To the Editor,
Atopic eczema (or atopic dermatitis) is a chronic relapsing inflammatory skin disease affecting 15–30% of children worldwide1. Although previous studies have attempted to link higher prenatal exposure to particulate matter with childhood eczema,2–6 most studies examined particulate matter with an aerodynamic diameter of 10 μm or less (PM10), but not particulate matter with an aerodynamic diameter of 2.5 μm or less (PM2.5), and yielded inconsistent results. Sensitive windows of prenatal exposure to PM2.5 for eczema development remain unclear. Here, we aimed to explore sensitive windows for effects of weekly average PM2.5 exposure during gestation on the development of childhood eczema.
In this case-control study, we used the data derived from the study population including 1,128 full-term children (mean age 6.4 years, 56% boys) who participated in the Longitudinal Investigation of Global Health in Taiwanese Schoolchildren (LIGHTS) cohort. Eczema was defined as having physician-diagnosed eczema and presence of eczema in the last 12 months through a modified International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire provided by parents/guardians of the study participants.7 We applied a distributed lag nonlinear model to examine the exposure-lag-response association of eczema with mean weekly PM2.5 estimates using a highly spatial-temporal resolution hybrid kriging/land-use regression model. The flow diagram for subject recruitment is shown in Figure S1. Seasonal averaged PM2.5 concentrations surface maps corresponding to residential address during pregnancy are shown in Figure S2. Detailed methods are provided in the Supporting information.
Table 1 shows the characteristics of 1,128 study children; 216 (19.1%) had eczema. Age at onset of eczema was distributed as follows: 67 (31.5%) during the first year of life; 41 (19.2%) during 1–2 years of age; 69 (32.4%) during 2–5 years of age; and 36 (16.9%) after 5 years of age. Figure 1 depicts the main findings of this study. We observed a significant association of childhood eczema with increased exposure to PM2.5 during gestational weeks 7 to 17, with the highest risk at gestational week 12 (adjusted odds ratios [AOR]=1.10 per 10 μg/m3; 95% CI=1.03–1.18) (Figure 1), after adjustment for child’s age, sex, body mass index (BMI), atopy, parental allergic disease, birth season, ambient temperature and relative humidity. In gestational week 12, the concentration-response relationship indicated that the AOR of childhood eczema was significantly higher than 1.0 at PM2.5 concentrations greater than 21.2 μg/m3 (Figure S3).
Table 1.
Characteristics of study children (n=1,128).
| Characteristic | All children | Eczema (n=216) | No eczema (n= 912) | P |
|---|---|---|---|---|
| Sex, boy, n (%) | 632/1,128 (56.0) | 121/216 (56.0) | 511/912 (56.0) | 0.99 |
| Age, years (mean ± SD) | 6.4±0.4 | 6.4±0.4 | 6.4±0.4 | 0.35 |
| Height, cm (mean ± SD) | 118.7±5.6 | 118.5±5.9 | 118.7±5.5 | 0.68 |
| Weight, kg (mean ± SD) | 22.4±4.7 | 22.9±5 | 22.3±4.7 | 0.10 |
| Body mass index, kg/m2 (mean ± SD) | 15.8±2.4 | 16.2±2.7 | 15.7±2.3 | 0.01 |
| Atopy, n (%) | 716/1,089 (65.8) | 163/205 (79.5) | 553/912 (62.6) | <0.001 |
| Breastfeeding, n (%) | 566/1,128(50.2) | 114/216(52.8) | 452/912(49.6) | 0.39 |
| Prenatal environmental tobacco smoke, n (%) | 472/1,128 (41.8) | 84/216 (38.9) | 388/912 (42.5) | 0.32 |
| Parental history of asthma, n (%) | 125/1,121 (11.2) | 37/213 (17.4) | 88/908 (9.7) | 0.001 |
| Parental history of allergic rhinitis, n (%) | 715/1,127 (63.4) | 159/216 (73.6) | 556/911 (61.0) | 0.001 |
| Parental history of atopic dermatitis, n (%) | 301/1,112 (27.1) | 81/214 (37.9) | 220/898 (24.5) | <0.001 |
| Winter (December to February) | 269/1,128(23.85) | 52/216 (24.1) | 217/912 (23.8) | |
| PM2.5, μg/m3 (median/IQR) | 26.1/4.3 | 26.7/4.6 | 26.0/4.3 | 0.08 |
| Ambient temperature, °C (median/IQR) | 22.3/2.9 | 22.4/2.9 | 22.3/3.0 | 0.95 |
| Relative humidity, % (median/IQR) | 76.5/1.6 | 76.6/1.8 | 76.4/1.5 | 0.33 |
Abbreviation: SD: standard deviation; PM2.5: particulate matter with an aerodynamic diameter of 2.5 μm or less; IQR: interquartile range.
