Abstract
Background:
Early discontinuation is a substantial barrier to the delivery of endocrine therapies (ET) and may influence recurrence and survival. We investigated the association between early discontinuation of ET and social determinants of health including insurance coverage and neighborhood deprivation index (NDI)-measured based on patients’ zip codes- in breast cancer.
Methods:
In this retrospective analysis of TAILORx prospective randomized clinical trial, women with hormone-receptor +, HER 2- breast cancer who started ET within a year of study entry were included. Early discontinuation was calculated as stopping ET within 4 years of start for reasons other than distant recurrence or death using Kaplan-Meier estimates. Cox proportional hazards joint model was used to analyze the association between the study entry insurance and NDI with ET early discontinuation adjusting for other variables.
Results:
Of included 9,475 women (mean age: 55.6; 84% white), 58.0% had private insurance, while 11.7% had Medicare, 5.8% had Medicaid, 3.8% were self-pay, and 19.1% were treated at international sites. The early discontinuation rate was 12.3%. Compared to private insurance, patients with Medicaid (HR 1.53; 95% CI 1.23-1.92) and self-pay (HR 1.65; 95% CI 1.25-2.17) had higher early discontinuation. Participants with first quartile NDI (highest deprivation) had a higher probability of discontinuation compared to those with fourth quartile (lowest deprivation) (HR, 1.34; 95% 1.11-1.62).
Conclusions:
Patients’ insurance and zip code at study entry play a role in adherence to ET, with uninsured and underinsured having a high rate of treatment non-adherence. Early identification of patients at risk may improve adherence to therapy.
Keywords: Breast Cancer, Social determinants of Health, endocrine therapy, adherence, Insurance
Lay Summary:
In this retrospective analysis of 9,475 breast cancer women participating in TAILORx clinical trial, patients with Medicaid and self-pay (compared to private insurance) and those in the highest quartile of neighborhood deprivation scores (compared to the lowest) had a higher probability of early discontinuation of endocrine therapy. These social determinants of health assume larger importance with the expected increase in unemployment rates and loss of insurance coverage in the aftermath of the COVID-19 pandemic. Early identification of patients at risk and enrollment in insurance optimization programs may improve the persistence of therapy.
Precise:
Patients’ insurance status and geographic residence play an important role in the persistence of endocrine therapy use. Early identification of patients at risk may improve adherence to therapy.
Introduction
Breast cancer is the most common cancer in American women with over 268,600 new cases in 2019.1 Approximately 75% of patients present with hormone receptor-positive breast cancers, for whom 5 years of adjuvant endocrine therapy (ET) substantially reduces the risks of locoregional and distant recurrence, contralateral breast cancer, death from breast cancer, and therefore death from any cause.2-5
The Trial Assigning Individualized Options for Treatment (TAILORx) was a prospective trial designed to assess the application of the 21-gene recurrence score in hormone receptor positive, human epidermal growth factor receptor 2 (HER2) negative, axillary node negative breast cancer.6 The results showed that ET alone was non-inferior to adjuvant chemotherapy plus ET in patients with a recurrence score (RS) of 11 to 25.6
Nonadherence and early discontinuation are substantial barriers to the delivery of ETs and may result in recurrence and mortality.2 Non-adherence is defined as any incident when doses are missed, extra doses are taken, or doses are taken in the wrong quantity or at the wrong time. Early discontinuation is defined as when a patient stops a medication earlier than the period for which it is prescribed.7 Approximately 30% to 60% of patients receiving ET are non-adherent to some degree,8-15 and non-adherence may increase over time.14 In adjuvant breast cancer clinical trials with longer (≥4 years) follow-up, ET was prematurely discontinued by about 23–36% of the study participants.16, 17
Several factors have been suggested as predictors of nonadherence and early discontinuation of ET including extremes of age,18, 19 African American race, 20-24 greater comorbidities,18, 19, 25 post-menopausal status,12 cognitive impairment, disease-related and treatment-related factors (e.g., type of surgery, receipt of adjuvant chemotherapy, 26 greater treatment side effects),18, 19 low recurrence risk perception,19 lack of provider communication regarding the importance of ET,18, 19 follow-up care with a general practitioner versus a cancer specialist,18, 19 increased out-of-pocket costs,18 low social support,18, 19, 25 and low socioeconomic status (SES).27-29 Social determinants of health incorporate economic stability (e.g. income, and insurance), the built environment (e.g. zip code/geography), and health system factors (access to, and quality of care).30 These have been hypothesized to shape health behavior and they contribute to health outcomes. For example, affordability is a significant factor in patients’ non-adherence to medication regimens or early discontinuation of therapy, as evidenced by higher rates of non-adherence and early discontinuation among patients with higher ET prescription copayment or those who received brand-name drugs compared to generic equivalents.31
We aim to investigate the association between early discontinuation of ET and social determinants of health, namely insurance coverage and neighborhood deprivation index (NDI), at study entry among patients enrolled in the TAILORx trial.
