Summary
Background:
Pneumococcus remains an important cause of morbidity in pregnant women with HIV and their infants. We compared the safety and immunogenicity of PCV-10 and PPV-23 with placebo administered in pregnancy.
Methods:
Double-blind, multi-site study of women with HIV on antiretrovirals randomized 1:1:1 to PCV-10, PPV-23 or placebo at 14–34 weeks gestational age (NCT02717494). The study was conducted in Brazil, between 01/04/2016 and 30/11/2017. Antibodies against 7 vaccine serotypes in PCV-10 and PPV-23 were measured by ELISA. The primary outcomes were maternal adverse events grade ≥3 in the first 4 weeks after vaccination and infant at birth; maternal seroresponse (≥2-fold increase in antibodies from pre- to post-vaccination) against ≥5 serotypes; and infant seroprotection (antibodies ≥0.35μg/ml) against ≥5 serotypes through 8 weeks of life. The study was powered to detect differences ≥20% in the primary immunologic outcomes between treatment groups.
Findings:
Adverse events were similar across treatment groups. Seroresponses against ≥5 serotypes were present in 74/114 (65%) PCV-10, 72/110 (65%) PPV-23 and 0/113 (0%) placebo recipients at 4 weeks post-vaccination (p<0.0001). Seroresponse differences ≥20% in vaccine compared with placebo recipients persisted up to 24 weeks postpartum. At birth, 76/113 (67%) infants in PCV-10, 62/109 (57%) in PPV-23 and 19/115 (17%) in placebo groups had seroprotection against ≥5 serotypes (p<0.0001). At 8 weeks, the outcome was met by 20/108 (19%) infants in PCV-10, 24/104 (23%) in PPV-23 and 1/109 (1%) in placebo groups (p<0.0001). Although a difference ≥20% compared with placebo was observed only in PPV-23 infants at 8 weeks of life, the difference between the two vaccine groups was not appreciable.
Interpretation:
PCV-10 and PPV-23 were equally safe and immunogenic in pregnant women with HIV and conferred similar levels of seroprotection to their infants. In areas where childhood PCV administration decreased the circulation of PCV serotypes, PPV-23 administration to pregnant women with HIV may be more advantageous compared to PCV by virtue of including a broader range of serotypes.
Introduction
Pneumococcus (PNC) has been a leading cause of bacterial pneumonia in pregnant and non-pregnant adults with HIV1. Even with the widespread use of antiretroviral therapy (ART), the incidence of invasive pneumococcal disease (IPD) and pneumonia remains higher in people with HIV compared with same-age adults without HIV2. IPD and PNC pneumonia are vaccine-preventable conditions to a large extent3. There are currently two types of vaccines available against PNC: a polysaccharide vaccine represented by the 23-valent (PPV-23) and two conjugated vaccines, 10- or 13-valent (PCV-10 or -23). The efficacy of PPV-23 in preventing IPD and all-cause pneumonia in people with HIV was shown in several non-randomized studies, but the only prospective, randomized, placebo-controlled trial showed a decrease in all-cause mortality but not in all-cause pneumonia4,5. The efficacy of PCV was demonstrated both in children and adults with HIV6,7. Both PPV-23 and PCV were shown to be safe and immunogenic in people with HIV8. However, individuals with lower CD4+ T cells and/or higher HIV plasma RNA had higher antibody responses to PCV compared with PPV-238. Pregnancy is associated with immunosuppression, which is designed to protect the fetus against allogeneic rejection. However, prior to this study, there was one report on the immunogenicity of PPV-23 in pregnant women with HIV and no data for PCV9.
Although the administration of PNC vaccines to adults with HIV has been recommended in the US for over 25 years, a survey of the International Maternal, Pediatric and Adolescent AIDS Clinical Trials (IMPAACT) clinical sites conducted prior to the inception of this study showed that pneumococcal vaccination was generally not performed during pregnancy3. Notably, ~25% women are first diagnosed with HIV in the US during pregnancy10. This constitutes a missed opportunity, because bacterial pneumonias are more common in pregnant women than in non-pregnant adults with HIV and are associated with increased risk of maternal morbidity and mortality and adverse pregnancy outcome1. The most common barrier against any vaccine administration during pregnancy is the potential risk of adverse pregnancy outcome perceived by mothers and their health care providers11.
In addition to maternal protection against infection, vaccination of pregnant women results in transplacental transport of maternal antibodies that may protect infants in the first few months of life. This has been clearly demonstrated for influenza and pertussis and has been proposed for other infections, including PNC12,13. Although the introduction of PCV in the childhood schedule of immunization has generally decreased the incidence of severe PNC disease in children ≤2 years of age, infants exposed in utero to HIV still have an increased risk of severe pneumonia and IPD irrespective of their HIV infection status14. Transplacental transport of pathogen-specific maternal antibodies decreases in the context of HIV infection and this may contribute to the high risk of IPD and severe pneumonia in HIV-exposed infants15. This could potentially be mitigated by increasing maternal anti-PNC antibody concentrations through immunization.
To address the important knowledge gap surrounding anti-PNC immunization in pregnant women with HIV, we designed a randomized, double-blind, placebo-controlled trial to determine the safety, immunogenicity and transplacental transport of maternal antibodies in women with HIV vaccinated against PNC during pregnancy. The underlying hypotheses were that PPV-23 and PCV-10 were safe and increased maternal PNC antibodies compared to placebo.
Methods
Study design and participants.
This was a 1:1:1 randomized, double-blind trial of PCV-10 (Glaxo Smith-Kline), PPV-23 (Merck) and placebo (NCT02717494; status completed). The study was conducted at 8 NICHD clinical trial sites in Brazil under approval of national and local regulatory review boards. All participants signed informed consents before enrollment. The study planned to enroll 345 pregnant women with HIV, between ≥14 and <34 weeks gestational age. Key eligibility criteria were HIV infection, pregnancy between ≥14 and <34 weeks gestational age, being on ART at study entry, and ability to give informed consent. The gestational age at entry was assessed by ultrasound or date of last menstrual period. Immunization status was assessed by review of medical history and vaccination record. Data were not collected on study candidates who declined participation.
Randomization and masking.
Randomization used permuted block allocation (block size 4) generated by a web-based, central computer system. Syringes filled with the study product were wrapped in aluminum foil and labeled with the participant’s study identification by the pharmacist before delivering the product to the clinic staff. This ensured that participants and study teams, with the exception of the pharmacist, were unaware of treatment allocation. After unblinding, the site principal investigators received information on treatment allocations and letters were sent to participants notifying them of the treatment received.
Procedures.
