Abstract
This brief definitive review of Herpes Zoster (HZ) will cover the current state of knowledge and questions that remain to be answered regarding HZ in general, as well as Herpes Zoster Ophthalmicus (HZO) in particular. A question-and-answer format will be used to address various important topics related to this common and serious disease. Questions to be addressed relate to common misconceptions, contagiousness of infection, unknowns regarding pathogenesis, rising incidence, risk factors and complications, relationship with temporal arteritis, vaccination, and current and future antiviral treatment. In addition, the importance of the Zoster Eye Disease Study (ZEDS) to determine the efficacy of suppressive valacyclovir treatment in preventing complications of HZO and the need to support enrollment will be discussed.
Keywords: Herpes Zoster, Herpes Zoster Ophthalmicus, vaccination, Zoster Eye Disease Study
Introduction
Herpes zoster (HZ) is caused by localized reactivation of the varicella-zoster virus (VZV) resulting in a unilateral painful rash in a dermatomal distribution in patients who have had chickenpox or varicella. herpes zoster ophthalmicus (HZO) is a term used to describe HZ when it involves the first and/or sometimes second division of the fifth trigeminal cranial nerve supplying the face and is associated with ocular involvement. HZ is a very common disease with over 1 million new cases per year in the United States (US). It has been estimated that 10 to 20% of cases involve the trigeminal nerve and put the eye at risk, although a very recent report suggests a figure of 7.9% (1). HZO is often complicated by acute, recurrent and/or chronic eye disease with many diverse manifestations; postherpetic neuralgia (PHN), a chronic pain syndrome; and life-threatening stroke, though rare. This review will focus on our current state of knowledge and remaining questions regarding this important disease that threatens vision, quality of life, and life itself. A question-and-answer format has been chosen to address commonly asked questions.
1. What are common misconceptions about HZ?
The first common misconception is that HZ is a disease of the elderly (typically defined as 65 years and older). While the rate of disease does increase with age, the number of cases is greatest among people in their 50s (2). In the most recent published data, the median age at onset is 56 years, and one-quarter of the cases occurred in persons age 43 to 56 years old (3). A second common misconception is that HZ is a disease of immunocompromised patients. While these individuals are at increased risk for HZ, including more severe disease, over 90% of patients with HZ are not immunosuppressed (4).
A third common misconception of HZ is that vaccination against varicella/chickenpox is associated with the rising incidence of HZ, when the evidence does not support this. The Hope Simpson hypothesis stated years ago that exogenous exposure to chickenpox boosts immunity and delays the onset of zoster (5). However, data has shown that the rising incidence of HZ began before chickenpox vaccination was introduced in the United States in 1995 and has continued afterwards without acceleration (4, 6).
2. How contagious is HZ?
Unlike chicken pox, shingles is not very contagious. Chicken pox is highly contagious by airborne spread. If someone has been exposed and does not develop chicken pox, it is likely that they already had it, or they developed a mild case that was not diagnosed. Shingles spreads by direct contact with fluid in vesicles of the acute rash to persons without a past history of chicken pox, who then develop chicken pox and not zoster. In the US, the vast majority of people have had chicken pox, or have been vaccinated against varicella if born after 1995, and according to the Centers for Disease control (CDC) are not at risk (https://www.cdc.gov/shingles/about/transmission.html). However, direct contact with fluid from vesicles should be avoided in general, and especially by persons without a history of chicken pox or vaccination against it who are pregnant or immunocompromised and therefore at risk for developing severe chicken pox.
3. What are some major unknowns about herpes zoster?
It is commonly stated that HZ results from reduced cell-mediated immunity to the varicella-zoster virus (VZV) with increasing age or immunosuppression resulting in localized unilateral reactivation of latent VZV virus. It is known that latent VZV exists in many ganglia. Herpes virus latency and reactivation is an area of active scientific research and is complex. But why in most cases does zoster occur in the distribution of a single dermatome if the cause is reduced immunity to VZV? Occasionally HZ occurs in 2 adjacent dermatomes, but very rarely in severely immunocompromised patients, is it disseminated. Why is it almost always unilateral, a feature so characteristic that it greatly facilitates the diagnosis? And given the widespread latency of VZV in ganglia, why does HZ occur in some locations much more often than in others? Local factors in addition to the process of latent viral reactivation are questions remaining regarding HZ. In addition, why is HZO more common in women and less common in Blacks and Hispanic persons than in whites (1)? All of these are questions merit further research.
4. Why is the incidence of zoster still rising?
Although the Hope Simpson hypothesis that exposure to chickenpox boosts immunity to VZV and delays the onset of zoster has led to the opinion that vaccination against chickenpox is associated with the increasing incidence of zoster, the evidence does not support this (4, 5, 6). The rising incidence of zoster began before the introduction of chickenpox vaccination in the US in 1995 and has continued afterwards (4).
According to recent publications reporting data from 1994 through 2018, the incidence of HZO has increased 3.6% per year (1, 3). Although the incidence of HZ and HZO has decreased since 2007 in persons less than 21 and greater than 60 years old, the increase in the 31 to 60 year age group has resulted in a continued overall increase. The data is consistent with vaccination against chicken pox and zoster being associated with a decrease in HZ among age groups with recommended vaccinations, and it suggests that expansion of vaccination against zoster to younger age groups would likely be beneficial. Currently, vaccination against zoster is approved by the US Food and Drug Administration (FDA) for the Recombinant Zoster Vaccine (RZV, Shingrix, GlaxoSmithKline, Research Triangle Park, NC) for adults aged 50 years and older. However, vaccination of adults age 30 to 50 years old should be studied since RZV appears to have a long duration of effectiveness, and it is generally now widely available in the US. These recent publications also confirm the decreased rate of disease among men (0.74) compared to women, and Blacks (0.75) and Hispanics (0.64) compared to Whites, for unknown reasons.
One can speculate that increasing use of statins, or even possibly the decreasing rate of smoking might be contributing to the increasing rate of HZ, but there is more speculation than evidence-based answers to this important question (7, 8).
5. What are new risk factors for herpes zoster?
Everyone with a history of chickenpox, whether they know it or not, is at risk for zoster. In addition to the widely recognized risk factors of increasing age, immunocompromise, and female sex, there is an ever-growing list of additional risk factors (9). These include depression, family history of HZ, stress, traumatic brain injury, and heart failure, which have all been reported to increase the risk by 2 or more times. In addition, immune-mediated diseases, acute kidney disease, asthma, diabetes, and chronic obstructive pulmonary disease have been reported to significantly increase the risk of developing HZ. A number of recent papers have reported that use of statins is a risk factor for the development of HZ, and that the risk is dose dependent (7). The Increased risk associated with statin use has been reported among patients less than age 49 years, leading to the question of whether vaccination against HZ should be evaluated in this population. The mechanism of the association of various risk factors with HZ is unclear. Rather than trying to remember the importance of vaccination against zoster in patients with various medical conditions that are risk factors for zoster, it is preferable to follow current CDC recommendations for vaccination of immunocompetent adults age 50 or older. Awareness of increased risk among populations of patients should lead to further study of vaccination at age younger than age 50 in these populations.
