Abstract
Background
Reducing disparities in men with prostate cancer (PCa) that may be due racial and socioeconomic differences is a major public health priority. Few reports have studied whether these disparities have changed over time.
Methods
Men diagnosed with PCa from January 1, 2000 to December 31, 2015 were identified from the Massachusetts and Pennsylvania cancer registries. All-cause and prostate- and cardiovascular cause-specific mortality was assessed. To estimate neighborhood socioeconomic position (nSEP), a summary score was generated using census tract-level measures of income, wealth, educational attainment, and racial and income segregation. We grouped participants by diagnosis year (2000–2003, 2004–2007, 2008–2011, 2012–2015) and estimated changing trends in the mortality rate ratio by race and nSEP using covariate adjusted Cox models with follow-up for up to 10 years, until death, or censoring on January 1, 2018.
Results
There were 193,883 PCa cases with 43,661 deaths over 1,404,131 person-years of follow-up. The Black-White adjusted hazard ratio (aHR) from 2000–2003 to 2012–2015 was stable for all-cause mortality (aHR: 1.14 to 0.97, Phet=0.42), decreased for prostate cancer-specific mortality (aHR: 1.38 to 0.93, Phet=0.005), and increased for cardiovascular mortality (1.09 to 1.28, Phet=0.034). The aHR comparing those in the lowest to highest nSEP quintile increased significantly for all-cause (1.54 to 1.79, Phet = 0.008) but not prostate-specific mortality (1.60 to 1.72, Phet=0.40) and cardiovascular (1.72 to 1.89, Phet=0.085).
Conclusion
Although Black-White disparities in prostate mortality declined in Massachusetts and Pennsylvania over the study period, nSEP mortality disparity trends were stagnant or increased, warranting further attention.
Keywords: Prostatic Neoplasms, registries, African Americans / statistics & numerical data, Male, Middle Aged, Socioeconomic Factors, Health Status Disparities
Lay Summary
Few reports have examined whether racial and socioeconomic disparities in prostate cancer mortality have widened or narrowed in recent years. Using data from two state registries (Massachusetts, Pennsylvania) with differing intensities of government-mandated health insurance, we studied trends in racial and neighborhood socioeconomic disparities among Black and White men diagnosed from 2000 to 2015. Overall, trends in racial disparities were stagnant for all-cause mortality, shrank for prostate mortality, and widened for cardiovascular mortality. Disparities associated with neighborhood socioeconomic status either were stagnant or widened across all mortality endpoints. In general, disparities were more pronounced in Pennsylvania than Massachusetts.
Precis
Trends in racial disparities among Black and White men in Pennsylvania and Massachusetts diagnosed from 2000 to 2015 were stagnant for all-cause mortality, shrank for prostate mortality, and widened for cardiovascular mortality.
Disparities associated with neighborhood socioeconomic status either were stagnant or widened across all mortality endpoints.
Background
Prostate cancer (PCa) is the second leading cause of non-cutaneous cancer-related mortality among men in the United States of America (US), resulting in an expected 33,330 deaths in 20201. Black men with PCa in the US are twice as likely to die from their disease relative to non-Hispanic White men, a disparity which has persisted for several decades2. Causes of these racial disparities are multifactorial, and may arise from historic racial segregation and discrimination, differential access to PCa screening and treatment, and genetic factors3–5. Disparities in PCa by socioeconomic position have also been reported at state and national levels6,7. Although studies have used different socioeconomic measures ranging from individual components (education, marital status, income, segregation indices) to summary scores, and at different levels (individual- and/or neighborhood-level), findings are remarkably consistent. Three-quarters of 40 studies cited in a review found significant evidence of a socioeconomic gradient in PCa survival, with men of lower socioeconomic position experiencing poorer outcomes7.
Reducing racial/ethnic and socioeconomic disparities is a major public health priority because these inequalities are assumed to be unjust and preventable8–10. Few studies have examined whether racial and socioeconomic disparities in mortality among men with PCa widened or narrowed during the decade from 2010 to 2019. Two major policy changes occurred during this period which could have influenced disparities. First, the United States Preventive Task Force advocated against routine prostate-specific antigen screening for PCa in 2012, potentially contributing to higher stage at diagnosis and consequently exacerbating mortality disparities among disadvantaged and/or Black patients11. Second, the federal government passed the Affordable Care Act (ACA) in March 2010, increasing insurance coverage for many low-income Americans, which could have reduced disparities12. However, states introduced these policies at different times. Medicaid expansion was introduced in the state of Massachusetts in 2006, before the ACA was passed, while Pennsylvania introduced Medicaid expansion in 2015. In 2010, 4.4% of the population of Massachusetts was uninsured compared to 10.2% of the population in Pennsylvania13. Comparing trends in racial and socioeconomic disparities between states with different policies affecting access to early diagnosis and financial support for PCa care could reveal policy environments that could be leveraged to reduce these disparities.
We examined trends in Black-White racial disparities and disparities by neighborhood socioeconomic position (nSEP) among men diagnosed with PCa in Massachusetts and Pennsylvania. We hypothesized that racial and socioeconomic disparities in mortality among men with PCa would decrease over time.
Methods
Study Population and Outcomes
We constructed a cohort of PCa cases using data from the Massachusetts and Pennsylvania Cancer registry datasets. Populations of PCa cases from these two states were chosen because they are similar in terms of geographic location and racial/ethnic composition14,15, but introduced universal government-sponsored health insurance with differing intensity and timing. Therefore, differences in mortality disparities by race/ethnicity and nSEP could be partially attributable to those different policy environments. Both population-based registries are accredited through the North American Association of Central Cancer Registries and the National Program of Cancer Registries and collect reports of new cases from state-based cancer care facilities and laboratories. Black and White men with PCa diagnosed from January 1, 2000 to December 31, 2015 were followed for up to 10 years, until death, or censoring on January 1, 2018, whichever came first. We chose to censor at 10 years to balance follow-up time across participants entering the cohort at different times, and for consistency with long-term follow-up reported in prior studies. Starting with a total of 216,921 eligible participants, we excluded men with in situ PCa (n=80), and men who were missing data on clinical, sociodemographic, or geographic variables (n=22,860). We further restricted the population to men with age ≥40 years at diagnosis for consistency with previous registry-based studies (n=98). This resulted in a study population of 193,883 (89%).