Method:
Study Protocol
TAILORx was a prospective National Cancer Institute (NCI)-funded clinical trial that was coordinated by the Eastern Cooperative Oncology Group (ECOG) and subsequently ECOG-ACRIN Cancer Research Group. It was approved by the NCI Central institutional review board and appropriate local institutional review board. TAILORx is registered at ClinicalTrials.gov (NCT00310180). Data from ECOG-ACRIN clinical trials are available to researchers by directly contacting ECOG-ACRIN.
Women were required to provide written informed consent, including a willingness to have treatment assigned or randomized based on the recurrence score results. Women with a recurrence score of 10 or lower were assigned to receive ET only (Arm A), and women with a score of 26 or higher were assigned to receive chemotherapy plus ET (Arm D). Women with a midrange score of 11 to 25 underwent randomization and were assigned to receive either ET alone (Arm B) or chemotherapy plus ET (Arm C). Additional details regarding the study protocol have been previously reported.6, 32, 33 For current study, which is a post-hoc analysis of the TAILORx trial, breast cancer patients enrolled in the TAILORx trial who started ET within one year and 3 weeks of study entry were included. The additional 3 weeks conservatively accounted for variations in the initiation of the second follow-up reporting period for patients in arms A and D. This time frame resulted in the inclusion of an additional 50 participants.
Sociodemographic and Clinical Variables
Insurance status at study entry was determined using standard categories collected for all National Clinical Trials Network studies at the time of registration. The NDI was calculated using the Agency for Healthcare Research and Quality (AHRQ) SES index.34 The index is a weighted combination of the percentage of households with a mean number of 1 person or more per room, the median value of owner-occupied values, the percentage living below the poverty level, the median household income, the percentage 25 years or older with a bachelor’s degree or higher, the percentage 25 years or older with less than a 12th-grade education, and the percentage 16 years or older in the labor force who are unemployed. It is scaled to the US population to lie between 0 and 100, with a higher number indicative of greater neighborhood deprivation.34 Previous studies have used this index to represent a geographical area–based measure of the socioeconomic deprivation experienced according to the neighborhood.35, 36 The NDI was computed by linking patients’ 5-digit zip codes at time of registration, for a subset of cases for whom the data were available, to county-level data using 2016-2017 Health Resource and Services Administration Area Health Resources File, which includes data on population characteristics and economics.37 When a zip code represented multiple counties, for each component variable in NDI, aggregate means, and totals from those multiple counties were used to represent the county level estimates for that zip code. Participants were grouped by NDI quartile. The index was not calculated for cases with unknown zip codes as well as those from Puerto Rico or international sites. Lower values for NDI represent lower neighborhood aggregate SES, and higher neighborhood deprivation.
Additional variables included arm assignment, age, race, menopausal status, tumor size, histologic grade, progesterone receptor expression, recurrence score, and first ET medication.
Study Endpoint
The primary endpoint was early discontinuation of ET, defined as stopping medication within 4 years of start (1,416 days) for reasons other than distant recurrence or death. The start time of ET was not always similar to the time of study entry in the TAILORx trial. Since patients could have started ET up to 1 year after study entry, some early termination events could have occurred up to 5 years after registration. Patients who were lost to follow-up or withdrew from the study within 4 years of starting therapy, but reported receipt of ET on their last follow-up were analyzed as still on ET with duration censored. Given that many patients appear to have enrolled in TAILORx to test for Oncotype score, and the treatments involved were widely available standard of care, withdrawal from the study was not assumed to imply stopping treatment. However, we further performed sensitivity analysis categorizing patients who were lost to follow-up or withdrew from the study within 4 years of starting ET as those with early treatment discontinuation. Since exact durations and reasons for discontinuation of medication were not recorded, if distant recurrence or death occurred within 3 months of the last dose, then the reason for discontinuation was assumed to be distant recurrence/death, and duration was censored at that point.
Statistical Analyses
For categorical variables frequencies and percentages and for continuous variables mean and standard deviation (SD) are reported. The early discontinuation rate was calculated using Kaplan-Meier estimates and reported as frequencies and percentages. Cox proportional hazards models were used after ensuring the proportional hazard assumption was met, to analyze the association between various factors individually and the rate of early discontinuation of therapy. A joint model was incorporating the major factors including insurance, NDI, arm assignment, age, race, menopausal status, tumor size, progesterone receptor expression, recurrent score, and first ET medication was also fit. Overall tests comparing the levels, hazard ratios (HR), and 95% confidence intervals (CI) are reported for each factor. P values < 0.05 were considered statistically significant.