In the first part of the study, which is reported in this manuscript, participants received one dose of PCV, PPV or placebo at entry. In the second part, reported elsewhere, placebo recipients were randomized at 24 weeks postpartum to receive PPV-23 or PCV-10. Adverse events were collected at each study visit. Maternal blood obtained at entry, 4 weeks after immunization, at delivery and 24 weeks postpartum was used to measure anti-PNC antibodies. Complete blood cell counts, CD4 cell numbers and HIV plasma RNA were assessed at entry and week 4. Infant blood obtained at 0, 8, 16 and 24 weeks of life was used for anti-PNC antibodies. Nasopharyngeal swabs collected in STGG broth at entry and delivery in mothers and at 8 weeks and 16 weeks of life in infants were used to measure PNC colonization.
Serum antibodies for serotypes 4, 7F, 23F and 33F were measured by enzyme-linked immunosorbent (ELISA) in all participants and by opsonophagocytic assays (OPA) in a subset of 60 randomly selected mother-infant pairs at the WHO reference laboratory at the University College of London using methods previously described16. Antibodies for serotypes 1, 5, 6B and 14 were measured at the University of Colorado Denver Anschutz Medical Campus using a chemiluminescent multiplex assay (MesoScaleDiscovery) as previously described17.
Nasopharyngeal swabs collected in skim milk, tryptone, glucose, and glycerin medium were used for PNC isolation as previously described18. Serotypes were determined by Quellung reaction using serotype-specific antisera (Serum Statens Institute) on pure isolate cultures.
Outcomes.
The primary outcomes were safety, defined by the incidence of grade ≥3 AEs in mothers or in neonates, and immunogenicity in women and infants. The primary immunologic outcome in mothers was seroresponse defined by a ≥2-fold increase in anti-PNC antibody concentrations against ≥5 serotypes from pre- to post-immunization. In infants, the primary immunologic outcome was seroprotection defined by anti-PNC antibody concentrations ≥0.35 μg/ml against ≥5 serotypes up to 8 weeks of life. Secondary immunologic outcomes were antibody concentrations, transplacental transport of maternal antibodies measured by the ratio between antibody concentrations in infants at birth and mothers at delivery (B:M), maternal antibody decay in infants and correlation between OPA and ELISA antibodies in mothers and infants. We examined maternal and infant PNC nasopharyngeal colonization as an exploratory objective.
Statistical analysis
The study was powered (≥80% with α=0.05) to detect ≥20% differences in maternal seroresponse and infant seroprotection between any 2 treatment groups. All women who received study treatment and all infants who were born alive on study were included in the safety summaries. Analyses of antibody data for women were restricted to women who delivered ≥4 weeks after vaccination. For the primary outcomes, we performed overall comparisons among all three treatment arms using a Chi-square test. When these overall tests were significant, we examined pairwise comparisons, using Fisher’s Exact test, due to low counts in many cells. We used Spearman correlations to assess relationships between ELISA-measured IgG and OPA antibodies. Statistical analyses were performed using SAS (SAS Institute, Inc., Cary, NC, USA) version 9.4.
Role of the funding source
The funder of the study had no role in study design, data collection, analysis, interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between 01/04/2016 and 30/11/2017, we enrolled 347 pregnant women with HIV, including 346 who received study treatment (Fig 1). The number of study candidates who declined participation was not recorded. At entry, participants had mean age of 27.6 years, median 26 weeks gestational age, and median of 547.5 CD4+ T cells per μL, including 95% with CD4+ T cells >200 cells per μL and 64% with plasma HIV RNA below the lower level of quantitation of 40 copies per mL (Table 1). All women received ART with ≥3 drugs from ≥2 classes per inclusion criteria.
Figure 1.

Consort diagram
Table 1:
Maternal Characteristics at Enrollment
| Treatment arm | |||||
|---|---|---|---|---|---|
| Characteristic | PCV-10 (N=115) | PPV-23 (N=115) | Placebo (N=116) | Total (N=346) | |
| Race | Black | 51 (44%) | 57 (50%) | 61 (53%) | 169 (49%) |
| White | 24 (21%) | 19 (17%) | 25 (22%) | 68 (20%) | |
| American Indian | 0 (0%) | 0 (0%) | 1 (1%) | 1 (0%) | |
| Other | 40 (35%) | 36 (31%) | 28 (24%) | 104 (30%) | |
| Unknown | 0 (0%) | 3 (3%) | 1 (1%) | 4 (1%) | |
| Ethnicity | Hispanic or Latino | 112 (97%) | 110 (96%) | 113 (97%) | 335 (97%) |
| Not Hispanic or Latino | 1 (1%) | 1 (1%) | 1 (1%) | 3 (1%) | |
| Unknown | 2 (2%) | 4 (3%) | 2 (2%) | 8 (2%) | |
| Age at randomization (years) | Mean (SD) | 27.3 (6.1) | 27.7 (6.2) | 27.8 (6.2) | 27.6 (6.1) |
| Gestational age (wks) | Median (Q1-Q3) | 26 (22–30) | 26 (21–30) | 25.5 (22–30) | 26 (22–30) |
| CD4+ T cells per μL | Median (Q1-Q3) | 568 (433–742) | 528 (405–745) | 513.5 (366–715.5) | 547.5 (399–732) |
| CD4+ T cells >200 cells per μL | Number (%) | 109 (95%) | 113 (98%) | 107 (92%) | 329 (95%) |
| RNA copies per mL of plasma | Median (Q1-Q3) | 39 (39–96) | 39 (39–103) | 39 (39–57) | 39 (39–90) |
| RNA < 40 copies per mL of plasma | Number (%) | 72 (63%) | 69 (62%) | 81 (71%) | 222 (64%) |
The incidence of grade ≥3 adverse events, including those considered by the team as treatment-related before the unblinding, was similar in vaccine and placebo recipients (Tables 2 and S1; page 1). However, injection site and systemic grade 2 adverse reactions were reported more frequently in the first 4 weeks after treatment by vaccine compared with placebo recipients (Table 2). The CD4 counts did not decrease and the HIV plasma RNA copies/ml did not increase after vaccination in any of the treatment groups (data not depicted). Pregnancy outcomes were not appreciably different across groups, with the exception of an unexpectedly low rate of preterm birth of 2% in the PCV-10 group compared with 13% and 12% in the PPV-23 and placebo groups. There was one case of pneumonia in a placebo recipient and no IPD reported in mothers. None of the infants acquired HIV infection. The incidence of infant grade ≥3 adverse events, congenital anomalies and pneumonia and/or sepsis were similar across treatment groups. The infant cases of pneumonia or sepsis did not have etiologic diagnoses.
