Abstract
Abstract
Cardiomyopathies are an important cause of heart failure and sudden cardiac death. Little is known about the role of rare genetic variants in inflammatory cardiomyopathy. Chronic Chagas disease cardiomyopathy (CCC) is an inflammatory cardiomyopathy prevalent in Latin America, developing in 30% of the 6 million patients chronically infected by the protozoan Trypanosoma cruzi, while 60% remain free of heart disease (asymptomatic (ASY)). The cytokine interferon-γ and mitochondrial dysfunction are known to play a major pathogenetic role. Chagas disease provides a unique model to probe for genetic variants involved in inflammatory cardiomyopathy.
Methods
We used whole exome sequencing to study nuclear families containing multiple cases of Chagas disease. We searched for rare pathogenic variants shared by all family members with CCC but absent in infected ASY siblings and in unrelated ASY.
Results
We identified heterozygous, pathogenic variants linked to CCC in all tested families on 22 distinct genes, from which 20 were mitochondrial or inflammation-related – most of the latter involved in proinflammatory cytokine production. Significantly, incubation with IFN-γ on a human cardiomyocyte line treated with an inhibitor of dihydroorotate dehydrogenase brequinar (enzyme showing a loss-of-function variant in one family) markedly reduced mitochondrial membrane potential (ΔψM), indicating mitochondrial dysfunction.
Conclusion
Mitochondrial dysfunction and inflammation may be genetically determined in CCC, driven by rare genetic variants. We hypothesize that CCC-linked genetic variants increase mitochondrial susceptibility to IFN-γ-induced damage in the myocardium, leading to the cardiomyopathy phenotype in Chagas disease. This mechanism may also be operative in other inflammatory cardiomyopathies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-021-01000-y.
Keywords: Variants, chagas, cardiomyopathy, pathogenic, mitochondria, inflammation
Introduction
Cardiomyopathies are an important cause of cardiovascular death by heart failure and arrhythmia. Familial cardiomyopathies are a group of Mendelian genetic disorders associated with rare high-impact gene variants altering protein structure and function, mostly involving genes encoding sarcomeric/structural and calcium handling proteins. Among the acquired causes of cardiomyopathy, an estimated 30% have an infectious etiology, associated with myocarditis [1].
Little is known about the genetic underpinnings of infectious cardiomyopathy. Chagas disease (CD), caused by infection with the protozoan Trypanosoma cruzi, is the most common cause of nonischemic cardiomyopathy in Latin America, where 6 million people are infected, causing approximately 10,000 deaths/year due to cardiac compromise [2]. It is transmitted by the reduviid insect vector, by blood transfusion, congenitally, and by ingestion. An estimated 400,000 infected persons live in nonendemic countries, mainly the USA and Europe. Chronic CD cardiomyopathy (CCC) is a chronic inflammatory cardiomyopathy occurring decades after infection with T. cruzi [3] in up to 30% of CD patients. ECG abnormalities and heart conduction defects are associated with progressive inflammatory fibrotic and hypertrophic myocardial lesions including the conducting tissue [4] and precede ventricular arrhythmia/sudden cardiac death (SCD) and/or dilated cardiomyopathy with heart failure (HF), the major causes of death in CCC [5]. From the remaining CD patients, 60% persist asymptomatic form (ASY), and 10% develop gastrointestinal motility disorders [6, 7]. CD patients with ECG abnormalities typical of CCC show higher total and cardiac mortality [8]. No adequate treatment is available to prevent the development of chronic heart disease, whose prognosis is worse than dilated cardiomyopathies of other etiologies [3]. New approaches to the treatment of CCC are thus sorely needed. The pathogenesis of CCC is still incompletely understood, although myocardial inflammation and reduced mitochondrial energy metabolism are thought to play a major role in CD and cardiac remodeling and heart failure of any etiology [2, 3]. After acute infection, parasitism is partially controlled by the immune response, and low-grade parasite persistence fuels the production of inflammatory cytokines like IFN-γ and TNF-α, which is more intense in CCC than ASY patients [9]. A T cell and monocyte-rich chronic myocarditis with fibrosis and hypertrophy is the histopathological hallmark of CCC [10, 11].
IFN-γ is the most abundant cytokine expressed in the CCC myocardium, and transcriptomic analyses of the CCC myocardium show a significant IFN-γ transcriptional signature [10, 12]. IFN-γ treatment induces reduced contractility [13] and fatty acid metabolism of cardiomyocytes [14] and profibrotic changes in fibroblasts and transgenic mice overexpressing IFN-γ develop inflammatory cardiomyopathy [15, 16]. Taken together, evidence suggests IFN-γ is the culprit of CCC. In addition, the myocardium from CCC patients with ventricular dysfunction displays decreased levels of mitochondrial metabolism enzymes [17] and ATP production [18]. IFN-γ has multiple deleterious effects on the cardiomyocyte mitochondria. It induces TNF-alpha and potentiates TNF-alpha-mediated NF-kB signaling, leading to NOS2 production of NO [19, 20] which in the presence of IFN-γ-induced reactive oxygen species turns into peroxynitrite [21] and ensuing mitochondrial fragmentation and reduction of mitochondrial membrane potential, lipid beta-oxidation [14], and ATP generation [22].
High-impact rare gene variants altering protein structure and function underlie Mendelian disease and contribute to complex multifactorial disease. Approximately 10% of acute viral myocarditis (AVM) patients carried rare pathogenic homozygous variants in genes implicated in familial cardiomyopathy [23], suggesting an overlap between genetic and acquired forms of myocarditis and cardiomyopathy. However, the evidence for a role of such variants in the pathogenesis of AVM is circumstantial, as no genetic study has compared AVM patients with virus-infected patients that failed to develop myocarditis. In CD, however, serological tests can readily ascertain T. cruzi infection in patients who have developed cardiomyopathy or have remained asymptomatic, decades after the initial infection. This has allowed genetic association studies of common gene polymorphisms between the two T. cruzi-exposed groups with divergent cardiac phenotypes, CCC and ASY [2], which have a low individual effect on phenotype. CD thus provides a unique model to probe the effect of rare pathogenic genetic variants in the susceptibility towards developing postinfectious cardiomyopathy in humans.
We hypothesize here that rare genetic variants may lead to progression towards CCC by increasing cardiomyocyte susceptibility to inflammatory damage. Whole exome sequencing (WES) studies in families with multiple disease cases are an unbiased approach that has been used to identify rare pathogenic variants in Mendelian genetic disorders and complex multifactorial diseases. We used WES to search for rare, high-impact gene variants linked to CCC in nuclear families containing multiple cases of CD and involved in pathobiological processes involved in inflammatory cardiomyopathy. Our analysis disclosed rare heterozygous pathogenic variants in inflammation-related and mitochondrial genes linked to CCC cases. Functional testing indicated that IFN-γ caused significant mitochondrial dysfunction on a human cardiomyocyte line treated with an inhibitor of dihydroorotate dehydrogenase brequinar (enzyme showing a loss-of-function variant in one family).
Patients, Materials, and Methods
Ethical Issues
This protocol was approved by the INSERM Internal Review Board and by the Brazilian National Ethics in Research Commission (CONEP), and written informed consent was obtained from the patients. All patients enrolled in this study were over 21 years old. Investigations were conformed to the principles outlined in the declaration of Helsinki.
Patients
Probands and their nuclear family members were recruited from the Sami-Trop CD cohort, in rural Minas Gerais state [24], and the CD outpatient clinic at the Heart Institute/HCFMUSP.
Nuclear families typically center on a married couple which may have any number of children. Figure 1 shows the pedigrees of the 6 selected nuclear families with multiple CD cases (n = 25), and Table 1 depicts the clinical and demographic parameters of the studied subjects. Families 1 and 6 came from the Heart Institute, while families 2–5 came from the Sami-Trop CD cohort.
Fig. 1.
Pedigrees of the six nuclear families included in this study. All patients underwent detailed clinical interview and T. cruzi serological tests. T. cruzi-seropositive individuals were considered as CCC patients when presenting major ECG abnormalities according to the Minnesota Code classification, modified by Ribeiro et al. [8]. Major ECG abnormalities are cited in online Table 1. The presence of any one of these major ECG changes was associated with a twofold increase in mortality [8]. T. cruzi-seropositive individuals presenting major ECG abnormalities, according to the Minnesota Code classification, modified by Ribeiro et al. [8] were considered as CCC patients. T. cruzi-seropositive patients without such ECG findings, with normal echocardiography, and no clinical signs were considered indeterminate forms of CD (ASY) cases
Table 1.