Results
Study Population
A total of 10,253 eligible women were registered between April 7, 2006, and October 6, 2010. Among 9,719 eligible patients with follow-up information who were included in the main analysis set, 9,475 started ET within 1 year and 3 weeks of entry. Baseline demographics of included patients are shown in Table 1. A total of 58.0% had private insurance, while 11.7% had Medicare; 5.8% had Medicaid; 0.9% had military/VA insurance; 3.8% were self-pay, and 19.1% were recruited from international sites with unknown insurance statues including Canada (n=872), Ireland (n=663), Peru (n=254), Australia (n=20) and New Zealand (n=5).
Table 1.
Baseline Demographics of TAILORx cohort with endocrine therapy start within 1 year and 3 weeks of study entry.
| Characteristics | N=9,475 |
|---|---|
| Study Arms, n (%) | |
| Arm A (RS 0-10, assigned endocrine therapy) | 1,577 (16.6%) |
| Arm B (RS 11-25, randomized to endocrine therapy) | 3,361 (35.5%) |
| Arm C (RS 11-25, randomized to endocrine therapy + chemotherapy) | 3,221 (34.0%) |
| Arm D (RS 26-100, assigned endocrine therapy + chemotherapy) | 1,316 (13.9%) |
| Receipt of Chemotherapy, n (%) | |
| Arm C or D, Received Chemo | 3894 (41.1%) |
| Arm C or D, No Chemotherapy * | 643 (6.8%) |
| Arm A or B, Received Chemotherapy * | 188 (2.0%) |
| Arm A or B, No Chemotherapy received | 4750 (50.1%) |
| Mean age (SD), year | 55.6 (9.1) |
| Race, n (%) | |
| White | 7,992 (84.3%) |
| Black | 668 (7.1%) |
| Asian | 398 (4.2%) |
| Other/Not Specified | 417 (4.4%) |
| Ethnicity, n (%) | |
| Hispanic | 861 (9.1%) |
| Not Hispanic | 7,445 (78.6%) |
| Not specified | 1169 (12.3%) |
| Insurance status, n (%) | |
| Private | 5,491 (58.0%) |
| Medicare | 1,104 (11.7%) |
| Medicaid | 549 (5.8%) |
| Military/VA | 82 (0.9%) |
| None (self-pay) | 360 (3.8%) |
| International | 1814 (19.1%) |
| Other/Unknown | 75 (0.8%) |
| Neighborhood Deprivation Index (NDI, value range), n (%) | |
| Quartile 1, highest deprivation (≤ 51.53) | 1,907 (20.1%) |
| Quartile 2 (51.54-53.53) | 1,846 (19.5%) |
| Quartile 3 (53.54-56.48) | 1,873 (19.8%) |
| Quartile 4, lowest deprivation (> 56.48) | 1,873 (19.8%) |
| US zip code unknown or US territory (Puerto Rico) | 162 (1.7%) |
| International | 1,814 (19.1%) |
| Menopausal Status, n (%) | |
| Premenopausal | 3,202 (33.8%) |
| Postmenopausal | 6273 (66.2%) |
| Tumor size in the largest dimension, n (%) | |
| <=2cm | 7,085 (74.8%) |
| > 2cm | 2388 (25.2%) |
| Histologic grade of tumor, n (%) | |
| Low | 2,441 (25.8%) |
| Intermediate | 5132 (54.2%) |
| High | 1620 (17.1%) |
| Unknown | 282 (2.9%) |
| Progesterone receptor expression, n (%) | |
| Negative | 914 (9.6%) |
| Positive | 8,357 (88.2%) |
| Unknown | 204 (2.2%) |
| Oncotype Dx Recurrence Score (RS), n (%) | |
| <= 10 | 1577 (16.6%) |
| 11-25 | 6582 (69.6%) |
| > 25 | 1316 (13.8%) |
| First Endocrine Therapy, n (%) | |
| AI | 5546 (58.5%) |
| Tamoxifen | 3576 (37.7%) |
| Tamoxifen & AI | 68 (0.7%) |
| Ovarian Function Suppression | 249 (2.6%) |
| Other | 36 (0.4%) |
reflects patients who did not adhere to assigned treatment arm
Early Discontinuation Rate
Table 2 shows Kaplan-Meier early discontinuation rates of ET and early study withdrawals with ET reported to be taken in the last follow-up (censored ET durations). The discontinuation rate of ET increased over time from 2.6% during the first year to 4.0% during the fourth year after the start of treatment. Overall, the early discontinuation rate was 12.3% within 4 years of start. Of note, 6.2% of participants withdrew from the study or were lost to follow-up within 4 years after the start of ET. These patients were assumed to have continued their ET between their last follow-up and time of study withdrawal or lost to follow-up.
Table 2.