Table 2.
Incidence of maternal and infant serious adverse events and pregnancy outcomes
| Maternal Adverse Events | PCV-10 (N=115)* | PPV-23 (N=115) | Placebo (N=116) |
|---|---|---|---|
| Incidence (95% CI) of Grade 2, ≤4 weeks post-vaccination | 40% (32%,48%), | 38% (31%,46%) | 34% (26%,42%) |
| Injection site and systemic reactions | 14% (9%,20%) | 7% (4%,12%) | 3% (1%,7%) |
| Incidence (95% CI) of Grade ≥3, ≤4 weeks post-vaccination | 3% (1%,7%) | 2% (0%,5%) | 3% (1%,8%) |
| Treatment-related | 1% (0%,4%) | 1% (0%,4%) | 1% (0%,4%) |
| Incidence of (95% CI) Grade ≥4, >4 weeks post-vaccination | 2% (0%,5%) | 1% (0%,4%) | 3% (1%,8%) |
| Pregnancy outcomes | PCV-10 | PPV-23 | Placebo |
| Mean (s.d.) gestational weeks at birth | 38.7 (1.1) N=98 | 38.4 (1.8) N=98 | 38.1 (1.6) N=103 |
| N preterm birth/ N records (%) | 2/98 (2%) | 13/98 (13%) | 12/103 (12%) |
| N stillbirth or miscarriage | 0 | 2 | 0 |
| N small for gestational age/ N records (%) | 7/109 (6%) | 10/105 (10%) | 14/111 (13%) |
| Mean weight in grams (s.d.) at birth | 3110 (422.9) | 3074.1 (561.5) | 2949.6 (529.2) |
| Infant adverse events* | PCV-10 (N=116) | PPV-23 (N=112) | Placebo (N=119) |
| HIV infection | 0 | 0 | 0 |
| Incidence (95% CI) of Grade ≥3 | 20% (14%,27%) | 21% (14%,28%) | 20% (14%,27%) |
| Incidence (95% CI) of Congenital Anomalies | 22% (15%,29%) | 17% (11%,24%) | 13% (8%,19%) |
| Incidence (95% CI) of Pneumonia and Sepsis | 7% (3%,12%) | 4% (2%,9%) | 7% (3%,12%) |
Numbers in the column headers correspond to the number of infants born alive on study, including 1 pair of twins in PCV-10 arm and 3 pairs in the placebo arm.
The primary maternal immunologic outcome, seroresponse against ≥5 vaccine serotypes, was met by 74/114 (65%) women in PCV-10, 72/110 (65%) in PPV-23 and 0/113 (0%) in placebo group at 4 weeks after immunization (overall p<0.0001; Table 3). Seroresponses and differences across treatment groups persisted at delivery and 24 weeks postpartum (Fig S1; page 2). Since contrary to our expectation seroresponses did not differentiate PCV-10 and PPV-23 recipients, we placed additional emphasis on the analysis of the magnitude of antibody concentrations across treatment groups due to its increased granularity. Antibody concentrations were similar across groups at entry and became significantly higher in vaccine compared to placebo recipients for all serotypes in each vaccine at 4 weeks post-immunization (Fig 2). In vaccinees, antibody concentrations declined from week 4 post-immunization to delivery and week 24 postpartum. In placebo recipients, antibody concentrations did not appreciably change during pregnancy and slightly increased postpartum, most likely due to reversion of the dilutional effect of pregnancy on antibody concentrations. The comparison between the two vaccines at 4 weeks after immunization showed lower antibody concentrations against serotype 1 (p=0.003) and higher antibody concentrations against serotype 4 (p≤0.004) in PCV-10 compared with PPV-23 recipients. PCV-10 recipients also had lower antibody levels against serotype 33F (p<0.001) which is included only in PPV-23. The differences between PCV-10 and PPV-23 recipients persisted at delivery and 24 weeks postpartum.
Table 3.
Frequency of participants meeting the primary outcome measure of seroresponse* against ≥5 vaccine serotypes at 4 weeks after vaccination.
| Group | N | Frequency per group (95% CI) | Groups compared | % Difference (95% CI) | P value# |
|---|---|---|---|---|---|
| Vaccine PCV-10 | 114 | 65% (55%,74%) | PCV-23 vs. PCV-10 | 1% (−12%, 13%) | 1.00 |
| Vaccine PPV-23 | 110 | 65% (56%,74%) | PCV-10 vs. Placebo | 65% (55%,74%) | <0.0001 |
| Placebo | 113 | 0% (0%,3%) | PCV-23 vs. Placebo | 65% (56%,74%) | <0.0001 |
Seroresponse was defined by ≥2-fold increase in antibody concentrations at 4 weeks post-vaccination compared with pre-vaccination.
Fisher’s Exact Test.
Figure 2. Kinetics of the anti-PNC antibody serum concentrations in vaccine and placebo recipients.