Description of the study phenotypes
| Family | ID | Phenotype | Chagas serology | Age | Sex | LVEF |
|---|---|---|---|---|---|---|
| A. Chagas disease families | ||||||
| Family 1 | IC250E | CCC | Seropositive | 42 | F | 0.56 |
| IC252E | ASY | Seropositive | 50 | F | 0.68 | |
| IC253E | ASY | Seropositive | 54 | F | 0.61 | |
| IC254E | CCC | Seropositive | 49 | M | 0.30 | |
| Family 2 | 6151 | ASY | Seropositive | 63 | F | 0.69 |
| 6152 | CCC | Seropositive | 37 | F | 0.63 | |
| 6153 | ASY | Seropositive | 36 | F | 0.66 | |
| 6154 | ASY | Seropositive | 39 | F | 0.65 | |
| 6155 | CCC | Seropositive | 45 | F | 0.60 | |
| 6156 | CCC | Seropositive | 79 | F | 0.62 | |
| 6157 | CCC | Seropositive | 49 | F | 0.56 | |
| Family 3 | 6086 | ASY | Seropositive | 71 | F | 0.70 |
| 6087 | CCC | Seropositive | 68 | F | 0.69 | |
| 6088 | ASY | Seropositive | 69 | F | 0.69 | |
| Family 4 | 6075 | CCC | Seropositive | 67 | F | 0.69 |
| 6076 | CCC | Seropositive | 57 | M | 0.69 | |
| 6078 | CCC | Seropositive | 52 | F | 0.67 | |
| Family 5 | 6131 | CCC | Seropositive | 24 | F | 0.65 |
| 6133 | ASY | Seropositive | 69 | F | 0.63 | |
| 6134 | CCC | Seropositive | 42 | F | 0.50 | |
| 6135 | CCC | Seropositive | 38 | F | 0.65 | |
| 6136 | CCC | Seropositive | 68 | M | 0.62 | |
| Family 6 | IC174EC | CCC | Seropositive | 41 | F | 0.38 |
| IC190EC | CCC | Seropositive | 50 | F | 0.37 | |
| IC191EC | CCC | Seropositive | 69 | F | 0.45 | |
| 53.6 ± 14.6 | 0.59 ± 0.11 | |||||
| Seronegative siblings in family 5 | ||||||
| 6132 | Healthy | Seronegative | 32 | M | 0.59 | |
| 6137 | Healthy | Seronegative | 30 | M | 0.7 | |
| B. Unrelated ASY controls | ||||||
| Unrelated | IC33EI | ASY | Seropositive | 65 | M | 0.69 |
| IC45EI | ASY | Seropositive | 51 | F | 0.61 | |
| IC49EI | ASY | Seropositive | 57 | F | 0.70 | |
| IC69EI | ASY | Seropositive | 90 | F | 0.68 | |
| IC92EI | ASY | Seropositive | 62 | M | 0.66 | |
| IC95EI | ASY | Seropositive | 57 | F | 0.70 | |
| IC106EI | ASY | Seropositive | 44 | F | 0.55 | |
| IC111EI | ASY | Seropositive | 42 | F | 0.63 | |
| 6083 | ASY | Seropositive | 41 | F | 0.65 | |
| 6091 | ASY | Seropositive | 50 | F | 0.62 | |
| 6097 | ASY | Seropositive | 47 | F | 0.56 | |
| 6102 | ASY | Seropositive | 45 | M | 0.66 | |
| 6129 | ASY | Seropositive | 71 | F | 0.74 | |
| 6143 | ASY | Seropositive | 39 | F | 0.66 | |
| 54.3 ± 14 | 0.65 ± 0.05 | |||||
LVEF, left ventricular ejection fraction; ASY, asymptomatic form
Blood DNA Preparation and Whole Exome Sequencing
On EDTA vacutainer tubes, 10 ml of blood was collected. The genomic DNA of 25 CCC and ASY patients belonging to the 6 families, plus 14 genetically unrelated ASY controls, was isolated with the QIAamp DNA Blood Midi Kit (Qiagen, Hilden, Germany). Exome sequencing was performed using the Ion Proton platform (Life Technologies, Villebon sur Yvette, France) according to a protocol previously described [25].
Variant Prioritization
We excluded synonymous, non-exonic polymorphisms, keeping polymorphisms with a minor allele frequency (MAF) of <1% in at least one public database using the VARAFT filtering and annotation tool (https://varaft.eu) on vcf files. Variant calls with variant quality (QUAL) ≤60, depth of coverage (DP) <20, and mapping quality (MQ) ≤40 were filtered out. Only exonic nonsynonymous damaging variants were kept for downstream genetic analyses. We searched for variants, independently, in each family, under an autosomal dominant or under autosomal recessive models. In order to identify gene variants associated with CCC in each family, we selected variants that were shared by all CCC patients and absent in any and all ASY patients in a given nuclear family, as well as in 14 unrelated ASY controls. We assessed the pathogenic potential of missense variants using 4 algorithms embedded in VarAft. Only rare variants tagged as pathogenic (or damaging) were retained for downstream genetic analyses.
Polymerase Chain Reaction and Sanger Sequencing
For validation, we designed specific primers for each mutation of interest with Primer3 software (V4.0.0) to amplify genomic DNAs (online Table 3). PCR amplifications were carried out with GoTaq polymerase (Promega, Charbonnières-les-Bains, France) and 1uM of each primer. On Eppendorf thermocycler, 50 μl reactions were carried out. The PCR products were visualized on agarose gel (1.5% agarose TBE0.5X) and purified with QIAEXII gel extraction kit (Qiagen) before Sanger sequencing.
Mitochondrial Membrane Potential Assay
Total mitochondria were stained with MitoTracker green, and mitochondrial membrane potential (MMP) was evaluated using the TMRE dye that accumulates in fully polarized, but not in depolarized mitochondria [26]. We measured TMRE fluorescence on MitoTracker green-labeled mitochondria. Cell viability was assessed using LIVE/DEAD™ Fixable Aqua Dead Cell Stain Kit. All fluorescent dyes were from Thermo Fisher Scientific, and assays were performed using live microscopy with the ImageXpress Micro instrument (Molecular Devices).
Results
We performed whole exome sequencing and assessed rare pathogenic gene variants associated with CCC in six nuclear families containing multiple cases of CD families (25 patients) and in a group of unrelated ASY patients (n = 14) who came from CD-endemic rural areas in Brazil. The average age in the CCC and ASY patients in the family groups and the ASY unrelated control were similar, around 54 ± 14 years (Table 1). Pedigrees are shown in Fig. 1.