6. What are the most serious and common complications of HZO?
Serious and common complications of HZO include acute, chronic and/or recurrent keratitis and iritis, neurotrophic keratopathy, postherpetic neuralgia (PHN), and uncommon but potentially life-threatening stroke. Acutely, unilateral pain in the V1 distribution precedes the onset of the typical rash and the diagnosis is delayed. (10). The pain is often unlike anything the patient has experienced before and can be described as itching, stinging, lightning bolts, or a feeling of insects running on the forehead. Radicular pain in a unilateral dermatomal distribution without a rash due to HZ (herpes zoster sine herpete) can occur, and often the diagnosis is missed with the acute recommended high-dose oral antiviral treatment not being given (11). Extension of the rash to the tip of the nose, referred to as Hutchinson’s sign, increases the likelihood of the eye becoming involved due to shared innervation.
Acute eye disease may occur early or develop within one month after the onset of the rash, and as such, patients need to be followed even after the rash starts to improve (12). All tissues in the eye and orbit can be involved. Acute anterior segment disease includes dendriform epithelial keratitis (DEK: Fig 1 and 2), stromal keratitis (SK) with stromal infiltrates without (Fig 3) or with ulceration (Fig 4), endothelial keratitis (EK) with edema and keratic precipitates (KP), and/or iritis. Anterior segment manifestations can be categorized similarly to HSV eye disease (13). DEK is treatable with topical or systemic antivirals. SK, EK and iritis are treated with topical steroids requiring a very gradual taper to the lowest possible potency and frequency sometimes less than once daily that prevents recurrent inflammation. In very severe cases there may be involvement of the optic nerve, other cranial nerves, and orbit.
Figure 1:
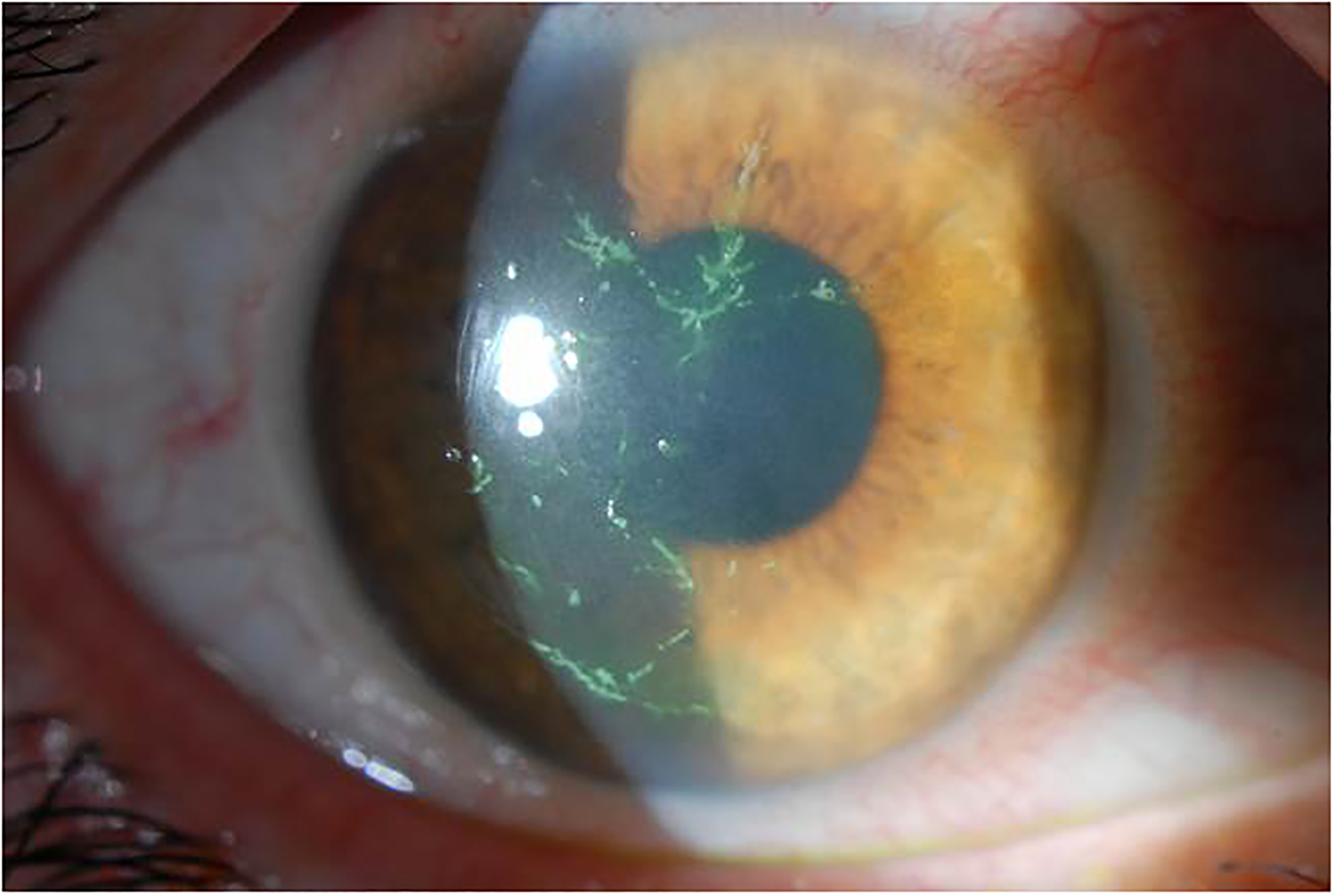
Dendriform epithelial keratitis. (Courtesy of Christopher J. Rapuano, MD)
Figure 2:
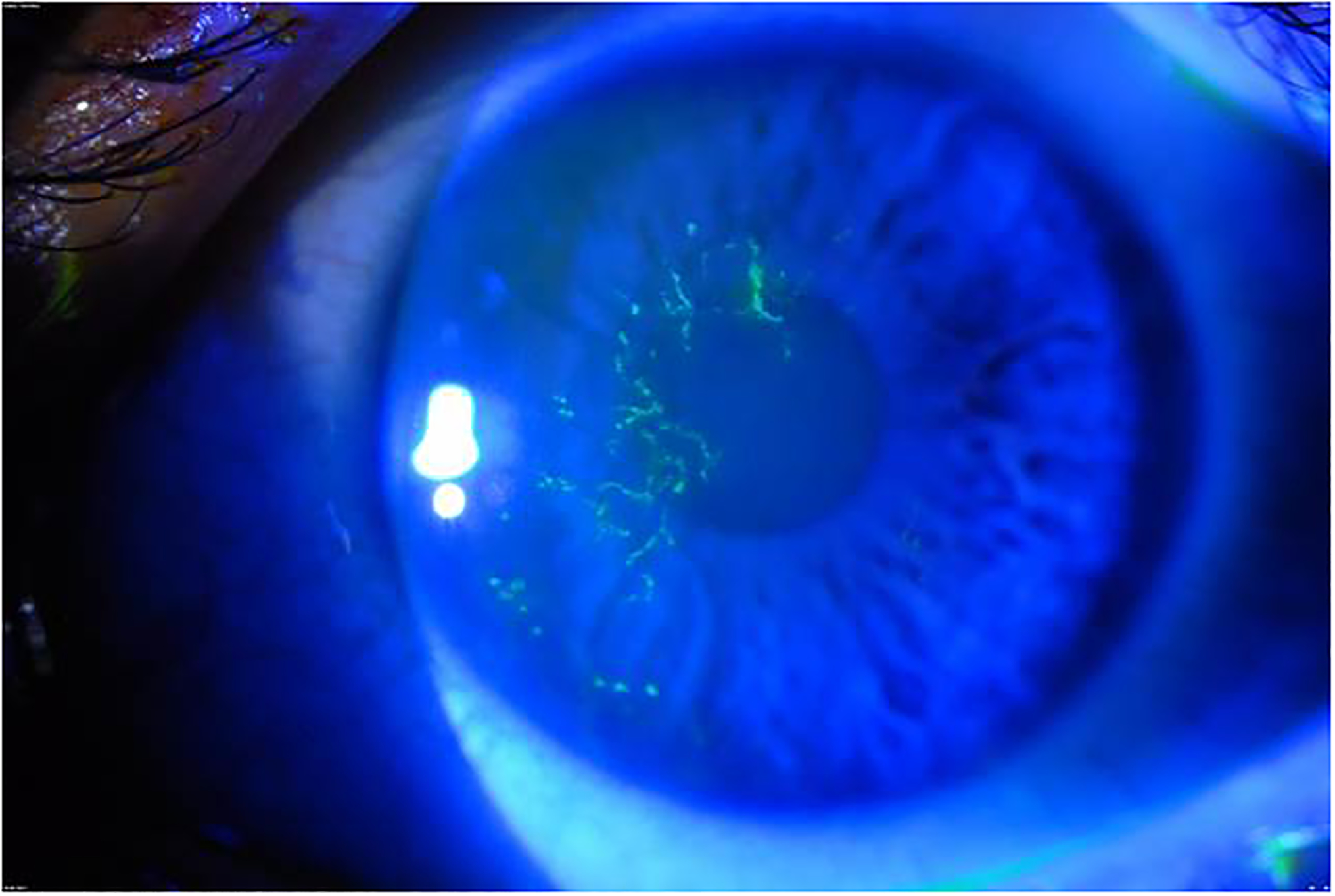
Fluorescein staining of dendriform epithelial keratitis seen under cobalt blue filter. (Courtesy of Christopher J. Rapuano MD)
Figure 3:
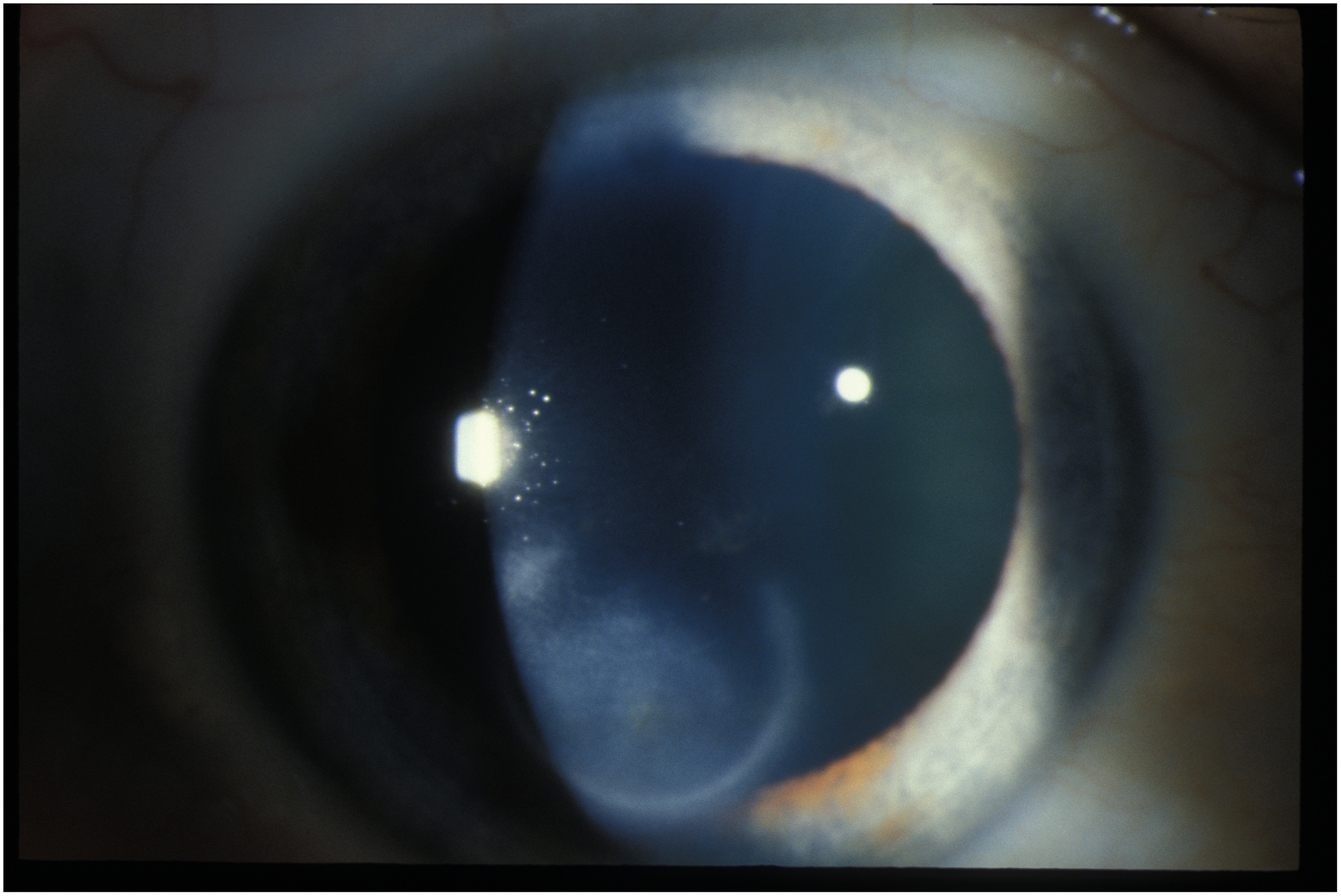
Herpes zoster stromal keratitis with infiltrates without ulceration.