Mortality was ascertained by the cancer registries through annual linkage with the National Death Index and state death registries. Prostate- and cardiovascular-specific causes of death were assigned using ICD-09 (prostate: 185, cardiovascular disease: 390–459) and ICD-10 codes (prostate: C61, cardiovascular disease: I00-I99). We chose to look at these two specific causes of death because these are the leading causes of death among men with PCa16. In addition, because many men are diagnosed with localized disease, cardiovascular deaths are more common than prostate-related deaths in this group17.
Measures
Perceived race/ethnicity (Black or White) for study participants were reported to registries from facilities in each state. Hispanic ethnicity was unavailable for all participants and so was not included in our analysis. We focused on disparities between these racial/ethnic groups because they present some of the largest disparities observed for any cancer4, and because Massachusetts and Pennsylvania have smaller populations of other racial/ethnic groups. To classify cases based on neighborhood socioeconomic position, a multi-dimensional construct that captures both resources and social privilege18–21, we constructed a neighborhood-level nSEP summary variable using data from the 2000 decennial census (for those diagnosed from 2000 to 2009) and the 2006–2010 American Community Survey and the 2010 decennial census (for those diagnosed from 2010 to 2015). Following methods developed by Messer and colleagues22, we selected a set of census tract-level measures (% poverty, median family income, % households with female head, % with income from interest or rent, % White, % Black, % unemployment, % male unemployment, % receiving welfare assistance, and the joint race-income Index of Concentration at Extremes) and used principal components analysis to create a summary nSEP score. Each variable was checked for skewness and log-transformed if appropriate, and then z-standardized. The nSEP summary score was calculated by summing each z-scaled measure, weighted by its loading onto the first principal component. We modeled nSEP using quintiles reflecting state-specific cutpoints. Further details regarding the statistical methods used to generate the nSEP score are included in the Supplementary Appendix.
Since Massachusetts and Pennsylvania exhibit different geographies and population density, we further controlled for a measure of geographic accessibility. Travel time to nearest cancer care facility estimated using cancer care facilities obtained from the Environmental Systems Research Institute (ESRI) business analyst database (2008) with North American Industry Classification System codes and the Network Analyst tool in ArcMap. We then estimated the origin-destination cost distance in minutes between participant address and closest cancer hospital using ArcMap 10.2 Network Analyst tool.
To model trends over time, we created a categorical variable using diagnosis year. Four categories (2000–2003, 2004–2007, 2008–2011, 2012–2015) were selected. While the final category (2012–2015) contained fewer observations than the other groups, this choice was justified based on changes in prostate-specific antigen screening policy that occurred in 201211. This policy advocated against routine screening, and was associated with changing clinical practice and increased stage at diagnosis for PCa patients23.
Statistical Analysis
We modeled disparities by race and nSEP two different ways. First, we plotted survival curves by race and by nSEP quintile using the Kaplan-Meier estimator with age as the time scale, stratifying by year of diagnosis (categorical) and state. For the Kaplan-Meier analysis, we restricted the cohort to men between 40 to 100 years old and censored follow-up at 100 years (n=193,871). Differences in survival were compared using median age at death and the log-rank test (two-sided, alpha=0.05). In order to isolate the role of race and nSEP, we modeled cause-specific mortality relative rate ratios by race/ethnicity and nSEP quintile using Cox Proportional hazards models, adjusted for age at diagnosis (deciles), state (Massachusetts, Pennsylvania), and census tract-level population density (binary: <1000 people/mi2, ≥1000 people/mi2) and travel time to closest cancer care facility. We adjusted for population density because racial/ethnic disparities in mortality among men with PCa could vary by urbanicity, with greater disparities in rural settings24. We interpreted the race/ethnicity coefficient and the coefficients corresponding to nSEP in our regression models as measures of the cause-specific mortality gap between the most privileged and most disadvantaged group along each disparity axis25. Proportional hazards were confirmed through assessment of Schoenfeld residuals plots26. We tested for trends in Black-White and nSEP mortality rate ratios over time by fitting a multiplicative interaction between diagnosis year modeled as an ordinal variable and the disparity measure of interest (Black-White race or a dummy variable for nSEP quintile 1 vs 5). The 0.05 two-sided p-value corresponding to this interaction term reflects significance of this heterogeneity over time (referred to hereafter as p-for-heterogeneity or Phet). Models where race/ethnicity was the primary disparity measure were adjusted for quintiles of nSEP, and models where nSEP was the primary disparity measure were adjusted for race. We assessed whether disparities were magnified jointly through race and nSEP using multiplicative interaction terms, but failed to find statistical evidence for these associations and so did not include them in our final models. For PCa-specific mortality and cardiovascular-specific mortality, we further stratified by PCa SEER summary stage (Localized compared to Regional/Distant)27.
As a sensitivity analysis, we fit Fine-Gray competing risks models for prostate- and cardiovascular-specific endpoints, specifying deaths from all other causes as a competing risk28. Under assumptions of (1) no unmeasured confounding of race/ethnicity and cause-specific mortality and (2) no unmeasured confounding of nSEP and cause-specific mortality, the race/ethnicity coefficients from our Cox regression models can be interpreted as the Black-White disparity that would be observed if everyone in the study resided in neighborhoods with the same neighborhood socioeconomic position25,29. This causal interpretation would suggest that the residual racial disparity then captures causal pathways unrelated to nSEP25, but does not capture the Black-White disparity that includes pathways associated with nSEP. For comparison, we included results from Cox regression models for Black-White cause-specific mortality disparities for the same set of covariates specified in our primary analysis, excluding nSEP. In order to ensure that the trends we observed were not dependent on the choice of cutpoints for the diagnosis year categories, we fit additional models with diagnosis year modeled non-parametrically using restricted cubic splines with five knots30,31. We fit Cox models parameterized as described above, and separately plotted the changing mortality rate ratio over time by race and by quintile of nSEP.
Results
After exclusions, 193,883 (89%) participants remained in the study population, with 43,661 deaths over 1,404,131 person-years of follow-up. There were 18,902 (10%) Black men with PCa, and the majority of cases (164,792, 85%) were diagnosed with localized SEER Summary Stage (Table 1). The proportion of men diagnosed with localized disease declined from 88% from 2000–2003 to 80% from 2012–2015, though overall case numbers also declined during this period. In both states, Black men with PCa were more likely than White men to reside in census tracts with the lowest quintile of nSEP (Massachusetts: 69% vs 16%, Pennsylvania: 77% vs 13%) (Supplementary Figure 1).
Table 1.