Whole exome sequencing disclosed that on average, each patient sample contained 41,780 gene variants. Among them, on average, 11,651 variants were located in coding (exonic or splicing) regions and nonsynonymous. We focused on variants characterized by a minor allele frequency < 1% in the databases (ESP6500, 1000G, and ExAC). Under a hypothesis of complete penetrance, for each given family, we selected nonsynonymous exonic or splicing variants shared by all family members with CCC but absent from ASY family members as well as by the unrelated ASY controls. For families 1 to 6, we found 39, 8, 108, 81, 9, and 76 variants fulfilling the above criteria, respectively, comprising a total of 321 CCC-specific nonsynonymous exonic rare variants. We performed a Reactome (reactome.org) pathways analysis of the 321 CCC-specific variants, prior to any prioritization. We found 8 enriched pathways with a significant false discovery ratio (FDR < 0.05). Pathways were based on a very limited number of genes (6/8 with one or two genes) and thus of limited relevance. At any event, the 8 pathways were related either to interferon signaling or to endosomal/antigen processing/presentation pathways (HLA molecules, BTK) (online Table 4). After the application of our pathogenicity filter with multiple algorithms, we found 102 CCC-specific rare pathogenic variants. After filtering for evolutionary conservation, we found 87 variants. At this point, we prioritized the 87 gene variants in 9 pathobiological processes associated to CD. It highlighted 23 of our candidate genes (corresponding to 25 variants). From these variants, 88% (22/25, contained in 20 genes) were confirmed by Sanger sequencing (Table 2, online Fig. 1). All these 22 variants showed CADD scores above 15, consistent with pathogenicity. Indeed, for information, the CADD score of each variant is also shown in Table 2b. Two variants have a CADD score above 15, while the other 20 variants have a CADD score above 20. Markers with a CADD score over 20 are usually included in the top 1% deleterious variants in the genome. For the remaining 62 genes, we performed a second gene set enrichment analysis using Ingenuity Pathway Analysis and Reactome, but no significant pathways were identified. Each family had CCC-associated 1 to 7 pathogenic variants in 1 to 6 genes. Each of the 22 CCC-specific variants only appeared in a single nuclear family. However, for the APOB gene, two variants were detected in two different families. Figure 2 shows the number of genes containing associated variants in each pathophysiological process. Some genes may be common to several pathways. Table 2 shows the detailed information on the 22 gene variants (2A), pathogenicity and conservation scores (2B), participation in pathobiological processes relevant for inflammatory cardiomyopathy (2C), and their frequency in the databases (2D). The frequencies of our variants of interest in Latino reference subpopulations is described in online Table 6. We found a striking accumulation of CCC-associated variants in inflammation-related and mitochondrial genes (17 out of the 20 genes). All families carried at least one variant in mitochondrial or inflammation-associated genes; five families carried variants in mitochondrial genes and 5 in inflammation-related genes. A total of 10 pathogenic variants were found in 9 mitochondrial genes (ADCY10, DHODH, GIT1, MRPS18B, RPUSD3, LEPR, UMPS, MOCS1, and OBSCN). A total of 11 pathogenic variants were located in 10 inflammation-associated genes (ADGRG6, AKAP13, LEPR, LILRA2, MAML1, MAP4K4, SLC11A1, TNFRSF4, APOB, and DHODH) (Figs. 2 and 3, Table 2). This accumulation of genes was not an artifact of selection, since the number of prioritized variants in each pathway was not proportional to the total number of genes in the pathway/process. For instance, while we found 10 variants in 1532 mitochondrial genes, there were 4 variants in the 951 genes belonging to the fibrosis/extracellular matrix process.
Table 2.
Description of pathogenic variants identified on the 6 nuclear families. A. Genetic data. B. pathogenicity and conservation. C. Participation in select pathobiological processes. D. Frequency of variants in different databases
| A | |||||||||||
| Family | Gene acronym | Chr | Start | End | Reference/mutated allele | Localization | Type of mutation | Nucleic acid change | Amino acid change | avsnp147 | |
| 1 | LEPR | 1 | 66,081,791 | 66,081,791 | C/T | Exonic | non syn | exon14 2096C > T (NM_001198687) | T699M | rs34499590 | |
| 1 | ADCY10 | 1 | 167,830,254 | 167,830,254 | T/C | Exonic | non syn | exon12 1205A > G (NM_001167749) | Y402C | rs140663029 | |
| 1 | MOCS1 | 6 | 39,877,666 | 39,877,666 | G/A | Exonic | non syn | exon8 1015C > T (NM_005943) | R339W | rs148579886 | |
| 1 | ADGRG6 | 6 | 142,724,940 | 142,724,940 | G/A | Exonic; splicing | non syn | exon13 1873G > A (NM_001032394) | A625T | rs184235213 | |
| 1 | AKAP13 | 15 | 86,124,694 | 86,124,694 | T/C | Exonic | non syn | exon7 3395 T > C (NM_006738) | L1132S | rs745783128 | |
| 2 | OBSCN | 1 | 228,464,267 | 228,464,267 | G/T | Exonic | non syn | exon22 6337G > T (NM_001098623) | G2113C | rs74623201 | |
| 3 | APOB | 2 | 21,247,996 | 21,247,996 | C/A | Exonic; splicing | non syn | exon16 2245G > T (NM_000384) | D749Y | . | |
| 3 | MRPS18B | 6 | 30,590,612 | 30,590,612 | G/A | Exonic; splicing | non syn | exon5 358G > A (NM_014046) | V120M | rs116524936 | |
| 3 | PKHD1 | 6 | 51,947,999 | 51,947,999 | G/A | Exonic | non syn | exon3 107C > T (NM_138694) | T36M | rs137852944 | |
| 3 | RNLS | 10 | 90,122,344 | 90,122,344 | C/T | Exonic | non syn | exon5 665G > A (NM_001031709) | R222H | rs191733133 | |
| 3 | GIT1 | 17 | 27,901,773 | 27,901,773 | C/T | Exonic | non syn | exon20 2233G > A (NM_014030) | A745T | . | |
| 3 | GIT1 | 17 | 27,910,559 | 27,910,559 | C/T | Exonic | non syn | exon2 128G > A (NM_001085454) | R43H | . | |
| 3 | LILRA2 | 19 | 55,098,715 | 55,098,715 | C/T | Exonic | non syn | exon6 1267C > T (NM_001290270) | R423C | rs149580797 | |
| 4 | MAP4K4 | 2 | 102,440,480 | 102,440,480 | A/G | Exonic | non syn | exon4 271A > G (NM_001242559) | K91E | . | |
| 4 | SLC11A1 | 2 | 219,257,728 | 219,257,728 | C/T | Exonic | non syn | exon12 1189C > T (NM_000578) | R397C | rs74906275 | |
| 4 | RPUSD3 | 3 | 9,880,802 | 9,880,802 | C/T | Exonic | stopgain | exon8 806G > A (NM_001351738) | W269X | rs142984515 | |
| 4 | UMPS | 3 | 124,449,406 | 124,449,406 | A/G | Exonic | non syn | exon1 88A > G (NM_000373) | S30G | rs17843776 | |
| 5 | MAML1 | 5 | 179,192,418 | 179,192,418 | G/A | Exonic | non syn | exon2 407G > A (NM_014757) | G136E | rs146382198 | |
| 5 | DHODH | 16 | 72,048,540 | 72,048,540 | C/T | Exonic | non syn | exon3 403C > T (NM_001361) | R135C | rs201230446 | |
| 6 | TNFRSF4 | 1 | 1,147,467 | 1,147,467 | G/C | Exonic | non syn | exon5 489C > G (NM_003327) | D163E | . | |
| 6 | APOB | 2 | 21,230,419 | 21,230,419 | G/C | Exonic | non syn | exon26 9321C > G (NM_000384) | N3107K | rs72653101 | |
| 6 | SERPINE2 | 2 | 224,866,427 | 224,866,427 | A/G | Exonic | non syn | exon2 191 T > C (NM_001136528) | M64T | rs34078713 | |
| B | |||||||||||
