Abstract
The response of native plants to allelopathic interference of invasive species may differ from species to species. In this study, the phytotoxic effects of Ageratina adenophora were tested on two native shrubs (Osbeckia stellata and Elsholtzia blanda) of Nepal. Both the shrubs were grown in pots under treatments of A. adenophora fresh leaves and root leachates, and litter. Then, the seedling length and biomass were compared among the treatments. The results show that A. adenophora litter has stimulatory effects but the leachates from fresh leaves and root are phytotoxic to the growth and development of native shrubs. Infrared Spectroscopy (IR) analysis confirmed the presence of O–H (Hydroxyl), N–H (Amines), C≡C (Alkynes), and C–H stretching (Aromatic) or C–O–C stretching (Ethers) in the leachates representing harmful allelochemicals. The invaded soil by A. adenophora had low pH and a high amount of organic matter, total nitrogen, phosphorus, and potassium than the uninvaded soil. The results indicate that the native O. stellata and E. blanda are harmed by A. adenophora in nature by leaching of allelochemicals and probably by reducing the soil pH. Overall, this study has provided valuable insights regarding the effects of A. adenophora invasion on native shrubs and revealing the potential mechanism of its invasiveness.
Subject terms: Ecophysiology, Invasive species, Ecology, Plant sciences
Introduction
Ageratina adenophora (Spreng.) R.M. King & H. Rob., a perennial shrub of family Asteraceae, is commonly called the Crofton weed. It is the native weed in Mexico1. It has become highly invasive and rapidly spread worldwide including Asia, Australia, and Africa1–3. In the invaded regions, it has threatened the biodiversity of native forests, rangelands, and farmlands4. This weed has the capability to regenerate by vegetative methods and also reproduce from its minute seeds which are produced in huge numbers. It proliferates rapidly in the invaded sites and forms its monoculture5,6.
Ageratina adenophora has been naturalized in Nepal where it was first reported in 19527. In Nepal, it is locally called ‘Kalo Banmara’ meaning the 'Forest Killer Plant' having dark green leaves. It has been spreading throughout the country from tropical regions to northern border crossing through the subtropical mountain region7. It has been spread along the trails, roads, disturbed sites, and margins or open canopy areas of the forests of Nepal8–14.
This plant is known to have negative impacts on native vegetation15–17. It affects plant community composition, species diversity, and abundance15. Thapa et al. (2020) reported that the plant is responsible to reduce the native species richness in the invaded sites in Nepal 17. Allelopathy has been one of the mechanisms affecting other plants by A. adenophora18. Negative impacts on physiology and morphology of some crops (e.g., rice), weeds (e.g., Lolium perenne, Trifolium repens, Galinsoga parviflora, and Medicago sativa) and native trees (e.g., Schima wallichii and Alnus nepalensis) by aerial parts (leaves and litter) and roots of this species have been reported previously16,19–22. Negative effects on native seed germination and growth by volatile compounds from the litter of A. adenophora have also been reported 17,23. In the inhibition, there is role of several allelochemicals present in the aerial and underground parts of this plant 23–25. The allelochemicals are the secondary metabolites that can be classified according to their carbon skeletal structure and type of functional groups such as alcohols, amines, carboxylic acids26. Thus, the functional group analysis is one of the most satisfactory and applicable methods of determining organic compounds which also lends towards the identification of organic compounds27. Such analyses have significance in understanding the chemical nature of invasive weed’s allelochemicals.
Although the previous studies have documented the negative impacts of A. adenophora on the native species, the insights regarding whether A. adenophora affects all the native plants always negatively or it may also have positive impacts on some native species are very scarce. For this, several experiments can be performed by testing the effect of A. adenophora on the native plants from invaded sites. We hypothesized that the native plants’ response to allelopathic interference of A. adenophora differs from species to species. Some native species are found frequently associated with A. adenophora in the invaded sites. There might be two reasons for such an association: one, the invasive species might have started colonization in the native region and the native species might be at the stage of replacement/inhibition by the invasive species. Another, the native species might have resisted the negative effects of invasion and therefore, the association has no significant negative relationship or sometimes there might be a positive interaction between them. Such phenomena cannot be predicted easily by simple field observations.
Two of the native species (Osbeckia stellata and Elsholtzia blanda) in the mid-hill region of central Nepal are frequently associated with A. adenophora invaded forests. Both of these species are the most common subtropical native shrubs in Nepal. As discussed above, it cannot be expected that these two native species are either tolerating the negative effects of A. adenphora or they are harming by the invasion. In such a condition, proper experiments should be designed to know the actual interactions among them. In this study, growth response of above mentioned native shrubs were tested against A. adenphora fresh leaves and roots leachates, and the litter. In addition, the chemical groups present in the leachates and the potential impact of A. adenophora invasion in soil was also explored. Testing phytotoxicity of A. adenophora on native shrubs of Nepal is a novel work. It would have significance to know the parts and chemicals found in A. adenphora contributing positively or negatively to the growth and development of native shrub species and soil properties.
Results
Shoot and root length
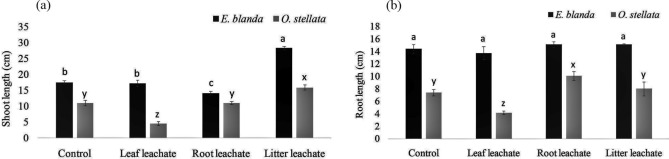
Shoots of both the native species E. blanda and O. stellata were longer in A. adenophora litter treatment compared to the control plants. A. adenophora root leachate reduced the shoot length of E. blanda while the leaf leachate did not show inhibitory or stimulatory effects. Interestingly, the shoot length of O. stellata was inhibited by the fresh leaf leachate, not by the root leachate of A. adenophora (Fig. 1a, df = 3, p < 0.001).
Figure 1.
