Abstract
Background:
Venetoclax (VEN) combined with the hypomethylating agent (HMA) azacitidine improves survival in patients ≥75 years of age with newly diagnosed acute myeloid leukemia (AML). Treatment with VEN-HMA can result in prolonged and often profound neutropenia, warranting antifungal prophylaxis. Azole antifungals inhibit CYP3A4, the primary enzyme responsible for VEN metabolism, resulting in VEN dose reductions for each concomitant antifungal. Limited clinical data exists on outcomes in patients treated with VEN-HMA and various azoles.
Methods:
Time to neutrophil recovery (ANC>1000 cells/mm3) and platelet recovery (PLT>100,000 cells/mm3) in 64 patients with newly diagnosed AML who achieved a response after course 1 VEN-HMA was evaluated. HMA therapy included azacitidine 75 mg/m2 IV/SC for 7 days or decitabine 20 mg/m2 IV for 5 or 10 days.
Results:
Forty-seven patients (73%) received an azole, including posaconazole (n=17; 27%), voriconazole (n=9; 14%), isavuconazole (n=20; 31%), or fluconazole (n=1; 2%). Median time to ANC recovery was similar between patients who did (37 days; 95%CI: 34, 38) or did not (39 days; 95%CI: 30, NA) receive an azole (p=0.8). Median time to PLT recovery was significantly longer in patients receiving azoles (28 vs 22 days, p=0.01). Median time to ANC (35 vs 38 days) and PLT (26 vs 32 days) recovery was similar between posaconazole and voriconazole.
Conclusion:
VEN-HMA results in neutropenia and thrombocytopenia, with the latter prolonged in patients receiving concomitant azoles. Concomitant posaconazole or voriconazole and VEN 100mg resulted in similar ANC and PLT recovery time, suggesting the safety of these dosage combinations during course 1.
Keywords: venetoclax, acute myeloid leukemia, azole antifungals, invasive fungal infection, prophylaxis
Table of Contents Precis
Prophylaxis with azole antifungal agents during VEN and HMA therapy impacted duration of thrombocytopenia but not neutropenia. The combination of VEN 100 mg daily and either posaconazole or voriconazole resulted in similar durations of cytopenias.
Introduction
Acute myeloid leukemia (AML) is the most common acute leukemia in adults, with an estimated 19,940 new cases in the year 20201. A disease of older adults, the median age at diagnosis is 68 years, necessitating tolerability in addition to efficacy be considered when determining optimal therapy. Venetoclax (VEN), an oral chemotherapy agent targeting BCL-2, has been studied in combination with hypomethylating agents (HMAs) and low-dose cytarabine in patients with newly diagnosed AML who are older or otherwise unfit for intensive chemotherapy2–4. In patients with a median age of 74 years, the combination of VEN with HMAs led to an overall response rate of 73% and median overall survival (OS) of 17.5 months, leading to Food and Drug Administration (FDA) approval of VEN in this setting2. Moreover, a recent phase III randomized trial demonstrated a survival benefit with the addition of VEN to azacitidine compared to azacitidine monotherapy, establishing VEN and HMA combinations as standard of care in the elderly and unfit population5. While the VEN and HMA combination is safe overall, neutropenia and thrombocytopenia are among the most common adverse events associated with this regimen.
Antimicrobial prophylaxis is a hallmark of AML therapy due to the increased risk of infections observed in this immunocompromised population. Patients with AML are at heightened risk of opportunistic infections, including invasive fungal infections (IFIs), due to prolonged myelosuppression caused by both AML-directed therapies as well as poor bone marrow reserve6–8. Prophylaxis with the broad-spectrum triazole antifungal agent posaconazole (PCZ) improved mortality in patients with newly diagnosed AML undergoing induction therapy with intensive chemotherapy regimens associated with prolonged myelosuppression7. Although PCZ is the only azole to have demonstrated a mortality benefit in this setting, routine antifungal prophylaxis with any mold-active azole or echinocandin is recommended for patients at risk for profound (absolute neutrophil count [ANC] < 100 cells/mm3) and prolonged (≥7 days) neutropenia9. While a paucity of literature exists to describe the anticipated risk of IFI in patients receiving VEN and HMA combinations10, the prolonged myelosuppression that is observed with VEN-based combinations often warrants broad-spectrum antifungal prophylaxis.
Azole antifungal agents inhibit cytochrome P450 3A4 (CYP3A4) to varying degrees. CYP3A4 is the primary enzyme responsible for the metabolism of VEN, therefore the addition of azole antifungals reduces its metabolism. This combination results in increased VEN exposure and potentially increased toxicity. A single pharmacokinetic analysis of 11 patients receiving the combination of PCZ with VEN 50 mg and 100 mg daily demonstrated an overall VEN exposure of 75% and 155%, respectively, when compared to exposure with standard VEN dosing of 400 mg daily11. These results led to the recommendation to reduce the dose of VEN by at least 75% in combination with PCZ. This pharmacokinetic study, limited by both a small sample and narrow focus on PCZ alone, also failed to provide clinical correlation with VEN exposure. While dose adjustment recommendations exist for combinations with other commonly used azole antifungals, such as voriconazole (VCZ) and isavuconazole (ISA), these recommendations are based upon the extrapolation of these pharmacokinetic data. Azole antifungals were utilized to varying degrees in the clinical trials evaluating VEN and HMA therapy,12, 13 and little real-world data exists to demonstrate the tolerability of VEN in combination with azoles in the setting of VEN and HMA therapy. This retrospective analysis sought to evaluate the duration of myelosuppression observed with VEN and HMA regimens in combination with different azole antifungal agents during course 1 of treatment.
Methods
This retrospective analysis included adult patients (≥18 years) with newly diagnosed AML treated at the University of Texas MD Anderson Cancer Center from November 2014 through December 2019 who achieved a response with HMA and VEN combination therapy. Responders were patients who achieved complete response with hematologic recovery (CR) or incomplete hematologic recovery (CRi) according to IWG criteria after course 114. Prior analyses did not demonstrate a difference in rate of CR/CRi versus morphologic leukemia free state (MLFS) or lack of response among patients receiving azole antifungals when treated with VEN and HMA therapy,13, 15 therefore only patients achieving a response who could be assessed for the primary outcome of blood count recovery were included. Patients were treated as standard of care or on clinical trial with VEN 400 mg dose equivalent and azacitidine 75 mg/m2 intravenously (IV) or subcutaneously (SC) daily for 7 days, decitabine 20 mg/m2 IV daily for 5 days2, or decitabine 20 mg/m2 IV daily for 10 days13. Venetoclax was administered days 1-28 but was stopped on day 21 if there was evidence of aplasia or 5% or fewer blasts on a day 21 bone marrow. Venetoclax may have been stopped sooner if deemed in best interest of the patient. Equivalent VEN dosage was considered any of the following: 400 mg daily without concomitant azole antifungal; 200 mg daily with the moderate CYP3A4 inhibitors ISA or fluconazole (FCZ); 100 mg daily with the strong CYP3A4 inhibitors PCZ or VCZ16. Patients received 100 mg daily with both PCZ and VCZ considering they are both strong CYP3A4 inhibitors, and these trials began prior to the FDA recommendation for an additional dose reduction with concomitant PCZ. Patients who received a concomitant antifungal for ≥ 5 days while also receiving VEN ≥ 7 days were included for analysis. The study was approved by the MD Anderson Institutional Review Board.