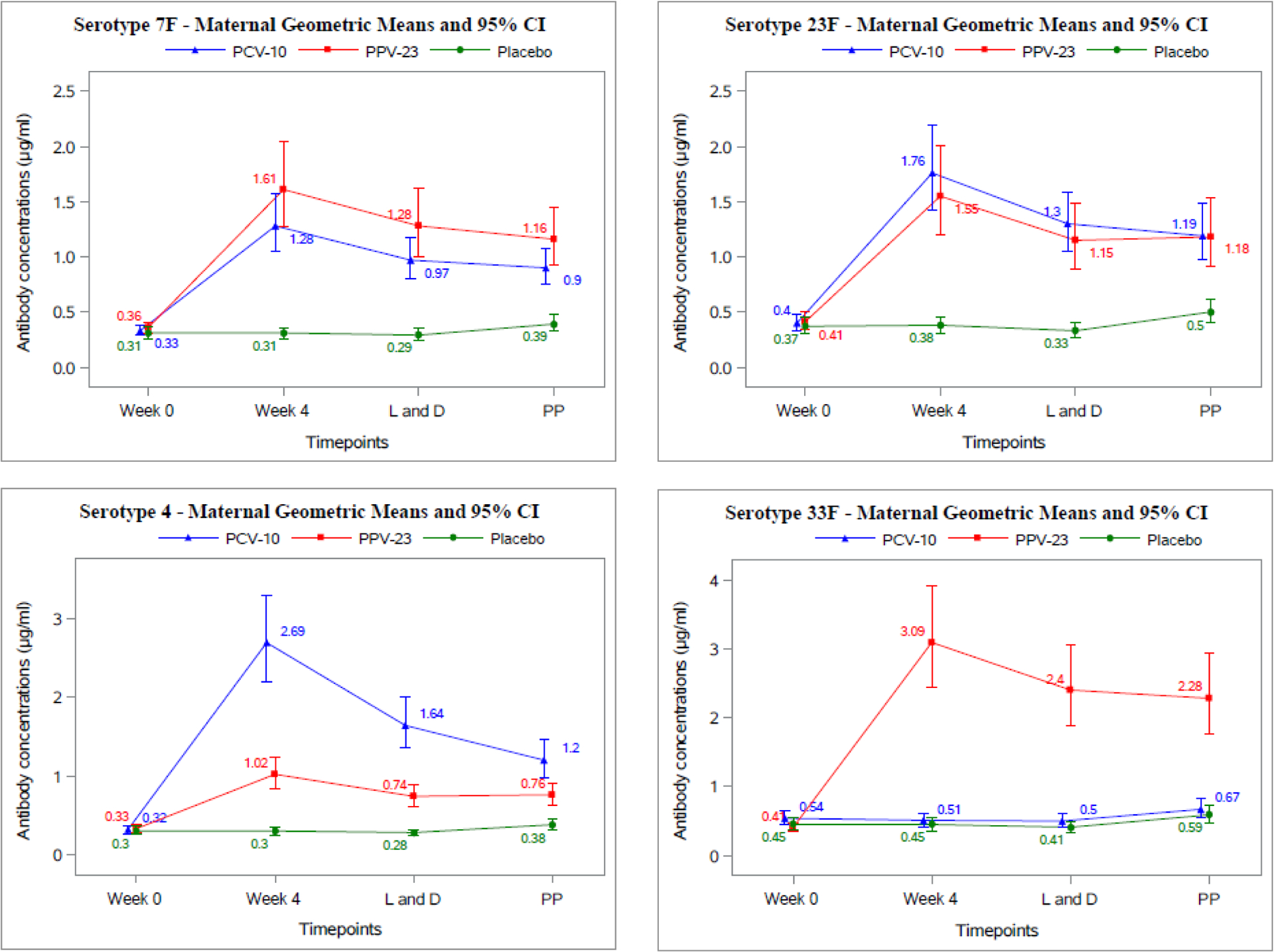
Data were derived from 346 pregnant women with HIV who received PCV-10, PPV-23 or placebo at week 0. The abscissa shows the time points of each measurement. The ordinate shows antibody concentrations in μg/ml. Abbreviations: L and D=delivery; PP= week 24 postpartum.
At birth, 76/113 (67%) infants in PCV-10, 62/109 (57%) in PPV-23 and 19/115 (17%) in placebo groups, had seroprotection against ≥5 serotypes (p<0.0001; Fig 3). However, at 8 weeks of life, due to the decay of maternal antibodies, only 19% of infants in the PCV-10, 23% in the PPV-23 and 1% in the placebo groups had seroprotection against ≥5 serotypes (p<0.0001; Table 4). Although a difference ≥20% in the primary outcome measure at 8 weeks of life was detected only in the PPV-23 group, it is important to note that there were no appreciable differences in the primary outcome measure between the 2 vaccine groups.
Figure 3. Proportions of infants with seroprotection at 0 and 8 weeks of life.
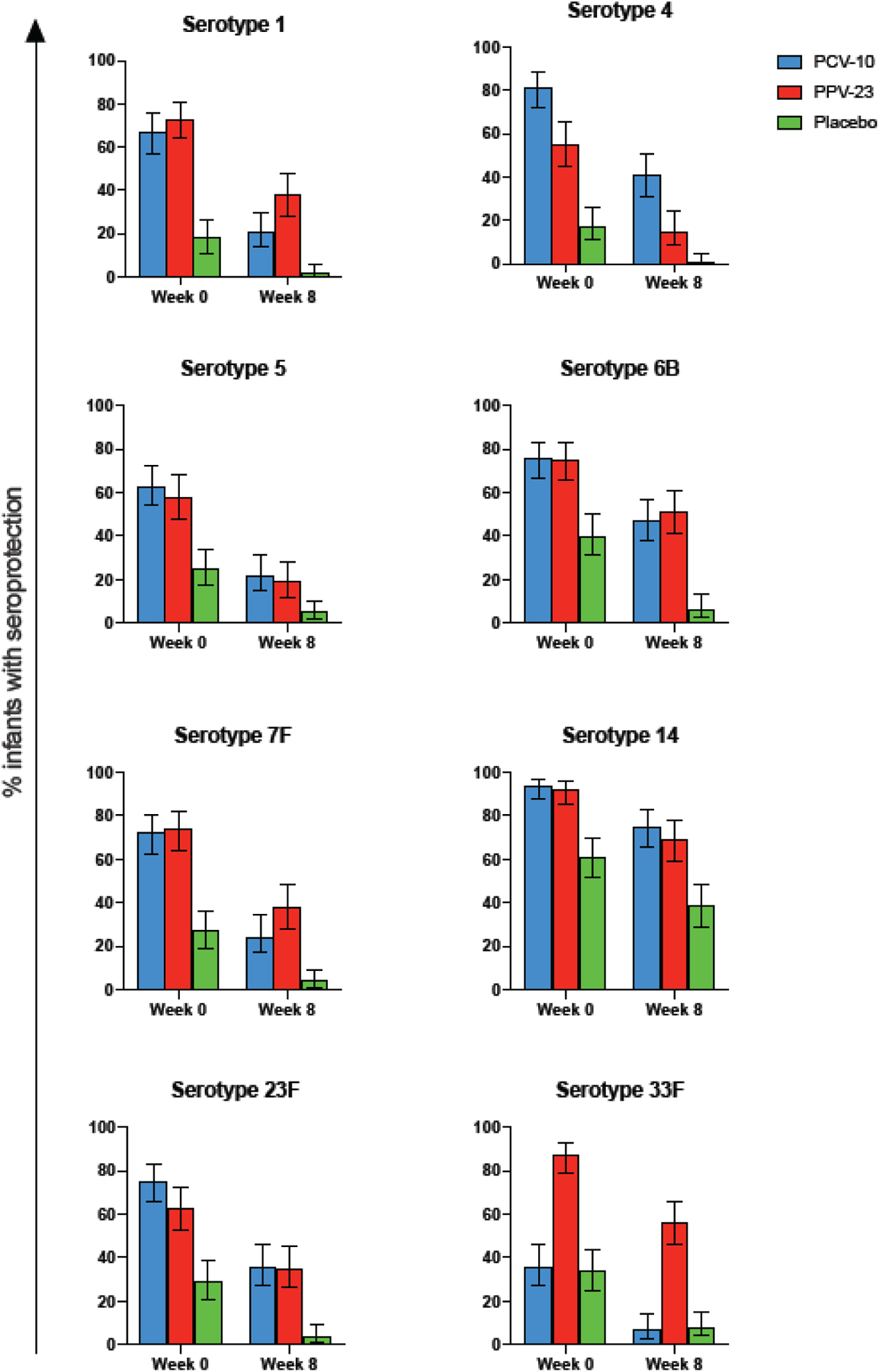
Data were obtained from 347 infants born to mothers who received PCV-10, PPV-23 or placebo during pregnancy. Bars show means and 95% CI of infants. The serotypes are indicated on the graph
Table 4.