Figure 4:
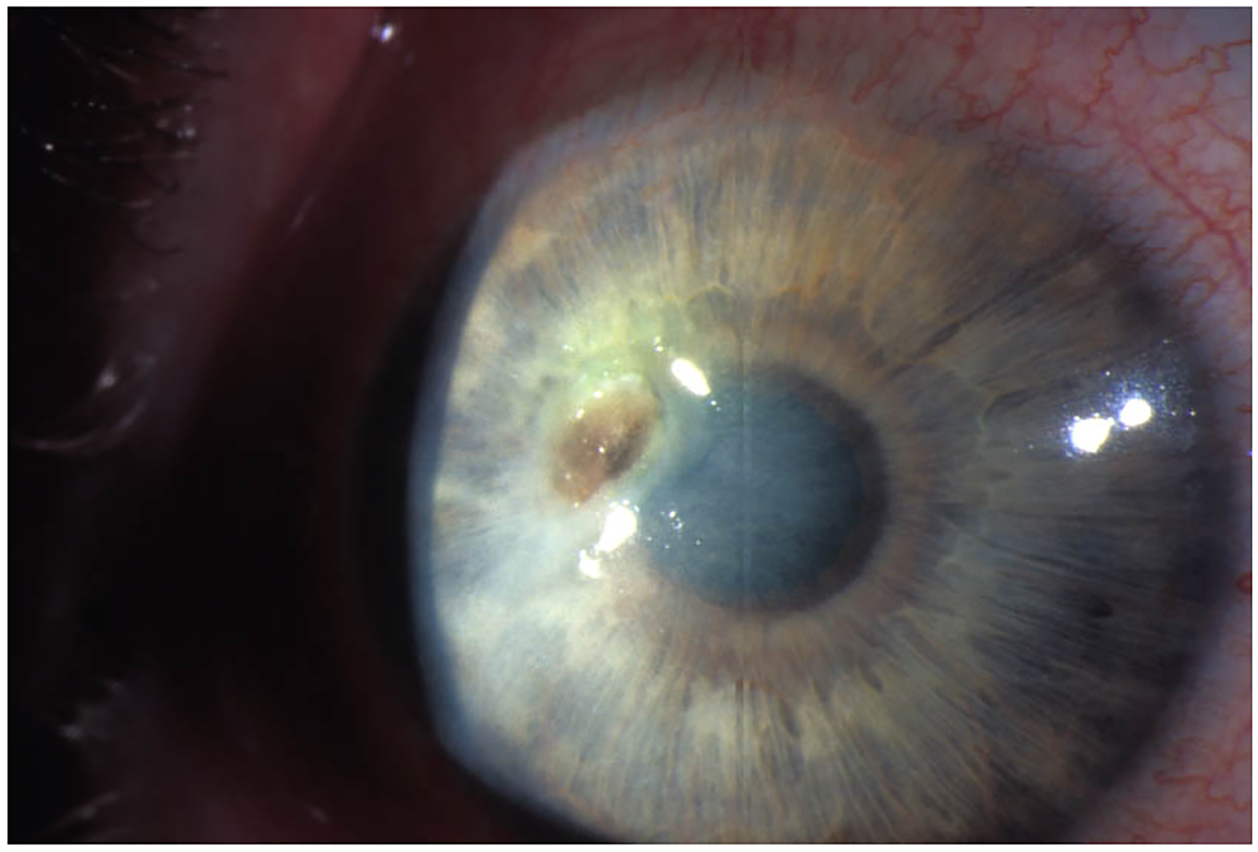
Herpes zoster stromal keratitis with ulceration.
Chronic eye disease, defined as disease persisting for 3 or more months, occurs in about one-quarter of cases, and recurrent eye disease increases with time and develops in about one-quarter of cases over 5 years. (14). Recommended acute oral antiviral treatment reduces chronic disease at 6 months from approximately 50% to 30% (15). The patients with chronic disease may require lifelong low-dose topical steroid treatment to prevent recurrent inflammation and monitoring for complications of topical steroids. Over 10% of HZO patients develop moderate (9.6%, less than or equal to 20/50) or severe (3.6%, less than or equal to 20/200) vision loss, most commonly due to scarring (12). Significant risk factors for severe vision loss are older age, immunocompromise, poor presenting vision, and uveitis. Cataract surgery in patients with a history of HZO may result in retinal complications reducing visual acuity or may instigate recurrent HZ ocular disease (16, 17). Penetrating keratoplasty in patients with HZO is complicated in about one-quarter of cases by ocular surface healing problems or glaucoma, negatively impacting visual outcomes. (18).
Neurotrophic keratopathy (NK) with or without melting, perforation or microbial superinfection is an especially challenging form of chronic eye disease in HZO (Fig 5). NK has become an increasingly recognized condition that has recently been most accurately defined as “a disease related to alterations in corneal nerves leading to impairment in sensory and trophic function with consequent breakdown of the corneal epithelium, affecting health and integrity of the tear film, epithelium and stroma (19). Herpes zoster is one of the more common causes of NK, occurring in 12.8% of HZ keratitis cases with a prevalence of 16 per 100,000 persons (20). Although “herpetic keratitis” from both herpes simplex and herpes zoster are oftentimes lumped together, it important to note that the NK that results from herpes zoster tends to occur more frequently, and is usually more severe (21). Regardless of the etiology, NK is a challenging condition to manage. However, recognizing that eyes with HZ keratitis are at risk for development of NK is important, as a heightened awareness will allow for prompt recognition and treatment.