| Family | Gene | Amino acid change | avsnp147 | Polyphen2 HDIV prediction | Polyphen2 HVAR prediction | SIFT prediction | UMD prediction | CADD prediction score | Consurf conservation score | ||
| 1 | LEPR | T699M | rs34499590 | Damaging | Damaging | Damaging | Prob Patho | 26.5 | 9 | ||
| 1 | ADCY10 | Y402C | rs140663029 | Damaging | Damaging | Damaging | Poly | 22.2 | 6 | ||
| 1 | MOCS1 | R339W | rs148579886 | Damaging | Damaging | Damaging | Patho | 33.0 | 8 | ||
| 1 | ADGRG6 | A625T | rs184235213 | Damaging | Damaging | Tolerate | Patho | 26.0 | 9 | ||
| 1 | AKAP13 | L1132S | rs745783128 | Damaging | Prob Dam | Damaging | Prob Patho | 15.6 | 7 | ||
| 2 | OBSCN | G2113C | rs74623201 | Damaging | Damaging | Damaging | Prob Poly | 25.0 | 9 | ||
| APOB | D749Y | . | Damaging | Damaging | Damaging | Patho | 28.5 | 7 | |||
| 3 | MRPS18B | V120M | rs116524936 | Damaging | Prob Dam | Damaging | Prob Patho | 29.4 | 3 | ||
| 3 | PKHD1 | T36M | rs137852944 | Damaging | Damaging | Damaging | Patho | 30.0 | 9 | ||
| 3 | RNLS | R222H | rs191733133 | Damaging | Damaging | Damaging | Prob Patho | 33.0 | 7 | ||
| 3 | GIT1 | A745T | . | Damaging | Damaging | Damaging | Patho | 33.0 | 9 | ||
| 3 | GIT1 | R43H | . | Damaging | Damaging | Damaging | Poly | 35.0 | 9 | ||
| 3 | LILRA2 | R423C | rs149580797 | Damaging | Damaging | Damaging | Prob Poly | 24.5 | 8 | ||
| 4 | MAP4K4 | K91E | . | Damaging | Damaging | Damaging | Patho | 24.4 | 3 | ||
| 4 | SLC11A1 | R397C | rs74906275 | Damaging | Damaging | Damaging | Poly | 33.0 | 6 | ||
| 4 | RPUSD3 | W269X | rs142984515 | Damaging | Damaging | Damaging | Prob Poly | 27.9 | 4 | ||
| 4 | UMPS | S30G | rs17843776 | Prob Dam | Prob Dam | Damaging | Poly | 23.4 | 9 | ||
| 5 | MAML1 | G136E | rs146382198 | Damaging | Prob Dam | Tolerate | Patho | 15.3 | 3 | ||
| 5 | DHODH | R135C | rs201230446 | Damaging | Damaging | Damaging | Patho | 34.0 | 9 | ||
| 6 | TNFRSF4 | D163E | . | Damaging | Damaging | Damaging | Poly | 22.3 | 9 | ||
| 6 | APOB | N3107K | rs72653101 | Damaging | Damaging | Damaging | Prob Poly | 22.9 | 7 | ||
| 6 | SERPINE2 | M64T | rs34078713 | Prob Dam | Prob Dam | Damaging | Prob Patho | 26.7 | 8 | ||
| C | |||||||||||
| Family | Gene | Amino acid change | avsnp147 | Inflammation | Mitochondrial genes | IFNγ modulated genes/Th1 response | Hypertrophy | Muscle contraction and contractility | Fibrosis extracellular matrix | Oxidative stress/antioxidant response | Familial CMP genes |
| 1 | LEPR | T699M | rs34499590 | X | X | X | X | ||||
| 1 | ADCY10 | Y402C | rs140663029 | X | |||||||
| 1 | MOCS1 | R339W | rs148579886 | X | |||||||
| 1 | ADGRG6 | A625T | rs184235213 | X | |||||||
| 1 | AKAP13 | L1132S | rs745783128 | X | X | X | |||||
| 2 | OBSCN | G2113C | rs74623201 | X | X | ||||||
| 3 | APOB | D749Y | . | X | X | ||||||
| 3 | MRPS18B | V120M | rs116524936 | X | |||||||
| 3 | PKHD1 | T36M | rs137852944 | X | |||||||
| 3 | RNLS | R222H | rs191733133 | X | X | ||||||
| 3 | GIT1 | A745T | . | X | X | X | |||||
| 3 | GIT1 | R43H | . | X | X | X | |||||
| 3 | LILRA2 | R423C | rs149580797 | X | |||||||
| 4 | MAP4K4 | K91E | . | X | X | ||||||
| 4 | SLC11A1 | R397C | rs74906275 | X | X | X | |||||
| 4 | RPUSD3 | W269X | rs142984515 | X | |||||||
| 4 | UMPS | S30G | rs17843776 | X | X | ||||||
| 5 | MAML1 | G136E | rs146382198 | X | |||||||
| 5 | DHODH | R135C | rs201230446 | X | X | X | |||||
| 6 | TNFRSF4 | D163E | . | X | |||||||
| 6 | APOB | N3107K | rs72653101 | X | X | ||||||
| 6 | SERPINE2 | M64T | rs34078713 | X | |||||||
| D | |||||||||||
| Family | Gene | Amino acid change | avsnp147 | Polymorphism frequency in the ESP6500 database | Polymorphism frequency in the 1000G database | Polymorphism frequency in theExAC database | Polymorphism frequency in the Brazilian genomic variant cohort (ABraOM) | ||||
| 1 | LEPR | T699M | rs34499590 | 1.28% | 0.98% | 0.32% | 0.82% | ||||
| 1 | ADCY10 | Y402C | rs140663029 | 0.04% | 0.080% | 0.02% | 0.16% | ||||
| 1 | MOCS1 | R339W | rs148579886 | 0.02% | 0.040% | 0.04% | NA | ||||
| 1 | ADGRG6 | A625T | rs184235213 | 0.24% | 0.26% | 0.07% | 0.08% | ||||
| 1 | AKAP13 | L1132S | rs745783128 | NA | NA | 0.0008% | NA | ||||
| 2 | OBSCN | G2113C | rs74623201 | 0.59% | 0.50% | 0.16% | 0.08% | ||||
| 3 | APOB | D749Y | . | NA | NA | NA | NA | ||||
| 3 | MRPS18B | V120M | rs116524936 | NA | 0.02% | 0.002% | NA | ||||
| 3 | PKHD1 | T36M | rs137852944 | 0.03% | 0.02% | 0.05% | NA | ||||
| 3 | RNLS | R222H | rs191733133 | NA | 0.02% | 0.002% | NA | ||||
| 3 | GIT1 | A745T | . | NA | NA | NA | NA | ||||
| 3 | GIT1 | R43H | . | NA | NA | NA | NA | ||||
| 3 | LILRA2 | R423C | rs149580797 | 0.21% | 0.30% | 0.09% | 0.33% | ||||
| 4 | MAP4K4 | K91E | . | NA | NA | NA | NA | ||||
| 4 | SLC11A1 | R397C | rs74906275 | 0.04% | 0.04% | 0.03% | NA | ||||
| 4 | RPUSD3 | W269X | rs142984515 | 0.008% | NA | 0.007% | NA | ||||
| 4 | UMPS | S30G | rs17843776 | 0.02% | 0.36% | 0.38% | NA | ||||
| 5 | MAML1 | G136E | rs146382198 | 0.008% | 0.08% | 0.07% | 0.49% | ||||
| 5 | DHODH | R135C | rs201230446 | 0.04% | 0.04% | 0.04% | 0.08% | ||||
| 6 | TNFRSF4 | D163E | . | NA | NA | NA | NA | ||||
| 6 | APOB | N3107K | rs72653101 | NA | NA | 0.0008% | NA | ||||
| 6 | SERPINE2 | M64T | rs34078713 | 0.78% | 0.30% | 0.75% | 1.15% | ||||
Prob, probable or probably; Patho, pathogenic; Poly = poly = polymorphism; Dam, damaging
CMP, cardiomyopathy
We excluded synonymous, non-exonic polymorphisms, keeping polymorphisms with a minor allele frequency (MAF) of <1% in at least one public databases (ESP6500; NHLBI GO Exome Sequencing Project (EVS, ESP6500SI-V2 release on http://evs.gs.washington.edu/EVS/); 1000 Genomes (April 2014 data release on http://browser.1000genomes.org); and Exome Aggregation Consortium (ExAC, January 2015 Version 0.3 data release onhttp://exac.broadinstitute.org)) using the VARAFT filtering and annotation tool (https://varaft.eu) on vcf files. Variant calls with Variant quality (QUAL) ≤60, depth of coverage (DP) <20, and mapping quality (MQ) ≤40 were filtered out. We also assessed an available Brazilian exome database of 609 elderly individuals from the city of Sao Paulo [18]. Only exonic nonsynonymous damaging variants were kept for downstream genetic analyses. We searched for variants, independently, in each family, under an autosomal dominant or under autosomal recessive models. In order to identify gene variants associated with CCC in each family, we selected variants that were shared by all CCC patients and absent in any and all IF patients in a given nuclear family, as well as in 14 unrelated IF controls. We assessed the pathogenic potential of missense variants using 4 algorithms embedded in VarAft: SIFT (https://sift.bii.a-star.edu.sg/), Polyphen 2 HumDiv and Polyphen 2 HumVar (http://genetics.bwh.harvard.edu/pph2/index.shtml), and UMD-Predictor (http://umd-predictor.eu/). Only rare variants tagged as pathogenic (or damaging) or possibly pathogenic (or probably damaging) in at least three databases and pathogenic/damaging in at least one algorithm were retained for downstream genetic analyses. We validated the abovementioned pathogenicity filter with the Combined Annotation-Dependent Depletion (CADD) tool (http://cadd.gs.washington.edu/score). Evolutionary conservation in the position was determined with the Consurf server (https://consurf.tau.ac.il)
Fig. 2.
