR maturation. For instance, Rhodin et al. indicated that GFR maturation before birth is best described using both BW and PMA, whereas De Cock et al. suggested that implementing BW alone suffices to describe antenatal GFR maturation as BW and PMA repeatedly use the same information [14, 18]. To account for prenatal GFR maturation, we decided to introduce both BW and GA (in this dataset de facto similar to PMA) as covariates on CL using an allometric three-quarters power function and a sigmoid hyperbolic function, respectively [14]. This decision was based on the observation that the covariate-η plots of BW and GA showed a clear trend (Fig. 1), whereas the correlation between BW and GA was rather weak (correlation coefficient = 0.34), suggesting that BW alone could only partly explain the inter-individual differences in prenatal GFR maturation. By simultaneously implementing BW and GA as covariates, the IIV on CL decreased considerably more (− 24.8 %) as compared with the ‘BW-only’ model (− 10.5%).
Glomerular filtration rate is known to increase rapidly in the first few days after birth [24]. This is commonly accounted for in PK models using either PNA and/or PMA as covariates on CL [14, 18, 25]. However, several studies have suggested that PMA is insufficient to describe postnatal GFR maturation as it ignores the impact of birth on GFR [18, 26]. Indeed, Salem et al. showed that in a group of neonates with the same PMA values, neonates with a higher PNA have higher GFR values as compared with more mature cases at birth, but with a lower PNA [26]. In other words, postnatal GFR maturation is best described using PNA rather than PMA. Consequently, we introduced PNA as a covariate on CL using both a linear and power function. However, the estimated effect of PNA on CL was negative in all tested cases, suggesting that GFR decreases with increasing PNA (data not shown). As this does not make sense from a physiological point of view, we decided to exclude PNA as a covariate on CL. We can only speculate, but one of the potential explanations might be that (although intended different, Table 1) there was some skewed sample collection over postnatal life in this cohort as all samples collected from 59 h (cf Fig. 2d) onwards were collected in cases that underwent HT. In addition, we did not implement LDH and ALT as covariates in the final model even though their covariate-η plots showed a clear trend. This decision was based on the observation that their overall trend was almost entirely determined by a single (same) outlier (Fig. 1d and e).
Our final model predicted a mannitol CL of 0.15 L/h for an asphyxiated neonate with a GA of 39.5 weeks and a BW of 3.25 kg who did not undergo TH. This is lower than the reported value of 0.33 L/h for a healthy neonate of approximately the same age and weight [10]. These results are to be expected as perinatal asphyxia is known to affect GFR, and thus mannitol CL [27].
The effect of hypothermia on renal drug CL in neonates with perinatal asphyxia has been investigated previously by several groups. For instance, Cristea et al. found that amikacin CL in neonates with perinatal asphyxia treated with TH was decreased by approximately 40% compared with controls (neonates treated for suspected early-onset sepsis), while a study conducted by Mark et al. found a 25.5% decrease in gentamicin CL following TH in 16 cases compared with seven cases with similar clinical characteristics but in whom HT were not provided [17, 28]. These findings are further supported by studies conducted in pigs that suggested that the intensity of the hypothermic treatment may determine the extent to which renal CL capacity decreases [29, 30]. However, these observations are contradicted by Liu et al. who failed to find an effect of TH on renal drug CL [31]. Because of these conflicting results, we decided to introduce TH as a binary covariate on CL as the η vs covariate plots showed a clear trend (Fig. 1f). We are hereby aware that TH was only initiated in cases with moderate-to-severe HIE. The IIV on CL decreased by approximately 20%, justifying our decision to include TH as a covariate. Our model predicted that mannitol CL, a surrogate for GFR, decreased by approximately 60% on average in neonates who underwent TH (Table 3). Moreover, we observed a large IIV (137%) in estimated mannitol CL. This is not unexpected as asphyxia severity is known to be correlated with kidney damage and our dataset contained neonates with perinatal asphyxia from mild (no TH) to moderate to severe as not all cases underwent TH, be it overweighted in TH cases (13/17) [4, 27]. This may also explain why we observed such a profound effect of TH on GFR. More specifically, we were unable to disentangle the effect of perinatal asphyxia from the effect of hypothermia on mannitol CL. The effect that we observed was therefore likely confounded with the severity of asphyxia. In an attempt to disentangle the impact of perinatal asphyxia from the impact of TH on mannitol CL, we explored whether introducing age at the start of cooling as a covariate would allow us to quantify CL before and after the initiation of TH. However, as only one measurement on average was available before the start of TH, mannitol CL before the initiation of TH could not be estimated.