Durations of neutropenia (ANC < 1000 cells/mm3) and thrombocytopenia (PLT < 100,000 cells/mm3) were calculated. Time to neutrophil recovery was counted from the date of ANC < 500 cells/mm3 until the first of two consecutive measurements of ANC > 500 cells/mm3 or ANC > 1000 cells/mm3, for each endpoint respectively. Similarly, time to platelet (PLT) count recovery was counted from date of PLT < 50,000 cells/mm3 until the first of two consecutive measurements of PLT > 50,000 cells/mm3 or PLT > 100,000 cells/mm3 without transfusion support. Additional safety outcomes including duration of hospitalization, incidence of febrile neutropenia, and incidence of documented infection were collected. Documented infections were considered infection with positive culture from sterile site or imaging consistent with pneumonia and associated documented symptoms. Invasive fungal infections (IFI) were diagnosed according to EORTC/MSG17 and breakthrough IFI (bIFI) according to ECMM criteria18.
The Kaplan-Meier method was used to estimate distributions of and median times to ANC and PLT recovery. Log rank test was used to compare these time-to-event outcomes among different subgroups. A p-value <0.05 was considered statistically significant. In addition to presence of azole antifungal, subgroups of interest included HMA schedule, disease subtype (de novo AML versus other), VEN duration, documented infection, and age. Cox regression analysis was conducted including any covariates of interest with p-value <0.1 on log rank analysis. All statistical analyses were performed using SPlus software v8.2 (TIBCO, Palo Alto, CA) and SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient Characteristics
Sixty-four patients were included in the analysis (Table 1). The median age was 72 years (range, 26-89), including 58 patients (91%) who were 65 years of age or older. Forty-four patients (69%) were treated for de novo AML, while the remaining 20 patients (31%) had secondary AML (sAML) or therapy-related AML (t-AML). All but 6 patients received decitabine for induction therapy, including 49 patients (77%) who received decitabine 20 mg/m2 IV daily for 10 days and 9 patients (14%) who received 20 mg/m2 IV daily for 5 days. Most often, VEN was administered for 22-28 days (n=31; 48%), followed by 15-21 days (n=24; 38%), and ≤ 14 days in 9 patients (14%). Patients were hospitalized for a median of 32 days (8-62) during induction. Forty-seven patients (73%) received an azole antifungal during induction therapy, including PCZ in 17 patients (27%), VCZ in 9 (14%), ISA in 20 (31%), and FCZ in 1 patient. The remaining 17 patients received an echinocandin.
Table 1.
Patient, Treatment, and Infection Characteristics
| Characteristic | N=64 |
|---|---|
| Male | 41 (64%) |
| Median age, years | 72 (26-89) |
| ≥ 65 years | 58 (91%) |
| ≥ 70 years | 46 (72%) |
| AML subtype | |
| de novo AML | 44 (69%) |
| Secondary AML, no prior therapy | 12 (19%) |
| Secondary AML, prior therapy for AHD | 4 (6%) |
| Therapy-related AML | 4 (6%) |
| Hypomethylating agent | |
| Decitabine | 58 (91%) |
| 20 mg/m2 daily x 10 days | 49 (77%) |
| 20 mg/m2 daily x 5 days | 9 (14%) |
| Azacitidine 75 mg/m2 daily x 7 days | 6 (9%) |
| Venetoclax dosage | |
| 400 mg without CYP3A4i | 17 (27%) |
| 200 mg with moderate CYP3A4i | 21 (33%) |
| 100 mg with strong CYP3A4i | 26 (41%) |
| Venetoclax duration, days | 21 (7-29) |
| >21 days | 31 (48%) |
| >14 to ≤21 days | 24 (38%) |
| ≤ 14 days | 9 (14%) |
| Concomitant antifungal therapy | |
| Posaconazole | 17 (27%) |
| Voriconazole | 9 (14%) |
| Isavuconazole | 20 (31%) |
| Fluconazole | 1 (2%) |
| Echinocandin | 17 (27%) |
| Neutropenic fever | 35 (55%) |
| Documented infection | 28 (44%) |
| Bloodstream infection, gram-positive | 3 (5%) |
| Bloodstream infection, gram-negative | 2 (3%) |
| Pneumonia | 21 (33%) |
| Probable invasive fungal infection | 3 (5%) |
| Possible invasive fungal infection | 3 (5%) |
| Cellulitis | 7 (11%) |
| Other | 2 (3%) |
Data expressed as median (range) or n(%); AHD: antecedent hematologic disorder; CYP3A4i: cytochrome P450 3A4 inhibitor; moderate CYP3A4i: fluconazole, isavuconazole; strong CYP3A4i: posaconazole, voriconazole
Other infections include n=1 each of pharyngitis and sinusitis
Myelosuppression
After course 1, 59 patients (92%) achieved ANC recovery to > 500 cells/mm3 (ANC500) and PLT > 50,000 cells/mm3 (PLT50), while 51 patients (80%) achieved ANC > 1000 cells/mm3 (ANC1000) and 56 (88%) PLT > 100,000 cells/mm3 (PLT100) prior to proceeding to cycle 2. The median time to ANC1000 and PLT100 was 35 days (range, 19-129) and 25 days (range, 12-207), respectively. Growth factor support was utilized in 17 patients (27%) a median of 31 days (range, 22-49) after initiating course 1 of therapy, primarily (65%) in patients with a documented infection. No impact of patient age, VEN duration, HMA schedule, the use of azole antifungals, or AML subtype was observed on time to ANC500 or ANC1000 (Table 2). When comparing individual azole antifungals, the median time to ANC1000 was 35 days (95% CI: 33, 40) with PCZ, 38 days (95% CI: 30, NA) with VCZ, and 37 days (95% CI: 33, NA) with ISA (Table 3). This was similar to a median of 39 days (95% CI: 30, NA) that was observed for those receiving a concomitant echinocandin (Figure 1). The one patient who received concomitant FCZ achieved ANC1000 after 22 days, which was significantly shorter than with any other antifungal (p<0.05).
Table 2.
Time to absolute neutrophil count and platelet count recovery by characteristic of interest
| Diagnosis | Hypomethylating agent schedule | Venetoclax duration (days) | Documented infection | ||||||
|---|---|---|---|---|---|---|---|---|---|
| sAML/t-AML (N=20) | De novo AML (N=44) | DAC 10 (N=49) | DAC 5 or AZA 7 (N=15) | >21 (N=31) | 15-21 (N=24) | ≤ 14 (N=9) | Yes (N=28) | No (N=36) | |
| ANC>500—n(%) | 19 (95%) | 40 (91%) | 46 (94%) | 13 (87%) | 27 (87%) | 23 (96%) | 9 (100%) | 27 (96%) | 32 (89%) |
| Median days to ANC>500 (95% CI) | 33 (31, 40) | 34 (33, 37) | 35 (33, 37) | 33 (28, NA) | 33 (30, 35) | 37 (33, 41) | 36 (29, NA) | 34 (31, 39) | 34 (32, 37) |
| ANC>1000—n(%) | 18 (90%) | 33 (75%) | 41 (84%) | 10 (67%) | 23 (74%) | 21 (88%) | 7 (78%) | 26 (93%) | 25 (69%) |
| Median days to ANC>1000 (95% CI) | 34 (33, 47) | 37 (34, 41) | 37 (34, 40) | 34 (30, NA) | 34 (32, 44) | 38 (37, 46) | 38 (33, NA) | 38 (33, 41) | 35 (33, 46) |
| PLT>50—n(%) | 15 (75%) | 44 (100%) | 46 (94%) | 13 (87%) | 29 (94%) | 22 (92%) | 8 (89%) | 24 (86%) | 35 (97%) |
| Median days to PLT>50 (95% CI) | 27 (21, NA)α | 23 (20, 26)α | 25 (22, 28) | 19 (16, 28) | 19 (17, 25)α | 26 (23, 31)α | 29 (22, NA)α | 28 (25, 34)α | 21 (18, 24)α |
| PLT>100—n(%) | 14 (70%) | 42 (95%) | 43 (88%) | 13 (87%) | 29 (94%) | 20 (83%) | 7 (78%) | 23 (82%) | 33 (92%) |
| Median days to PLT>100 (95% CI) | 29 (22, NA)β | 24 (22, 29)β | 27 (23, 33) | 22 (20, 32) | 22 (20, 27)β | 31 (24, 39)β | 32 (23, NA)β | 31 (26, 50)β | 23 (21, 27)β |
ANC: absolute neutrophil count (cells/mm3); PLT: platelet count (cells/mm3); sAML/t-AML: secondary AML, therapy-related AML; DAC 10: decitabine 20 mg/m2 IV daily x 10 days; DAC 5: decitabine 20 mg/m2 IV daily x 5 days; AZA 7: Azacitidine 75mg/m2 daily x 7 days
p<0.05 for difference in median days to PLT50 within group
p<0.05 for difference in median days to PLT100 within group; shaded cells represent p<0.05 for within group comparisons
Table 3.