Number of genes containing pathogenic variants for each biological process related to cardiomyopathy. Pathway analyses were performed with the Ingenuity Pathways Analysis (IPA®, Qiagen, Redwood City, USA) or Reactome (reactome.org); we also interrogated whether genes participated in 9 biological processes related to the pathophysiology of CCC: inflammation, IFNγ-modulated genes/Th1 response, fibrosis/extracellular matrix, contractility of heart, hypertrophy, arrhythmia, oxidative stress/antioxidant response, familial cardiomyopathy, and mitochondria-related genes. For these nine additional biological processes, we merged IPA Knowledge Base (IKB) gene lists and published gene lists including the IFNγ induced/repressed gene lists, and Nrf2-modulated genes where appropriate. Mitochondrial gene list was a combination of genes contained in the Mitochondrion Gene Ontology term and Mitocarta 2.0. The gene list for these pathways/processes in online Table 2
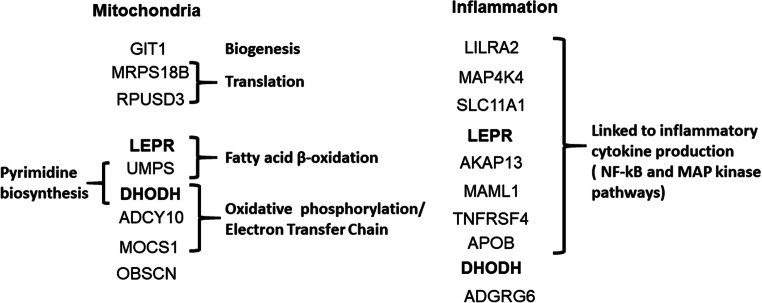
Fig. 3.
Features of the main mitochondrial and inflammation-related genes containing deleterious pathogenic variants associated to CCC
Conversely, 3 variants were found in the 178 genes belonging to the contraction/contractility process. Figure 3 shows the participation of the variant genes in different mitochondrial and inflammation-related pathways. Eight out of 9 mitochondrial genes with CCC-specific variants are involved directly or indirectly with energy generation, in processes including mitochondrial biogenesis, mitochondrial DNA-encoded gene translation, fatty acid oxidation, and oxidative phosphorylation/electron transfer chain; interestingly, two genes are involved in pyrimidine biosynthesis. Of note, 8 out of 10 inflammation-related genes are involved in proinflammatory cytokine production via activation of NF-kB and MAP kinase pathways. Two mitochondrial genes (DHODH and LEPR) are also involved in inflammation (Table 2, Fig. 3 in bold). On the whole, 5 of the inflammation-related genes and 5 of the mitochondrial genes also played roles in other cardiomyopathy-related pathobiological processes. The RPUSD3 gene, involved in the assembly of the mitochondrial ribosome, displayed a stopgain variant at exon 8 in family 4, creating a truncated version lacking 24% of its C-terminal sequence.
The GIT1 gene showed two variants in family 3 that segregated together, suggesting compound heterozygosity. Only 3 variants occurred in genes that were not mitochondrial and/or inflammation-related: PKHD1 and SERPINE2, which belonged to the ECM/fibrosis process, and RNLS, belonging to the hypertrophy and contraction processes. Only one variant gene, OBSCN, had previously been described in genetic/familial cardiomyopathy. No variants were detected in arrhythmia/ion channel–related genes. Patients carrying heterozygous gene variants had a normal childhood and reported no debilitating disease before developing CCC as adults, and we inferred that the variants by themselves alone were not able to induce childhood-onset mitochondriopathy. Detailed information on the variants is described in Table 2.
We have performed Sanger sequencing in two healthy, eutrophic, seronegative siblings from family 5 searching for the CCC-associated variants in genes DHODH and MAML1. One of them (subject 6137) carried the heterozygote variant DHODH C/T (R135C) shared by the CCC family members, while the other (subject 6132) carried the “wild-type” homozygous DHODH n.403 C/C (R135). Regarding MAML1, the second variant gene in Family 5, both 6132 and 6137 carried the “wild-type” homozygous MAML1 n. 407 G/G (G136).
Since both IFN-γ and loss-of-function mitochondrial mutations cause mitochondrial dysfunction, we studied the effect of IFN-γ on cardiomyocytes made deficient in mitochondrial enzyme dihydroorotate dehydrogenase (DHODH) activity with the inhibitor Brequinar. This enzyme is important for the electron transport chain and showed a loss-of-function mutation (DHODH R135C) linked with CCC in one of the studied families. We found that both IFN-γ and brequinar treatment significantly reduced mitochondrial membrane potential (ΔψM) as expressed by mitochondrial TMRE fluorescence on the AC16 human cardiomyocyte cell line and that cells treated simultaneously with IFN-γ and brequinar showed an even larger decrease on ΔψM (Fig. 4).
Fig. 4.
IFN-γ and brequinar treatment significantly reduced mitochondrial membrane potential. AC16 human cardiomyocyte cell line was stimulated with or without IFN-γ and with or without brequinar for 48 h. Total mitochondria were stained with MitoTracker green, and mitochondrial membrane potential (MMP) was evaluated using the TMRE dye that accumulates in fully polarized, but not in depolarized mitochondria. We measured TMRE fluorescence on MitoTracker green-labeled mitochondria. Cell viability was calculated as the ratio of the amount of live cells (propidium iodide-negative) and total cells (propidium iodide-negative plus propidium iodide-positive cells) × 100. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001
Discussion
In this study of whole exome sequencing of six nuclear families with multiple cases of CD, we found 22 CCC-associated rare heterozygous nonsynonymous high-impact pathogenic variants in 20 genes belonging to pathways relevant to inflammatory cardiomyopathy. Only individuals that were both seropositive and carriers of the heterozygous pathogenic variants developed CCC, but not seropositive patients carrying the wild-type sequences, nor seronegative siblings carrying the pathogenic variant. A striking accumulation of CCC-specific variants (86%) occurred in mitochondrial or inflammation-related genes, and all studied families displayed at least one CCC-associated variant gene belonging to these pathways. The finding that only a single family carried a variant in a gene previously associated with familial cardiomyopathy indicates the genetic landscape, and pathogenesis of CCC is distinct from that of familial cardiomyopathy. Importantly, we also found that IFN-γ potentiated mitochondrial dysfunction caused by an inhibitor of DHODH, a mitochondrial enzyme bearing a loss-of-function mutation (DHODH R135C) linked with CCC in one of our families, indicating synergy in the induction of mitochondrial dysfunction. To our knowledge, this is the first report that rare heterozygous pathogenic variants in mitochondrial and inflammation-related genes are linked to the development of complex multifactorial inflammatory cardiomyopathy.
Among the 9 mitochondrial genes showing CCC-specific pathogenic variants, 8 are involved in processes leading to mitochondrial ATP production (biogenesis, translation, fatty acid oxidation-FAOx, and the electron transfer chain/oxidative phosphorylation (OXPHOS)). The R135C variant observed in the DHODH gene was the only variant known to lead to complete loss-of-function and mitochondrial dysfunction [27]. Genetic truncation or expression of dominant-negative variants of DHODH, GIT1, RPUSD3, ADCY10, and MOCS1 in cells was shown to cause mitochondrial dysfunction, including reduced mitochondrial membrane potential, respiratory chain/oxidative phosphorylation (OXPHOS) activity, and ATP production [28–30]. Interestingly, patients carrying mutations or animals genetically deficient in 6 genes (DHODH, UMPS, MRPS18B, GIT1, OBSCN, and LEPR) developed cardiac phenotypes [27, 30–34]. Parents of patients with Miller syndrome (homozygous DHODH loss-of-function R135C or other variants; 70% with congenital cardiac abnormalities) carry one copy of the defective gene as members of family 5 but are nevertheless healthy [27]. Animals genetically deficient in MRPS18B and GIT1 showed evidence of mitochondrial dysfunction, altered mitochondrial morphology, and reduced myocardial contractility [35]. Details about the function of affected genes are shown in online Table 5.