Figure 5:
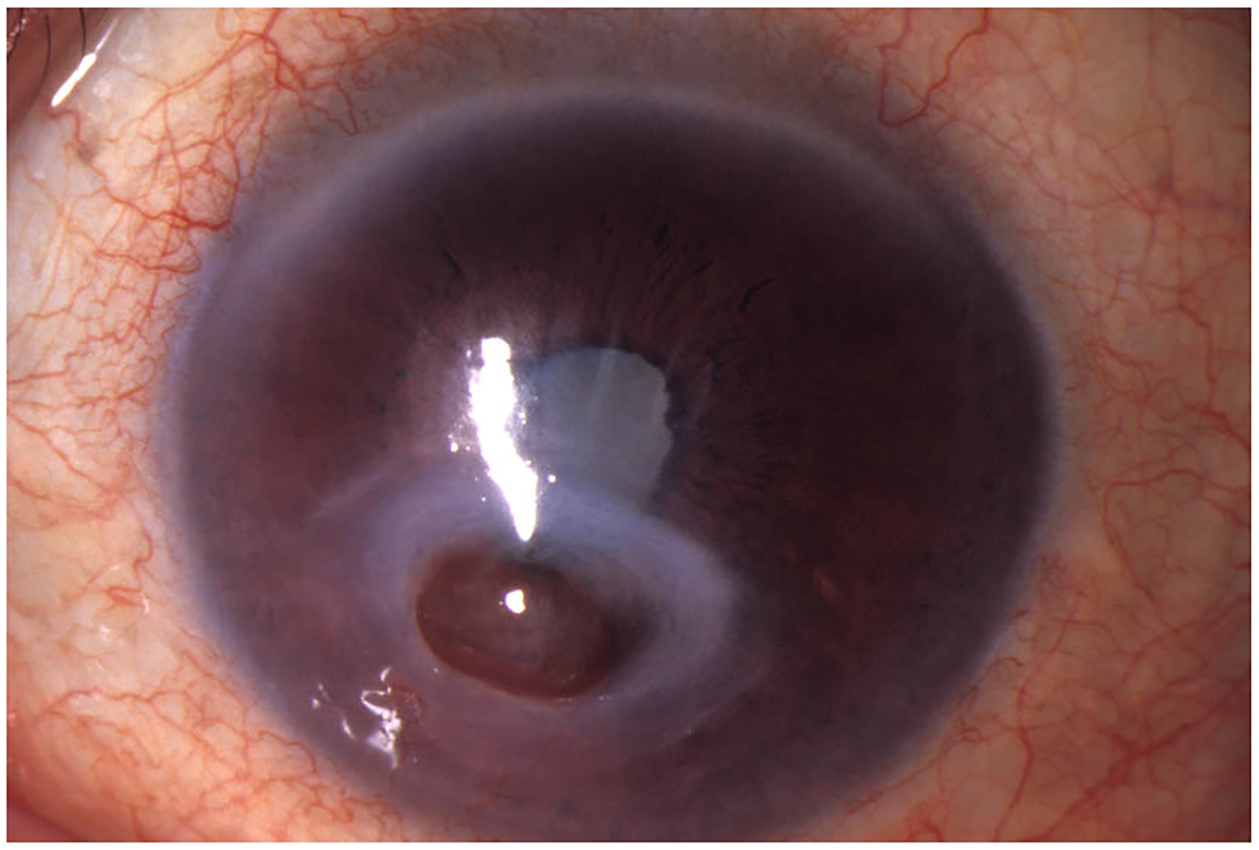
Neurotrophic keratopathy from herpes zoster with resultant perforation.
For eyes with HZ keratitis, corneal sensation should be checked (with cotton tip wisps, dental floss, or a Cochet-Bonnet esthsiometer) to determine if there is decreased sensation that will put the eye at risk for NK. Confocal imaging can also assist in the assessment, but interpretation of the images requires experience. When decreased corneal sensation is noted, aggressive measures to maintain the health of the ocular surface needs to be instituted, and careful monitoring for development of NK must occur. These conventional treatment measures include lubrication, reduction of toxic medications, amniotic membrane grafts, and tarsorrhaphy.
One of the newer treatments for NK is recombinant human nerve growth factor (cenegermin 0.002%, Oxervate, Dompe, Milan, Italy). In several published trials, eyes with herpetic etiologies (both herpes zoster and herpes simplex) for NK were included, but no significant differences were noted in success rates between these eyes and those without herpetic etiologies (22, 23). As such, it is likely for NK from zoster keratitis that cenegermin treatment is more effective than vehicle in healing nonhealing corneal defects. Although this treatment has been found to be effective in many circumstances, the cost of the drug is high, and some analyses have uncertainties with its clinical- and cost-effectiveness evidence (24).
Corneal neurotization is a newer surgical alternative to treating NK whereby contralateral (or ipsilateral) supraorbital or supratrochlear nerves are brought under the conjunctiva to re-innervate the cornea. An indirect method, using a segment of sural or greater auricular nerve as a conduit, has also been used. First described in 2009, this procedure has become increasingly popular, and to date, less than 100 cases have been reported (25, 26). Current techniques can involve using cadaveric nerves as an indirect conduit, as well. The results have been promising for restoring corneal innervation and sensation in eyes with NK, including those from herpes zoster. (26)
Postherpetic neuralgia (PHN), defined as pain or itch beyond 3 months after the onset of the zoster rash, is the most common complication of HZ. It occurs in approximately 30% of HZO patients and mostly in those with disease onset at age 65 or older (27). Risk factors for the development of PHN include increased age, severity of acute pain and rash, as well as HZO. The proportion of HZO patients with PHN has reported to be higher in recent years for unknown reasons, since the age at onset is decreasing (3). PHN may occur more frequently than evident by chart review when determined by self-report among older, male and unvaccinated patients who are less likely to care (28). HZ has been reported in population-based studies to be a risk factor for the development of major depression (29) and has also been reported to be the most common cause of suicide due to pain among people age 70 years and older (30). There are currently no very effective and well-tolerated treatments for PHN. If one wants to prevent PHN with its devastating consequences on the quality of life of older patients with zoster, it is necessary to prevent zoster through vaccination.
Zoster has long been known to be a risk factor for potentially fatal stroke. The greatest risk for stroke is within 3 months of the onset of zoster (31). A high risk of stroke soon after zoster has been reported in patients less than age 50 years old (32). Due to the risk of stroke after zoster in patients less than 50 years old, experts in the field have questioned whether these patients should have prophylactic antiviral treatment for 1 year following zoster (33). The risk of stroke after HZO is 2–4 times greater than the risk after zoster in other locations because the virus spreads from the nerves to nearby vessels. In recent years, zoster also has been recognized to be a risk factor for heart disease (34).
7. What is the relationship between VZV and temporal arteritis/giant cell arteritis (TA/GCA)?
In the neurology literature, there is compelling evidence that VZV is the likely trigger for giant cell/temporal arteritis. Through meticulous research in which 50 sections were analyzed per temporal artery biopsy and subsequently stained for VZV antigen, it was determined that this antigen was present in approximately 75% of the biopsies positive for giant cells (35). In addition, in sections adjacent to VZV antigen giant cells were present in almost 90% of cases. The paper concludes that GAC/TA should be considered VZV vasculopathy of the temporal artery, antiviral treatment may benefit steroid treated TA patients, and this should be studied in a randomized clinical trial. These findings, however, have not been confirmed in the ophthalmic literature where a minority of the temporal arteries have been observed to be positive for VZV antigen (36). This discrepancy may be because many more sections were examined in the neurology compared to ophthalmology studies (34). In a recent population-based study, the prevalence of GCA in HZ was significantly higher than the prevalence in the general population (37). The prevalence in HSV patients was also higher than the general population, but significantly lower than the HZ prevalence. The role of VZV in temporal arteritis remains a question that is worthy of further study to obtain high-quality data regarding the efficacy and safety of antiviral treatment of steroid treated TA patients. Hopefully, neuro-ophthalmologists will conduct a RCT to develop evidence-based recommendations that may improve outcomes in TA, a blinding disease with risk of stroke.