Effect of A. adenophora leachates on (a) shoot length and, (b) root length of native E. blanda and O. stellata. Different letters above the error bar indicate significant differences among the treatments (‘a’, ‘b’, and ‘c’ for E. blanda; ‘x’, ‘y’, and ‘z’ for O. stellata; p < 0.05).
In the case of root length, there was no stimulatory or inhibitory effects on E. blanda by all the treatments (Fig. 1b, df = 3, p = 0.44). The roots of O. stellata were longer in the treatment of A. adenophora root leachate comparing to the control plants while the leaf leachate showed inhibition to the root length (Fig. 1b, df = 3, p < 0.001).
Shoot and root dry weight (biomass)
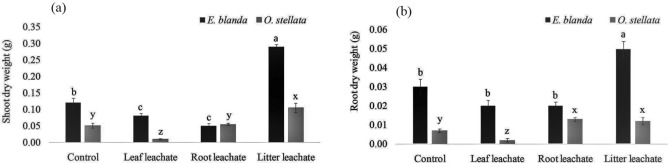
Similar to the shoot length, A. adenophora litter increased dry weight of shoots in both native species. Comparing to the control plants shoots were lighter in the treatments of A. adenophora leaf and root leachates. The root leachate did not inhibit the dry weight of O. stellata but the leaf leachate reduced the weight significantly (Fig. 2a, df = 3, p < 0.001).
Figure 2.
Effect of A. adenophora leachates on (a) shoot dry weight and (b) root dry weight of native E. blanda and O. stellata. Different letters above the error bar indicate significant differences among the treatments (‘a’, ‘b’, and ‘c’ for E. blanda; ‘x’, ‘y’, and ‘z’ for O. stellata; p < 0.05).
The dry weight of roots in both the native species was increased by A. adenophora litter. There was no significant effects of both the leaf and root leachate on the root dry weight of E. blanda. The root leachate also increased dry weight of roots in O. stellata while the leaf leachate reduced the dry weight (Fig. 2b, df = 3, p < 0.001).
Chlorophyll a and b
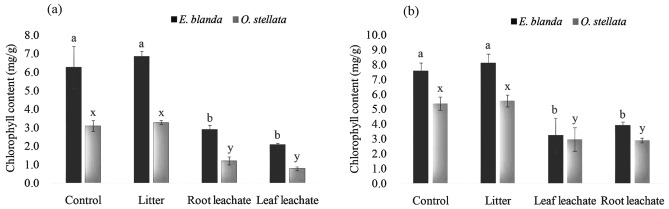
Ageratina adenophora litter did not increase or decrease the content of chlorophyll - a and b in both E. blanda and O. stellata but the leaf and root leachates inhibited chlorophyll content significantly [Fig. 3, df = 3, p < 0.01 (E. blanda) and p < 0.001 (O. stellata)].
Figure 3.
Effect of A. adenophora leachates on (a) chlorophyll - a and, (b) chlorophyll - b of native E. blanda and O. stellata. Different letters above the error bar indicate significant differences among the treatments (‘a’, ‘b’, and ‘c’ for E. blanda; and ‘x’, ‘y’, and ‘z’ for O. stellata; p < 0.05).
Infrared spectroscopy (IR) analysis
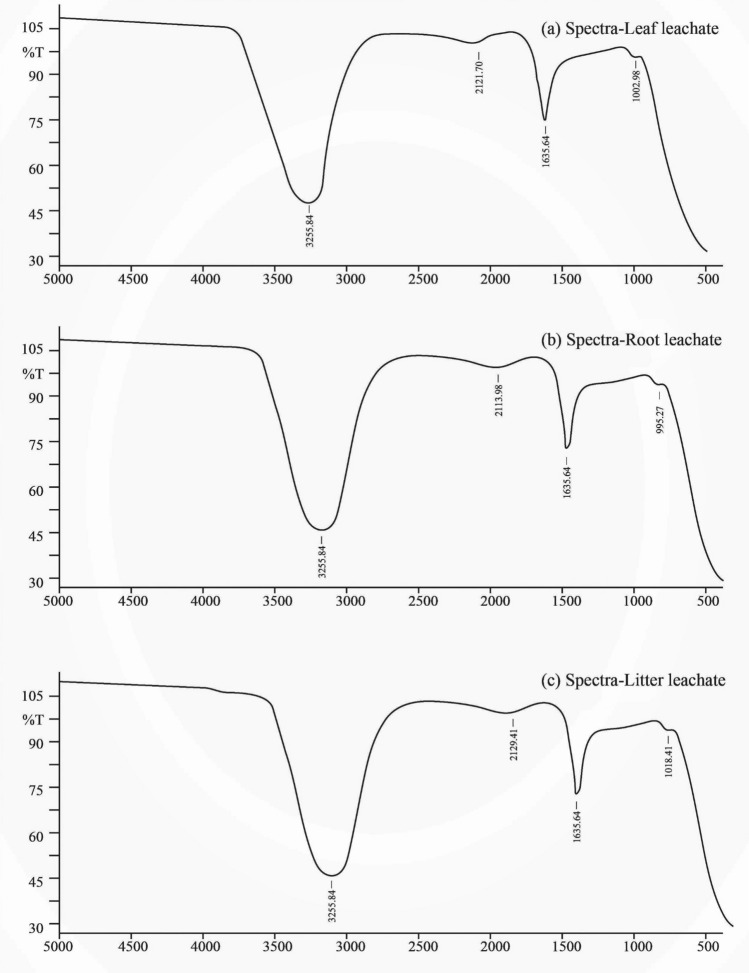
Infrared spectroscopy (IR) analysis showed that all the leachates (leaf, litter, and root) of A. adenophora have similar functional groups, that were O-H (Hydroxyl), N–H (Amine), C≡C (Alkynes), C–H stretching (Aromatic) or C–O–C stretching (Ethers) groups (Fig. 4, Table 1). The wavenumber of the hydroxyl group was 3255.84 cm−1, alkynes had wavenumbers ranged from 2113.98 cm−1 to 2129.41 cm−1, amines had 1635.64 cm−1, and the wavenumber of C-H stretching (Aromatic) or C–O–C stretching (Ethers) varied from 995.27 cm−1 to 1018.41 cm−1 (Fig. 4, Table 1).