Time to absolute neutrophil count and platelet count recovery by antifungal
| Concomitant azole | Antifungal agent | CYP3A4 inhibitor | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (N=47) | No (N=17) | PCZ (N=17) | VCZ (N=9) | ISA (N=20) | ECHINO (N=17) | None (N=17) | Moderate (N=21) | Strong (N=26) | |
| ANC>500—n(%) | 45 (96%) | 14 (82%) | 17 (100%) | 9 (100%) | 18 (90%) | 14 (82%) | 14 (82%) | 19 (90%) | 26 (100%) |
| Median days to ANC>500 (95% CI) | 34 (33, 37) | 34 (28, NA) | 35 (33, 39) | 35 (26, NA) | 34 (32, 47) | 34 (28, NA) | 34 (28, NA) | 34 (31, 45) | 34 (33, 38) |
| ANC>1000—n(%) | 41 (87%) | 10 (59%) | 17 (100%) | 7 (78%) | 16 (80%) | 10 (59%) | 10 (59%) | 17 (81%) | 24 (92%) |
| Median days to ANC>1000 (95% CI) | 37 (34, 38) | 39 (30, NA) | 35 (33, 40) | 38 (30, NA) | 37 (33, NA) | 39 (30, NA) | 39 (30, NA) | 34 (32, NA) | 37 (34, 40) |
| PLT>50—n(%) | 42 (89%) | 17 (100%) | 15 (88%) | 8 (89%) | 18 (90%) | 17 (100%) | 17 (100%) | 19 (91%) | 23 (89%) |
| Median days to PLT>50 (95% CI) | 26 (23, 29)α | 19 (16, 23)α | 25 (21, 50)α | 28 (25, NA)α | 25 (19, 29)α | 19 (16, 23)α | 19 (16, 23)α | 25 (19, 29)α | 27 (22, 37)α |
| PLT>100—n(%) | 39 (83%) | 17 (100%) | 14 (82%) | 8 (89%) | 16 (80%) | 17 (100%) | 17 (100%) | 17 (81%) | 22 (85%) |
| Median days to PLT>100 (95% CI) | 28 (23, 34)β | 22 (20, 25)β | 26 (23, NA) | 32 (30, NA) | 27 (21, 35) | 22 (20, 25) | 22 (20, 25)β | 27 (21, 35)β | 31 (23, 41)β |
ANC: absolute neutrophil count (cells/mm3); PLT: platelet count (cells/mm3); PCZ: posaconazole; VCZ: voriconazole; ISA: isavuconazole; ECHINO: echinocandin
p<0.05 for difference in median days to PLT50 within group
p<0.05 for difference in median days to PLT100 within group; shaded cells represent p<0.05 for within group comparisons