Descriptive Characteristics of Men with Prostate Cancer in Massachusetts and Pennsylvania, Diagnosed between 2000 and 2015
| Y00–Y03 | Y04–Y07 | Y08–Y11 | Y12–Y15 | Overall | |
|---|---|---|---|---|---|
| n | 52912 | 53037 | 50370 | 37564 | 193883 |
| Black race/ethnicity (%) | 4565 (8.6) | 4851 (9.1) | 5189 (10.3) | 4297 (11.4) | 18902 (9.7) |
| Age at diagnosis (median [IQR]) | 68.00 [61.00, 74.00] | 67.00 [60.00, 74.00] | 65.00 [59.00, 72.00] | 65.00 [59.00, 71.00] | 66.00 [60.00, 73.00] |
| Massachusetts (%) | 18778 (35.5) | 18005 (33.9) | 17100 (33.9) | 11539 (30.7) | 65422 (33.7) |
| SEER Summary Stage 2000 (%) | |||||
| Localized | 46539 (88.0) | 45780 (86.3) | 42594 (84.6) | 29879 (79.5) | 164792 (85.0) |
| Regional | 4580 (8.7) | 5366 (10.1) | 5948 (11.8) | 5557 (14.8) | 21451 (11.1) |
| Distant | 1793 (3.4) | 1891 (3.6) | 1828 (3.6) | 2128 (5.7) | 7640 (3.9) |
| Census tract neighborhood SEP | |||||
| % Black (median [IQR]) | 2 [1, 5] | 1 [1, 4] | 2 [1, 6] | 3 [1, 7] | 2 [1, 5] |
| % White (median [IQR]) | 96 [89, 98] | 96 [90, 98] | 94 [86, 98] | 93 [83, 97] | 95 [87, 98] |
| % foreign born (median [IQR]) | 4 [2, 8] | 4 [2, 7] | 4 [2, 9] | 5 [2, 10] | 4 [2, 8] |
| % under 5 (median [IQR]) | 6 [5, 7] | 6 [5, 7] | 6 [5, 7] | 5 [5, 6] | 6 [5, 7] |
| % over 65 (median [IQR]) | 15 [12, 19] | 15 [12, 19] | 15 [12, 19] | 15 [12, 19] | 15 [12, 19] |
| % female head of household (median [IQR]) | 17 [11, 26] | 16 [11, 25] | 17 [11, 28] | 20 [12, 31] | 17 [11, 27] |
| % over 25 with no high school (median [IQR]) | 15 [9, 21] | 15 [9, 21] | 12 [7, 18] | 10 [6, 15] | 13 [7, 19] |
| % over 25 with high school (median [IQR]) | 35 [26, 42] | 35 [26, 43] | 34 [26, 43] | 35 [25, 43] | 35 [26, 43] |
| % over 25 with college or more (median [IQR]) | 23 [14, 37] | 23 [13, 37] | 25 [15, 40] | 27 [16, 43] | 24 [14, 39] |
| Household (family) income (1000 USD, median [IQR]) | 54.26 [42.24, 69.06] | 54.36 [42.51, 69.41] | 61.56 [46.98, 82.00] | 70.48 [53.82, 92.38] | 58.54 [45.16, 77.01] |
| Family home value (1000 USD, median [IQR]) | 123.09 [84.30, 177.66] | 123.01 [85.03, 176.74] | 155.84 [98.50, 262.70] | 211.70 [124.40, 339.20] | 141.21 [92.30, 227.00] |
| % families receiving interest (median [IQR]) | 41 [31, 50] | 41 [32, 50] | 34 [24, 45] | 27 [20, 35] | 36 [26, 47] |
| % receiving welfare assistance (median [IQR]) | 5 [3, 8] | 5 [3, 7] | 3 [1, 6] | 2 [1, 3] | 4 [2, 6] |
| % poverty (median [IQR]) | 6 [4, 11] | 6 [4, 11] | 7 [4, 12] | 7 [4, 13] | 7 [4, 12] |
| % population 16+ years male unemployed (median [IQR]) | 31 [26, 38] | 31 [25, 38] | 30 [25, 37] | 30 [24, 37] | 31 [25, 38] |
| % population 16+ years unemployed (median [IQR]) | 4 [3, 6] | 4 [3, 6] | 5 [3, 8] | 6 [5, 9] | 5 [3, 7] |
| % occupied housing units (median [IQR]) | 96 [92, 97] | 96 [92, 97] | 95 [91, 97] | 94 [91, 96] | 95 [92, 97] |
| ICE (income) (median [IQR]) | 0.17 [−0.04, 0.38] | 0.17 [−0.04, 0.38] | 0.27 [0.04, 0.47] | 0.34 [0.14, 0.53] | 0.23 [0.01, 0.44] |
| ICE (Race) (median [IQR]) | 0.95 [0.87, 0.98] | 0.95 [0.88, 0.98] | 0.95 [0.85, 0.98] | 0.94 [0.83, 0.98] | 0.95 [0.86, 0.98] |
| ICE (Joint race and income) (median [IQR]) | 0.32 [0.19, 0.47] | 0.32 [0.19, 0.48] | 0.39 [0.24, 0.54] | 0.45 [0.30, 0.58] | 0.36 [0.22, 0.51] |
| Population density per mile2 (per 1000, median [IQR]) | 0.71 [0.19, 1.93] | 0.62 [0.15, 1.77] | 0.61 [0.15, 1.77] | 0.63 [0.16, 1.83] | 0.65 [0.16, 1.83] |
| NDVI (median [IQR]) | 0.55 [0.46, 0.62] | 0.59 [0.50, 0.64] | 0.54 [0.45, 0.61] | 0.57 [0.48, 0.63] | 0.56 [0.47, 0.63] |
| Travel time to closest cancer center (median [IQR]) | 5.52 [3.00, 10.13] | 5.80 [3.15, 10.76] | 5.88 [3.15, 10.83] | 7.63 [3.60, 19.49] | 5.99 [3.17, 11.50] |
| nSES Z-score (mean (SD)) | 0 (2.57) | 0.06 (2.55) | 0 (2.55) | −0.09 (2.55) | 0 (2.55) |
| nSES Z-score (quintiles)(%) | |||||
| Q1 | 10825 (20.5) | 10064 (19.0) | 10069 (20.0) | 7819 (20.8) | 38777 (20.0) |
| Q2 | 10763 (20.3) | 10734 (20.2) | 9834 (19.5) | 7444 (19.8) | 38775 (20.0) |
| Q3 | 10244 (19.4) | 10451 (19.7) | 10287 (20.4) | 7810 (20.8) | 38792 (20.0) |
| Q4 | 10384 (19.6) | 10685 (20.1) | 10153 (20.2) | 7518 (20.0) | 38740 (20.0) |
| Q5 | 10696 (20.2) | 11103 (20.9) | 10027 (19.9) | 6973 (18.6) | 38799 (20.0) |
Notes: nSEP = neighborhood socioeconomic position using United States Census tract-level data from 2000 for men diagnosed between 2000–2009, and 2010 for men diagnosed from 2010–2015. ICE=index of concentration at extremes, a metric that classifies neighborhoods based on the degree of homogeneity with respect to income and racial characteristics.