Figure 1.
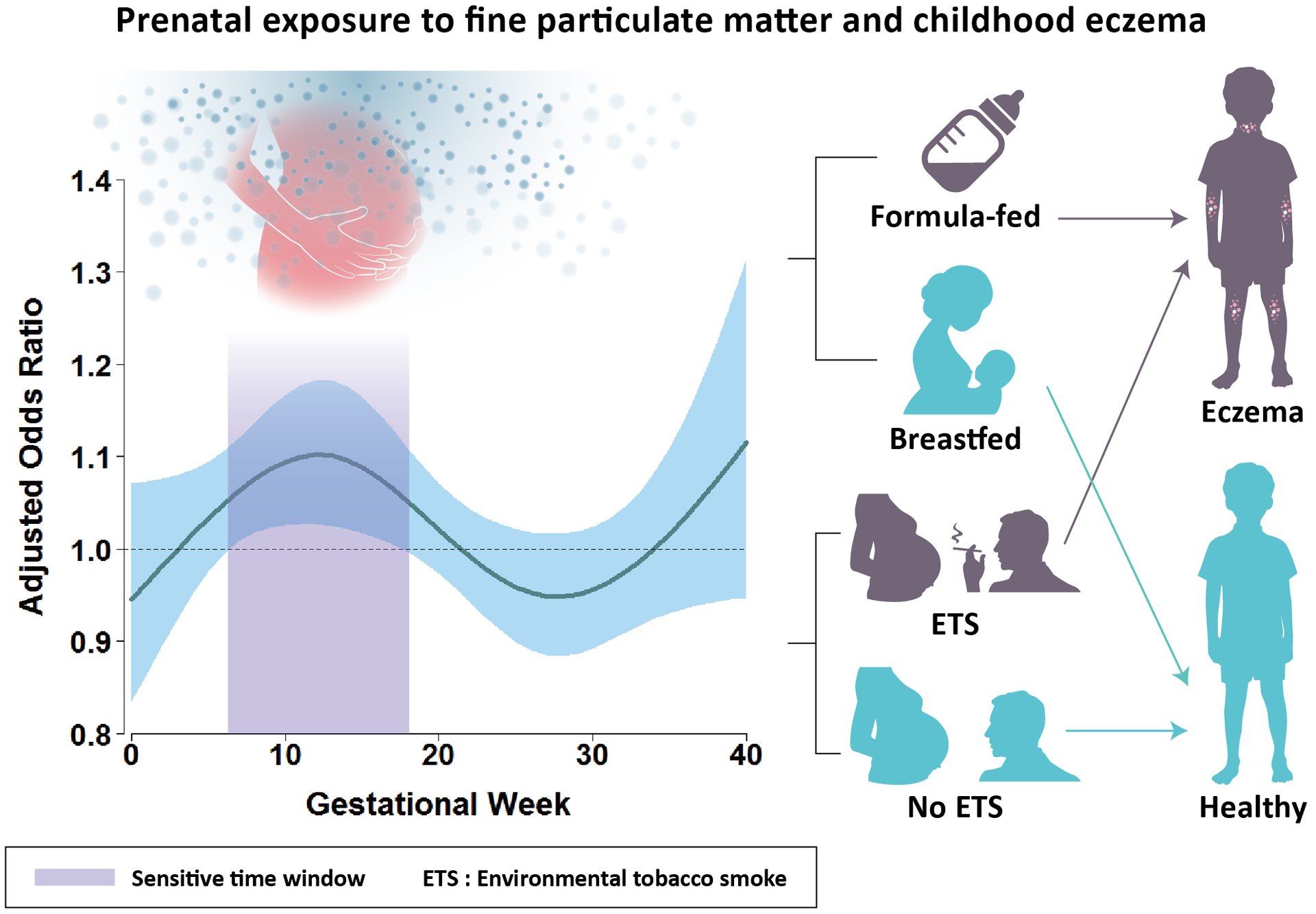
Increased exposure to PM2.5 during gestational weeks 7 to 17 was significantly associated with an increased risk of childhood eczema and this risk was largely confined to children who were not breastfed or exposed to prenatal environmental tobacco smoke. Note: A distributed lag nonlinear model was applied to explore sensitive windows for the effects of weekly average PM2.5 exposure during gestation on the development of eczema by age 6 years, adjusting for child’s age, gender, body mass index, atopy, parental allergic disease, birth season, ambient temperature, and relative humidity. The y-axis shows the adjusted odds ratio of eczema in relation to a 10 μg/m3 increase in prenatal PM2.5 exposure; the x-axis depicts gestational age in weeks. The solid line indicates the estimated odds ratio and the shading area represents the 95% confidence interval. A sensitive window is identified when the estimated pointwise 95% confidence interval of odds ratio does not include 1.0. Abbreviations: PM2.5: particulate matter with an aerodynamic diameter of 2.5 μm or less.