Figure 4.
Spectra of IR analysis in A. adenophora fresh leaf, root and litter leachates. The names of the functional groups are given in Table 1.
Table 1.
Functional groups of chemicals found in A. adenophora leachates.
| S.N. | FTIR peak value | Functional groups | ||
|---|---|---|---|---|
| Leaf leachate | Root leachate | Litter leachate | ||
| 1 | 3255.84 | 3255.84 | 3255.84 | Hydroxyl (O–H) |
| 2 | 2121.7 | 2129.41 | 2113.98 | Alkynes (C≡C) |
| 3 | 1635.64 | 1635.64 | 1635.64 | Amines (N–H) |
| 4 | 1002.98 | 1018.41 | 995.27 | C–H stretching (Aromatic) or C–O–C stretching (Ethers) |
Soil analysis
Soil analysis showed differences in pH, soil organic matter (OM), total nitrogen (N), available phosphorus (P) and potassium (K) between A. adenophora invaded and uninvaded sites. A. adenophora invaded soil had low pH (5.13 ± 0.06) than the uninvaded soil (5.61 ± 0.10). The concentrations of organic matter (OM), total nitrogen, available phosphorus and potassium were significantly high in the invaded soil comparing to the uninvaded soil (Table 2).
Table 2.
Data on soil analysis (A. adenophora invaded and uninvaded soils).
| Soil parameters | Invaded soil | Uninvaded soil | df | t-value | p value |
|---|---|---|---|---|---|
| pH | 05.13 ± 0.06 b | 05.61 ± 0.10 a | 18 | − 4.04 | 0.001 |
| OM (%) | 05.08 ± 0.15 a | 04.25 ± 0.15 b | 3.809 | 0.001 | |
| Total N (%) | 00.29 ± 0.01 a | 00.23 ± 0.01 b | 7.163 | < 0.001 | |
| K (kg/ha) | 83.92 ± 3.79 a | 73.07 ± 3.63 b | 2.064 | 0.050 |
| Soil parameters | Invaded soil | Uninvaded soil | df | Mann–Whitney U | p value |
|---|---|---|---|---|---|
| P (kg/ha) | 00.44 ± 0.06 a | 00.33 ± 0.10 b | 18 | 20 | 0.023 |
The letters ‘a’ and ‘b’ after the mean value ± SE indicate significant differences (p < 0.05).
Discussion
Ageratina adenophora litter showed positive effects on E. blanda seedling growth. Both the shoots and roots of native E. blanda were longer in A. adenophora litter treated plants compared to the control plants (Fig. 1). A similar effect was found in shoot and root biomass as well (Fig. 2). It confirms that A. adenophora may also have a supportive role in the growth and development of native E. blanda. On the other hand, shoot length and biomass were reduced by A. adenophora root leachate (Figs. 1, 2) but leaf leachate reduced only the biomass. From this result, it can be stated that E. blanda taking benefit from A. adenophora litter can be harmed in another way by roots or fresh leaves of A. adenophora. Overall, the impacts of A. adenophora to E. blanda could be negative because one component (litter) facilitates the growth whereas two-components (root and fresh leaves) have an inhibitory role. Simultaneously, the toxic effect of root and leaf leachates on chlorophyll contents (Fig. 3) may impair the light energy uptake during photosynthesis in E. blanda.
In the case of native O. stellata, the litter of A. adenophora increased shoot length but decreased the root length. The root leachate increased root length and biomass but decreased shoot length and biomass (Figs. 1, 2). This effect may create an abnormality in the root-shoot ratio as there is a stimulatory effect on one part (aerial or underground) and an inhibitory effect on another part (aerial or underground). The root-shoot ratio indicates overall health of plants28,29. Any change in normal root-shoot ratio plants would be an indication of a change in the overall health of plants30. The effect of the root and leaf leachates on chlorophylls of O. stellata was similar to the E. blanda. These results have confirmed that the invasive A. adenophora is capable to alter the root-shoot ratio and content of the photosynthetic pigments of native O. stellata.
There are not many validations showing the effects of A. adenophora on native herbs, shrubs, and tree species. Most commonly the researchers consider the crop plants and weeds as the test species. The results of some of the previous studies are in support of the findings of our study. For example, Das et al. (2018) tested the effect of this weed on seed germination and seedling growth of some crop plants and weeds31. The crop plants were Triticum aestivum and Brassica campestris and the weeds were Ageratum conyzoides, Bidens pilosa, Galinsoga parviflora and Cyperus rotundus. They have found that the extracts of A. adenophora inhibited the seed germination and seedling length of the tested plants. Similarly, Thapa et al. (2020) found that the growth and development of seedlings of native trees (Schima wallichii and Alnus nepalensis) were inhibited by A. adenophora fresh leaves and leaf extract16. Regarding the allelopathic mechanism of inhibition to native plants by invasive plants, the plant parts which are potential to produce harmful allelochemicals should be identified. Our study reveals that the fresh leaves and roots of A. adenophora are the parts which are the potential to produce harmful allelochemicals that can inhibit native species growth and development.