Kaplan-Meier early discontinuation and early withdrawal/lost to follow-up rates for study population (n=9,475).
| Time from start of endocrine therapy |
N (%) stopping Endocrine therapy |
N (%) withdrawal from study or lost to follow-up with report of endocrine therapy in the last follow-up |
|---|---|---|
| 0-1 years | 243 (2.6%) | 136 (1.4%) |
| 1-2 years | 260 (2.8%) | 145 (1.5%) |
| 2-3 years | 269 (2.9%) | 136 (1.4%) |
| 3-4 years | 353 (4.0%) | 168 (1.8%) |
| Total | 1125 (12.3%) | 585 (6.2%) |
Social determinants of health and non-adherence
Table 3 shows the Kaplan-Meier estimates of early discontinuation rates for two variables related to social determinants of health: insurance and NDI, adjusting for other variables. Compared to participants with private insurance, those with Medicaid (HR 1.53; 95% CI 1.23-1.92) or who self-pay (HR 1.65; 95%CI 1.25-2.17) had a higher probability of discontinuing ET within 4 years of start. There was no significant difference in early discontinuation rate between patients with Medicare and private insurance. Sensitivity analysis including the 6.2% (n=282) participants who withdrew from the study or were lost to follow-up within 4 years after the start of ET as early discontinuation of therapy did not change the result (data not presented).
Table 3.
Endocrine therapy Kaplan-Meier early discontinuation rates and hazard ratios from a joint model.
| Ratio | 95% CI | p-value | |
|---|---|---|---|
| Arm Assignment with receipt of Chemo | |||
| Arm C (RS 11-25) or D (RS 26-100) , Received Chemo | Ref | ||
| Arm C (RS 11-25) or D (RS 26-100), No Chemotherapy | 1.71 | (1.37, 2.14) | 0.000003 |
| Arm A (RS 0-10) or B (RS 11-25) , Received Chemotherapy | 1.03 | (0.66, 1.61) | 0.89 |
| Arm A (RS 0-10) or B (RS 11-25), No Chemotherapy received | 1.18 | (1.01, 1.38) | 0.03 |
| Age | |||
| 40 years or younger | Ref | ||
| 41 to 50 years old | 0.70 | (0.54, 0.89) | 0.005 |
| 51 to 60 years old | 0.52 | (0.39, 0.70) | 0.00001 |
| 61 to 70 | 0.46 | (0.34, 0.64) | 0.000003 |
| 71 and older | 0.57 | (0.38, 0.86) | 0.008 |
| Race | |||
| White | Ref | ||
| Black | 0.73 | (0.57, 0.93) | 0.01 |
| Asian | 0.50 | (0.34, 0.75) | 0.0007 |
| Other or unknown | 1.07 | (0.79, 1.45) | 0.65 |
| Ethnicity | |||
| Non-Hispanic | Ref | ||
| Hispanic | 0.87 | (0.70, 1.09) | 0.23 |
| Ethnicity not reported | 0.82 | (0.66, 1.02) | 0.07 |
| Insurance Type (US participants only) | |||
| Private | Ref | ||
| Medicare | 1.10 | (0.88, 1.37) | 0.41 |
| Medicaid | 1.53 | (1.23, 1.92) | 0.0002 |
| Military/VA | 0.80 | (0.38, 1.69) | 0.56 |
| None | 1.65 | (1.25, 2.17) | 0.0003 |
| Other or unknown | 0.84 | (0.40, 1.78) | 0.65 |
| Neighborhood Deprivation Index (NDI) | |||
| Quartile 1, highest deprivation | 1.34 | (1.11, 1.62) | 0.003 |
| Quartile 2 | 1.12 | (0.92, 1.36) | 0.26 |
| Quartile 3 | 1.23 | (1.01, 1.49) | 0.04 |
| Quartile 4, lowest deprivation | Ref | ||
| Unknown | 1.10 | (0.66, 1.85) | 0.71 |
| International participant (compared to US participant with private insurance and lowest deprivation quartile) | 0.98 | (0.79, 1.22) | 0.84 |
| Menopausal Status | |||
| Premenopausal | Ref | ||
| Postmenopausal | 1.08 | (0.87, 1.34) | 0.48 |
| Tumor Size in the largest dimension | |||
| Less than or equal to 2.0 cm | Ref | ||
| Greater than 2.0cm | 1.06 | (0.93, 1.22) | 0.38 |
| Progesterone receptor expression | |||
| Negative | Ref | ||
| Positive | 0.75 | (0.61, 0.91) | 0.004 |
| Unknown | 0.51 | (0.28, 0.92) | 0.03 |
| Oncotype Dx Recurrent Score (RS) | |||
| Less than or equal to 10 | Ref | ||
| 11-25 | 0.80 | (0.67, 0.94) | 0.007 |
| Greater than 25 | 0.83 | (0.63, 1.08) | 0.16 |
| First Endocrine Therapy | |||
| Aromatase Inhibitor (AI) | Ref | ||
| Tamoxifen | 1.00 | (0.84, 1.20) | 0.96 |
| Tamoxifen & AI | 1.27 | (0.70, 2.32) | 0.44 |
| Ovarian Function Suppression | 1.05 | (0.72, 1.54) | 0.81 |
| Other | 3.68 | (1.35,10.06) | 0.01 |
Further, participants with first quartile NDI (highest deprivation) had a higher probability of discontinuation compared to those with fourth quartile NDI (lowest deprivation) (HR, 1.34; 95%CI 1.11-1.62). Overall, the probability of early discontinuation increased as neighborhood deprivation increased.