Frequency of infants meeting the primary outcome measure of seroprotection* against ≥5 vaccine serotypes at 8 weeks of life.
| Group | N | Frequency per group (95% CI) | Groups compared | % Difference (95% CI) | P value# |
|---|---|---|---|---|---|
| Vaccine PCV-10 | 108 | 19% (12%, 27%) | PCV-23 vs. PCV-10 | 5% (−7%, 16%) | 0.5 |
| Vaccine PPV-23 | 104 | 23% (15%, 32%) | PCV-10 vs. Placebo | 18% (10%, 26%) | <0.0001 |
| Placebo | 109 | 1% (0%, 55) | PCV-23 vs. Placebo | 22% (14%, 31%) | <0.0001 |
Seroprotection was defined by antibody concentrations≥ 0.35 μg/ml.
Fisher’s Exact Test.
The transplacental transport of maternal antibodies was measured by the ratio between serum antibody concentrations in infants at birth and mothers at delivery (B:M). The individual B:M ratios for different serotypes/treatment groups ranged from 0.45 to 0.81 (Table S2; page 3). The medians of the B:M ratios across all serotypes were 0.66 in the PCV-10, 0.6 in the PPV-23 and 0.57 in the placebo groups. Serotype-specific B:M ratios were significantly higher in the PCV-10 group compared with placebo for serotypes 1, 5, 6B and 14 (p≤0.005, Table S3; page 3). The B:M ratio for serotype 5 was also significantly higher in PCV-10 compared with PPV-23 group (p=0.04) and the median against serotype 1 was higher in PPV-23 compared with placebo group (p=0.01). Transplacental transport varied by serotype in addition to vaccines, but there was no pattern to indicate that antibodies specific to any serotype were transported across the placenta better than others (data not depicted).
We performed correlation analyses of antibody responses to the vaccines and of transplacental transport of maternal antibodies with CD4+ and CD8+ T cell numbers, HIV plasma RNA, maternal age, and gestational age at vaccination, and gestational age at delivery. The results showed weak positive correlations (rho ≤ 0.24) of antibody responses to PCV-10 with CD4+ T cells and weak negative correlations (rho ≥ −0.23) of antibody responses to PPV-23 with CD8+ T cell numbers (Table S4; page 4). There were no correlations of antibody responses with maternal or gestational age at vaccination in either vaccine group. Transplacental transport of antibodies was not affected by the maternal CD4 cell count; HIV viral load; gestational age at vaccination or at delivery (Table S5; page 5).
The decay in maternal antibodies underlying the difference in seroprotection between birth and 8 weeks of life was calculated using serial measures of antibodies against PNC serotype 33F, present only in PPV-23 (Fig S2; page 6). The half-life of maternal antibodies in infants was 57 days.
OPA assays provide a measure of anti-PNC antibody functionality. We investigated OPA for PNC serotypes 4, 6B, 14 and 23F in 60 mothers at 4 weeks after immunization and their infants at birth and assessed correlations between OPA and ELISA results (Table 5). Maternal ELISA and OPA antibody concentrations were significantly and positively correlated in all treatment groups for serotype 14 and in vaccine recipients only for serotype 4. In contrast, all serotypes showed strong, significant OPA and ELISA positive correlations in infants born to vaccine recipients. Infants born to placebo recipients had a significant correlation between ELISA and OPA antibody only for serotype 14.
Table 5.
Correlations between OPA and ELISA antibodies
| Serotype | Treatment Group | Spearman Correlation coefficient | P val | N |
|---|---|---|---|---|
| Maternal | ||||
| 4 | PCV-10 | 0.474 | 0.03 | 20 |
| Placebo | 0.286 | 0.27 | 17 | |
| PPV-23 | 0.617 | 0.004 | 20 | |
| 6B | PCV-10 | 0.391 | 0.09 | 20 |
| Placebo | 0.197 | 0.43 | 18 | |
| PPV-23 | 0.389 | 0.09 | 20 | |
| 14 | PCV-10 | 0.708 | 0.0005 | 20 |
| Placebo | 0.613 | 0.005 | 19 | |
| PPV-23 | 0.753 | 0.0001 | 20 | |
| 23F | PCV-10 | −0.121 | 0.62 | 19 |
| Placebo | 0.117 | 0.64 | 18 | |
| PPV-23 | 0.216 | 0.37 | 19 | |
| Infant | ||||
| 4 | PCV-10 | 0.675 | 0.001 | 20 |
| Placebo | 0.257 | 0.27 | 20 | |
| PPV-23 | 0.817 | <0.0001 | 19 | |
| 6B | PCV-10 | 0.743 | 0.0003 | 19 |
| Placebo | 0.174 | 0.46 | 20 | |
| PPV-23 | 0.816 | <0.0001 | 19 | |
| 14 | PCV-10 | 0.734 | 0.0002 | 20 |
| Placebo | 0.802 | <0.0001 | 20 | |
| PPV-23 | 0.913 | <0.0001 | 20 | |
| 23F | PCV-10 | 0.788 | <0.0001 | 20 |
| Placebo | 0.095 | 0.69 | 20 | |
| PPV-23 | 0.935 | <0.0001 | 20 | |
Bold font indicates statistically significant associations
This study was not powered for efficacy, but we investigated in an exploratory fashion PNC colonization, which generally precedes invasive disease19. Nasopharyngeal cultures revealed PNC colonization in 25 mothers at entry and 19 at delivery (Table S6, page 7). At entry, 2 out of 342 mothers (1%) were colonized with PCV-10 and 12 (4%) with PPV-23 vaccine serotypes. Fourteen participants acquired new serotypes between entry and delivery. None of the newly acquired serotypes were included in PCV-10, but 1 mother in each vaccine group (1%) and 5 placebo recipients (4%) acquired new serotypes included in PPV-23. In infants, we measured nasopharyngeal colonization with PNC vaccine serotypes at 8 weeks of life, before the first childhood dose of PCV-10, but also at 16 weeks of life, before the second dose of PCV-10, on the assumption that protection conferred by a single dose of PCV-10 might be incomplete. At 8 weeks of life, 8 out of 324 infants (2%) had PNC colonization with vaccine serotypes in PCV-10 and 26 (8%) with serotypes in PPV-23 (Table S6, page 7). Colonization with PCV-10 vaccine serotypes was not appreciably different across treatment groups. However, infant colonization with PPV-23 vaccine serotypes at 8 weeks of life was lower in the PPV-23 group (4%) compared with PCV-10 (11%; p=0.047) or placebo (9%; p=0.11). Infant colonization at 16 weeks of life showed 1 to 4% colonization with PCV-10 serotypes across groups. PPV-23 serotype colonization was lower, but statistically nonsignificant, in infants in the PPV-23 group (3%) compared with infants in the PCV-10 (7%; p=0.13) or placebo (8%; p=0.09) groups. Complete listings of vaccine and non-vaccine PNC serotypes detected at 8 and 16 weeks of life in infants are presented in Tables S7 and S8 (page 8–9). To explore the hypothesis that maternal PNC vaccination might prevent infant PNC colonization by decreasing the exposure of the infant to maternal PNC colonization, we investigated the association between maternal and infant PNC colonization. At 8 weeks of life, a single infant shared maternal nasopharyngeal PNC serotypes present at delivery and at 16 weeks of life, two infants shared maternal PNC serotypes (Table S9; page 10).