Diverse chemical compounds such as monoterpenes, sesquiterpenes, diterpenes and triterpenes, phenylpropanoids, flavonoids, coumarins, sterols, and alkaloids have been reported from A. adenophora. As examples, Zhou et al. (2013) have identified eleven phenolic compounds such as 7-hydroxy-8,9-dehydrothymol 9-O-trans-ferulate; 7-hydroxythymol 9-O-trans-ferulate; 7,8-dihydroxythymol 9-O-trans-ferulate; 7,8-dihydroxythymol 9-O-cis-ferulate; methyl (7R)-3-deoxy-4,5-epoxy-D-manno-2-octulosonate 8-O-trans-p-coumarate; methyl (7R)-3-deoxy-4,5-epoxy-D-manno-2-octulosonate 8-O-cis-p-coumarate; and o-coumaric acid, etc. having inhibitory effects on Arabidopsis seed germination32.
Quinic acid derivative (5-O-trans-o-coumaroylquinic acid methyl ester; chlorogenic acid methyl ester; macranthoin F and macranthoin G were isolated by Zhang et al. (2013) from the aerial parts of A. adenophora33. Similarly, thymol derivatives such as 7,9-diisobutyryloxy-8-ethoxythymol; 7-acetoxy-8-methoxy-9-isobutyryloxythymol and 7,9-di-isobutyryloxy-8-methoxythymol; 9-oxoageraphorone; (−)-isochaminic acid and (1α,6α)-10-hydroxycar-3-ene-2-one were identified by Dong et al. (2017) from roots of A. adenophora34. Including these chemical compounds and other monoterpenes from the aerial parts of A. adenophora are reported to have allelopathic potential33,35.
For the identification of at least the functional groups in chemicals found in the leachates of A. adenophora, Infrared Spectroscopy (IR) analysis was carried out in this study. The IR analysis is the most common and widely used spectroscopic technique for determining functional groups of chemical compounds existing in the leachates. The analyses had confirmed four functional groups in the leachates. The wavenumbers 3255.84 cm−1 and 1635.64 cm−1 indicated the presence of O–H (Hydroxyl) group and N-H (Amine) group, respectively in all types of A. adenophora leachates (root, fresh leaves, and litter) (Table 1). The wavenumbers ranged from 2113.98 to 2129.41 cm−1 found in the leachates indicated alkynes. Similarly, the wavenumbers ranged from 995.27 to 1018.41 cm−1 indicates the presence of C–H stretching (Aromatic) or C–O–C stretching (Ethers) in the leachates (Table 1).
Existence of these functional groups confirms that the allelochemicals identified by previous researchers are present in the root, leaf, and litter leachates and they are almost similar based on the functional groups. But, the treatments of the root leachate, fresh leaf leachate, and litter have shown different effects on the tested native species i.e., one can inhibit only aerial parts (shoot) or belowground part (root) and another can inhibit both shoot and root of native species (Figs. 1, 2). Hence, it can be expected that the concentrations of allelochemicals may vary in different parts of A. adenophora and the effects on growth and development of native species may depend on the concentrations. Also, further analysis of chemical compounds and their allelopathic effects should be explored to understand the exact effect on particular native species.
The study on allelopathic inhibition of the invasive species on the selected native species markedly shows that aerial and belowground parts (leaves and roots) are phytotoxic to the native plants. Fresh leaves, litter, and root extracts of A. adenophora were found to be toxic to the growth and development of native trees such as Schima wallichii and Alnus nepalensis in Nepal16,19. Phenologically, A. adenophora produces new leaves starting from pre-monsoon and its luxuriant growth is seen in the monsoon16. Meanwhile, the native species E. blanda and O. stellata also germinate during pre-monsoon to early monsoon. This coincidence has a high probability of facing allelopathic effect by the native seedlings because the rainwater washes the allelochemicals from aerial parts of A. adenophora and mix into the soil. Therefore, it is recommended that the whole body of A. adenophora should be removed before starting pre-monsoon in Nepal. This could prevent the release of allelochemicals from invasive species and mix them into the soil. If the underground parts are left unremoved there will be new sprouts. The removed plant materials should be managed properly, for example, the burial of the removed parts could be one option of the management36. The alternate options might be the utilization of removal parts for composting as the application of compost from A. adenophora may be beneficial for some crop plants37.
We have tested A. adenophora uninvaded and invaded soils to know whether there is any change caused by invasions in the soil properties. It is well known that A. adenophora can bring changes in soil physicochemical parameters38. Impacts of A. adenophora on soil organic matters, soil nitrogen, soil phosphorus, and soil potassium may vary with seasons, exposure, and degrees invasion level and therefore, there is no unified rule on how A. adenophora alter soil parameters39. Hence, it is suggested that the influence of particular invasive species on the soil should be studied at different invaded locations.
Soil analysis shows differences in pH, soil organic matter, total nitrogen, total phosphorus, and potassium (Table 2). A. adenophora had reduced the soil pH. It could be one of the mechanisms to inhibit seed germination, seedling growth, and development of native species19. Soils may become acidic as a result of leaching allelochemicals through rainwater. Thapa et al. (2017) expected that the reduced pH in the A. adenophora may reduce the seedling growth and development of native Schima wallichii19. Here also, it is likely that the growth and development of native E. blanda and O. stellata might have affected by reduced pH. Nirola and Jha (2011) have found that the soil pH in the sites having high importance value index (IVI) of O. stellata was 5.6040 which is similar to our results. Previous studies regarding the soil pH for E. blanda are deficient.
The organic matter, total nitrogen, and available phosphorus in the A. adenophora invaded soil were high as compared to the uninvaded soil. The available potassium was also increased by the invasion of A. adenophora (Table 2). This result was contrasting with the finding of previous studies, for instance, Thapa et al. (2017) found that there were no significant changes in these parameters in Schima-Alnus forest in Nepal19. Soil samples in this study were taken from a mixed plant community of the uninvaded sites. Therefore, during comparison of these parameters, type of uninvaded sites should also be characterized. Moreover, the contradictory results on soil parameters may mislead the readers or researchers to understand the actual pattern of invasive species interactions. Hence, it is suggested that the plant community and history of alien plant invasion as well as the degree of invasion level should be considered to know the actual pattern of changes in soil properties by invasive plants. Also, updating the soil status of native plant communities on the periodic basis would have a great significance for future predictions.