Other Factors associated with Early Discontinuation Rate
In addition to insurance status and NDI at study entry, there were several other factors associated with early discontinuation rate (Table 3). No receipt of chemotherapy was significantly associated with a higher probability of early discontinuation of ET. Increasing age compared to age 40 or lower, Black and Asian race (compared to white), and higher recurrence score were significantly associated with lower probability of early discontinuation of ET.
Discussion
Our retrospective analysis of women with breast cancer enrolled in the TAILORx trial and who started on ET, showed that patients’ insurance type and NDI at study entry are independent factors associated with early discontinuation. Among US participants, patients who were self-pay or had Medicaid or those who lived in neighborhoods with the highest deprivation level (i.e. first quartile of NDI) were more likely to stop ET early. Of note, patients were recruited between April 2006 to October 2010. With the Affordable Care Act (ACA) going into effect in January 2010, only a small proportion of the participants likely had ACA exchange insurance coverage and such information is not available.
Our study showed a 12.3% early discontinuation rate of ET within 4 years of start in patients participating in the TAILORx trial. The parent study did not collect patient-reported reasons for early discontinuation. Prior studies report a 5-year early discontinuation rate ranging between 20% to 48%.18, 19, 38 The variability in the method for measuring early discontinuation among other reasons may contribute to a wide range of reported rates.
Our results for economic factors associated with early discontinuation is mostly consistent with prior studies with self-pay and Medicaid insurance and residence in neighborhoods with higher deprivation levels associated with higher rates of early discontinuation.27-29 The uninsured face barriers for prescription medications due to high out-of-pocket responsibilities. The average out-of-pocket cost for a month of supply for ET ranges between $70 (Tamoxifen) to $505 (aromatase inhibitors) for self-pay patients without coupons or prescription assistance.39 As literature has established, the Medicaid population is also at higher risk for early discontinuation due to lesser coverage of routine care, heterogeneous coverage of costs of clinical trial participation,40 and potential lower financial or other reserves among this population. 27-29
TAILORx did not include data on income or education. Neighborhood deprivation may be a proxy for participant SES. In addition, neighborhood influences access to care. In the absence of patient-level SES, we cannot tease apart the contribution of patient- vs neighborhood- SES on early discontinuation of ET in this population. However, after controlling for insurance status, a more direct proxy for individual-level resources, NDI remained a significant correlate of early hormone therapy discontinuation, suggesting independent effects of the built environment on health behavior and consequently, health outcome.
TAILORx trial did not include patients older than 75 years old. However, unlike prior studies, 12, 26 the current study found that post-menopausal status and receipt of chemotherapy was associated with lower rates of early discontinuation. We assume some degree of higher rates of early discontinuation in pre-menopausal women likely correlates with higher rates of early discontinuation seen in patients younger than 50 years old. However, in the multivariable model, the association of both factors with early discontinuation remains independently significant. Higher probability of early discontinuation of ET among patients with lower recurrence scores in our study might be explained by lower recurrence risk perception among these patients, consistent with prior studies.19 Conversely, receipt of chemotherapy exerts an opposite effect, reducing early discontinuation, perhaps due to higher recurrence risk perception or desire to avoid chemotherapy in the future.
Finally, in the current study, Black and Asian participants (compared to White participants), had a lower probability of early discontinuation of ET. Prior studies have shown among patients with sporadic (non-high risk) breast cancer, Asians have higher odds of using ET compared to non-Hispanic Whites and African Americans.21, 41 While in some studies Black women compared to White women have been shown to have a higher likelihood of early discontinuation, 20-24 we observed the opposite correlation after controlling for the neighborhood and insurance effects. We hypothesize that the previously demonstrated race effect on early discontinuation may be due to or otherwise accounted for in the neighborhood deprivation index in our multivariable analysis.42 In a recent study of TAILORx participants compared to White women, Black women had worse clinical outcomes (e.g., recurrence rates and survival).43 Our study findings suggest that these disparities do not seem to be due to differences in ET adherence. Future analyses of clinical outcomes that include NDI may be informative. The striking reversal of the expected pattern of early discontinuation of hormone therapy among Black women after controlling for neighborhood characteristics strongly suggests the need for a routine longitudinal collection of variables representing social determinants of health. Such clinical and trial data may more fully explain the variations in health behavior and health outcome currently attributed to race.