Trends in absolute racial and nSEP disparities in median age at death
Curves comparing survival by race, nSEP, year of diagnosis and state over ages of follow-up are provided in Figure 1 (numeric estimates of median age at diagnosis in each group and log-rank p-values are presented in Supplementary Table 1). Among men diagnosed between 2000 to 2003, the median age at death was 6 years greater for White (79 years) compared to Black (73 years) men with PCa (P<0.0001). The overall median age at death among men diagnosed between 2012–2015 was higher and among White (84 years) and Black (85 years) men compared to those diagnosed between 2000 to 2003, and there was less of racial disparity (p=0.055). There was no significant difference in the median age at death comparing Black and White men diagnosed from 2000–2003 (P=0.086) or 2012–2015 (P=0.11) in Massachusetts, but median age at death was significantly higher among White men at both time points in Pennsylvania (2000–2003: P<0.0001; 2012–2015: P=0.0005). Median age at death was significantly higher among men with nSEP Q5 compared to Q1 at both time points (2000–2003: 9 years, P <0.0001; 2012–2015: 4 years, P <0.0001). These gaps were more pronounced in Pennsylvania compared to Massachusetts.
Figure 1.
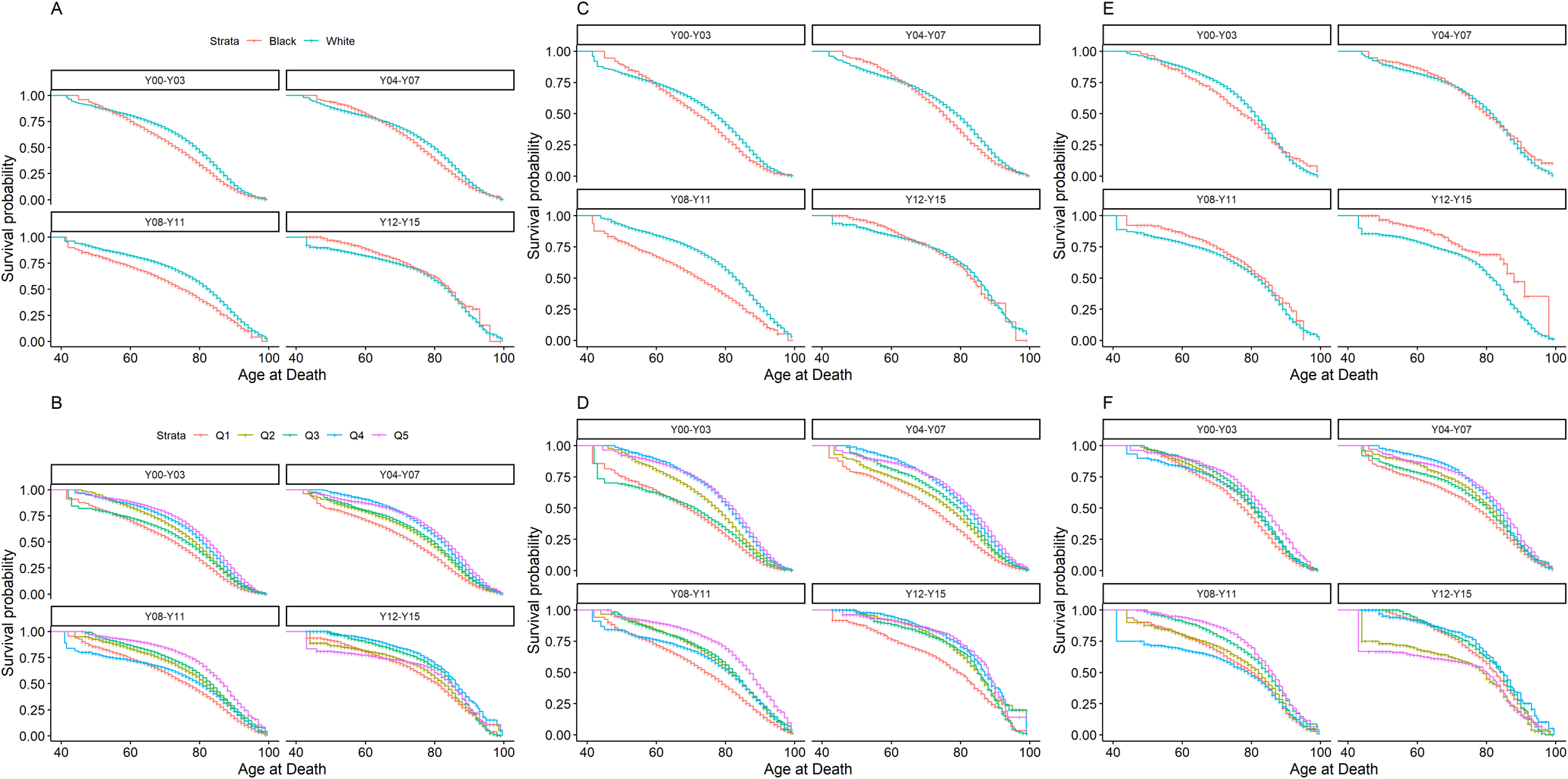
Survival by age at death stratified by Race, quintile of Neighborhood Socioeconomic Position (nSEP), and diagnosis year among Black (Red) and White (Blue) men with prostate cancer in Massachusetts and Pennsylvania. Panel A: Survival by Race and Diagnosis Year Category in Total Population; Panel B: Survival by quintile of nSEP (Q1=Red, Q2=Yellow, Q3=Green, Q4=Blue, Q5=Purple) and Diagnosis Year Category in Total Population; Panel C: Survival by Race and Diagnosis Year Category in Pennsylvania; D: Survival by quintile of nSEP and Diagnosis Year Category in Pennsylvania; Panel E: Survival by Race and Diagnosis Year Category in Massachusetts; Panel F: Survival by quintile of nSEP and Diagnosis Year Category in Massachusetts
Trends in relative racial and nSEP cause-specific mortality disparities
Figure 2 displays covariate adjusted hazard ratios for race and nSEP disparities in all-cause, prostate- and cardiovascular-specific mortality (Q1 v. Q5) over time (numeric results are presented in Supplementary Table 2). Though the Black-White all-cause mortality disparity for all participants declined from 2000–2003 (aHR: 95% CI: 1.14, 95% CI: 1.08, 1.20) to 2012–2015 (aHR: 0.97, 95% CI: 0.85, 1.10), this trend was not significant (Phet=0.42). However, state-level analyses revealed evidence of a reversal in