From the results, it is obvious that increasing the content of organic matter, total nitrogen and phosphorus, and potassium in the invaded sites may support the native species. However, the allelopathic effects has an equal chance to harm the native species. It is necessary to evaluate the contribution of such nutrients by invasive plants and the other allelochemical dose released by them in the invaded sites. If the dose of harmful allelochemicals released is higher than the nutrient contribution to the soil, the native plants may not take benefits from the nutrients provided by invasive alien plants.
Based on the field observation, it was difficult to predict the reasons behind association between the native species (O. stellata and E. blanda) and invasive A. adenophora. Our results clarify that the duration of A. adenphora invasion is not much longer to replace these native species but it can be expected that if the process of inhibition due to allelochemicals washed by rainwater continues, these native species might be replaced in the future. The contribution of A. adenophora litter might be lesser than the contribution of fresh parts (leaves) because the litters (dry leaves) are seen only during the post-winter season and the amount is relatively less, while the fresh leaves sprout enormous from pre-monsoon that remain throughout the year. The current study highlights that the interaction between A. adenophora and native O. stellata and E. blanda is negative and based on the results it can be expected that the population of these native shrubs would be diminished gradually in the invaded sites. Hence, regular observation and abundance measurement of the native species are recommended to confirm our prediction.
In conclusion, the impacts of A. adenophora to E. blanda is negative because one component (litter) has an advantage whereas the two-components (root and fresh leaves) have inhibitory role. Regarding the native O. stellata, there is a stimulatory effect of A. adenophora on one part (aerial or underground) and an inhibitory effect on another part (aerial or underground). This effect may create abnormality in the root-shoot ratio. The results also show that the fresh leaves and root of A. adenophora are the parts which are the potential to produce harmful allelochemicals that can inhibit native species. The IR analysis confirmed four functional groups in the A. adenophora leachates that are O–H Stretching (Hydroxyl), N-H (Amines), C–H stretching (Aromatic), and C–O–C stretching (Ethers). This result indicates that A. adenophora allelochemicals belong to these functional groups. Their concentrations may differ based on the vegetative parts of A. adenophora which may produce positive and negative effects on the growth and development of native species. Removal of the whole body of A. adenophora could prevent the release of allelochemicals from aerial and underground parts and mix them into the soil.
Soil analysis shows differences in pH, soil organic matter, total nitrogen, total phosphorus, and potassium between A. adenophora invaded and uninvaded soils. Reduction in the soil pH by A. adenophora can be a mechanism to inhibit the native species. Comparing the concentrations of organic matter, total nitrogen, and total phosphorus in the invaded soil with previous findings, it is suggested that the plant community and history of alien invasion as well as the degree of invasion level should be considered to know the actual pattern of changes in soil properties by an alien invasion. Monitoring the soil status frequently in the invaded regions have great significance for the future predictions. Additionally, it is necessary to evaluate the positive contribution of nutrients and the negative effects of allelochemicals released from invasive plants to estimate the net effect on the growth and development of native species.
Materials and methods
Test species
Two native shrubs Osbeckia stellata Buchanan-Hamilton ex Kew Gawler and Elsholtzia blanda (Benth.) Benth. were the native test species for the phytotoxicity of invasive A. adenophora. These species are found associated with A. adenophora in the invaded sites. E. blanda represents a member of the family Lamiaceae and O. stellata of the family Melastomataceae. Both reach a height up to 2 m. E. blanda is an aromatic plant and yields essential oil. Both species are the highly valuable medicinal plants. E. blanda is used in cuts and wounds, cough, choleric diarrhoea, fever, hepatitis, nephritis, pharyngitis, and cardiovascular disorders41,42. O. stellata is used in the treatment of diarrhoea, dysentery, and juice of leaves is used for scabies treatment43. In addition, both the species are important elements of native species composition in subtropical regions of Nepalese forests.
Pot experiment
The native plants were grown in pots containing soil collected from the uninvaded area and treated with A. adenophora leachates and litter. Seeds of native E. blanda and O. stellata, and the soils were collected in February 2018 from the Takhtar Community Forest (27° 24′ 59.99′′ N and 85° 01′ 60.00′′ E, elevation: 1750–1900 masl.). The community forest is located in Thaha Municipality -9, Chitlang village of Makawanpur district, Bagmati Province, Nepal. The average annual temperature in the Chitlang village area is 16.2 °C and the average annual rainfall is 2812 mm44. The seeds were brought to the Central Department of Botany, Tribhuvan University, Kirtipur, and stored at 4 °C in the airtight plastic bag until use.
Polyethylene pots (size 6 × 10 cm2) were filled with 200 g of soil and the soil was moistened by 200 ml distilled water. The seeds of native species were placed on moist filter paper at room temperature (25 ± 5 °C) and allowed to germinate in dark. After the 7th day of seed soaking, the seedlings grown were about 1 to 1.5 cm long. The seedlings of homogenous size were gently picked up and transplanted to the pots prepared. Six seedlings were transplanted to each pot containing the moist soil.
The native seedlings were grown in the pots with the following treatments (i) Control (distilled water), (ii) A. adenophora leachate (leachate obtained from 10 g leaves/100 ml distilled water), (iii) A. denophora root leachate (leachate obtained from 10 g root/100 ml distilled water), and (iv) A. adenophora litter (1 cm thick layer on pot surface). Each treatment had 6 replicated pots. Altogether there were 48 pots (4 treatments × 6 replicates × 2 native species = 48).