Genetic disorders involving mitochondrial genes are the most common congenital genetic syndromes. Mitochondriopathies are caused by homozygous pathogenic rare variants in nuclear-encoded mitochondrial genes or in mitochondrial DNA. Homozygous pathogenic variants of more than 250 of the ~1500 nuclear-encoded mitochondrial genes were identified as causally related to mitochondrial disorders [35]. Each leads to drastic mitochondrial dysfunction and energetic and functional impairment in tissues of high energy demand, causing syndromes affecting single organs or organ combinations that usually present in early childhood; each clinical syndrome is associated with variants in specific mitochondrial genes [35], and clinical penetrance is variable. The diverse clinical presentation of mitochodriopathies is related to genetic heterogeneity and the central role of mitochondria in multiple processes, including energy generation, antioxidant defenses, Ca++ homeostasis, intracellular signaling, macromolecule and nucleotide biosynthesis, reprogramming nuclear gene expression, and cell death. Interestingly, up to 30% of mitochondriopathy patients develop cardiomyopathy, heart conduction defects, ventricular arrhythmia or sudden cardiac death, and autonomic nervous system imbalance [36], while up to 15% develop gastrointestinal motility disorders including achalasia/megaesophagus and megacolon [37]. The striking similarity between the clinical presentation and proportion of cardiac and digestive disorders in mitochondriopathies and the clinical spectrum of CD [3] suggested the pathogenesis of CCC may be dependent on mitochondrial dysfunction. Indeed, CCC myocardium displays signs of reduced mitochondrial activity and energy production. Decreased mitochondrial rRNA [10], rDNA [38], and in vivo ATP production [18] were observed in the CCC myocardium. Myocardial levels and activity of mitochondrial energy metabolism enzymes ATP synthase and creatine kinase are even lower than in CCC than other cardiomyopathies [17], which might contribute to the worse prognosis of CCC. This is in line with Nisha Garg’s group findings implicating myocardial mitochondrial dysfunction and oxidative stress in the pathogenesis of murine models of CCC (reviewed in [39]). IFN-γ and brequinar treatment significantly reduced mitochondrial membrane potential (ΔψM) on the AC16 human cardiomyocyte cell line. Significantly, cells treated simultaneously with IFN-γ, and brequinar showed an even larger decrease on ΔψM. This result is consistent with an increased susceptibility of cells with reduced mitochondrial enzyme activity to IFN-γ. Even though the identified genes play important roles in mitochondrial energy generation and physiology, the heterozygous mitochondrial variants did not cause childhood signs of altered development typical of mitochondriopathies and were only associated with progression to cardiac disease in T. cruzi-seropositive patients, whose age group was typical from CCC patients in general. Taken together, our results suggest that T. cruzi-induced IFN-γ is necessary to cause clinically significant disease in patients with heterozygous variants. We propose that long-term exposure to high levels IFN-γ associated with CD – particularly high in CCC myocardium [40] – is the additional mitochondrial insult suffered by carrier of heterozygous mitochondrial variants that leads them to mitochondrial dysfunction. IFN-γ has multiple deleterious effects on cardiomyocyte mitochondria. It induces TNF-alpha and potentiates TNF-alpha-mediated NF-kB signaling, leading to NOS2 production of NO [41], which in the presence of IFN-γ-induced reactive oxygen species turns into peroxynitrite [21], and ensuing mitochondrial fragmentation and reduction of mitochondrial membrane potential, lipid beta-oxidation [14], and ATP generation [22], leading to cardiomyocyte dysfunction and apoptosis [15]. Variants in the inflammation-related genes may also lead to a mitochondrial dysfunction phenotype. Gain-of-function variants of genes linked to NF-kB or MAP kinase signaling could lead to increased proinflammatory cytokine production, which might lead to further mitochondrial dysfunction in cardiomyocytes [22], and this mitochondrial damage can be further fueled by IFN-γ [42]. It is thus possible that the common final pathogenic mechanism for mitochondrial and inflammation-associated gene variants might involve enhancement of IFN-γ-induced cardiac mitochondrial damage. As mitochondrial damage and release of damage-associated molecular patterns can itself induce the production of proinflammatory cytokines, a self-perpetuating positive feedback loop between inflammation and mitochondrial damage [43] may contribute to the chronicity of inflammation in CCC heart tissue.
Our study has limitations. CCC patients included in the pedigrees mostly have major diagnostic ECG changes without left ventricular dysfunction. However, follow-up studies show that asymptomatic chagasic individuals with ECG abnormalities typical of CCC show higher total and cardiac mortality in relation to chagasic individuals with normal ECG; up to 50% of deaths in this groups are due to SCD [6, 8, 44–46]. Similarly, conduction defects and arrhythmias can be the presenting symptoms of genetic mitochondriopathies [47]. Even though CCC has a progressive character and inflammatory mediators and mitochondrial dysfunction are key participants of arrhythmia [48] and heart failure [43], we can only hypothesize that the genetic variants identified in our study may play a direct role in the inflammation [10, 11] and mitochondrial dysfunction [17, 38] observed in end-stage CCC.
Conclusion
Results indicate that the genetic contribution to CCC is polygenic and driven by several rare variants in genes that differ between families but are related to mitochondria and inflammation. Results imply that mitochondrial dysfunction and inflammation, key processes in the pathophysiology of CCC, are at least in part genetically determined. To our knowledge, this is the first report that rare variants in mitochondrial and inflammation-related genes are linked to complex multifactorial cardiomyopathy. Our results also support the notion of a two-hit mechanism where IFN-γ and proinflammatory cytokines induced by chronic infection trigger mitochondrial dysfunction and clinical disease in carriers of heterozygous mitochondrial gene variants. Indeed, modulation of mitochondrial damage induced by IFN-γ and other cytokines could perhaps be a suitable therapeutic target in CCC. Treatment with mitochondria-protective agents such as antioxidants or agonists of sirtuin-1 and AMP-activated protein kinase (AMPK) was found to attenuate or even reverse cardiac damage in mouse models of CCC, by reducing NF-kB activation and the intensity of chronic myocarditis (reviewed in [38]). To conclude, it is possible that a similar two-hit mechanism, whereby genetic variants may increase mitochondrial susceptibility to inflammatory cytokine-induced dysfunction, may be relevant for the pathogenesis of other inflammatory cardiomyopathies and degenerative diseases associated with mitochondrial dysfunction.
Supplementary Information
Confirmation of the pathogenic variants by Sanger sequencing (PDF 2238 kb)
(DOCX 12 kb)
(DOCX 16 kb)
(DOCX 37 kb)
(DOCX 13 kb)
(DOCX 23 kb)
(DOCX 13 kb)
Acknowledgments
We thank all the patients and their relatives.
Availability of Data and Material
Raw data will be available on request.
Abbreviations
- T. cruzi
Trypanosoma cruzi
- CCC
Chronic Chagas cardiomyopathy
- CD
Chagas disease
- ASY
Asymptomatic form
- DCM
Dilated cardiomyopathy
- ECG
Electrocardiography
- IFN
Interferon
- TNF
Tumor necrosis factor
- NO
Nitrite oxide
- ATP
Adenosine triphosphate
- AVM
Acute viral myocarditis
- WES
Whole exome sequencing
- SCD
Sudden cardiac death
- HF
Heart failure
- LEPR
Leptin receptor
- ADCY10
Adenylate cyclase 10
- MOCS1
Molybdenum cofactor synthesis 1
- ADGRG6
Adhesion G protein-coupled receptor G6
- AKAP13
AKinase-anchoring protein 13
- OBSCN
Obscurin
- APOB
Apolipoprotein B
- MRPS18B
Mitochondrial ribosomal protein S18B
- PKHD1
Polycystic kidney and hepatic disease 1
- RNLS
Renalase, FAD-dependent amine oxidase
- GIT1
G protein-coupled receptor kinase interactor 1
- LILRA2
Leukocyte immunoglobulin–like receptor A2
- MAP 4 K4
Mitogen-activated protein kinase kinase kinase kinase 4
- SLC11A1
Solute carrier family 11 member 1
- RPUSD3
RNA pseudouridine synthase D3
- UMPS
Uridine monophosphate synthetase
- MAML1
Mastermind like transcriptional coactivator 1
- DHODH
Dihydroorotate dehydrogenase
- TNFRSF4
TNF receptor superfamily member 1
- SERPINE2
Serpin family E member 2
Authors’ Contribution
Designed the study: JK, LAR, ECS, ECN, and CC.
Supervised the study as a whole: ECN and CC.
Participated in the recruitment of patients and sample collection: AFF, BI, AMF, CM, PB,
ClC, ORSJ, LCO, CDLO, MDCN, and LAR.
Performed experiments: MO, SM, RRA, JPN, LRPF, SB, DL, VOCR, DC, RCFZ, MT, FG, and PA.
Performed and supervised computational studies: LA, AC, ECN, and CC.
Wrote the manuscript: ECN and CC.
All authors reviewed the results and approved the final version of the manuscript.