the Black-White disparity in Massachusetts (Phet=0.047), but stagnant elevated relative mortality rates for Black men over time in Pennsylvania (2000–2003 aHR: 1.17, 95% CI: 1.10, 1.25; 2012–2015 aHR: 1.10, 95% CI: 0.95, 1.28, Phet=0.38). The hazard ratio for prostate-specific mortality comparing Black to White men declined over time (2000–2003 aHR: 1.38, 95% CI: 1.23, 1.54; 2012–2015 aHR: 0.93, 95% CI: 0.75, 1.16, Phet=0.005). Reductions in the racial disparity in prostate-specific mortality appeared to be driven by narrowing over time among cases with distant/regional summary stage (Supplementary Table 3; 2000–2003 aHR: 1.30, 95% CI: 1.12, 1.51; 2012–2015 aHR: 0.90, 95% CI: 0.70, 1.15, Phet=0.046). While there was no evidence of declines in Pennsylvania (Phet=0.36), the racial disparity narrowed over time in Massachusetts (2000–2003 aHR: 1.32, 95% CI: 1.05, 1.65; 2012–2015 aHR: 0.74, 95% CI: 0.48, 1.12, Phet=0.008). The racial disparity in cardiovascular mortality widened over time (2000–2003 aHR: 1.09, 95% CI: 0.98, 1.21; 2012–2015 aHR: 1.28, 95% CI: 0.96, 1.72, Phet=0.034). The increasing trend in cardiovascular mortality was more pronounced in Pennsylvania (2000–2003 aHR: 1.11, 95% CI: 0.99, 1.25; 2012–2015 aHR: 1.63, 95% CI: 1.19, 2.24, Phet=0.009). Increasing trends in Black-White cardiovascular mortality disparities were more pronounced among men diagnosed with localized disease (Supplementary Table 3). In separate models estimating Black-White cause-specific disparities over time without adjustment for nSEP, the magnitude of disparities was greater, but inference regarding trends remained unchanged from our primary analysis (Supplementary Table 4).
Figure 2. Hazard Ratios corresponding to Racial and Neighborhood Socioeconomic Position (nSEP) Disparities in Cause-specific Mortality stratified by Diagnosis Year and State among Black and White men with prostate cancer in Massachusetts and Pennsylvania.
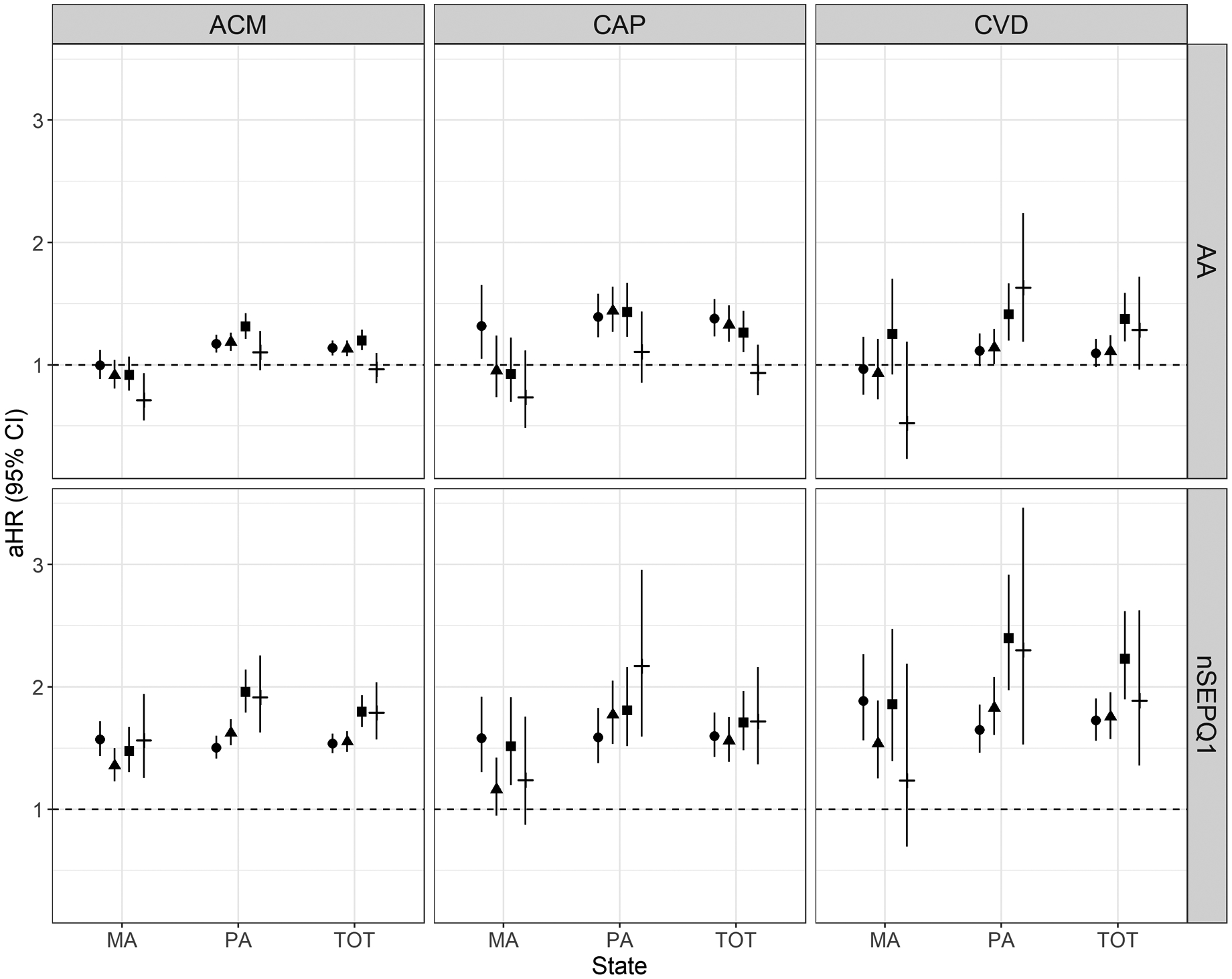
Note: Legend: Diagnosis Year Category 2000–2003 (circle); 2004–2007 (triangle); 2008–2011 (square); 2012–2015 (dash); ACM = all-cause mortality, CAP = prostate mortality, CVD = cardiovascular mortality, aHR = adjusted hazard ratios. Hazard ratios for racial disparities (Black vs White, labeled “AA”) and neighborhood socioeconomic disparities (nSEP Quintile 1 vs 5) were estimated using Cox proportional hazards models adjusted for race, nSEP quintile, age at diagnosis, population density (≥ 1000 people/mi2), and travel time to closest cancer care center, and fit with interactions between disparity measure and categorical diagnosis year.