We further stratified the analysis by breastfeeding and exposure to prenatal environmental tobacco smoke (ETS). When stratified by breastfeeding, we observed a significant sensitive exposure window between 8 and 18 gestational weeks among children who were not breastfed, but not among children who were breastfed (Figure S4A & S4B). Exposure to prenatal ETS might accentuate the harmful effect of PM2.5, as a significant sensitive exposure window between 6 and 11 gestation weeks was found among children exposed to prenatal ETS, but not among children not exposed to prenatal ETS (Figure S4C & S4D).
This study has identified, for the first time, a sensitive window of exposure to PM2.5 at gestational weeks 7 to 17 on the risk of developing childhood eczema, which may provide insight into underlying mechanisms. Developmental periods of skin have been documented as follows: embryonic (gestational weeks 5–8), epidermal stratification (gestational weeks 9–14), follicular keratinization (gestational weeks 14–24), and interfollicular keratinization (after gestational week 24) periods.8 The epidermal barrier does not form in the human fetus until 20–24 gestational weeks,9 making the period before gestational week 20 as a critical window where the fetus may be highly vulnerable to the harmful effects of PM2.5 diffusing into the placental barrier. The sensitive window of exposure identified in the present study coincides with the embryonic, epidermal stratification, and follicular keratinization periods of skin development, and particularly, coincides with the crucial period of vulnerability from the beginning of embryonic skin development at gestational week 5 to the initiation of epidermal barrier formation at gestational weeks 20–24. One potential explanation might be due to dysregulation of filaggrin, a key protein involved in skin barrier function and maintenance of skin integrity. Human studies have demonstrated that filaggrin expresses simultaneously with the morphologic occurrence of keratinization at gestational week 15 in follicles and gestational weeks 22–24 in the interfollicular epidermis.8 Since the expression of filaggrin is influenced by exogenous stressors, such as systemic inflammatory mediators, oxidative stress and Th2 inflammatory responses, it is possible that filaggrin expression might be dysregulated by prenatal exposure to particulate matter during the sensitive time-window, subsequently, contributing to the development of childhood eczema.10
This is the first study to provide evidence linking prenatal exposure to ambient PM2.5 above a threshold concentration of 21.2 μg/m3 to the development of childhood eczema. In a meta-analysis of more than 46,100 subjects from 13 studies, human skin could be adversely affected when PM2.5 concentrations reached upwards 26.04 μg/m3.11 Our findings provide further evidence for the adverse effects of particulate matter on skin in developing fetus at a slightly lower threshold concentration, compared to previously reported threshold concentrations for the detrimental effects of PM2.5 on human skin in children and adults.11
This study adds new evidence to the literature by suggesting that exclusive breastfeeding during the first 3 months of life or longer may reduce the risk of developing eczema from prenatal exposure to PM2.5. One possible explanation is that breast milk contains many immunomodulatory factors, which may more effectively promote the programming of the infant’s developing immune system than infant formula and countervail the harmful effects of air pollution. Mukherjee and colleagues have reported that breastfeeding modified the effect of smoking during pregnancy on eczema in offspring in the Isle of Wight birth cohort.12 Zhang et al. have found that breastfeeding was associated with lower risk of lung function impairment among children exposed to air pollution.13
Our results suggest that prenatal exposure to ETS may attenuate host response to fine particulate matter pollution, leading to the development of childhood eczema, as the threshold of prenatal exposure to PM2.5 on eczema risk decreased from 21.2 μg/m3 to 12 μg/m3 in the presence of prenatal ETS exposure. Similar to our findings, a synergistic effect of combined exposure to prenatal PM2.5 and postnatal ETS on the development of infantile eczema was reported in a previous birth cohort.3
This study primarily focus on investigating the influence of prenatal PM2.5 exposure on risk of childhood eczema. Further investigation will be needed to uncover sensitive windows of PM2.5 exposure during the postnatal period. A limitation of this study is that we did not examine the associations of prenatal exposure to PM2.5 with severity or duration of eczema because detailed data on severity and age at onset of eczema were not available.