8. Should individuals be vaccinated against zoster with the Recombinant Zoster Vaccine (RZV, Shingrix, GlaxoSmithKline, Research Triangle Park, NC)?
In 2017, the US FDA approved RZV for all adults age 50 years and older after it was shown in landmark studies to prevent HZ in approximately 90% of vaccine recipients across all age groups with 85% persistent efficacy after 4 years (38,39). In 2018, the Advisory Committee on Immunization Practices (ACIP) of the CD) recommended RZV for immunocompetent adults aged 50 years and older, including adults who had previously received the Zoster Vaccine Live (ZVL, Zostavax, Merck & Co. Inc., Whitehouse Station, NJ) and persons with a previous history of HZ. The CDC recommended RZV as preferred over ZVL (40). As of November 2020, ZVL, the original vaccine containing a live attenuated virus, is no longer available in the US. The CDC limited their recommendation to immunocompetent adults because the RCTs did not include immunocompromised persons. In recent years there have been a number of publications reporting the safety and efficacy of RZV in various immunocompromised populations age 18 years and older (41, 42). Recent publications also confirm the effectiveness of the RCV in real world settings, although it is somewhat less than in the RCTs (42, 43). Completing the 2-dose regimen was associated with greater effectiveness even when the second dose was delayed beyond 6 months (42). Effectiveness was also comparable in persons with a past history of ZVL (43). Unfortunately, only 3.6% of the large eligible population studied received recommended RZV, a stark reminder of the importance of improving vaccine uptake (43).
The American Academy of Ophthalmology (AAO), in a policy statement updated in 2018, encouraged ophthalmologists to strongly recommend RZV to all persons age 50 years and above without contraindications, and to work with other health care professionals to do the same (44). Contraindications according to the FDA label are limited to those individuals with a history of anaphylaxis to a component of the vaccine or to the first dose. Post licensure safety surveillance and safety studies conducted in study participants who were randomized to placebo in the original trials and then offered RZV confirm that systemic flulike reactions are more common in persons under age 70, local adverse events at the injection site are more common in people age 70 and above, and overall very few potentially immune mediated diseases have been reported (45, 46). Patients should be informed regarding the likelihood of acute systemic and local reactions lasting 2 to 3 days, and should time their vaccinations accordingly. The novel immunogenic adjuvant in RZV is of potential concern regarding immune mediated diseases and persons at very high risk for these should consult their medical doctor as the benefit usually outweighs the risk.
An important question for ophthalmologists is what should one recommend about vaccination against HZ for patients with HZO. An episode of HZ stimulates VZV cellular immunity for an unknown period of time, and there is a 6% rate of another episode of zoster over 8 years (47). Unfortunately, there are no recommendations from the CDC regarding this. As expected, there have been case reports of reactivation of HZO after RZV (48, 49). In our opinion, although many HZ patients are highly motivated to avoid a second episode of zoster and are willing to take a small risk of recurrent eye inflammation, one should delay vaccination until the eye is stable, and monitor the patient both before and after each of the 2 RZV doses for stability and possible recurrence of eye disease. It is important that ophthalmologists report complications to the Vaccine Adverse Event Reporting System (VAERS) at info@vaers.org so more information on the risk of recurrent inflammation in HZO patients after RZV vaccination becomes available.
During the COVID-19 pandemic, it is worth noting that vaccination against coronavirus is not recommended by the CDC within 2 weeks of vaccination with RZV even though neither contains a live virus.
In conclusion, all adults age 50 years and older should be vaccinated with RZV against HZ unless there are compelling contraindications, and vaccination of younger high-risk patients should be further studied and considered on a case-by-case basis. It is possible that the FDA will extend their approval with regard to immunocompromised persons.
The bottom line is that vaccination against HZ is an ounce of prevention that can save your vision, quality of life, and life, so every effort shout be made to increase its use.
9. What is current recommended antiviral treatment?
Approved and recommended treatment for acute herpes zoster consists of valacyclovir 1000 mg 3 times daily, acyclovir 800 mg 5 times daily, or famciclovir 500 mg 3 times daily for 7 to 10 days. This dosage for HZ therapy is twice the dosage for herpes simplex virus disease, and this treatment can shorten the duration of the rash and reduce the occurrence of chronic eye disease, although it does not prevent postherpetic neuralgia. Suppressive antiviral treatment for HSV genital disease consisting of valacyclovir 1000 mg daily is FDA approved, and suppressive antiviral treatment is the standard of care for current HSV keratitis based on the Herpetic Eye Disease Study (50). However, there is no evidence-based recommendation for the use of suppressive antiviral treatment for HZO. There is a retrospective study that reported the efficacy of suppressive antiviral treatment for HZO and HSV eye disease (51). However, the increasingly widespread belief among cornea specialists that suppressive antiviral treatment is effective in HZO (52) is not supported by any high-quality evidence.
10. What is the Zoster Eye Disease Study (ZEDS) and why should one strongly encourage enrollment?
The Zoster Eye Disease Study (ZEDS), funded by the National Eye Institute of the National Institutes of Health (NEI/NIH) is a multicenter randomized placebo-controlled clinical trial to determine whether prolonged suppressive valacyclovir treatment reduces complications of HZO including eye disease and/or postherpetic neuralgia. Its purpose is to develop high-quality evidence-based new standard of care regarding suppressive antiviral treatment for HZO. The rationale of ZEDS is the relatively recent knowledge of the infectious pathogenesis of complications of HZ and HZO and the significant benefit of suppressive antiviral treatment in reducing recurrent HSV eye disease. HZO and HSV keratitis are caused by different herpes viruses but are analogous in many ways. Active infection contributes to HZO eye disease including dendriform epithelial keratitis (DEK) and iritis, which have been shown to be PCR positive for VZV (53, 54). The HEDS Acyclovir Prevention Trial provided quality evidence that long-term suppressive treatment with oral acyclovir resulted in a 45% reduction in recurrent HSV disease at 1 year (50). This is standard of care for HSV keratitis and has improved outcomes.