An additional relevant limitation of our model is its limited applicability. More specifically, because of the conflicting reports in the literature regarding the effect of TH on GFR and the fact that TH as an independent covariate did not affect mannitol CL to a statistically significant extent in our model, we suggest that the results in the current study should not be extrapolated to other renally cleared drugs. However, our results do provide additional evidence that perinatal asphyxia affects GFR compared to healthy controls. Consequently, care should be taken when dosing this patient population with renally cleared drugs, be it that we are unable to provide any guidance in relevant covariates within this population. In addition, future studies should be conducted that pool data on GFR estimates from different centers to further investigate the effect of TH on GFR.
Conclusions
We developed a model that accurately described the pharmacokinetics of mannitol, a surrogate for GFR, in neonates with perinatal asphyxia. Gestational age and BW were implemented as covariates to account for prenatal GFR maturation, whereas TH was introduced as a binary covariate on CL to quantify the impact of hypothermia on mannitol CL. Therapeutic hypothermia was found to decrease mannitol CL by approximately 60%, although this effect is likely confounded with asphyxia severity.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Spaghetti plot of mannitol concentration–time profiles after intravenous administration stratified by patient (ID) (JPG 62 KB)
Acknowledgements
The ALBINO coordinating investigators are Axel R. Franz (University Hospital Tübingen, Tübingen, Germany) and Mario Rüdiger (University Hospital CG Carus, Medizinische Fakultät der TU Dresden, Dresden, Germany). The beneficiaries and national coordinators are Axel R. Franz and Christian F. Poets (Tübingen, Germany), Mario Rüdiger (Dresden, Germany), Manon Benders and Frank van Bel (Utrecht, the Netherlands), Karel Allegaert and Gunnar Naulaers (Leuven, Belgium), Dirk Bassler (Zurich, Switzerland), Katrin Klebermass-Schrehof (Vienna, Austria), Maximo Vento (Valencia, Spain), Hercilia Guimaraes (Porto, Portugal), Tom Stiris (Oslo, Norway), Isabella Mauro (Udine, Italy), Marjo Metsäranta (Helsinki, Finland), Sampsa Vanhatalo (Helsinki, Finland), Jan Mazela (Poznan, Poland), Tuuli Metsvaht (Tartu, Estonia), and Yannique Jacobs (ACE Pharmaceuticals, Zeewolde, the Netherlands).
Declarations
Funding
The ALBINO project is funded under the Horizon 2020 Framework EU Program call H2020-PHC-2015-two-stage, grant 667224. The research on the pharmacokinetics during whole body hypothermia (Karel Allegaert, Pieter Annaert, Anne Smits) is further supported by the iPREDICT project (FWO Senior research project, fundamental research, G0D0520N).
Conflicts of Interest/Competing Interests
Neel Deferm, Kim V. Annink, Ruben Faelens, Michael Schroth, Christian A. Maiwald, Loubna el Bakkali, Frank van Bel, Manon J.N.L. Benders, Mirjam M. van Weissenbruch, Anja Hagen, Anne Smits, Pieter Annaert, Axel R. Franz, and Karel Allegaert have no conflicts of interest that are directly relevant to the content of this article. Yannique Jacobs is a collaborator within the ALBINO project and is an employee of ACE Pharmaceuticals, Zeewolde, the Netherlands, which holds the Dutch marketing authorization registration for Acepurin® (allopurinol 1 g/100 mL) for intravenous application for the treatment of gout.
Ethics Approval
The Medical Ethics Committee of the University Medical Centre Utrecht and the Central Committee on Human Research approved the study including the specific pharmacokinetic sub-study for the Netherlands (NL57237.041.16). The German Federal Authority (EudractCT 2016-000222-19) as well as the leading Ethics Committee of Tübingen approved the study for Germany.
Consent to Participate
Oral consent was obtained from at least one parent before administering the study medication. After the first dose, but before the (potential) second dose, full written parental consent was obtained. In case parents did not sign the informed consent, blood samples already collected shortly after birth were destroyed.
Availability of Data and Material
The raw data are available upon reasonable request by an e-mail to the corresponding author, while blinding for group allocation should be respected until the primary outcome analysis has been finalized.
Authors’ Contributions
Protocol development and study conduct: KVA, MS, CM, LEB, FVB, MB, MVW, AH, AF; ethics and study registration: KVA, FVB, MB, AF; study recruitment and data collection: KVA, MS, CM, LEB, FVB, MB, MVW, AH, AF; modeling: ND, RF, AS, PA, KA; drafting of the manuscript: ND, RF, AS, PA, AF, KA. All authors have read and commented on the paper, and have approved the submitted version.