Figure 1. Time to absolute neutrophil count (ANC) recovery based on concomitant CYP3A4 inhibitor (CYP3A4i) and azole antifungal.
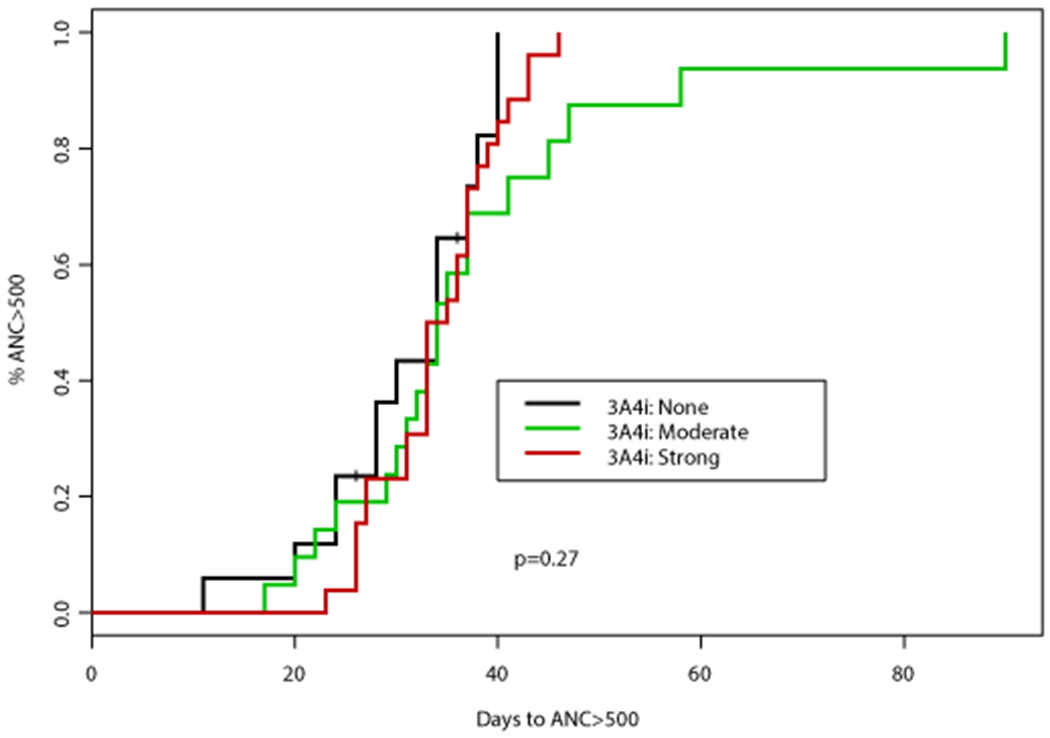
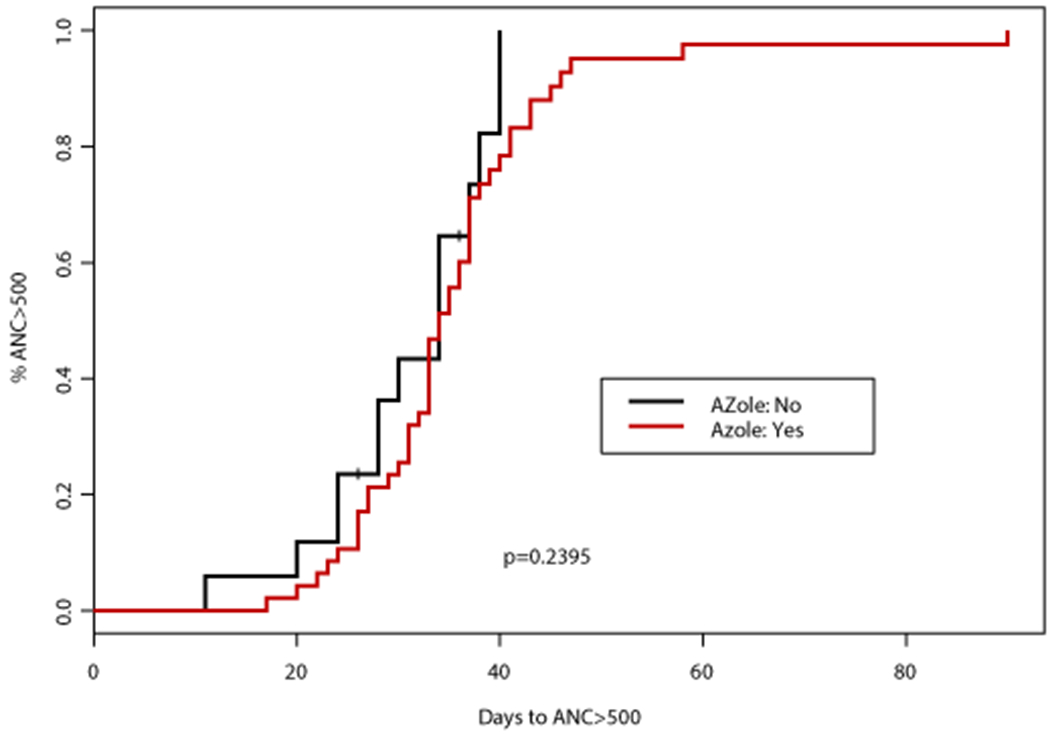
(A) Time to ANC >500 cells/mm3 was not significantly different according to concomitant CYP3A4i (p=0.27) or use of azole antifungal (p=0.2395). (B) Similarly, time to ANC >1000 cells/mm3 was not significantly different according to concomitant CYP3A4i (p=0.623) or use of azole antifungal (p=0.7948)
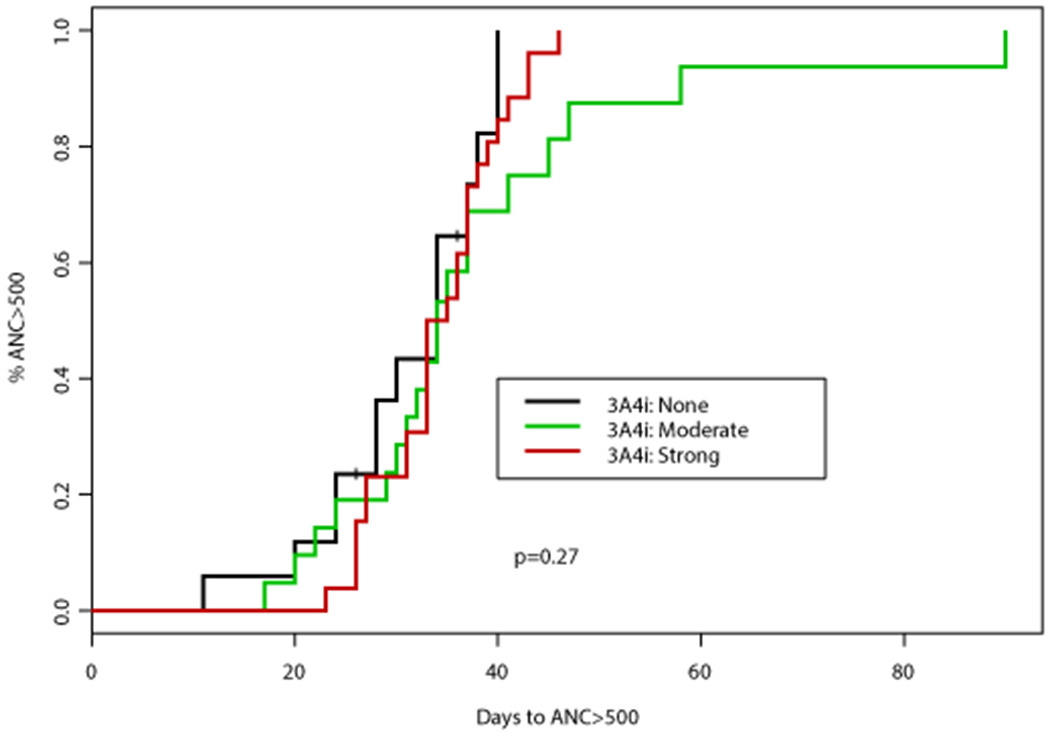
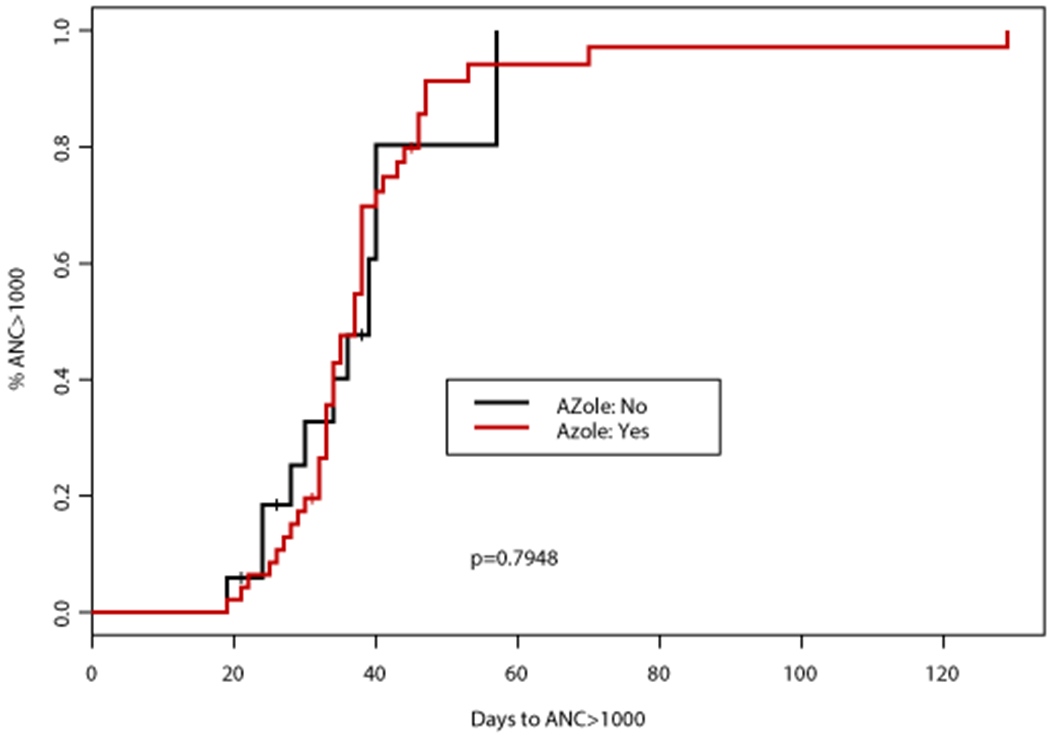
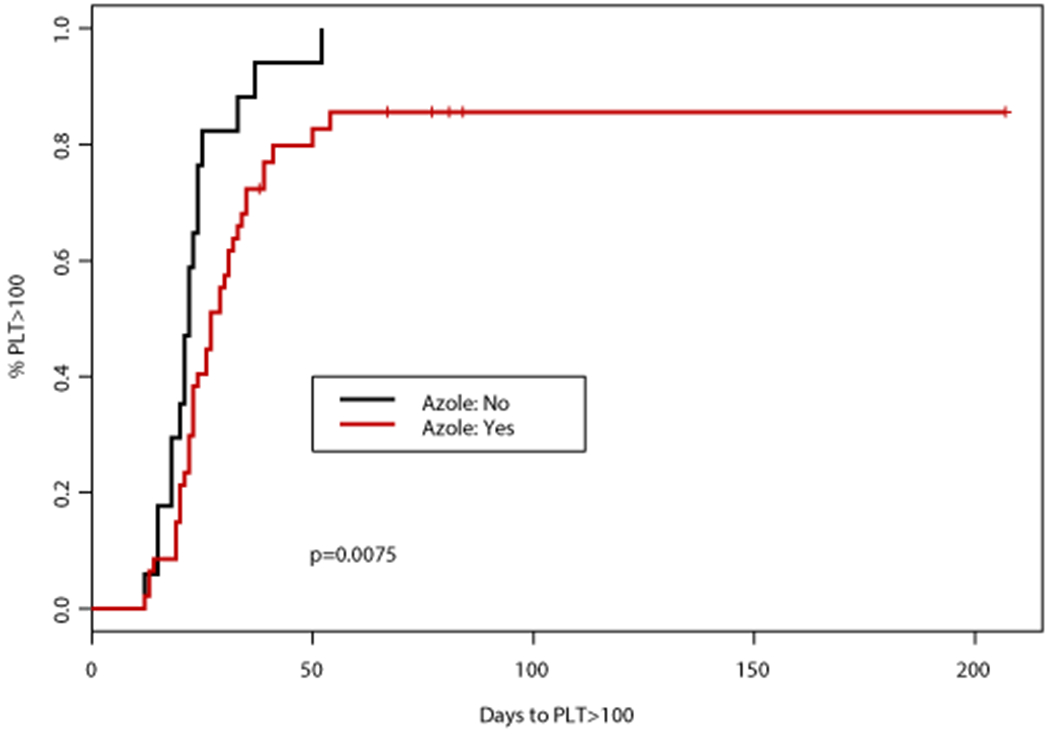
An association between numerous factors including antifungal agent, VEN duration, AML subtype, and documented infection with time to PLT50 and PLT100 was observed (Tables 2–3). Concomitant VCZ resulted in the longest median time to PLT100 of 32 days (95%CI: 30, NA) compared to 26 days (95%CI: 23, NA) with PCZ, and 27 days (95%CI: 21, 35) with ISA, p=0.067 (Table 3). When analyzed by intensity of CYP3A4 inhibition, patients who did not receive a concomitant CYP3A4 inhibitor had the shortest median time to PLT100 (22 days; 95%CI: 20, 25), followed by those who received a moderate CYP3A4 inhibitor (27 days; 95%CI: 21, 35), and finally strong CYP3A4 inhibitor (31 days; 95%CI: 23, 41), p=0.026. Altogether, patients receiving a concomitant azole antifungal had a longer median time to PLT100 of 27 days (95%CI: 23, 34) compared to a median of 22 days (95%CI: 20, 25) in those receiving an echinocandin only, p=0.008 (Figure 2). Patients with de novo AML achieved PLT100 after a median of 24 days (95%CI: 22, 29) compared to 