Our study has implications for clinical practice. To counteract increasing medication costs, pharmacy benefit plans have added additional tiers of drugs, increased copayment rates, and deductibles, excluded some drugs from coverage, and increased preauthorization requirements.44 Additional efforts should be made to implement initiatives such as price transparency, insurance optimization, and financial navigation to mitigate the patient-borne effects of cost-sharing.45-47 Enactment of parity laws and efforts to limit ET out-of-pocket costs may also impact medication adherence.48 Additional efforts such as coordination of transportation for clinic visits, and use of telemedicine and personalized culturally-tailored interventions may improve early discontinuation due to limited access to healthcare. Finally, culturally sensitive outreach and education on consequences of early discontinuation, as well as available resources on improving insurance coverage and access might be helpful in mitigating early discontinuation as well as building trust in the health system, particularly in communities of color. These recommendations may be particularly salient with the economic shock related to COVID-1949 and expected increase in unemployment rates, insurance coverage loss, and potential downward social mobility further influencing early discontinuation.
Our study has several limitations. First, the early discontinuation rate was calculated based on the report of receipt or non-receipt of medication in the last follow-up. Since exact durations and reasons for discontinuation of ET were not recorded, if distant recurrence or death occurred within 3 months of last ET, then the reason for discontinuation was assumed to be distant recurrence/death. Second, we only analyzed the association between early discontinuation and insurance and NDI at study entry. It is possible that a patient’s insurance or place of residence has changed over the 5 years and the impact of these changes on discontinuation rate is not assessed. In other settings, transitions between coverage types can positively (e.g., individuals transitioning from Medicaid-only to more generous dual Medicare/Medicare coverage) or negatively (e.g., women with the spousal transition to Medicare) influence health access and quality.50, 51 Liu et al estimated that 25.6% relocate after their first cancer diagnosis.52 Although the relative neighborhood deprivation levels before and after relocation were not assessed, poverty and lack of health insurance correlated with relocation, suggesting downward neighborhood mobility. TAILORx did not collect data of treatment side effects. Therefore, we did not assess the correlation between side effects and ET early discontinuation. Participants in clinical trials differ from the general patient population, limiting generalizability of our results.
In summary, patients’ insurance status and zip code play an important role in the persistence of ET use with uninsured and Medicaid patients and participants who live in neighborhoods with high deprivation levels having a high rate of early discontinuation of therapy. Early identification of patients at risk, enrollment in insurance optimization programs, and culturally appropriate recurrence risk reduction education may improve the persistence of therapy.
Figure 1.
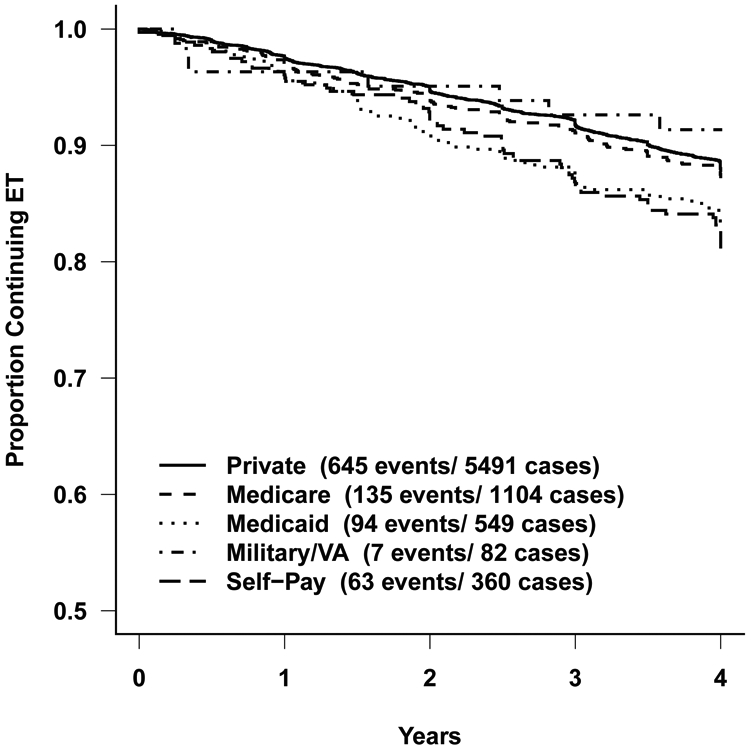
Early discontinuation rates for endocrine therapy by type of insurance.