Footnotes
The ALBINO Study Group coordinating investigator names are present in Acknowledgements section.
Contributor Information
Karel Allegaert, Email: karel.allegaert@uzleuven.be.
the ALBINO Study Group:
Axel R. Franz, Mario Rüdiger, Christian F. Poets, Manon Benders, Frank van Bel, Karel Allegaert, Gunnar Naulaers, Dirk Bassler, Katrin Klebermass-Schrehof, Maximo Vento, Hercilia Guimaraes, Tom Stiris, Isabella Mauro, Marjo Metsäranta, Sampsa Vanhatalo, Jan Mazela, Tuuli Metsvaht, and Yannique Jacobs
References
- 1.Smits A, Annaert P, Van Cruchten S, Allegaert K. A physiology-based pharmacokinetic framework to support drug development and dose precision during therapeutic hypothermia in neonates. Front Pharmacol. 2020;11:587. doi: 10.3389/fphar.2020.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azzopardi D, Brocklehurst P, Edwards D, Halliday H, Levene M, Thoresen M, TOBY Study Group et al. The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr. 2008;8:17. doi: 10.1186/1471-2431-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allegaert K, Smits A, van Donge T, van den Anker J, Sarafidis K, Levtchenko E, et al. Renal precision medicine in neonates and acute kidney injury: how to convert a cloud of creatinine observations to support clinical decisions. Front Pediatr. 2020;8:366. doi: 10.3389/fped.2020.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur S, Jain S, Saha A, Chawla D, Parmar VR, Basu S, et al. Evaluation of glomerular and tubular renal function in neonates with birth asphyxia. Ann Trop Paediatr. 2011;31:129–134. doi: 10.1179/146532811X12925735813922. [DOI] [PubMed] [Google Scholar]
- 5.Selewski DT, Jordan BK, Askenazi DJ, Dechert RE, Sarkar S. Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. J Pediatr. 2013;162(725–29):e1. doi: 10.1016/j.jpeds.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Kirkley MJ, Boohaker L, Griffin R, Soranno DE, Gien J, Askenazi D, Neonatal Kidney Collaborative (NKC) et al. Acute kidney injury in neonatal encephalopathy: an evaluation of the AWAKEN database. Pediatr Nephrol. 2019;34:169–176. doi: 10.1007/s00467-018-4068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mok TYD, Tseng M-H, Lee J-C, Chou Y-C, Lien R, Lai M-Y, et al. A retrospective study on the incidence of acute kidney injury and its early prediction using troponin-I in cooled asphyxiated neonates. Sci Rep. 2020;10:15682. doi: 10.1038/s41598-020-72717-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiss K, Molnár M, Söndergaard S, Molnár G, Ricksten S-E. Mannitol clearance for the determination of glomerular filtration rate-a validation against clearance of 51 Cr-EDTA. Clin Physiol Funct Imaging. 2018;38:10–16. doi: 10.1111/cpf.12374. [DOI] [PubMed] [Google Scholar]
- 9.Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther. 2014;19:262–276. doi: 10.5863/1551-6776-19.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayton WL. Maturation and growth of renal function: dosing renally cleared drugs in children. AAPS PharmSci. 2000;2:E3. doi: 10.1208/ps020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin MI, Bruck E, Rapoport M. Maturation of renal function in childhood; clearance studies. J Clin Invest. 1949;28:1144–1162. doi: 10.1172/JCI102149. [DOI] [PubMed] [Google Scholar]
- 12.Maiwald CA, Annink KV, Rüdiger M, Benders MJNL, van Bel F, Allegaert K, ALBINO Study Group et al. Effect of allopurinol in addition to hypothermia treatment in neonates for hypoxic-ischemic brain injury on neurocognitive outcome (ALBINO): study protocol of a blinded randomized placebo-controlled parallel group multicenter trial for superiority (phase III) BMC Pediatr. 2019;19:210. doi: 10.1186/s12887-019-1566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Cock RFW, Piana C, Krekels EHJ, Danhof M, Allegaert K, Knibbe CAJ. The role of population PK-PD modelling in paediatric clinical research. Eur J Clin Pharmacol. 2011;67(Suppl. 1):5–16. doi: 10.1007/s00228-009-0782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24:67–76. doi: 10.1007/s00467-008-0997-5. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson EN, Karlsson MO. Automated covariate model building within NONMEM. Pharm Res. 1998;15:1463–1468. doi: 10.1023/A:1011970125687. [DOI] [PubMed] [Google Scholar]