29 days (95%CI: 22, NA) for patients with sAML or t-AML, p=0.0439. Of interest, patients receiving VEN for ≤14 days had the longest median time to PLT100 (32 days; 95%CI: 23, NA) compared to those receiving VEN for 15-21 (31 days; 95%CI: 24, 39) or 22-28 days (22 days; 95%CI: 20, 27), p=0.047. A significant enrichment for concomitant strong CYP3A4 inhibitors (78%) as well as documented infections (67%) was noted in this subgroup.
Figure 2. Time to platelet (PLT) count recovery based on concomitant CYP3A4 inhibitor (CYP3A4i) and azole antifungal.
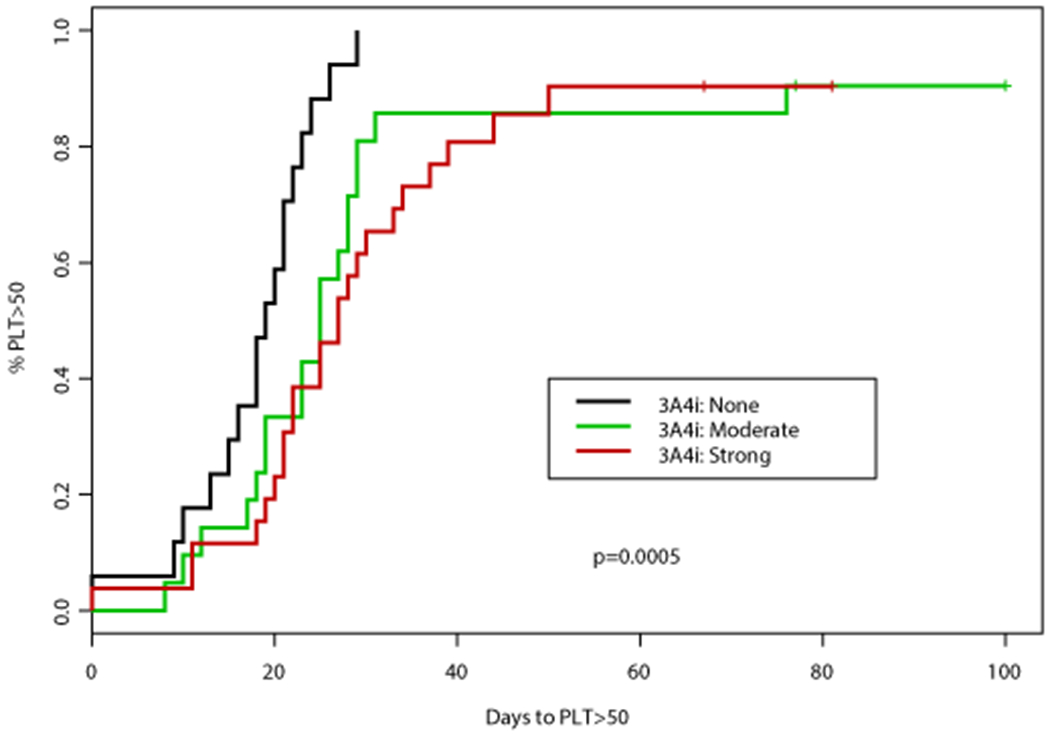
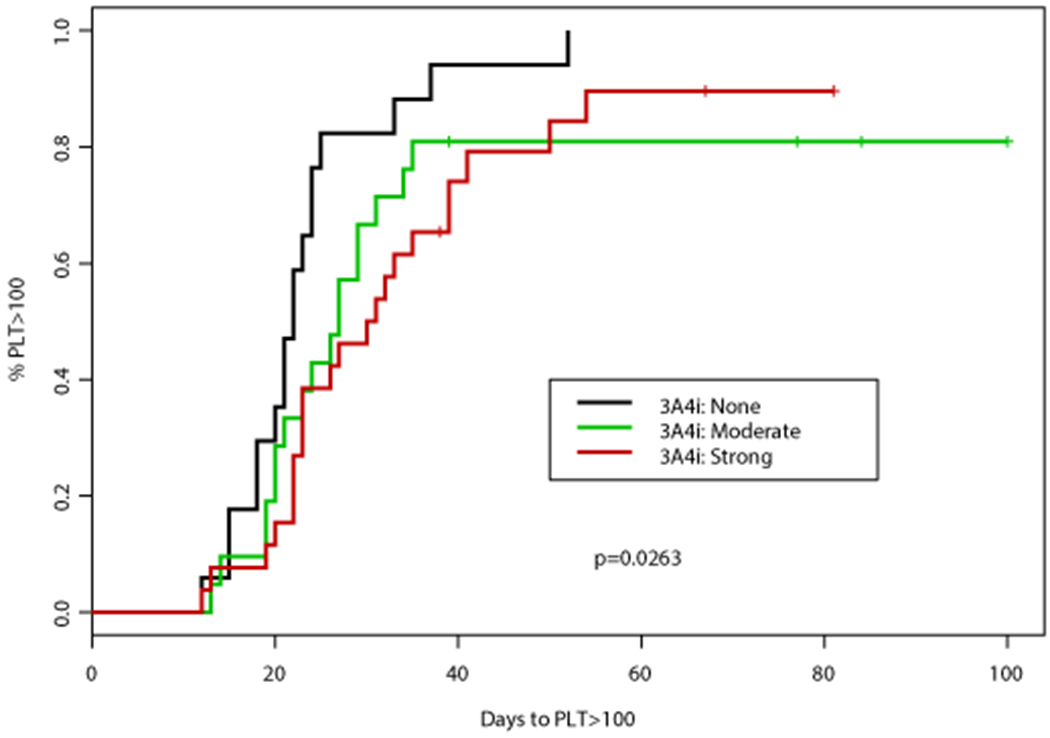
(A) Time to PLT >50,000 cells/mm3 was significantly different according to concomitant CYP3A4i (p=0.0005), patients receiving a concomitant azole antifungal had a significantly longer time to PLT >50,000 cells/mm3 (median 26 days vs 19 days, p=0.0001). (B) Time to PLT >100,000 cells/mm3 was also significantly different according to concomitant 3A4i (p=0.0263), patients receiving a concomitant azole antifungal had a significantly longer time to PLT >100,000 cells/mm3 (median 28 vs 22 days, p=0.0075)
Infectious Complications
Infectious complications during induction were also collected. Neutropenic fever occurred in a majority of patients (55%) with infection documented in 28 patients (44%), including pneumonia (n=21), cellulitis (n=7), and bacteremia due to gram-positive (n=3) or gram-negative (n=2) organisms. Other infections included pharyngitis (n=1) and sinusitis (n=1). No difference in rate of neutropenic fever, documented infection, or type of infection was observed in patients treated with 10 days of decitabine compared to standard HMA dosing (Supplemental Table 1).