The control pots were watered (10 ml) using distilled water on an alternate day. Similarly, A. adenophora fresh leaf and root leachates were poured into the respective pots. The litter treatment pots were watered using 10 ml distilled water over the litter. The pots were placed in the glasshouse of the Central Department of Botany, Tribhuvn University, Kathmandu, Nepal and allowed the seedlings to grow. The positions of the pots were randomly changed regularly in the glasshouse to minimize the positional effect. The temperature of the house ranged between 20 and 38 °C and moisture between 50 and 88%. Plants were harvested on the 48th days after seedling transplantation. After harvesting, the length and dry weight of roots and shoots were taken separately. The roots and shoots were dried in a hot air oven at 80 °C for 24 h for the dry weight.
Infrared spectroscopy (IR) analysis
Infrared spectroscopy (IR) analysis of A. adenophora leaf, root, and litter leachates was done for determining the functional groups of chemicals present in the extract. A small quantity of each leachate was separately poured on the Attenuated Total Reflection (ATR) Diamond puck at ATR crystal of Fourier Transform Infrared Spectrometer (FTIR). The ATR is the method that allows the direct measurement of samples for FTIR. The IR spectrum was obtained using SHIMADZU IRPrestige-21, FTIR Spectrometer, Department of Plant Resource, Ministry of Forests and Environment, Government of Nepal, Thapathali, Kathmandu, Nepal. The samples were scanned 25 times with a resolution of 16 from 5000 cm−1 to 500 cm−1 wavenumber range. The spectrum of peaks with wavenumbers was recorded.
Soil analysis and chlorophyll estimation
Ageratina adenophora invaded and uninvaded soils were collected from Takhtar Community Forest, Chitlang, Makwanpur (surface soil from 5 to 15 cm). Two-line transects were made, one along each invaded and uninvaded site in the forest. Ten plots of size 1 m2 were sampled at each transect. Altogether 20 soil samples were collected (10 from invaded and 10 from uninvaded plots). The uninvaded plots were free of A. adenophora whereas the cover of A. adenophora,45 and the total nitrogen was estimated by the Kjeldahl method46. Estimation of phosphorus and potassium was followed by Olsen’s bicarbonate method47 and the Flame photometry48, respectively. Chlorophyll contents were estimated in the leaves sampled from the plants grown in pots under different treatments as mentioned in the pot experiment. Acetone (80%) was used for extraction and the contents were measured using the method described by Bajracharya (1999)49.
Statistical analyses
The growth parameters (root shoot length and dry weight) and chlorophyll content among different treatments (root leachate, leaf leachate and litter of A. adenophora) were compared using one-way ANOVA. Soil parameters (pH, OM, total N and K) between the A. adenophora invaded and uninvaded sites were compared using independent sample t-test but the phosphorus was compared using the Mann–Whitney U test as the data was not normal. The p-value < 0.05 was considered statistically significant differences in the plant growth and soil parameters.
Ethics and research guideline statement
Research permission including the collection of plant materials was taken from the Department of Plant Resources (DPR), Ministry of Forests and Environment, Government of Nepal, Thapathali, Kathmandu, Nepal. The experiments were conducted following relevant guidelines and regulations.
Acknowledgements
This study was supported by University Grants Commission, Bhaktapur, Nepal (FRG-73/74-S&T-01). We are grateful to the Department of Plant Resources (DPR), Ministry of Forests and Environment, Government of Nepal, Thapathali, Kathmandu, Nepal and Central Department of Environmental Science, Institute of Science and Technology, Tribhuvan University, Kirtipur, Kathmandu, Nepal for providing laboratory facilities. We are thankful to Prof. Emeritus Dr. Pramod Kumar Jha, Prof. Dr. Mohan Siwakoti, Prof. Dr. Rejina Maskey, and Prof. Dr. Ram Kailashi Prasad Yadav for every support during the study.
Author contributions
L.B.T. and R.R.P. designed the experiments and obtained funding. T.B.D., B.A., S.P., S.N. and K.B. performed the experiments, analyzed the data, and drafted the manuscript. T.D.B, G.P., and K.B.P. participated in data analysis and interpretations. All authors contributed to the revising of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cronk QCB, Fuller JL. Plant Invaders: The Threat to Natural Ecosystems. Chapman and Hall; 1995. [Google Scholar]
- 2.Tererai F, Wood AR. On the present and potential distribution of Ageratina adenophora (Asteraceae) in South Africa. S. Afr. J. Bot. 2014;95:152–158. doi: 10.1016/j.sajb.2014.09.001. [DOI] [Google Scholar]
- 3.Yu F, Akin-Fajiye M, Thapa Magar K, Ren J, Gurevitch J. A global systematic review of ecological field studies on two major invasive plant species, Ageratina adenophora and Chromolaena odorata. Divers. Distrib. 2016;22:1174–1185. doi: 10.1111/ddi.12481. [DOI] [Google Scholar]