Comparing nSEP quintile 1 to 5 in the total population, disparities widened over time (2000–2003, aHR: 1.54, 95% CI: 1.46, 1.62; 2012–2015: aHR 1.79, 95% CI: 1.57, 2.04, Phet=0.008). Socioeconomic disparities were stagnant in Massachusetts (Phet=0.57) but increased in Pennsylvania (2000–2003: aHR: 1.50, 95% CI: 1.41, 1.60; 2012–2015: aHR: 1.92, 95% CI: 1.63, 2.26, Phet<0.0001). Socioeconomic disparities in prostate-specific mortality (2000–2003: aHR: 1.60, 95% CI: 1.43, 1.79; 2012–2015: aHR: 1.65, 95% CI: 1.37, 2.16, Phet = 0.40) and cardiovascular-specific mortality (2000–2003: aHR: 1.72, 95% CI: 1.56, 1.91; 2012–2015: aHR: 1.89, 95% CI: 1.36, 2.62, Phet = 0.085) persisted over time.
In sensitivity analysis, competing risks models yielded similar hazard ratio estimates for disparities (Supplementary Table 5). Using splines to model year at diagnosis as a continuous variable, we observed more rapid declines in mortality rates for Black compared to White men with respect to all-cause and prostate-specific, but not cardiovascular-specific mortality (Supplementary Figure 2 Panel A), and similar rates of decline for all causes of mortality for men with prostate cancer in nSEP Q5 compared to Q1 (Supplementary Figure 2 Panel B).
Discussion
Using data from population-based registries in two Northeastern US states, we examined trends in racial and socioeconomic disparities in mortality among men diagnosed with PCa between 2000 and 2015. Trends for cause-specific outcomes revealed diverging patterns with respect to Black-White disparities in cause-specific mortality: no change for all-cause mortality, statistically significant decline for prostate-specific mortality, and statistically significant increase for cardiovascular-specific mortality. State-level trends revealed important differences, with reversing trends in Black-White disparities observed in Massachusetts but not Pennsylvania in more recent years. Studies comparing cardiovascular mortality rates between Black and White Americans suggest that different lifestyle risk factor profiles and socioeconomic factors between groups could explain race/ethnicity differences in cardiovascular mortality risk, which may also be true for men with PCa32,33. Another possible explanation could be increased use over time of androgen-deprivation therapy, for which certain treatments are associated with higher cardiovascular mortality34. Structural racism, which refers to systems that maintain inequities between a dominant and marginalized racial group in society35, and perceived racism could also contribute to higher cardiovascular mortality among Black men with PCa. Racial segregation concentrates disadvantage through fewer safe areas for physical activity, fewer healthy dietary options, and limited socioeconomic opportunities and is associated with higher mortality among Black adults and children36,37. Perceived racism is associated with higher prevalence of cardiovascular risk factors38. Though the primary goal of this analysis was to compare trends in consistent measures of racial and socioeconomic disparities in mortality among men with PCa over time, our findings suggest that differences in nSEP at diagnosis between Black and White men do not completely explain the observed racial disparities in cause-specific mortality, supporting the hypothesis that other causal pathways are involved36. Trends in nSEP disparities for all-cause mortality increased significantly over time, driven by increases in cardiovascular-specific mortality. There were no changes in nSEP disparities in prostate-specific mortality over the study period. Relative rates of all-cause and cardiovascular-specific mortality among men in nSEP Q1 compared to Q5 were significantly higher in both states across time periods.
Racial and nSEP disparities were less pronounced in Massachusetts than in Pennsylvania. This may reflect state-level differences in access to health services for Black men and those living in lower nSEP. Levels and trends of mortality disparities could also reflect impacts following earlier adoption of universal health coverage in Massachusetts compared to Pennsylvania. Current evidence suggests that the Medicaid expansion provision of the ACA succeeded in increasing insurance coverage, as well as access to ambulatory and surgical cancer care and possibly led to lower stage at presentation among low-income populations12,39,40, but had less impacts on use of cancer prevention services and race/ethnicity disparities in access39,41,42. Massachusetts is a wealthier and more densely populated state than Pennsylvania, which could also have resulted in smaller absolute disparities based on race/ethnicity and nSEP. Adoption of screening guidelines and novel treatments may therefore have spread more quickly in Massachusetts than Pennsylvania, leading to less variability in care-related outcomes43.
Study findings reflect regional patterns in Northeastern states that might differ from the population of PCa cases from other US states. Even though our geographic focus was limited, we observed stage migration consistent with national reports, with higher prevalence of regional and distant stage PCa among men diagnosed more recently23. Despite increasing prevalence of advanced PCa over time, the decline in racial disparities for prostate-specific mortality was greatest among men presenting with regional and distant PCa. This may be because of improvements in screening and treatment offered by physicians to Black men, or better access to PCa care among Black men over time44,45. A study using SEER registry data with follow-up from 2007–2014 reported larger Black-White disparities in prostate-specific mortality among cases from Southern US states, which could reflect broader regional patterns in state-level differences in racialized access to PCa care46. Earlier nation-wide studies of trends in racial disparities in PCa reported stagnant mortality disparities by race2, though few reported on men diagnosed later than 2010. A nation-wide study using county-level data from 1996–2006 found stronger socioeconomic gradients among Black compared to White men with PCa, suggesting that patterns of disparities by both race and socioeconomic status may exhibit more variability within smaller geographic areas across a larger set of states47.