Six patients (9%) had a possible (n=3) or probable (n=3) fungal pneumonia. One patient had a possible fungal pneumonia at initial presentation, the remaining 5 were breakthrough IFIs (bIFI), occurring while patients were receiving either triazole or echinocandin primary prophylaxis. Among the 3 patients with a probable bIFI, 2 were receiving ISA, and the other VCZ prophylaxis. The 2 possible bIFI occurred in patients receiving ISA (n=1) and caspofungin (n=1). Patients with a documented infection during induction had longer median time to PLT50 (28 versus 21 days, p=0.0019) and PLT100 (31 days versus 23 days, p=0.0127) compared to those who did not have a documented infection. Among the 28 patients with a documented infection, 5 (18%) were receiving VCZ, 6 (21%) an echinocandin, and 8 patients each (29%) were receiving ISA or PCZ. The 1 patient receiving FCZ for prophylaxis also had a documented infection.
Multivariable Analysis
Variables with p-value < 0.1 on log rank analysis were included in multivariable Cox regression analysis for time to PLT50 and time to PLT100 (Table 4) including documented infection, concomitant azole antifungal, VEN duration, and AML subtype. Documented infection (HR: 0.43 95%CI: 0.25, 0.73, p=0.002) and concomitant azole antifungal (HR: 0.36 95%CI 0.19, 0.69, p=0.002) were found to be significantly associated with a longer time to PLT50 on multivariable analysis. Duration of VEN had an inverse relationship with time to PLT50 (HR: 1.06; 95% CI: 1.00, 1.11, p=0.038) which also maintained significance on multivariable analysis. Secondary and t-AML was not significant on multivariable analysis for either PLT50 (HR: 1.83 95%CI: 0.95, 3.5, p=0.07) or PLT100 (HR: 1.56 95%CI: 0.81,3.02, p=019). Documented infection (HR: 0.5; 95%CI: 0.29, 0.87, p=0.013) and duration of VEN (HR: 1.07; 95%CI: 1.01, 1.13, p=0.015) remained significant on multivariable analysis for time to PLT100, however concomitant azole antifungal did not (HR: 0.7; 95%CI: 0.34, 1.43, p=0.322).
Table 4.
Select univariate and multivariable analysis results
| Variable | Median days to PLT > 50 (95%CI) | Univariate p-value | HR (95%CI), p-value | Median days to PLT > 100 (95%CI) | Univariate p-value | HR (95%CI), p-value |
|---|---|---|---|---|---|---|
| AML subtype | 1.83 (0.95, 3.50), p=0.07 | 1.56 (0.81, 3.02), p=0.19 | ||||
| sAML/t-AML | 27 (21, NA) | 0.014 | 29 (22, NA) | 0.044 | ||
| de novo AML | 23 (20, 26) | 24 (22, 29) | ||||
| Concomitant azole antifungal | 0.36 (0.19, 0.69), p=0.002 | 0.70 (0.34, 1.43), p=0.32 | ||||
| No | 19 (16, 23) | 0.0001 | 22 (20, 25) | 0.008 | ||
| Yes | 26 (23, 29) | 28 (23, 34) | ||||
| Venetoclax duration, days | 1.06 (1.00, 1.11), p=0.04† | 1.07 (1.01, 1.13), p=0.01† | ||||
| >21 | 19 (17, 25) | 0.042 | 22 (20, 27) | 0.047 | ||
| 15-21 | 26 (23, 31) | 31 (24, 39) | ||||
| ≤ 14 | 29 (22, NA) | 32 (23, NA) | ||||
| Documented infection | 0.43 (0.25, 0.73), p=0.002 | 0.50 (0.29, 0.87), p=0.01 | ||||
| No | 21 (18, 24) | 0.002 | 23 (21, 27) | 0.013 | ||
| Yes | 28 (25, 34) | 31 (26, 50) | ||||
PLT: platelet count (cells/mm3); sAML/t-AML: secondary AML, therapy-related AML
Analyzed as continuous variable
Discussion
Venetoclax in combination with HMA improves remission rates and leads to prolonged overall survival in elderly patients with newly diagnosed AML, and has already become a new standard of care in this patient population5. Overall this combination is well tolerated, however febrile neutropenia and grade ≥3 infection are common, occurring in 35%-61% of patients2, 5, 19, 20, demonstrating the need for antimicrobial prophylaxis, including a mold-active antifungal agent due to prolonged severe neutropenia. As a significant interaction mediated by the CYP3A4 enzyme exists between the CYP3A4 inhibiting azole antifungals and VEN, a CYP3A4 substrate11, dose reductions for VEN are recommended when given in combination with azole antifungals depending upon the assumed degree of CYP3A4 inhibition by each azole. Despite these recommendations, there is little clinical data describing patient outcomes with these combinations. This retrospective review describes the duration of myelosuppression observed in patients receiving VEN and HMA therapy while receiving concomitant azole antifungals during the first cycle of therapy.
This analysis included an elderly population in which HMA and VEN combination therapy was the treatment of choice. Of the 64 patients included, the median age was 72 years, 91% were ≥ 65 years and 72% were ≥ 70 years, with most patients (69%) receiving treatment for de novo AML. Although patients were treated with either azacitidine or decitabine, it is important to note that 75% received decitabine 20 mg/m2 IV daily for 10 days during induction in combination with VEN. Concomitant azole antifungals were used in 48 patients (78%) who were receiving VEN and HMA therapy, consistent with the recommendation to use azole antifungals in patients undergoing induction therapy for AML known to result in prolonged and profound neutropenia9.
Multiple patient and disease-related factors have the potential to impact duration of myelosuppression in AML patients, including age and disease subtype (i.e. de novo AML vs sAML/t-AML), in addition to treatment-based factors including HMA and VEN dosing and schedule. The majority of patients (75%) in this analysis received decitabine 20 mg/m2 IV daily for 10 days, a regimen often associated with prolonged myelosuppression, particularly neutropenia21, 22. With the addition of VEN to 10 days of decitabine, the median time to ANC > 500 cells/mm3 was 42 days (95%CI: 37-46) after course 1 in patients with newly diagnosed AML, similar to the median time of 34 days observed in this cohort20. The prolonged neutropenia anticipated with 10 days of decitabine in combination with VEN likely prevented the ability to discern any specific factors to be associated with duration of neutropenia in this cohort.