Discussion
This was the first randomized, double-blind, placebo-controlled study comparing PCV-10 with PPV-23 and both vaccines with placebo in pregnancy. We showed that PCV-10 and PPV-23 were generally safe and highly immunogenic in pregnant women with HIV. Compared to placebo, both vaccines significantly increased the antibody concentrations against all the vaccine serotypes tested and 65% of the mothers had seroresponse against ≥5 of 7 serotypes common to both vaccines. Moreover, the seroresponse was maintained for the entire duration of the study, which ended at 6 months postpartum. This indicates that both PPV-23 and PCV-10 administered during pregnancy are likely to confer durable increased protection against IPD in women with HIV without prior PNC immunization.
The magnitude and kinetics of the antibodies generated by PCV-10 and PPV-23 measured by ELISA in pregnant women with HIV were not appreciably different. OPA antibodies have been proposed as a better predictor of protection against PNC disease compared with ELISA20. The vaccine groups showed similar correlation of OPA with ELISA titers. Collectively, these observations indicate no difference in the immunogenicity of PCV-10 and PPV-23 in pregnant women with HIV. This observation is in agreement with the results of previous studies conducted in young adults with similar HIV disease characteristics compared to our study population, but differs from adults with relatively advanced HIV-associated immunosuppression, who generally have higher antibody responses to PCV compared with PPV8,21. This indicates that although pregnancy is an immunosuppressive condition, it does not differentially affect the immunogenicity of PNC vaccines. Moreover, the responses to PPV-23 in pregnant women with HIV in our study and in pregnant women without HIV previously described did not appreciably differ, suggesting that HIV infection does not impair responses to PPV-23 in young women on ART12,22,23. The immunogenicity of PCV in pregnant women without HIV has not been sufficiently studied to allow meaningful comparisons with our results.
Anti-PNC antibodies were transported across the placenta in all groups, but with higher efficiency in the PCV-10 compared to the other two groups. Several factors may modulate the transplacental transport of maternal antibodies, including antibody titers at delivery, immunoglobulin class and subclass, glycans associated with the maternal fragment crystallizable (Fc) region of the antibody, antigen specificity and CD4 counts and HIV viral load in mothers with HIV15,24. In our study, placebo recipients had lower antibody titers at delivery compared with vaccinees, but these were not associated with higher B:M ratios25. Only IgG is transported across the placenta by the neonatal Fc receptor (FcRn), which appears to have highest affinity for IgG1, followed by IgG4, IgG3 and IgG225. Previous studies showed that PCV predominantly generated IgG1, which may account for the superior transplacental transfer efficiency in PCV-10 compared with other groups26. However, Munoz et al. showed equally efficient transport of anti-PNC IgG1 and IgG2 antibodies in mothers without HIV immunized with PPV-23 during pregnancy and in unvaccinated controls, resulting in similar PNC serotype-specific IgG1:IgG2 ratios in mothers and their infants12. The exclusive transplacental transport of IgG antibodies may also account for strong OPA and ELISA correlations in infants, but not in mothers, whose sera also contain opsonizing IgA and IgM anti-PNC antibodies27. In contrast to previous reports, the transplacental transport of maternal antibodies in our study was not associated with maternal CD4 cell counts HIV plasma RNA or with gestational age at vaccination or delivery15,28. Moreover, the transplacental transport of maternal antibodies in our study population did not appreciably differ from that described in mother-infant dyads without HIV22,23,29. This might be due to the fact that our population was relatively homogeneous and included mostly women with controlled viral replication and high CD4+ cell counts.
Infants born to vaccine recipients had seroprotection at birth and 8 weeks against significantly higher numbers of vaccine serotypes compared with infants born to placebo recipients. However, the prespecified primary outcome of a difference ≥20% in the proportion of children with seroprotection against ≥5 serotypes in each vaccine compared with placebo group was achieved only for PPV-23. The first few weeks of life is the period when protection from maternal antibodies is most needed, since infant PCV immunization begins at 6 to 8 weeks of life. In Brazil, a second dose of PCV is administered 2 months after the first dose.
Our study was not designed to test the efficacy of either vaccine against maternal or infant PNC disease. However, we investigated in an exploratory fashion PNC colonization, which is the precursor of invasive disease and an important vehicle of transmission19. Overall, 1% of mothers and ≤2% of infants were colonized with PCV-10 serotypes at any time point and there were no new acquisitions of PCV-10 serotypes in mothers post-immunization. This finding was consistent with the decrease in PCV-10 serotype circulation reported in most countries after the introduction of the vaccine in the childhood immunization schedule, including in Brazil where the vaccine was introduced in 201030. In contrast, PPV-23 serotypes were present in 4% of mothers at entry and 2% acquired new PPV-23 between entry and delivery. Moreover, in infants PPV-23 serotype colonization was more frequent compared with PCV-10 serotype colonization and was lower in infants born to mothers who received PPV-23 during pregnancy compared with the other two groups. This could not be ascribed to cocooning, since <1% infants were colonized with vaccine or non-vaccine serotypes found in mothers at delivery. Considering also that the immunogenicity of PPV-23 and PCV were similar in pregnant women with HIV without prior PNC immunization, it may be reasonable to prioritize administration of PPV-23 over PCV during pregnancy. There is an ample debate on whether it is worth vaccinating adults highly susceptible to PNC disease with the same PCV preparation used in the primary immunization schedule altogether. Recently, the Advisory Committee on Immunization Practices has changed its recommendations regarding the use of PCV-13 in older adults in the US from recommended to optional. A similar consideration might be appropriate for people with HIV.