Funding
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM); the Aix-Marseille University (grant number: AMIDEX “International_2018” MITOMUTCHAGAS); the French Agency for Research (Agence Nationale de la Recherche-ANR (grant numbers: “Br-Fr-Chagas,” “landscardio”); the CNPq (Brazilian Council for Scientific and Technological Development); the FAPESP (São Paulo State Research Funding Agency Brazil (grant numbers: 2013/50302–3, 2014/50890–5); and the National Institutes of Health/USA (grant numbers: 2 P50 AI098461–02 and 2U19AI098461–06). This project has received funding from the Excellence Initiative of Aix-Marseille University (A*Midex) a French “Investissements d’Avenir programme”- Institute MarMaRa AMX-19-IET-007. ECN, JK, ALR, and ECS are recipients of productivity awards by CNPq. The funders do not play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edecio Cunha-Neto and Christophe Chevillard contributed equally to this work.
Contributor Information
Edecio Cunha-Neto, Email: edecunha@gmail.com.
Christophe Chevillard, Email: christophe.chevillard@univ-amu.fr.
References
- 1.Schultheiss HP, Fairweather D, Caforio ALP, Escher F, Hershberger RE, Lipshultz SE, Liu PP, Matsumori A, Mazzanti A, McMurray J, Priori SG. Dilated cardiomyopathy. Nat Rev Dis Primers. 2019;5(1):32. doi: 10.1038/s41572-019-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chevillard C, Nunes JPS, Frade AF, Almeida RR, Pandey RP, Nascimento MS, Kalil J, Cunha-Neto E. Disease tolerance and pathogen resistance genes may underlie trypanosoma cruzi persistence and differential progression to Chagas disease cardiomyopathy. Front Immunol. 2018;9:2791. doi: 10.3389/fimmu.2018.02791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bocchi EA, Bestetti RB, Scanavacca MI, Cunha Neto E, Issa VS. Chronic Chagas heart disease management: from etiology to cardiomyopathy treatment. J Am Coll Cardiol. 2017;70(12):1510–1524. doi: 10.1016/j.jacc.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Casado J, Davila DF, Donis JH, Torres A, Payares A, Colmenares R, Gottberg CF. Electrocardiographic abnormalities and left ventricular systolic function in Chagas' heart disease. Int J Cardiol. 1990;27(1):55–62. doi: 10.1016/0167-5273(90)90191-7. [DOI] [PubMed] [Google Scholar]
- 5.Barbosa MP, da Costa Rocha MO, de Oliveira AB, Lombardi F, Ribeiro AL. Efficacy and safety of implantable cardioverter-defibrillators in patients with Chagas disease. Europace. 2013;15(7):957–962. doi: 10.1093/europace/eut011. [DOI] [PubMed] [Google Scholar]
- 6.Baroldi G, Oliveira SJ, Silver MD. Sudden and unexpected death in clinically ‘silent’ Chagas’ disease. A hypothesis. Int J Cardiol. 1997;58(3):263–268. doi: 10.1016/s0167-5273(96)02878-1. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi ML, De Morais CF, Pereira Barreto AC, Lopes EA, Stolf N, Bellotti G, et al. The role of active myocarditis in the development of heart failure in chronic Chagas’ disease: a study based on endomyocardial biopsies. Clin Cardiol. 1987;10(11):665–670. doi: 10.1002/clc.4960101113. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro AL, Marcolino MS, Prineas RJ, Lima-Costa MF. Electrocardiographic abnormalities in elderly Chagas disease patients: 10-year follow-up of the Bambui cohort study of aging. J Am Heart Assoc. 2014;3(1):e000632. doi: 10.1161/JAHA.113.000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abel LC, Rizzo LV, Ianni B, Albuquerque F, Bacal F, Carrara D, et al. Chronic Chagas' disease cardiomyopathy patients display an increased IFN-gamma response to trypanosoma cruzi infection. J Autoimmun. 2001;17(1):99–107. doi: 10.1006/jaut.2001.0523. [DOI] [PubMed] [Google Scholar]
- 10.Cunha-Neto E, Dzau VJ, Allen PD, Stamatiou D, Benvenutti L, Higuchi ML, Koyama NS, Silva JS, Kalil J, Liew CC. Cardiac gene expression profiling provides evidence for cytokinopathy as a molecular mechanism in Chagas' disease cardiomyopathy. Am J Pathol. 2005;167(2):305–313. doi: 10.1016/S0002-9440(10)62976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogueira LG, Santos RH, Fiorelli AI, Mairena EC, Benvenuti LA, Bocchi EA, et al. Myocardial gene expression of T-bet, GATA-3, Ror-gammat, FoxP3, and hallmark cytokines in chronic Chagas disease cardiomyopathy: an essentially unopposed TH1-type response. Mediat Inflamm. 2014;2014:914326. doi: 10.1155/2014/914326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laugier L, Rodrigues Pinto Ferreira L, Moraes Ferreira F, Cabantous S, Farage Frade A, Ribeiro Almeida R, et al. miRNAs may play a major role in the control of gene expression in key pathobiological processes in Chagas disease cardiomyopathy. Plos Negl Trop Dis. 2020; In press. [DOI] [PMC free article] [PubMed]
- 13.Patten M, Kramer E, Bunemann J, Wenck C, Thoenes M, Wieland T, et al. Endotoxin and cytokines alter contractile protein expression in cardiac myocytes in vivo. Pflugers Arch. 2001;442(6):920–927. doi: 10.1007/s004240100612. [DOI] [PubMed] [Google Scholar]
- 14.Ni C, Ma P, Wang R, Lou X, Liu X, Qin Y, Xue R, Blasig I, Erben U, Qin Z. Doxorubicin-induced cardiotoxicity involves IFNgamma-mediated metabolic reprogramming in cardiomyocytes. J Pathol. 2019;247(3):320–332. doi: 10.1002/path.5192. [DOI] [PubMed] [Google Scholar]
- 15.Levick SP, Goldspink PH. Could interferon-gamma be a therapeutic target for treating heart failure? Heart Fail Rev. 2014;19(2):227–236. doi: 10.1007/s10741-013-9393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soares MP, Gozzelino R, Weis S. Tissue damage control in disease tolerance. Trends Immunol. 2014;35(10):483–494. doi: 10.1016/j.it.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Teixeira PC, Santos RH, Fiorelli AI, Bilate AM, Benvenuti LA, Stolf NA, et al. Selective decrease of components of the creatine kinase system and ATP synthase complex in chronic Chagas disease cardiomyopathy. PLoS Negl Trop Dis. 2011;5(6):e1205. doi: 10.1371/journal.pntd.0001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leme AM, Salemi VM, Parga JR, Ianni BM, Mady C, Weiss RG, et al. Evaluation of the metabolism of high energy phosphates in patients with Chagas' disease. Arq Bras Cardiol. 2010;95(2):264–270. doi: 10.1590/s0066-782x2010005000099. [DOI] [PubMed] [Google Scholar]
- 19.Silva JS, Vespa GN, Cardoso MA, Aliberti JC, Cunha FQ. Tumor necrosis factor alpha mediates resistance to trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect Immun. 1995;63(12):4862–4867. doi: 10.1128/iai.63.12.4862-4867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vila-del Sol V, Punzon C, Fresno M. IFN-gamma-induced TNF-alpha expression is regulated by interferon regulatory factors 1 and 8 in mouse macrophages. J Immunol. 2008;181(7):4461–4470. doi: 10.4049/jimmunol.181.7.4461. [DOI] [PubMed] [Google Scholar]