Immunocompetent HZO patients age 18 years and older with a history of a typical unilateral HZO rash and an episode in the past year of active keratitis or iritis are eligible for enrollment (55). Exclusion criteria include immunocompromise using the CDC criteria for impaired cellular immunity contraindications for the ZVL, renal insufficiency, pregnancy, history of penetrating keratoplasty, or inability to give informed consent and comply with the study protocol. Study participants are randomized 1:1 to double-masked valacyclovir 1000 mg or placebo daily for 1 year. After enrollment there are study visits every 3 months for 18 months
The primary objective is to evaluate whether suppressive valacyclovir treatment compared to placebo delays the time to first occurrence during 12 months of treatment of new or worsening dendriform epithelial keratitis (DEK), stromal keratitis without (SK) or with ulceration (SKU), endothelial keratitis (EK), iritis (IR). These disease manifestations are carefully defined with treatment requirements necessary for an endpoint. Secondary objectives include whether or not there is a persistent treatment benefit for 6 months following treatment and if treatment reduces PHN. In addition, the impact of vaccinations against HZ on study outcomes and Covid–19 will be explored.
As of the time of this writing, enrollment is less than 50% of expected in over 80 clinical centers in the USA, Canada and New Zealand. Adequate enrollment is necessary to obtain statistically significant results and to determine an evidence-based new standard of care. One challenge to enrollment is that many investigators think that suppressive antiviral treatment is effective despite the absence of good evidence supporting this belief (56). The protocol does allow for study participants who develop new or worsening HZO disease to have open label antiviral treatment if the investigator believes it is indicated. Preferred practices are determined by evidence and not by expert opinion. It is therefore of critical importance that we support enrollment in ZEDS so that high-quality evidence can be obtained to determine a new standard of care of HZO regarding suppressive antiviral treatment and thereby improve outcomes. Please contact the ZEDS Coordinating Center at NYU Grossman School of Medicine (zeds.cta@nyuylangone.org) or refer to our website (https://med.nyu.edu/research/zoster-eye-disease-study/) if you are interested in further information on how to refer HZO patients for possible enrollment in ZEDS. ZEDS is a unique opportunity that deserves your support.
Acknowledgments
Supported by NEI/NIH cooperative agreement U10 EY 026869
Footnotes
The authors do not have any conflicts of interest.
References
- 1.Kong CL, Thompson RR, Porco TC, et al. Incidence Rate of Herpes Zoster Ophthalmicus: A Retrospective Cohort Study from 1994 through 2018. Ophthalmology. 2020. March; 127(3):324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insinga RP, Itzler RF, Pellissier JM, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med, 20:748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson RR, Kong CL, Porco TC, et al. Herpes Zoster and Post-Herpetic Neuralgia: Changing incidence rates from 1994 to 2018 in the United States Clin Infect Dis. 2020. August 23:ciaa1185. doi: 10.1093/cid/ciaa1185. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai K, Yawn BP, Wollan P, and Harpaz R. Increasing incidence of herpes zoster over a 60-year pFrom a population-based study. Clin Infect Dis. 2016;63:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hope-Simpson RE. The nature of herpes zoster: A long-term study and a new hypothesis. Proc R Soc Med. 1965; 58(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harpaz R, van Hoek AJ, Harpaz R, et al. Point-Counterpoint: The Hope-Simpson hypothesis and its implications regarding an effect of routine varicella vaccination on Herpes Zoster incidence. J Infect Dis. 2018; 218(suppl_2):S57–S62. [DOI] [PubMed] [Google Scholar]
- 7.Zuin M, Rigatelli G, L’Erario R, et al. Herpes zoster infection and statins: which implications in clinical practice? Eur J Clin Microbiol Infect Dis. 2019;38:93–99. [DOI] [PubMed] [Google Scholar]
- 8.Ban J, Takao Y, Okuno Y, et al. Association of cigarette smoking with a past history and incidence of herpes zoster in the general Japanese population: the SHEZ Study. Epidemiol Infect. 2017; 145:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawai K, Yawn B. Risk factors for herpes zoster: Systemic review and meta-analysis. Mayo Clinic Proceedings. 2017; 92: 1806–1821. [DOI] [PubMed] [Google Scholar]
- 10.Lee HL, Yeo M, Choi GH, et al. Clinical characteristics of headache or facial pain prior to the development of acute herpes zoster of the head. Clin Neurol Neurosurg. 2017; 152:90–94. [DOI] [PubMed] [Google Scholar]
- 11.Lewis GW. Zoster sine herpete Br Med J. 1958; 2(5093):418–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niederer RL, Meyer JJ, Liu K, et al. Herpes zoster ophthalmicus clinical presentation and risk factors for loss of vision. Am J Ophthalmol. 2021. February 8:S0002–9394(21)00064–7. doi: 10.1016/j.ajo.2021.02.002. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.White.2014, Jul http://one.aao.org/clinical-statement/herpes-simplex-virus-keratitis-treatment-guideline
- 14.Tran KD, Falcone MM, Choi DSi et al. Epidemiology of herpes zoster ophthalmicus: recurrence and chronicity. Ophthalmology. 2016; 123:1469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoang-Xuan T, Büchi ER, Herbort CP, et al. Oral acyclovir for herpes zoster ophthalmicus. Ophthalmology. 1992; 99:1062–70; discussion 1070–1. [DOI] [PubMed] [Google Scholar]
- 16.He Y, de Melo Franco R, Kron-Gray MM, et al. Outcomes of cataract surgery in eyes with previous herpes zoster ophthalmicus. J Cataract Refract Surg. 2015; 41:771–7 [DOI] [PubMed] [Google Scholar]
- 17.Lu LM, McGhee CNJ, Sims JL, Niederer RI. High rate of recurrence of herpes zoster-related ocular disease after phacoemulsification cataract surgery. J Cataract Refract Surg. 2019; 45:810–815. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka TS, Hood CT, Kriegel MF, et al. Long-term outcomes of penetrating keratoplasty for corneal complications of herpes zoster ophthalmicus. Br J Ophthalmol. 2019; 103:1710–1715s [DOI] [PubMed] [Google Scholar]
- 19.Dua H, Said DG, Messmer EM, et al. Neurotrophic keratopathy. Prog Ret Eye Res 2018; 66:107–131. [DOI] [PubMed] [Google Scholar]
- 20.Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44(suppl. 1):S1–S26. [DOI] [PubMed] [Google Scholar]
- 21.Margolis TP. Neurotrophic keratopathy: Ophthalmology’s diabetic foot problem. Eye Contact Lens 2021; 47:136–139. [DOI] [PubMed] [Google Scholar]