Limitations of this study included the small number of participants with PNC colonization, the inclusion of participants from a single geographic location, and the low representation of Asians and American Indians.
In conclusion, administration of PCV-10 or PPV-23 in pregnant women with HIV was safe and equally increased maternal antibodies and infant seroprotection against vaccine serotypes. PCV-10 serotype colonization was low in mothers and infants indicating that the administration of PCV-10 during pregnancy may not play a role in protection of mothers or infants after the introduction of PCV in the childhood schedule of immunization. In contrast, PPV-23 administration during pregnancy tended to decrease infant PNC colonization in the first weeks of life. Although our study had insufficient number of participants to draw definitive conclusions on protection conferred by PPV-23 maternal immunization, it provided the information necessary to design definitive studies.
Supplementary Material
Evidence before this study
Pneumococcus (PNC) remains an important cause of morbidity in people with HIV, including pregnant women, in spite of the widespread use of antiretroviral therapy. In addition, infants exposed in utero to HIV have an increased risk of severe pneumonia and invasive pneumococcal disease irrespective of their HIV infection status. This observation has held true even after the introduction of PNC vaccines in the childhood schedule of immunizations, although vaccination reduced the incidence of invasive disease in children with and without HIV. Several studies showed that PNC vaccination using conjugated (PCV) or polysaccharide (PPV) vaccines prevented severe complications of PNC infection in people with HIV. Some of these studies also showed that PCV was more immunogenic than PPV in people with significant immune deficiency resulting from HIV infection, which led to the recommendation of sequential use of PCV and PPV in people with HIV in some countries. Although pregnancy is associated with immune suppression, a Pubmed search of publications performed as recently as July 1, 2020 using the terms ((HIV) AND (Pregnancy)) AND (Pneumococcal vaccine) or ((HIV) AND (Pregnancy)) AND (Pneumococcal conjugate vaccine) without any time interval or language restrictions revealed a single study of PPV-23 and no studies of PCV.
Vaccination of pregnant mothers has been successfully used to prevent maternal and/or infant morbidity due to tetanus, influenza and pertussis and has been proposed for the prevention of severe PNC infection in infants. Several studies investigated the safety, immunogenicity, transplacental transport of maternal antibodies and prevention of infant invasive disease through administration of PNC vaccines in pregnant women without HIV in PNC hyperendemic areas. However, our literature search revealed only one study of PPV-23 immunization in pregnant women with HIV, which was relatively small and was conducted before the universal recommendation of 3-drug antiretroviral therapy during pregnancy. We did not find any studies of PCV in pregnancy complicated by HIV infection.
Added value of this study
To provide scientific evidence for future recommendations on the use of PNC vaccines in pregnant women with HIV, we conducted the first randomized, double-blind, placebo-controlled study comparing the safety, immunogenicity and transplacental transport of maternal antibodies after administration of PCV-10, PPV-23 or placebo in pregnant women with HIV on 3-drug ART in Brazil, where there was no formal recommendation to immunize against PNC individuals with HIV. The study, which enrolled 346 women randomized 1:1:1, showed that PCV-10 and PPV-23 had excellent safety profiles and significantly increased antibody concentrations against tested PNC vaccine serotypes in mothers and infants compared with placebo. The comparison between the two vaccines did not reveal any appreciable differences with respect to the magnitude or persistence of the maternal antibody responses, the transplacental transport of antibodies, or the persistence of maternal antibodies in infants. An exploratory analysis of the PNC nasopharyngeal carriage showed higher frequency of PPV-23 compared with PCV-10 vaccine serotypes in mothers and infants, which was consistent with the widespread use of PCV-10 in children in Brazil. We observed lower PPV-23 serotype carriage in infants born to PPV-23 vaccine recipients compared with placebo or PCV-10 recipients, but no difference in the carriage of PCV-10 serotypes. However, the study was not powered for this analysis and its results were hypothesis-generating.
Implication of all available evidence
Our data indicate that administration of PNC vaccines during pregnancy should be encouraged in women with HIV without prior immunization. Considering the similar safety and immunogenicity of the two vaccines in pregnant women with HIV, PPV-23 administration offers the advantage of covering a larger number of PNC serotypes compared with currently available PCV preparations. The protective effect of maternal PNC vaccination against PNC carriage in infants needs to be further studied. Our study provides effect estimates for PPV-23 efficacy that can be used to power future studies in areas of low PCV serotype circulation.
Acknowledgments:
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development through contract HHSN275201800001I. We thank Dr. Helena Keiko Sato from the Centro de Vigilancia Epidemiologica da Secretaria da Saude do Estado de Sao Paulo for donating the PCV-10 doses used for maternal immunization; Drs. George Siberry, Heather Watts and Elizabeth Smith, and the IMPAACT network for their support in the early development of this study.
Declaration of interests.
AW receives grants from NIH, GSK, Janssen and Merck. TF and PM receive grants from NICHD. SIP receives personal fees from Merck, Sanofi and Pfizer and grants from NIH and Pfizer. DG receives personal fees from Merck and grants from GSK and Merck. MMP, GD, NC, MJJ, JC, LL, LN, JK, BZ, FB, ECJ, BRS, ESM and JAP did not declare any conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data sharing statement: Data obtained in this study, including deidentified individual participant data reported in this article and a data dictionary defining each field in the set, will be made available to other investigators. The study protocol will be made available as well. Data will be made available immediately following publication, with no end date, to researchers who provide a methodologically sound proposal to the NICHD. Data will be available at https://dash.nichd.nih.gov/.