- 16.Favié LMA, de Haan TR, Bijleveld YA, Rademaker CMA, Egberts TCG, Nuytemans DHGM, et al. Prediction of drug exposure in critically ill encephalopathic neonates treated with therapeutic hypothermia based on a pooled population pharmacokinetic analysis of seven drugs and five metabolites. Clin Pharmacol Ther. 2020;108:1098–1106. doi: 10.1002/cpt.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cristea S, Smits A, Kulo A, Knibbe CAJ, van Weissenbruch M, Krekels EHJ, et al. Amikacin pharmacokinetics to optimize dosing in neonates with perinatal asphyxia treated with hypothermia. Antimicrob Agents Chemother. 2017;61:e01282–e1317. doi: 10.1128/AAC.01282-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Cock RFW, Allegaert K, Schreuder MF, Sherwin CMT, de Hoog M, van den Anker JN, et al. Maturation of the glomerular filtration rate in neonates, as reflected by amikacin clearance. Clin Pharmacokinet. 2012;51:105–117. doi: 10.2165/11595640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed. 2008;90:154–166. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Brendel K, Comets E, Laffont C, Mentré F. Evaluation of different tests based on observations for external model evaluation of population analyses. J Pharmacokinet Pharmacodyn. 2010;37:49–65. doi: 10.1007/s10928-009-9143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutz IC, Allegaert K, de Hoon JN, Marynissen H. Pharmacokinetics during therapeutic hypothermia for neonatal hypoxic ischaemic encephalopathy: a literature review. BMJ Paediatr Open. 2020;4:e000685. doi: 10.1136/bmjpo-2020-000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samant TS, Mangal N, Lukacova V, Schmidt S. Quantitative clinical pharmacology for size and age scaling in pediatric drug development: a systematic review. J Clin Pharmacol. 2015;55:1207–1217. doi: 10.1002/jcph.555. [DOI] [PubMed] [Google Scholar]
- 23.Holford N, Heo Y-A, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102:2941–2952. doi: 10.1002/jps.23574. [DOI] [PubMed] [Google Scholar]
- 24.Wilson CG, Sarkar PK, Mazumdar J, Bharadhwaj B. Study of glomerular functions in neonates. Med J Armed Forces India. 1999;55:183–186. doi: 10.1016/S0377-1237(17)30436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Kumar SS, Sherwin CM, Ward R, Baer G, Burckart GJ, et al. Renal clearance in newborns and infants: predictive performance of population-based modeling for drug development. Clin Pharmacol Ther. 2019;105:1462–1470. doi: 10.1002/cpt.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salem F, Johnson TN, Hodgkinson ABJ, Ogungbenro K, Rostami-Hodjegan A. Does, “birth” as an event impact maturation trajectory of renal clearance via glomerular filtration? Reexamining data in preterm and full-term neonates by avoiding the creatinine bias. J Clin Pharmacol. 2021;61(2):159–171. doi: 10.1002/jcph.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alaro D, Bashir A, Musoke R, Wanaiana L. Prevalence and outcomes of acute kidney injury in term neonates with perinatal asphyxia. Afr Health Sci. 2014;14:682–688. doi: 10.4314/ahs.v14i3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mark LF, Solomon A, Northington FJ, Lee CK. Gentamicin pharmacokinetics in neonates undergoing therapeutic hypothermia. Ther Drug Monit. 2013;35:217–222. doi: 10.1097/FTD.0b013e3182834335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satas S, Hoem NO, Melby K, Porter H, Lindgren CG, Whitelaw A, Thoresen M. Influence of mild hypothermia after hypoxia-ischemia on the pharmacokinetics of gentamicin in newborn pigs. Biol Neonate. 2000;77:50–57. doi: 10.1159/000014195. [DOI] [PubMed] [Google Scholar]
- 30.Koren G, Barker C, Bohn D, Kent G, Biggar WD. Influence of hypothermia on the pharmacokinetics of gentamicin and theophylline in piglets. Crit Care Med. 1985;13:844–847. doi: 10.1097/00003246-198510000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Borooah M, Stone J, Chakkarapani E, Thoresen M. Serum gentamicin concentrations in encephalopathic infants are not affected by therapeutic hypothermia. Pediatrics. 2009;124:310–315. doi: 10.1542/peds.2008-2942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Spaghetti plot of mannitol concentration–time profiles after intravenous administration stratified by patient (ID) (JPG 62 KB)