This study has some limitations. Misclassification may have arisen due to discrepancies between registry-reported race/ethnicity and self-identified race/ethnicity48. We could not assess proximate causes of mortality among men with PCa, including individual-level risk factors such as smoking, physical activity, and obesity. It is unlikely that changes in these risk factors over time could completely explain the reported declines in PCa-specific disparities in mortality because they are only modestly associated with PCa mortality49, although they would be expected to play a larger role in cardiovascular disease mortality. Deaths were ascertained on an annual basis and may be less complete for the more recent diagnoses50. If mortality was underreported for more recent diagnoses of PCa, failure to account for missing deaths in later periods could exaggerate the reported trends in disparity reductions. Reassuringly, we failed to find evidence of non-linearities later in follow-up in our sensitivity analysis. Study follow-up through 2018 may not have fully captured mortality effects of screening policies implemented in 2012. Our nSEP measures reflect residential, area-level SEP and may not perfectly capture individual-level SEP. However, evidence from other registries suggests that nSEP and individual-SEP are highly correlated and analyses using either measure often yield similar results51. Finally, data presented here using two population-based state registries may not reflect patterns of disparities in other US states. However, our observation of stage migration implies that our results may partially reflect changes in disparities arising from national policies for screening adopted in 2012, and so may apply to other US contexts.
In conclusion, we report that among men with PCa in two northeastern US states, there have been recent declines in Black-White racial disparities in prostate-specific mortality, but concurrent increases in cardiovascular mortality disparities. We further report that disparities associated with nSEP appear to be increasing across both states, despite implementation of government policies to increase equitable access to health care services. Incorporating neighborhood socioeconomic measures into routine investigation of disparities, greater collaboration between state-level registries and policy researchers, and further consideration of causes of and means for preventing cardiovascular mortality could accelerate progress towards reducing racial and socioeconomic disparities among men with PCa.
Supplementary Material
Acknowledgements
We are grateful to staff at the Massachusetts and Pennsylvania Cancer Registry for facilitating acquisition of the data used in this study. We are also grateful to the men with prostate cancer who agreed to contribute their personal information enabling us to conduct this study.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (T32 CA009001 to HSI, U01 CA184374 to TRR).
Role of the funder
The funder had no role in the design, conduct, analysis, review or decision to submit the manuscript for publication.
Footnotes
Disclosures
The authors declare no competing interests.
Data Availability Statement
The data underlying this article cannot be shared publicly due to cancer registry policies regarding data sharing and patient confidentiality. Please contact the corresponding author for more information about how to acquire these data.
Reference
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: A Cancer Journal for Clinicians. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Kelly SP, Rosenberg PS, Anderson WF, et al. Trends in the Incidence of Fatal Prostate Cancer in the United States by Race. Eur Urol. 2017;71(2):195–201. doi: 10.1016/j.eururo.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamins MR, Hunt BR, Raleigh SM, Hirschtick JL, Hughes MM. Racial Disparities in Prostate Cancer Mortality in the 50 Largest US Cities. Cancer Epidemiology. 2016;44:125–131. doi: 10.1016/j.canep.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 4.Rebbeck TR. Prostate Cancer Disparities by Race and Ethnicity: From Nucleotide to Neighborhood. Cold Spring Harb Perspect Med. Published online December 11, 2017:a030387. doi: 10.1101/cshperspect.a030387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zavala VA, Bracci PM, Carethers JM, et al. Cancer health disparities in racial/ethnic minorities in the United States. British Journal of Cancer. Published online September 9, 2020:1–18. doi: 10.1038/s41416-020-01038-6 [DOI] [Google Scholar]
- 6.Coughlin SS. A review of social determinants of prostate cancer risk, stage, and survival. Prostate International. Published online August 27, 2019. doi: 10.1016/j.prnil.2019.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein J, von dem Knesebeck O. Socioeconomic inequalities in prostate cancer survival: A review of the evidence and explanatory factors. Social Science & Medicine. 2015;142:9–18. doi: 10.1016/j.socscimed.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 8.Marmot M Achieving health equity: from root causes to fair outcomes. The Lancet. 2007;370(9593):1153–1163. doi: 10.1016/S0140-6736(07)61385-3 [DOI] [PubMed] [Google Scholar]
- 9.Williams DR, Lawrence JA, Davis BA. Racism and Health: Evidence and Needed Research. Annu Rev Public Health. 2019;40:105–125. doi: 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braveman P Defining equity in health. Journal of Epidemiology & Community Health. 2003;57(4):254–258. doi: 10.1136/jech.57.4.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moyer VA, U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–134. doi: 10.7326/0003-4819-157-2-201207170-00459 [DOI] [PubMed] [Google Scholar]
- 12.Griffith K, Evans L, Bor J. The Affordable Care Act Reduced Socioeconomic Disparities In Health Care Access. Health Affairs. 2017;36(8):1503–1510. doi: 10.1377/hlthaff.2017.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keisler-Starkey K, Bunch LN. Health Insurance Coverage in the United States: 2019. U.S. Government Publishing Office; 2020. [Google Scholar]
- 14.US Census Bureau. Massachusetts: 2000. U.S. Department of Commerce. Economics and Statistics Administration.; 2002. [Google Scholar]
- 15.US Census Bureau. Pennsylvania: 2000. U.S. Department of Commerce. Economics and Statistics Administration.; 2002. [Google Scholar]
- 16.Epstein MM, Edgren G, Rider JR, Mucci LA, Adami H-O. Temporal Trends in Cause of Death Among Swedish and US Men with Prostate Cancer. J Natl Cancer Inst. 2012;104(17):1335–1342. doi: 10.1093/jnci/djs299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Hemelrijck M, Folkvaljon Y, Adolfsson J, et al. Causes of death in men with localized prostate cancer: a nationwide, population‐based study. BJU Int. 2016;117(3):507–514. doi: 10.1111/bju.13059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341 [DOI] [PubMed] [Google Scholar]