- 4.Niu HB, Liu WX, Wan FH, Liu B. An invasive aster (Ageratina adenophora) invades and dominates forest understories in China: Altered soil microbial communities facilitate the invader and inhibit natives. Plant Soil. 2007;294:73–85. doi: 10.1007/s11104-007-9230-8. [DOI] [Google Scholar]
- 5.Wang JJ. Ageratina adenophora (Spreng.) In: Wan FH, Zheng XB, Guo JY, editors. Biology and Management of Invasive Alien Species in Agriculture and Forestry. Science Press; 2005. pp. 651–661. [Google Scholar]
- 6.Yang G, Gui F, Liu W, Wan F. Crofton weed Ageratina adenophora (Sprengel) In: Wan F, Jiang M, Zhan A, editors. Biological Invasions and Its Management in China. Springer; 2017. pp. 111–129. [Google Scholar]
- 7.Shrestha BB. Invasive alien plant species in Nepal. In: Jha PK, Siwakoti M, Rajbhandary S, editors. Frontiers of Botany. Tribhuvan University; 2016. pp. 269–284. [Google Scholar]
- 8.Alka C, Adhikari BS, Joshi NC, Rawat GS. Patterns of invasion by crofton weed (Ageratina adenophora) in Kailash sacred landscape region of western Himalaya (India) Environ. Conserv. J. 2019;20:9–17. [Google Scholar]
- 9.Balami S, Thapa LB. Herbivory damage in native Alnus nepalensis and invasive Ageratina adenophora. Bot. Orient. 2017;11:7–11. doi: 10.3126/botor.v11i0.21026. [DOI] [Google Scholar]
- 10.Thapa LB, Kaewchumnong K, Sinkkonen A, Sridith K. Plant communities and Ageratina adenophora invasion in lower montane vegetation, central Nepal. Int. J. Ecol. Dev. 2016;31:35–49. [Google Scholar]
- 11.Thapa LB, Thapa H, Magar BG. Perception, trends and impacts of climate change in Kailali District, Far West Nepal. Int. J. Environ. 2015;4:62–76. doi: 10.3126/ije.v4i4.14099. [DOI] [Google Scholar]
- 12.Thapa N, Maharjan M. Invasive alien species: Threats and challenges for biodiversity conservation (A case study of Annapurna Conservation Area, Nepal) In: Thapa GJ, Subedi N, Pandey MR, Thapa SK, Chapagain NR, Rana A, editors. Proc. International Conference on Invasive Alien Species Management, Chitwan, March 25–27, 2014. National Trust for Nature Conservation; 2014. pp. 18–22. [Google Scholar]
- 13.Tiwari S, Adhikari B, Siwakoti M, Subedi K. An Inventory and Assessment of Invasive Alien Plant Species of Nepal. IUCN Nepal; 2005. [Google Scholar]
- 14.Tripathi RS, Yadav AS, Kushwaha SPS. Biology of Chromolaena odorata and Ageratina adenophora. In: Bhatt JR, Singh JS, Singh SP, Tripathi RS, Kohli RK, editors. Invasive Alien Plants: An Ecological Appraisal for the Indian Subcontinent. CAB International Publishing; 2012. pp. 43–56. [Google Scholar]
- 15.Fu D, Wu X, Huang N, Duan C. Effects of the invasive herb Ageratina adenophora on understory plant communities and tree seedling growth in Pinus yunnanensis forests in Yunnan, China. J. For. Res. 2018;23:112–119. doi: 10.1080/13416979.2018.1429202. [DOI] [Google Scholar]
- 16.Thapa LB, Kaewchumnong K, Sinkkonen A, Sridith K. “Soaked in rainwater” effect of Ageratina adenophora on seedling growth and development of native tree species in Nepal. Flora. 2020;263:151554. doi: 10.1016/j.flora.2020.151554. [DOI] [Google Scholar]
- 17.Thapa LB, Kaewchumnong K, Sinkkonen A, Sridith K. Airborne and belowground phytotoxicity of invasive Ageratina adenophora on native species in Nepal. Plant Ecol. 2020;221:883–892. doi: 10.1007/s11258-020-01048-7. [DOI] [Google Scholar]
- 18.Wan F, Liu W, Guo J, Qiang S, Li B, Wang J, Yang G, Niu H, Gui F, Huang W, Jiang Z. Invasive mechanism and control strategy of Ageratina adenophora (Sprengel) Sci. China Life Sci. 2010;53:1291–1298. doi: 10.1007/s11427-010-4080-7. [DOI] [PubMed] [Google Scholar]
- 19.Thapa LB, Kaewchumnong K, Sinkkonen A, Sridith K. Plant invasiveness and target plant density: High densities of native Schima wallichii seedlings reduce negative effects of invasive Ageratina adenophora. Weed Res. 2017;57:72–80. doi: 10.1111/wre.12238. [DOI] [Google Scholar]
- 20.Wan H, Liu W, Wan F. Allelopathic effect of Ageratina adenophora (Spreng.) leaf litter on four herbaceous plants in invaded regions. Chin. J. Eco-Agric. 2011;19:130–134. doi: 10.3724/SP.J.1011.2011.00130. [DOI] [Google Scholar]
- 21.Yang GQ, Wan FH, Guo JY, Liu WX. Cellular and ultrastructural changes in the seedling roots of upland rice (Oryza sativa) under the stress of two allelochemicals from Ageratina adenophora. Weed Biol. Manage. 2011;11:152–159. doi: 10.1111/j.1445-6664.2011.00413.x. [DOI] [Google Scholar]
- 22.Zhang F, Guo J, Chen F, Liu W, Wan F. Identification of volatile compounds released by leaves of the invasive plant croftonweed (Ageratina adenophora, Compositae), and their inhibition of rice seedling growth. Weed Sci. 2012;60:205–211. doi: 10.1614/WS-D-11-00156.1. [DOI] [Google Scholar]
- 23.Inderjit EH, et al. Volatile chemicals from leaf litter are associated with invasiveness of a Neotropical weed in Asia. Ecology. 2011;92:316–324. doi: 10.1890/10-0400.1. [DOI] [PubMed] [Google Scholar]
- 24.Yang GQ, Qiu WR, Jin YN, Wan FH. Potential allelochemicals from root exudates of invasive Ageratina adenophora. Allelopathy J. 2013;32:233. [Google Scholar]
- 25.Zhu XZ, Guo J, Shao H, Yang GQ. Effects of allelochemicals from Ageratina adenophora (Spreng.) on its own autotoxicity. Allelopathy J. 2014;34:253. [Google Scholar]