Acknowledgments
Funding:
This study was conducted by the ECOG-ACRIN Cancer Research Group (Peter J. O'Dwyer, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers U10CA180820, U10CA180794, UG1CA189828, UG1CA189859, UG1CA233160, UG1CA233320, UG1CA233247. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Conflict of Interests:
DC is the president of FACIT.ORG. RCC receives salary support as editor-in-chief of Journal of American College of Radiology (JACR) and travel support from the GE Radiology Research Academic Fellowship (GERRAF) as Board of Review Chair. All other authors do not report any conflict of interests.
References:
- 1.American Cancer Society. Cancer Facts & Figures 2019. Atlanta: American Cancer Society; 2019. [Google Scholar]
- 2.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28: 3784–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365: 1687–1717. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative G, Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378: 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists' Collaborative G. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386: 1341–1352. [DOI] [PubMed] [Google Scholar]
- 6.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2018;379: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59: 56–66. [DOI] [PubMed] [Google Scholar]
- 8.Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer. 2007;109: 832–839. [DOI] [PubMed] [Google Scholar]
- 9.Miaskowski C, Shockney L, Chlebowski RT. Adherence to oral endocrine therapy for breast cancer: a nursing perspective. Clin J Oncol Nurs. 2008;12: 213–221. [DOI] [PubMed] [Google Scholar]
- 10.Ma AM, Barone J, Wallis AE, et al. Noncompliance with adjuvant radiation, chemotherapy, or hormonal therapy in breast cancer patients. Am J Surg. 2008;196: 500–504. [DOI] [PubMed] [Google Scholar]
- 11.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19: 322–328. [DOI] [PubMed] [Google Scholar]
- 12.Simon R, Latreille J, Matte C, Desjardins P, Bergeron E. Adherence to adjuvant endocrine therapy in estrogen receptor-positive breast cancer patients with regular follow-up. Can J Surg. 2014;57: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fyles AW, McCready DR, Manchul LA, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med. 2004;351: 963–970. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Anderson S, Tan-Chiu E, et al. Tamoxifen and chemotherapy for axillary node-negative, estrogen receptor-negative breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-23. J Clin Oncol. 2001;19: 931–942. [DOI] [PubMed] [Google Scholar]
- 15.Hershman D, Moseley A, Arnold KB, Hillyer G, Gralow J, Henry NL, Neugut AI, Ramsey SD, Unger JM. Predictive model of aromatase inhibitor non-adherence using patient-reported outcomes in women with breast cancer (SWOG S1105). J Clin Oncol 38: 2020. (suppl; abstr 12019). [Google Scholar]
- 16.Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71: 1–9. [DOI] [PubMed] [Google Scholar]
- 17.Hutchins LF, Green SJ, Ravdin PM, et al. Randomized, controlled trial of cyclophosphamide, methotrexate, and fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil with and without tamoxifen for high-risk, node-negative breast cancer: treatment results of Intergroup Protocol INT-0102. J Clin Oncol. 2005;23: 8313–8321. [DOI] [PubMed] [Google Scholar]
- 18.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134: 459–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chlebowski RT, Kim J, Haque R. Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res (Phila). 2014;7: 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29: 2534–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28: 4120–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Short LJ, Fisher MD, Wahl PM, et al. Disparities in medical care among commercially insured patients with newly diagnosed breast cancer: opportunities for intervention. Cancer. 2010;116: 193–202. [DOI] [PubMed] [Google Scholar]
- 23.Prehn AW, Topol B, Stewart S, Glaser SL, O'Connor L, West DW. Differences in treatment patterns for localized breast carcinoma among Asian/Pacific islander women. Cancer. 2002;95: 2268–2275. [DOI] [PubMed] [Google Scholar]
- 24.Wu XC, Lund MJ, Kimmick GG, et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30: 142–150. [DOI] [PubMed] [Google Scholar]
- 25.Gotay C, Dunn J. Adherence to long-term adjuvant hormonal therapy for breast cancer. Expert Rev Pharmacoecon Outcomes Res. 2011;11: 709–715. [DOI] [PubMed] [Google Scholar]
- 26.Kimmick GG, Li X, Fleming ST, et al. Risk of cancer death by comorbidity severity and use of adjuvant chemotherapy among women with locoregional breast cancer. J Geriatr Oncol. 2018;9: 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yung RL, Hassett MJ, Chen K, et al. Initiation of adjuvant hormone therapy by Medicaid insured women with nonmetastatic breast cancer. J Natl Cancer Inst. 2012;104: 1102–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner VL, Jing W, Boscoe FP, Schymura MJ, Roohan PJ, Gesten FC. Improving Adjuvant Hormone Therapy Use in Medicaid Managed Care-Insured Women, New York State, 2012-2014. Prev Chronic Dis. 2016;13: E120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheeler SB, Kohler RE, Reeder-Hayes KE, et al. Endocrine therapy initiation among Medicaid-insured breast cancer survivors with hormone receptor-positive tumors. J Cancer Surviv. 2014;8: 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Artiga S, Hinton E. Beyond Health Care: The Role of Social Determinants in Promoting Health and Health Equity. May 10, 2018. Available at: https://www.kff.org/racial-equity-and-health-policy/issue-brief/beyond-health-care-the-role-of-social-determinants-in-promoting-health-and-health-equity/. [Google Scholar]