- 22.Bonini S, Lambiase A, Rama P, et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology 2018; 125:1332–1343. [DOI] [PubMed] [Google Scholar]
- 23.Pflugfelder SC, Massaro-Giordano M, Perez VL, et al. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy. Ophthalmology 2020; 127:14–26. [DOI] [PubMed] [Google Scholar]
- 24.Fleeman N, Mahon J, Nevitt S, et al. Cenegermin for treating neurotrophic keratitis: an evidence review group perspective of a NICE single technology appraisal. Pharmacoecon Open 2019; 3:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terzis JK, Dryer MM, Bodner BI. Corneal neurotization: a novel solution to neurotrophic keratopathy. Plast Reconstr Surg 2009; 123:112–120. [DOI] [PubMed] [Google Scholar]
- 26.Park JK, Charlson ES, Leyngold I, Kossler AL. Corneal neurotization: a review of pathophysiology and outcomes. Ophthalmic Plast Reconstr Surg 2020; 36:431–437. [DOI] [PubMed] [Google Scholar]
- 27.Borkar DS, Tham VM, Esterberg E, et al. Incidence of herpes zoster ophthalmicus: results from the Pacific Ocular Inflammation Study. Ophthalmology. 2013; 120:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanenbaum HC, Lawless A, Sy LS, et al. Differences in estimates of post-herpetic neuralgia between medical chart review and self-report. J Pain Res. 2020; 13:1757–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen MH, Wei HT, Su TP et al. Risk of depressive disorder among patients with herpes zoster: a 29. nationwide population-based prospective study. Psychosom Med. 2014; 76:285–91. [DOI] [PubMed] [Google Scholar]
- 30.Hess TM, Lutz LJ, Nauss LA, Lamer TJ. Treatment of acute herpetic neuralgia. A case report and review of the literature. Minn Med. 1990; 73:37–40. [PubMed] [Google Scholar]
- 31.Yawn BP, Wollan PC, Nagel MA, Gilden D. Risk of stroke and myocardial infarction after hZoster in older adults in a US community population. Mayo Clin Proc. 2016; 91:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patterson BJ, Rausch DA, Irwin DE, et al. Analysis of vascular event risk after herpes zoster from 2007 to 2014 US insurance claims data. Mayo Clin Proc. 2019; 94:763–775. [DOI] [PubMed] [Google Scholar]
- 33.Nagel MA, Bubak AN. Herpes Zoster, a rash of cerebrovascular events. Mayo Clin Proc. 2019; 94:742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagel MA, Bubak AN. Varicella Zoster Virus vasculopathy. J Infect Dis. 2018; 218 (suppl 2):S107–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilden D, White T, Khmeleva N, et al. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology. 2015; 84:1948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckingham EM, Foley MA, Grose C, et al. Identification of Herpes Zoster-associated temporal arteritis among cases of giant cell arteritis. Am J Ophthalmol. 2018; 187:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee DH, Iovieno A, Sheldon CA. Is there an association between herpetic infections and giant cell arteritis? A population-based study. J Clin Med. 2020; 10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015; 372:2087–96. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the Herpes Zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016; 375:1019–32. [DOI] [PubMed] [Google Scholar]
- 40.Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018; 67:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Racine É, Gilca V, Amini R, et al. A systematic literature review of the recombinant subunit herpes zoster vaccine use in immunocompromised 18–49 year old patients. Vaccine. 2020; 38:6205–6214. [DOI] [PubMed] [Google Scholar]
- 42.Izurieta HS, Wu X, Forshee R, et al. Recombinant Zoster Vaccine (Shingrix) real-world effectiveness in the first two years post-licensure. Clin Infect Dis. 2021. February 13;ciab125. doi: 10.1093/cid/ciab125. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Sun Y, Kim E, Kong CL, et al. Effectiveness of the recombinant zoster vaccine in adults aged 50 and older in the United States: a claims-based cohort study. Clin Infect Dis. 2021. February 13; ciab121. doi: 10.1093/cid/ciab121. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Policy Statement. Recommendations for herpes zoster vaccine for patients 50 years of age and older. Ophthalmology 2018; 125: 1813–6. [Google Scholar]
- 45.Hesse EM, T Shimabukuro TT, Su JR, et al. Postlicensure safety surveillance of recombinant zoster vaccine (Shingrix) - United States, October 2017-June 2018. MMWR Morb Mortal Wkly Rep. 2019; 68:91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ocran-Appiah J, Boutry C, Hervé C, et al. Safety of the adjuvanted recombinant zoster vaccine in adults aged 50 years or older. A phase IIIB, non-randomized, multinational, open-label study in previous ZOE-50 and ZOE-70 placebo recipients. Vaccine. 2021; 39:6–10. [DOI] [PubMed] [Google Scholar]
- 47.Yawn BP, Wollan PC, Kurland MJ, et al. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011; 86:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehmann A, Matoba A. Reactivation of herpes zoster stromal keratitis after HZ/su adjuvanted herpes zoster subunit vaccine. Ophthalmology. 2018; 125:1682. [DOI] [PubMed] [Google Scholar]
- 49.Jabbour S, Shekhawat NS, Chen A, Woreta FA. Presumed herpes zoster ophthalmicus reactivation following recombinant zoster vaccination. Cornea. 2021; 40:248–250. [DOI] [PubMed] [Google Scholar]
- 50.No authors listed. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. N Engl J Med. 1998; 339:300–6 [DOI] [PubMed] [Google Scholar]
- 51.Miserocchi E, Fogliato G, Bianchi I, et al. Clinical features of ocular herpetic infection in an Italian referral center. Cornea, 2014; 33:565–70. [DOI] [PubMed] [Google Scholar]
- 52.Lo DM, Jeng BH, Gillespie C, et al. Current practice patterns and opinions in the management of recent onset or chronic herpes zoster ophthalmicus of Zoster Eye Disease Study investigators. Cornea 2019; 38:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu AY, Strauss EC, Holland GN, et al. Late varicella-zoster virus dendriform keratitis in patients with histories of herpes zoster ophthalmicus. Am J Ophthalmol, 2010; 149:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takase H, Kubono R, Terada Y, et al. Comparison of the ocular characteristics of anterior uveitis caused by herpes simplex virus, varicella-zoster virus, and cytomegalovirus. Jpn J Ophthalmol, 2014; 58:473–82. [DOI] [PubMed] [Google Scholar]
- 55.Cohen EJ, Hochman JS, Troxel AB, Colby KA, Jeng BH. The Zoster Eye Disease Study (ZEDS): Rationale and design. Cornea. Accepted 2.27.2021. [DOI] [PubMed] [Google Scholar]
- 56.Cohen EJ, Jeng BH, Troxel AB, et al. Enrollment in the Zoster Eye Disease Study. Cornea. 2020; 39:1480–1484. [DOI] [PubMed] [Google Scholar]