Unlike neutropenia, differences in time to PLT count recovery were observed based on antifungal usage, VEN duration, and documented infection. Median time to achieve PLT > 50,000 cells/mm3 was 7 days longer for patients receiving azole antifungals compared to those receiving an echinocandin (p<0.001), and 6 days longer for PLT > 100,000 cells/mm3 (p=0.0062). Patients receiving either PCZ or VCZ achieved PLT >100,000 cells/mm3 after a median of 31 days (95%CI: 23, 41) compared to 27 days (95%CI: 31, 35) with ISA or FCZ, and 22 days (95%CI: 20, 25) with echinocandin prophylaxis (p=0.022). Hypomethylating agents alone typically result in a shorter duration of thrombocytopenia versus neutropenia, possibly allowing for the differences in time to PLT recovery to be more readily observed. Azole antifungal agents as a class are not known to cause thrombocytopenia, therefore the prolonged time to PLT recovery observed in patients receiving these agents is most likely due to higher VEN exposure that is achieved when given in combination with CYP3A4 inhibitors.
Venetoclax dosing recommendations are 400 mg once daily in patients not receiving a concomitant CYP3A4 inhibitor, such as the azole antifungals. Patients receiving a concomitant moderate inhibitor (i.e. FCZ, ISA) should receive 200 mg of VEN daily, while those receiving a strong inhibitor such as VCZ should receive 100 mg daily. The dose reduction for VEN with PCZ specifically is 70 mg once daily16. This finding is based on the pharmacokinetic analysis that in combination with PCZ there is greater VEN exposure in patients receiving 100 mg of VEN, but insufficient exposure in those receiving 50 mg of VEN compared to the standard 400 mg dosage11. Despite this dosing recommendation, the availability of VEN as 10 mg, 50 mg, or 100 mg tablets requires patients to take multiple tablets per day to create the 70 mg dosage. Having multiple tablet sizes increases the risk for medication errors in addition to higher copays. As a result, the 100 mg dosage is often utilized for patients receiving PCZ. Due to this common practice, this analysis considered VEN 100 mg daily with PCZ to be equivalent to 400 mg VEN without a concomitant azole. While the use of azole antifungals and strong CYP3A4 inhibitors were associated with longer time to PLT recovery, significantly longer myelosuppression was not observed in patients receiving PCZ compared to VCZ or any other azole. In fact, the combination with VCZ was found to result in the longest time to PLT recovery. This likely indicates that VEN may be dosed safely at 100 mg in patients receiving either VCZ or PCZ in the setting of VEN and HMA therapy.
Documented infections occurred in 28 (44%) of patients during cycle 1, similar to what has been previously reported in patients treated with HMA and VEN2, with pneumonia being the most common. While the anticipated rate of IFI in patients treated with VEN and HMA therapy is relatively unknown, the incidence of bIFI in this cohort was 7.8%, similar to what has been reported in patients with AML undergoing remission induction chemotherapy receiving mold-active antifungal prophylaxis7, 23. A retrospective analysis of 116 patients reported a 13% incidence of IFI among patients with newly diagnosed or relapsed/refractory AML treated with VEN and HMA therapy10. The majority of patients included were receiving prophylaxis with an echinocandin (38%) or azole antifungal (41%), resulting in a bIFI rate of 13.8%10. One prospective study with VEN and HMA therapy reported an 8% rate of grade ≥ 3 IFI with an additional 3 patients whose 30-day mortality was attributed to pneumonia or lung infection2. Given the difficulty in diagnosing IFIs, it is unknown whether these patients truly also had an IFI. Although azole antifungals were not allowed, 46% of patients received echinocandin prophylaxis2. Similarly, 41% of patients in VIALE-A received antifungal prophylaxis with a mold-active azole (21%), echinocandin (15%), or amphotericin B (5%)12 and all patients treated with VEN and 10-day decitabine received echinocandin or mold-active azole antifungal prophylaxis13, although the incidence of IFI is not reported in either of these trials. The bIFI rate observed in our cohort as well as that reported by Aldoss et al. is similar to what is has been observed among patients undergoing intensive remission induction chemotherapy, warranting anti-mold prophylaxis in patients receiving VEN and HMA therapy. While initial investigation of VEN in AML avoided the combination of CYP3A4 inhibitors given the lack of pharmacokinetic data, this report provides clinical data to support the safety of combining azole antifungals with VEN at appropriate dose reductions when combined with HMA.
Unexpectedly, duration of VEN therapy had an inverse relationship on PLT count recovery. The reason for this observation is likely multifactorial. Duration of VEN was largely dependent upon a mid-cycle bone marrow biopsy, often conducted around day 21. Patients with no evidence of leukemia stopped further VEN in accordance with clinical trial recommendations13 and other published HMA and VEN combination strategies in an effort to improve count recovery2, 5. Therefore, patients tolerating therapy well and achieving blast clearance typically received 21-23 days of VEN depending upon the timing of biopsy results. On the other hand, patients with declining clinical status or significant infections could have VEN stopped early if in the best interest of the patient. As administering VEN for ≤14 days during AML induction is not standard practice, likely patients receiving this short course had a poor performance status, comorbidities, were elderly, or had concomitant infections precluding the continuation of VEN. While age was not associated with significantly longer time to ANC or PLT recovery amongst the entire cohort, it is worth noting among these 9 patients receiving a shorter course of VEN, the median age was 73 years (range, 45-89), and 33% were 80 years of age or older. In addition, 67% of this subset of patients had a documented infection, and all but 2 patients were receiving a concomitant strong CYP3A4 inhibitor, likely also contributing to the prolonged thrombocytopenia. Thus, this unexpected finding is likely related to competing patient characteristics rather than the duration of VEN itself.
Given the retrospective nature of this analysis, a number of limitations are present. Patients were included if they were treated with either azacitidine or decitabine, given at any dosing schedule. The majority of patients in this cohort received decitabine for 10 days, which may have prevented a difference in neutropenia from being observed in patients receiving an azole antifungal, or other subgroups of interest such as older patients and those with sAML/t-AML. Although HMA schedule was evaluated, this limits the applicability to patients receiving azacitidine and decitabine for 5 days. Only a minority of patients received standard HMA dosing which precluded additional analyses, however when evaluated separately similar associations in this subset were observed including an effect of concomitant azole antifungal on time to PLT recovery and not ANC recovery, and should be explored in a larger dataset. Similarly, patients received VEN at various durations. However, this appropriately reflects the changes that occur in clinical practice and is a real-world representation of this therapy. Providers may hold VEN in the context of declining clinical status or in elderly and frail patients. Given the prolonged time to PLT recovery observed in patients receiving azole antifungals, it is worth noting that bleeding events were not collected and therefore could not be evaluated in patients receiving azole antifungals. Despite this, prospective studies of VEN and HMA that allow the inclusion of azole antifungals have not described increased rates of bleeding events in patients receiving a concomitant azole antifungal2, 13, 19, 20.
In conclusion, prolonged myelosuppression is anticipated with the use of VEN and HMA therapy. Prophylaxis with azole antifungals are preferred for patients with AML undergoing induction therapy expected to result in prolonged neutropenia. Therefore, the combination of VEN with azole antifungals is inevitable, creating a need to understand the effect of this combination on both ANC and PLT recovery. Time to ANC recovery was not impacted, while time to PLT recovery was modestly prolonged in those receiving concomitant azole antifungals. Concomitant PCZ and VCZ with VEN 100 mg had similar time to ANC and PLT recovery, indicating VEN may be administered at the same dosage with either PCZ or VCZ during course 1 of therapy with VEN and HMA. Pharmacokinetic analyses of plasma VEN concentrations in patients receiving concomitant VCZ as well as other azole antifungals should be evaluated as previously described with PCZ11. Conducting these analyses in a large dataset with the goal to associate an obtainable value (e.g. peak, trough, or AUC) with clinical outcomes including both efficacy and safety would be ideal. These studies are warranted and could provide a target venetoclax level to be incorporated into future clinical practice.
Supplementary Material
Funding/Acknowledgement
This research was supported in part by the Research Project Grant Program (R01CA235622) from the National Institutes of Health.