References
- 1.Ramogale MR, Moodley J, Sebiloane MH. HIV-associated maternal mortality--primary causes of death at King Edward VIII Hospital, Durban. S Afr Med J. 2007;97(5):363–366. [PubMed] [Google Scholar]
- 2.Yin Z, Rice BD, Waight P, et al. Invasive pneumococcal disease among HIV-positive individuals, 2000–2009. AIDS. 2012;26(1):87–94. [DOI] [PubMed] [Google Scholar]
- 3.Pilishvili T, Bennett NM. Pneumococcal Disease Prevention Among Adults: Strategies for the Use of Pneumococcal Vaccines. Am J Prev Med. 2015;49(6 Suppl 4):S383–390. [DOI] [PubMed] [Google Scholar]
- 4.French N, Moore M, Haikala R, Kayhty H, Gilks CF. A case-control study to investigate serological correlates of clinical failure of 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults. J Infect Dis. 2004;190(4):707–712. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Barradas MC, Goulet J, Brown S, et al. Impact of pneumococcal vaccination on the incidence of pneumonia by HIV infection status among patients enrolled in the Veterans Aging Cohort 5-Site Study. Clin Infect Dis. 2008;46(7):1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med. 2010;362(9):812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klugman KP, Madhi SA, Huebner RE, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349(14):1341–1348. [DOI] [PubMed] [Google Scholar]
- 8.Feikin DR, Elie CM, Goetz MB, et al. Specificity of the antibody response to the pneumococcal polysaccharide and conjugate vaccines in human immunodeficiency virus-infected adults. Clin Diagn Lab Immunol. 2004;11(1):137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida Vde C, Mussi-Pinhata MM, De Souza CB, et al. Immunogenicity of 23-valent pneumococcal polysaccharide vaccine in HIV-infected pregnant women and kinetics of passively acquired antibodies in young infants. Vaccine. 2009;27(29):3856–3861. [DOI] [PubMed] [Google Scholar]
- 10.Floridia M, Ravizza M, Tamburrini E, et al. Diagnosis of HIV infection in pregnancy: data from a national cohort of pregnant women with HIV in Italy. Epidemiol Infect. 2006;134(5):1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naleway AL, Smith WJ, Mullooly JP. Delivering influenza vaccine to pregnant women. Epidemiol Rev. 2006;28:47–53. [DOI] [PubMed] [Google Scholar]
- 12.Munoz FM, Englund JA, Cheesman CC, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the third trimester of gestation. Vaccine. 2001;20(5–6):826–837. [DOI] [PubMed] [Google Scholar]
- 13.Madhi SA, Nunes MC, Cutland CL. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;371(24):2340. [DOI] [PubMed] [Google Scholar]
- 14.Adler C, Haelterman E, Barlow P, Marchant A, Levy J, Goetghebuer T. Severe Infections in HIV-Exposed Uninfected Infants Born in a European Country. PLoS One. 2015;10(8):e0135375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez DR, Fong Y, Li SH, et al. Fc Characteristics Mediate Selective Placental Transfer of IgG in HIV-Infected Women. Cell. 2019;178(1):190–201 e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton RL, Antonello J, Cooper D, et al. Assignment of Opsonic Values to Pneumococcal Reference Serum 007sp for Use in Opsonophagocytic Assays for 13 Serotypes. Clin Vaccine Immunol. 2017;24(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchese RD, Puchalski D, Miller P, et al. Optimization and Validation of a Multiplex, Electrochemiluminescence-Based Detection Assay for the Quantitation of Immunoglobulin G Serotype-Specific Antipneumococcal Antibodies in Human Serum. Clinical and Vaccine Immunology. 2009;16(3):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finkelstein JA, Huang SS, Daniel J, et al. Antibiotic-Resistant Streptococcus pneumoniae in the Heptavalent Pneumococcal Conjugate Vaccine Era: Predictors of Carriage in a Multicommunity Sample. Pediatrics. 2003;112(4):862. [DOI] [PubMed] [Google Scholar]
- 19.Simell B, Auranen K, Käyhty H, Goldblatt D, Dagan R, O’Brien KL. The fundamental link between pneumococcal carriage and disease. Expert Review of Vaccines. 2012;11(7):841–855. [DOI] [PubMed] [Google Scholar]
- 20.Schuerman L, Wysocki J, Tejedor JC, Knuf M, Kim KH, Poolman J. Prediction of pneumococcal conjugate vaccine effectiveness against invasive pneumococcal disease using opsonophagocytic activity and antibody concentrations determined by enzyme-linked immunosorbent assay with 22F adsorption. Clin Vaccine Immunol. 2011;18(12):2161–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu CL, Chang SY, Chuang YC, et al. Revaccination with 7-valent pneumococcal conjugate vaccine elicits better serologic response than 23-valent pneumococcal polysaccharide vaccine in HIV-infected adult patients who have undergone primary vaccination with 23-valent pneumococcal polysaccharide vaccine in the era of combination antiretroviral therapy. Vaccine. 2014;32(9):1031–1035. [DOI] [PubMed] [Google Scholar]
- 22.Quiambao BP, Nohynek HM, Kayhty H, et al. Immunogenicity and reactogenicity of 23-valent pneumococcal polysaccharide vaccine among pregnant Filipino women and placental transfer of antibodies. Vaccine. 2007;25(22):4470–4477. [DOI] [PubMed] [Google Scholar]
- 23.Shahid NS, Hoque SS, Begum T, Steinhoff MC, Thompson C, Siber GR. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. The Lancet. 1995;346(8985):1252–1257. [DOI] [PubMed] [Google Scholar]
- 24.Salicio VMM, Fett CA, Salicio MA, et al. The Effect of Caffeine Supplementation on Trained Individuals Subjected to Maximal Treadmill Test. Afr J Tradit Complement Altern Med. 2017;14(1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilcox CR, Holder B, Jones CE. Factors Affecting the FcRn-Mediated Transplacental Transfer of Antibodies and Implications for Vaccination in Pregnancy Frontiers in Immunology. 2017;8(1294). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uddin S, Borrow R, Haeney MR, et al. Total and serotype-specific pneumococcal antibody titres in children with normal and abnormal humoral immunity. Vaccine. 2006;24(27–28):5637–5644. [DOI] [PubMed] [Google Scholar]
- 27.Park S, Nahm MH. Older Adults Have a Low Capacity To Opsonize Pneumococci Due to Low IgM Antibody Response to Pneumococcal Vaccinations. Infection and Immunity. 2011;79(1):314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eberhardt CS, Blanchard-Rohner G, Lemaitre B, et al. Pertussis Antibody Transfer to Preterm Neonates After Second- Versus Third-Trimester Maternal Immunization. Clin Infect Dis. 2017;64(8):1129–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz FM, Weisman LE, Read JS, et al. Assessment of safety in newborns of mothers participating in clinical trials of vaccines administered during pregnancy. Clin Infect Dis. 2014;59 Suppl 7:S415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandileone MC, Zanella RC, Almeida SCG, et al. Long-term effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae in children in Brazil. Vaccine. 2019;37(36):5357–5363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