- 31.Hershman DL, Tsui J, Meyer J, et al. The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. J Natl Cancer Inst. 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sparano JA, Gray RJ, Makower DF, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015;373: 2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and Genomic Risk to Guide the Use of Adjuvant Therapy for Breast Cancer. N Engl J Med. 2019;380: 2395–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapter 3: Creation of New Race-Ethnicity Codes and SES Indicators for Medicare Beneficiaries - Chapter 3. January 2008. Agency for Healthcare Research and Quality, Rockville, MD. http://archive.ahrq.gov/research/findings/final-reports/medicareindicators/medicareindicators3.html. [Google Scholar]
- 35.Bhavsar NA, Gao A, Phelan M, Pagidipati NJ, Goldstein BA. Value of Neighborhood Socioeconomic Status in Predicting Risk of Outcomes in Studies That Use Electronic Health Record Data. JAMA Netw Open. 2018;1: e182716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berkowitz SA, Traore CY, Singer DE, Atlas SJ. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50: 398–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Area Health Resources Files. Available at: https://data.hrsa.gov/topics/health-workforce/ahrf. Accessed on June 19, 2020. [Google Scholar]
- 38.Huiart L, Ferdynus C, Giorgi R. A meta-regression analysis of the available data on adherence to adjuvant hormonal therapy in breast cancer: summarizing the data for clinicians. Breast Cancer Res Treat. 2013;138: 325–328. [DOI] [PubMed] [Google Scholar]
- 39.Stephen P The Cost of Tamoxifen vs. Aromatase Inhibitors. Available at: https://www.verywellhealth.com/compared-cost-effectiveness-of-tamoxifen-and-arimidex-430669. Accessed on May 8, 2020. [Google Scholar]
- 40.Obeng-Gyasi S, Kircher SM, Lipking KP, et al. Oncology clinical trials and insurance coverage: An update in a tenuous insurance landscape. Cancer. 2019;125: 3488–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Livaudais JC, Li C, John EM, et al. Racial and ethnic differences in adjuvant hormonal therapy use. J Womens Health (Larchmt). 2012;21: 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mode NA, Evans MK, Zonderman AB. Race, Neighborhood Economic Status, Income Inequality and Mortality. PLoS One. 2016;11: e0154535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albain KS, Gray RJ, Makower DF, et al. Race, ethnicity and clinical outcomes in hormone receptor-positive, HER2-negative, node-negative breast cancer in the randomized TAILORx trial. J Natl Cancer Inst. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foundation KF. Prescription Drug Trends. In: Washington: Kaiser Family Foundation; 2010. [Google Scholar]
- 45.Sadigh G, Gallagher K, Obenchain J, et al. Pilot Feasibility Study of an Oncology Financial Navigation Program in Brain Cancer Patients. J Am Coll Radiol. 2019;16: 1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadigh G, Carlos RC, Krupinski EA, Meltzer CC, Duszak R Jr. Health Care Price Transparency and Communication: Implications for Radiologists and Patients in an Era of Expanding Shared Decision Making. AJR Am J Roentgenol. 2017;209: 959–964. [DOI] [PubMed] [Google Scholar]
- 47.Sherman DE. Transforming Practices Through the Oncology Care Model: Financial Toxicity and Counseling. J Oncol Pract. 2017;13: 519–522. [DOI] [PubMed] [Google Scholar]
- 48.Chin AL, Bentley JP, Pollom EL. The impact of state parity laws on copayments for and adherence to oral endocrine therapy for breast cancer. Cancer. 2019;125: 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carlos RC, Lowry KP, Sadigh G. The Coronavirus Disease 2019 (COVID-19) Pandemic: A Patient-Centered Model of Systemic Shock and Cancer Care Adherence. J Am Coll Radiol. 2020;17: 927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burns ME, Huskamp HA, Smith JC, Madden JM, Soumerai SB. The Effects of the Transition From Medicaid to Medicare on Health Care Use for Adults With Mental Illness. Med Care. 2016;54: 868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schumacher JR, Smith MA, Liou JI, Pandhi N. Insurance disruption due to spousal Medicare transitions: implications for access to care and health care utilization for women approaching age 65. Health Serv Res. 2009;44: 946–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu B, Lee FF & Boscoe F Residential mobility among adult cancer survivors in the United States. BMC Public Health 20, 1601 (2020). 10.1186/s12889-020-09686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