Conflicts of Interest
CDD reports personal fees from AbbVie, Agios, Novartis, ImmuneOnc, Daiichi Sankyo, Celgene, Jazz Pharmaceuticals, and Notable Labs, outside the submitted work. AM reports research funding from Celgene. GB reports research funding from AbbVie, Incyte, Janssen, GSK, Cyclacel, and Oncoceutics; consultancy and research funding from BioLine; and consultancy from NKarta and PTC Therapeutics, outside the submitted work. NP reports personal fees from Pacylex Pharmaceuticals, Incyte, LFB Biotechnologies, Roche Diagnostics, and Blueprint Medicines; grants from Affymetrix and SagerStrong Foundation; personal fees and other from Novartis; non-financial support and other from Stemline Therapeutics; personal fees and non-financial support from Celgene, MustangBio, and DAVA Oncology; personal fees, non-financial support, and other from AbbVie; and other from Samus Therapeutics, Cellectis, Daiichi Sankyo, and Plexxikon, outside the submitted work. ND reports grants from AbbVie, Genentech, Astellas, Daiichi-Sankyo, Pfizer, Bristol-Myers Squibb (BMS), Immunogen, Novimmune, and Forty Seven; and personal fees from AbbVie, Genentech, Astellas, Daiichi-Sankyo, Pfizer, BMS, Immunogen, Jazz Pharmaceuticals, Trillium, Forty Seven, Gilead, Kite, and Novartis, outside the submitted work. KS reports honoraria from Otsuka; is on the advisory board for Daiichi-Sankyo and Pfizer Japan; and reports research funding and is on the advisory board for Novartis, outside the submitted work. GCI reports research funding from Celgene and served on an advisory board for Novartis. KT reports personal fees for service on advisory boards of Symbio Pharmaceuticals, GSK, and Celgene, outside the submitted work. DPK reports personal fees and other from Merck & Co., Gilead Sciences, and United Medical; other from Astellas Pharma, Pharma Pharmaceutical Industries, Cidara Therapeutics, Amplyx Pharmaceuticals, and Mayne Pharma, outside the submitted work. FR reports personal fees and research grants from AbbVie, outside the submitted work. HK reports grants and other from AbbVie, Agios, Amgen, Immunogen, and Pfizer; grants from Ariad, Astex, BMS, Cyclacel, Daiichi-Sankyo, Jazz Pharma, and Novartis; and other from Actinium and Takeda, outside the submitted work. MK reports grants from NIH, NCI, Abbvie, Genentech, Stemline Therapeutics, Forty Seven, Eli Lilly, Cellectis, Calithera, Ablynx, and Astra Zeneca; consulting or honorarium from AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty Seven, and Kisoji; clinical trial support from Ascentage; and has stocks or royalties in Reata Pharmaceutical, outside the submitted work. All other authors declare no competing conflicts of interest.
References
- 1.N H, AM N, M K, et al. SEER Cancer Statistics Review, 1975-2013. Accessed December, 2016.
- 2.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. January 2019;133(1):7–17. doi: 10.1182/blood-2018-08-868752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei AH, Strickland SA, Hou JZ, et al. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J Clin Oncol. May 2019;37(15):1277–1284. doi: 10.1200/JCO.18.01600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for patients with untreated AML ineligible for intensive chemotherapy: phase 3 randomized placebo-controlled trial. Blood. March 2020; doi: 10.1182/blood.2020004856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. August 2020;383(7):617–629. doi: 10.1056/NEJMoa2012971 [DOI] [PubMed] [Google Scholar]
- 6.Bow EJ, Loewen R, Cheang MS, Schacter B. Invasive Fungal Disease in Adults Undergoing Remission-Induction Therapy for Acute Myeloid Leukemia: The Pathogenetic Role of the Antileukemic Regimen. Clinical Infectious Diseases. 1995;21(2):361–369. doi: 10.1093/clinids/21.2.361 [DOI] [PubMed] [Google Scholar]
- 7.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. January 2007;356(4):348–59. doi: 10.1056/NEJMoa061094 [DOI] [PubMed] [Google Scholar]
- 8.Biehl LM, Vehreschild JJ, Liss B, et al. A cohort study on breakthrough invasive fungal infections in high-risk patients receiving antifungal prophylaxis. J Antimicrob Chemother. September 2016;71(9):2634–41. doi: 10.1093/jac/dkw199 [DOI] [PubMed] [Google Scholar]
- 9.Taplitz RA, Kennedy EB, Bow EJ, et al. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J Clin Oncol. October 2018;36(30):3043–3054. doi: 10.1200/JCO.18.00374 [DOI] [PubMed] [Google Scholar]
- 10.Aldoss I, Dadwal S, Zhang J, et al. Invasive fungal infections in acute myeloid leukemia treated with venetoclax and hypomethylating agents. Blood Adv. December 2019;3(23):4043–4049. doi: 10.1182/bloodadvances.2019000930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal SK, DiNardo CD, Potluri J, et al. Management of Venetoclax-Posaconazole Interaction in Acute Myeloid Leukemia Patients: Evaluation of Dose Adjustments. Clin Ther. February 2017;39(2):359–367. doi: 10.1016/j.clinthera.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 12.DiNardo C, Jonas B, Pullarkat V, et al. A randomized, double-blind, placebo-controlled study of venetoclax with azacitidine vs azacitidine in treatment-naive patients with acute myeloid leukemia ineligible for intensive therapy-VIALE-A. EHA Library 2020. [Google Scholar]
- 13.DiNardo CD, Maiti A, Rausch CR, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol. September 2020; doi: 10.1016/S2352-3026(20)30210-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. December 2003;21(24):4642–9. doi: 10.1200/JCO.2003.04.036 [DOI] [PubMed] [Google Scholar]
- 15.Rausch CR, DiNardo CD, Maiti A, et al. Venetoclax Dosing in Combination with Antifungal Agents: Real World Experience in Patients with Acute Myeloid Leukemia. Blood. 2019;134(Supplement_1):2640–2640. doi: 10.1182/blood-2019-131988 [DOI] [Google Scholar]
- 16.Venclexta (venetoclax) [package insert]. Chicago, IL: AbbVie; 2018. [Google Scholar]
- 17.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. June 2008;46(12):1813–21. doi: 10.1086/588660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornely OA, Hoenigl M, Lass-Flörl C, et al. Defining breakthrough invasive fungal infection-Position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses. September 2019;62(9):716–729. doi: 10.1111/myc.12960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiti A, DiNardo CD, Cortes JE, et al. Interim Analysis of Phase II Study of Venetoclax with 10-Day Decitabine (DEC10-VEN) in Acute Myeloid Leukemia and Myelodysplastic Syndrome. Blood. 2018;132(Suppl 1):286–286. doi: 10.1182/blood-2018-99-113749 [DOI] [Google Scholar]
- 20.Maiti A, DiNardo CD, Rausch CR, et al. Ten-Day Decitabine with Venetoclax (DEC10-VEN) in Acute Myeloid Leukemia: Updated Results of a Phase II Trial. Blood. 2019;134(Supplement_1):2637–2637. doi: 10.1182/blood-2019-127803 [DOI] [Google Scholar]
- 21.Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A. April 2010;107(16):7473–8. doi: 10.1073/pnas.1002650107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Short NJ, Kantarjian HM, Loghavi S, et al. Treatment with a 5-day versus a 10-day schedule of decitabine in older patients with newly diagnosed acute myeloid leukaemia: a randomised phase 2 trial. Lancet Haematol. January 2019;6(1):e29–e37. doi: 10.1016/S2352-3026(18)30182-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bose P, McCue D, Wurster S, et al. Isavuconazole as Primary Anti-Fungal Prophylaxis in Patients with Acute Myeloid Leukemia or Myelodysplastic Syndrome: An Open-Label, Prospective, Phase II Study. Clin Infect Dis. April 2020; doi: 10.1093/cid/ciaa358 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