- 26.Latif S, Chiapusio G, Weston LA. Allelopathy and the role of allelochemicals in plant defence. Adv. Bot. Res. 2017;82:19–54. doi: 10.1016/bs.abr.2016.12.001. [DOI] [Google Scholar]
- 27.Siggia S. Importance of functional group determination in organic quantitative analysis. J. Chem. Educ. 1950;27(3):141. doi: 10.1021/ed027p141. [DOI] [Google Scholar]
- 28.Rogers ER, Zalesny RS, Hallett RA, Headlee WL, Wiese AH. Relationships among root–shoot ratio, early growth, and health of hybrid poplar and willow clones grown in different landfill soils. Forests. 2019;10:49. doi: 10.3390/f10010049. [DOI] [Google Scholar]
- 29.Thornley JHM. A balanced quantitative model for root: Shoot ratios in vegetative plants. Ann. Bot. 1972;36:431–441. doi: 10.1093/oxfordjournals.aob.a084602. [DOI] [Google Scholar]
- 30.Mašková T, Herben T. Root: Shoot ratio in developing seedlings: How seedlings change their allocation in response to seed mass and ambient nutrient supply. Ecol. Evol. 2018;8:7143–7150. doi: 10.1002/ece3.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das MBB, Acharya BD, Saquib M, Chettri MK. Effect of aqueous extract and compost of invasive weed Ageratina adenophora on seed germination and seedling growth of some crops and weeds. J. Biodivers. Conserv. Bioresour. Manage. 2018;4:11–20. doi: 10.3329/jbcbm.v4i2.39843. [DOI] [Google Scholar]
- 32.Zhou ZY, Liu WX, Pei G, Ren H, Wang J, Xu QL, Xie HH, Wan FH, Tan JW. Phenolics from Ageratina adenophora roots and their phytotoxic effects on Arabidopsis thaliana seed germination and seedling growth. J. Agric. Food Chem. 2013;61:11792–11799. doi: 10.1021/jf400876j. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M, Liu WX, Zheng MF, Xu QL, Wan FH, Wang J, Lei T, Zhou ZY, Tan JW. Bioactive quinic acid derivatives from Ageratina adenophora. Molecules. 2013;18:14096–14104. doi: 10.3390/molecules181114096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong LM, Zhang M, Xu QL, Zhang Q, Luo B, Luo QW, Liu WB, Tan JW. Two new thymol derivatives from the roots of Ageratina adenophora. Molecules. 2017;22:592. doi: 10.3390/molecules22040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao X, Zheng GW, Niu XM, Li WQ, Wang FS, Li SH. Terpenes from Eupatorium adenophorum and their allelopathic effects on Arabidopsis seeds germination. J. Agric. Food Chem. 2009;57:478–482. doi: 10.1021/jf803023x. [DOI] [PubMed] [Google Scholar]
- 36.Kollmann J, Brink-Jensen K, Frandsen SI, Hansen MK. Uprooting and burial of invasive alien plants: A new tool in coastal restoration? Restor. Ecol. 2011;19(3):371–378. doi: 10.1111/j.1526-100X.2009.00569.x. [DOI] [Google Scholar]
- 37.Jiao Y, Jia R, Sun Y, Yang G, Li Y, Huang J, Yuan L. In situ aerobic composting eliminates the toxicity of Ageratina adenophora to maize and converts it into a plant-and soil-friendly organic fertilizer. J. Hazard. Mater. 2021;410:124554. doi: 10.1016/j.jhazmat.2020.124554. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Liu Y, Liu H, Wang H, Yang D, Huangfu C. (2015) Impacts of four invasive Asteraceae on soil physico-chemical properties and AM fungi community. Am. J. Plant Sci. 2009;6:2734. doi: 10.4236/ajps.2015.617274. [DOI] [Google Scholar]
- 39.Yu FK, Huang XH, Duan CQ, He SZ, Zhang GS, Liu CE, Fu DG, Shao HB. Impacts of Ageratina adenophora invasion on soil physical–chemical properties of Eucalyptus plantation and implications for constructing agro-forest ecosystem. Ecol. Eng. 2014;64:130–135. doi: 10.1016/j.ecoleng.2013.12.050. [DOI] [Google Scholar]
- 40.Nirola R, Jha PK. Phytodiversity and soil study of Shiwalik Hills of Ilam, Nepal: An ecological perspective. Ecoprint. 2011;18:77–83. doi: 10.3126/eco.v18i0.9414. [DOI] [Google Scholar]
- 41.Lu JS, Shen T, Guo Z, Shen XW, Zheng SZ. The chemical constituents of Elsholtzia blanda. Acta Bot. Sin. 2001;43:545–550. [Google Scholar]
- 42.Singh TT, Sharma HM, Devi AR, Sharma HR. Plants used in the treatment of piles by the scheduled caste community of Andro village in Imphal East District, Manipur (India) J. Plant Sci. 2014;2:113–119. [Google Scholar]
- 43.Malla B, Chhetri RB. Indigenous knowledge on medicinal non-timber forest products (NTFP) in Parbat district of Nepal. Indo. Glob. J. Pharm. Sci. 2012;2:213–225. [Google Scholar]
- 44.Climate-data.org. Chitlang Climate (Nepal) (2021). https://en.climate-data.org/asia/nepal/central-development-region/chitlang-1061755/ (Accessed 2 April 2021).
- 45.Walkley A, Black IA. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37(1):29–38. doi: 10.1097/00010694-193401000-00003. [DOI] [Google Scholar]
- 46.Bremner JM, Mulvaney CS. Nitrogen-total. In: Page AL, Miller RH, Keeney DR, editors. Methods of Soil Analysis, Part 2. American Society of Agronomy; 1982. pp. 595–624. [Google Scholar]
- 47.Olsen SR, Cole CV, Watanable FS, Dean LA. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate. USDA Circular 939. U.S. Govt Printing Office; 1954. [Google Scholar]
- 48.Toth SJ, Prince AL. Estimation of cation-exchange capacity and exchangeable Ca, K, and Na contents of soils by flame photometer techniques. Soil Sci. 1949;67(6):439–446. doi: 10.1097/00010694-194906000-00003. [DOI] [Google Scholar]
- 49.Bajracharya, D. Experiments in Plant Physiology. 51-52 (Narosa Publishing House, New Delhi, India, 1999).