In conclusion, this study lends further evidence linking prenatal exposure to PM2.5 during 7 to 17 gestational weeks to an increased risk of developing childhood eczema by age 6 years, with the risk largely confined to children who were not breastfed or exposed to prenatal ETS.
Supplementary Material
Acknowledgements:
The authors thank the study participants and their parents for their active participation in the study. The authors also thank Po-Hsiu Lin for preparing figures.
Funding sources:
This work was supported by the Ministry of Science and Technology of Taiwan (PI: Tsung-Chieh Yao, MOST 106-2314-B-182-051-MY3 and MOST 104-2314-B-182-046-MY2; Hui-Ju Tsai, MOST 107-2314-B-400-031-MY3), by Chang Gung Medical Foundation, Taiwan (PI: Tsung-Chieh Yao, CMRPG3E1201~5, CMRPG3F1711~3, CORPG3F0361, CMRPG3J0121, and CMRPG3J1711), and by the National Institutes of Health, U.S.A. (PI: Yvonne J. Huang, R01AI129958 and R03HL138310).
Footnotes
Conflict of interest: The authors declare no conflict of interest.
REFERENCES
- 1.Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361(9352):151–160. [DOI] [PubMed] [Google Scholar]
- 2.Lee JY, Lamichhane DK, Lee M, et al. Preventive Effect of Residential Green Space on Infantile Atopic Dermatitis Associated with Prenatal Air Pollution Exposure. Int J Environ Res Public Health. 2018;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jedrychowski W, Perera F, Maugeri U, et al. Effects of prenatal and perinatal exposure to fine air pollutants and maternal fish consumption on the occurrence of infantile eczema. Int Arch Allergy Immunol. 2011;155(3):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang CC, Wen HJ, Chen PC, Chiang TL, Lin SJ, Guo YL. Prenatal air pollutant exposure and occurrence of atopic dermatitis. Br J Dermatol. 2015;173(4):981–988. [DOI] [PubMed] [Google Scholar]
- 5.Liu W, Cai J, Huang C, et al. Associations of gestational and early life exposures to ambient air pollution with childhood atopic eczema in Shanghai, China. Sci Total Environ. 2016;572:34–42. [DOI] [PubMed] [Google Scholar]
- 6.Lu C, Deng L, Ou C, Yuan H, Chen X, Deng Q. Preconceptional and perinatal exposure to traffic-related air pollution and eczema in preschool children. J Dermatol Sci. 2017;85(2):85–95. [DOI] [PubMed] [Google Scholar]
- 7.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–491. [DOI] [PubMed] [Google Scholar]
- 8.Dale BA, Holbrook KA, Kimball JR, Hoff M, Sun TT. Expression of epidermal keratins and filaggrin during human fetal skin development. J Cell Biol. 1985;101(4):1257–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardman MJ, Moore L, Ferguson MW, Byrne C. Barrier formation in the human fetus is patterned. J Invest Dermatol. 1999;113(6):1106–1113. [DOI] [PubMed] [Google Scholar]
- 10.Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014;134(4):792–799. [DOI] [PubMed] [Google Scholar]
- 11.Ngoc LTN, Park D, Lee Y, Lee YC. Systematic Review and Meta-Analysis of Human Skin Diseases Due to Particulate Matter. International Journal of Environmental Research and Public Health. 2017;14(12):1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukherjee N, Sutter TR, Arshad SH, Holloway JW, Zhang H, Karmaus W. Breastfeeding duration modifies the effect of smoking during pregnancy on eczema from early childhood to adolescence. Clin Exp Allergy. 2018;48(12):1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Guo Y, Xiao X, et al. Association of Breastfeeding and Air Pollution Exposure With Lung Function in Chinese Children. JAMA Netw Open. 2019;2(5):e194186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