- 19.Krieger N, Feldman JM, Kim R, Waterman PD. Cancer Incidence and Multilevel Measures of Residential Economic and Racial Segregation for Cancer Registries. JNCI Cancer Spectr. 2018;2(1). doi: 10.1093/jncics/pky009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieger N, Wright E, Chen JT, Waterman PD, Huntley ER, Arcaya M. Cancer Stage at Diagnosis, Historical Redlining, and Current Neighborhood Characteristics: Breast, Cervical, Lung, and Colorectal Cancers, Massachusetts, 2001–2015. Am J Epidemiol. doi: 10.1093/aje/kwaa045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieger N, Waterman PD, Spasojevic J, Li W, Maduro G, Van Wye G. Public Health Monitoring of Privilege and Deprivation With the Index of Concentration at the Extremes. Am J Public Health. 2016;106(2):256–263. doi: 10.2105/AJPH.2015.302955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messer LC, Laraia BA, Kaufman JS, et al. The Development of a Standardized Neighborhood Deprivation Index. J Urban Health. 2006;83(6):1041–1062. doi: 10.1007/s11524-006-9094-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler SS, Muralidhar V, Zhao SG, et al. Prostate cancer incidence across stage, NCCN risk groups, and age before and after USPSTF Grade D recommendations against prostate-specific antigen screening in 2012. Cancer. 2020;126(4):717–724. doi: 10.1002/cncr.32604 [DOI] [PubMed] [Google Scholar]
- 24.Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, Rural-Urban, and Racial Inequalities in US Cancer Mortality: Part I-All Cancers and Lung Cancer and Part II-Colorectal, Prostate, Breast, and Cervical Cancers. Journal of cancer epidemiology. 2011;2011:107497. doi: 10.1155/2011/107497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanderWeele TJ, Robinson WR. On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology. 2014;25(4):473–484. doi: 10.1097/EDE.0000000000000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoenfeld D Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. doi: 10.1093/biomet/69.1.239 [DOI] [Google Scholar]
- 27.Young J, Roffers S, Ries L, Fritz A, Hurlbut A. SEER Summary Staging Manual – 2000 Codes and Coding Instructions. National Cancer Institute; 2001. [Google Scholar]
- 28.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 29.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. doi: 10.1037/a0031034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504 [DOI] [PubMed] [Google Scholar]
- 31.Govindarajulu US, Malloy EJ, Ganguli B, Spiegelman D, Eisen EA. The comparison of alternative smoothing methods for fitting non-linear exposure-response relationships with Cox models in a simulation study. Int J Biostat. 2009;5(1):Article 2. doi: 10.2202/1557-4679.1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas AJ, Eberly LE, Davey Smith G, Neaton JD, Stamler J. Race/Ethnicity, Income, Major Risk Factors, and Cardiovascular Disease Mortality. Am J Public Health. 2005;95(8):1417–1423. doi: 10.2105/AJPH.2004.048165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovesdy CP, Norris KC, Boulware LE, et al. Association of Race with Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015;132(16):1538–1548. doi: 10.1161/CIRCULATIONAHA.114.015124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Jiun-Ruey, Duncan Meredith S., Morgans Alicia K., et al. Cardiovascular Effects of Androgen Deprivation Therapy in Prostate Cancer: Contemporary Meta-Analyses. Arteriosclerosis, Thrombosis, and Vascular Biology. 2020;40(3):e55–e64. doi: 10.1161/ATVBAHA.119.313046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. The Lancet. 2017;389(10077):1453–1463. doi: 10.1016/S0140-6736(17)30569-X [DOI] [PubMed] [Google Scholar]
- 36.Williams DR, Collins C. Racial Residential Segregation: A Fundamental Cause of Racial Disparities in Health - David R. Williams, Chiquita Collins, 2001. Public Health Reports. Published online September 1, 2001. Accessed January 18, 2021. http://journals.sagepub.com/doi/abs/10.1093/phr/116.5.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White K, Borrell LN. Racial/ethnic residential segregation: Framing the context of health risk and health disparities. Health & Place. 2011;17(2):438–448. doi: 10.1016/j.healthplace.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shariff-Marco S, Klassen AC, Bowie JV. Racial/Ethnic Differences in Self-Reported Racism and Its Association With Cancer-Related Health Behaviors. Am J Public Health. 2010;100(2):364–374. doi: 10.2105/AJPH.2009.163899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon K, Soni A, Cawley J. The Impact of Health Insurance on Preventive Care and Health Behaviors: Evidence from the First Two Years of the ACA Medicaid Expansions. Journal of Policy Analysis and Management. 2017;36(2):390–417. doi: 10.1002/pam.21972 [DOI] [PubMed] [Google Scholar]
- 40.Jemal A, Lin CC, Davidoff AJ, Han X. Changes in Insurance Coverage and Stage at Diagnosis Among Nonelderly Patients With Cancer After the Affordable Care Act. JCO. 2017;35(35):3906–3915. doi: 10.1200/JCO.2017.73.7817 [DOI] [PubMed] [Google Scholar]
- 41.McCormick D, Hanchate AD, Lasser KE, et al. Effect of Massachusetts healthcare reform on racial and ethnic disparities in admissions to hospital for ambulatory care sensitive conditions: retrospective analysis of hospital episode statistics. BMJ. 2015;350. doi: 10.1136/bmj.h1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moss HA, Wu J, Kaplan SJ, Zafar SY. The Affordable Care Act’s Medicaid Expansion and Impact Along the Cancer-Care Continuum: A Systematic Review. J Natl Cancer Inst. 2020;112(8):779–791. doi: 10.1093/jnci/djaa043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boscoe FP, Zhang X. Visualizing the Diffusion of Digital Mammography in New York State. Cancer Epidemiol Biomarkers Prev. 2017;26(4):490–494. doi: 10.1158/1055-9965.EPI-16-0928 [DOI] [PubMed] [Google Scholar]
- 44.Shenoy D, Packianathan S, Chen AM, Vijayakumar S. Do African-American men need separate prostate cancer screening guidelines? BMC Urol. 2016;16(1):19. doi: 10.1186/s12894-016-0137-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts MC, Khoury MJ, Mensah GA. Perspective: The Clinical Use of Polygenic Risk Scores: Race, Ethnicity, and Health Disparities. Ethn Dis. 2019;29(3):513–516. doi: 10.18865/ed.29.3.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fletcher SA, Marchese M, Cole AP, et al. Geographic Distribution of Racial Differences in Prostate Cancer Mortality. JAMA Netw Open. 2020;3(3). doi: 10.1001/jamanetworkopen.2020.1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krieger N, Chen JT, Kosheleva A, Waterman PD. Shrinking, widening, reversing, and stagnating trends in US socioeconomic inequities in cancer mortality for the total, black, and white populations: 1960–2006. Cancer Causes Control. 2012;23(2):297–319. doi: 10.1007/s10552-011-9879-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rebbeck TR, Halbert CH, Sankar P. Genetics, Epidemiology, and Cancer Disparities: Is it Black and White? JCO. 2006;24(14):2164–2169. doi: 10.1200/JCO.2005.05.1656 [DOI] [PubMed] [Google Scholar]
- 49.Pernar CH, Ebot EM, Wilson KM, Mucci LA. The Epidemiology of Prostate Cancer. Cold Spring Harb Perspect Med. 2018;8(12):a030361. doi: 10.1101/cshperspect.a030361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson CJ, Weir HK, Fink AK, et al. The impact of National Death Index linkages on population-based cancer survival rates in the United States. Cancer Epidemiol. 2013;37(1):20–28. doi: 10.1016/j.canep.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krieger N Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–710. doi: 10.2105/AJPH.82.5.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