- 21.Rakshit S, Chandrasekar BS, Saha B, Victor ES, Majumdar S, Nandi D. Interferon-gamma induced cell death: regulation and contributions of nitric oxide, cJun N-terminal kinase, reactive oxygen species and peroxynitrite. Biochim Biophys Acta. 2014;1843(11):2645–2661. doi: 10.1016/j.bbamcr.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Buoncervello M, Maccari S, Ascione B, Gambardella L, Marconi M, Spada M, Macchia D, Stati T, Patrizio M, Malorni W, Matarrese P, Marano G, Gabriele L. Inflammatory cytokines associated with cancer growth induce mitochondria and cytoskeleton alterations in cardiomyocytes. J Cell Physiol. 2019;234(11):20453–20468. doi: 10.1002/jcp.28647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belkaya S, Kontorovich AR, Byun M, Mulero-Navarro S, Bajolle F, Cobat A, Josowitz R, Itan Y, Quint R, Lorenzo L, Boucherit S, Stoven C, di Filippo S, Abel L, Zhang SY, Bonnet D, Gelb BD, Casanova JL. Autosomal recessive cardiomyopathy presenting as acute myocarditis. J Am Coll Cardiol. 2017;69(13):1653–1665. doi: 10.1016/j.jacc.2017.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardoso CS, Sabino EC, Oliveira CD, de Oliveira LC, Ferreira AM, Cunha-Neto E, et al. Longitudinal study of patients with chronic Chagas cardiomyopathy in Brazil (SaMi-trop project): a cohort profile. BMJ Open. 2016;6(5):e011181. doi: 10.1136/bmjopen-2016-011181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marquet S, Bucheton B, Reymond C, Argiro L, El-Safi SH, Kheir MM, et al. Exome sequencing identifies two variants of the alkylglycerol monooxygenase gene as a cause of relapses in visceral leishmaniasis in children, in Sudan. J Infect Dis. 2017;216(1):22–28. doi: 10.1093/infdis/jix277. [DOI] [PubMed] [Google Scholar]
- 26.Khan R, Lee JE, Yang YM, Liang FX, Sehgal PB. Live-cell imaging of the association of STAT6-GFP with mitochondria. PLoS One. 2013;8(1):e55426. doi: 10.1371/journal.pone.0055426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang J, Uchiumi T, Yagi M, Matsumoto S, Amamoto R, Takazaki S, Yamaza H, Nonaka K, Kang D. Dihydro-orotate dehydrogenase is physically associated with the respiratory complex and its loss leads to mitochondrial dysfunction. Biosci Rep. 2013;33(2):e00021. doi: 10.1042/BSR20120097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arroyo JD, Jourdain AA, Calvo SE, Ballarano CA, Doench JG, Root DE, Mootha VK. A genome-wide CRISPR death screen identifies genes essential for oxidative phosphorylation. Cell Metab. 2016;24(6):875–885. doi: 10.1016/j.cmet.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grings M, Seminotti B, Karunanidhi A, Ghaloul-Gonzalez L, Mohsen AW, Wipf P, Palmfeldt J, Vockley J, Leipnitz G. ETHE1 and MOCS1 deficiencies: disruption of mitochondrial bioenergetics, dynamics, redox homeostasis and endoplasmic reticulum-mitochondria crosstalk in patient fibroblasts. Sci Rep. 2019;9(1):12651. doi: 10.1038/s41598-019-49014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang J, Xu X, Getman MR, Shi X, Belmonte SL, Michaloski H, Mohan A, Blaxall BC, Berk BC. G protein coupled receptor kinase 2 interacting protein 1 (GIT1) is a novel regulator of mitochondrial biogenesis in heart. J Mol Cell Cardiol. 2011;51(5):769–776. doi: 10.1016/j.yjmcc.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abella V, Scotece M, Conde J, Pino J, Gonzalez-Gay MA, Gomez-Reino JJ, et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. 2017;13(2):100–109. doi: 10.1038/nrrheum.2016.209. [DOI] [PubMed] [Google Scholar]
- 32.Ding Y, Liu W, Deng Y, Jomok B, Yang J, Huang W, Clark KJ, Zhong TP, Lin X, Ekker SC, Xu X. Trapping cardiac recessive mutants via expression-based insertional mutagenesis screening. Circ Res. 2013;112(4):606–617. doi: 10.1161/CIRCRESAHA.112.300603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marston S. Obscurin variants and inherited cardiomyopathies. Biophys Rev. 2017;9(3):239–243. doi: 10.1007/s12551-017-0264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyazawa S, Furuta S, Hashimoto T. Reduction of beta-oxidation capacity of rat liver mitochondria by feeding orotic acid. Biochim Biophys Acta. 1982;711(3):494–502. [PubMed] [Google Scholar]
- 35.Kohda M, Tokuzawa Y, Kishita Y, Nyuzuki H, Moriyama Y, Mizuno Y, Hirata T, Yatsuka Y, Yamashita-Sugahara Y, Nakachi Y, Kato H, Okuda A, Tamaru S, Borna NN, Banshoya K, Aigaki T, Sato-Miyata Y, Ohnuma K, Suzuki T, Nagao A, Maehata H, Matsuda F, Higasa K, Nagasaki M, Yasuda J, Yamamoto M, Fushimi T, Shimura M, Kaiho-Ichimoto K, Harashima H, Yamazaki T, Mori M, Murayama K, Ohtake A, Okazaki Y. A comprehensive genomic analysis reveals the genetic landscape of mitochondrial respiratory chain complex deficiencies. PLoS Genet. 2016;12(1):e1005679. doi: 10.1371/journal.pgen.1005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finsterer J, Kothari S. Cardiac manifestations of primary mitochondrial disorders. Int J Cardiol. 2014;177(3):754–763. doi: 10.1016/j.ijcard.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Finsterer J, Frank M. Gastrointestinal manifestations of mitochondrial disorders: a systematic review. Ther Adv Gastroenterol. 2017;10(1):142–154. doi: 10.1177/1756283X16666806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan X, Gupta S, Zago MP, Davidson MM, Dousset P, Amoroso A, et al. Defects of mtDNA replication impaired mitochondrial biogenesis during trypanosoma cruzi infection in human cardiomyocytes and chagasic patients: the role of Nrf1/2 and antioxidant response. J Am Heart Assoc. 2012;1(6):e003855. doi: 10.1161/JAHA.112.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez M, Tanowitz HB, Garg NJ. Pathogenesis of chronic Chagas disease: macrophages, mitochondria, and oxidative stress. Curr Clin Microbiol Rep. 2018;5(1):45–54. [PMC free article] [PubMed] [Google Scholar]
- 40.Reis MM, Higuchi Mde L, Benvenuti LA, Aiello VD, Gutierrez PS, Bellotti G, et al. An in situ quantitative immunohistochemical study of cytokines and IL-2R+ in chronic human chagasic myocarditis: correlation with the presence of myocardial trypanosoma cruzi antigens. Clin Immunol Immunopathol. 1997;83(2):165–172. doi: 10.1006/clin.1997.4335. [DOI] [PubMed] [Google Scholar]
- 41.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25(10):1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Lee HJ, Oh YK, Rhee M, Lim JY, Hwang JY, Park YS, Kwon Y, Choi KH, Jo I, Park SI, Gao B, Kim WH. The role of STAT1/IRF-1 on synergistic ROS production and loss of mitochondrial transmembrane potential during hepatic cell death induced by LPS/d-GalN. J Mol Biol. 2007;369(4):967–984. doi: 10.1016/j.jmb.2007.03.072. [DOI] [PubMed] [Google Scholar]
- 43.Zhou B, Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 2018;128(9):3716–3726. doi: 10.1172/JCI120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manzullo EC, Chuit R. Risk of death due to chronic chagasic cardiopathy. Mem Inst Oswaldo Cruz. 1999;94(Suppl 1):317–320. doi: 10.1590/S0074-02761999000700060. [DOI] [PubMed] [Google Scholar]
- 45.Pimenta J, Valente N, Miranda M. Long-term follow up of asymptomatic chagasic individuals with intraventricular conduction disturbances, correlating with non-chagasic patients. Rev Soc Bras Med Trop. 1999;32(6):621–631. doi: 10.1590/s0037-86821999000600003. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez-Salas LA, Klein E, Acquatella H, Catalioti F, Davalos VV, Gomez-Mancebo JR, et al. Echocardiographic and clinical predictors of mortality in chronic Chagas' disease. Echocardiography. 1998;15(3):271–278. doi: 10.1111/j.1540-8175.1998.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 47.Bonnet D, Martin D, Pascale De L, Villain E, Jouvet P, Rabier D, et al. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. 1999;100(22):2248–2253. doi: 10.1161/01.cir.100.22.2248. [DOI] [PubMed] [Google Scholar]
- 48.Montaigne D, Marechal X, Lacroix D, Staels B. From cardiac mitochondrial dysfunction to clinical arrhythmias. Int J Cardiol. 2015;184:597–599. doi: 10.1016/j.ijcard.2015.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Confirmation of the pathogenic variants by Sanger sequencing (PDF 2238 kb)
(DOCX 12 kb)
(DOCX 16 kb)
(DOCX 37 kb)
(DOCX 13 kb)
(DOCX 23 kb)
(DOCX 13 kb)
Data Availability Statement
Raw data will be available on request.